Abstract
Objective
To examine whether domain-specific patterns of cognitive impairment and trajectories of decline differed in patients with clinically diagnosed Parkinson disease dementia (PDD) (N = 29) and autopsy-confirmed dementia with Lewy bodies (DLB) (N = 58) or Alzheimer disease (AD) (N = 174) and to determine the impact of pooling patients with PDD and DLB in clinical trials targeting cognition.
Methods
Patients were matched on demographics and level of global cognitive impairment. Patterns of cross-sectional performance and longitudinal decline were examined in 4 cognitive domains: Visuospatial, Memory, Executive, and Language. Power analyses were performed to determine the numbers of participants needed to adequately power a hypothetical clinical trial to slow cognitive decline in pure PDD, pure DLB, or a mixed PDD/DLB group.
Results
Both DLB and PDD were more impaired and declined more rapidly than AD in the Visuospatial domain. Patients with PDD exhibited the most impairment and fastest decline in Executive, although patients with DLB also declined faster than AD. Memory was more impaired in AD than DLB and in both compared with PDD; however, all 3 groups declined at comparable rates. In contrast, PDD declined at a slower rate on Language measures than DLB or AD. Power analyses suggest that Visuospatial and Executive outcome measures would be most sensitive in PDD, but Memory and Language in DLB.
Conclusion
DLB and PDD differ from each other, and from AD, in a cognitive domain-specific manner. As such, different outcome measures may be most sensitive to detecting changes in DLB vs PDD, suggesting that the 2 should be analyzed separately in clinical trials.
Parkinson disease dementia (PDD) and dementia with Lewy bodies (DLB) are disorders characterized by cognitive impairment and motor symptoms associated with α-synuclein pathology in the brainstem nuclei, neocortex, and paralimbic regions.1–4 Although subtle neuropathologic differences may exist, no hallmark features easily distinguish the 2, so the disorders are often grouped as Lewy body disease (LBD).5,6 The clinical distinction is primarily order of symptom onset: in PDD, dementia begins at least 1 year after onset of Parkinson disease (PD), whereas in DLB, dementia precedes or co-occurs with parkinsonism. When both motor and cognitive symptoms are present, the disorders appear remarkably similar, and both may exhibit psychiatric symptoms, autonomic symptoms, REM sleep behavior disorder, and cognitive fluctuations.7–12 There are, however, potential differences in patterns of cognitive deficits in PDD and DLB,9,13–18 although the extent of these differences and how they evolve over time remains largely unknown. This presents a pressing problem as anti-synuclein therapeutic trials applicable to both disorders develop.19 If the conditions differ substantially in profiles of cognitive impairment and decline, pooling patients with PDD and DLB in an LBD trial may substantially reduce power to detect a targeted cognitive response by increasing within-group variability. With these issues in mind, the present study compares cognitive profiles and trajectories of decline in PDD and DLB and assesses the impact of pooling these groups in a hypothetical LBD clinical trial. Comparisons are also made to Alzheimer disease (AD), as concomitant AD is more common in DLB than PDD and may affect cognitive decline.15,20,21
Methods
Standard protocol approvals, registrations, and patient consents
The research protocol was reviewed and approved by the human subject's review board at the University of California, San Diego (UCSD). Informed consent was obtained at the point of entry into the Alzheimer's Disease Research Center (ADRC) longitudinal study from all patients or their caregivers consistent with California state law. Informed consent for autopsy was obtained at the time of death from the next of kin.
Participants
Participants for this study were selected from the longitudinal study and brain bank of the UCSD Shiley-Marcos Alzheimer's Disease Research Center, recruited from 1985 to 2014. A baseline visit was identified for all potential cases as the first ADRC evaluation that warranted a diagnosis of dementia. Cases with significant concomitant pathologic diagnoses (e.g., frontotemporal lobar degeneration and hippocampal sclerosis) were excluded from the selection process.
We identified 29 patients who met the clinical diagnostic criteria for PDD2: all initially presented with PD defined by the presence of 2 of 3 cardinal features22 and developed dementia more than 1 year later. The diagnosis of PD is likely to be highly accurate in these patients, given the excellent sensitivity and specificity provided by the clinical criteria.23,24 Other neurologic conditions that could produce parkinsonism were ruled out by neurologic examination. The average interval between the onset of parkinsonism and development of dementia was 8.9 years (SD = 8.0). Twelve of the 29 PDD cases were autopsied, and all had neuropathologic changes consistent with idiopathic PD.
