Abstract
This was a nation-wide retrospective study in Japan examining women who underwent radical hysterectomy for clinical stage IB–IIB cervical cancer with pelvic and/or para-aortic lymph node metastasis between 2004 and 2008. Time to recurrence or death and patterns of disease recurrence were compared based upon the adjuvant treatment pattern: whole pelvic radiotherapy alone (n = 253), concurrent chemoradiotherapy (CCRT, n = 502) and chemotherapy alone (n = 319). Women who received chemotherapy alone had similar recurrence (5-year rates, 36.6% vs. 34.1%, adjusted-hazard ratio [HR] 0.95, 95% confidence interval [CI] 0.70–1.28, P = 0.72) and cervical cancer mortality (24.7% vs. 21.8%, adjusted-HR 0.96, 95% CI 0.67–1.38, P = 0.83) rates compared to those who received CCRT on multivariate analysis. However, when recurrence patterns were stratified, chemotherapy treatment was independently associated with decreased risk of distant recurrence (5-year cumulative rates, 19.2% vs. 24.6%, adjusted-HR 0.47, 95% CI 0.31–0.71, P < 0.001) but increased risk of local recurrence (23.9% vs. 14.3%, adjusted-HR 2.03, 95% CI 1.34–3.08, P = 0.001) compared to CCRT. Non-squamous histology, parametrial involvement and high lymph node ratio were independent predictors for local recurrence, and presence of multiple risk factors was associated with high 5-year cumulative local recurrence rate in the chemotherapy group: no risk factor 3.9%, single factor 14.2–22.1%, and multiple risk factors 27.8–71.9% (P < 0.001). In conclusion, while exhibiting different recurrence patterns, systemic chemotherapy may be as effective a postoperative treatment as radiation-based therapy in node-positive high-risk stage IB–IIB cervical cancer. When tumor exhibits certain risk factors, chemotherapy alone is likely insufficient for local control and adding pelvic irradiation to systemic chemotherapy is recommended in this subgroup.
Keywords: cervical cancer, radical hysterectomy, early stage, nodal metastasis, chemotherapy
Cervical cancer remains the most common gynecologic malignancy worldwide.1 While advanced-stage disease is often difficult to cure, early-stage disease generally has a good prognosis and cure is highly achievable with treatment (5-year overall survival rate, 94.8–97.5% for stage IA, 75.7–89.1% for stage IB, 65.8–73.4% for stage II, 39.7–41.5% for stage III and 9.3–22.0% for stage IV diseases).2 Surgery remains the mainstay of treatment for early-stage cervical cancer, consisting of radical hysterectomy and pelvic lymphadenectomy.3 Surgical-pathological risk factors are histological findings from the tumor specimen, and these are historically classified into intermediate- and high-risk groups based on the type of tumor factors.4–6 Pelvic lymph node involvement is considered a high-risk factor associated with adverse survival outcome.3,6
Per the National Comprehensive Cancer Network guidelines, use of pelvic irradiation with concurrent administration of weekly chemotherapy (CCRT) is recommended as adjuvant therapy for node-positive early-stage cervical cancer.7 However, a recent meta-analysis showed that CCRT is associated with increased risk of severe toxicity.8 Owing to this toxicity profile which is mostly related to radiotherapy, systemic chemotherapy has been considered as an alternative treatment option for adjuvant therapy after primary surgical treatment for this high-risk group. While some studies suggested a possible utility of systemic chemotherapy, others concluded that systemic chemotherapy does not improve survival outcome.9–14 However, these studies were either performed with relatively small sample sizes or lacked specific data for node-positive high-risk disease making the results difficult to adopt.
The objective of this study was to examine survival outcomes and recurrence patterns of women with node-positive stage IB–IIB cervical cancer who received only postoperative systemic chemotherapy after radical hysterectomy as compared to those women who received radiation-based therapy.
Patients and Methods
Eligibility
This was a nation-wide large-scale retrospective observational study conducted in 116 Japanese Gynecologic Oncology Group (JGOG) designated institutions. The survey collected consecutive cases of clinical stage IB–IIB cervical cancer treated with primary radical hysterectomy between January 1, 2004 and December 31, 2008. The survey period for the data acquisition was between October 1, 2012 and February 28, 2013. Institutional Review Board approval was obtained at Tottori University and served as the host institution, and the JGOG-participating institutions reviewed the protocol and obtained the Institutional Review Board approval as indicated.
Eligibility criteria for this study were node-positive high-risk stage IB–IIB cervical cancer treated with adjuvant therapy after upfront type III radical hysterectomy and pelvic lymphadenectomy. Node metastasis included pelvic and/or para-aortic lymph nodes. Women who received neoadjuvant therapy prior to hysterectomy had distant metastasis other than para-aortic lymph node and microscopic findings of malignant cells in peritoneal cytology, and who did not receive adjuvant treatment were excluded from the analysis. The STROBE guidelines for a retrospective observational study were consulted to outline this study.15
Clinical information
Clinical and tumor information was abstracted from archived medical and pathological records by investigators in each participating institution. These included age, histologic subtype, clinical and pathological stages, tumor size, pelvic/para-aortic lymph node status (sampled number and tumor-involved number), parametrial involvement, deep stromal invasion (outer half), lymphovascular space invasion, uterine corpus involvement, peritoneal cytology results, ovarian involvement and presence of distant metastasis at surgery. LNR was defined as the percent proportion of tumor-involved lymph node among total sampled lymph nodes, dichotomized with the median value based on a previous study (high vs. low, ≥6.6% vs. <6.6%).16
Adjuvant treatment information included the three modalities: whole pelvic radiotherapy alone (RT group), concurrent chemoradiotherapy which consisted of pelvic irradiation and weekly chemotherapy administration (CCRT group) and systemic chemotherapy alone (chemotherapy group). Use of additional radiotherapy for aortic boost and vaginal brachytherapy were also recorded. Regimen and administered cycles of chemotherapy were abstracted.
