Abstract
Objective
To evaluate whether structural and functional changes occur in the afferent visual system of patients with stiff-person syndrome (SPS) and whether these changes correlate with disease burden, given the high concentration of γ-aminobutyric acid receptors, which are generally thought to be involved in SPS pathogenesis, in the retina.
Methods
In this single-center, cross-sectional study, patients with SPS and healthy controls (HCs) underwent optical coherence tomography (OCT), with a subset undergoing high- and low-contrast visual acuity (VA) assessments. Burden of disease was assessed via the number of body regions affected. Individuals with uncontrolled hypertension or comorbid neurologic or ophthalmologic disorders were excluded. Statistical analyses were performed using mixed-effects linear regression models.
Results
Thirty-five patients with SPS and 40 age- and sex-matched HCs underwent OCT. A subset of 23 patients with SPS and 28 HCs underwent VA assessments. Relative to HCs, patients with SPS had lower ganglion cell + inner plexiform layer (GCIPL) thicknesses (SPS: 74.36 µm [SD 5.7]; HCs: 76.33 µm [SD 4.2]; p = 0.005), inner nuclear layer thicknesses (SPS: 44.37 µm [SD 2.7]; HCs: 45.18 µm [SD 2.2]; p = 0.042), and 100% (SPS: 53 [SD 9.6]; HCs: 57.5 [SD 6.1]; p = 0.005), 2.5% (SPS: 24.35 [SD 10.1]; HCs: 30.16 [SD 7.7]; p = 0.006), and 1.25% contrast (SPS: 16.41 [SD 10.6]; HCs: 20.84 [SD 8.6]; p = 0.034) letter acuity scores. GCIPL thicknesses correlated with the number of body regions affected in SPS (decrease of 1.25 µm [95% confidence interval, −2.2 to −0.3 µm; p = 0.008] per additional body region affected).
Conclusions
Retinal neuronal pathology can occur in SPS. OCT may have utility as a biomarker of disease burden in SPS.
Stiff-person syndrome (SPS) is a rare neuroimmunologic disorder characterized by spasms and rigidity, predominantly of the axial and lower extremity muscles. Although the primary etiology of SPS is unknown, anti–glutamic acid decarboxylase (anti-GAD65) antibodies purportedly targeting inhibitory γ-aminobutyric acid (GABAergic) neurons have been reported in 60%–80% of cases, and the muscle hyperactivity observed is attributed to impaired GABA descending pathways.1 The retina is highly enriched with GABAergic neurons—while amacrine cells that are immunoreactive to GABA and express the isoform GAD65 are located primarily in the inner nuclear layer (INL), the inner plexiform layer comprises a large number of dendrites of these amacrine cells—these include starburst amacrine cells, which synapse directly on ganglion cells.2,3 Although visual dysfunction has been observed in isolated cases of SPS, to date, visual outcome measures have not been evaluated at a cohort level.2,4 Optical coherence tomography (OCT) has emerged as a reliable and reproducible high-resolution imaging technique that facilitates quantification of discrete axonal and neuronal retinal layers.5 In particular, the composite thickness of the ganglion cell + inner plexiform layer (GCIPL) has been observed to correlate strongly with visual loss and disability across a spectrum of optic neuropathies.6,7 Hence, we sought to assess structural and functional changes in the afferent visual system of patients with SPS, and determine whether these changes correlate with disease burden.
Methods
Standard protocol approvals, registrations, and patient consents
The study was approved by the Johns Hopkins institutional review board. All participants provided written informed consent.
Data availability
Anonymized data used for this study are available from the corresponding authors on reasonable request.
Study design and participants
An SPS cohort and an age- and sex-matched healthy control (HC) cohort were recruited by convenience sampling from the Johns Hopkins SPS Center from 2009 to 2016. Cross-sectional comparisons of visual outcome measures were performed between patients with SPS and HCs. Individuals with refractive errors greater than ±6 diopters, history of ocular surgery, glaucoma, uncontrolled hypertension, or other neurologic or ophthalmologic disorders were excluded.
Data acquisition
Participants underwent spectral-domain OCT (Cirrus HD-OCT; Carl Zeiss Meditec, Dublin, CA), with retinal layer thicknesses derived utilizing a validated, automated segmentation algorithm, as previously described.8 Scans with signal strength below 7/10, or with artifact, were excluded, in accordance with OSCAR-IB criteria.9 A subset of participants underwent 100% visual acuity (VA) assessment using Early Treatment Diabetic Retinopathy Study charts and 2.5% and 1.25% contrast VA assessments using Sloan letter charts. Disease involvement of 6 distinct body regions—torso, legs, arms, face, oculomotor, and cerebellum—was recorded systematically as clinical surrogates of disease burden. Serum anti-GAD65 antibody titers were measured in patients with SPS within 6 months of visual assessments.
