ABSTRACT
Tumor necrosis factor-α (TNF-α)-inhibiting agents are a standard therapy for moderate-to-severe inflammatory bowel disease (IBD). IgA nephropathy in the setting of prolonged exposure to TNF-α inhibitors is a rare, clinically significant adverse event often overlooked by gastroenterologists but well documented in the rheumatologic literature. We present a case series of 3 patients with IBD on TNF-α inhibitors who developed biopsy-proven IgA nephropathy. Clinicians prescribing TNF-α inhibitors to patients with IBD need to be aware of this potential side effect. Therapies with alternative mechanisms of action should instead be considered.
INTRODUCTION
Inflammatory bowel disease (IBD) traditionally comprises the following 2 conditions: Crohn's disease (CD) and ulcerative colitis. CD is characterized by a transmural, granulomatous inflammation of the gastrointestinal tract. Ulcerative colitis typically only involves the colonic mucosa and submucosa. Their pathogenesis is believed to be related to immune system dysregulation, possibly triggered by environmental or microbial factors, in the background of a genetic predisposition. Tumor necrosis factor-α (TNF-α) is a central mediator in the inflammatory response to antigens in the gastrointestinal mucosa, and its high levels are implicated in the immune dysregulation associated with IBD. TNF-α inhibitors, including adalimumab and infliximab, are a mainstay of treatment for induction and maintenance therapy of moderate-to-severe IBD. Some estimates report that 10%–25% of patients with IBD are on a TNF-α inhibitor.1,2
IgA nephropathy (IgAN) is a primary glomerulonephritis diagnosed by renal biopsy and characterized by deposition of IgA complexes within a proliferating glomerular mesangium. Patients may present with hematuria, renal failure, flank pain, and hypertension. Management consists of blood pressure control and immunosuppressive therapy. Patients with IBD develop IgAN at a significantly higher rate than the general population, and a shared genetic locus has been demonstrated.3–6 The prevalence of concurrence was previously found to be around 24%.3 In addition, infliximab and adalimumab have been associated with IgAN.3,7–14 To our knowledge, there are no reported cases of IgAN associated with golimumab or certolizumab. We describe 3 cases of new-onset IgAN in patients with IBD on TNF-α inhibitors.
CASE REPORTS
Patient 1
A 19-year-old woman with ileocolonic CD diagnosed at age 10 who initially failed azathioprine and mesalamine therapy achieved clinical remission on infliximab monotherapy. She later developed fever and fatigue and was found to have disseminated histoplasmosis. Her infliximab was discontinued, and she completed 18 months of itraconazole therapy. Resolution of her infection was confirmed by a negative urine antigen. Eight months later, while off medical therapy for CD, she developed a flare. Infliximab was resumed, and she again achieved clinical remission. Three years later, she developed refractory hypertension. During an infliximab infusion, she was noted to have severe symptomatic hypertension with a headache and chest tightness. Laboratory test results did not reveal antidrug antibodies or an elevation in serum creatinine. She was referred to nephrology for the evaluation of refractory hypertension and microscopic hematuria. Laboratory test results were revealing of hypocomplementemia (C3) and elevated serum antinuclear antibody titer. Renal biopsy and immunofluorescence (IF) staining revealed IgA-predominant glomerular mesangial deposits. Electron microscopy revealed characteristic dense mesangial electron deposits, consistent with IgAN (Figure 1). Infliximab was discontinued, and therapy was transitioned to vedolizumab. Her hypertension improved, and she was able to be managed on single agent therapy. Follow-up ileocolonoscopy on vedolizumab was consistent with endoscopic remission.
Figure 1.