Survival information included time to recurrence and death from cervical cancer. Time to recurrence was defined as the time interval between the hysterectomy date and the first recurrent date. Time to death was defined as the time interval between the date of hysterectomy and the date of death due to cervical cancer. The patients were censored if alive at the last follow-up or died of another cause. Among recurrent cases, locations of recurrence were grouped into local recurrence (vaginal cuff and/or pelvis) and distant recurrence (any site other than local).
Statistical analysis
The primary interest of analysis was to examine survival outcome of women with chemotherapy-treated node-positive stage IB–IIB cervical cancer. The secondary interest of analysis was to profile recurrence patterns based on types of adjuvant therapy.
Kaplan–Meier method was used to construct cumulative incidence curves for recurrence and cervical cancer death,17 and statistical significance between the curves was determined by log-rank test. Cox proportional hazard regression models were used for multivariate analysis.18 First, an association of adjuvant treatment patterns and survival outcomes were adjusted by a priori survival factors such as age, histology, parametrial involvement, deep stromal invasion, tumor size, lymphovascular space invasion, uterine corpus involvement, ovarian involvement, peritoneal cytology and lymph node status. Second, an association of adjuvant treatment pattern and recurrence pattern (local vs. distant) was adjusted by significant survival factors as above. Magnitude of statistical significance was expressed with adjusted HR and 95% CI.
As an alternative corroboration to examine the association of adjuvant chemotherapy and survival outcome of women with node-positive clinical stage IB–IIB cervical cancer, we performed propensity score matching to adjust the background differences between the chemotherapy group and the CCRT group. Propensity score for chemotherapy treatment was computed for each case determined by multivariate logistic regression analysis (chemotherapy vs. CCRT). Patient demographics and tumor factors were entered in the propensity score model. One-to-one propensity score matching between the chemotherapy group and the CCRT group was performed via an automated algorithm with the propensity score difference cut-off being 1%.
Additionally, a subgroup analysis was performed to examine histology subtypes (squamous vs. non-squamous). For each histology subtype, association of treatment modality (chemotherapy vs. radiotherapy) and recurrence pattern was examined on multivariate analysis as described above. The ratio of events of interest per the adjusting covariates was assessed for overfitting (cut-off level <10). All statistical analyses were two-sided, and a p value of <0.05 was considered significant. Statistical Package for Social Sciences (IBM SPSS Statistics version 24.0) was used for the analysis.
Results
The selection schema is shown in Figure 1. Among 6,003 cases in the study cohort, there were 1,074 cases eligible for this analysis. These included the CCRT group (n = 502), the chemotherapy group (n = 319) and the RT group (n = 253). Demographics across the three groups and chemotherapy regimens are shown in Table 1 (Supporting Information, Table S1). A taxane/platinum-doublet was the most common choice among chemotherapy regimens (56.4%). The median number of administered chemotherapy cycles was 6, and 51.7% received 6 cycles of chemotherapy.
Figure 1.
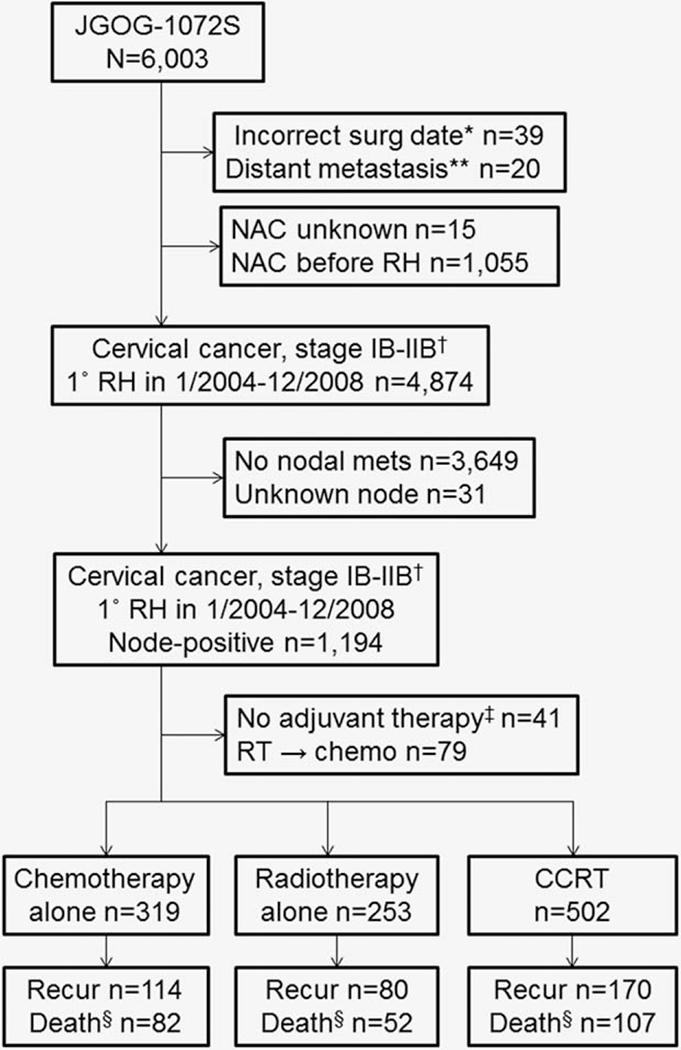
Study selection schema. *Date of surgery was not reported as 1/1/2004–12/31/2008. **Not including para-aortic lymph node metastasis. †Clinical stage for the study cohort. ‡Including unknown adjuvant therapy type. §Death due to cervical cancer. Abbreviations: JGOG, Japanese Gynecologic Oncology Group; NAC, neoadjuvant chemotherapy; RH, radical hysterectomy; RT, radiotherapy; CCRT, concurrent chemoradiotherapy; WPRT, whole pelvic radiotherapy.
