Abstract
Objective:
Adrenal gland secretes stress-induced glucocorticoids (iGC) in order to coping with stress. Previous study showed that scavenger receptor BI null (SR-BI−/−) mice failed to generate iGC in stress conditions, suggesting that SR-BI-mediated cholesterol uptake from HDL is a key regulator for iGC production. However, the LDL/LDL receptor (LDL/LDLr) pathway can also provide cholesterol for iGC synthesis, but rodents have limited LDL levels in circulation. Here, we generated SR-BI−/−ApoBtg mice with normal LDL levels in circulation to determine the relative contribution of the HDL/SR-BI and LDL/LDLr pathways to iGC production in stress conditions.
Approach and Results:
To obtain mouse models with normal LDL levels, SR-BI−/− mice were bred to ApoBtg mice. Then, the F1 SR-BI+/−ApoBtg mice were backcrossed to SR-BI−/− to obtain SR-BI−/−ApoBtg, SR-BI−/−ApoBwt and SR-BI+/+ApoBtg mice. We first examined the lipoprotein profile, which shows a 6.5-fold increase in LDL levels in SR-BI−/−ApoBtg mice compared to SR-BI−/−ApoBwt mice. Then, we induced stress with adrenocorticotropic hormone (ACTH) and cecal ligation and puncture (CLP). One hour after ACTH stimulation, SR-BI+/+ApoBtg control mice produced iGC (14.9 folds), but both SR-BI−/−ApoBwt and SR-BI−/−ApoBtg showed no iGC production (p < 0.001). Three hours after CLP treatment, SR-BI+/+ApoBtg control mice showed iGC production (6.4 folds), but both SR-BI−/−ApoBwt and SR-BI−/−ApoBtg mice showed no iGC production (p < 0.001).
Conclusions:
SR-BI−/−ApoBtg mice fail to produce iGC in stress conditions even though with restored LDL levels in circulation. These findings clarify that the HDL/SR-BI, not LDL/LDLr, pathway is responsible for iGC production in stress conditions.
Keywords: ACTH, sepsis, ApoB, HDL, glucocortitoid, LDL, LDL receptor, steroidogenesis, scavenger receptor BI
AHA Journals Subject Terms: Basic Science Research
Graphical Abstract
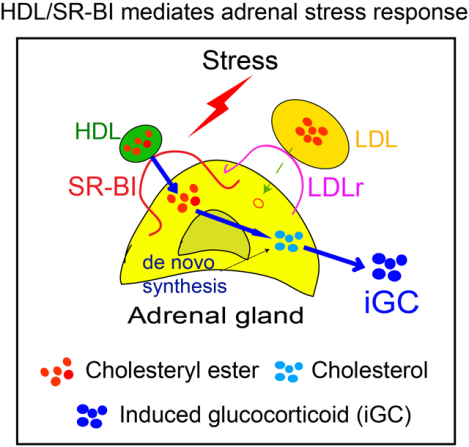
INTRODUCTION
Stress activates the hypothalamic–pituitary–adrenal axis and adrenal gland secretes stress-induced glucocorticoids (iGC) as an essential host response to cope with stress.1 While GC is a well-studied molecule and well-known for its immunosuppressive properties,2–4 the functions of iGC and how iGC production is regulated remain largely unknown. Numerous laboratories including ours recently showed that iGC protects against sepsis through suppression of inflammatory cytokine production and promotion of phagocytosis.5–9 Understanding the mechanism of iGC production will provide further insights into GC biology and host response.
Adrenal cells use cholesterol as the substrate for GC synthesis. Low density lipoprotein receptor (LDLr) and scavenger receptor class B type I (SR-BI, a major HDL receptor) have been found in adrenal gland, suggesting that both the LDL/LDLr and HDL/SR-BI pathways can provide cholesterol for iGC production. Using mouse adrenal cells (Y1 cells), Goldstein and Brown’s group first showed that mouse or human LDL binds to LDLr on Y1 cells with high affinity and delivers cholesterol to the cells at unstimulated or adrenocorticotropic hormone-stimulated conditions (ACTH-stimulated conditions).10 They further showed that high density lipoprotein (HDL) does not increase the cholesterol content in Y1 cells. However, using rat adrenal gland, Gwynne et. al. showed that, upon incubation of adrenal gland with human LDL or HDL, the rate of transfer of labeled cholesterol from HDL into the adrenal gland was two to three times greater than from LDL. Interestingly, ACTH stimulation further increased the transfer of cholesterol from HDL but not from LDL.11 Using primary rat adrenal cells, another study by Gwynne et. al. showed that rat or human HDL or LDL enhances steroid production and increases cellular cholesterol content.12 In vivo studies also showed controversial findings. Two clinical studies showed that family hyperlipidemia patients (deficient in LDLr) displace normal iGC production in the early time points of ACTH stimulation. However, there were only three patients in each study, and one study lacked normal controls and another showed impaired iGC production upon repeated ACTH stimulations.13, 14 Using LDLr null mouse models, Kraemer et al reported that the LDLr is not required for acute adrenal steroidogenesis,15 but a study using Watanabe heritable hyperlipidemic rabbits, which lack LDLr, showed subnormal corticosterone production in response to ACTH stimulation.16 Nevertheless, the contribution of the LDL/LDLr pathway to iGC production remains elusive.
