Abstract
Objective
To examine the trends of epithelial ovarian cancer histologic subtypes in Japan.
Methods
A nationwide retrospective registry study was performed between 2002 and 2015 (Japan cohort, n =48,640). Trends were also examined in The Surveillance, Epidemiology, and End Results Program (US cohort, n = 49,936). Time-specific proportional changes of four major histological subtypes (serous, clear cell, endometrioid, and mucinous) were examined.
Results
The Japan cohort had more stage I disease (44.1% versus 24.9%) and less stage IV disease (10.0% versus 23.1%) than the US cohort (P < 0.001). The Japan cohort had more non-serous histology, particularly clear cell carcinoma (26.9% versus 8.4%), than the US cohort (P < 0.001). In the Japan cohort, proportion of clear cell carcinoma increased significantly from 23.4% to 29.1% between 2002 and 2010 (P < 0.001). Among stage I disease, clear cell carcinoma increased significantly in the Japan cohort from 32.9% to 40.3% between 2002 and 2015 (P < 0.001), whereas mucinous carcinoma increased significantly in the US cohort from 15.0% to 24.8% (P = 0.01). In 2015, clear cell carcinoma was most common among women aged <50 years from the Japan cohort (30.2%) versus serous carcinoma in the US cohort (50.8%). In the Japan cohort, the peak age was 75 years for serous, 57 for clear cell, and 45 for endometrioid carcinoma (P < 0.001). Mucinous carcinoma decreased until 43 years and increased again after age 73 years (P < 0.001).
Conclusion
Characteristics of epithelial ovarian cancer in Japan are largely different compared to the US. In Japan, clear cell carcinoma has increased significantly in recent years to account for nearly 30% of epithelial ovarian cancer.
Keywords: Ovarian cancer, Trends, Stage, Histology, Clear cell, Survival
1. Introduction
Ovarian cancer remains the third most common gynecologic malignancy in the world, and it is the seventh most common female malignancy with 238,700 estimated new cases worldwide in 2012 [1]. Multiple factors are associated with a greater risk of developing ovarian cancer; these factors include age, obesity, cigarette smoking, nulliparity, hormone therapy for menopause, endometriosis, and genetics [2–8]. Moreover, genetic and socio-environmental factors influence the incidence of ovarian cancer in different populations [9]. Presently, the age-adjusted incidence rate of ovarian cancer has increased over the past decades in Japan from 4.0 to 15.0 per 100,000 women between 1975 and 2013 [10], whereas that in the United States has decreased from 16.3 to 11.4 per 100,000women during the same time period [11].
Ovarian cancer demonstrates histological heterogeneity and has various histologic features [12]. The most common histological subtypes of epithelial ovarian cancer are serous, clear cell, endometrioid, and mucinous [9]. Histological subtype has been recognized as one of the important prognostic factors in ovarian cancer [13]. However, the frequencies and trends in the various histological subtypes of ovarian cancer vary among different populations, and there is currently a lack of population-based statistics in Japan. This study aimed to examine the trends in the characteristics of the various histological subtypes of ovarian cancer in Japan.
2. Materials andmethods
2.1. Study design and eligibility criteria
This is a retrospective observational study examining the gynecologic tumor registry database of the Japan Society of Obstetrics and Gynecology (JSOG) between 2002 and 2015 (Japanese cohort). This study was conducted as a Japan Society of Gynecologic Oncology (JSGO) project, and the data set for Japanese cohort was provided by the gynecologic tumor committee of JSOG. The JSOG database is an organ-based cancer registry for gynecologic malignancy that records cancer subtypes, characteristics, treatments, and survivals [14]. The registry, maintained by the gynecologic tumor committee of JSOG, comprises 388 local and regional leading hospitals and covers approximately 50% of all new patients with gynecological malignancy in Japan [15].
This study utilized the Surveillance, Epidemiology, and End Results (SEER) program as the control group for comparison (US cohort). The SEER registry is a population-based database launched in 1973 that is supported and managed by the National Cancer Institute in the US. It covers approximately 34.6% of the US population from 11 states and 7 areas in the most recent registry [16]. SEER registries collect data on patient demographics, primary tumor site, tumor morphology, stage at diagnosis, and first course of treatment and follow-up with patients for vital status. In this study, SEER cases between 2002 and 2015 were abstracted from the database using SEER*Stat 8.3.5 (IMS Inc., Calverton, MD, USA).
Institutional Review Board exempted the current study due to the use of publicly available deidentified data. Cases were identified by searching for “Ovary” limited to malignancy from both databases. Women with epithelial ovarian cancer with serous, clear cell, endometrioid, and mucinous histology were eligible for analysis, whereas those with epithelial histology other than these four types, germ cell tumor, sex cord-stromal tumor, sarcoma, metastatic tumor from another origin, and tumors of unknown histology (including unspecified adenocarcinoma) were excluded from the analysis.
