Abstract
Objective
To investigate the association of triglyceride (TG) principal component scores with Alzheimer disease (AD) and the amyloid, tau, neurodegeneration, and cerebrovascular disease (A/T/N/V) biomarkers for AD.
Methods
Serum levels of 84 TG species were measured with untargeted lipid profiling of 689 participants from the Alzheimer's Disease Neuroimaging Initiative cohort, including 190 cognitively normal older adults (CN), 339 with mild cognitive impairment (MCI), and 160 with AD. Principal component analysis with factor rotation was used for dimension reduction of TG species. Differences in principal components between diagnostic groups and associations between principal components and AD biomarkers (including CSF, MRI and [18F]fluorodeoxyglucose-PET) were assessed with a generalized linear model approach. In both cases, the Bonferroni method of adjustment was used to correct for multiple comparisons.
Results
The 84 TGs yielded 9 principal components, 2 of which, consisting of long-chain, polyunsaturated fatty acid–containing TGs (PUTGs), were significantly associated with MCI and AD. Lower levels of PUTGs were observed in MCI and AD compared to CN. PUTG principal component scores were also significantly associated with hippocampal volume and entorhinal cortical thickness. In participants carrying the APOE ε4 allele, these principal components were significantly associated with CSF β-amyloid1–42 values and entorhinal cortical thickness.
Conclusion
This study shows that PUTG component scores were significantly associated with diagnostic group and AD biomarkers, a finding that was more pronounced in APOE ε4 carriers. Replication in independent larger studies and longitudinal follow-up are warranted.
Triglycerides (TGs) may represent a risk factor for Alzheimer disease (AD), yet this relationship is not well understood. TGs are lipids that are made up of 3 fatty acids (FAs). Blood total TG levels are measured in routine clinical checkups. Depending on the FAs that contribute to TG, many combinations of carbon chains and double bonds result, leading to >6,000 species encompassed by the term TG. Conflicting reports exist in the literature regarding TG homeostasis in AD. No relationship between AD and total TGs has been reported,1 while others suggest that elevated TGs early in life represented a risk factor for increased amyloidosis 20 years later.2,3 Finally, others found that individuals with probable AD had significantly decreased serum TG levels.4,5
APOE ε4 carrier status may serve as a risk factor for altered TG levels in AD. TGs are transported by lipoproteins, due to their lipophilic nature. ApoE regulates TG homeostasis by acting as a ligand for the TG-rich lipoproteins. The APOE ε4 allele is a major genetic risk factor for sporadic AD and is associated with a significant decrease in blood TG levels in AD.5 Here, we examined the association between TGs and AD biomarkers, with and without medication adjustment. We also investigated the effect of APOE ε4 by stratifying on APOE ε4 carrier status. This study shows that serum-based TG principal components (PCs) differ as a function of diagnostic group and are associated with AD biomarkers.
Methods
Study sample
All individuals used in this study were participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Phase 1 (ADNI-1), which is a longitudinal study aimed to explore clinical, genetic, imaging, and biological biomarkers for early AD progression. ADNI-1 is a multicenter consortium across 59 sites in the United States and Canada composed of ≈200 cognitively normal (CN) older adults, 400 adults diagnosed with mild cognitive impairment (MCI), and 200 adults diagnosed with probable AD, ranging in age from 47 to 91 years.6,7 In partnership with ADNI, the Alzheimer's Disease Metabolomics Consortium (ADMC) provided serum metabolic data for this group of participants. Demographic information, APOE ε4 carrier status, clinical information, neuroimaging, and CSF biomarker data were downloaded from the ADNI data repository (adni.loni.usc.edu).
Lipid analysis
Lipid analyses were performed as previously described.8 In summary, an untargeted lipidomics dataset was generated by the NIH West Coast Metabolomics Center (metabolomics.ucdavis.edu) using an ultra-high performance liquid chromatography–quadrupole time-of-flight mass spectrometer (Agilent, Santa Clara, CA) for 807 baseline serum samples from ADNI-1 participants of the ADMC initiative. Lipid species were annotated by matching accurate mass, isotope abundance, retention times, and tandem mass spectroscopy fragmentation spectra in LipidBlast9 in silico mass spectral library and measured by signal intensities on the precursor mass level. After data processing, the quality of data was assessed, and low-quality lipid species were removed. These quality control (QC) analyses resulted in 349 annotated lipids, including 84 TG species. The TG values obtained from the QC step were unadjusted and adjusted10 for the effect of medication use at baseline.
Neuroimaging analysis
MRI scans were processed before download as previously described.11,12 These scans were further processed locally with FreeSurfer version 5.1.13–15 Regions of interest were extracted, including the bilateral hippocampal volumes, entorhinal cortical thickness, and total intracranial volume (ICV). [18F]fluorodeoxyglucose (FDG) PET scans were preprocessed before download.13,16 Standardized uptake value ratio images were created by intensity normalization with the use of a pons reference region. Mean standardized uptake value ratio values were extracted for each participant from an overall cortical region representing regions where CN > AD from the full ADNI-1 cohort. White matter hyperintensity volumes were assessed with previously described methods.17,18
CSF biomarker analysis
The CSF biomarker data were downloaded from the ADNI data repository. As previously described,19 CSF measurements for β-amyloid (Aβ)1–42 peptide, total tau (t-tau), and tau phosphorylated at threonine 181 (p-tau) were obtained by the validated and highly automated Roche Elecsys (Basel, Switzerland) electrochemiluminescence immunoassays.