Table 1.
Patient demographics (N = 1,074)
| Characteristic No. | Radiotherapy alone n = 253 | CCRT n = 502 | Chemotherapy alone n = 319 | p value |
|---|---|---|---|---|
| Year | <0.001 | |||
| 2004 | 78 (30.8%) | 77 (15.3%) | 48 (15.0%) | |
| 2005 | 66 (26.1%) | 107 (21.3%) | 52 (16.3%) | |
| 2006 | 44 (17.4%) | 95 (18.9%) | 67 (21.0%) | |
| 2007 | 36 (14.2%) | 111 (22.1%) | 79 (24.8%) | |
| 2008 | 29 (11.5%) | 112 (22.3%) | 73 (22.9%) | |
| Age | 49.4 (±12.2) | 48.1 (±11.9) | 46.7 (±11.8) | 0.024 |
| Clinical stage | 0.65 | |||
| IB1 | 111 (43.9%) | 199 (39.6%) | 141 (44.2%) | |
| IB2 | 39 (15.4%) | 95 (18.9%) | 61 (19.1%) | |
| IIA | 28 (11.1%) | 64 (12.7%) | 34 (10.7%) | |
| IIB | 75 (29.6%) | 144 (28.7%) | 83 (26.0%) | |
| Histology | <0.001 | |||
| Squamous | 215 (85.0%) | 382 (76.1%) | 156 (48.9%) | |
| Adenocarcinoma | 21 (8.3%) | 94 (18.7%) | 109 (34.2%) | |
| Adenosquamous | 15 (5.9%) | 24 (4.8%) | 40 (12.5%) | |
| Others | 2 (0.8%) | 2 (0.4%) | 14 (4.4%) | |
| Parametrium | 0.06 | |||
| Not involved | 142 (56.1%) | 289 (57.6%) | 207 (64.9%) | |
| Involved | 111 (43.9%) | 213 (42.4%) | 112 (35.1%) | |
| Deep stromal invasion | 0.08 | |||
| No | 38 (17.6%) | 99 (21.9%) | 44 (15.4%) | |
| Yes | 178 (82.4%) | 353 (78.1%) | 241 (84.6%) | |
| Tumor size | 0.58 | |||
| ≤2.0 cm | 31 (12.7%) | 66 (13.7%) | 39 (12.7%) | |
| 2.1–4.0 cm | 119 (48.8%) | 227 (47.2%) | 158 (51.3%) | |
| 4.1–6.0 cm | 84 (34.4%) | 153 (31.8%) | 90 (29.2%) | |
| >6.0 cm | 10 (4.1%) | 35 (7.3%) | 21 (6.8%) | |
| LVSI | 0.60 | |||
| Not present | 25 (10.7%) | 57 (11.9%) | 42 (13.5%) | |
| Present | 209 (89.3%) | 424 (88.1%) | 270 (86.5%) | |
| Uterine corpus | 0.001 | |||
| Not involved | 192 (76.2%) | 412 (82.4%) | 218 (71.5%) | |
| Involved | 60 (23.8%) | 88 (17.6%) | 87 (28.5%) | |
| Pelvic washing | 0.06 | |||
| No malignancy | 136 (92.5%) | 240 (93.0%) | 173 (86.9%) | |
| Malignancy | 11 (7.5%) | 18 (7.0%) | 26 (13.1%) | |
| Ovary | <0.001 | |||
| Not involved | 243 (98.0%) | 488 (98.2%) | 275 (93.2%) | |
| Involved | 5 (2.0%) | 9 (1.8%) | 20 (6.8%) | |
| Pelvic lymph nodes | 36 (22) | 31 (20) | 33 (21) | 0.47 |
| Not involved | 0 | 2 (0.4%) | 2 (0.6%) | |
| Involved | 253 (100%) | 500 (99.6%) | 317 (99.4%) | |
| Para-aortic lymph nodes | 9 (11) | 7 (10) | 14 (17) | 0.003 |
| Not involved | 59 (85.5%) | 76 (75.2%) | 58 (61.7%) | |
| Involved | 10 (14.5%) | 25 (24.8%) | 36 (38.3%) | |
| Lymph node ratio (%) | ||||
| Pelvic | 5.0 (7.2) | 7.1 (8.8) | 7.1 (10.7) | <0.001 |
| Para-aortic | 66.7 (52.2) | 23.2 (82.2) | 31.0 (40.7) | 0.015 |
| Additional radiotherapy | ||||
| Aortic boost | <0.001 | |||
| No | 221 (87.4%) | 428 (85.3%) | 319 (100%) | |
| Yes | 32 (12.6%) | 74 (14.7%) | 0 | |
| Vaginal brachytherapy | <0.001 | |||
| No | 208 (85.6%) | 401 (83.9%) | 256 (97.3%) | |
| Yes | 35 (14.4%) | 77 (16.1%) | 7 (2.7%) |
Mean (±SD), median (interquartile range), or number (%) per column are shown. Abbreviations: CCRT, concurrent chemoradiotherapy; LVSI, lymphovascular space invasion.