SR-BI is a physiological receptor of HDL and most abundantly expressed in adrenal gland.17, 18 Adrenal SR-BI expression is upregulated by ACTH stimulation both in vivo and in adrenal cells, suggesting a role of SR-BI in iGC production. In vitro study showed that HDL provides cholesterol for GC synthesis via SR-BI,19 but the known two natural mutations of SR-BI in human provide contradictory observations. The carriers of SR-BI P297S mutant, which losses 50% of HDL cholesterol uptake activity, had attenuated adrenal steroidogenesis as evidenced by a decreased urinary excretion of sterol metabolites, a decreased iGC production to ACTH stimulation, and symptoms of diminished adrenal function; while the carriers of SR-BI P376L mutant, which completely losses HDL cholesterol uptake activity, displayed normal GC production.20, 21 The role of SR-BI in iGC production in mice seemed well-illustrated. A number of laboratories including ours reported that mice deficient in SR-BI have normal basal GC levels but fail to generate iGC in stress conditions, including LPS-induced endotoxemia,5 cecal ligation and puncture (CLP)-induced sepsis7–9 and long-term fasting.22, 23 These mouse studies demonstrated that the HDL/SR-BI pathway is not needed for GC production in basal conditions but required for iGC production in stress conditions. However, a limitation with these studies is that rodents have low LDL levels in circulation, thus, the relative contribution of LDL/LDLr in iGC production remains to be clarified. Here, we generated SR-BI−/−Apolipoprotein B transgenic mice (SR-BI−/−ApoBtg) with high plasma LDL levels and found that SR-BI−/−ApoBtg mice failed to produce iGC in stress conditions. These findings clarify that the HDL/SR-BI, not LDL/LDLr, pathway is responsible for iGC production in stress conditions.
MATERIALS AND METHODS
The manuscript adheres to the AHA Journals’ implementation of the Transparency and Openness Promotion (TOP) Guidelines.
Mice:
ApoBtg mice were purchased from Taconic Biosciences. SR-BI+/− mice on C57BL/6Jx129 background were obtained from the Jackson Laboratory. SR-BI−/− and SR-BI+/+ mice were generated by breeding with SR-BI+/− mice. SR-BI−/− mice were bred to ApoBtg mice, then, the F1 SR-BI+/−ApoBtg mice were backcrossed to SR-BI−/− to get the F2 SR-BI−/−ApoBtg and SR-BI−/−ApoBwt littermates. To generate SR-BI+/+ApoBtg mice, SR-BI+/+ mice were bred to the F1 SR-BI+/−ApoBtg mice. All the mice used for breeding were housed in the University of Kentucky Animal Care Facility, following institutional and National Institutes of Health guidelines after approval by the Institutional Animal Care and Use Committee. We used both of male and female, because there were no significant sex difference in iGC production in sepsis.8 In total, 35 male and 36 female mice at 6–10 weeks were used.
Lipoprotein profiling and cholesterol measurement:
Lipoproteins were separated with FPLC and total and free cholesterol were measured using the Wako Cholesterol E and Free Cholesterol E kit (Cat No. 999–02601 and No. 993–02501, respectively, FUJIFILM Wako Diagnostics, USA). The levels of LDL were determined by calculating the area under the curve (AUC). Briefly, to calculate AUC, the peak of LDL was identified by findPeaks function from R package quantmod (Jeffrey A. Ryan and Joshua M. Ulrich (2020). quantmod: Quantitative Financial Modelling Framework. R package version 0.4–16. https://CRAN.R-project.org/package=quantmod). Then the AUC of LDL was calculated using fitpeaks function from R package Alsace (Ron Wehrens (2019). alsace: ALS for the Automatic Chemical Exploration of mixtures. R package version 1.20.0. https://github.com/rwehrens/alsace). The detailed programing is listed in the Supplemental Materials section.
Oil Red O staining:
The adrenal glands were mounted in Tissue-Tek OCT compounds (Sakura Finetek). For histology, the 10 μm sections were fixed in 4% paraformaldehyde in 1xPBS for 5 min at room temperature (RT). Incubating the slides with 60% isopropanol for 5min, the slides were stained with freshly prepared Oil Red O (0.5% in triethylphophate) working solution for 10 min. Then, the slides were rinsed with 60% isopropanol for 2min repeatedly, following the HE staining for 10s. The slides were rinsed with 1xTBS for 3 times and with dH2O before mounting a cover-slip onto the slides with warmed glycerol gelatin. Images were obtained by ECLIPSE Ti-S Inverted Microscope System (Nikon).