Cognitive assessment
We used the modified Alzheimer's Disease Assessment Scale cognition subscale (ADAS-Cog 13)20 and memory (ADNI-MEM) as indices of general cognitive performance.21,22 ADAS-Cog includes 11 items assessing memory, language, praxis, and orientation. ADAS-Cog 13 includes all items from ADAS-Cog plus delayed recall and cancellation tasks. ADNI-MEM is a memory composite score calculated from the items in several memory tasks, including the Rey Auditory Verbal Learning Test, ADAS-Cog, Logical Memory (passage recall), and the Mini-Mental Status Examination. Alternative forms were accounted for when applicable.21
A/T/N/V biomarkers
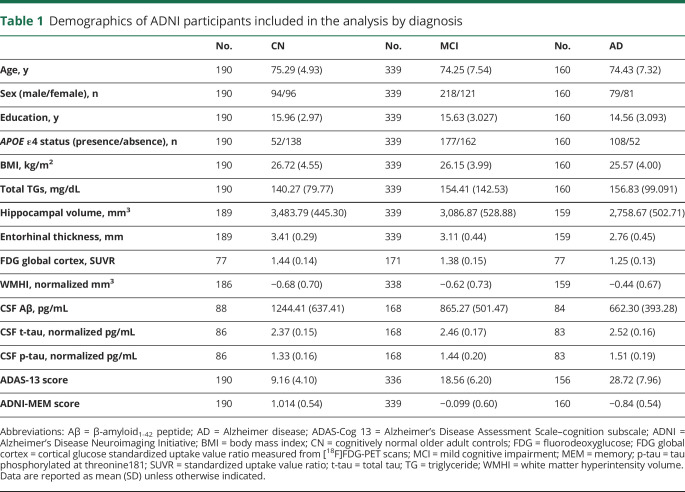
We used CSF Aβ1-42 levels as a biomarker of Aβ (A), CSF phosphorylated tau levels as a biomarker of tau (T), structural atrophy on MRI, FDG PET metabolism, and CSF t-tau levels as biomarkers of neurodegeneration (N) and white matter hyperintensity volume as a biomarker for microvascular disease burden (V), as described in the National Institute on Aging–Alzheimer's Association Research Framework.23 Some ADNI participants had missing data for specific A/T/N/V biomarkers. The specific number available for each biomarker is presented in table 1.
Table 1.
Demographics of ADNI participants included in the analysis by diagnosis
Medication adjustment
Triglyceride data were corrected for 41 major medication classes, including psychiatric, cardiovascular, antidiabetic, antihyperlipidemia, and dietary supplements. Medication intake was coded as present or absent, and a model-based evaluation determined the association of each drug with TG levels, which resulted in drug-corrected serum TG levels.
Dimension reduction was then performed on the 84 medication-adjusted TGs with PC analysis (PCA) as implemented in SPSS (version 24; SPSS Inc, Chicago, IL). A complete list of medications and procedures was provided previously.10
Education
Education was measured in years and used as a covariate for MRI endophenotypes and memory tests.
Statistical analysis
Dimension reduction was performed on the original 84 TGs using PCA. Initial PC extraction was followed by orthogonal rotation to yield PC-based factor scores. The number of PCs extracted was prespecified using the standard eigenvalues >1 criterion. Top contributors of each rotated PC were defined as those with a factor loading >0.8. All other contributors for each rotated PC (with a factor loading <0.8) were excluded from further consideration. A series of 6 separate generalized linear models (GLMs) were performed to assess diagnostic group (CN, MCI, AD) differences across all PCs. Bonferroni adjustment was used to correct for significance of diagnostic group differences across the 9 PCs of TGs . In reality, only 6 of the 9 TG PCs were brought forward for further consideration on the basis of our prespecified preprocessing criteria. Therefore, correcting for 9 PCs of analytes was considered conservative. A series of 14 separate post hoc linear regressions were performed to assess the association of 7 A/T/N/V biomarkers with PC3 and PC5. Bonferroni adjustment was used to correct for the 14 association analyses between PC3, PC5, and 7 A/T/N/V endophenotypes. A series of 14 separate post hoc linear regressions were used to assess the association of 7 A/T/N/V biomarkers with PC3 and PC5 in APOE ε4 carriers and noncarriers, separately. Bonferroni adjustment was used to correct for the 14 association analyses between PC3, PC5, and 7 A/T/N/V endophenotypes in APOE ε4 carriers and noncarriers, separately. Covariates of interest in analyses of the PCA results included age, sex, body mass index (BMI), total TGs, and APOE ε4 carrier status for linear regression. Additional covariates included years of education for cognitive performance and years of education and ICV for MRI biomarkers. For example, statistical models for PC5 and diagnosis, CSF Aβ1-42, and hippocampal volume appeared as follows:
Dependent variable (DV) = PC5; independent variable (IV) = diagnosis (CN, MCI, AD); covariates = age, sex, BMI, total TGs, and APOE ε4 carrier status;
DV = PC5; IV = CSF Aβ1-42; covariates = age, sex, BMI, total TGs, and APOE ε4 carrier status; and
DV = PC5; IV = hippocampal volume; covariates = age, sex, BMI, total TGs, APOE ε4 carrier status, ICV, and years of education.
We did not use total serum cholesterol levels as a covariate because total serum cholesterol levels had no effect on all 6 PCs. We did not use systolic and diastolic blood pressures as covariates because neither variable differed as a function of diagnosis or was associated with A/T/N/V biomarkers in our dataset. Significant associations were defined as p < 0.05 after Bonferroni adjustment to correct for multiple testing in individual analyses.
Whole-brain surface-based analysis
As previously described, we performed a series of GLM analyses of cortical thickness over the whole brain for PC3 and PC5 using SurfStat software (math.mcgill.ca/keith/surfstat/). We constructed a GLM using age, sex, APOE ε4 status, BMI, total TGs, years of education, and ICV as covariates. We corrected for multiple comparisons using the random field theory correction method at the p < 0.05 significance level.24
Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained at the time of enrollment for imaging and sample collection, and protocols of consent forms were approved by the Institutional Review Board at each participating site.
Data availability
All data used in the analyses reported here are available in the ADNI data repository (adni.loni.usc.edu).
