Abstract
Background:
Information about longer-term functional outcomes following lower extremity amputation for peripheral vascular disease and diabetes remains limited. This study examined factors associated with mobility success during the first year following amputation.
Methods:
Prospective cohort study of 87 amputees experiencing a first major unilateral amputation surgery. Seventy-five (86%) participants completed 12-month follow-up interview.
Results:
Twenty-eight subjects (37%) achieved mobility success, defined as returning to or exceeding a baseline level of mobility on the locomotor capability index (LCI-5). Forty-three subjects (57%) were satisfied with their mobility. Individuals who were 65 years of age and older (risk difference [RD] = −0.52; 95% confidence interval [CI]: −0.75, −0.29), reported a current alcohol use disorder (RD = −0.37; 95% CI: −0.48, −0.26), had a history of hypertension (RD = −0.23; 95% CI: −0.43, −0.03) or treatment for anxiety or depression (RD = −0.39; 95% CI: −0.50, −0.28) were less likely to achieve mobility success. Mobility success was associated with mobility satisfaction (RD = 0.36; 95% CI: 0.20, 0.53) and satisfaction with life (RD = 0.28; 95% CI: 0.06, 0.50). Although higher absolute mobility at 12 months was also associated with mobility satisfaction and overall life satisfaction, 50% of individuals who achieved success with low to moderate 12-month mobility function reported they were satisfied with their mobility.
Conclusion:
Defining success after amputation in relation to an individual’s specific mobility prior to the development of limb impairment which led to amputation provides a useful, patient-centered measure that takes other aspects of health, function, and impairment into account.
Challenges in quantifying and predicting outcome after lower extremity amputation (LEA) secondary to peripheral vascular disease (PVD) and/or diabetes can limit effective and consistent health care decision-making. Defining successful outcome is particularly challenging when superimposed upon aging and impairments related to the underlying disease processes.1 Additionally, various stakeholders (eg, patient, surgeon, third party payer) may prioritize outcomes differently (eg, mortality, hospitalization, mobility, self-care, social integration, and satisfaction with life and/or quality of life).2–7 In an initial effort to address these complexities, Taylor defined successful outcome based on a combination of survival, amputation wound healing, and mobility (defined as daily ambulation with a prosthesis for 1 year or until death).8
To date, mobility has been the functional outcome most commonly examined postamputation. However, new methods are needed to ensure that measures quantify aspects of mobility that are important to the patient, incorporate the patient’s premorbid functional status, and provide health care providers with useful information at key decision points in the amputation continuum of care.9 To address these challenges, we propose a new definition of mobility outcome, “mobility success,” based on the rehabilitation tenet that amputation is a functionally restoring procedure and that success is defined not only relative to the impairment necessitating the surgical procedure, but also relative to the additional impairments that affect mobility at baseline.10 Thus, patients with relatively low levels of underlying mobility can be viewed as having a successful outcome if they return to this level of function after amputation.
The objectives of this study were (1) to utilize the novel definition of “mobility success” to describe mobility outcome after LEA due to PVD and diabetes; (2) to compare rates of mobility success between various amputation levels; (3) to evaluate factors, independent of amputation level, associated with mobility success; (4) to explore the definition of mobility success by examining its association with satisfaction with mobility and satisfaction with life; and (5) to determine if the new mobility success variable adds to the utility of more traditional approaches to measuring mobility in this population.
METHODS
Study design
We performed a multisite prospective cohort study of individuals undergoing major lower extremity amputation due to complications of PVD or diabetes at two-VA medical centers (VA Medical Center, Puget Sound and Denver), a university hospital (University of Colorado Medical Center, Denver), and a level I trauma center (Harborview Medical Center, Seattle). The decision to perform transmetatarsal (TM), transtibial (TT), or transfemoral (TF) amputation was made at each site per usual care. Participants were assessed via in-person or telephone interview at four time points: presurgically (if available), 6 weeks, 4 months, and 12 months postsurgically. Patients not available presurgically were enrolled at 6 weeks (Fig 1). Additional data were gathered via systematic review of the medical records. Only the premorbid data obtained at 6 weeks and the 12-month follow-up data are presented here. The 6-week and 4-month outcomes/follow-up data were not included in this report because these were considered intermediate outcomes rather than the ultimate level of functional mobility. All assessments were performed by a trained study coordinator designated for each site who was responsible for recruitment, interviews and completion of case report forms, and routine monitoring of all enrolled patients. This study was conducted in accordance with the procedures approved by local human subjects review boards.
Fig 1.