The proportion of women who received chemotherapy increased significantly during the study period (15.0% in 2004 to 22.9% in 2008, P < 0.001; Table 1). Women who received adjuvant chemotherapy were more likely to be young, and have non-squamous histology, uterine corpus involvement, and ovarian metastasis (all, P < 0.05). Chemotherapy group had the highest number of sampled para-aortic lymph nodes among the groups (P = 0.003). Para-aortic lymph node metastasis, seen in 71 (6.6%) cases, was significantly associated with use of chemotherapy (P = 0.003). Women who had malignant cells in pelvic cytology were more likely to receive systemic chemotherapy although it did not reach statistical significance.
There were 364 recurrences and 241 deaths due to cervical cancer identified in the study. Median follow-up time of the cases without any events of recurrence or cervical cancer death was 64.5 months for the entire cohort. The chemotherapy group had a similar 5-year cumulative recurrence rate (34.1% for CCRT group, 29.1% for RT group and 36.6% for chemotherapy group, P = 0.49) and cervical cancer mortality rate (21.8% for CCRT group, 21.7% for RT group and 24.7% for chemotherapy group, P = 0.29) compared to the CCRT and RT groups (Figs. 2a and 2b). After propensity score matching (Supporting Information, Table S2), women who received chemotherapy had similar recurrence (5-year cumulative rates, 34.2% vs. 35.1%, P = 0.94) and cervical cancer mortality (21.9% vs. 24.7%, P = 0.48) rates relative to those who received CCRT.
Figure 2.
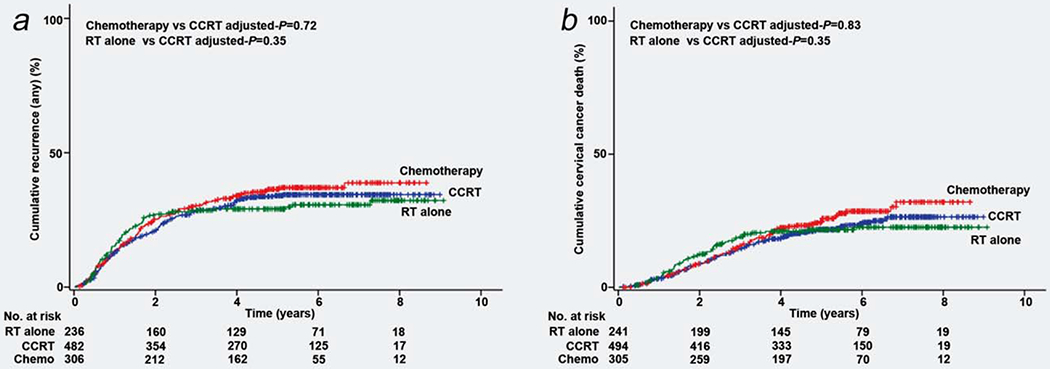
Cumulative incidence curves for recurrence and cervical cancer death based on adjuvant treatment types. Cumulative incidence curves based on adjuvant therapy types are shown for (a) recurrence and (b) death from cervical cancer. Abbreviations: CCRT, concurrent chemoradiotherapy; RT, radiation-based therapy. [Color figure can be viewed at wileyonlinelibrary.com]
On multivariate analysis controlling for survival factors (Table 2), the chemotherapy group had similar recurrence (adjusted HR 0.95, 95% CI 0.70–1.28, P = 0.72) and cervical cancer mortality (adjusted HR 0.96, 95% CI 0.67–1.38, P = 0.83) risks relative to the CCRT group. After propensity score matching (Supporting Information, Table S3), women who received chemotherapy had similar recurrence (adjusted HR 1.19, 95% CI 0.82–1.74, P = 0.36) and cervical cancer mortality (adjusted HR 1.04, 95% CI 0.66–1.64, P = 0.87) risks compared to those who received CCRT. Among women with clinical stage I disease (n = 646) and stage II disease (n = 428), the results were virtually identical to the main cohort (Supporting Information, Tables S4 and S5).
Table 2.
Survival outcomes based on adjuvant therapy (N = 1,074)
| Characteristic | No. | Recurrence | Cervical cancer death | ||
|---|---|---|---|---|---|
| Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | ||
| Adjuvant type | |||||
| CCRT | 502 | 1 | 1 | ||
| RT alone | 253 | 18.17 (0.84–1.62) | 0.35 | 1.20 (0.81–1.78) | 0.35 |
| Chemo alone | 319 | 0.95 (0.70–1.28) | 0.72 | 0.96 (0.67–1.38) | 0.83 |
| Age (continuous) | 1,074 | 0.99 (0.98–0.99) | 0.011 | 0.98 (0.97–0.99) | 0.003 |
| Histology | |||||
| SCC | 753 | 1 | 1 | ||
| Non-SCC | 321 | 2.23 (1.71–2.91) | <0.001 | 2.44 (1.77–3.35) | <0.001 |
| Parametrium | |||||
| Not involved | 638 | 1 | 1 | ||
| Involved | 436 | 1.95 (1.49–2.56) | <0.001 | 1.80 (1.29–2.50) | <0.001 |
| Deep stromal invasion | |||||
| No | 181 | 1 | 1 | ||
| Yes | 772 | 1.44 (0.95–2.20) | 0.09 | 1.63 (0.95–2.77) | 0.07 |
| Tumor size | |||||
| ≤4.0 cm | 640 | 1 | 1 | ||
| >4.0 cm | 393 | 1.09 (0.84–1.40) | 0.52 | 1.03 (0.76–1.40) | 0.86 |
| LVSI | |||||
| Not present | 124 | 1 | 1 | ||
| Present | 903 | 1.60 (0.94–2.72) | 0.08 | 1.42 (0.75–2.68) | 0.28 |
| Uterine corpus | |||||
| Not involved | 822 | 1 | 1 | ||
| Involved | 235 | 1.10 (0.82–1.47) | 0.54 | 1.35 (0.96–1.91) | 0.09 |
| Ovary | |||||
| Not involved | 1006 | 1 | 1 | ||
| Involved | 34 | 1.87 (1.08–3.26) | 0.027 | 1.82 (0.92–3.57) | 0.09 |
| Peritoneal cytology | |||||
| No malignancy | 549 | 1 | 1 | ||
| Malignancy present | 55 | 1.49 (0.93–2.38) | 0.10 | 0.91 (0.49–1.70) | 0.77 |
| Not examined | 470 | 1.13 (0.87–1.47) | 0.37 | 1.04 (0.75–1.43) | 0.82 |
| Pelvic LNR (continuous) | 1,048 | 1.03 (1.02–1.03) | <0.001 | 1.03 (1.02–1.03) | <0.001 |
| Para-aortic lymph nodes | |||||
| Not involved | 193 | 1 | 1 | ||
| Involved | 71 | 1.13 (0.65–1.97) | 0.68 | 0.93 (0.47–1.86) | 0.85 |
| Not examined | 810 | 1.12 (0.79–1.59) | 0.51 | 1.03 (0.68–1.57) | 0.90 |
An association of adjuvant treatment type and survival outcome was adjusted by a priori survival factors in Cox proportional-hazards regression models. All covariates in this table are entered in the final model. The ratio of event number per the number of covariates was ≥10 indicating the absence of overfitting in the multivariate model.