Results
In the analysis, we included 689 ADNI participants who had baseline data for the 84 TGs (190 CN older adults, 339 with MCI, and 160 with AD), after QC procedures we removed participants with nonfasting status (n = 69). Demographic information is shown in table 1.
PCA for dimension reduction of TGs
Dimension reduction with PCA resulted in 9 PCs with eigenvalues >1 (data available from Dryad, table e-1, doi.org/10.5061/dryad.1dp8r8s). After selecting for the top contributors with a factor loading ≥0.8 in each component, 6 of 9 components remained for further analysis (data available from Dryad, table e-2).
Between-group differences in TG PCs
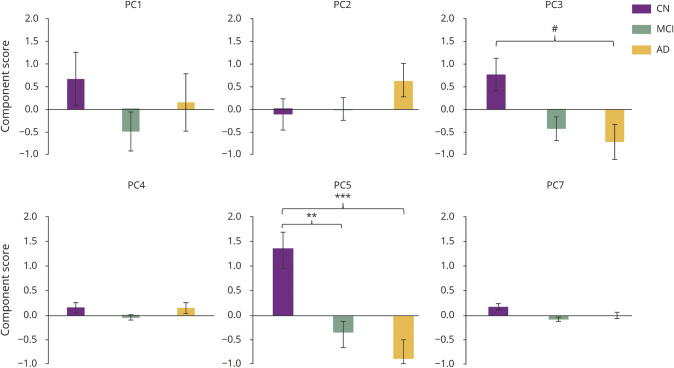
Figure 1 illustrates the profile of group differences between CN and MCI and AD for PCs (PC3 and PC5) after adjustment for multiple testing and covariates. We identified significant group differences in PC5 between CN and AD (p = 4.34E-04, Cohen d = 0.386) and between CN and MCI (p value = 1.83E-03, Cohen d = 0.313; figure 1). MCI and AD did not differ significantly for either PC3 or PC5. PC5 consists of 6 long-chain, polyunsaturated TGs (PUTGs), with all species containing ≥8 double bonds. Lower levels of PUTGs belonging to PC5 are seen in MCI and AD compared to CN (data available from Dryad, figure e-1 and table e-3, doi.org/10.5061/dryad.1dp8r8s). In addition, among the 6 PUTGs, 4 (TG 60:11, TG 58:9, TG 58:8, and TG 56:8) were significantly lower in MCI and AD compared to CN (p < 0.05) (data available from Dryad, figure e-1). We also identified suggestive group differences in PC3 between CN and AD (p = 0.0533, Cohen d = 0.179; figure 1). PC3 consisted of 7 PUTGs, with almost all species containing ≥2 double bonds. Similar to PC5, patients with AD compared to CN individuals showed lower component scores for PC3 (data available from Dryad, table e-4).
Figure 1. Group differences of PCs of TGs with diagnosis groups (CN, MCI, and AD).
A series of 6 separate generalized linear models were performed to assess diagnostic group (cognitively normal [CN], mild cognitive impairment [MCI], Alzheimer disease [AD]) differences across all principal components (PCs). Bonferroni adjustment was used to correct for significance of diagnostic group differences across the 9 PCs of triglycerides (TGs). Covariates included age, sex, body mass index, total TGs, and APOE ε4 status. Values are mean ± standard error. #p = 0.0533, *p < 0.05, **p < 0.01, ***p < 0.001.
Association of TG PCs with AD biomarkers
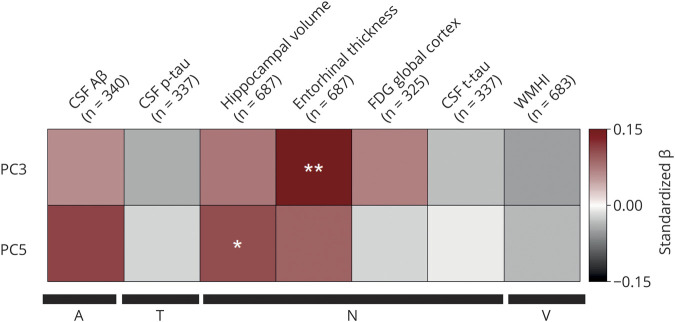
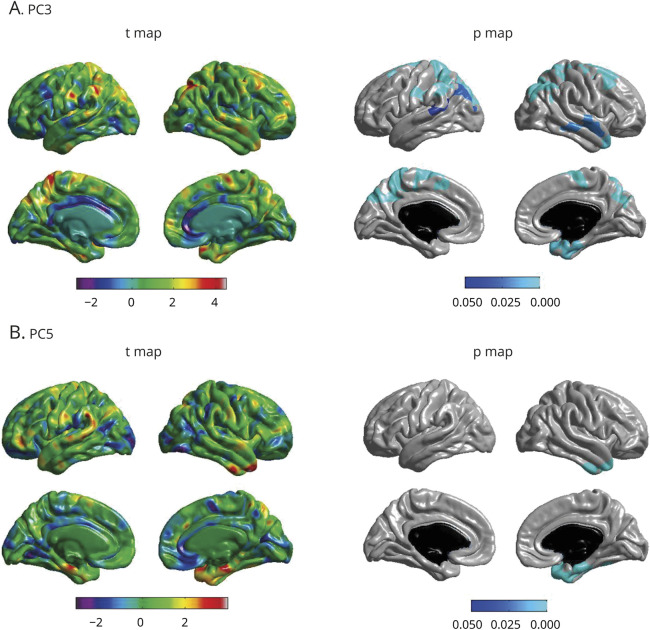
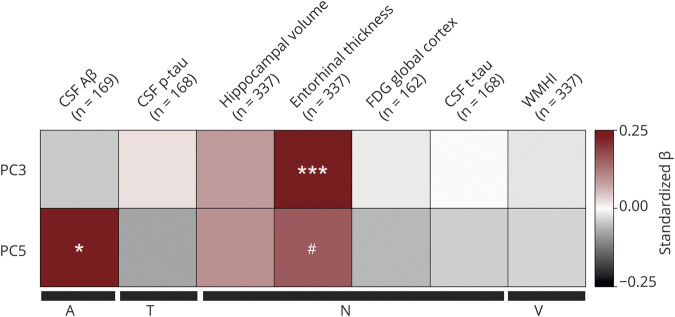
The 2 PCs that showed significant (PC5) or suggestive (PC3) diagnosis group differences were further investigated to assess their associations with continuous A/T/N/V biomarkers for AD. Figure 2 shows associations of PC3 and PC5 with AD biomarkers. Linear regression analysis indicated a significant association between PC5 and hippocampal volume (N) (p = 0.0243, standardized β = 0.135, adjusted R2 = 0.0683; figure 2) and PC3 and entorhinal thickness (N) (p value = 0.00363, standardized β = 0.132, adjusted R2 = 0.188; figure 2). Lower PC5 scores were associated with greater brain atrophy. Because PC5 is associated with hippocampal volume, we investigated the association of PC5 with cognitive performance using ADAS-Cog13 scores and ADNI-MEM. The analysis revealed associations between ADAS- Cog13 (p = 0.012, standardized β = −0.099, adjusted R2 = 0.0579) and ADNI-MEM (p = 0.00443, standardized β = 0.115, adjusted R2 = 0.0613) with PC5. We then performed a detailed whole-brain surface-based analysis of cortical thickness to investigate the effects of PC3 and PC5 on cortical atrophy in a spatially unbiased manner. Lower component scores of PC3 were significantly associated with reduced cortical thickness in bilateral frontal and parietal lobes and right temporal lobe, including the entorhinal cortex (p < 0.05; figure 3A). In addition, lower component scores of PC5 were significantly associated with reduced cortical thickness in right temporal lobe, including the entorhinal cortex (p < 0.05; figure 3B).
Figure 2. Association of TG PC3 and PC5 with A/T/N/V biomarkers for Alzheimer disease.