Quantification of total and free cholesterol contents in adrenal gland by gas chromatography:
Adrenal glands were dissected to remove any adherent adipose tissue and weighed. Lipids were extracted by incubating adrenals in 2:1 chloroform/methanol at 60°C for 3 hours in a glass culture tube. The lipid chloroform/methanol phase was transferred to a clean glass tube and the solvent was evaporated under a steady stream of N2 in a 55°C water bath. Lipids were then re-dissolved in hexane and the free cholesterol contents were determined by gas chromatography system (Agilent Technology 7890B, Santa Clara, CA, USA). Then, the lipid extract was transferred back to the glass tube and the solvent was evaporated under N2. The remaining lipids were saponified by incubation with 1ml of ethanol and 0.1 ml of 50% potassium hydroxide at 60°C for 2 hours. The nonsaponifiable lipids were separated by adding 1ml of hexane and 1 ml of deionized water. Lipids in hexane phase were collected and the total cholesterol contents were determined by gas chromatography. The mass of total and free cholesterol contents in the adrenal glands was calculated by comparing the AUC of the cholesterol peak to the AUC of the internal control of 5-alpha cholestane peak.
Adrenocorticotropic hormone (ACTH)-induced stress:
Two IU ACTH was injected subcutaneously. The plasma was collected via tail cut 1 hour after injection (Cat No. A6303, Sigma, USA).
CLP-induced septic stress:
CLP was conducted as previously described.7
Corticosterone Measurement:
Corticosterone was measured using 100 folds dilution plasma with an ELISA kit (Cat No. ADI-900–097, Enzo Life Sciences, USA).
Real-time quantitative PCR:
Total RNA was extracted from adrenal gland using RNeasy Mini Kit (Cat No. 74104, Qiagen, USA), according to the instructions of the kit. Briefly, after determining the concentration with Nanodrop 8000, equal amount (1ug) of RNA from each sample was reversely transcribed using iScript cDNA synthesis kit (Cat No. 1708890, Bio-Rad, USA). Real-time quantitative PCR was performed with SYBR green mix on CFX96 machine (Bio-Rad). The primers used in this study were listed in Table 1.
Table 1.
Primers for real-time quantitative RT-PCR.
| Gene | Sequence |
|---|---|
| LDLR | 5’-GATGGCTATACCTACCCCTCAA-3’ |
| 5’-TGCTCATGCCACATCGTC-3’ | |
| HMG-CoA | 5’-TGATTGGAGTTGGCACCAT-3’ |
| 5’-TGGCCAACACTGACATGC-3’ | |
| CYP11A1 | 5’-AAGTATGGCCCCATTTACAGG-3’ |
| 5’-TGGGGTCCACGATGTAAACT-3’ | |
| StAR | 5’-ATGTTCCTCGCTACGTTCAAG-3’ |
| 5’-CCCAGTGCTCTCCAGTTGAG-3’ |
Quantification and statistical analysis:
Data were represented as mean ± SEM. Student’s t-test (two-sided) was used to compare two-group data with normal distribution and equivalent variance; for multiple-group with two independent factors, two-way ANOVA with Tukey’s multiple comparison test was applied for normally distributed variables. All statistical analyses were conducted on biological replicates in the SPSS version 17.0.2 software package (SPSS Inc., Chicago, IL, USA).
Disclosure of the data:
As following TOP Guidelines, the data that support the findings of this study are available upon reasonable requests. Because of the sensitive nature of the data collected for this study, requests to access the dataset may be sent to Xiangan Li at xli2@email.uky.edu.
RESULTS
SR-BI−/−ApoBtg mice exhibit high plasma LDL levels.
ApoBtg mice have been used to increase LDL levels in circulation.24 To investigate the relative contribution of HDL/SR-BI and LDL/LDLr pathway in iGC production, we generated whole body SR-BI knockout mice with transgenic expression of human ApoB. Lipoprotein profile analysis showed that SR-BI−/−ApoBtg mice have a profound increase in LDL fraction (Figure 1A) and a 6.5-fold increase in LDL-C levels as calculated from AUC (Figure 1B). In consistent with the previous report,24 transgenic expression of human ApoB in SR-BI+/+ mice (SR-BI+/+ApoBtg) also showed a profound increase in plasma LDL levels (Figure 1, A and B). There were no significant differences in plasma total and free cholesterol concentration between SR-BI−/−ApoBtg and SR-BI−/−ApoBwt mice (Figure 1, C and D). Previous report showed that SR-BI-/mice have enlarged adrenal glands, SR-BI−/−ApoBtg mice did not show reduce adrenal weight compared to SR-BI−/−ApoBwt mice (Figure 1E). Despite a significant increase in plasma LDL levels in SR-BI−/−ApoBtg mice, SR-BI−/−ApoBtg mice did not restore lipid storage in adrenal gland as shown by the Oil Red O staining (Figure 1F) and by quantification of total or free cholesterol contents (Figure 1, G and H). Taken together, we successfully introduced high levels of LDL in SR-BI−/−ApoBtg mice.
Figure 1. SR-BI−/−ApoBtg mice display a human-like lipoprotein profile.