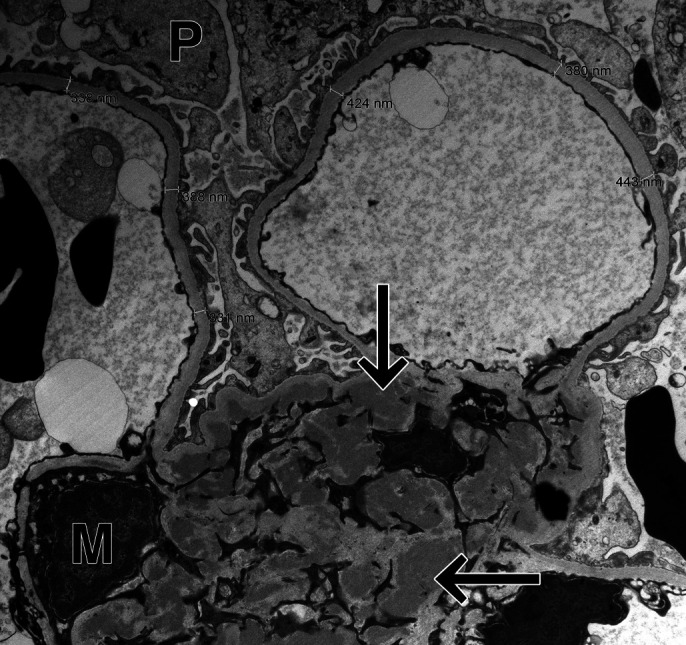
Ultramicrograph shows mesangial electron dense deposits (arrows). M, mesangium; P, podocyte.
Patient 2
A 74-year-old man with stricturing ileal CD diagnosed at age 15 requiring multiple small bowel resections was started on adalimumab therapy. Findings on surveillance ileocolonoscopy 11 months later showed endoscopic remission. In the interim, he developed subacute, progressively worsening renal failure in the setting of chronic obstructive uropathy. His urinalysis was significant for proteinuria, hematuria, and pyuria, and his serum creatinine increased to 1.22 mg/dL from a baseline of 0.90 mg/dL. He was referred to nephrology for workup of suspected glomerulonephritis. Given the timing of the initiation of adalimumab in relation to his worsening renal function, therapy was discontinued. A renal biopsy was performed, and IF stain revealed IgA/IgM codominant mesangial deposits in the glomerulus, consistent with IgAN. Two years after cessation of adalimumab therapy, his CD remained in clinical remission and his renal function continued to gradually decline. He later developed septic shock secondary to a urinary tract infection requiring an intensive care unit admission and eventually passed away.
Patient 3
A 45-year-old man was diagnosed with ulcerative pancolitis at age 30 and started on induction and maintenance dose mesalamine. He later developed recurrent flares while on-and-off therapy requiring prednisone. After a second disease flare, repeat ileocolonoscopy revealed aphthous ulcers in the terminal ileum consistent with ileocolonic CD. He was started on induction and maintenance adalimumab monotherapy and achieved clinical and endoscopic remission. On routine monitoring 4 years later, he was noted to have hypertension and elevation of his serum creatinine to 1.81 mg/dL from a baseline of 1.2 mg/dL. A urinalysis was notable for hematuria. He was referred to nephrology and underwent renal biopsy. IF staining showed IgA-predominant glomerular mesangial deposits, consistent with IgAN. Adalimumab was discontinued, and he was started on vedolizumab induction and maintenance therapy. His creatinine level peaked at 2.03 mg/dL and then gradually declined to a level of 1.57 mg/dL. Surveillance ileocolonoscopy on vedolizumab was consistent with endoscopic and histologic remission.
DISCUSSION
We present 3 patients with IBD who developed hypertension and hematuria secondary to IgAN occurring 1–4 years after starting or restarting anti-TNF therapy. Two patients also developed acute kidney injury. Previous case reports show that patients with rheumatologic conditions develop IgAN while on TNF-α inhibitors.7–14 This adverse therapeutic event is less reported in patients with IBD. Stokes et al demonstrated a relationship between IgAN and TNF-α inhibitors in 5 patients with rheumatoid arthritis.7 These patients recovered after switching immunosuppressive regimens. A mechanism of this relationship proposed by Di Lernia is based on aberrant IgA molecules cross-reacting with antidrug antibodies and forming complexes that deposit in the glomerular mesangium, disrupting normal filtration.15
In a case, a patient with CD who developed adalimumab-induced IgAN recovered after switching to infliximab.16 However, our series demonstrates a possible class effect. With the advent of novel biologics with alternative mechanisms of action, it may be preferable to choose an alternative class agent. We caution substituting TNF-α inhibitors in patients with documented drug-related IgAN unless alternative therapies are deemed inappropriate. This report aims to raise awareness of IgAN as an increasingly recognized adverse effect of TNF-α inhibitors. Clinicians who prescribe these agents should routinely monitor patient renal function and blood pressure and be aware of this potentially reversible adverse event that requires urgent nephrology evaluation, renal biopsy, and drug cessation.