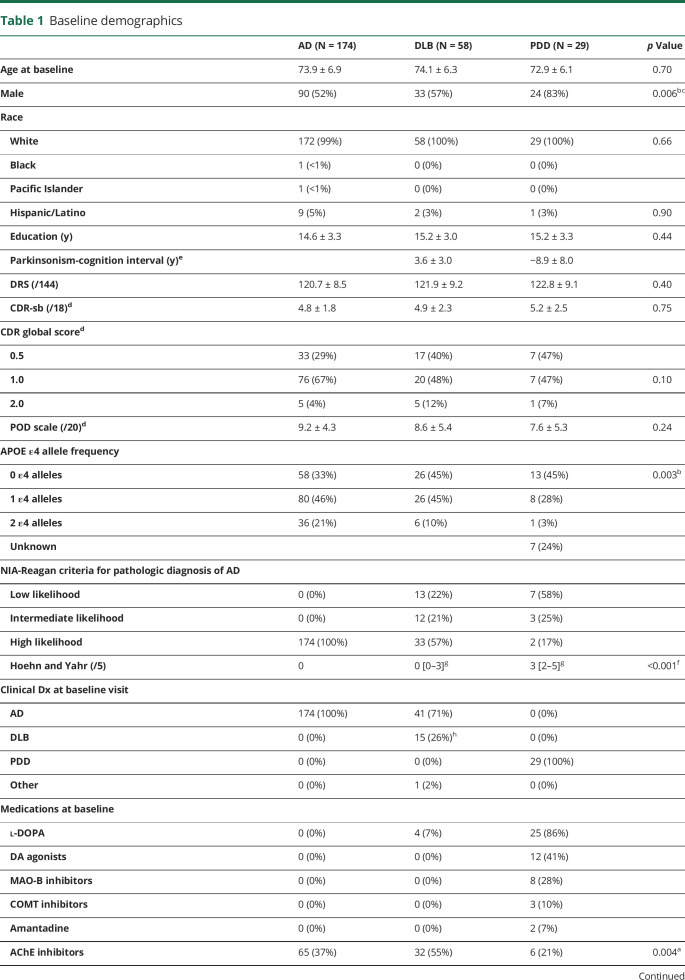
Autopsy-confirmed cases with DLB were matched 2:1 to PDD cases on demographics (age and education) and global cognitive performance at baseline using nearest neighbor propensity score matching.25 This resulted in 58 DLB cases. All initially presented with dementia only, and those that developed parkinsonism (i.e., at least 2 cardinal signs of PD) did so 4.7 years (SD = 3.6) after dementia onset. Autopsy-confirmed patients with AD were similarly matched 2:1 to the combined PDD/DLB groups, resulting in 174 AD cases (table 1).
Table 1.
Baseline demographics
Neuropathology
The brain was divided sagittally, and the left hemibrain was fixed in 10% buffered formalin, whereas the right hemibrain was sectioned coronally and then frozen at −70°C. Right hemibrain tissue blocks from the midfrontal, inferior parietal, and superior temporal cortices, primary visual cortex in the occipital cortex, hippocampus, amygdala, basal ganglia, substantia nigra, and cerebellum were removed and placed in 2% paraformaldehyde for subsequent thick sectioning by vibratome. Tissue blocks adjacent to these were stored at −70°C for subsequent immunoblot analysis for synaptic proteins and Aβ species (soluble and oligomers). Vibratome sections (40 μm thick) were stored in cryoprotective medium at −20°C for subsequent immunochemical studies. The formalin-fixed left hemibrain was serially sectioned in 1-cm slices, and tissue blocks from the regions described above were processed for histopathologic examination by hematoxylin-eosin (H&E), thioflavin S, and immunohistochemistry with antibodies to detect tau and β-amyloid deposits.
Brains were staged for degree of neurofibrillary tangle pathology by 1 pathologist (L.A.H.) using a modification of the Braak staging scheme.26 Estimates of neuritic plaque density were calculated using methods recommended by the Consortium to Establish a Registry for Alzheimer's Disease (CERAD).27 AD was operationalized using the NIA-Reagan consensus criteria for the postmortem diagnosis of AD, wherein Braak stage V-VI with moderately to severely dense neuritic plaques corresponds to high likelihood that dementia is due to AD.28 None of the AD cases had Lewy bodies or abnormal a-synuclein immunostaining in the neocortex or pigmented brainstem nuclei.
The DLB cases fell into either the limbic (transitional) or neocortical subtypes proposed in the 1996 consensus guidelines for the pathologic diagnosis of DLB,4 based on H&E staining and immunostaining with antibodies against α-synuclein.1,3,4 Cases were not classified as DLB if Lewy bodies were found only in the amygdala. Some of the DLB cases had sufficient concomitant AD pathology to warrant a secondary diagnosis of AD (historically called Lewy body variant of AD20). In a secondary analysis, cases were divided by the likelihood that a given combination of DLB subtype and Braak stage would result in a typical clinical DLB syndrome, determined according to the latest DLB criteria.1 Twelve patients with PDD from this cohort were autopsied, and in all cases, Lewy bodies were found in the locus ceruleus, substantia nigra, and/or nucleus basalis of Meynert, as well as in the neocortex.
Clinical evaluation
Participants had annual standardized clinical, neurologic, and neuropsychological evaluations as previously described.29,30 The clinical evaluation included review of history with the patient and/or informant, mental status testing, assessment of psychiatric symptoms (e.g., depression and psychosis including hallucinations), and assessment of functional impairment using the Pfeffer Outpatient Disability (POD) scale31 or the Functional Assessment Questionnaire (converted to corresponding POD scores). The Clinical Dementia Rating (CDR) total score and scores for each of 6 subdomains were computed (i.e., CDR sum of boxes). Hoehn and Yahr32 staging scores were determined for those with PDD or DLB.
