ABSTRACT
For unclear reasons, there has been an increasing number of reported cases of Sarcina infections in the gastrointestinal tract over the past several years. Associated clinical conditions with the infection most commonly include delayed gastric emptying from diabetes mellitus, a history of previous gastrointestinal surgery, and ulcer disease. The precise pathogenetic role of Sarcina infection in humans remains unclear. Because of the ubiquitous environmental presence of Sarcina and limited previously reported clinical cases, the link between symptoms along with endoscopic findings to Sarcina can be associative at best. When found in the upper GI tract, the decision to treat along with the chosen regimen remains debatable. Sarcina, however, has rarely been seen in the esophagus. We report the third case of Sarcina of the esophagus associated with Helicobacter pylori gastritis.
INTRODUCTION
Sarcina is a Gram-positive, nonmotile, anaerobic coccus that relies exclusively on the fermentative metabolism which was first identified in the gastric contents of human beings by John Goodsir in 1842.1,2 Since then, its precise pathogenetic role in human beings still remains unclear because clinical observations have ranged from asymptomatic to life-threatening complications, such as gastric perforation and emphysematous gastritis.2,3 To date, less than 30 cases of Sarcina-associated gastrointestinal pathology have been published in the literature, with most of them presenting with symptoms of abdominal pain, nausea, vomiting, and delayed gastric emptying.4 Our review of literature yielded a total of 7 case reports that reported 8 patients with Sarcina on esophageal biopsies.5–11 Two of those had concomitant Helicobacter pylori gastritis.11 We report the third case of Sarcina of the esophagus associated with H. pylori gastritis.
CASE REPORT
A 70-year-old woman presented to the gastroenterology outpatient clinic with an increase in abdominal discomfort, heartburn, postprandial nausea, and intermittent episodes of nonbilious, nonbloody vomiting over the past several months. She had previously been taking omeprazole daily for reflux symptoms without relief and naproxen daily for joint pain. She underwent an esophagogastroduodenoscopy that showed erythematous mucosa in the distal esophagus, gastropathy, and a hiatal hernia (Figure 1). Targeted esophageal and gastric mucosal biopsies showed erosive esophagitis with many colonies of Sarcina species and chronic gastritis with H. pylori infection, respectively (Figure 2). The diagnosis of Sarcina was made with hematoxylin-eosin stain and Gram stain.
Figure 1.
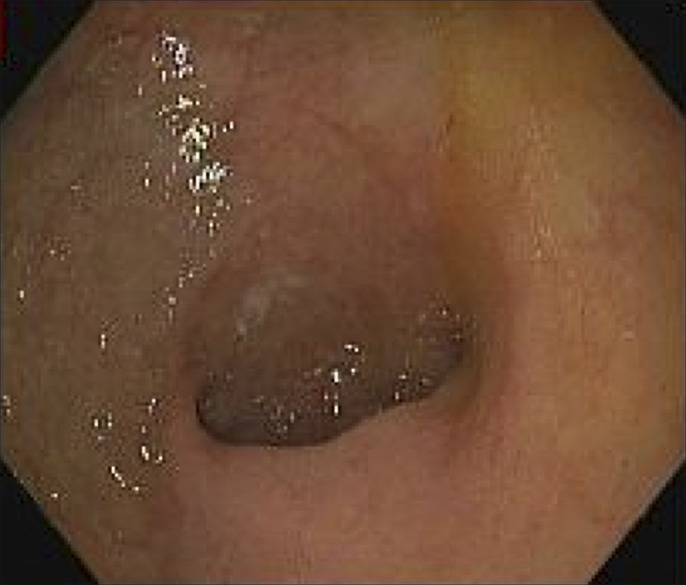
Esophagogastroduodenoscopy showing gastric erosions.
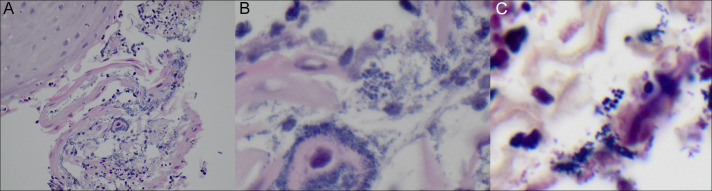
Figure 2.