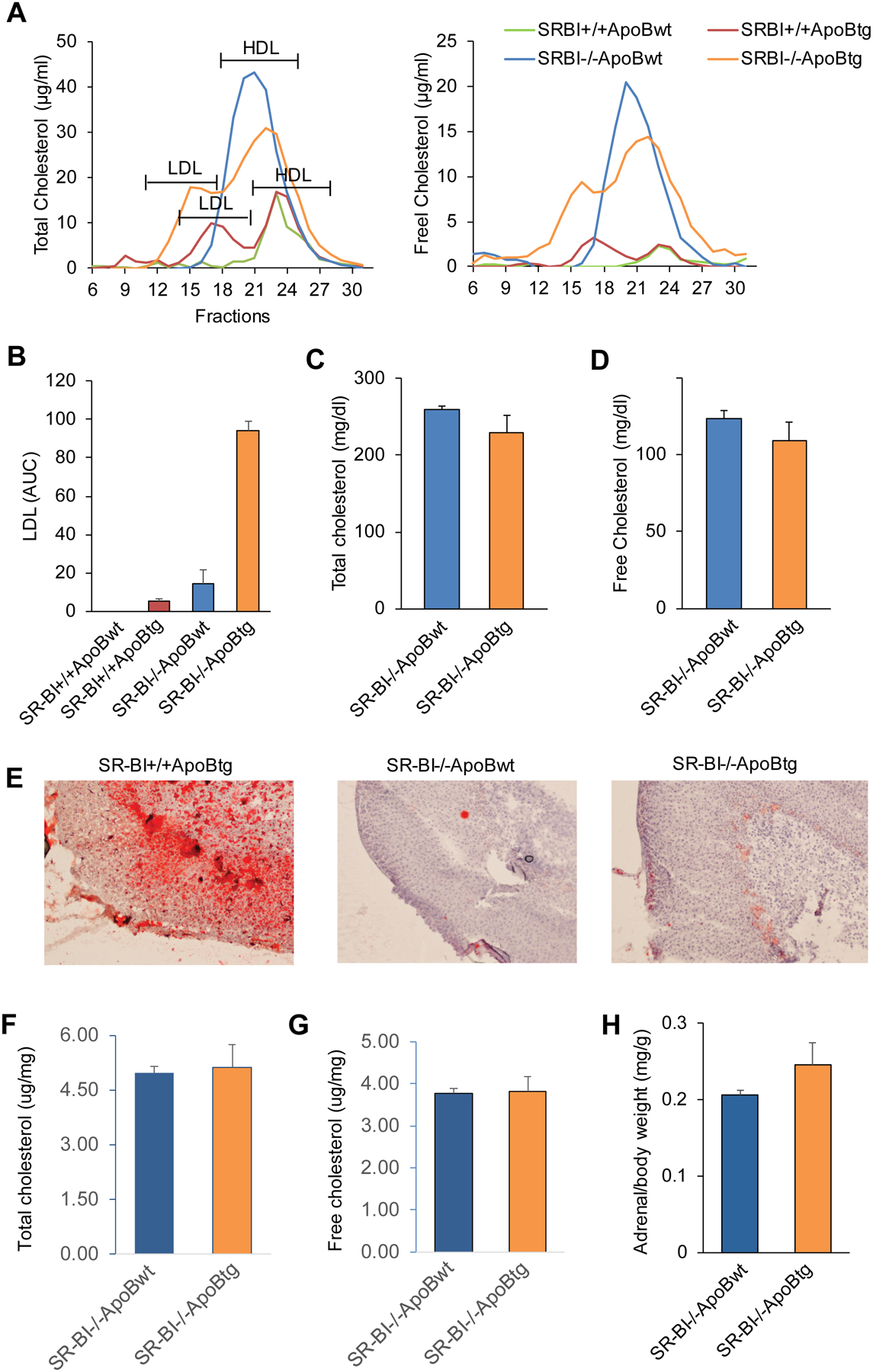
(A) Plasma from SR-BI−/−ApoBwt or SR-BI−/−ApoBtg mice was fractionated with FPLC and the lipoprotein profiles were analyzed by measuring total cholesterol and free cholesterol, respectively in each fraction. n = 2 per group (all females); (B) Area under curve (AUC) of LDL, n = 2 per group; (C and D) The total cholesterol and free cholesterol concentrations in plasma, n = 4–5 per group (all females). (E) The Oil Red O staining of adrenal gland, n=3 per group (all males), representative data; (F and G) Quantification of total and free cholesterol contents in adrenal gland (μg/mg tissue), n=2–3 per group (all males); (H) adrenal weight (mg/g body weight), n= 3 per group (all males). Data represent the mean ± SEM.
The HDL/SR-BI pathway, not the LDL/LDLr pathway, mediates adrenal stress response
In stress conditions, the activated hypothalamus-pituitary-adrenal stress axis releases ACTH into circulation that induces iGC production in adrenal gland. Therefore, we subcutaneously injected ACTH to stimulate iGC production. ACTH induced a 14.9-fold increase in iGC production in SR-BI+/+ApoBtg mice but no iGC production in both SR-BI−/−ApoBtg and SR-BI−/−ApoBwt mice (Figure 2A). We also verified iGC production in septic conditions using a CLP sepsis model. CLP induced a 6.4-fold increase in iGC production in SR-BI+/+ApoBtg mice but no iGC production in both SR-BI−/−ApoBtg and SR-BI−/−ApoBwt mice (Figure 2A).