The physical portion of the structured ADRC neurologic examination was used to assess degree of motor impairment. Many patients in the cohort were examined before the implementation of the Uniform Parkinson's Disease Rating Scale (UPDRS), but the vast majority of the ADRC structured neurologic examination overlapped features of the UPDRS. A 20-point motor impairment scale33 was derived from the ADRC examination based on the presence (1 point) or absence (0 points) of parkinsonian features (table 2).
Table 2.
Motor symptoms by diagnostic group
Global cognitive function was assessed with the Dementia Rating Scale.34 Further neuropsychological assessment included standardized measures of Memory (Wechsler Memory Scale [WMS] Visual Reproduction Test immediate and delayed recall; WMS-Revised Logical Memory Test; and Verbal List Learning Test), Language (30-item Boston Naming Test; Letter Fluency Test (F-A-S); Category Fluency Test [“animals,” “fruits,” and “vegetables”]; and Wechsler Adult Intelligence Scale–Revised [WAIS-R] Vocabulary Test), Executive functions (modified Wisconsin Card Sorting Test; Trail Making Test Parts A and B; and WAIS-R Digit Symbol Substitution Test), and Visuospatial abilities (Wechsler Intelligence Scale for Children–Revised Block Design Test; Visual Reproduction Test copy; Clock Drawing Test; and Cube Drawing Test). The Verbal List Learning Test was derived as the z-score for the immediate recall condition (summed across trials) of the Buschke Selective Reminding Task (12% of cases), the CERAD Word List Learning Test (2% of cases), or the California Verbal Learning Test (86% of cases). Performance on all measures was transformed to z-scores using reference values from a pool of 228 robust normal controls who were diagnosed as normal on their first ADRC evaluation and remained normal for the duration of their participation in the ADRC longitudinal study.
Consensus clinical diagnoses based on published criteria were made by 2 or more board-certified neurologists with expertise in dementia and movement disorders. Diagnosing neurologists were informed whether the neuropsychological assessment identified deficits in 2 or more domains of cognition, but not of individual test or cognitive domain scores. Probable DLB was diagnosed clinically based on the presence of dementia and at least 2 of 3 additional core features of mild parkinsonism, well-formed visual hallucinations, and fluctuations in consciousness or attention.3,4 Rapid eye movement (REM) sleep behavior disorder was also considered, but was not systematically assessed before its inclusion in the latest DLB guidelines.1 Cognitive decline had to precede or occur in conjunction with mild parkinsonism. The clinical diagnosis of PDD was based on the presence of at least 2 of the cardinal motor signs of PD, as well as objective cognitive deficits on neuropsychological tests and functional decline due to cognitive problems.2 Motor signs had to precede cognitive decline by more than 1 year. Probable AD was diagnosed according to National Institute of Neurological and Communicative Disorders-Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA)35 or National Institute on Aging-Alzheimer's Association (NIA-AA) criteria.36
Principal component analysis and generation of participant-level domain scores
The Very Simple Structure criteria37 suggested 4 as the optimal number of interpretable factors to extract from the baseline scores for the selected cognitive measure of the entire sample (n = 261). Principal component analysis (PCA) with varimax rotation resulted in 4 orthogonal rotated components, which were conceptually labeled “Visuospatial,” “Memory,” “Executive,” and “Language” based on the highest loadings for each measure (table 3).
Table 3.
Principal component analysis factor loadings
The PCA loadings matrix was used to generate individual domain scores for each participant at their baseline visit. A small number of missing values (less than 5% per test, except for Logical Memory, Cube, and Vocabulary, which were missing up to 13% of values) were imputed using fully conditional specification38 as implemented by the mice R statistical package.39 Five parallel imputations were performed and carried forward through the modeling analysis before being pooled for the final result. Each imputation was guided by diagnostic grouping, participant demographics, global cognitive test scores, and scores on other cognitive measures in the test's domain.
Although imputation of a small portion of baseline data adds little bias to the analysis,38 we were concerned about longitudinal imputation of missing values because the amount of missing data increased when multiple evaluations were considered. Thus, we did not generate longitudinal PCA-derived domain scores. We used an alternative approach wherein each test at each visit was assigned to only 1 cognitive domain, guided by the highest PCA loadings. Z-scores for all tests in that domain were averaged to create a domain composite score. If less than half of measures in any given domain were available for a patient, the visit for that patient was dropped from the analysis. The rate of dropped visits did not differ by diagnostic group.
Statistical analysis
Demographics and clinical characteristics were compared across groups using a 3-group analysis of variance for continuous variables with post hoc Tukey honest significant difference tests for significant results or a 3-group Fisher exact test for categorical variables with post hoc pairwise Fisher exact comparisons for significant results. Cross-sectional comparisons of cognitive domain scores across groups were performed using linear least squares regression adjusting for age, sex, and education.