Statistical analyses
Statistical analyses were performed using STATA version 13 (StataCorp, College Station, TX). Comparisons of demographic characteristics were performed using Kruskal-Wallis test (age) and Fisher exact test (sex, race, concurrent diabetes mellitus). Comparisons of SPS and HC visual outcome measures were performed using mixed-effects linear regression models, accounting for within-subject intereye correlations. Unadjusted analyses were performed, as well as multivariable analyses adjusting for age, sex, and diabetes history. In addition, multivariable analyses restricted to the SPS cohort were performed, comparing disease burden to visual outcome measures, adjusting for age, sex, diabetes history, disease duration, and history of immunomodulatory therapy. We defined statistical significance as p < 0.05.
Results
Characteristics of the study population
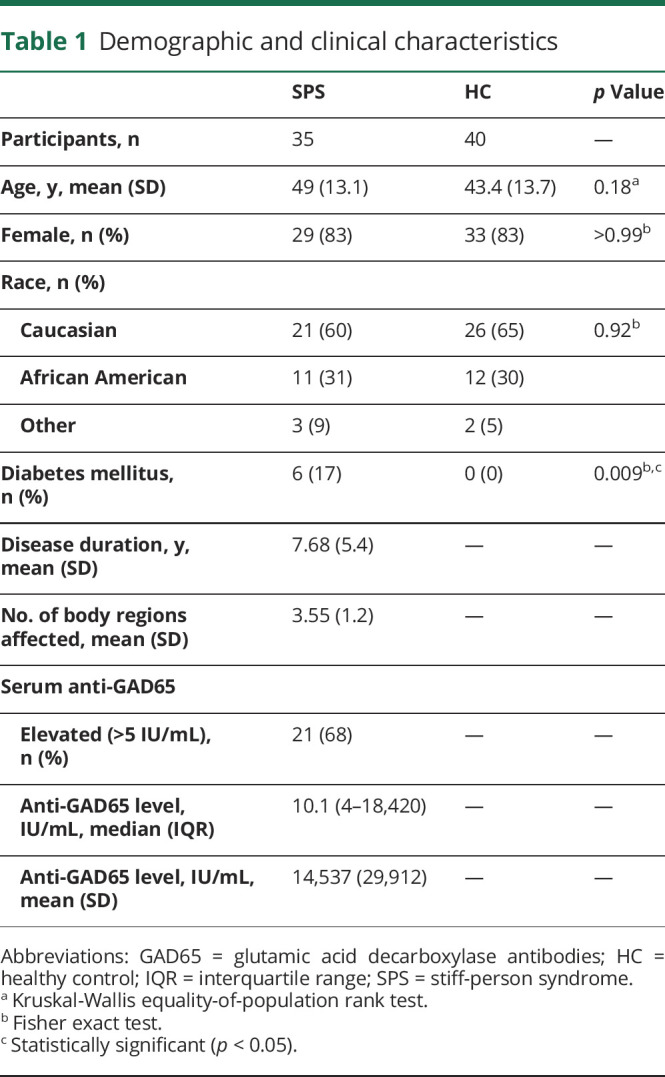
Thirty-five patients with SPS (mean [SD] age, 49 years [13.1]; 29 [83%] female) and 40 HCs (mean [SD] age, 43.4 years [13.7]; 33 [83%] female) underwent OCT. Ten OCT images (7 eyes from patients with SPS; 3 eyes from HCs) were excluded from analyses due to inadequate scan quality. A subset of 23 patients with SPS and 28 HCs underwent VA assessments. A summary of demographic and clinical characteristics is shown in table 1.
Table 1.