Abbreviations: HR, hazard ratio; CI, confidence interval; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; SCC, squamous cell carcinoma; LVSI, lymphovascular space invasion; LNR, lymph node ratio.
Patterns of recurrence were examined (Supporting Information, Table S6). Local recurrence was seen in 172 (16.0%) cases including 57 (5.3%) cases of vaginal cuff recurrence. Distant recurrence was seen in 239 (22.3%) cases with para-aortic lymph nodes (n = 100, 9.3%) being the most common anatomical sites of recurrence followed by lung (n = 81, 7.5%) and thoracic/cervical lymph nodes (n = 59, 5.5%). The chemotherapy group had higher local recurrence (5-year cumulative rate, 14.3% for CCRT group, 13.3% for RT group and 23.9% for chemotherapy alone group, P < 0.001) but lower distant recurrence (24.6% for CCRT group, 22.1% for RT group and 19.2% for chemotherapy alone group, P = 0.05) rates compared to the CCRT group (Figs. 3a and 3b). Similar results were observed after propensity score matching.
Figure 3.
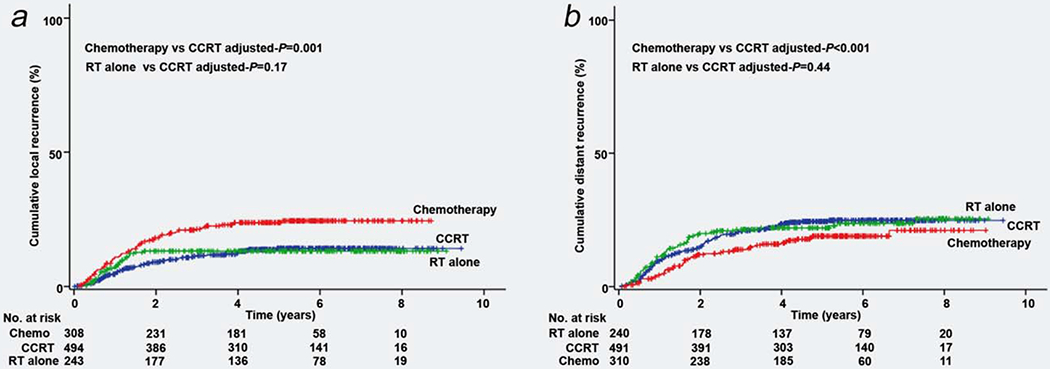
Cumulative incidence curves for local and distant recurrences based on adjuvant treatment types. Cumulative incidence curves based on adjuvant therapy types are shown for (a) local recurrence and (b) distant recurrence. Abbreviations: CCRT, concurrent chemoradiotherapy; RT, radiation-based therapy. [Color figure can be viewed at wileyonlinelibrary.com]
On multivariate analysis (Table 3), chemotherapy treatment was independently associated with increased local recurrence (adjusted HR 2.03, 95% CI 1.34–3.08, P = 0.001) but decreased distant recurrence (adjusted HR 0.47, 95% CI 0.31–0.71, P < 0.001) risks compared to CCRT. Similarly, after propensity score matching (Supporting Information, Tables S7 and S8), the chemotherapy group had an independently increased local recurrence (adjusted HR 1.94, 95% CI 1.17–3.22, P = 0.01) but decreased distant recurrence (adjusted HR 0.61, 95% CI 0.38–0.98, P = 0.041) risks compared to the CCRT group.
Table 3.
