Abstract
PURPOSE
In advanced gastrointestinal stromal tumor (GIST), there is an unmet need for therapies that target both primary and secondary mutations of pathogenic KIT/PDGFRA oncoproteins. Ripretinib is a novel switch-control kinase inhibitor designed to inhibit a wide range of KIT and PDGFRA mutations.
PATIENTS AND METHODS
This first-in-human, to our knowledge, phase I study of ripretinib (ClinicalTrials.gov identifier: NCT02571036) included a dose-escalation phase and subsequent expansion phase at the recommended phase II dose (RP2D). Eligible patients included those with advanced GIST, intolerant to or experienced progression on ≥ 1 line of systemic therapy, and other advanced malignancies. Safety, dose-limiting toxicities (DLTs), maximum-tolerated dose (MTD), and preliminary antitumor activity were evaluated.
RESULTS
At data cutoff (August 31, 2019), 258 patients (n = 184 GIST) were enrolled, with 68 patients in the dose-escalation phase. Three DLTs were reported: grade 3 lipase increase (n = 2; 100 mg and 200 mg twice a day) and grade 4 increased creatine phosphokinase (n = 1; 150 mg once daily). MTD was not reached (maximum dose evaluated, 200 mg twice a day); 150 mg once daily was established as the RP2D. The most frequent (> 30%) treatment-emergent adverse events in patients with GIST receiving ripretinib 150 mg once daily (n = 142) were alopecia (n = 88 [62.0%]), fatigue (n = 78 [54.9%]), myalgia (n = 69 [48.6%]), nausea (n = 65 [45.8%]), palmar-plantar erythrodysesthesia (n = 62 [43.7%]), constipation (n = 56 [39.4%]), decreased appetite (n = 48 [33.8%]), and diarrhea (n = 47 [33.1%]). Objective response rate (confirmed) of 11.3% (n = 16/142) ranging from 7.2% (n = 6/83; fourth line or greater) to 19.4% (n = 6/31; second line) and median progression-free survival ranging from 5.5 months (fourth line or greater) to 10.7 months (second line), on the basis of investigator assessment, were observed.
CONCLUSION
Ripretinib is a well-tolerated, novel inhibitor of KIT and PDGFRA mutant kinases with promising activity in patients with refractory advanced GIST.
INTRODUCTION
Activating genomic alterations in KIT or PDGFRA drive cellular growth in most gastrointestinal stromal tumors (GISTs) and other cancer types, including systemic mastocytosis and some glioblastomas.1-4 There is no curative therapy for metastatic GIST. First-line treatment with the tyrosine kinase inhibitor (TKI) imatinib has a median progression-free survival (mPFS) of 1.7-2 years.5-7 Progression is largely due to the development of secondary resistance mutations in the ATP-binding domain or activation loop of KIT/PDGFRA.1,5-13 Secondary mutations can sterically disrupt binding of some TKIs or result in kinase activation.13 The mPFS with approved second-line (sunitinib) and third-line (regorafenib) therapies is approximately 5.6 months and 4.8 months, respectively—outcomes that likely reflect incomplete inhibition of secondary resistance mutations.8,9,14-16 Avapritnib is only approved for treatment of GIST with PDGFRA exon 18 mutations, which currently account for approximately 6% of the overall GIST population.13,17,18 Consequently, an unmet medical need exists for treatments effective against a broad range of KIT/PDGFRA mutations in advanced GIST, as well as other cancers that have biologically relevant KIT/PDGFRA mutations.
CONTEXT
Key Objective
Treatments against a broad range of KIT/PDGFRA mutations in advanced gastrointestinal stromal tumor (GIST) are needed. Efficacy for second-line sunitinib and third-line regorafenib are minimal and avapritinib is limited to GIST with PDGFRA exon 18 mutations. This study determined the recommended phase II dose (RP2D) and preliminary safety and efficacy of ripretinib, a novel tyrosine kinase inhibitor, in patients with advanced gastrointestinal stromal tumor (GIST).
Knowledge Generated
Ripretinib 150 mg daily (RP2D) was well tolerated in patients with advanced GIST; no maximum tolerated dose was reached. Preliminary efficacy in patients with advanced GIST was promising as second-, third-, and fourth-line or greater therapy.
Relevance
Our study results support the further development of ripretinib as an active and well-tolerated therapy in advanced GIST in the second-, third-, and fourth-line or greater, including the initiation of INVICTUS, a phase III study that showed significantly improved median progression-free survival with ripretinib compared with placebo as fourth-line or greater therapy in advanced GIST, and INTRIGUE, a phase III study currently evaluating ripretinib versus sunitinib as second-line therapy. In May 2020, ripretinib was approved by the US FDA for the treatment of adult patients with advanced GIST who have received prior treatment with three or more kinase inhibitors, including imatinib.
Ripretinib (DCC-2618) is a switch-control TKI designed to broadly inhibit KIT and PDGFRA kinase signaling through a dual mechanism of action (MoA).19 As dual-switch kinases, KIT and PDGFRA contain an inhibitory switch and an activation loop that can occupy the switch pocket and determine an inactive or active kinase conformation, respectively. Ripretinib is designed to precisely and durably bind to both the switch pocket and the activation loop to prevent kinase activation and stabilize the kinase in the inactive state, preventing downstream signaling and cell proliferation.19 This MoA provides broad inhibition of KIT/PDGFRA kinase activity, including KIT/PDGFRA wild type and multiple primary and secondary mutations. Ripretinib also inhibits other kinases in vitro, including PDGFRB, TIE2, VEGFR2, and BRAF.19 A comparison of the MoA of ripretinib with that of other TKIs currently approved for the treatment of advanced GIST is provided in the Appendix (online only).
We report results from, to our knowledge, the first-in-human phase I study of ripretinib (ClinicalTrials.gov identifier: NCT02571036) in patients with advanced GIST and other malignancies with a focus on patients with GIST receiving 150 mg once daily.