Biopsy showing (A) squamous mucosa with acute inflammation and reactive cellular changes that includes parakeratosis and fibrinopurulent exudate indicative of esophageal erosion (hematoxylin and eosin stain, 40× magnification) and (B) polymorphous population of cocci, the largest in tetrads characteristic of Sarcina organisms (hematoxylin and eosin stain, 60× magnification) and Gram stain showing Gram-positive cocci (60× magnification).
The patient was prescribed tetracycline, metronidazole, and bismuth subsalicylate for 2 weeks, and her omeprazole increased to twice daily for treatment of both infections. After completing the treatment regimen, the patient reported having little improvement in symptoms. Repeat esophagogastroduodenoscopy showed normal-appearing mucosa of the esophagus, stomach, and duodenum. Esophageal biopsies showed normal squamous mucosa. Gastric biopsies showed persistent H. pylori gastritis. She was started on a salvage treatment regimen of levofloxacin 500 mg daily, amoxicillin 1 g twice daily, and omeprazole 20 mg twice daily for a total of 14 days. Repeat testing was noted for persistent H. pylori infection. At this point, we planned to await culture and antibiotic sensitivity report before considering retreatment.
DISCUSSION
Sarcina has been well documented in the veterinary literature as being a causative organism of gastric dilatation and death in sheep, goats, cats, and horses.12,13 The bacteria have been found in the soil, air, and stagnant water, and even in the feces of human beings who have been noted to eat primarily a plant-based diet.14 However, there is currently no clear understanding of whether it has a precise mechanism for causing direct disease in human beings. Most cases of infection have been seen in patients with a history of gastric outlet obstruction, diabetic gastroparesis, pyloric stenosis, and gastrointestinal surgeries.4,14 It has been believed that these pre-existing conditions serve as a prime environment for rapid growth of the anaerobic organism, which in turn produces extensive carbon dioxide through fermentative metabolism that leads to patients reporting symptoms of bloating and abdominal discomfort.2
Sarcina has also been implicated in human cases of gastric perforation, emphysematous gastritis, and gastric adenocarcinomas.15 However, there have been reports of asymptomatic patients with Sarcina found incidentally on gastric biopsies.13 In our case, Sarcina was found on esophageal biopsy, and not on gastric biopsy. Based on our review of the literature, there have been 8 patients noted to have Sarcina identified on esophageal biopsies.5–11 Common presenting symptoms included epigastric pain, dysphagia, and vomiting. Of the 8 cases, 3 had endoscopic findings of erosive esophagitis.9,11 Of the 8 cases, 2 had concomitant H. pylori gastritis.11 Of the 8 cases, 2 had concomitant alternative esophageal etiologies that included Candida and Cytomegalovirus infection.5,7 Of the 8 cases, only 2 were provided treatment for Sarcina.8,9
In our case, minimal improvement in patient symptoms despite successful Sarcina eradication and persistent H. pylori infection suggests that although there was endoscopic and histologic evidence of esophagitis, most patient symptoms were likely due to H. pylori or noninfective etiology. This finding supports those who favor Sarcina to be a benign pathogen in the GI tract. Furthermore, there is currently no consensus on a standard regimen or duration for the treatment of Sarcina infection. Because of its implication in cases of life-threatening complications, such as gastric perforation and emphysematous gastritis, some have decided to treat patients with a regimen consisting of antibiotics, a proton-pump inhibitor, and a prokinetic.12 In our case, our patient was treated with a similar regimen that covered for both Sarcina and H. pylori infections. Although persistent H. pylori infection was noted on repeat biopsy, Sarcina was not. This result illustrates endoscopic and histological improvement with treatment, but our patient did not improve symptomatically. It is unclear whether H. pylori gastritis in any way can be associated with Sarcina esophagitis, but this case certainly raises a question.
In conclusion, our case is the third-ever report of concomitant Sarcina esophagitis and H. pylori gastritis. In the absence of known risk factors for Sarcina infection of the upper GI tract such as gastric outlet obstruction or gastroparesis, the possibility that active H. pylori infection is a risk factor for Sarcina infection cannot be refuted. Whether Sarcina has a direct pathogenetic role in gastric and esophageal diseases along with it warranting antibiotic treatment remains unknown. Few prefer to treat it because of the risk of associated life-threatening complications.