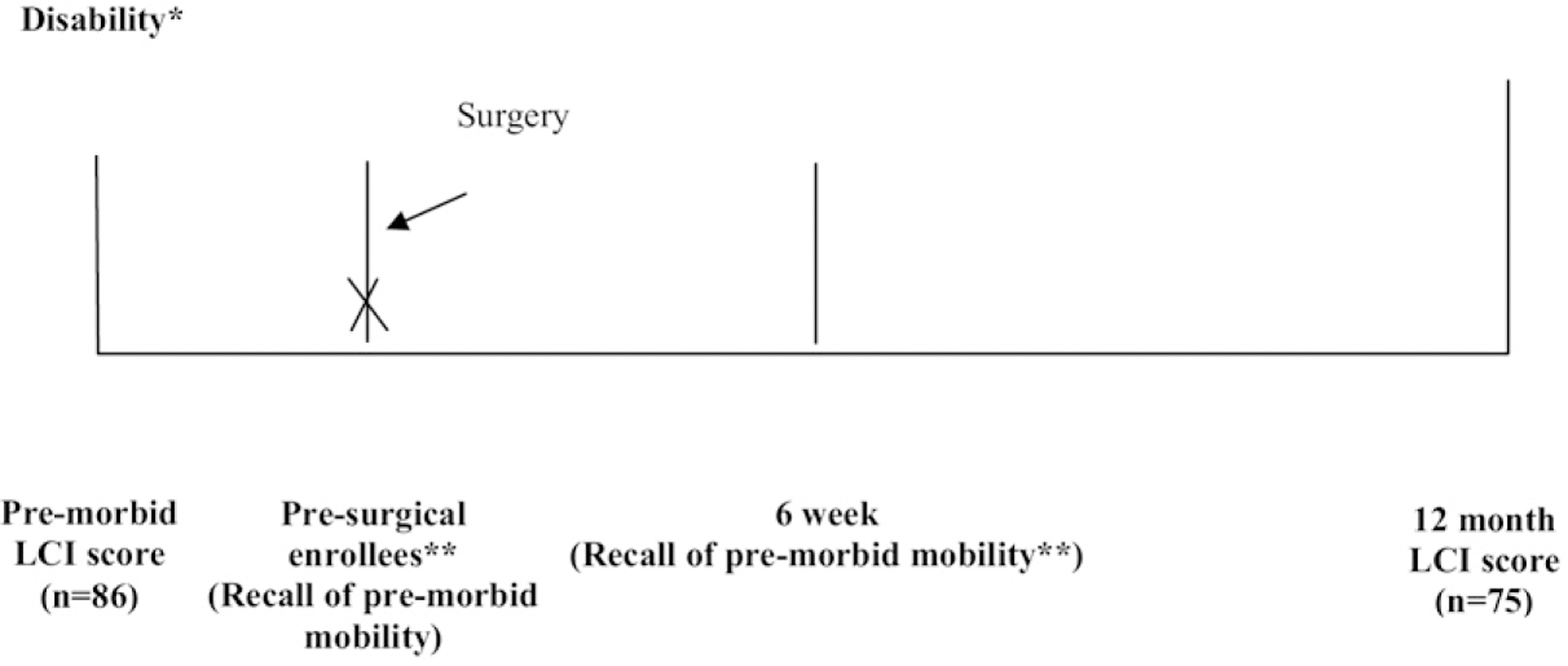
Time points for assessing level of mobility from premorbid function to 12 months after amputation. *The patient’s recall of his or her mobility before a decline in function due to disability in the limb that underwent amputation. One subject died after enrollment before the locomotor capability index (LCI) score could be assessed. **Subjects who were enrolled presurgically completed the LCI questionnaire then and at 6 weeks to assess their premorbid mobility. These two scores demonstrated strong agreement with a Spearman correlation coefficient of 0.83 (P < .003).
Participants
Two hundred thirty-nine individuals were screened for participation between September 2005 and December 2008. Subjects were considered eligible if (1) they were age 18 years or older, (2) they were awaiting (or underwent in the last 6 weeks) a first major amputation (defined as TM level or higher), and (3) the primary cause of amputation was complications of diabetes or PVD. Criteria for a dysvascular amputation etiology were met if the medical record confirmed that the amputation was necessitated by peripheral vascular disease and/or diabetes, and there was no significant history of extremity trauma or tumor. Subjects were excluded if: (1) they had inadequate cognitive or language function to consent or participate, defined by ≥6 errors on the Short Portable Mental Status Questionnaire, or (2) they were nonambulatory before the amputation for reasons unrelated to PVD or diabetes. Of the 239 individuals screened, 136 (57%) met study criteria. Thirteen subjects were excluded as we were unable to verify eligibility due to privacy standards at one facility. Eighty-seven subjects (64%) participated (Fig 2).
Fig 2.