A series of 14 separate post hoc linear regressions were performed to assess the association of 7 amyloid, tau, neurodegeneration, and cerebrovascular disease (A/T/N/V) biomarkers with principal component (PC) 3 and PC5. Bonferroni adjustment was used to correct for the 14 association analyses between PC3, PC5, and 7 A/T/N/V endophenotypes. Covariates included age, sex, body mass index, total triglycerides (TGs), and APOE ε4 status for all A/T/N/V phenotypes. For MRI biomarkers, we also included years of education and intracranial volume as additional covariates. The y-axis colors represent standardized β values from the linear regression analysis, with shades of red indicating a positive standardized β value and gray scale indicating a negative standardized β value. Aβ = β-amyloid1-42 peptide; FDG global cortex = cortical glucose standardized uptake value ratio measured from [18F]fluorodeoxyglucose-PET scans; p-tau = tau phosphorylated at threonine 181; t-tau = total tau; WMHI = white matter hyperintensity total volume. *p < 0.05, **p < 0.01.
Figure 3. Whole-brain surface-based analysis of cortical thickness for PC3 and PC5.
(A) Whole-brain analysis of cortical thickness across the brain surface was performed to identify the association of 2 principal components (PCs) ([A] PC3 and [B] PC5) with brain structure shown as a t value map and a p value map. Statistical maps were thresholded using a random field theory for a multiple testing adjustment to a significance level of 0.05. Positive t values (red, yellow) indicate thicker cortical thickness. The p value for clusters indicates significant p values with the lightest blue color. Covariates included age, sex, body mass index, total triglycerides, APOE ε4 status, years of education, and intracranial volume.
Effect of APOE ε4 on TGs
To investigate the effect of APOE ε4 on TGs, we first investigated the presence of an interaction between APOE ε4 status and diagnosis and A/T/N/V biomarkers for AD with PCs. We did not find evidence of significant interactions for any PCs with APOE ε4 carrier status. We then performed an association analysis of PCs with diagnosis and A/T/N/V biomarkers for AD after stratifying on APOE ε4 carrier status. In both the APOE ε4 carrier group and APOE ε4 noncarrier group, we did not find any significant associations of PCs with diagnosis (data available from Dryad, figure e-2, doi.org/10.5061/dryad.1dp8r8s).
However, in the APOE ε4 carrier group, PC5 was significantly associated with CSF Aβ1-42 levels (p = 0.0359, standardized β = 0.228, adjusted R2 = 0.101; figure 4) and marginally associated with entorhinal cortical thickness (p value = 0.0537, standardized β = 0.156, adjusted R2 = 0.073; figure 4). PC3 was also significantly associated with entorhinal cortical thickness (p = 9.66E-04, standardized β = 0.192, adjusted R2 = 0.267) (figure 4). In the APOE ε4 noncarrier group, we did not identify any significant associations of PCs with A/T/N/V biomarkers for AD.
Figure 4. Association of PC3 and PC5 with A/T/N/V biomarkers for Alzheimer disease in the APOE ε4 carrier group.
A series of 14 separate post hoc linear regressions were used to assess the association of 7 amyloid, tau, neurodegeneration, and cerebrovascular disease (A/T/N/V) biomarkers with principal component (PC) 3 and PC5 in APOE ε4 carriers. Bonferroni adjustment was used to correct for the 14 association analyses between PC3, PC5, and 7 A/T/N/V endophenotypes. Covariates included age, sex, body mass index, total triglycerides, and APOE ε4 carrier status for all A/T/N/V phenotypes. For MRI biomarkers, we also included years of education and intracranial volume as additional covariates. The y-axis colors represent standardized β values from the linear regression analysis, with shades of red indicating a positive standardized β value and gray scale indicating a negative standardized β value. Aβ = β-amyloid1-42 peptide; FDG global cortex = cortical glucose standardized uptake value ratio measured from [18F]fluorodeoxyglucose-PET scans; p-tau = tau phosphorylated at threonine 181; t-tau = total tau; WMHI = white matter hyperintensity total volume. #p = 0.0537, *p < 0.05, **p < 0.01, ***p < 0.001.