PATIENTS AND METHODS
Patients
Patients were ≥ 18 years of age with a histologically confirmed diagnosis of GIST with a KIT or PDGFRA mutation and disease progression or intolerance to ≥ 1 line of systemic anticancer therapy or other malignancies with amplifications and/or mutations in KIT or PDGFRA, or other mutations that conferred sensitivity to ripretinib (eg, PDGFRB, TIE2, or VEGFR2). Initially, patients with KIT/PDGFRA wild-type mutational status were permitted; however, after protocol amendments, these patients were excluded. Other eligibility criteria included Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤ 2 and adequate organ function and bone marrow reserve. Complete inclusion and exclusion criteria are available in the Data Supplement.
Study Design
This was a multicenter phase I dose-escalation study of single-agent oral ripretinib with an expansion phase at the recommended phase II dose (RP2D). Primary objectives were to determine the safety and tolerability, maximum tolerated dose (MTD), and RP2D in the dose-escalation phase and to further evaluate safety and early efficacy in the dose-expansion phase. Secondary objectives included pharmacokinetic (PK) profile assessment. A pharmacologically guided 3 + 3 design was used to determine the MTD of ripretinib administered once or twice daily. Patients received ripretinib 20-200 mg twice a day or 100-250 mg once daily in repeated 28-day cycles until disease progression, unacceptable toxicity, or consent withdrawal. The MTD was defined as the highest dose level immediately below the dose level that resulted in dose-limiting toxicity (DLT) in ≥ 33% of patients during the first treatment cycle. If a dose level was declared safe (ie, zero DLTs in three patients or one DLT in six patients), the cohort may have been expanded to an additional six patients to further investigate PKs, pharmacodynamics, tolerability, or antitumor activity. Throughout the dose-escalation phase, data were routinely reviewed and monitored by a safety review team composed of principal investigators and key Deciphera personnel.
In the expansion phase, patients were enrolled into molecularly defined (KIT or PDGFRA) disease-specific cohorts, including GIST, and received the RP2D of ripretinib 150 mg once daily. Patients with GIST receiving ripretinib 150 mg once daily with disease progression were allowed to escalate to 150 mg twice a day after cycle 2; patient outcomes were collected according to the initial dose assigned. This article presents results focused on patients with GIST in the expansion phase and those in the dose-escalation phase who received 150 mg once daily.
This study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization Guidelines for Good Clinical Practice. Patients provided written informed consent to participate in this study, and the protocol, protocol amendments, and informed-consent documents were approved by institutional review boards at each study site and by appropriate regulatory authorities before the start of the study.
Safety and Efficacy Assessments
Routine clinical and laboratory assessments, physical examination, ECOG PS, echocardiograms/multigated acquisition scans, as well as dermatologic and ophthalmologic examinations were conducted at baseline and at prespecified intervals (Data Supplement). Adverse events (AEs) were captured continuously from the signing of the informed consent until 30 days after the last administration of ripretinib and were graded by the investigators using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Efficacy was evaluated in patients with GIST receiving 150 mg once daily in the dose-escalation and expansion phases as a preplanned subanalysis. Tumor imaging was performed at screening, after cycles 2, 4, and 6, every three cycles thereafter, and at final study visit. Objective response in both phases was confirmed approximately 28 days later and assessed by the investigator using RECIST version 1.1. An objective response rate (ORR) was defined as the proportion of patients with a confirmed complete response (CR) or partial response (PR). Other efficacy end points included time to best response (defined as time from cycle 1 day 1 to PR or CR), duration of objective response (time from a confirmed CR or PR to disease progression or death), and PFS (time from cycle 1 day 1 to disease progression or death).
Pharmacokinetic Assessments
All PK samples were analyzed by a central laboratory. PK parameters included time to maximum observed concentration (Tmax), maximum observed concentration (Cmax), area under the concentration-time curve (AUC), and half-life.
Statistical Analyses
Safety analyses were performed using the safety population (all patients who received a ripretinib dose). Clinical activity analyses, including efficacy, were performed using the intent-to-treat population (patients in the safety population, excluding two patients who only received one single ripretinib dose on day −7). PK analyses were performed on all patients in the safety population who had ≥ 1 PK concentration.
Baseline demographics, patient characteristics, plasma concentrations, and PK parameters were summarized using descriptive statistics. ORR was summarized using two-sided 95% exact binomial CIs. PFS and duration of response were summarized using the Kaplan-Meier method; time to response was summarized descriptively for confirmed responders.
RESULTS
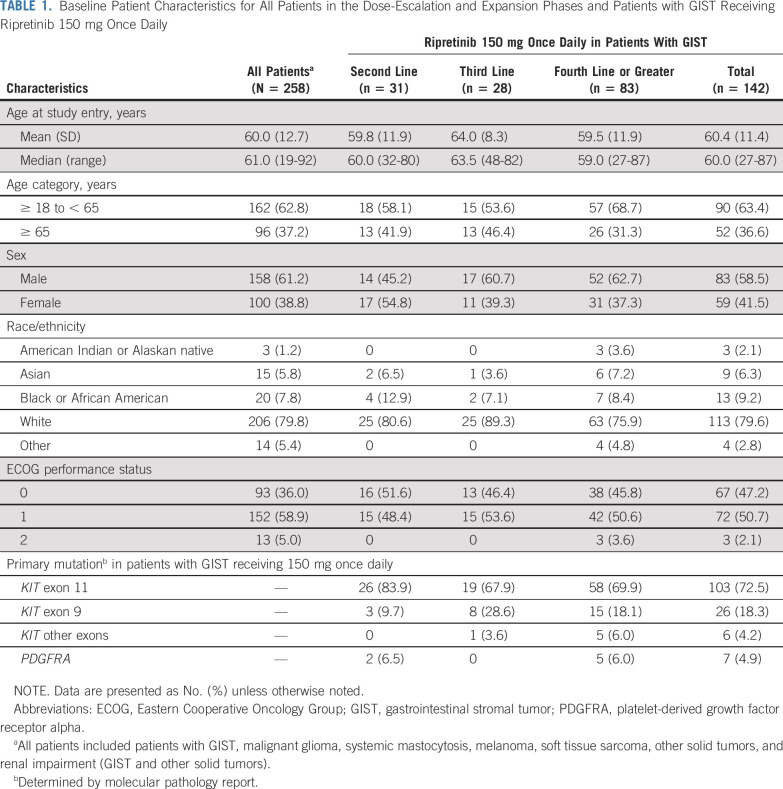
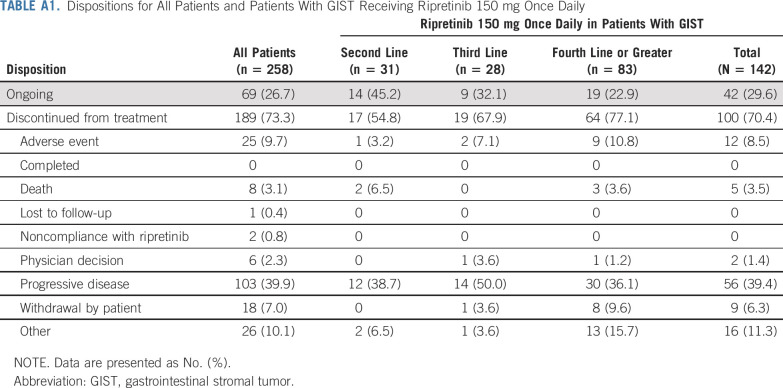
From November 12, 2015 to August 31, 2019 (data cutoff), 258 patients (n = 184 GIST) were enrolled and received ≥ 1 dose of ripretinib during the dose-escalation (n = 68) and expansion (n = 190) phases (Appendix Fig A1, online only). At the time of data cutoff, 69 (26.7%) patients remained on study treatment (n = 12, dose-escalation phase; n = 57, expansion phase). Reasons for treatment discontinuation are listed in Appendix Table A1 (online only). The median (range) follow-up time was 6.2 (0.1-45.6) months. Overall baseline patient characteristics are listed in Table 1.