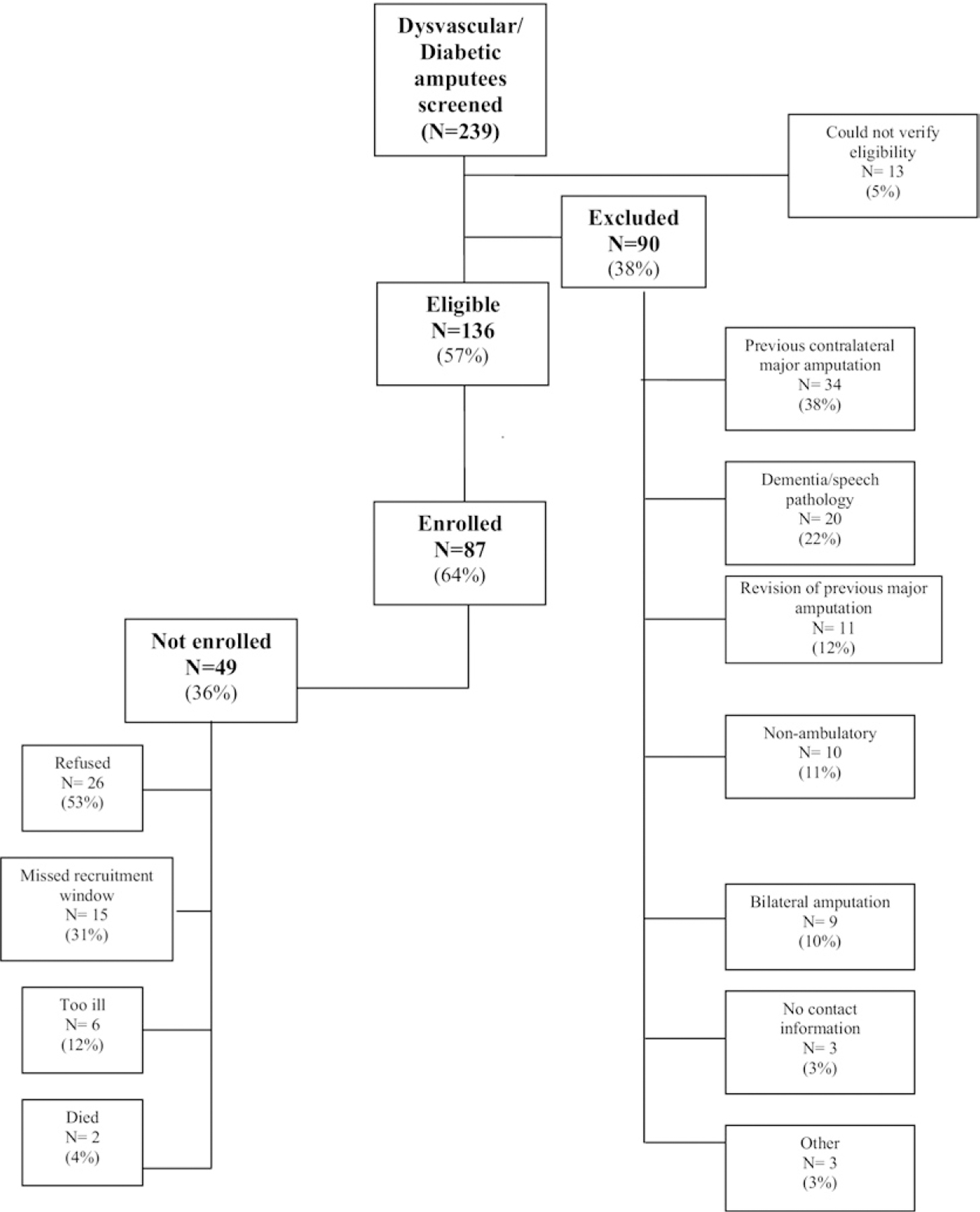
Diagram depicting screening and enrollment numbers.
Procedures
Presurgical assessment.
The presurgical status assessment was comprised of information collected via interview and verified via medical record review. Measures included demographics, information about the index amputation, health factors, and mobility. Time between the decision to amputate and surgery was often brief, and we were unable to enroll all subjects prior to surgery. Subjects enrolled prior to surgery (n = 29, 33%) completed the presurgical status assessment at the time of enrollment, while those enrolled 6 weeks postsurgically completed a retrospective assessment of their presurgical status. Retrospective questions were as identical as possible to questions on the presurgical battery. The study coordinator prefaced the questions by saying, “In the following questions, we are asking you to tell us about your life and health in the week BEFORE your amputation.”
The primary etiology and the anatomic level of amputation was categorized as TM, TT, or TF as reported in the medical record and confirmed during interview. The Charlson comorbidity index11 was used to determine the presence of presurgical comorbid conditions. Additional comorbid conditions hypothesized to be relevant in these populations were also assessed, see Table I. Smoking status was assessed by three standard questions from the VA Large Health Survey. Subjects were considered smokers if they endorsed smoking “every day” or “some days” prior to amputation, and non-smokers if they endorsed the remaining category “not smoke at all.” A three-item version of the Alcohol Use Disorders Identification Test (AUDIT-C) was used to assess alcohol consumption patterns in the past year.12 Possible scores range from 0 to 12, with higher scores indicating greater alcohol misuse severity, and a score of “4 or more” and “3 or more” identifying alcohol misuse in men and women, respectively. To ensure the reliability of presurgical recall, we evaluated the test–retest reliability comparing presurgical with 6 week postsurgery self-report among the participants enrolled presurgically. The κ coefficient for smoking was 0.53 (P = .03) and the intraclass correlation coefficient for total AUDIT-C score was 0.98 (P < .001), suggesting that retrospective recall 6 weeks after surgery is a reliable way to assess premorbid status, especially for alcohol use. All presurgical assessment measures are presented in Table I.
Table I.