Figure 2. SR-BI−/−ApoBtg mice failed to generate iGC in stress conditions.
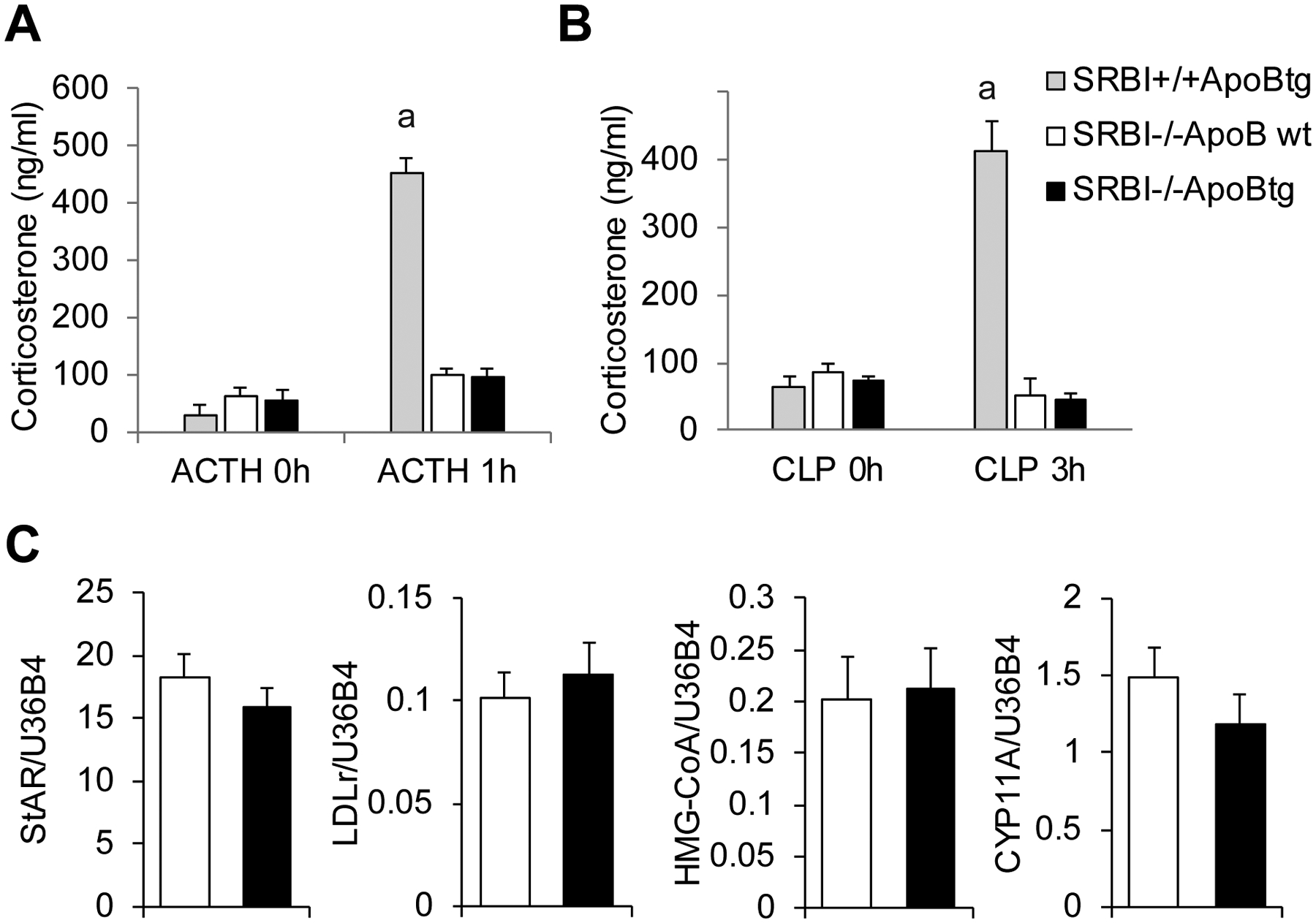
(A) The corticosterone levels before and after ACTH stimulation or CLP challenge in SR-BI+/+ApoBtg (3F), SR-BI−/−ApoBwt (2M+5F) and SR-BI−/−ApoBtg (6M+1F) mice. Statistical significance was analyzed by two-way ANOVA followed by Tukey’s multiple comparison test. a denotes p < 0.001. n = 3–7 per group. (B) The gene expressions of molecules involved in steroidogenesis, including HMG-CoA reductase (de novo cholesterol synthesis), LDLr (intracellular cholesterol uptake from LDL), StAR (cholesterol transport to mitochondria), and CYP11A1 (converting cholesterol to GC). Gene expression was measured in adrenal glands of SR-BI−/−ApoBwt (5F) and SR-BI-/ApoBtg (1M+5F) mice by real-time quantitative RT-PCR. n=5–6 per group. Data represent the mean ± SEM.