TABLE 1.
Baseline Patient Characteristics for All Patients in the Dose-Escalation and Expansion Phases and Patients with GIST Receiving Ripretinib 150 mg Once Daily
In the dose-escalation phase, the doses tested included 20 (n = 4), 30 (n = 4), 50 (n = 11), 100 (n = 12), 150 (n = 6), and 200 (n = 7) mg twice a day and 100 (n = 6), 150 (n = 12), and 250 (n = 6) mg once daily. Patients in the expansion phase received the RP2D of 150 mg once daily. A total of 142 patients with advanced GIST from both phases (n = 12, dose-escalation phase; n = 130, expansion phase) were initially assigned to receive ripretinib 150 mg once daily. Of those patients, 42 (29.6%) remained on treatment; 100 (70.4%) had discontinued treatment at data cutoff. Primary reasons for treatment discontinuation were similar to those in the overall group (Appendix Table A1). The median (range) follow-up time in patients with GIST receiving 150 mg once daily was 11.5 (0.1-33.0) months; baseline characteristics were similar to those of all patients (Table 1).
DLTs and MTD
Three DLTs were reported in the dose-escalation phase, with one each occurring in the 100 mg twice a day (n = 12), 200 mg twice a day (n = 7), and 150 mg once daily (n = 12) dose cohorts. At 100 mg twice a day, a DLT of asymptomatic grade 3 lipase elevation occurred. Ripretinib treatment was interrupted in this patient; the dose was reduced to 100 mg once daily and subsequently increased to 100 mg twice a day. At 200 mg twice a day, a DLT of asymptomatic grade 3 lipase elevation occurred, which normalized within 5 days, and the ripretinib dose was reduced to 150 mg twice a day. At 150 mg once daily, a DLT of asymptomatic grade 4 creatine phosphokinase increase occurred. Ripretinib treatment was held and then continued at a reduced dose of 100 mg once daily.
No MTD was reached, as < 33% of patients at each dose level experienced a DLT. The presumed threshold for efficacy for ripretinib is an AUC0-24 h of 10,000 ng × h/mL (ripretinib and its active metabolite, DP-5439), which produced > 90% KIT inhibition in a preclinical murine cancer model and complete tumor growth inhibition at this target AUC. On the basis of in vivo and in vitro pharmacology