Baseline socio-demographic and general health data by amputation levela
| Variable | TM (n = 27) | TT (n = 52) | TF (n = 8) | P valuec |
|---|---|---|---|---|
| Age | 63.0 ± 7.8 | 61.5 ± 9.1 | 62.5 ± 10.0 | .61 |
| Female | 0 (0) | 6 (12) | 1 (13) | .18 |
| BMI | 29.8 ± 6.0 | 31.6 ± 7.8 | 34.0 ± 9.0 | .15 |
| Marital status | .35 | |||
| Not married/partner | 9 (33) | 25 (48) | 4 (57) | |
| Married/partner | 18 (67) | 27 (52) | 3 (43) | |
| Race | .23 | |||
| Caucasian | 19 (70) | 46 (88) | 8 (100) | |
| Black | 6 (22) | 3 (6) | 0 (0) | |
| Other | 2 (7) | 3 (6) | 0 (0) | |
| Employment status | .10 | |||
| Not employed | 27 (100) | 44 (85) | 6 (86) | |
| Employed | 0 (0) | 8 (15) | 1 (14) | |
| Education level | .85 | |||
| Some high school | 2 (7) | 18 (67) | 7 (26) | |
| High school grad | 3 (6) | 39 (75) | 10 (19) | |
| College grad | 0 (0) | 6 (86) | 1 (14) | |
| Living status | .69 | |||
| Home alone | 9 (33) | 13 (25) | 4 (57) | |
| Home with spouse/other | 16 (59) | 33 (64) | 3 (43) | |
| SNF/nursing home | 1 (4) | 4 (8) | 0 (0) | |
| Other | 1 (4) | 2 (4) | 0 (0) | |
| Socioeconomic status | .57 | |||
| ≤25,000 | 12 (44) | 24 (46) | 2 (29) | |
| 25,001–50,000 | 8 (30) | 21 (40) | 3 (43) | |
| >50,000 | 7 (26) | 7 (14) | 2 (29) | |
| Charlson | .83 | |||
| Low | 8 (30) | 9 (17) | 1 (14) | |
| Moderate | 6 (22) | 16 (31) | 2 (29) | |
| High | 9 (33) | 15 (29) | 2 (29) | |
| Very high | 4 (15) | 12 (23) | 2 (29) | |
| Diabetesb | 27 (100) | 44 (85) | 4 (50) | .001 |
| Strokeb | 5 (19) | 11 (21) | 1 (13) | .84 |
| Heart attackb | 9 (33) | 17 (33) | 3 (38) | .97 |
| Dialysisb | 11 (41) | 19 (37) | 2 (29) | .83 |
| Chronic obstructive pulmonary diseaseb | 1 (4) | 7 (13) | 1 (13) | .39 |
| Lower extremity arterial reconstruction | 11 (41) | 19 (37) | 2 (29) | .83 |
| Traumatic brain injury | 8 (30) | 11 (21) | 3 (43) | .39 |
| Joint replacement | 2 (7) | 5 (10) | 1 (14) | .85 |
| Hypertension | 19 (70) | 33 (64) | 7 (88) | .38 |
| Posttraumatic stress disorder | 3 (11) | 8 (15) | 1 (14) | .87 |
| Smoker | 5 (19) | 22 (42) | 5 (71) | .02 |
| Alcohol consumption | .54 | |||
| Negative screen | 24 (89) | 42 (81) | 5 (71) | |
| Positive or serious disorder | 3 (11) | 10 (19) | 2 (29) | |
| Premorbid LCI score | 49.1 ± 9.8 | 47.1 ± 12.3 | 47.9 ± 8.9 | .76 |
LCI, Locomotor capability index; SNF, skilled nursing facility; TF, transfemoral; TM, transmetatarsal; TT, transtibial.
Incomplete numbers represent missing values.
Comorbidities obtained from the Charlson comorbidity index.
P value based on analysis of variance (ANOVA) for age and body mass index (BMI), and on χ2 test for the categorical variables.
Follow-up assessments.
Follow-up interviews were obtained 12 months postamputation by in-person interview if possible, otherwise via telephone. Global comparisons of telephone and in-person interviews have consistently demonstrated strong agreement across many types of measures, such as health behaviors and mental health issues, suggesting this is a reasonable method.13–15
Primary outcome measure: Mobility success
Mobility success was defined dichotomously. Success occurred when the level of mobility at 12 months was the same as or greater than the premorbid mobility level. The level of mobility at each time period was measured using the locomotor capability index (LCI-5). The LCI-5 consists of 14 items graded on a five-level ordinal scale ranging from unable to perform the activity (0), to able to perform alone without aids.4,16 Scores for the LCI-5 range from 0 to 56 with higher scores representing higher function. The LCI-5 has well-established internal consistency, test–retest reliability, and validity. Additionally, it has a lower ceiling and a larger effect size than the original LCI, making it an appropriate tool for detecting functional change in this population.16
Premorbid mobility was defined as the level of mobility prior to the development of disability (eg, ulcer, edema, associated pain) in the limb undergoing amputation. Subjects who reported disability in their amputated limb were asked to complete the LCI-5 based on their function “immediately prior to developing any limitations in your leg awaiting amputation.”
Premorbid mobility was collected 6 weeks after surgery for all enrolled subjects (n = 86; one subject passed away after enrollment before function could be assessed). For the 29 subjects recruited presurgically, premorbid mobility was also collected prior to amputation, at the time of enrollment. To assess the reliability of our retrospective premorbid mobility measure, the responses at the two time periods were compared. The intraclass correlation of the 6-week postsurgically assessed premorbid score (agreement if recall was within three points) to the presurgical recall of the premorbid score was 0.87 (P = .003). To further ensure the reliability of the mobility measure, the 6-week recall of premorbid mobility was compared between those recruited presurgically and those recruited post surgically, and the mean difference was only 1.6 points (P = .52). The 6-week recall of premorbid mobility was therefore used as the basis for the determination of mobility success for all subjects.