Effect of medication use on TGs
Using TG values adjusted for the effect of medication use at baseline as a potential confounder, we repeated all analyses. All key findings remained significant after adjustment for medication use (data available from Dryad, figures e-3 and e-4, doi.org/10.5061/dryad.1dp8r8s).
Discussion
In this study, we found that long-chain PUTGs showed significant differences between diagnostic groups (CN, MCI, AD; data available from Dryad, tables e-3 and e-4, doi.org/10.5061/dryad.1dp8r8s). Lower PUTG component scores in MCI suggest that the changes occur during prodromal stages of disease, although longitudinal studies will be required to confirm this cross-sectional analysis. PUTG component scores were significantly associated with early-AD biomarkers, including hippocampal volume and entorhinal cortical thickness measured from MRI scans. In addition, we observed a significant APOE ε4 effect on PUTG components. In APOE ε4 carriers, we found significant positive associations between component scores of PUTGs and entorhinal cortical thickness and CSF Aβ1-42 levels but no significant associations in APOE ε4 noncarriers. The pattern of observed PC scores suggests that a reduction of PUTGs is associated with early-stage changes in AD.
The association of PUTGs with atrophy in the entorhinal cortex and hippocampus is noteworthy because these regions are affected in early stages of AD pathophysiology. Aβ accumulation is an important early change in AD and is associated with APOE ε4 carrier status. TGs have been shown to be particularly associated with Aβ. For example, mouse studies showed that serum TG levels were elevated before amyloid deposition,25 while human studies showed that increased serum TGs were associated with increased amyloidosis in CN individuals.3 Longitudinal studies showed that increased midlife TGs predicted amyloidosis and tau pathology 20 years later,2 providing additional evidence for the early influence of TGs in AD. Thus, it is particularly interesting that we found a selective association of PUTGs with CSF Aβ only in APOE ε4 carriers. The specific mechanistic role underlying the association of decreased PUTGs and early processes involved in AD pathogenesis remains to be determined, but our results suggest a relationship between a decrease in component scores of PUTGs and early-stage biomarkers in AD. Results remained significant after adjustment for medication use.
Thus, exposure to medication by AD and some patients with MCI does not appear to account for these results, although some roles cannot be entirely ruled out.
The relationship between TGs and Aβ may be explained through the role of TGs in the lipoprotein peripheral transport of Aβ. ApoE is a plasma protein that is involved in lipid transport and metabolism in lipoproteins, including TG-rich lipoproteins.26 Studies suggest that TG-rich lipoproteins may participate in peripheral Aβ transport and delivery, as evidenced by a study that showed that Aβ accumulated in TG-rich lipoproteins.27 As discussed, ApoE exists in 3 major isoforms, ApoE ε4, ApoE ε3, and ApoE ε2. ApoE ε4 has been shown to be unable to complex with Aβ and, peripherally, to associate with TG-rich very-low-density lipoproteins in contrast to high-density lipoproteins.28,29 These studies suggest an important relationship between Aβ and peripheral TG lipoprotein carriers that may provide further insight into aberrant serum PUTG levels in AD.
Omega-3 polyunsaturated FAs such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) have anti-inflammatory and neuroprotective roles, as evidenced by their associations with cognitive improvement in the elderly.30 In blood, polyunsaturated FAs travel in different states: esterified, bound to complex lipids (such as TGs) or lipoproteins, or free-floating. TGs are created by incorporating 3 FAs onto a carbon backbone, which can then serve to transport and store FAs. A previous study using the same TG dataset identified EPA and DHA as the primary polyunsaturated FAs contributing to PUTG FAs of PC5.31 Recently published studies have shown that serum DHA and EPA levels were decreased in AD32 and that polyunsaturated FA intake acted to reduce the risk for AD.33 We found that lower component scores of PUTGs were associated with poorer cognitive performance. Due to the importance of these polyunsaturated FAs in cognition and neuroinflammation, we believe our findings need further study to determine the overlapping mechanism that may underlie PUTG and polyunsaturated FA aberrations. PUTGs and, by extension, polyunsaturated FAs represent links in the gut-liver-brain axis. TGs are synthesized in the liver or absorbed from the gut and have recently been found to cross the blood-brain barrier and accumulate in the brain.34 PUFAs are rapidly and preferentially incorporated into TGs after postprandial ingestion, suggesting an important role for TGs in PUFA supplementation.35 The gut, liver, and brain communicate through neuronal, neuroimmune, and neuroendocrine pathways. These pathways overlap polyunsaturated FAs and AD and may serve an interesting direction to study PUTGs.
A lipid hypothesis has been proposed, suggesting that lipid oxidation is the initiating factor for late-onset AD.36 Lipid oxidation is a key early event in AD that precedes amyloid and neurofibrillary tangle deposition. When lipids are exposed to free radicals, they progress through autoperoxidation, when reactive oxidative species are released. Previous studies have shown that Aβ aggregation occurred more readily in membranes composed of oxidized lipids37 and that polyunsaturated lipids were most vulnerable to oxidative stress.38 Early-stage AD is characterized by an accumulation of Aβ, potentially resulting from impaired clearance of pathogenic species by microglia. Early microglial activity is neuroprotective, but progressive cytokine production from microglia, due to aging and disease progression, can reduce Aβ clearance and promote its accumulation. Peripheral inflammatory cytokines have been shown to interact with or pass through endothelial cells on the blood-brain barrier to activate microglia. It is well established that polyunsaturated FA intake is associated with an anti-inflammatory effect. Polyunsaturated FAs have been directly linked to the regulation of cytokines by decreasing the expression of proinflammatory pathways. Moreover, polyunsaturated FA intake has been shown to decrease microglial inflammatory activation of Aβ, supporting a neuroprotective role.39 Decreases in PUTGs could presumably result in decreased availability for polyunsaturated FAs and loss of neuroprotective metabolites, thereby suggesting the need for further study of this relationship.