studies predicting that 150 mg once daily was an efficacious dose, as well as interim PK analysis in this study, the ripretinib 150 mg once daily dose was predicted to maintain the PK exposure above this presumed threshold in > 90% of patients. In addition, exposure and safety data from the dose-escalation phase of this study supported the selection of the RP2D of 150 mg once daily in the expansion phase.
Safety and Efficacy of Ripretinib 150 mg Once Daily in Patients With GIST
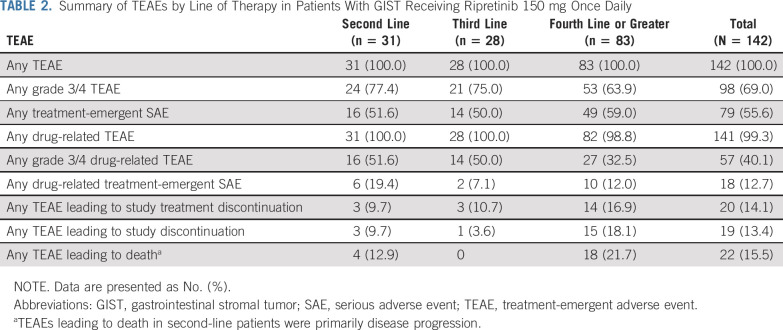
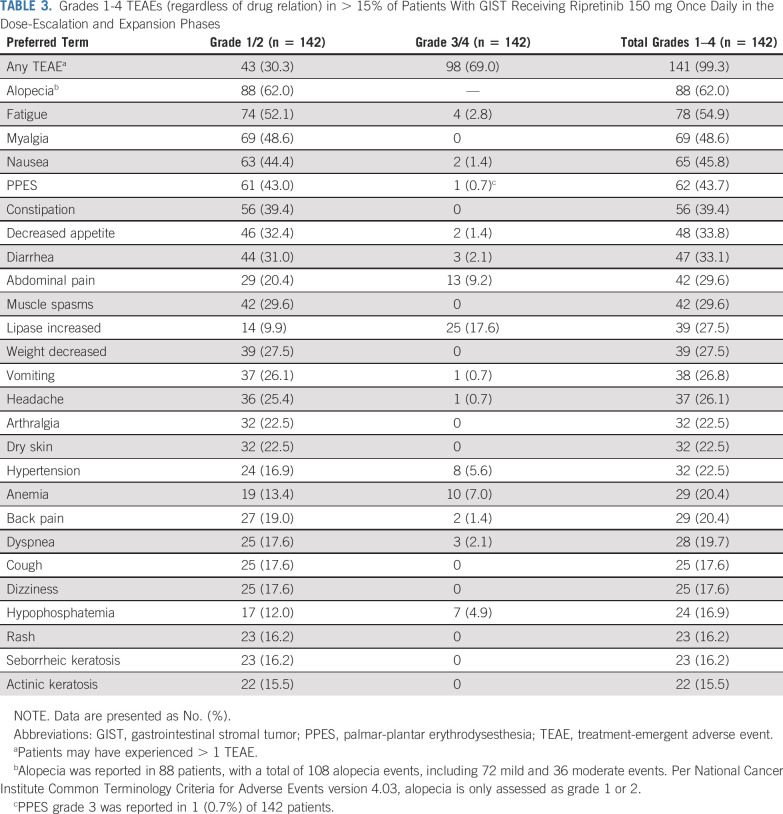
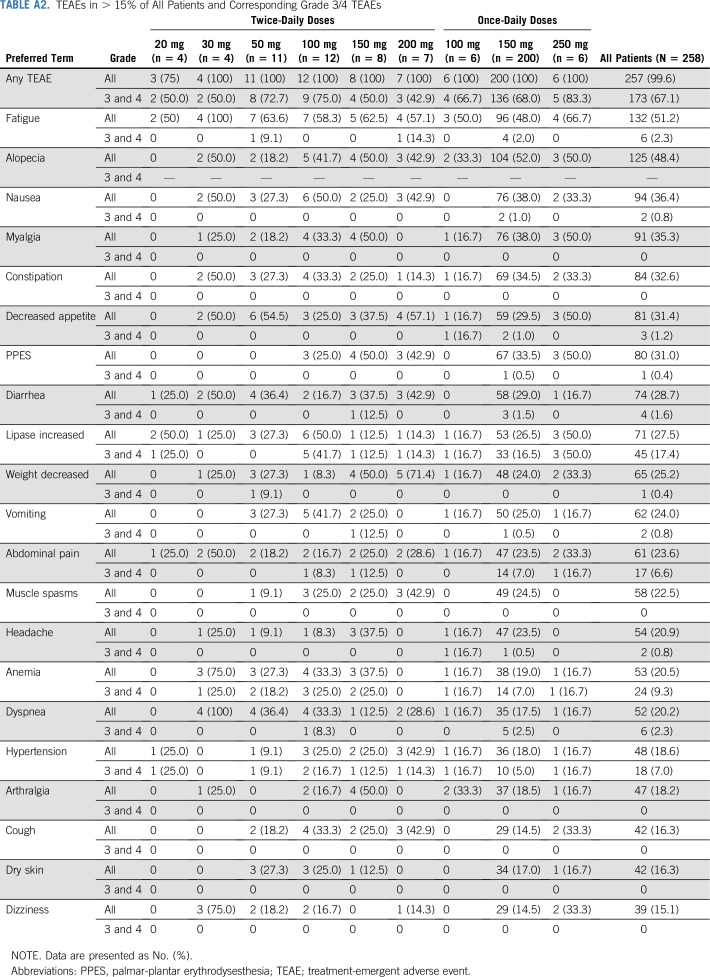
Ripretinib was generally well tolerated in patients with advanced GIST receiving 150 mg once daily despite treatment-emergent adverse events (TEAEs) being reported in 142 (100%) patients (Table 2). The most common overall TEAEs (> 15% of patients, regardless of drug relation) and corresponding grade 3/4 TEAEs are reported in Table 3. Treatment-emergent serious adverse events (regardless of drug relation) in > 3% of patients with GIST receiving 150 mg once daily included abdominal pain (n = 14 [9.9%]), death (n = 11 [7.7%]), cancer surgery (n = 7 [4.9%]), and sepsis (n = 6 [4.2%]). TEAEs leading to a dose reduction occurred in 26 (18.3%) patients, and TEAEs leading to treatment interruption occurred in 77 (54.2%) patients. Only eight (5.6%) patients with GIST receiving 150 mg once daily discontinued study treatment secondary to a drug-related TEAE. Safety data for all patients are included in the Appendix and in Appendix Table A2 (online only).
TABLE 2.
Summary of TEAEs by Line of Therapy in Patients With GIST Receiving Ripretinib 150 mg Once Daily
TABLE 3.
Grades 1-4 TEAEs (regardless of drug relation) in > 15% of Patients With GIST Receiving Ripretinib 150 mg Once Daily in the Dose-Escalation and Expansion Phases
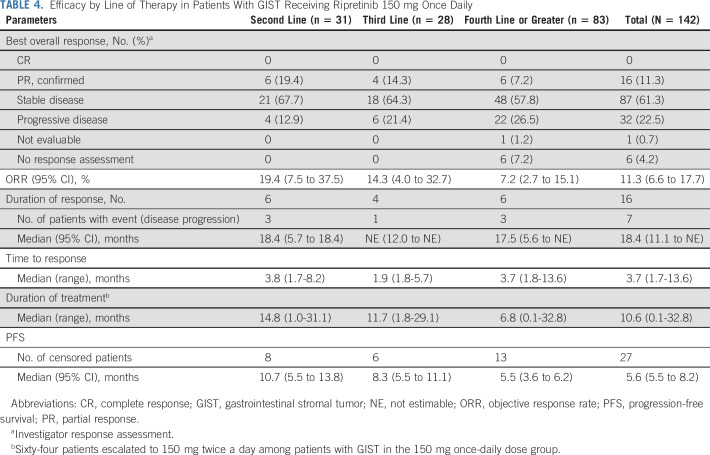
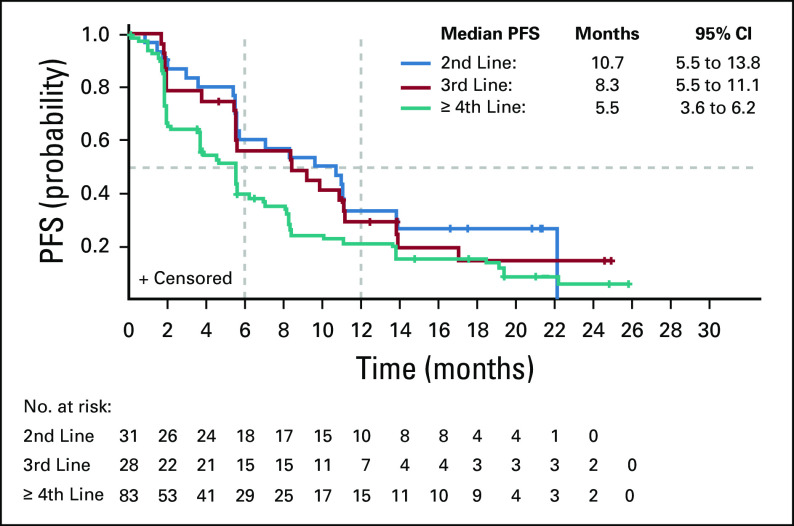
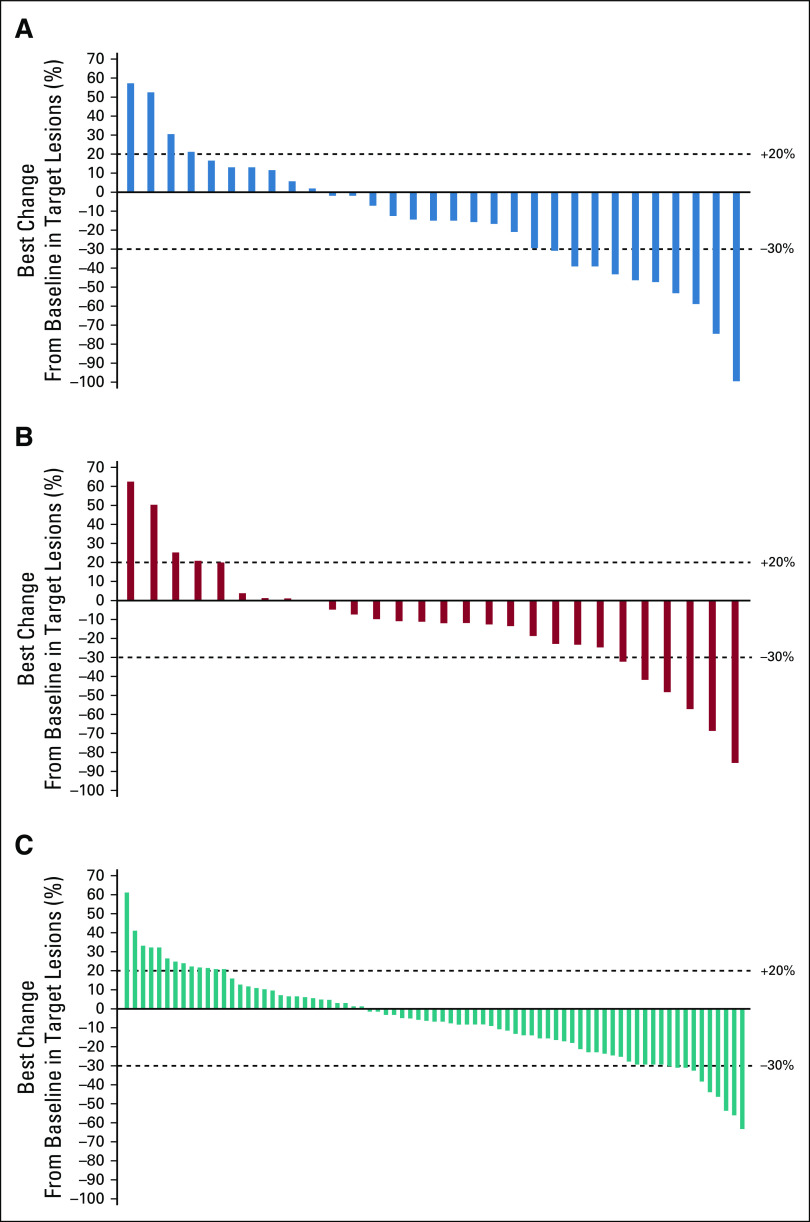