Secondary outcome measures: Satisfaction with mobility and satisfaction with life
Satisfaction with mobility at 12 months was determined through a single item created for this study: “How satisfied are you with your current walking ability?” Subjects responded using a 0 to 10 point Likert scale where 0 represented “not at all satisfied” and 10 “extremely satisfied.” A mean score of ≥6 on this scale was defined as “satisfied.” To measure more global life satisfaction, we used the Satisfaction with Life Scale (SWLS).17 This five-item measure is designed to measure the judgmental or cognitive component of subjective well-being. Each item is scored on a Likert scale, with response options ranging from 1 (strongly disagree) to 7 (strongly agree). Items are summed, such that possible scores range from 5 to 35, and higher scores indicate greater satisfaction. The measure possesses good internal consistency, test–retest reliability, and validity.18 The 12-month median score was 19; therefore, we considered a score of 20 points or above as “satisfied” and a score of less than 20 points as “unsatisfied.”
Data analysis
Descriptive statistics of presurgical variables are presented in Table I. Differences by amputation level for categorical and continuous variables were made using Pearson χ2 tests and analysis of variance (ANOVA), respectively. Multivariate associations of presurgical factors with mobility success were examined using forward stepwise negative binomial regression with risk differences (RD) and 95% confidence intervals (CI). Only variables with adjusted P values <.05 were included in the final models. Amputation level was included in the final model regardless of statistical significance. All variables listed in Table I were assessed.
Utility of mobility success outcome measure
We examined the utility of “mobility success” as an outcome measure through a series of analyses. First, we examined if there was a positive association between mobility success and both satisfaction with mobility and satisfaction with life. Second, we determined if the mobility success measure was able to identify individuals with low to moderate levels of mobility at 12 months who might still be perceived as having a successful outcome because they returned to their previous level of function and were satisfied with mobility. To accomplish this, we categorized the LCI 12-month scores into three levels based on the total score for each column of response categories. We created categories described as “needing help from others,” “needing help from ambulation aids,” and “independent” using the following cutoffs, respectively: 0 to 27, 28 to 42, and 42 to 56 points, respectively.
RESULTS
Baseline characteristics.
Among the 87 participants enrolled, four participants (5%) formally withdrew, two were lost to follow-up (2%), and six participants (7%) passed away over 12-month follow-up period. Seventy-five participants completed their 12-month interview (86%) (Fig 2). The majority of the 87 subjects enrolled in the study were TT (60%) followed by TM (31%) amputees. One participant had a knee disarticulation that was classified as a TF amputation. Most baseline demographics and health factors were similar across amputation levels. However, all subjects were unemployed (retired or not employed) in the TM group while a small proportion were employed in the TT and TF groups. Additionally, there was a significantly (P < .01) larger proportion of individuals with diabetes in the TM group (100%) compared with the TT (85%) and TF groups (50%). The proportion of smokers increased with higher levels of amputation: 19%, 42%, and 71% for TM, TT, and TF amputees, respectively (P < .05). It is also noteworthy that premorbid mobility (as measured by the LCI score prior to disability) was similar between all amputation levels, Table II (P = .76).
Table II.
Success rates and LCI-5 baseline, 12-month follow-up, and change scores among those who achieved their 12-month follow-up
| Mobility | TM (n = 26) | TT (n = 42) | TF (n = 7) | P value |
|---|---|---|---|---|
| Success rate (%) | 35 | 41 | 29 | .78a |
| Premorbid LCI-5 score | 49.1 ± 9.8 | 47.1 ± 12.3 | 47.9 ± 8.9 | |
| 12-month LCI-5 score | 43.2 ± 12.5 | 40.7 ± 16.2 | 33.0 ± 20.9 | |
| Change score | −5.9 ± 11.5 | −6.4 ± 14.7 | −14.9 ± 7.3 | .19b |
LCI, Locomotor capability index; TF, transfemoral; TM, transmetatarsal; TT, transtibial.
Pearson χ2 test.
Comparing between group change scores using analysis of variance.
Mobility success rates by amputation level.
As shown in Table II, the mean change in mobility from premorbid function to 12 months after surgery for each amputation group was negative, ie, there was a decline in mobility over time. The differences were not statistically significant, despite a large decrease in mobility among the TF group. This is likely due to the small sample of TF subjects. Overall, 28 subjects (37%) achieved mobility success, and rates of mobility success were comparable across amputation levels (35% TM, 41% TT, and 29% TF, P = .78, see Table II).
Factors associated with mobility success.
As shown in Table III, controlling for amputation level, those subjects who were 65 years or older had a 52% lower success rate than subjects 45 to 54 years of age (RD = −0.52; 95% CI: −0.75, −0.29, P < .001). Subjects with an alcohol disorder had a 37% lower success rate than those without a disorder (RD = −0.37; 95% CI: −0.48, −0.26, P < .001). Subjects with hypertension had a 23% lower success rate than those without hypertension (RD = −0.23; 95% CI: −0.43, −0.3, P = .02), and those who had been treated previously for anxiety or depression had a 39% lower success rate than those who had not (RD = −0.39; 95% CI: −0.50, −0.28, P < .001).