2.2. Clinical information
Among cases that met the inclusion criteria, patient demographics (age, calendar year at diagnosis, race/ethnicity, and registry area), tumor characteristics (histological subtype and cancer stage), and treatment type (performance of surgical treatment including hysterectomy, salpingo-oophorectomy, and lymphadenectomy) were abstracted from both databases.
Age at diagnosis (<50 vs. ≥50 years) and registry areas (north, west, central, or east) were defined as in a previous study [17,18]. The histological subtypes were divided into serous, clear cell, endometrioid, and mucinous as above. The recorded cancer stage was reclassified according to the 2014 International Federation of Gynecology and Obstetrics staging system [19]. The ICD-0–3 site/histology validation list and World Health Organization histological classification were used for grouping the histological subtypes (Supplemental Table S1) [20].
2.3. Statistical analysis
The primary objective of this study was to examine the temporal trends in the different histological subtypes of primary epithelial ovarian cancer over time in Japan. The secondary objective was to compare the histological subtypes of epithelial ovarian cancer between the Japanese and US cohorts.
Continuous variables were reported as median (interquartile range) and the difference were assessed by the Mann-Whitney U test. Ordinal and categorical variables were expressed with number and (percent per group) and the difference was assessed with the chi-square test.
The Joinpoint Regression Program 4.6.0.0 provided by the National Cancer Institute was used to determine potential changes in the temporal trends in the incidence rate [21]. The trends in the histological subtypes of ovarian cancer were examined for every calendar year or every patient age. Linear segmented regression analysis was utilized for the model, and log transformation of the data was performed to determine the annual percentage change in the slope along with a 95% confidence interval.
All analyses were based upon two-tailed hypothesis, and a P-value of <0.05 was considered to indicate statistical significance. Analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS, version 25.0, Armonk, NY, USA). The Strengthening the Reporting of Observational studies in Epidemiology guideline was referred to when planning this study [22].
3. Results
3.1. Patient demographics
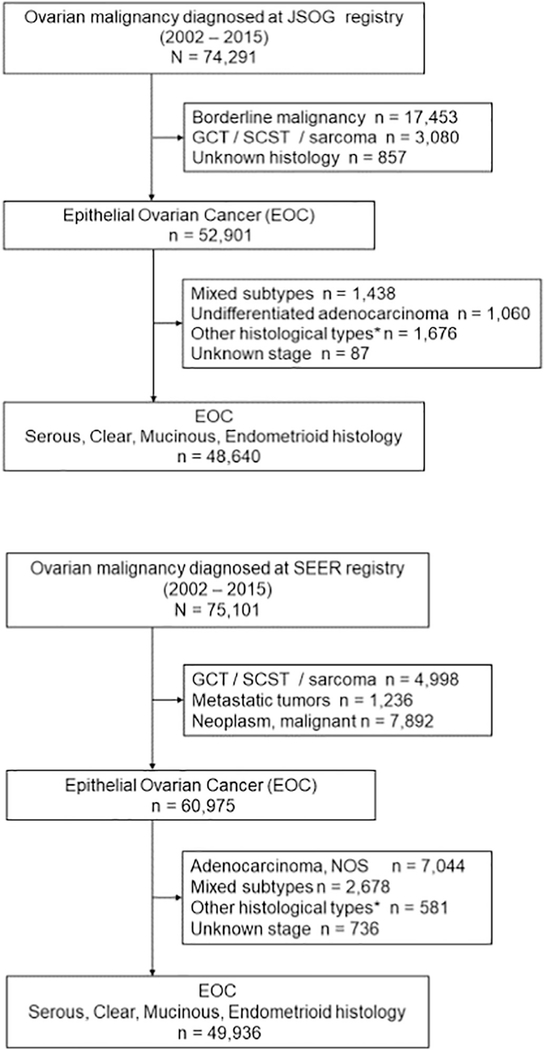
Patient selection schema is shown in Fig. 1. During the study period, 74,291 cases of women with ovarian malignancies were recorded in the JSOG tumor registry. The final study population for the Japanese cohort comprised 48,640 women with the four major subtypes of epithelial ovarian cancer. Similarly, 75,101 women with ovarian malignancy were identified from the SEER registry, and 49,936 women with the four major subtypes of epithelial ovarian cancer represented the US cohort.
Fig. 1.