Intracellular cholesterol comes from three resources. In addition to the HDL/SR-BI or the LDL/LDLr pathways, de novo cholesterol synthesis can also provide cholesterol for GC production. To exclude the possibility that the introduction of LDL in circulation affects gene expression of hydroxymethylglutaryl-CoA reductase (HMG-CoA reductase, a key regulator for de novo cholesterol synthesis), we conducted quantitative RT-PCR for HMG-CoA reductase, which showed no difference in the expression of HMG-CoA reductase between SR-BI−/−ApoBtg and SR-BI−/−ApoBwt mice (Figure 2B). We also measured the gene expression of LDLr and key enzymes involved in steroidogenesis. The gene expressions of LDLr, steroidogenic acute regulatory protein (StAR; key molecule for transporting cholesterol to mitochondria) and Cytochrome P450 Family 11 Subfamily A Member 1 (CYP11A1; key enzyme for converting cholesterol to GC) were identical between SR-BI−/−ApoBtg and SR-BI−/−ApoBwt mice (Figure 2B). Taken together, our findings clarified that HDL/SR-BI, not LDL/LDLr, mediates iGC production in stress conditions.
DISCUSSION
Although numerous earlier studies clearly showed that the HDL/SR-BI pathway is indispensable for iGC production using SR-BI null mice,5, 7–9, 22, 23 a critical limitation for these studies is that rodents have low LDL levels in circulation. To clarify the contribution of the LDL/LDLr and HDL/SR-BI pathways to iGC production in stress conditions, we generated SR-BI−/−ApoBtg mice with high LDL levels in circulation. We found that normalization of LDL levels in SR-BI−/−ApoBtg mice does not restore iGC production in response to ACTH stimulation or CLP-induced septic stress. Our findings demonstrate that HDL/SR-BI, not LDL/LDLr, mediates iGC production in stress conditions.
An early in vitro study using mouse skin fibroblast cells showed that human LDL binds to mouse LDLr with low affinity.25 This raises a concern that a lack of iGC production observed in SR-BI−/−ApoBtg mice may be caused by low binding between human LDL and mouse LDLr. While fibroblast cells are well-used for the study of LDLr-mediated LDL metabolism, the cells do not have the machinery required for steroidogenesis. Thus, it may not be an optimal model for steroidogenesis. Indeed, using mouse adrenal cell Y1, Goldstein and Brown’s group clearly showed that mouse or human LDL binds to LDLr on adrenal cells with similar high affinity and can deliver cholesterol to the adrenal cells for steroidogenesis at unstimulated or ACTH-stimulated conditions.10
To address a role of LDL/LDLr in iGC production, van der Sluis et al. generated adrenal LDLr wild type and adrenal LDLr KO mice in LDLr null background by transplanting adrenal gland from wild type or LDLr null mice to adult LDLr null recipients.26 They found that LDLr null mice with wild type adrenal gland had a significant decrease in iGC production compared to LDLr null mice with LDLr KO adrenal gland in response to fasting. Based on this observation, they concluded that LDLr negatively regulates iGC production in response to stress. We would like to point out that transplantation of wild type adrenal gland to LDLr null recipient may not be a sound technical approach, which may render their conclusion invalid. Eight weeks after transplantation, the transplanted wild type adrenal gland expresses LDLr protein, but the adult recipient LDLr null mice will recognize the LDLr protein as foreign protein and generate antibodies against it. This will not only neutralize LDLr protein but also induce immune cells to attack adrenal cells, which will impair the function of adrenal cells. While the authors conducted RT-PCR and HE staining trying to exclude a graft-versushost response, they found a ~ 50%, 60% and 30% increase (no statistical significance) in CD8, CD14 and CD68 expression in wild type adrenal gland transplantation to LDLr null recipients compared to LDLr KO adrenal gland transplantation to LDLr null recipients. These data did not exclude an immune response against LDLr expression in adrenal gland, rather, suggested such a possibility. As shown in Supplemental Figure 1, we conducted adrenal transplantation experiments by transplant an adrenal gland from wild type mice to wild type or to SRBI null recipients. Interestingly, we observed that, in contrast to nice iGC production in SRBI+/+ adrenal to SRBI+/+ recipients in response to septic stress, most SRBI+/+ adrenal to SRBI−/− recipients displaced poor iGC production in response to septic stress. Taken together, transplantation of wild type adrenal gland to either LDLr or SR-BI null recipients leads to impaired iGC production in KO recipients. Obviously, it is unlikely that both LDLr and SR-BI negatively regulate iGC production. A plausible explanation is that this impaired iGC production was caused by the immune response against adrenal expression of LDLr or SRBI in LDLr null or SRBI null recipients, respectively. In another report by the same group,23 the authors generated adrenal spefic SR-BI null mice by adrenal transplantation. They found that the adrenal SR-BI null mice fed an atherigenic diet had a 50% reduction in iGC production compared to wild type controls upon overnight fasting at hyperlipidemic conditions. While this observation suggests that LDL may not contribute to iGC production under stress conditions, the authors did not show LDL levels, because the large VLDL peak overshowed LDL fraction. Interestingly, the adrenal SR-BI null mice also had a 50% reduction in VLDL plus LDL fractions compared to wild type controls. This raises another possibility that the 50% less iGC production may be caused by the 50% reduction in VLDL plus LDL levels. Thus, the true contribution of LDL to iGC production remains to be determined.