The mPFS was 10.7 months (95% CI, 5.5 to 13.8 months) for patients on second-line therapy, 8.3 months (95% CI, 5.5 to 11.1 months) for third-line, and 5.5 months (95% CI, 3.6 to 6.2 months) for fourth-line or greater therapy (Table 4; Fig 1). The probability of PFS at 12 months, by line of therapy, was 33.5% (95% CI, 17.6% to 50.1%), 29.2% (95% CI, 13.5% to 46.9%), and 21.2% (95% CI, 12.8% to 31.1%) for second-, third-, and fourth-line or greater therapy, respectively. ORRs for second-, third-, and fourth-line or greater therapies were 19.4% (n = 6/31), 14.3% (n = 4/28), and 7.2% (n = 6/83), respectively (Table 4). The best percentage change in target lesions in patients with GIST on second-, third-, or fourth-line or greater therapy is shown in Figure 2. The median time (range) to response among responders was 3.7 (1.7-13.6) months, and the median duration of response was 18.4 (95% CI, 11.1 to not estimable [NE]) months in the 16 responders receiving ripretinib 150 mg once daily, with nine patients continuing to respond as of the data cutoff. The median duration of response for patients on second-line therapy (n = 6) was 18.4 months and was NE for patients on third-line therapy (n = 4). In fourth-line or greater therapy, the median duration of response was 17.5 months in six responders, with three patients continuing to respond as of the data cutoff. As described in the methods, patients who experienced progression and continued to receive clinical benefit were allowed to stay on treatment with the option of dose escalation and therefore may have remained on treatment post progression. The median (range) duration of treatment of second-, third-, and fourth-line or greater therapy were 14.8 (1.0-31.1) months, 11.7 (1.8-29.1) months, and 6.8 (0.1-32.8) months, respectively.
TABLE 4.
Efficacy by Line of Therapy in Patients With GIST Receiving Ripretinib 150 mg Once Daily
FIG 1.
Kaplan-Meier plot of progression-free survival (PFS) by line of therapy in patients with gastrointestinal stromal tumors receiving an initially assigned dose of 150 mg once daily in dose-escalation and expansion phases.
FIG 2.
The best percentage change in target lesions in patients with gastrointestinal stromal tumors receiving ripretinib 150 mg once daily as (A) second-line therapy, (B) third-line therapy, or (C) fourth-line or greater therapy. The waterfall plot shows the best percentage change in target lesions regardless of confirmation of response.