Selection criteria. Abbreviations: JSOG, Japan Society of Obstetrics and Gynecology; EOC, epithelial ovarian cancer; GCT, germ cell tumor; SCST, sex cord stromal tumor; and SEER, Surveillance, Epidemiology, and End Results Program. *Other than serous, clear, mucinous, and endometrioid histology.
Demographics of the two cohorts are described in Table 1. In the Japanese cohort, the most common histological subtype was serous (40.8%), followed by clear cell (26.9%), endometrioid (19.2%), and mucinous (13.1%). Compared with women with the US cohort, those in the Japan cohort were significantly younger (median age: 57 vs. 61 years), more likely to have non-serous histology particularly clear cell carcinoma (26.9% vs. 8.4%), more likely to have a recent diagnosis (proportion of cases diagnosed in 2011–2015: 49.5% vs. 37.1%), and more likely to undergo additional lymphadenectomy at surgery (52.0% vs. 41.4%) (all, P < 0.001). In the Japanese cohort, the number of women with stage I disease was significantly more (44.1% vs. 24.9%) and those with stage IV disease (10.0% vs. 23.1%) was lower compared with those in the US cohort (P < 0.001).
Table 1.
Demographic profile of the two cohorts.
| Characteristics | JSOG | SEER | P-value |
|---|---|---|---|
| No. of patients | n = 48,640 | n = 49,936 | |
| Age (years) | 57 (IQR 48–66) | 61 (IQR 52–71) | <0.001 |
| <40 | 4027 (8.3%) | 2735 (5.5%) | |
| 40–49 | 9873 (20.3%) | 7312 (14.6%) | |
| 50–59 | 14,320 (29.4%) | 13,018 (26.1%) | |
| 60–69 | 12,559 (22.8%) | 12,819 (25.7%) | |
| ≥70 | 7861 (16.2%) | 14,052 (28.1%) | |
| Race/ethnicity | n/a | ||
| White | – | 36,680 (73.5%) | |
| Black | – | 3440 (6.9%) | |
| Hispanic | – | 5350 (10.7%) | |
| Asian | – | 3711 (7.4%) | |
| Others | – | 755 (1.5%) | |
| Registry area | n/a | ||
| North | 4286 (8.8%) | – | |
| Central | 6479 (13.3%) | 9120 (18.2%) | |
| East | 19,717 (40.5%) | 13,613 (27.3%) | |
| West | 18,158 (37.3%) | 27,203 (54.5%) | |
| Year of diagnosis | <0.001 | ||
| 2002–2005 | 8612 (17.7%) | 13,804 (27.6%) | |
| 2006–2010 | 15,945 (32.8%) | 17,646 (35.3%) | |
| 2011–2015 | 24,083 (49.5%) | 18,486 (37.1%) | |
| Stage | <0.001 | ||
| I | 21,450 (44.1%) | 12,452 (24.9%) | |
| II | 4716 (9.7%) | 4640 (9.3%) | |
| III | 17,595 (36.2%) | 21,284 (42.6%) | |
| IV | 4879 (10.0%) | 11,560 (23.1%) | |
| Histology | <0.001 | ||
| Serous | 19,900 (40.9%) | 34,404 (68.9%) | |
| Clear cell | 13,065 (26.9%) | 4171 (8.4%) | |
| Endometrioid | 9321 (19.2%) | 7369 (14.8%) | |
| Mucinous | 6354 (13.1%) | 3992 (8.0%) | |
| Hysterectomy | <0.001 | ||
| Performed | 35,459 (72.9%) | 38,899 (77.9%) | |
| Not performed/unknown | 13,181 (27.1%) | 11,037 (22.1%) | |
| Salpingo-oophorectomy | <0.001 | ||
| Performed | 42,849 (88.1%) | 44,839 (89.8%) | |
| Not performed/unknown | 5791 (11.9%) | 5097 (10.2%) | |
| Lymphadenectomy | <0.001 | ||
| Performed | 25,276 (52.0%) | 20,649 (41.4%) | |
| Not performed/unknown | 23,364 (48.0%) | 29,287 (58.6%) |
Data are shown as number (percent per column) or median (interquartile range). Abbreviations: JSOG, Japan Society of Obstetrics and Gynecology; and SEER, Surveillance, Epidemiology, and End Results Program.
The utilization rate of chemotherapy was examined across histological types in the Japanese cohort (Supplemental Table S2). Among those with stage I disease, the utilization rate of chemotherapy was the highest for clear cell histology (72.2%), whereas it was lowest for mucinous histology (38.1%; P < 0.001). In contrast, in higher-stage diseases (stages II–IV), the utilization rate of chemotherapy was the highest for serous histology (84.6%–90.0%), but it was least for mucinous histology (73.4%–79.0%; all, P < 0.001).