The known two natural mutations of SR-BI in human provided inconsistent observations regarding the role of SR-BI in steroidogenesis. Vergeer et al. reported that the carriers of SR-BI P297L mutant had impaired iGC production in response to ACTH stimulation, reduced urinary steroid secretion and symptoms due to diminished adrenal function20, while Zanoni et al. reported that the carriers of SR-BI P376L mutant had GC levels in normal range.21 It is worth noting that the Zanoni’s report only tested GC levels at physiological conditions. Whether the carriers of SR-BI P376L mutant have impaired iGC production remains to be determined. The adrenal glands are responsible not only for iGC production in stress conditions but also for physiological levels of GC production at normal conditions. The iGC production is an acute response that occurs immediately upon stress and the iGC levels are 10–100 folds higher than physiological GC levels. Thus, we speculate that the adrenal cells use different mechanisms to provide intracellular cholesterol for GC synthesis in basal physiological and stress conditions. The SR-BI−/−ApoBtg mice had normal levels of GC at physiological conditions but lacked iGC production in response to stress, suggesting that the HDL/SR-BI pathway is responsible for iGC production while de novo cholesterol synthesis is critical for GC production under physiological conditions. It is worth noting that the synthesis of steroid hormone in adrenal gland is a multi-step oxidation process, involving a variety of enzymes. Human and mouse express different levels of these enzymes, as a result, human produces cortisol and mouse produces corticosterone as the major stress hormone, respectively. SR-BI mediates intracellular cholesteryl ester uptake from HDL to provide cholesterol for steroid hormone synthesis, which is upstream of the oxidation steps. Therefore, even though human and mouse produce different major stress hormones, SR-BI should play similar critical roles in regulating adrenal stress response in both human and mouse.
Supplementary Material
HIGHLIGHTS.
The relative contribution of the high density lipoprotein/scavenger receptor BI (HDL/SR-BI) and the low density lipoprotein/low density lipoprotein receptor (LDL/LDLr) pathways to stress-induced glucocorticoids (iGC) production in stress conditions remains to be clarified.
We generated SR-BI−/−ApoBtg mice with restored LDL levels in circulation.
SR-BI−/−ApoBtg mice failed to produce iGC in stress conditions induced with adrenocorticotropic hormone (ACTH) and cecal ligation and puncture (CLP).
These findings clarify that the HDL/SR-BI, not LDL/LDLr, pathway is responsible for iGC production in stress conditions.
ACKNOWLEDGMENTS
X.L. designed experiments; X.Y., Q.W., L.G., performed experiments and data analysis; D.H. and A.D. conducted HPLC; LC and RT quantified cholesterol in adrenal gland. M.I. and X.Y. verified the data and wrote the manuscript; All authors have read and agreed for the submission of the manuscript.
SOURCES of FUNDING
This study was supported by Grants NIH R01GM113832, NIH R01GM121796 and VA 1I01BX004639 (to X-A Li). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or VA.
ABBRIVIATIONS:
- ACTH
adrenocorticotropic hormone
- ApoBtg
apolipoprotein B transgenic
- ApoBwt
apolipoproteinB wild type
- AUC
area under curve
- CLP
cecal ligation and puncture
- CYP11A1
cytochrome P450 family 11 subfamily A member 1
- HDL
high density lipoprotein
- HMG-CoA reductase
hydroxymethylglutaryl-CoA reductase
- iGC
stress-induced glucocorticoids
- LDL
low density lipoprotein
- LDLr
low density lipoprotein receptor
- StAR
steroidogenic acute regulatory protein
- RT
room temperature
- SR-BI
scavenger receptor class B type I
Footnotes
DISCLOSURES
The authors declare no competing interests.
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by Xiangan Li.