Neural connections between the brain and the gut exist through the vagus nerve and enteric system. The vagus nerve originates in the brainstem and complexes onto the enteric plexus in the gut. These neural connections are important for gastric motility and have been implicated in relaying inflammatory, microbial, and nutrient information from the gut to the brain.40 Dietary fat has been shown to activate the vagus nerve and neuroimmunologic pathways.41 Recent work in Parkinson disease found that severance of the vagal trunk was protective against PD, suggesting that CNS invasion may occur from the gut through the vagus nerve.42 This study provides an interesting avenue of study for AD due to many links between PD and AD.
The gastrointestinal tract is home to >10 times the number of bacteria than cells in the human body, functioning in a mutualistic relationship with their human host.43 Gut microbiota are known to function in immune responses and nutrient absorption and to regulate gut motility.44,45
When gut microbiota are no longer homeostatic (gut dysbiosis), the CNS receives signals to activate inflammatory processes. Alterations in gut microbiota have been linked to neurologic conditions, including AD, in which gut dysbiosis was associated with memory dysfunction and decreased hippocampal neurogenesis.46 Polyunsaturated FA intake has been associated with changes in gut microbiota and intestinal excretion of mucosal defense factors that exhibit anti-inflammatory effects. The data suggest an interesting relationship between polyunsaturated FAs, gut microbiota, and AD and an interesting direction to study PUTGs.
We did not see a significant association between saturated TGs and AD, although we did find a significant difference in PUTG component scores between diagnostic groups. A recent meta-analysis revealed that, over time, saturated fat intake was associated with an increase in AD risk.47 It is possible that blood TG levels do not accurately represent long-term saturated TG intake and, rather, that BMI may better represent the effects of highly saturated diets over time.48 Further studies are required to investigate these findings.
We hypothesized that APOE ε4 carrier status may influence how TGs associate with AD endophenotypes, given evidence that APOE ε4 polymorphisms influence fasting serum TG levels and play a significant role in AD.49 We did see a significant association of PUTGs with AD endophenotypes in analyses stratified for APOE ε4 carrier status, but there was not an overall modifying effect of APOE ε4 carrier status on the association of PUTGs with AD endophenotypes. Although the absence of an overall main effect of APOE ε4 carrier status limits generalizability, the association of serum TG subspecies with AD endophenotypes in APOE ε4 carriers is of heuristic value and warrants further investigation in a larger, independent study.
There are some limitations to our study. The ADNI observational cohort was designed to be typical of participants who enroll in clinical trials but is not necessarily representative of the broader community as would be found in epidemiologically derived samples. Because ADNI is a largely white sample with a high mean education, the present results should not be generalized to community-based populations without further investigation. It will be important to repeat these analyses in more socioeconomically, educationally, and racially diverse samples.
The present study of cross-sectional associations is unable to support causal inferences regarding directionality. For example, it is plausible that dietary habits, nutritional intake, and neurodegeneration influence serum levels of these lipids. Longitudinal assays and clinical follow-up of the present cohort and replication in independent samples will be important to establish potential causal relationships between PUTGs and AD endophenotypes. In addition, functional experiments with lipidomic readouts will ultimately help to clarify underlying mechanisms. Model system studies are required to determine the role and mechanisms of specific classes of TGs in AD initiation and progression. Mechanistic investigations into the cause of decreased PUTG component scores using mouse models of AD may provide insight relevant to early detection and treatment for AD. Although we report modest effect sizes, these effects may indicate important biological relationships that warrant future pursuit with alternative or complementary study design. In multifactorial diseases (such as AD), high levels of discrimination and confidence are achieved through the combination of multiple weak individual markers into a single strong multivariate model, a well-known approach in drug and biomarker discovery.50 Future studies are warranted to investigate the potential role of PUTGs as early detection biomarkers and as targets for therapeutic development. Replication studies, longitudinal analyses, and functional experimental evaluation in model systems are all future directions for the PUTGs in this study.
Our study investigated the relationship between TG species, AD risk, and biomarkers for AD. This study showed decreased levels of highly unsaturated, long-chain TGs in MCI and AD compared to CN older adults. We also observed an association between decreased PUTG component scores and increased brain atrophy, decreased CSF Aβ concentration, and the effect of APOE ε4 carrier status on PUTG components in AD. Our study aimed to increase biological understanding of AD pathophysiology and mechanisms through association studies of TG profiles with AD endophenotypes. Our findings identify a specific subcategory of TGs, namely the PUTGs, that appear mechanistically relevant and provide the foundation for future work in therapeutic development. We report evidence that PUTGs are associated with an early prodromal stage of cognitive impairment (i.e., MCI) and early-stage biomarkers for AD. Replication in independent samples and longitudinal analysis is needed to elucidate any causal relationships.
Glossary
- A/T/N/V
amyloid, tau, neurodegeneration, and cerebrovascular disease
- Aβ
amyloid-β
- AD
Alzheimer disease
- ADAS-Cog13
Alzheimer's Disease Assessment Scale cognition subscale
- ADMC
Alzheimer's Disease Metabolomics Consortium
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- ADNI-MEM
ADNI memory
- ADNI-1
ADNI Phase 1
- BMI
body mass index
- CN
cognitively normal
- DHA
docosahexaenoic acid
- DV
dependent variable
- EPA
eicosapentaenoic acid
- FDG
[18F]fluorodeoxyglucose
- GLM
generalized linear model
- ICV
intracranial volume
- IV
independent variable
- MCI
mild cognitive impairment
- PC
principal component
- PCA
PC analysis
- PUTG
polyunsaturated TG
- QC
quality control
- t-tau
total tau
- TG
triglyceride
Appendix 1. Authors
Appendix 2.