Demographics among Asians in the two cohorts are compared (Supplemental Table S3). Asian women in the US cohort were younger than those in the Japanese cohort (median: 55 vs. 57 years) and were more likely to have stage IV disease (18.6% vs. 10.0%) and serous histology (53.5% vs. 40.9%) (all, P < 0.001). Clear cell carcinoma was significantly more frequent in Asian women than in non-Asian women in the US cohort (17.3% vs. 7.6%, P < 0.001); however, the frequency was even higher (26.9%) in Asian women in the Japanese cohort than in those in the US cohort.
3.2. Age-specific trends
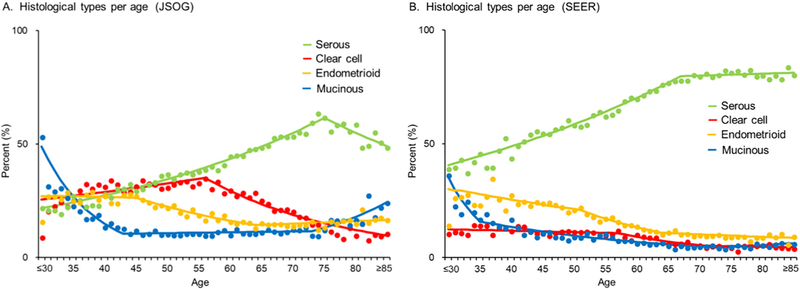
The histological subtypes of ovarian cancer were investigated in relation to the age at diagnosis (Fig. 2). In the Japanese cohort, the peak age at diagnosis was lower for clear cell carcinoma than for serous carcinoma (56 vs. 75 years, P < 0.001). The frequency of clear cell carcinoma significantly increased up to the age of 56 years (9.0% at ≤30 years to 35.0% at 56 years, P = 0.001) and decreased thereafter (35.0% at 56 years to 10.6% at ≥85 years, P < 0.001).
Fig. 2.
Age-specific temporal trends of ovarian cancer histology. Four histological subtypes of epithelial ovarian cancer were stratified by age at diagnosis. Lines are modeled estimates and points represent actual data. Abbreviations: JSOG, Japan Society of Obstetrics and Gynecology; and SEER, Surveillance, Epidemiology, and End Results Program.
The frequency of mucinous carcinoma increased significantly after the age of 73 years in women in the Japanese cohort (9.7% at 73 years to 24.1% at ≥85 years, P < 0.001), and similar results were observed in women in the US cohort (4.3% at 73 years to 6.1% at ≥85 years, P = 0.047). In both cohorts, the frequency of serous carcinoma increased significantly up to the age of 70 years (Japanese cohort, from 21.9% at ≤30 years to 61.7% at 75 years, P < 0.001; US cohort, from 39.7% at ≤30 years to 78.0% at 67 years, P < 0.001).
3.3. Histological type-specific trends
The temporal trend of annual frequency of each histological subtype is shown in Fig. 3. A significant increase in the incidence rate of clear cell carcinoma in the Japanese cohort was observed between 2002 and 2010 (from 23.4% to 29.1%, 24.4% relative increase, P < 0.001), after which the frequency remained fairly stable between 2010 and 2015 (27.7%; range 25.9–29.5, P = 0.19) (Fig. 3A). Conversely, the US cohort showed a significant decrease in the incidence rate of clear cell carcinoma between 2002 and 2007 (from 10.0% to 7.7%, 23.0% relative decrease, P = 0.001) and a stable frequency between 2007 and 2015 (8.0%; range 6.8–9.3, P = 0.12; Fig. 3B).
Fig. 3.
Temporal trends of ovarian cancer histology. A: The Y-axis is truncated to 0–50%. B: The Y-axis is truncated to 0–80%. The annual percentage of each histological subtype among the four major primary epithelial ovarian cancers is shown. Lines are modeled estimates and points represent actual data. Abbreviations: JSOG, Japan Society of Obstetrics and Gynecology; and SEER, Surveillance, Epidemiology, and End Results Program.
Among women aged <50 years in the Japanese cohort, the incidence rates of both clear cell and endometrioid carcinomas have significantly increased between 2002 and 2015 (clear cell, from 23.8% to 30.2%, 26.9% relative increase, P < 0.001; endometrioid, from 24.4% to 27.5%, 12.7% relative increase, P = 0.001; Fig. 4A). To those aged <50 years, the frequency of the incidence of these two cancer types was stable between 2005 and 2015 in the US cohort (clear cell, 10.5% range 7.4–13.8, P = 0.71; endometrioid, 22.8% range 18.5–27.2, P = 0.34; Fig. 4B).