Analyses of trajectories of cognitive decline across groups used data from baseline and 2 annual follow-up evaluations. Longitudinal linear mixed-effects models were used to assess how performance in each cognitive domain composite declined with time. Performance was modeled with fixed effects of diagnostic group, sex, years of education, age at baseline, baseline score on the measure of interest, and each term's interaction with time. Participant-specific intercepts and slopes were included as random effects. All analyses were performed in R version 3.6.0 using the lme4 package with restricted maximum likelihood estimation. Degrees of freedom for fixed effects were estimated by the Satterthwaite approximation as implemented in the package lmerTest.
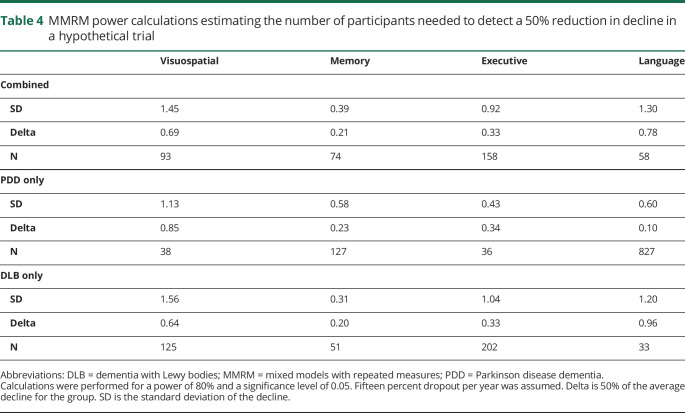
Power analyses for mixed models with repeated measures (MMRM) were performed as implemented in the longpower package.40 We determined the sample sizes needed in a hypothetical 2-year trial to detect a 50% reduction in decline on each cognitive domain composite (power 0.8, significance 0.05) if the sample consisted of a pooled group of patients with DLB and PDD (in a 1:1 ratio) or separate groups of patients with DLB or PDD. Each analysis assumed 15% attrition per year.
Data availability
Anonymized data and related documents such as study protocols and statistical analysis plans will be shared with any qualified investigator on request.
Results
Participant demographics
The PDD, DLB, and AD groups did not differ significantly in age, education, or global cognition at baseline (table 1). The PDD group had a higher median Hoehn and Yahr PD staging score than the DLB group. On our 20-point motor impairment scale, patients with AD averaged 1.5 (SD = 2.7), patients with DLB 4.5 (SD = 5.1), and patients with PDD 14.7 points (SD = 3.0) (tabulations in table 2). The PDD group had a higher percentage of males than the other groups, consistent with the known greater prevalence of PD in males than females.41 The AD group had a higher percentage of individuals with 1 or more APOE ε4 allele than the PDD group, but did not differ from the DLB group. All patients with PDD were on dopaminergic medication at baseline, with 86% taking l-DOPA, compared with only 7% of patients with DLB and no patients with AD. The percentage of patients with DLB taking acetylcholine esterase inhibitors at baseline was higher than in AD, and the percentage taking NMDA antagonists was higher than in the PDD group (in which no one was prescribed this medication). Antidepressant and antipsychotic use did not differ across groups.
Because many participants enrolled before the development of the DLB diagnostic criteria,4 only 26% of the pathologically confirmed DLB cases were clinically diagnosed with probable or possible DLB at baseline. However, retrospective chart review of all DLB cases revealed that 31% met the diagnostic criteria for probable DLB and 12% for possible DLB (i.e., presence of 1 core feature4) at baseline, and 35% meet these criteria at a subsequent visit, bringing the total of those ever meeting clinical criteria for DLB to 78%.
Cross-sectional cognitive profiles
Separate regression analyses for each domain at baseline revealed significant group differences in the Visuospatial, Memory, and Executive domains, but not in the Language domain (figure 1). PDD (β ± standard error [SE] = −0.81 ± 0.27, p = 0.003) and DLB (β ± SE = −1.11 ± 0.27, p = 0.003) were more impaired than AD in the Visuospatial domain, but did not differ from each other. In contrast, AD performed worse than DLB (β ± SE = 0.26 ± 0.27, p = 0.016), and much worse than PDD (β ± SE = 1.26 ± 0.15, p = 2.6 × 10−15), in the Memory domain. Furthermore, DLB performed worse than PDD (β ± SE = −0.98 ± 0.16, p = 5.9 × 10−9) in the Memory domain. PDD was more impaired than DLB (β ± SE = 0.59 ± 0.27, p = 0.029) or AD (β ± SE = −0.84 ± 0.24, p = 4.7 × 10−4) in the Executive domain, whereas AD and DLB did not differ from each other (p = 0.14).
Figure 1. Cross-sectional cognitive profiles.
Cross-sectional cognitive domain scores of the DLB, PDD, and AD groups at baseline, matched on demographics and global cognitive impairment. Statistical comparisons are made based on linear least squares regression adjusted for age, sex, and education. *p < 0.05; **p < 0.01; ***p < 0.001. AD = Alzheimer disease; DLB = dementia with Lewy bodies; PDD = Parkinson disease dementia.