Appendix 3.

Study funding
Funding for ADMC (led by Dr R. Kaddurah-Daouk at Duke University) was provided by National Institute on Aging grant R01AG046171, a component of the Accelerated Medicines Partnership for AD Target Discovery and Preclinical Validation Project (nia.nih.gov/research/dn/amp-ad-target-discovery-and-preclinical-validation-project), and National Institute on Aging grant RF1 AG0151550, a component of the Molecular Mechanisms of the Vascular Etiology of AD Consortium nia.nih.gov/news/decoding-molecular-ties-between-vascular-disease-and-alzheimers). Data collection and sharing for this project were funded by the ADNI (NIH grant U01 AG024904) and Department of Defense ADNI (Department of Defense award W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging and the National Institute of Biomedical Imaging and Bioengineering and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc; Biogen; Bristol-Myers Squibb Co; CereSpir, Inc; Cogstate; Eisai Inc; Elan Pharmaceuticals, Inc; Eli Lilly and Co; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc; Fujirebio; GE Healthcare; IXICO Ltd; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co, Inc; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corp; Pfizer Inc; Piramal Imaging; Servier; Takeda Pharmaceutical Co; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Samples from the National Cell Repository for AD, which receives government support under a cooperative agreement grant (U24 AG21886) awarded by the National Institute on Aging, were used in this study. The work of consortium investigators was also supported by multiple National Institute on Aging grants (U01AG024904-09S4, P50NS053488, R01AG19771, P30AG10133, P30AG10124, R03AG054936, K01AG049050), the National Library of Medicine (R01LM011360, R01 LM012535), and the National Institute of Biomedical Imaging and Bioengineering (R01EB022574). Additional support came from the Alzheimer's Association, the Indiana Clinical and Translational Science Institute, and the Indiana University-IU Health Strategic Neuroscience Research Initiative. Finally, this work was supported by the Indiana Clinical and Translational Sciences Institute funded by grant UL1TR002529 from NIH, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award, and the National Institute on Aging of the NIH under award F30AG063449. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
M.M. Bernath, S. Bhattacharyya, K. Nho, D.K. Barupal, O. Fiehn, R. Baillie, S.L. Risacher, M. Arnold, and T. Jacobson report no relevant disclosures. J.Q. Trojanowski may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is a coinventor and received revenue from the sale of Avid to Eli Lily as a coinventor on imaging-related patents submitted by the University of Pennsylvania. L.M. Shaw receives research funding from NIH (U01 AG024904; R01 MH 098260; R01 AG 046171; 1RF AG 051550) and Michael J. Fox Foundation for Parkinson’s Research; is a consultant for Eli Lilly, Novartis, and Roche; and provides QC oversight for the Roche Elecsys immunoassay as part of responsibilities for the ADNI study. M.W. Weiner reports stock/stock options from Elan and Synarc and travel expenses from Novartis, Tohoku University, Fundacio Ace, Travel eDreams, MCI Group, NSAS, Danone Trading, ANT Congress, NeuroVigil, CHRU-Hopital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Lilly, University of California, San Diego–ADNI, Paris University, Institut Catala de Neurociencies Aplicades, University of New Mexico School of Medicine, Ipsen, Clinical Trials on Alzheimer's Disease, Pfizer, and AD PD meeting. P.M. Doraiswamy received research grants from Lilly, Neuronetrix, Avanir, Alzheimer's Drug Discovery Foundation, ADNI, Department of Defense, Office of Naval Research, NIH, and Bausch; received speaking or advisory fees from Anthrotronix, Neuroptix, Genomind, MindLink and CEOs Against Alzheimer's; owns shares in Muses Labs, Anthrotronix, Evidation Health, Turtle Shell Technologies, and Advera Health Analytics; serves on the board of Baycrest, Apollo Hospitals, Goldie Hawn Foundation, and Live Love Laugh Foundation; and is a coinventor (through Duke) on patents relating to dementia biomarkers and therapies. R. Kaddurah-Daouk is inventor on key patents in the field of metabolomics, including applications for Alzheimer disease. A.J. Saykin reports investigator-initiated research support from Eli Lilly; received consulting fees and travel expenses from Eli Lilly and Siemens Healthcare; is a consultant to Arkley BioTek; and receives support from Springer-Nature Publishing as editor in chief of Brain Imaging and Behavior. Go to Neurology.org/N for full disclosures.
Publication history
This manuscript was previously published in bioRxiv: doi: 10.1101/441394. Received by Neurology April 2, 2019. Accepted in final form November 19, 2019.