Pharmacokinetics
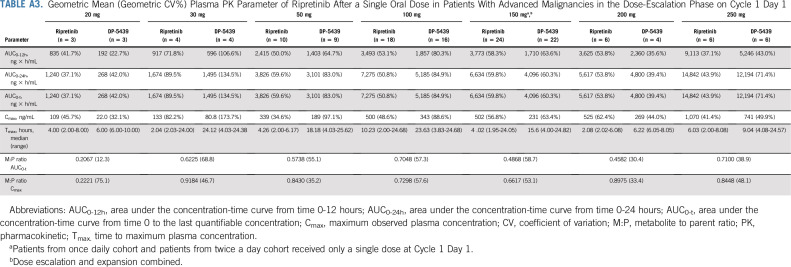
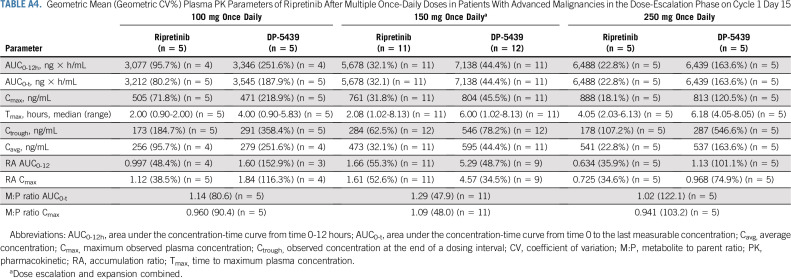
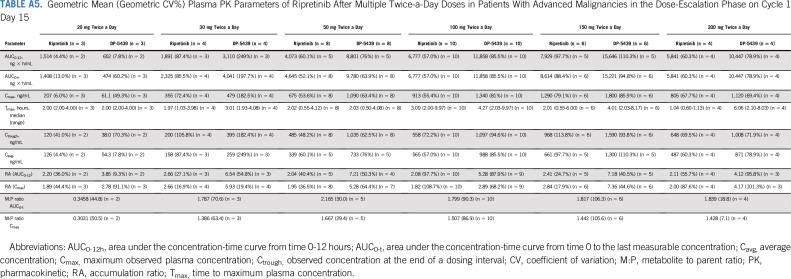
Ripretinib was absorbed after single and multiple doses across the dose ranges studied (single doses from 20-250 mg; Appendix Table A3, online only) and multiple doses from 20-200 mg twice a day; Tables A4 and A5, online only). The time to Tmax ranged from 2-10 hours after single doses administered under fasting conditions on cycle 1 day 1. After a single dose of ripretinib 150 mg on cycle 1 day 1, mean Cmax (coefficient of variation [CV%]), AUC0-12h (CV%), and AUC0-24h (CV%) were 502 ng/mL (56.8%), 3,773 ng × h/mL (58.3%), and 6634 ng × h/mL (59.8%), respectively. However, ripretinib PK parameters after single doses were highly variable between patients, with the CV% for Cmax and AUC0-24h ranging from 35%-60% at ripretinib doses with PK data for at least 10 patients. In most patients, the terminal half-life on the basis of noncompartmental analysis could not be estimated with the PK sampling time points included in the protocol. At cycle 1 day 15, the mean Cmax (CV%) and AUC0-12h (CV%) were 761 ng/mL (31.8%) and 5,678 ng × h/mL (32.1%), respectively, after administration of ripretinib 150 mg once daily. On the basis of AUC0-12h, 66% accumulation was observed, whereas Cmax was 61% higher compared with that at cycle 1 day 1 for ripretinib 150 mg once daily.
The exposure (ie, AUC) of DP-5439 (active metabolite of ripretinib) was approximately 49% of the parent ripretinib after single 150-mg doses and approximately 129% after 15 days of dosing at 150 mg once daily. After a single dose of ripretinib 150 mg on cycle 1 day 1, DP-5439 mean Cmax (CV%), AUC0-12h (CV%), and AUC0-24h (CV%) were 231 ng/mL (63.4%), 1,710 ng × h/mL (63.6%), and 4,096 ng × h/mL (60.3%), respectively. At cycle 1 day 15, the DP-5439 mean Cmax (CV%) and AUC0-12h (CV%) were 804 ng/mL (45.5%) and 7,138 ng × h/mL (44.4%), respectively, after administration of ripretinib 150 mg once daily; accumulation of DP-5439 was 4.6- to 5.3-fold.
DISCUSSION
Efficacy for second-line sunitinib and third-line regorafenib are limited, with an mPFS of approximately 5.6 months and ORR of 6.8% for sunitinib and an mPFS of 4.8 months and an ORR of 4.5% for regorafenib.8,9 In addition, treatment with avapritinib is limited to GIST with PDGFRA exon 18 mutations.17 Therefore, an unmet medical need remains for tolerable and effective targeted therapies in patients with imatinib-resistant advanced GIST. In this phase I study, ripretinib had a favorable safety profile and exhibited preliminary efficacy in advanced GIST.