Table III.
Multivariate results for 12-month mobility success
| Risk factor | RDa (95% CI) | P Value |
|---|---|---|
| Amputation levelb | ||
| TT | 0.17 (−0.02, 0.36) | .08 |
| TF | 0.06 (−0.13, 0.26) | .50 |
| Agec | ||
| 55–64 years | −0.10 (−0.36, 0.15) | .43 |
| 65 years | −0.52 (−0.75, −0.29) | <.001 |
| HTN (yes) | −0.23 (−0.43, −0.03) | .02 |
| AUD (yes)d | −0.37 (−0.48, −0.26) | <.001 |
| MH TX (yes) | −0.39 (−0.50, −0.28) | <.001 |
AUD, Alcohol use disorder; CI, confidence interval; HTN, hypertension; MH TX, prior treatment for anxiety or depression; RD, risk difference; TF, transfemoral; TM, transmetatarsal; TT, transtibial.
Risk differences generated from a negative binomial regression model represent an increase (or decrease if negative) in the success rate relative to reference category.
TM = reference category.
45 to 54 years = reference category.
positive screen or serious alcohol disorder; negative for alcohol disorder = reference category.
Association between mobility success and mobility satisfaction and satisfaction with life.
Among subjects who reached the 12-month follow-up, 57% (n = 43) were satisfied with their mobility (62%, 50%, and 29% for TM, TT, and TF, respectively). Among the subjects who achieved mobility success (n = 28), 20 (71%) were satisfied with their mobility and eight (29%) were dissatisfied. When controlling for amputation level, age, and alcohol disorder, those who achieved mobility success had a 36% higher mobility satisfaction rate than those who did not achieve success (RD = 0.36; 95% CI: 0.20, 0.53, P < .001). Transfemoral amputees had a 32% lower mobility satisfaction rate than TM amputees (RD = −0.32; 95% CI: −0.60, −0.04, P = .03). There was no statistically significant difference between TT and TM amputees.
Similarly, those who achieved mobility success at 12 months were more likely to be satisfied with life than those that did not (61% vs 34%, respectively; P = .02) and similarly had higher mean SWLS scores (22.8 ± 8.4 vs 17.7 ± 8.3, respectively; P = .01). When controlling for amputation level, those who achieved mobility success had a 28% higher satisfaction with life rate than those who did not (RD = 0.28; 95% CI: 0.06, 0.50, P = .01).
Association between 12-month LCI scores and satisfaction scores.
When controlling for amputation level, age, baseline mobility, prior stroke, and presence of chronic obstructive pulmonary disease, 12-month satisfaction with mobility was significantly associated with the 12-month LCI score (coefficient = 15.186; P < .001), suggesting that those with higher mobility scores were more likely to be satisfied with their mobility.
When controlling for amputation level, age, baseline mobility, prior stroke, and presence of chronic obstructive pulmonary disease, 12-month satisfaction with life was significantly associated with the 12-month LCI score (coefficient = 8.159; P < .01), suggesting that those with higher mobility scores were more likely to be satisfied with life.
Benefits gained with the mobility success outcome versus mobility score.
Since both mobility success and 12-month LCI scores were significantly associated with mobility satisfaction and life satisfaction, we attempted to determine if any benefit is gained by using the mobility success score. We were interested in subjects with low to moderate mobility levels at 12 months who also met the criteria for mobility success. These were subjects who one might assume would not be satisfied with mobility or life based on their lower 12-month mobility scores. If a reasonable proportion of these subjects were also satisfied, then perhaps our measure of mobility success adds additional value to the assessment of outcome after amputation. Six of the 28 who achieved mobility success (21%) were categorized as “needing help from others” (<28 points) to “needing help from ambulation aids” (28–42 points) 12 months after surgery. Three of these six (50%) were satisfied with their mobility. Two of the six (33%) were satisfied with life so we did identify individuals who ordinarily may have been considered failures or poor outcomes due to their lower 12-month LCI scores.
DISCUSSION
The primary goal of this investigation was to describe a novel approach to the measurement of “mobility success” that could account for the sum total of underlying impairments and comorbidities predating the amputation. Further, this definition does not base success simply on how well the patient is functioning 12 months after surgery, rather, it quantifies success based on the patient’s final level of mobility relative to disability preceding the amputation. In exploring the utility of this approach, we examined the relationship between mobility success, satisfaction with mobility, and satisfaction with life. Additionally, this investigation compared “mobility success” across amputation levels and identified patient-related variables associated with mobility success.