Demographic and clinical characteristics
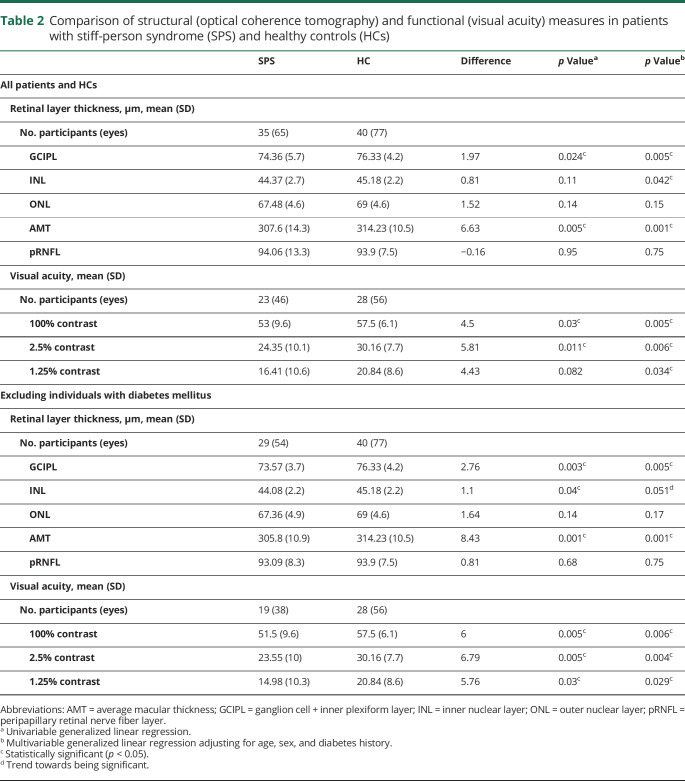
Comparison of visual outcome measures between patients with SPS and HCs
In multivariable models, patients with SPS had lower GCIPL (p = 0.005) and INL (p = 0.042) thicknesses compared to HCs. Patients with SPS had lower scores on VA testing at 100% (p = 0.005), 2.5% (p = 0.006), and 1.25% contrast (p = 0.034) (table 2).
Table 2.
Comparison of structural (optical coherence tomography) and functional (visual acuity) measures in patients with stiff-person syndrome (SPS) and healthy controls (HCs)
We performed sensitivity analyses excluding individuals with concurrent diabetes mellitus (DM; n = 6) to reduce the potentially confounding effect of DM on visual measures. These analyses demonstrated lower GCIPL thicknesses (p = 0.005) and lower scores on 100% (p = 0.006), 2.5% (p = 0.004), and 1.25% (p = 0.029) VA testing in patients with SPS relative to HCs.
Correlation of visual outcome measures with SPS disease burden
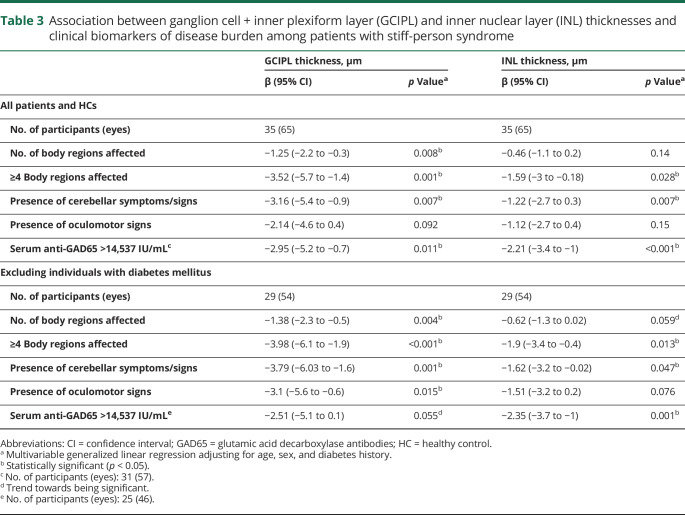
In multivariable models among patients with SPS, each additional body region affected was associated with a mean decrease in GCIPL thickness of 1.25 µm (95% CI, −2.2 to −0.3; p = 0.008). Patients with ≥4 body regions affected (the mean number of body regions affected) had lower GCIPL (p = 0.001) and INL (p = 0.028) thicknesses relative to patients with <4 regions affected. In addition, GCIPL and INL thicknesses were associated with signs/symptoms of cerebellar dysfunction (p = 0.007 for GCIPL; p = 0.007 for INL). No significant relationships were observed between retinal thickness measures and other individual body regions, or between VA measures and body regions affected. Similar results were observed in sensitivity analyses excluding individuals with DM (table 3).
Table 3.
Association between ganglion cell + inner plexiform layer (GCIPL) and inner nuclear layer (INL) thicknesses and clinical biomarkers of disease burden among patients with stiff-person syndrome
Higher anti-GAD65 levels were associated with lower thicknesses of INL (R2 = 0.25; p = 0.006). Patients with SPS with an anti-GAD65 titer above the mean of 14,537 IU/mL (n = 10) had significantly reduced GCIPL (p = 0.011) and INL (p < 0.001) thicknesses compared to patients with a titer below the mean. VA did not demonstrate significant relationships with serum anti-GAD65 antibody titers. Similar relationships were observed in sensitivity analyses excluding patients with DM (table 3).
Discussion
Patients with SPS in the cohort studied had lower GCIPL and INL thicknesses, and lower high- and low-contrast VA scores, as compared to HCs. In addition, GCIPL and INL thicknesses were associated with number of body regions affected and serum anti-GAD65 antibody levels among patients with SPS. Our novel findings are important as they may provide insight into the pathophysiology of SPS, and suggest a potential role for OCT in reflecting burden of disease, which is an area of unmet need in SPS.