In the dose-escalation phase of the study, the MTD was not reached. The assessment of the safety, PK, and preliminary pharmacodynamic data resulted in determination of an RP2D of ripretinib 150 mg once daily. This dose was further evaluated in the expansion phase. Safety and efficacy results for patients with GIST receiving ripretinib 150 mg once daily were combined from the dose-escalation and expansion phases. Ripretinib 150 mg once daily was generally well tolerated, with only eight of 142 (5.6%) patients discontinuing the study secondary to a drug-related TEAE. One of the most common TEAEs reported in patients with GIST receiving 150 mg once daily was alopecia, occurring in 88 (62.0%) patients, majority grade 1. The pathogenesis of ripretinib-associated alopecia is unclear but may be secondary to the inhibition of kinases associated with alopecia (eg, KIT, PDGRA, VEGFR2, BRAF).19-21 Alopecia has been reported with other TKIs in patients with advanced GIST; however, the incidence in our study was higher.9,17,22 Similarly, palmar-plantar erythrodysesthesia (PPES; reported with other TKIs as hand-foot skin reaction or hand-foot syndrome) was reported in 62 of 142 (43.7%) patients with GIST receiving ripretinib 150 mg once daily, with grade 3 in only one (0.7%) patient; no patients discontinued study treatment because of PPES. Syndromes such as PPES have been reported as grade 3 in 4% and 19.7% with sunitinib and regorafenib, respectively.8,9
Grade 3 or 4 lipase increase was reported in 25 (17.6%) patients with GIST receiving ripretinib 150 mg once daily and was typically asymptomatic and not clinically significant. The two patients with a DLT of lipase increased were asymptomatic, with no diagnosis of pancreatitis. In two separate patients receiving 150 mg once daily, pancreatitis was diagnosed based on abdominal pain and lipase elevation (mild pancreas inflammation on computed tomography scan in one patient). In both cases, symptoms and lipase elevation improved after a dosing interruption; ripretinib was restarted at a reduced dose in both cases without recurrence of pancreatitis. Although the mechanism of increased lipase levels in patients treated with TKIs is unclear, increases are also reported with other TKIs such as imatinib in patients with chronic myeloid leukemia and with regorafenib in patients with colorectal cancer.14,22
Preliminary efficacy results in patients with GIST receiving ripretinib 150 mg once daily showed promising activity across all lines of therapy included in this study. In patients receiving fourth-line or greater therapy, the ORR (all confirmed PR) was 7.2%; mPFS was 5.5 months. Early results of this study supported the initiation of INVICTUS (ClinicalTrials.gov identifier: NCT0335373), a phase III study of ripretinib in patients with advanced GIST who experienced treatment failure with at least imatinib, sunitinib, and regorafenib.23 INVICTUS demonstrated a significant improvement in mPFS compared with placebo (6.3 v 1 month, respectively; hazard ratio, 0.15; 95% CI, 0.09 to 0.25; P < .0001); the ORR was 9.4% versus 0.0% (P = .0504) for ripretinib and placebo, respectively. The ORR and mPFS reported in this phase I study for second-line (19.4% and 10.7 months) and third-line (14.3% and 8.3 months) therapies are encouraging. Although formal cross-trial comparisons are not possible, when taken in context of prior reports, ORR and PFS of ripretinib in this study exceed those historically reported in centrally read registrational trials for second-line sunitinib therapy (6.8% and approximately 5.6 months) and third-line regorafenib therapy (4.5% and 4.8 months).9,14,15 These results also support the ongoing phase III study (INTRIGUE; ClinicalTrials.gov identifier: NCT03673501) in which ripretinib is being evaluated as a second-line therapy compared with sunitinib in patients with advanced GIST.24 In May 2020, the US FDA approved ripretinib for the treatment of adult patients with advanced GIST who have received previous treatment with three or more kinase inhibitors including imatinib.25
In summary, ripretinib has a favorable safety profile and demonstrated substantial promising efficacy in patients with advanced GIST previously treated with imatinib across all lines of therapy.
DATA AVAILABILITY STATEMENT
Deciphera will share the redacted Phase I study protocol in the publication supplement. Qualified scientific and medical researchers may make requests for individual participant data that underlie the results (text, tables, figures, and appendices) reported in this article, after de-identification, at info@deciphera.com.
Methodologically sound proposals for such data will be evaluated and approved by Deciphera in its sole discretion. All approved researchers must sign a data access agreement prior to accessing the data. Data will be available as soon as possible but no later than within 1 year of the acceptance of the article for publication, and for 3 years following article publication. Deciphera will not share identified participant data or a data dictionary.
DATA AVAILABILITY STATEMENT
Deciphera will share the redacted Phase I study protocol in the Data Supplement. Qualified scientific and medical researchers may make requests for individual participant data that underlie the results (text, tables, figures, and appendices) reported in this article, after de-identification, at info@deciphera.com.Methodologically sound proposals for such data will be evaluated and approved by Deciphera in its sole discretion. All approved researchers must sign a data access agreement prior to accessing the data. Data will be available as soon as possible but no later than within 1 year of the acceptance of the article for publication, and for 3 years following article publication. Deciphera will not share identified participant data or a data dictionary.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers, the investigators, and the investigational site staff of the phase I study. Medical writing assistance was provided by Laura Jung, PharmD of PRECISIONscientia in Yardley, PA, which was supported financially by Deciphera Pharmaceuticals, LLC, in compliance with international Good Publication Practice guidelines. Oliver Rosen, MD, is currently with SQZ Biotech, Watertown, MA.
APPENDIX
Ripretinib Mechanism of Action
Similar to imatinib and regorafenib, ripretinib is a type II tyrosine kinase inhibitor (TKI) and binds to both the ATP hinge region and the adjacent allosteric pockets of the inactive kinase, stabilizing KIT and PDGFRA in inactive conformations. Ripretinib sterically blocks the activation loop from switching into an active conformation by binding to the switch pocket, as well as directly binding and stabilizing the activation loop in its inactive conformation. This dual binding mechanism allows for antagonism of activation loop mutations comparable with or exceeding that with type I inhibitors (bind in the ATP-binding pocket of the active kinase) like avapritinib. Sunitinib is a type II TKI in its binding to KIT but does not bind into the switch pocket, nor does it effectively antagonize activation loop mutations (Roskoski R Jr: Pharmacol Res 103:26-48, 2016).8,19
Overall Safety
Overall, ripretinib was well tolerated, despite 257 (99.6%) patients reporting a TEAE; 137 (53.1%) had a treatment-emergent serious adverse event (SAE), and 173 (67.1%) had a grade 3/4 TEAE. The most common TEAEs reported in > 15% of patients and corresponding grade 3/4 TEAEs are listed in Appendix Table A2. Treatment-emergent SAEs in > 3% of patients were abdominal pain (17 [6.6%]), death (16 [6.2%]), cancer surgery (10 [3.9%]), and dyspnea (8 [3.1%]). TEAEs leading to a dose reduction occurred in 38 (14.7%) patients, and TEAEs leading to treatment interruption occurred in 127 (49.2%) patients. Only 16 (6.2%) patients discontinued study treatment secondary to a drug-related TEAE.
FIG A1.