References
- 1.Østergaard SD, Mukherjee S, Sharp SJ, et al. Associations between potentially modifiable risk factors and Alzheimer disease: a mendelian randomization study. PLoS Med 2015;12:e1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nägga K, Gustavsson AM, Stomrud E, et al. Increased midlife triglycerides predict brain β- amyloid and tau pathology 20 years later. Neurology 2018;90:e73–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi HJ, Byun MS, Yi D, et al. Association between serum triglycerides and cerebral amyloidosis in cognitively normal elderly. Am J Geriatr Psychiatry 2016;24:604–612. [DOI] [PubMed] [Google Scholar]
- 4.Lepara O, Valjevac A, Alajbegović A, Zaćiragić A, Nakas-Ićindić E. Decreased serum lipids in patients with probable Alzheimer's disease. Bosn J Basic Med Sci 2009;9:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall K, Murrell J, Ogunniyi A, et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology 2006;66:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saykin AJ, Shen L, Foroud TM, et al. Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement 2010;6:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saykin AJ, Shen L, Yao X, et al. Genetic studies of quantitative MCI and AD phenotypes in ADNI: progress, opportunities, and plans. Alzheimers Dement 2015;11:792–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cajka T, Fiehn O. LC-MS-Based lipidomics and automated identification of lipids using the LipidBlast in-silico MS/MS library. Methods Mol Biol 2017;1609:149–170. [DOI] [PubMed] [Google Scholar]
- 9.Kind T, Liu KH, Lee DY, DeFelice B, Meissen JK, Fiehn O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat Methods 2013;10:755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toledo JB, Arnold M, Kastenmüuller G, et al. Metabolic network failures in Alzheimer's disease: a biochemical road map. Alzheimers Dement 2017;13:965–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jack CR, Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement 2010;6:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risacher SL, Kim S, Shen L, et al. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI). Front Aging Neurosci 2013;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis, II: inflation, flattening, and a surface-based coordinate system. NeuroImage 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 15.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. NeuroImage 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 16.Jagust WJ, Bandy D, Chen K, et al. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimer's Demen 2010;6:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer Disease Neuroimaging Initiative. Arch Neurol 2010;67:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz C, Fletcher E, DeCarli C, Carmichael O. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without FLAIR. Inf Process Med Imaging 2009;21:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-β PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;14:1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope: the Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(suppl 2):S13–S21. [PubMed] [Google Scholar]
- 21.Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 2012;6:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbons LE, Carle AC, Mackin RS, et al. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 2012;6:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nho K, Risacher SL, Crane PK, et al. Voxel and surface-based topography of memory and executive deficits in mild cognitive impairment and Alzheimer's disease. Brain Imaging Behav 2012;6:551–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess BL, McIsaac SA, Naus KE, et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer's disease mouse models with abundant A beta in plasma. Neurobiol Dis 2006;24:114–127. [DOI] [PubMed] [Google Scholar]
- 26.Mensenkamp AR, Jong MC, van Goor H, et al. Apolipoprotein E participates in the regulation of very low density lipoprotein-triglyceride secretion by the liver. J Biol Chem 1999;274:35711–35718. [DOI] [PubMed] [Google Scholar]
- 27.James AP, Pal S, Gennat HC, Vine DF, Mamo JCL. The incorporation and metabolism of amyloid-beta into chylomicron-like lipid emulsions. J Alzheimers Dis 2003;5:179–188. [DOI] [PubMed] [Google Scholar]
- 28.Weisgraber KH. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res 1990;31:1503–1511. [PubMed] [Google Scholar]
- 29.Zhou Z, Smith JD, Greengard P, Gandy S. Alzheimer amyloid-beta peptide forms denaturant-resistant complex with type epsilon 3 but not type epsilon 4 isoform of native apolipoprotein E. Mol Med 1996;2:175–180. [PMC free article] [PubMed] [Google Scholar]
- 30.Kesse-Guyot E, Péneau S, Ferry M, et al. Thirteen-year prospective study between fish consumption, long-chain n-3 fatty acids intakes and cognitive function. J Nutr Health Aging 2011;15:115–120. [DOI] [PubMed] [Google Scholar]
- 31.Barupal DK, Fan S, Wancewicz B, et al. Generation and quality control of lipidomics data for the Alzheimer's Disease Neuroimaging Initiative cohort. Scientific Data 2018;5:180263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shang J, Yamashita T, Fukui Y, et al. Different associations of plasma biomarkers in Alzheimer's disease, mild cognitive impairment, vascular dementia, and ischemic stroke. J Clin Neurol 2018;14:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr 2016;103:330–340. [DOI] [PubMed] [Google Scholar]
- 34.Banks WA, Farr SA, Salameh TS, et al. Triglycerides cross the blood–brain barrier and induce central leptin and insulin receptor resistance. Int J Obes 2018;42:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plourde M, Chouinard-Watkins R, Vandal M, et al. Plasma incorporation, apparent retroconversion and β-oxidation of 13C-docosahexaenoic acid in the elderly. Nutr Metab 2011;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper JL. Dietary lipids in the aetiology of Alzheimer's disease. Drugs Aging 2003;20:399–418. [DOI] [PubMed] [Google Scholar]
- 37.Koppaka V, Axelsen PH. Accelerated accumulation of amyloid beta proteins on oxidatively damaged lipid membranes. Biochemistry 2000;39:10011–10016. [DOI] [PubMed] [Google Scholar]
- 38.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, α- tocopherol, and ascorbate. Arch Biochem Biophys 1993;300:535–543. [DOI] [PubMed] [Google Scholar]
- 39.Hopperton KE, Trépanier MO, Giuliano V, Bazinet RP. Brain omega-3 polyunsaturated fatty acids modulate microglia cell number and morphology in response to intracerebroventricular amyloid-β 1-40 in mice. J Neuroinflammation 2016;13:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miao FJP, Green PG, Levine JD. Mechanosensitive duodenal afferents contribute to vagal modulation of inflammation in the rat. J Physiol 2004;554:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luyer MD, Greve JWM, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med 2005;202:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svensson E, Horváth-Puhó E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol 2015;78:522–529. [DOI] [PubMed] [Google Scholar]
- 43.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005;307:1915–1920. [DOI] [PubMed] [Google Scholar]
- 44.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012;336:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004;101:15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desbonnet L, Clarke G, Traplin A, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun 2015;48:165–173. [DOI] [PubMed] [Google Scholar]
- 47.Ruan Y, Tang J, Guo X, Li K, Li D. Dietary fat intake and risk of Alzheimer's disease and dementia: a meta-analysis of cohort studies. Curr Alzheimer Res 2018;15:869–876. [DOI] [PubMed] [Google Scholar]
- 48.Hariri N, Gougeon R, Thibault L. A highly saturated fat-rich diet is more obesogenic than diets with lower saturated fat content. Nutr Res 2010;30:632–643. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho-Wells AL, Jackson KG, Gill R, et al. Interactions between age and apoE genotype on fasting and postprandial triglycerides levels. Atherosclerosis 2010; 212:481–487. [DOI] [PubMed] [Google Scholar]
- 50.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 2013;9:280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in the analyses reported here are available in the ADNI data repository (adni.loni.usc.edu).