Recurrence patterns based on adjuvant therapy (N = 1,074)
| Characteristic | No. | Local recurrence | Distant recurrence | ||
|---|---|---|---|---|---|
| Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | ||
| Adjuvant type | |||||
| CCRT | 502 | 1 | 1 | ||
| RT alone | 253 | 1.41 (0.86–2.30) | 0.17 | 1.16 (0.79–1.71) | 0.44 |
| Chemo alone | 319 | 2.03 (1.34–3.08) | 0.001 | 0.47 (0.31–0.71) | <0.001 |
| Age (continuous) | 1,074 | 0.98 (0.96–0.99) | 0.005 | 0.99 (0.98–1.01) | 0.21 |
| Histology | |||||
| SCC | 753 | 1 | 1 | ||
| Non-SCC | 321 | 2.04 (1.40–2.97) | <0.001 | 1.91 (1.37–2.67) | <0.001 |
| Parametrium | |||||
| Not involved | 638 | 1 | 1 | ||
| Involved | 436 | 2.53 (1.72–3.74) | <0.001 | 1.53 (1.09–2.14) | 0.015 |
| Deep stromal invasion | |||||
| No | 181 | 1 | 1 | ||
| Yes | 772 | 1.31 (0.70–2.43) | 0.39 | 1.43 (0.86–2.39) | 0.17 |
| Tumor size | |||||
| ≤4.0 cm | 640 | 1 | 1 | ||
| >4.0 cm | 393 | 1.06 (0.75–1.51) | 0.75 | 1.16 (0.84–1.59) | 0.37 |
| LVSI | |||||
| Not present | 124 | 1 | 1 | ||
| Present | 903 | 1.59 (0.76–3.34) | 0.22 | 1.43 (0.76–2.72) | 0.27 |
| Uterine corpus | |||||
| Not involved | 822 | 1 | 1 | ||
| Involved | 235 | 1.26 (0.85–1.89) | 0.25 | 1.03 (0.71–1.51) | 0.87 |
| Ovary | |||||
| Not involved | 1006 | 1 | 1 | ||
| Involved | 34 | 1.84 (0.91–3.69) | 0.09 | 2.74 (1.35–5.56) | 0.005 |
| Peritoneal washing | 0.28 | 0.99 | |||
| No malignancy | 549 | 1 | 1 | ||
| Malignancy present | 55 | 1.58 (0.87–2.86) | 0.14 | 0.97 (0.50–1.91) | 0.93 |
| Not examined | 470 | 0.97 (0.66–1.42) | 0.87 | 0.87 (0.70–1.35) | 0.87 |
| Pelvic LNR (continuous) | 1,048 | 1.02 (1.01–1.03) | 0.001 | 1.03 (1.02–1.04) | <0.001 |
| Para-aortic lymph nodes | 0.28 | 0.09 | |||
| Not involved | 193 | 1 | 1 | ||
| Involved | 71 | 0.93 (0.58–1.48) | 0.75 | 2.15 (1.09–4.23) | 0.027 |
| Not examined | 810 | 0.53 (0.23–1.21) | 0.13 | 1.39 (0.88–2.20) | 0.16 |
An association of adjuvant treatment type and recurrence patterns was adjusted by a priori survival factors in Cox proportional-hazards regression models. The ratio of event number per the number of covariates was ≥10 indicating the absence of overfitting in the multivariate model. Abbreviations: HR, hazard ratio; CI, confidence interval; CCRT, concurrent chemoradiotherapy; RT, radiotherapy; SCC, squamous cell carcinoma; LVSI, lymphovascular invasion; LNR, lymph node ratio.
Local recurrence risk in the chemotherapy group was stratified based on the patterns of independent tumor factors for local recurrence such as non-squamous histology, parametrial involvement and high LNR (Table 3). In the absence of these tumor factors, the 5-year cumulative local recurrence rate was 3.9%. However, with the presence of multiple risk factors, local recurrence risks were significantly increased (5-year cumulative risk for single factor 14.2–22.1%, two risk factors 27.8–71.9% and three risk factors 58.5%; Supporting Information, Table S9).
Recurrence patterns were examined per histology subtype (Supporting Information, Table S10). Across the histology subtypes, a multivariate analysis showed that chemotherapy use was independently associated with decreased distant recurrence risk compared to radiotherapy: 5-year cumulative rates 13.2% vs. 21.7%, adjusted HR 0.35, 95% CI 0.19–0.65, P = 0.001 for squamous type; and 25.8% vs. 33.8%, adjusted HR 0.54, 95% CI 0.31–0.94, P = 0.028 for non-squamous type. Contrary, the chemotherapy group had a higher local recurrence risk compared to the radiotherapy group in the non-squamous group (5-year cumulative rates 32.7% vs. 22.5%, adjusted HR 2.21, 95% CI 1.25–3.89, P = 0.006). In the squamous group, this increased risk of local recurrence with chemotherapy was marginal and the difference did not reach statistical significance (15.6% vs. 11.8%, adjusted HR 1.61, 95% CI 0.95–2.75, P = 0.08).
Discussion
This study found that systemic chemotherapy alone had a comparable survival outcome compared to CCRT for surgically treated women with node-positive early-stage cervical cancer. Specifically, systemic chemotherapy treatment decreased distant recurrence but increased local recurrence when compared to CCRT.
Utility of systemic chemotherapy as an adjuvant therapy in high-risk early-stage cervical cancer has been an issue of longstanding debate over the past decades but an ultimate answer has been missing. Our study results are similar to prior studies that demonstrated comparable survival outcomes between systemic chemotherapy and pelvic irradiation as post-hysterectomy adjuvant therapy.11,13,14 Because these prior studies were either small in sample size or did not solely examine node-positive high-risk cases, our data are more definitive to clarify this research question.
A hypothesis generated from our results is that this study functions as a “proof-of-principal” study in that (i) nodal metastasis is a surrogate marker for distant recurrence, and pelvic irradiation alone is not adequate to control such distant recurrence outside the radiation field, and that (ii) cervical cancer is sensitive to radiation. This hypothesis is based on the notion in this study that systemic chemotherapy decreased distant recurrence while there was more risk of local recurrence when pelvic irradiation was not administered in early-stage cervical cancer with nodal metastasis.