Longitudinal cognitive decline
Linear mixed-effects modeling, adjusted for demographics and baseline performance, identified differences in the slope of 2-year decline in Visuospatial, Executive, and Language domain composites, but not the Memory domain (figure 2). DLB (β ± SE = −0.52 ± 0.14, p = 0.001) and PDD (β ± SE = −0.85 ± 0.20, p = 1.6 × 10−4) declined more rapidly than AD in the Visuospatial domain, but did not differ from each other. In contrast, PDD declined more rapidly than DLB (β ± SE = 0.41 ± 0.18, p = 0.024) or AD (β ± SE = −0.66 ± 0.16, p = 1.3 × 10−4) in the Executive domain, and DLB also declined more rapidly than AD (β ± SE = −0.25 ± 0.11, p = 0.027). DLB declined more rapidly than PDD (β ± SE = −0.62 ± 0.28, p = 0.04) in the Language domain. Despite large cross-sectional differences in the Memory domain composite, rates of decline in Memory did not differ across groups.
Figure 2. Longitudinal decline.
Longitudinal cognitive decline on each domain composite score in DLB, PDD, and AD over 2 years. Statistical comparisons are made between the slopes of decline, rather than absolute values, based on mixed-effects models adjusted for age, sex, education, and baseline performance. *p < 0.05; **p < 0.01; ***p < 0.001. AD = Alzheimer disease; DLB = dementia with Lewy bodies; PDD = Parkinson disease dementia.
Power calculations for a 2-year treatment trial with cognitive outcomes in LBD
Power calculations for MMRM analyses suggest that different numbers of participants with LBD would be needed to detect a 50% reduction in decline in cognition over 2 years (power = 0.80; p = 0.05) depending on the cognitive domain assessed and the make-up of the LBD sample (table 4). A 50% reduction in decline on the Visuospatial composite score could be reliably detected with 38 participants with PDD per group, whereas 125 participants with DLB or 93 participants with a 1:1 mixture of PDD and DLB would be needed per group. The Executive domain composite score would require only 36 patients with PDD per group, but 202 patients with DLB or 158 patients with PDD and DLB with a 1:1 mixture. In contrast, despite a lack of difference in the average rate of decline between groups, an outcome based on the Memory composite score would require 127 patients with PDD per group to detect a 50% reduction in decline, but only 51 patients with DLB or 74 patients with PDD and DLB with a 1:1 mixture. This is due to the much larger variance in the PDD group trajectories compared with DLB. Similarly, the Language composite score would require 827 patients with PDD per group compared with only 33 patients with DLB or 58 patients with PDD and DLB with a 1:1 mixture.
Table 4.
MMRM power calculations estimating the number of participants needed to detect a 50% reduction in decline in a hypothetical trial
Secondary analyses
Secondary analyses were performed to assess how stricter definitions of groups affected the results. First, if the analysis was restricted to pathologically confirmed PDD cases, the pattern of cross-sectional results remained unchanged; however, differences in decline between DLB and PDD no longer reached significance. Similarly, if the analysis was restricted to the 78% of DLB cases that met the clinical DLB criteria in life, the only changes were the loss of significance in the cross-sectional difference in memory between DLB and AD (now p = 0.09) and loss of the cross-sectional and longitudinal differences in executive function between DLB and PDD (now p = 0.08 and 0.10).
Finally, we divided the DLB group based on likelihood of each combination of DLB stage and Braak stage being associated with a typical DLB clinical syndrome.1 This resulted in significant differences such that Visuospatial ability was more impaired in high-likelihood DLB (p = 0.03), whereas Memory was more impaired in low/intermediate-likelihood DLB (p = 0.02). However, both DLB groups remained more impaired than the PDD group in Memory (both p < 1 × 10−10). Meanwhile, only high-likelihood DLB and PDD differed from AD on Visuospatial, Memory, and Executive abilities (all p < 0.05).
Longitudinally, the 2 DLB groups did not differ from each other in any rates of decline. However, only PDD and high-likelihood DLB differed from AD in Visuospatial (both p = 0.0001), whereas low/intermediate likelihood DLB declined more rapidly than AD in Memory (p = 0.03). All 3 other groups declined less rapidly on Executive function than PDD (all p < 0.05) and did not differ from each other. Finally, PDD declined marginally less rapidly than each of the other 3 groups on Language (all p = 0.05–0.06).