Fig. 4.
Temporal trends of ovarian cancer histology stratified by age. The Y-axis is truncated according to the distribution. The annual percentage of each histological subtype among the four major primary epithelial ovarian cancers is shown with stratification by age (<50 vs. ≥50 years). Lines are modeled estimates and points represent actual data. Abbreviations: JSOG, Japan Society of Obstetrics and Gynecology; and SEER, Surveillance, Epidemiology, and End Results Program.
However, among women aged ≥50 years in the Japanese and US cohorts, the frequency of incidence of clear cell carcinoma remained stable throughout the study period (Japanese cohort, 25.6% range 23.1–28.2, P = 0.10; and US cohort, 7.5% range 6.2–8.9, P = 0.72; Fig. 4C–D).
3.4. Stage-specific trends
The temporal trend of annual frequency of each cancer stage is shown in Supplemental Fig. S1. There was a significant increase in the number of women with stage I disease in both cohorts throughout the study period (Japanese cohort, from 40.7% to 44.3%, 8.8% relative increase, P = 0.005; US cohort, from 23.9% to 25.7%, 7.5% relative increase, P = 0.001).
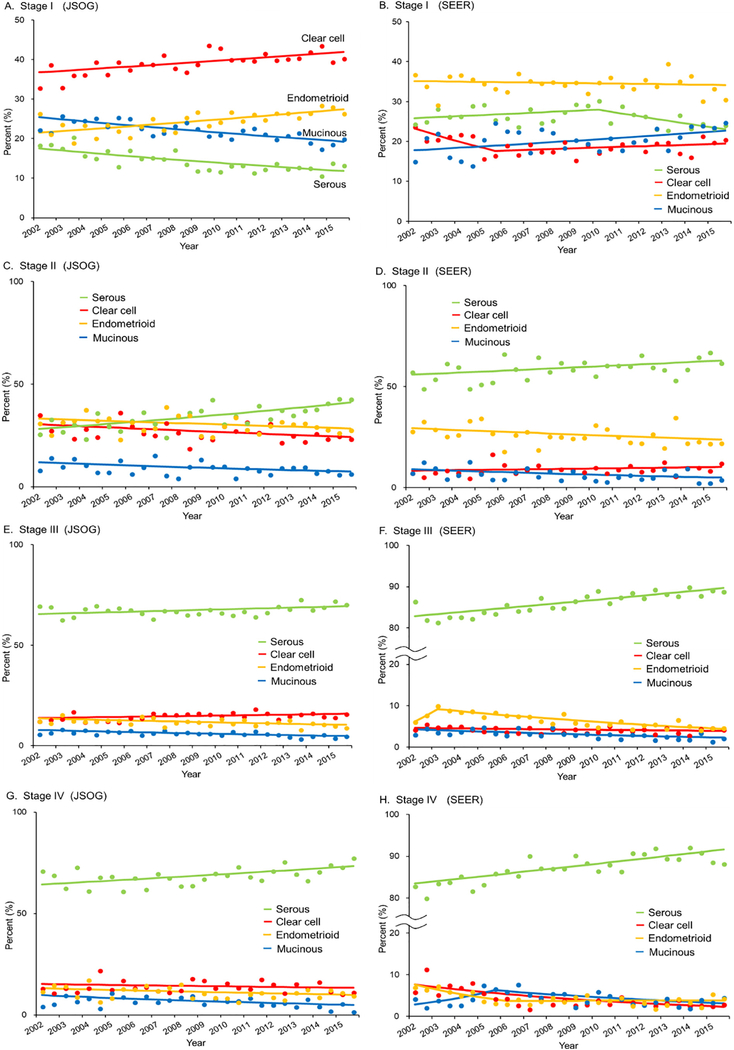
The temporal trend of annual frequency of each histological subtype was investigated stratified by stage (Fig. 5). Among women with stage I disease in the Japanese cohort, the frequency of the incidence of both clear cell and endometrioid carcinomas increased significantly between 2002 and 2015 (from 32.9% to 40.3% for clear cell, 22.5% relative increase, P < 0.001; from 23.8% to 27.2% for endometrioid, 14.3% relative increase, P < 0.001), whereas the frequency of the incidence of mucinous carcinoma showed a significant increase between 2002 and 2015 among women with stage I disease in the US cohort (from 15.0% to 24.8%, 65.3% relative increase, P = 0.01). Among women with stage II–IV disease, serous carcinoma increased significantly between 2002 and 2015 in both cohorts (all P < 0.05).
Fig. 5.