Rationale of preferring systemic chemotherapy over radiotherapy is to avoid complications related to pelvic irradiation after radical pelvic surgery. This was based on a randomized clinical trial demonstrating high complication rates after post-hysterectomy pelvic irradiation compared to surgery alone.19 While there is no head-to-head clinical trial directly comparing efficacy and adverse events between adjuvant chemotherapy and radiotherapy in high-risk early-stage cervical cancer, current available evidence points toward fewer intestinal and urinary complication rates with chemotherapy treatment.9–11
There are potential clinical implications drawn from our study results. First, systemic chemotherapy can be an alternative adjuvant treatment option to CCRT in high-risk early-stage disease. This can be particularly applicable to women who are not a candidate for pelvic radiotherapy after surgery, for example, due to extensive pelvic adhesive disease or intraoperative substantial pelvic organ injury (urinary tract, intestinal and vascular). Notably we found that non-squamous histology, parametrial involvement and high LNR are independent predictors for local failure, and that the presence of multiple factors had a disproportionally high risk of local recurrence in the chemotherapy group (Supporting Information, Table S9). This result implies that chemotherapy alone is likely insufficient for local control when the tumor exhibits multiple factors especially with non-squamous histology and parametrial involvement, and it is reasonable to recommend pelvic irradiation in addition to systemic chemotherapy when these factors are present. On the same line, chemotherapy without radiotherapy may be safely offered in the absence of these tumor factors. Further studies are warranted to outline the optimal candidates for adjuvant systemic chemotherapy after radical hysterectomy.
Our results showed that treatment responses were different across the histology subtypes. This finding is consistent with the current view of literature in that squamous and non-squamous types have a different treatment response.20 Specifically, histology-specific analysis of GOG-92 trial demonstrated that the reduction in the rate of recurrence after pelvic irradiation was markedly larger in the adenocarcinomas as compared to squamous carcinomas, implying that cervical adenocarcinoma is more radiosensitive than squamous carcinoma.5,21 Our study supports this hypothesis in that radiotherapy had a lower rate of local recurrence compared to chemotherapy among the non-squamous cases, whereas this association was not clearly seen in the squamous cases.
With regards to histology-specific chemotherapy response, various studies have suggested that chemotherapy may be more effective in non-squamous type than squamous type.22–24 In our study, chemotherapy was effective in reducing distant recurrence observed in both histology subtypes but it seems that the squamous type may respond better than non-squamous type (adjusted HR 0.35 vs. 0.54). However, as this comparison was between chemotherapy and radiotherapy and not a direct comparison between squamous and non-squamous histology subtypes, the interpretation of these different observations between ours and others needs to be distinguished. In addition, difference in the targeted patient population may be the causality of these findings. While past studies were conducted in the salvage setting for metastatic or recurrent disease reflecting different tumor biology after multiple treatments, our study was conducted in the setting of upfront treatment. Further study is warranted to examine the histology-specific treatment response in cervical cancer.
Limitations of this study are that this is a retrospective study that may miss confounding factors. For instance, there was no standard objective measurement to assess surgical radicality and completeness of hysterectomy and lymphadenectomy. In addition, the exact indication for treatment allocation to chemotherapy versus radiotherapy was not abstracted. The study population was of Asian ethnicity, and generalizability and reproducibility in different study populations is unknown. Last, our study did not have information on complications related to surgery, chemotherapy and radiotherapy, so this study could not evaluate the safety of adjuvant therapy. Strengths of this study include one of the largest sample sizes in the literature and inclusion of a homogenous study population. That is, this study only examined node-positive early-stage cervical cancer, reflecting the largest magnitude of survival significance amongst the surgical-pathological risk factors.25 Propensity score matching enriched the statistical analysis in the study.
In Japan, infrastructural support and resource for radiotherapy has long been limited in availability.26,27 For this reason, gynecologic oncologists heavily rely on treatment modality without radiotherapy such as surgery and chemotherapy in the management of stage IB–IIB cervical cancer. In fact, more than a quarter of women with stage II cervical cancer underwent surgery-based treatment without radiotherapy shown in the 2013 national statistics.28 In addition, a concern for radiation toxicity after radical pelvic surgery remains a salient factor to avoid postoperative radiotherapy in Japan because Japanese women with cervical cancer are generally thin and such body habitus is associated with increased radiation toxicity.29,30 In consideration of these circumstances in Japan and based our results, the JGOG is going to launch a phase III study comparing postoperative CCRT to chemotherapy alone in surgically treated high-risk stage IB–IIB cervical cancer in the future (AFTER trial), and this trial will ultimately provide the rationale of effectiveness and adverse effects of postoperative systemic chemotherapy in high-risk early-stage cervical cancer.
In summary, systemic chemotherapy may be an effective postoperative treatment choice for women with surgically treated node-positive stage IB–IIB cervical cancer by reducing distant recurrence risk. As systemic chemotherapy seems inferior to control local recurrence compared to pelvic radiotherapy, adding radiotherapy to systemic chemotherapy would be most effective to reduce local recurrence when certain tumor factors associated with local recurrence risk are present.
Supplementary Material
What’s new?
Treatment for early-stage cervical cancer commonly involves removal of the uterus and pelvic lymph nodes. To improve survival, adjuvant therapy, using either systemic chemotherapy or concurrent chemoradiation therapy (CCRT) may also be considered. Here, in a cohort of 1,074 Japanese women with node-positive early-stage (IB-IIB) cervical cancer who underwent primary radical hysterectomy, similar survival outcomes were observed for women who received postoperative systematic chemotherapy compared to those who received CCRT. The two modalities, however, had distinct recurrence patterns, with systemic chemotherapy associated with increased risk of local recurrence and decreased risk of distant recurrence compared to CCRT.