Discussion
Although DLB and PDD may be nearly indistinguishable pathologically, our work adds to the small literature suggesting that the 2 may differ cognitively in important ways, adding to the current debate on whether the conditions should be pooled or treated separately.5–13 Our findings showed that patients with DLB and PDD with comparable levels of global cognition differed in their domain-specific profiles of impairment and trajectories of decline. Specifically, we replicated previous work suggesting greater impairment and/or decline in Executive function in both DLB and PDD relative to AD.15 However, we also observed a previously unreported greater impairment and more rapid decline (adjusted for baseline score) of Executive function in PDD than DLB (PDD < DLB < AD). PDD and DLB were relatively more impaired and declined more rapidly than AD in Visuospatial ability, but did not differ from each other (DLB ≈ PDD < AD). These findings are in line with previous work suggesting that visuospatial impairments are similar in PDD and DLB, but are less pronounced in AD42; however, we did not observe the greater visuospatial impairments in DLB compared with PDD reported in other studies.43,44
Although there were large cross-sectional differences in Memory with AD and DLB worse than PDD (AD < DLB << PDD) consistent with previous reports,13,43,44 the 3 groups declined at nearly identical rates on average. This may be a function of the relatively mild level of dementia of the participants—a point when AD and DLB have already experienced most of their early memory decline, but PDD has not reached the memory deficits associated with later stages of this disorder.45 Conversely, although the 3 groups performed similarly in Language ability cross-sectionally at this mild level of global impairment, the patients with PDD declined less rapidly than either the patients with DLB or AD, in line with previous reports of greater language/verbal memory impairments in DLB and AD vs PDD in the later stages of disease.46
The observed double dissociations in both our cross-sectional and longitudinal results (i.e., worse Executive in PDD than DLB; worse Language and Memory in DLB than PDD) are likely a reflection of the subtle differences in pathology between DLB and PDD. Of interest, both cortical and subcortical pathology appear at comparable rates in the disorders, and as a result, the disorders do not differ in the proportion that may be characterized with a cortical or subcortical cognitive presentation.47 Nevertheless, the generally more severe brainstem pathology in PDD may account for the disproportionate impairments in Executive ability. In contrast, the greater concomitant AD pathology of the DLB cases may shift the cognitive impairment profile to be more similar to AD with greater Memory and Language impairments.
It should be noted, however, that differences in degree of AD pathology do not account for all of the observed cognitive differences between DLB and PDD. Despite a substantial reduction in statistical power, in secondary analyses separating the DLB group by level of concomitant AD pathology into those with high likelihood or low/intermediate likelihood of expressing a typical DLB syndrome, both remained more impaired cross-sectionally in Memory and declined slower in Executive and (marginally) Language than the PDD group. Furthermore, the 2 DLB groups did not differ from each other in any rates of decline, diverging only in cross-sectional Visuospatial and Memory impairments at baseline.
The observed differences in cognitive patterns and rates of decline are essential to consider in the design of clinical trials for LBD that may be targeting the common underlying α-synuclein pathology of PDD and DLB.48 If the baseline impairments and longitudinal trajectories of cognitive decline differ by domain, any clinical response to a compound may be apparent in one group but not the other, depending on the domain weighting of the cognitive outcome measure—resulting in a loss of statistical power to detect a change. To assess the impact of pooling DLB and PDD in clinical trials, we performed power calculations for the sample sizes that would be needed to detect a 50% reduction in decline over 2 years in only patients with DLB, only patients with PDD, and a 1:1 mixture of the 2. We find that because of the higher variability (SD) in the rates of decline in DLB over PDD in the Executive and Visuospatial domains, far fewer participants were needed to reach the desired power in a pure PDD sample than in either a pure DLB or a combined cohort. In contrast, the tiny effect size in the Language domain would require massive numbers of patients with PDD, despite the lower variability in PDD, whereas only a small handful of patients with DLB would be required to reach the same power. A somewhat similar picture emerged with Memory, where both groups had nearly identical rates of decline, but the greater variability in the patients with PDD resulted in the need for more than twice as many patients to reach the same power as for patients with DLB.
Our power analysis indicated that the most efficient approach to detecting changes in cognition is to focus on Visuospatial and Executive measures in PDD, but Memory and Language in DLB. For example, based on the high PCA loadings in both Visuospatial and Executive domains (table 3), the Wisconsin Card Sorting Test, Digit Symbol Substitution, and the Trail Making Test would make good choices to track changes in PDD. In contrast, measures with high loadings on Memory (e.g., Logical Memory) or Language (e.g., Category Fluency and Confrontation Naming) would provide the most power to track changes in DLB. Thus, despite the similarity in underlying pathology and drug targets, separating DLB and PDD groups and examining appropriate outcome measures should substantially improve the power of a trial to detect an effect on cognition.
Because a portion (22%) of our pathologically confirmed DLB group was never clinically diagnosed with DLB in life, it is unlikely they would be selected for a clinical trial of LBD. Therefore, we repeated our primary analysis restricting to the 78% of DLB cases that met the DLB criteria during life. The pattern of results and effect sizes was largely unchanged, albeit with reduced statistical power. We note that with promising work on effective α-synuclein biomarkers,49,50 it will soon be possible to restrict the DLB group in a clinical trial to those accurately diagnosed during life, and our full primary analysis is representative of the cognitive profiles and rates of decline that can be expected.
A major strength of this study is the use of autopsy-confirmed cases of DLB and AD given the marginal performance of the DLB clinical diagnostic criteria. The majority of previous work relied on these clinical diagnoses resulting in nearly guaranteed inclusion of pure AD cases in their DLB groups and inclusion of DLB cases with very subtle neurologic features of DLB in their AD groups. An additional strength of this study is the examination of multiple cognitive domains using multiple measures, which have not previously been studied longitudinally. Finally, longitudinal analyses allowed us to perform power calculations with direct implications for clinical trial design and patient care.