Although serum anti-GAD65 titers do not consistently correlate with disease burden in SPS, anti-GAD65 antibodies are generally thought to be involved in SPS disease pathogenesis. Our findings therefore seem relevant and plausible given, as discussed above, the high concentration of GABAergic neurons in the retina, in particular the INL and inner plexiform layer.2,3 The reduced thicknesses of the INL and GCIPL observed in our SPS cohort may therefore result from an autoimmune retinopathy; however, further studies are needed to evaluate this proposed mechanism. Furthermore, our observation that GCIPL and INL thinning were associated with cerebellar dysfunction in particular is interesting, as cerebellar features, such as gait or focal limb ataxia, nystagmus, and hypometric saccades, have been observed with increased frequency in phenotypes of SPS that demonstrate a worse functional prognosis.10
Limitations of this study include its cross-sectional design and small sample size; however, it is worth noting that SPS is a rare disease, for which this is among the largest cohort studies reported to date. Although efforts were made to exclude individuals with ophthalmologic disorders, not all participants underwent formal ophthalmologic assessment to exclude occult pathology. In addition, we did not exclude patients with concurrent DM; hence, the high frequency of DM among SPS populations may complicate interpretation of an abnormal OCT measurement. For both OCT and VA measurements, greater differences were observed between the SPS and HC cohorts in analyses excluding patients with comorbid DM. Additional neuro-ophthalmologic/retinal assessments such as visual evoked potential or electroretinogram testing were not performed, and may have provided further evidence of primary retinal pathology. Further larger and longitudinal studies are needed to evaluate the association of structural and functional visual outcome measures with SPS clinical phenotypes and disease burden.
Glossary
- DM
diabetes mellitus
- GABA
γ-aminobutyric acid
- GAD65
glutamic acid decarboxylase
- GCIPL
ganglion cell + inner plexiform layer
- HC
healthy control
- INL
inner nuclear layer
- OCT
optical coherence tomography
- SPS
stiff-person syndrome
- VA
visual acuity
Appendix. Authors
Study funding
This study was funded by NIH/NINDS (R01NS082347 to P.A.C.).
Disclosure
J. Lambe and A. Rothman report no disclosures relevant to the manuscript. J. Prince is a founder of Sonovex, Inc. and serves on its Board of Directors; has received consulting fees from JuneBrain LLC; and is PI on research grants to Johns Hopkins from 12Sigma Technologies and Biogen. S. Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Biogen, Genzyme, Genentech Corporation, EMD Serono, and Celgene; is the PI of investigator-initiated studies funded by Genentech Corporation and Biogen Idec; received support from the Race to Erase MS Foundation; has received equity compensation for consulting from JuneBrain LLC, a retinal imaging device developer; and is the site investigator of a trial sponsored by MedDay Pharmaceuticals. P. Calabresi has received consulting fees from Disarm and Biogen and is PI on grants to JHU from Biogen and Annexon. S. Newsome has received consultant fees for scientific advisory boards from Biogen, Genentech, Celgene, and EMD Serono; is an advisor for Gerson Lehrman Group; is a clinical adjudication committee member for a MedDay Pharmaceuticals clinical trial; and has received research funding (paid directly to his institution) from Biogen, Novartis, Genentech, National MS Society, Department of Defense, and Patient Centered Outcomes Institute. Go to Neurology.org/N for full disclosures.
References
- 1.Sarva H, Deik A, Ullah A, Severt WL. Clinical spectrum of stiff person syndrome: a review of recent reports. Tremor Other Hyperkinet Mov 2016;6:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steffen H, Menger N, Richter W, et al. Immune-mediated retinopathy in a patient with stiff-man syndrome. Graefes Arch Clin Exp Ophthalmol 1999;237:212–219. [DOI] [PubMed] [Google Scholar]
- 3.Crooks J, Kolb H. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol 1992;315:287–302. [DOI] [PubMed] [Google Scholar]
- 4.Economides JR, Horton JC. Eye movement abnormalities in stiff person syndrome. Neurology 2005;65:1462–1464. [DOI] [PubMed] [Google Scholar]
- 5.Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol 2008;4:664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JJ, Costello F. The role of optical coherence tomography in neuro-ophthalmology. Ann Eye Sci 2018;3:35. [Google Scholar]
- 7.Ratchford JN, Saidha S, Sotirchos ES, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 2013;80:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang A, Carass A, Hauser M, et al. Retinal layer segmentation of macular OCT images using boundary classification. Biomed Opt Express 2013;4:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 2012;7:e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakocevic G, Raju R, Semino-Mora C, Dalakas MC. Stiff person syndrome with cerebellar disease and high-titer anti-GAD antibodies. Neurology 2006;67:1068–1070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data used for this study are available from the corresponding authors on reasonable request.