Stage-specific temporal trends of ovarian cancer histology. The annual percentage of each histological subtype among the four major primary epithelial ovarian cancers is shown per stage. Lines are modeled estimates and points represent actual data. Abbreviations: JSOG, Japan Society of Obstetrics and Gynecology; and SEER, Surveillance, Epidemiology, and End Results Program.
4. Discussion
This study demonstrated significant differences in the distribution of the four major histological subtypes of ovarian cancer between the Japanese and US cohorts over time. Notably, the frequency of the incidence of clear cell carcinoma showed a significant increase in Japan but not in the US.
Variations in the histologic subtypes of ovarian cancer between the Japanese and US cohorts could be partly explained by differences in the environmental and genetic factors [23,24]. The proportion of clear cell carcinoma incidence at 26.9% in the Japanese cohort seems to be surprisingly higher than that in a previous study in the US cohort, which accounts for approximately 4–6% of epithelial ovarian cancer [13].
This high prevalence of clear cell carcinoma in the Japanese cohort would be associated with the following three social-environmental factors: (i) changes in the age at first menarche and menopause among Japanese women; (ii) changes in diet and marital status and lower use of smoking and oral contraceptives; (iii) and a remarkably decreased pregnancy rate [25–28]. These factors increase the number of ovulations and menstruations in women’s lifetime, and a longer duration of menstruation substantially increases the risk of developing endometriosis, which is a known precursor of endometrioid and clear cell carcinomas [29].
In fact, the number of women with endometriosis has been increasing in Japan, with estimated 247,000 cases [30], and approximately 0.7% of these women are reported to develop endometriosis-related ovarian cancer every year [31]. Those social-environmental factors seemed to make a large contribution to the increased occurrence of endometriosis-related ovarian cancer in Japan. Moreover, in this study, women in the Japanese cohort have a higher frequency of clear cell carcinoma occurrence than those of Asian ethnicity in the US cohort. The difference in frequency of clear cell carcinoma occurrence between the two cohorts clearly suggests the involvement of the environmental factor.
A previous study estimated that genetic mutations account for up to a quarter of patients with ovarian cancer [32]. The histological subtypes of ovarian cancer were characterized by a different mutational spectrum. BRCA mutation is one of the most common genetic mutation in ovarian cancer, especially in high-grade serous carcinoma. Germline BRCA mutations have been identified in approximately 13–14% of Japanese women with ovarian cancer, which is comparable to the US population [33]. The clear cell type has distinct genetic, epigenetic, and immunological aspects compared to other histological types [34]. However, for the difference in genetic factors in ovarian cancer, whether the frequency of other genetic variants may vary between ethnic groups and geographical locations is still unknown.
Another interesting finding was the increased frequency of mucinous carcinoma among older women in the Japanese cohort. Primary mucinous carcinoma is a relatively rare subtype of ovarian cancer. Recent molecular study has suggested that mucinous carcinoma arises from an ovarian cortical inclusion cyst and has acquired a gastrointestinal phenotype through metaplasia [35]. The gene expression profile of mucinous carcinoma, including upregulation of the tumor suppressor gene TP53 and activation of K-RAS mutation [36], appears to be distinct from other histological subtypes of ovarian cancer and has been reported to resemble that of normal colonic mucosa [37]. The process of colon carcinogenesis is time-dependent, and this cancer is more common in elderly patients; similarly, the increase in the incidence of mucinous carcinoma in ovarian cancer is more common among elderly women.
Differentiation between primary and metastatic involvement of the ovary is important problem for optimal patient management. Even among skilled pathologists, this distinction can be problematic, as can the distinction between primary mucinous ovarian carcinoma and mucinous carcinoma metastatic to the ovary [38]. With the application of histopathological techniques and a better understanding of ovarian cancer biology, the incidence rate of primary mucinous carcinoma has fallen over recent years from 12% to 3% in the US [38,39]. However, even in recent multicenter clinical trials such as GOG 182, an expert pathological review suggests that 60% of tumors originally classified as primary mucinous ovarian carcinoma were in fact those of metastatic cancer [40].
A limitation of our study is its retrospective design and lack of central pathology review. Therefore, we do not know if mucinous carcinoma truly originates from the ovary in both cohorts. Similarly, the temporal trend observed in clear cell histology is opposite to that observed in serous histology in the Japanese cohort (Fig. 3A). Possibly, the histopathological classification may have changed over time, but this study lacks information regarding the diagnostic criteria for each histological type.
The strengths of our study include its nationwide research and likely the largest sample size in Japan reported in the literature. However, the JSOG database is focused on leading hospitals in Japan, such as university hospitals and cancer centers, raising the possibility of sectional bias. Another limitation of our study is the lack of data on ethnicity in the Japanese cohort. This may influence the frequency of the histological subtypes owing to genetic factors. However, foreigners only account for 1.2% of the total population in Japan, and many are from other Asian countries; therefore, JSOG data are overwhelmingly based on Asian patients [41].