Several caveats should be considered. First, although the groups were carefully matched on demographics and global cognition, there were differences in degree of motor impairment and the use of parkinsonian and cognitive medications. It should be noted, however, that baseline performance and rate of decline for each cognitive domain did not correlate with degree of motor impairment in the combined DLB/PDD group. Furthermore, many of the participants with DLB enrolled before the most recent DLB criteria, and some even before the first DLB criteria were published. A chart review was preformed to retrospectively apply criteria to all DLB cases, but some information (especially REM behavior disorder) may not have been systematically collected. Finally, regarding power calculations, we note that neuropathologic diagnosis will not be available to clinical trials, and specificity of diagnoses in trials will generally be lower than those used here. Hence, power of clinical trials in practice will be lower than suggested by our power calculations. We also note the domain-specific composite scores reported here have not been validated as primary end points for clinical trials of neurodegenerative diseases. The purpose of the power calculation exercise is simply to characterize the potential improvement in trial efficiency to be gained by choosing the domains of cognitive function most sensitive to the respective neurodegenerative processes. Although specific sample sizes reported here are not reliable, the relative efficiency of domain-specific end point scales is well represented by this analysis.
In summary, this work characterized both the cross-sectional and longitudinal differences in the cognitive profiles of patients with PDD, DLB, and AD and examined the resulting effects on a hypothetical clinical trial. Our findings suggest that there may be substantial benefits to a trial by separately examining patients with DLB and PDD with outcome measures targeting the cognitive processes most affected in each.
Glossary
- ADRC
Alzheimer's Disease Research Center
- AD
Alzheimer disease
- CDR
Clinical Dementia Rating
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- DLB
dementia with Lewy bodies
- H&E
hematoxylin-eosin
- LBD
Lewy body disease
- MMRM
mixed models with repeated measures
- POD
Pfeffer Outpatient Disability
- PCA
principal component analysis
- PD
Parkinson disease
- PDD
Parkinson disease dementia
- UCSD
University of California, San Diego
- UPDRS
Uniform Parkinson's Disease Rating Scale
- WAIS-R
Wechsler Adult Intelligence Scale–Revised
- WMS
Wechsler Memory Scale
Appendix. Authors
Footnotes
Editorial, page 858
CME Course: NPub.org/cmelist
Study funding
This work was funded by NIH P30AG062429, R01AG049810, and UL1 TR001442.
Disclosure
D.S. Smirnov reports no disclosures. D. Galasko serves as editor for Alzheimer's Research and Therapy and as a paid consultant on Data Safety Monitoring Boards for Pfizer, Inc., Elan, Inc., and Balance Pharmaceuticals, Inc. S.D. Edland serves as a paid consultant on Data Safety Monitoring Boards for Lilly USA, LLC, and Suven Life Sciences Ltd. J.V. Filoteo and L.A. Hansen report no disclosures. D.P. Salmon serves as a paid consultant for Takeda Pharmaceuticals, Inc., Aptinyx, Inc., and Biogen, Inc. Go to Neurology.org/N for full disclosures.
References
- 1.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 3.McKeith I, Dickson D, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 4.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB). Neurology 1996;47:1113–1124. [DOI] [PubMed] [Google Scholar]
- 5.Tsuboi Y, Uchikado H, Dickson DW. Neuropathology of Parkinson's disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord 2007;13:S221–S224. [DOI] [PubMed] [Google Scholar]
- 6.Lippa C, Duda J, Grossman M, et al. DLB and PDD boundary issues. Neurology 2007;68:812–819. [DOI] [PubMed] [Google Scholar]
- 7.Richard I, Papka M, Rubio A, Kurlan R. Parkinson's disease and dementia with Lewy bodies: one disease or two? Mov Disord 2002;17:1161–1165. [DOI] [PubMed] [Google Scholar]
- 8.Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson's disease dementia the same disease? BMC Med 2018;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fields JA. Cognitive and neuropsychiatric features in Parkinson's and Lewy body dementias. Arch Clin Neuropsych 2017;32:786–801. [DOI] [PubMed] [Google Scholar]
- 10.Takemoto M, Sato K, Hatanaka N, et al. Different clinical and neuroimaging characteristics in early stage Parkinson's disease with dementia and dementia with Lewy bodies. J Alzheimer's Dis 2016;52:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuboi Y, Dickson DW. Dementia with Lewy bodies and Parkinson's disease with dementia: are they different? Parkinsonism Relat Disord 2005;11:S47–S51. [DOI] [PubMed] [Google Scholar]
- 12.Aarsland D, Ballard C, Halliday G. Are Parkinson's disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psych Neur 2004;17:137–145. [DOI] [PubMed] [Google Scholar]
- 13.Park K, Kim H, Cheon SM, Cha JK, Kim SH, Kim J. Dementia with Lewy bodies versus Alzheimer's disease and Parkinson's disease dementia: a comparison of cognitive profiles. J Clin Neurol Seoul Korea 2011;7:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filoteo JV, Maddox TW, Salmon DP, Song DD. Implicit category learning performance predicts rate of cognitive decline in nondemented patients with Parkinson's disease. Neuropsychology 2007;21:183–192. [DOI] [PubMed] [Google Scholar]