Acknowledgements
The authors thank all the JGOG institutions participated in this study and the JGOG Cervical Cancer Committee members for administrative work for the study. They also thank Dr Lynda D. Roman for her scientific input to the study.
Grant sponsor: None DOI: 10.1002/ijc.30793
Abbreviations
- CCRT
concurrent chemo-radiotherapy
- JGOG
Japanese Gynecologic Oncology Group
- LNR
lymph node ratio
- HR
hazard ratio
- CI
confidence interval
- GOG
Gynecologic Oncology Group
Footnotes
Disclosure of Potential Conflict of Interest: None declared.
Abstract of the study was presented at 48th Annual Meeting on Women’s Cancer, National Harbor, MD, March 11–15, 2017.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 2006;95:S43–103. [DOI] [PubMed] [Google Scholar]
- 3.Waggoner SE. Cervical cancer. Lancet 2003;361:2217–25. [DOI] [PubMed] [Google Scholar]
- 4.Delgado G, Bundy B, Zaino R, et al. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 1990;38:352–7. [DOI] [PubMed] [Google Scholar]
- 5.Sedlis A, Bundy BN, Rotman MZ, et al. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol 1999;73:177–83. [DOI] [PubMed] [Google Scholar]
- 6.Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol 2000;18:1606–13. [DOI] [PubMed] [Google Scholar]
- 7.Greer BE, Koh WJ, Abu-Rustum NR, et al. Cervical cancer. J Natl Compr Canc Netw 2010;8:1388–416. [DOI] [PubMed] [Google Scholar]
- 8.Rosa DD, Medeiros LR, Edelweiss MI, et al. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev 2012; CD005342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeshima N, Umayahara K, Fujiwara K, et al. Treatment results of adjuvant chemotherapy after radical hysterectomy for intermediate- and high-risk stage IB-IIA cervical cancer. Gynecol Oncol 2006;103:618–22. [DOI] [PubMed] [Google Scholar]
- 10.Hosaka M, Watari H, Takeda M, et al. Treatment of cervical cancer with adjuvant chemotherapy versus adjuvant radiotherapy after radical hysterectomy and systematic lymphadenectomy. J Obstet Gynaecol Res 2008;34:552–6. [DOI] [PubMed] [Google Scholar]
- 11.Hosaka M, Watari H, Kato T, et al. Clinical efficacy of paclitaxel/cisplatin as an adjuvant chemotherapy for patients with cervical cancer who underwent radical hysterectomy and systematic lymphadenectomy. J Surg Oncol 2011;105:612–6. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Song X, Liu R, et al. Chemotherapy versus radiotherapy for FIGO stages IB1 and IIA1 cervical carcinoma patients with postoperative isolated deep stromal invasion: a retrospective study. BMC Cancer 2016;16:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung PS, Kim DY, Lee SW, et al. Clinical role of adjuvant chemotherapy after radical hysterectomy for FIGO stage IB–IIA cervical cancer: comparison with adjuvant RT/CCRT using inverse-probability-of-treatment weighting. PLoS One 2015;10:e0132298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Hu T, Chen Y, et al. Adjuvant chemotherapy, a valuable alternative option in selected patients with cervical cancer. PLoS One 2013;8:e73837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming ND, Frumovitz M, Schmeler KM, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol 2014;136:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 18.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol 1972;34:187–220. [Google Scholar]
- 19.Landoni F, Maneo A, Colombo A, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet 1997; 350:535–40. [DOI] [PubMed] [Google Scholar]
- 20.Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: a unique cervical cancer. Gynecol Oncol 2009;116:140–6. [DOI] [PubMed] [Google Scholar]
- 21.Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 2006;65:169–76. [DOI] [PubMed] [Google Scholar]
- 22.Curtin JP, Blessing JA, Webster KD, et al. Paclitaxel, an active agent in nonsquamous carcinomas of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol 2001;19:1275–8. [DOI] [PubMed] [Google Scholar]
- 23.Kastritis E, Bamias A, Efstathiou E, et al. The outcome of advanced or recurrent non-squamous carcinoma of the uterine cervix after platinum-based combination chemotherapy. Gynecol Oncol 2005;99:376–82. [DOI] [PubMed] [Google Scholar]
- 24.Nagao S, Fujiwara K, Oda T, et al. Combination chemotherapy of docetaxel and carboplatin in advanced or recurrent cervix cancer. A pilot study. Gynecol Oncol 2005;96:805–9. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo K, Mabuchi S, Okazawa M, et al. Utility of risk-weighted surgical-pathological factors in early-stage cervical cancer. Br J Cancer 2013;108:1348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teshima T Patterns of care study in Japan. Jpn J Clin Oncol 2005;35:497–506. [DOI] [PubMed] [Google Scholar]
- 27.Tomita N, Toita T, Kodaira T, et al. Patterns of radiotherapy practice for patients with cervical cancer in Japan, 2003–2005: changing trends in the pattern of care process. Int J Radiat Oncol Biol Phys 2012;83:1506–13. [DOI] [PubMed] [Google Scholar]
- 28.Saito T, Katabuchi H. Annual Report of the Committee on Gynecologic Oncology, Japan Society of Obstetrics and Gynecology: patient Annual Report for 2013 and Treatment Annual Report for 2008. J Obstet Gynaecol Res 2016;42:1069–79. [DOI] [PubMed] [Google Scholar]
- 29.Asano H, Todo Y, Watari H. Adjuvant chemotherapy for early-stage cervical cancer. Chin J Cancer Res 2016;28:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kizer NT, Thaker PH, Gao F, et al. The effects of body mass index on complications and survival outcomes in patients with cervical carcinoma undergoing curative chemoradiation therapy. Cancer 2010;117:948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