In conclusion, women with epithelial ovarian cancer in Japan have distinct characteristics compared with those in the US. Particularly, clear cell carcinoma has increased in recent years, reaching to nearly 30% of ovarian cancer cases in Japan. In addition, mucinous carcinoma has increased in elderly individuals with bidirectional changes in Japan. Demonstrated changes in temporal trend shown in this study are clinically meaningful that may influence the future direction of investigation.
Supplementary Material
HIGHLIGHTS.
The trends in four major histological subtypes of epithelial ovarian cancer (EOC) in Japan and the US were examined.
The characteristics of the histological subtypes of EOC in Japan are distinct from those in the US.
The frequency of clear cell carcinoma has increased in Japan, accounting for almost 30% of EOC in recent years.
The frequency of mucinous carcinoma has increased significantly among elderly in both countries.
Acknowledgments
The authors thank the member institutions of the JSOG for their cooperation in providing data on patients with gynecological tumors. The authors also thank all members of the Committee on Gynecological Oncology of JSOG.
Footnotes
Conflict of interest statement
Honorarium, Chugai and Astra Zeneca (T.E.); Honorarium, Chugai, book editorial, Springer, and meeting expense, OVAL (K.M.); none for others.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ygyno.2019.03.243.
References
- [1].Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A, Global cancer statistics, 2012, CA Cancer J. Clin 65 (2015) 87–108. [DOI] [PubMed] [Google Scholar]
- [2].Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, Body fatness and cancer—viewpoint of the IARC Working Group, N. Engl. J. Med 375 (2016) 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Faber MT, Kjaer SK, Dehlendorff C, Chang-Claude J, Andersen KK, Hogdall E, et al. Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies. Cancer Causes & Control: CCC. 2013;24:989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adami HO, Hsieh CC, Lambe M, Trichopoulos D, Leon D, Persson I, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet (London, England). 1994;344: 1250–4. [DOI] [PubMed] [Google Scholar]
- [5].Modugno F, Ness RB, Allen GO, Schildkraut JM, Davis FG, Goodman MT, Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis, Am. J. Obstet. Gynecol 191 (2004) 733–740. [DOI] [PubMed] [Google Scholar]
- [6].Salehi F, Dunfield L, Phillips KP, Krewski D, Vanderhyden BC, Risk factors for ovarian cancer: an overview with emphasis on hormonal factors, Journal of Toxicology and Environmental Health Part B, Critical Reviews 11 (2008) 301–321. [DOI] [PubMed] [Google Scholar]
- [7].Melin A, Sparen P, Persson I, Bergqvist A, Endometriosis and the risk of cancer with special emphasis on ovarian cancer, Human Reproduction (Oxford, England) 21 (2006) 1237–1242. [DOI] [PubMed] [Google Scholar]
- [8].King MC, Marks JH, Mandell JB, Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2, Science (New York, N.Y.) 302 (2003) 643–646. [DOI] [PubMed] [Google Scholar]
- [9].Coburn SB, Bray F, Sherman ME, Trabert B, International patterns and trends in ovarian cancer incidence, overall and by histologic subtype, Int. J. Cancer 140 (2017) 2451–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project, Jpn. J. Clin. Oncol 45 (2015) 884–891. [DOI] [PubMed] [Google Scholar]
- [11].https://seer.cancer.gov/faststats/selections.php?#Output (Results were generated from the website on 10/15/2018).
- [12].Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, et al. Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer. Clinical Cancer Research: an official journal of the American Association for Cancer Research. 2005;11:6422–30. [DOI] [PubMed] [Google Scholar]
- [13].Oliver KE, Brady WE, Birrer M, Gershenson DM, Fleming G, Copeland LJ, et al. An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: an NRG Oncology/Gynecologic Oncology Group experience. Gynecol. Oncol.. 2017;147:243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. Clinical statistics of gynecologic cancers in Japan. J. Gynecol. Oncol. 2017;28:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].project JSoOaGGOCr, 68th Annual Congress of the Japan Society of Obstetrics and Gynecology Tokyo, April 21–24, 2016. [Google Scholar]
- [16].National Cancer Institute, Surveillance E, and End Results program, Available at: http://seer.cancer.gov/, Accessed date: 15 October 2018.