There is increasing recognition of the importance of patient-reported measures when examining lower extremity amputation outcomes.9 The surgical care and subsequent rehabilitation process after amputation seeks to optimize and restore the physical, mental, emotional, social, vocational, and economic outcome of each patient.19 To our knowledge, there are no measures of adequate complexity and comprehensiveness to evaluate all of these domains, and efforts to define successful outcome have received very limited study. Although focused on surgical outcome for critical limb ischemia, Landry argues that improvements in functional status are difficult to define because of the numerous and often severe comorbid conditions in this patient population.9 He and others20 suggest that it is the improvement in function relative to a baseline functional status that is critical. Mobility has been one of the key outcomes used to quantify functional improvement after amputation. A number of investigators have attempted to quantify differences between pre- and post-amputation mobility21–23 but have not identified returning to or exceeding pre-amputation mobility as a measure of success. In this study, mobility success allows mobility to be assessed in the context of background psychological, physical, and social factors that may contribute to mobility impairment. At 12 months, only 37% of patients achieved mobility success. The factors associated with a failure to achieve mobility success controlling for amputation level were age 65 years or older, alcohol disorder, hypertension, and previous history of being treated for anxiety or depression. The contributions of these factors to mobility outcome have been evaluated by previous studies using other mobility measures, often using cross-sectional or retrospective study designs and often without multivariate analyses. They have confirmed the adverse effect of increasing age on outcome,22–24 as well as the association between anxiety and depression and ongoing activity limitation.25,26 The association between alcohol use disorder and mobility in amputees has received little study. One of the major advantages of this prospective study over cross-sectional studies is that we can be sure these exposures preceded the outcome.
In contrast to previous investigations that suggest the more distal the amputation level the better the mobility outcome,4,22 this investigation did not show a significant relationship between amputation level and mobility success. The absence of a significant difference in mobility outcome after TF amputation compared with the more distal amputation levels is likely related to the very small number of TF amputees in the study population, and the variability in their mobility measures. There was a nonsignificant trend suggesting the mobility success rate for TT amputees was 17% higher than in TM amputees (P = .08). This finding is counterintuitive given the current emphasis on preserving as much limb length as possible to maximize the restoration of mobility. Baseline demographic data showed that TM amputees were less likely to be smokers but more likely to have diabetes than those who underwent more proximal amputation levels. The increased frequency of diabetes may have been associated with the presence of multiple diabetes-related comorbid medical conditions that were not accounted for in the Charlson comorbidity index, which was designed primarily to predict the effect of comorbid medical conditions on mortality.
One of the key considerations in evaluating any outcome measure is whether or not it is valid and relevant to patients. The relationship between level of mobility and patient satisfaction in lower extremity amputees has received limited study. Some suggest a relationship between walking ability and life satisfaction,27 while others show no relationship.28 Our study did identify a relationship between mobility success and mobility satisfaction as well as satisfaction with life. Those who achieved mobility success were 36% more likely to be satisfied with their mobility and 28% more likely to be satisfied with life than those who did not. The significant correlations suggest that mobility success is a measure that is relevant and important from a patient perspective.
Several limitations of the current study are worthy of note. The sample was restricted to patients with at least a minimum level of ambulatory function prior to amputation and with the cognitive capacity to participate in an interview, limiting generalization. In addition, the primary ambulation outcome was collected through interview and may be subject to the limitations of self-report. Concerns about this issue are at least partially mitigated by the positive psychometric properties of the LCI, and a strong movement toward patient reported outcomes for outcomes research. Additionally, recent investigation has shown that there is a high level of correlation between capacity and performance measures in lower extremity amputees. Specifically the LCI-5 used in this study was strongly correlated with the 2-minute walk test and the timed get up and go test.29 We based our definition of mobility success on what we defined as premorbid mobility. This represented mobility prior to a decline in function that commonly occurs as a result of disability in the limb awaiting amputation and comorbid disease. It is possible that asking subjects to recall their premorbid mobility 6 weeks after surgery was subject to recall bias, although the time period was brief, and a test–retest reliability subsample of our presurgical enrollees demonstrated strong agreement between time points. We also had subjects recall some of their presurgical risk factors to include smoking and alcohol. With alcohol, which was a covariate in our final model, there was near perfect agreement. The majority of the other presurgical factors we were able to verify in the medical record. Our overall sample size limited the ability to examine characteristics of specific amputation types (most notably TFs) in greater detail but we believe the final number we achieved was noteworthy given the prospective nature of the study.
CONCLUSION
We have reported a novel method for measuring mobility success that allows the evaluation of mobility in the context of a patient’s premorbid mobility level. This allows the incorporation of the effects of premorbid mobility impairments in this population with a high incidence of comorbidities. The utility of this proposed measure is strengthened by its significant relationship with satisfaction with mobility and satisfaction with life. We have also shown amputation level is not significantly associated with mobility success and have determined the importance of various presurgical risk factors on mobility success. These include subjects 65 years of age and older, those with an alcohol disorder, hypertension, and previous history of being treated for anxiety or depression.
Acknowledgments
Supported by the US Department of Veterans Affairs, Office of Research and Development, Rehabilitation Research and Development (Merit Review A41241 Joseph Czerniecki, PI, and Career Development Award B4927W Aaron Turner, PI).