REFERENCES
- 1.Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends in pharmacological sciences. 2013;34:518–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandevyver S, Dejager L, Tuckermann J, Libert C. New insights into the anti-inflammatory mechanisms of glucocorticoids: An emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology. 2013;154:993–1007 [DOI] [PubMed] [Google Scholar]
- 4.Busillo JM, Cidlowski JA. The five rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24:109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai L, Ji A, de Beer FC, Tannock LR, van der Westhuyzen DR. Sr-bi protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest. 2008;118:364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XA, Guo L, Asmis R, Nikolova-Karakashian M, Smart EJ. Scavenger receptor bi prevents nitric oxide-induced cytotoxicity and endotoxin-induced death. Circ Res. 2006;98:e60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo L, Song Z, Li M, Wu Q, Wang D, Feng H, Bernard P, Daugherty A, Huang B, Li XA. Scavenger receptor bi protects against septic death through its role in modulating inflammatory response. J Biol Chem. 2009;284:19826–19834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ai J, Guo L, Zheng Z, Wang S-X, Huang B, Li X-A. Corticosteroid therapy benefits septic mice with adrenal insufficiency but harms septic mice without adrenal insufficiency*. Critical Care Medicine. 2015;43:e490–e498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilibert S, Galle-Treger L, Moreau M, Saint-Charles F, Costa S, Ballaire R, Couvert P, Carrie A, Lesnik P, Huby T. Adrenocortical scavenger receptor class b type i deficiency exacerbates endotoxic shock and precipitates sepsis-induced mortality in mice. J Immunol. 2014;193:817–826 [DOI] [PubMed] [Google Scholar]
- 10.Faust JR, Goldstein JL, Brown MS. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured mouse adrenal cells. J Biol Chem. 1977;252:4861–4871 [PubMed] [Google Scholar]
- 11.Gwynne JT, Mahaffee D, Brewer HB Jr., Ney RL. Adrenal cholesterol uptake from plasma lipoproteins: Regulation by corticotropin. Proc Natl Acad Sci U S A. 1976;73:4329–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwynne JT, Hess B. The role of high density lipoproteins in rat adrenal cholesterol metabolism and steroidogenesis. J Biol Chem. 1980;255:10875–10883 [PubMed] [Google Scholar]
- 13.Allen JM, Thompson GR, Myant NB. Normal adrenocortical response to adrenocorticotrophic hormone in patients with homozygous familial hypercholesterolaemia. Clin Sci (Lond). 1983;65:99–101 [DOI] [PubMed] [Google Scholar]
- 14.Illingworth DR, Lees AM, Lees RS. Adrenal cortical function in homozygous familial hypercholesterolemia. Metabolism. 1983;32:1045–1052 [DOI] [PubMed] [Google Scholar]
- 15.Kraemer FB, Shen WJ, Patel S, Osuga J, Ishibashi S, Azhar S. The ldl receptor is not necessary for acute adrenal steroidogenesis in mouse adrenocortical cells. Am J Physiol Endocrinol Metab. 2007;292:E408–412 [DOI] [PubMed] [Google Scholar]
- 16.Hoeg JM, Loriaux L, Gregg RE, Green WR, Brewer HB, Jr. Impaired adrenal reserve in the watanabe heritable hyperlipidemic rabbit: Implications for ldl-receptor function in steroidogenesis. Metabolism: clinical and experimental. 1985;34:194–197 [DOI] [PubMed] [Google Scholar]
- 17.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor sr-bi in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387 [DOI] [PubMed] [Google Scholar]
- 18.Zheng Z, Ai J, Li XA. Scavenger receptor class b type i and immune dysfunctions. Curr Opin Endocrinol Diabetes Obes. 2014;21:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imachi H, Murao K, Sayo Y, Hosokawa H, Sato M, Niimi M, Kobayashi S, Miyauchi A, Ishida T, Takahara J. Evidence for a potential role for hdl as an important source of cholesterol in human adrenocortical tumors via the cla-1 pathway. Endocr J. 1999;46:27–34 [DOI] [PubMed] [Google Scholar]
- 20.Vergeer M, Korporaal SJ, Franssen R, Meurs I, Out R, Hovingh GK, Hoekstra M, Sierts JA, Dallinga-Thie GM, Motazacker MM, Holleboom AG, Van Berkel TJ, Kastelein JJ, Van Eck M, Kuivenhoven JA. Genetic variant of the scavenger receptor bi in humans. N Engl J Med. 2011;364:136–145 [DOI] [PubMed] [Google Scholar]
- 21.Zanoni P, Khetarpal SA, Larach DB et al. Rare variant in scavenger receptor bi raises hdl cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoekstra M, Meurs I, Koenders M, Out R, Hildebrand RB, Kruijt JK, Van Eck M, Van Berkel TJ. Absence of hdl cholesteryl ester uptake in mice via sr-bi impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J Lipid Res. 2008;49:738–745 [DOI] [PubMed] [Google Scholar]
- 23.Hoekstra M, van der Sluis RJ, Van Eck M, Van Berkel TJ. Adrenal-specific scavenger receptor bi deficiency induces glucocorticoid insufficiency and lowers plasma very-low-density and low-density lipoprotein levels in mice. Arterioscler Thromb Vasc Biol. 2013;33:e39–46 [DOI] [PubMed] [Google Scholar]
- 24.Linton MF, Farese RV Jr., Chiesa G, Grass DS, Chin P, Hammer RE, Hobbs HH, Young SG. Transgenic mice expressing high plasma concentrations of human apolipoprotein b100 and lipoprotein(a). The Journal of clinical investigation. 1993;92:3029–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corsini A, Mazzotti M, Villa A, Maggi FM, Bernini F, Romano L, Romano C, Fumagalli R, Catapano AL. Ability of the ldl receptor from several animal species to recognize the human apo b binding domain: Studies with ldl from familial defective apo b-100. Atherosclerosis. 1992;93:95–103 [DOI] [PubMed] [Google Scholar]
- 26.van der Sluis RJ, Van Eck M, Hoekstra M. Adrenocortical ldl receptor function negatively influences glucocorticoid output. J Endocrinol. 2015;226:145–154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