- 15.Janvin C, Larsen J, Salmon DP, Galasko D, Hugdahl K, Aarsland D. Cognitive profiles of individual patients with Parkinson's disease and dementia: comparison with dementia with Lewy bodies and Alzheimer's disease. Mov Disord 2006;21:337–342. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton JM, Salmon DP, Galasko D, et al. A comparison of episodic memory deficits in neuropathologically-confirmed Dementia with Lewy bodies and Alzheimer's disease. J Int Neuropsych Soc 2004;10:689–697. [DOI] [PubMed] [Google Scholar]
- 17.Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y. Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord 2004;19:60–67. [DOI] [PubMed] [Google Scholar]
- 18.Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen J. Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer's disease. J Neurol Neurosurg Psychiatry 2003;74:1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brundin P, Dave KD, Kordower JH. Therapeutic approaches to target alpha-synuclein pathology. Exp Neurol 2017;298:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen L, Salmon D, Galasko D, et al. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology 1990;40:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Merdes A, Hansen L, Jeste D, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 2003;60:1586–1590. [DOI] [PubMed] [Google Scholar]
- 22.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol 1993;50:140–148. [DOI] [PubMed] [Google Scholar]
- 23.Larsen JP, Dupont E, Tandberg E. Clinical diagnosis of Parkinson's disease. Proposal of diagnostic subgroups classified at different levels of confidence. Acta Neurol Scand 1994;89:242–251. [DOI] [PubMed] [Google Scholar]
- 24.Hughes A, Daniel S, Kilford L, Lees A. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011;42:1–28. [Google Scholar]
- 26.Hansen L, Terry R. Position paper on diagnostic criteria for Alzheimer disease. Neurobiol Aging 1997;18:S71–S73. [DOI] [PubMed] [Google Scholar]
- 27.Mirra S, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 28.National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging 1997;18:S1–S2. [PubMed] [Google Scholar]
- 29.Galasko D, Hansen LA, Katzman R, et al. Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Arch Neurol 1994;51:888–895. [DOI] [PubMed] [Google Scholar]
- 30.Salmon DP, Butters N. Neuropsychological assessment of dementia in the elderly. In: Katzman R, Rowe JW, editors. Principles of Geriatric Neurology. Philadelphia: FA Davis Company; 1992:144–163. [Google Scholar]
- 31.Pfeffer R, Kurosaki T, Harrah C, et al. A survey diagnostic tool for senile dementia. Am J Epidemiol 1981;114:515–527. [DOI] [PubMed] [Google Scholar]
- 32.Hoehn MM, Yahr MD. Parkinsonism onset, progression, and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton JM, Salmon DP, Raman R, et al. Accounting for functional loss in Alzheimer's disease and dementia with Lewy bodies: beyond cognition. Alzheimers Dement 2014;10:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattis S. Dementia Rating Scale (DRS). Odessa: Psychological Assessment Resources; 1988:1–2. [Google Scholar]
- 35.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 36.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Revelle W, Rocklin T. Very Simple structure: an alternative procedure for estimating the optimal number of interpretable factors. Multivar Behav Res 1979;14:403–414. [DOI] [PubMed] [Google Scholar]
- 38.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 39.van Buuren S, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 40.Lu K, Luo X, Chen PY. Sample size estimation for repeated measures analysis in randomized clinical trials with missing data. Int J Biostat 2008;4:1–16. [DOI] [PubMed] [Google Scholar]
- 41.Miller IN, Cronin-Golomb A. Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord 2010;25:2695–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosimann U, Mather G, Wesnes K, O'Brien J, Burn D, McKeith I. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology 2004;63:2091–2096. [DOI] [PubMed] [Google Scholar]
- 43.Mondon K, Gochard A, Marqué A, et al. Visual recognition memory differentiates dementia with Lewy bodies and Parkinson's disease dementia. J Neurol Neurosurg Psychiatry Res 2007;78:738–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brønnick K. Cognitive profile in Parkinson's disease dementia. In: Emre M, editor. Cognitive Impairment and Dementia in Parkinson's Disease, 2nd ed. Oxford: University Press; 2015:27–45. [Google Scholar]
- 45.Stern Y, Richards M, Sano M, Mayeux R. Comparison of cognitive changes in patients with Alzheimer's and Parkinson's disease. Arch Neurol 1993;50:1040–1045. [DOI] [PubMed] [Google Scholar]
- 46.Filoteo JV, Salmon DP, Schiehser DM, et al. Verbal learning and memory in patients with dementia with Lewy bodies or Parkinson's disease with dementia. J Clin Exp Neuropsyc 2009;31:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salmon D, Filoteo J. Neuropsychology of cortical versus subcortical dementia syndromes. Semin Neurol 2007;27:7–21. [DOI] [PubMed] [Google Scholar]
- 48.Wesnes KA, Aarsland D, Ballard C, Londos E. Memantine improves attention and episodic memory in Parkinson's disease dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry 2015;30:46–54. [DOI] [PubMed] [Google Scholar]
- 49.Groveman BR, Orru CD, Hughson AG, et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang UJ, Boehme AK, Fairfoul G, et al. Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson's disease. Mov Disord 2019;34:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data and related documents such as study protocols and statistical analysis plans will be shared with any qualified investigator on request.