DISCLOSURES
Author contributions: M. Chan wrote the manuscript. D. Jain and A. Platt edited the manuscript. C. Minimo provided the pathology images. M. Chan is the article guarantor.
Financial disclosure: None to report.
Previous presentation: This case was presented at the American College of Gastroenterology Annual Scientific Meeting; October 25–30, 2019; San Antonio, Texas.
Informed consent was obtained for this case report.
REFERENCES
- 1.Ferrier D. The constant occurrence of Sarcina ventriculi (Goodsir) in the blood of man and the lower animals: With remarks on the nature of sarcinous vomiting. Br Med J. 1872;1(578):98–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolentino LF, Kallichanda N, Javier B, Yoshimori R, French SW. A case report of gastric perforation and peritonitis associated with opportunistic infection by Sarcina ventriculi. Lab Med. 2003;34(7):535–7. [Google Scholar]
- 3.Laass MW, Pargac N, Fischer R, Bernhardt H, Knoke M, Henker J. Emphysematous gastritis caused by Sarcina ventriculi. Gastrointest Endosc. 2010;72(5):1101–3. [DOI] [PubMed] [Google Scholar]
- 4.Rizwan M, Al Rasheed H, Senseng CG. Sarcina ventriculi : Review of the literature. Arch Pathol Lab Med. 2016;140:1441–5. [DOI] [PubMed] [Google Scholar]
- 5.Neppl C, Friedli B, Hewer E. Esophageal cytology: A tale of shish kebab and roman legionaries. Gastroenterology. 2018;155(1):e14–5. [DOI] [PubMed] [Google Scholar]
- 6.Dolganiuc A, Liu X, Sharma A. Dysphagia with unusual esophageal plaques. Gastroenterology. 2017;152(4):e7–8. [DOI] [PubMed] [Google Scholar]
- 7.Li, Feng; Arnold, Christina; Hart P. Unlikely organisms during an evaluation for abdominal pain in an immunosuppressed patient. Am J Gastroenterol. 2017;112:S1313–4. [Google Scholar]
- 8.Behzadi J, Modi RM, Goyal K, Chen W, Pfeil S. Sarcina ventriculi as an unknown culprit for esophageal stricturing. ACG Case Rep J. 2017;4:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Meij TGJ, van Wijk MP, Mookhoek A, Budding AE. Ulcerative gastritis and esophagitis in two children with Sarcina ventriculi infection. Front Med. 2017;4:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrigan S, Grin A, Al-Haddad S, et al. Emphysematous oesophagitis associated with Sarcina organisms in a patient receiving anti-inflammatory therapy. Histopathology. 2015;67(2):270–2. [DOI] [PubMed] [Google Scholar]
- 11.Sauter JL, Nayar SK, Anders PD, D'Amico M, Butnor KJ, Wilcox RL. Co-existence of Sarcina organisms and Helicobacter pylori gastritis/duodenitis in pediatric siblings. J Clin Anat Pathol. 2013;1:103. [PMC free article] [PubMed] [Google Scholar]
- 12.Lam-Himlin D, Tsiatis AC, Montgomery E, et al. Sarcina organisms in the gastrointestinal tract: A clinicopathologic and molecular study. Am J Surg Pathol. 2011;35(11):1700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratuapli SK, Lam-Himlin DM, Heigh RI. Sarcina ventriculi of the stomach: A case report. World J Gastroenterol. 2013;19(14):2282–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sopha SC, Manejwala A, Boutros CN. Sarcina, a new threat in the bariatric era. Hum Pathol. 2015;46(9):1405–7. [DOI] [PubMed] [Google Scholar]
- 15.Bhagat P, Gupta N, Kumar M, Radotra BD, Sinha SK. A rare association of Sarcina with gastric adenocarcinoma diagnosed on fine-needle aspiration. J Cytol. 2015;32(1):50–2. [DOI] [PMC free article] [PubMed] [Google Scholar]