- [17].ITO K, Inter-metropolitan migration and regional economic differentials in postwar Japan, Studies in Regional Science 22 (1991) 19–36. [Google Scholar]
- [18].Matsuo K, Machida H, Takiuchi T, Grubbs BH, Roman LD, Sood AK, et al. Role of hysterectomy and lymphadenectomy in the management of early-stage borderline ovarian tumors. Gynecol. Oncol. 2017;144:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zeppernick F, Meinhold-Heerlein I, The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer, Arch. Gynecol. Obstet 290 (2014) 839–842. [DOI] [PubMed] [Google Scholar]
- [20].http://seer.cancer.gov/icd-o-3, Accessed date: 15 October 2018.
- [21].Kim HJ, Fay MP, Feuer EJ, Midthune DN, Permutation tests for joinpoint regression with applications to cancer rates, Stat. Med 19 (2000) 335–351. [DOI] [PubMed] [Google Scholar]
- [22].von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, BMJ (Clinical Research Ed) 335 (2007) 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the Ovarian Cancer Cohort Consortium. Journal of Clinical Oncology: official journal of the American Society of Clinical Oncology. 2016;34:2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gates MA, Rosner BA, Hecht JL, Tworoger SS, Risk factors for epithelial ovarian cancer by histologic subtype, Am. J. Epidemiol 171 (2010) 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kono S, Sunagawa Y, Higa H, Sunagawa H, Age of menopause in Japanese women: trends and recent changes, Maturitas 12 (1990) 43–49. [DOI] [PubMed] [Google Scholar]
- [26].Nagata C, Takatsuka N, Kawakami N, Shimizu H, Association of diet with the onset of menopause in Japanese women, Am. J. Epidemiol 152 (2000) 863–867. [DOI] [PubMed] [Google Scholar]
- [27].Sakauchi F, Khan MM, Mori M, Kubo T, Fujino Y, Suzuki S, et al. Dietary habits and risk of ovarian cancer death in a large-scale cohort study (JACC study) in Japan. Nutr. Cancer 2007;57:138–45. [DOI] [PubMed] [Google Scholar]
- [28].Yoshida H, Sakamoto H, Leslie A, Takahashi O, Tsuboi S, Kitamura K, Contraception in Japan: current trends, Contraception 93 (2016) 475–477. [DOI] [PubMed] [Google Scholar]
- [29].Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. The Lancet Oncology. 2012;13:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arakawa I, Momoeda M, Osuga Y, Ota I, Koga K, Cost-effectiveness of the recommended medical intervention for the treatment of dysmenorrhea and endometriosis in Japan, Cost Effectiveness and Resource Allocation: C/E 16 (2018) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kobayashi H, Sumimoto K, Kitanaka T, Yamada Y, Sado T, Sakata M, et al. Ovarian endometrioma—risks factors of ovarian cancer development. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;138:187–93. [DOI] [PubMed] [Google Scholar]
- [32].Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited mutations in women with ovarian carcinoma. JAMA oncology. 2016;2:482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sakamoto I, Hirotsu Y, Nakagomi H, Ouchi H, Ikegami A, Teramoto K, et al. BRCA1 and BRCA2mutations in Japanese patients with ovarian, fallopian tube, and primary peritoneal cancer. Cancer. 2016;122:84–90. [DOI] [PubMed] [Google Scholar]
- [34].Oda K, Hamanishi J, Matsuo K, Hasegawa K, Genomics to immunotherapy of ovarian clear cell carcinoma: unique opportunities for management, Gynecol. Oncol 151 (2018) 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clinical Cancer Research: an official journal of the American Association for Cancer Research. 2005; 11:6116–26. [DOI] [PubMed] [Google Scholar]
- [36].Mueller JJ, Schlappe BA, Kumar R, Olvera N, Dao F, Abu-Rustum N, et al. Massively parallel sequencing analysis of mucinous ovarian carcinomas: genomic profiling and differential diagnoses. Gynecol. Oncol. 2018;150:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Alexandre J, Ray-Coquard I, Selle F, Floquet A, Cottu P, Weber B, et al. Mucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experience. Annals of Oncology: official journal of the European Society for Medical Oncology. 2010;21: 2377–81. [DOI] [PubMed] [Google Scholar]
- [38].Perren TJ, Mucinous epithelial ovarian carcinoma, Annals of Oncology: official journal of the European Society for Medical Oncology 27 (Suppl. 1) (2016) i53–i57. [DOI] [PubMed] [Google Scholar]
- [39].Schiavone MB, Herzog TJ, Lewin SN, Deutsch I, Sun X, Burke WM, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am. J. Obstet. Gynecol. 2011; 205:480.e1–8. [DOI] [PubMed] [Google Scholar]
- [40].Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA, Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study, Cancer 117 (2011) 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/tokusyu/gaikoku14/dl/02.pdf, Accessed date: 15 October 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.