DISCLOSURES
Author contributions: T. Strobel wrote the manuscript and reviewed the literature. W. Ahmed edited the manuscript and reviewed the literature. C. De la Sancha provided the pathology images. M. Bohm and M. Fischer edited the manuscript. W. Ahmed is the article guarantor.
Acknowledgments: The authors would like to acknowledge Dr. Carrie Phillips who helped with the pathology specimens in this report.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.Targownik LE, Tennakoon A, Leung S, et al. Factors associated with discontinuation of anti-TNF inhibitors among persons with IBD: A population-based analysis. Inflamm Bowel Dis. 2017;23(3):409–20. [DOI] [PubMed] [Google Scholar]
- 2.Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut. 2020;69:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambruzs JM, Walker PD, Larsen CP. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9(2):265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filiopoulos V, Trompouki S, Hadjiyannakos D, Paraskevakou H, Kamperoglou D, Vlassopoulos D. IgA nephropathy in association with Crohn's disease: A case report and brief review of the literature. Ren Fail. 2010;32(4):523–7. [DOI] [PubMed] [Google Scholar]
- 5.Saha MK, Julian BA, Novak J, Rizk DV. Secondary IgA nephropathy. Kidney Int. 2018;94(4):674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi D, Zhong Z, Wang M, et al. Identification of susceptibility locus shared by IgA nephropathy and inflammatory bowel disease in a Chinese Han population. J Hum Genet. 2020;65(3):241–9. [DOI] [PubMed] [Google Scholar]
- 7.Stokes MB, Foster K, Markowitz GS, et al. Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant. 2005;20(7):1400–6. [DOI] [PubMed] [Google Scholar]
- 8.Sakellariou GT, Vounotrypidis P, Berberidis C. Infliximab treatment in two patients with psoriatic arthritis and secondary IgA nephropathy. Clin Rheumatol. 2007;26(7):1132–3. [DOI] [PubMed] [Google Scholar]
- 9.Jacquet A, Francois H, Frangie C, et al. IgA nephropathy associated with ankylosing spondylitis is not controlled by infliximab therapy. Nephrol Dial Transplant. 2009;24(11):3540–2. [DOI] [PubMed] [Google Scholar]
- 10.Marocchi E, Spadaro A, Giannakakis K, Priori R, Valesini G. Infliximab in a patient with ankylosing spondylitis and secondary IgA nephropathy requiring haemodialysis. Clin Exp Rheumatol. 2010;28:440. [PubMed] [Google Scholar]
- 11.Sokumbi O, Wetter DA, Makol A, Warrington KJ. Vasculitis associated with tumor necrosis factor-alpha inhibitors. Mayo Clin Proc. 2012;87(8):739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozcakar L, Ekiz T, Yalcin S, Akinci A. IgA nephropathy in an ankylosing spondylitis patient during infliximab therapy: Chicken, egg or mother and child reunion? Acta Reumatol Port. 2013;38(4):310. [PubMed] [Google Scholar]
- 13.Wei SS, Sinniah R. Adalimumab (TNF alpha inhibitor) therapy exacerbates IgA glomerulonephritis acute renal injury and induces lupus autoantibodies in a psoriasis patient. Case Rep Nephrol. 2013;2013:812781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluger N, Du-Thanh A, Bessis D, Servel MF, Mourad G. Psoriasis-associated IgA nephropathy under infliximab therapy. Int J Dermatol. 2015;54(3):e79–80. [DOI] [PubMed] [Google Scholar]
- 15.Di Lernia V. IgA nephropathy during treatment with TNF-alpha blockers: Could it be predicted? Med Hypotheses. 2017;107:12–3. [DOI] [PubMed] [Google Scholar]
- 16.Bhagat Singh AK, Jeyaruban AS, Wilson GJ, Ranganathan D. Adalimumab-induced IgA nephropathy. BMJ Case Rep. 2019;12(3):e226442. [DOI] [PMC free article] [PubMed] [Google Scholar]