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
REFERENCES
- 1.Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J 2002;95:875–83. [DOI] [PubMed] [Google Scholar]
- 2.Campbell WB, St Johnston JA, Kernick VF, Rutter EA. Lower limb amputation: striking the balance. Ann R Coll Surg Engl 1994;76: 205–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz CP, Eidt JF, Capps C, Kirtley L, Moursi MM. Major lower extremity amputations at a Veterans Affairs Hospital. Am J Surg 2003; 186:449–54. [DOI] [PubMed] [Google Scholar]
- 4.Larsson J, Agardh CD, Apelqvist J, Stenstrom A. Long-term prognosis after healed amputation in patients with diabetes. Clin Orthop Relat Res 1998;350:149–58. [PubMed] [Google Scholar]
- 5.Narang IC, Mathur BP, Singh P, Jape VS. Functional capabilities of lower limb amputees. Prosthet Orthot Int 1984;8:43–51. [DOI] [PubMed] [Google Scholar]
- 6.Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, et al. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg 2003;38:7–14. [DOI] [PubMed] [Google Scholar]
- 7.Pernot HF, Winnubst GM, Cluitmans JJ, De Witte LP. Amputees in Limburg: incidence, morbidity and mortality, prosthetic supply, care utilisation and functional level after one year. Prosthet Orthot Int 2000;24:90–6. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SM, Kalbaugh CA, Cass AL, Buzzell NM, Daly CA, Cull DL, et al. “Successful outcome” after below-knee amputation: an objective definition and influence of clinical variables. Am Surg 2008;74:607–12. [DOI] [PubMed] [Google Scholar]
- 9.Landry GJ. Functional outcome of critical limb ischemia. J Vasc Surg 2007;45:A141–8. [DOI] [PubMed] [Google Scholar]
- 10.van Velzen JM, van Bennekom CA, Polomski W, Slootman JR, van der Woude LH, Houdijk H. Physical capacity and walking ability after lower limb amputation: a systematic review. Clin Rehabil 2006;20:999–1016. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 12.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789–95. [DOI] [PubMed] [Google Scholar]
- 13.Aziz MA, Kenford S. Comparability of telephone and face-to-face interviews in assessing patients with posttraumatic stress disorder. J Psychiatr Pract 2004;10:307–13. [DOI] [PubMed] [Google Scholar]
- 14.Midanik LT, Greenfield TK. Telephone versus in-person interviews for alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depend 2003;72:209–14. [DOI] [PubMed] [Google Scholar]
- 15.Pridemore WA, Damphousse KR, Moore RK. Obtaining sensitive information from a wary population: a comparison of telephone and face-to-face surveys of welfare recipients in the United States. Soc Sci Med 2005;61:976–84. [DOI] [PubMed] [Google Scholar]
- 16.Franchignoni F, Orlandini D, Ferriero G, Moscato TA. Reliability, validity, and responsiveness of the locomotor capabilities index in adults with lower-limb amputation undergoing prosthetic training. Arch Phys Med Rehabil 2004;85:743–8. [DOI] [PubMed] [Google Scholar]
- 17.Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction with Life Scale. J Personal Assess 1985;49:71–5. [DOI] [PubMed] [Google Scholar]
- 18.Pavot W, Diener E, Colvin CR, Sandvik E. Further validation of the Satisfaction with Life Scale: evidence for the cross-method convergence of well-being measures. J Personal Assess 1991;57:149–61. [DOI] [PubMed] [Google Scholar]
- 19.Geertzen JH, Martina JD, Rietman HS. Lower limb amputation. Part 2: rehabilitation--a 10-year literature review. Prosthet Orthot Int 2001; 25:14–20. [DOI] [PubMed] [Google Scholar]
- 20.Kent R, Fyfe N. Effectiveness of rehabilitation following amputation. Clin Rehabil 1999;13(Suppl 1):43–50. [DOI] [PubMed] [Google Scholar]
- 21.Johnson VJ, Kondziela S, Gottschalk F. Pre- and post-amputation mobility of trans-tibial amputees: correlation to medical problems, age and mortality. Prosthet Orthot Int 1995;19:159–64. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, et al. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg 2005;42:227–35. [DOI] [PubMed] [Google Scholar]
- 23.Sansam K, Neumann V, O’Connor R, Bhakta B. Predicting walking ability following lower limb amputation: a systematic review of the literature. J Rehabil Med 2009;41:593–603. [DOI] [PubMed] [Google Scholar]
- 24.Oneill C, Jamison J, McCulloch D, Smith D. Age-related macular degeneration: cost-of-illness issues. Drugs Aging 2001;18:233–41. [DOI] [PubMed] [Google Scholar]
- 25.Callaghan B, Condie E, Johnston M. Using the common sense self-regulation model to determine psychological predictors of prosthetic use and activity limitations in lower limb amputees. Prosthet Orthot Int 2008;32:324–36. [DOI] [PubMed] [Google Scholar]
- 26.Schoppen T, Boonstra A, Groothoff JW, de Vries J, Goeken LN, Eisma WH. Physical, mental, and social predictors of functional outcome in unilateral lower-limb amputees. Arch Phys Med Rehabil 2003;84:803–11. [DOI] [PubMed] [Google Scholar]
- 27.Deans SA, McFadyen AK, Rowe PJ. Physical activity and quality of life: a study of a lower-limb amputee population. Prosthet Orthot Int 2008;32:186–200. [DOI] [PubMed] [Google Scholar]
- 28.Remes L, Isoaho R, Vahlberg T, Viitanen M, Koskenvuo M, Rautava P. Quality of life three years after major lower extremity amputation due to peripheral arterial disease. Aging Clin Exp Res 2010;22:395–405. [DOI] [PubMed] [Google Scholar]
- 29.Parker K, Kirby RL, Adderson J, Thompson K. Ambulation of people with lower-limb amputations: relationship between capacity and performance measures. Arch Phys Med Rehabil 2010;91:543–9. [DOI] [PubMed] [Google Scholar]