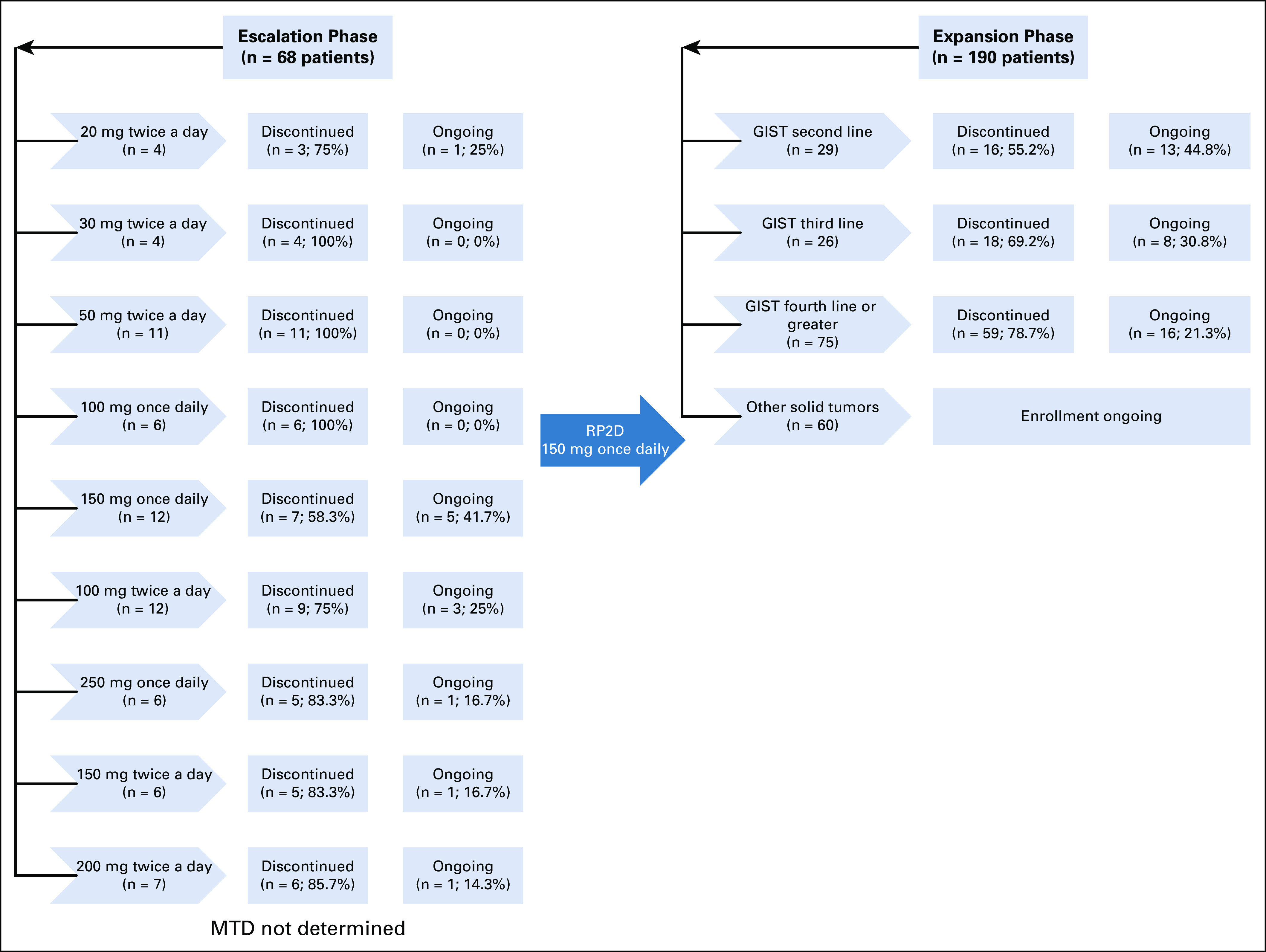
Study overview. GIST, gastrointestinal stromal tumor; MTD, maximum tolerated dose; RP2D, recommended phase II dose.
TABLE A1.
Dispositions for All Patients and Patients With GIST Receiving Ripretinib 150 mg Once Daily
TABLE A2.
TEAEs in > 15% of All Patients and Corresponding Grade 3/4 TEAEs
TABLE A3.
Geometric Mean (Geometric CV%) Plasma PK Parameter of Ripretinib After a Single Oral Dose in Patients With Advanced Malignancies in the Dose-Escalation Phase on Cycle 1 Day 1
TABLE A4.
Geometric Mean (Geometric CV%) Plasma PK Parameters of Ripretinib After Multiple Once-Daily Doses in Patients With Advanced Malignancies in the Dose-Escalation Phase on Cycle 1 Day 15
TABLE A5.
Geometric Mean (Geometric CV%) Plasma PK Parameters of Ripretinib After Multiple Twice-a-Day Doses in Patients With Advanced Malignancies in the Dose-Escalation Phase on Cycle 1 Day 15
PRIOR PRESENTATION
Presented in part at the American Association for Cancer Research (AACR)–National Cancer Institute (NCI)– European Organisation for Research and Treatment of Cancer (EORTC) International Conference on Molecular Targets and Cancer Therapeutics, Boston, MA, October 26-30, 2019; the American Association for Cancer Research Annual Meeting, Atlanta, GA, March 29-April 3, 2019; the European Society for Medical Oncology Congress, Munich, Germany, October 19-23, 2018; the Connective Tissue Oncology Society Annual Meeting, Rome, Italy, November 14-17, 2018; the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2018; the American Association for Cancer Research Annual Meeting, Chicago, IL, April 14-18, 2018; the Connective Tissue Oncology Society Annual Meeting, Maui, Hawaii, November 8-11, 2017; the European Society for Medical Oncology Congress, Madrid, Spain, September 8-12, 2017; the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2-6, 2017; the American Association for Cancer Research Annual Meeting, Washington, DC, April 1-5, 2017; and the EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, Munich, Germany, November 29-December 2, 2016. Data (pharmacokinetics and clinical course) for 2 patients were previously published as part of a larger manuscript, Smith BD, et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell 35:738-751, 2019
SUPPORT
Supported by Deciphera Pharmaceuticals, LLC and a Department of Veterans Affairs Merit Review Grant No. I01 BX000338 (M.C.H.).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Filip Janku, Michael C. Heinrich, Robin L. Jones, Jonathan Trent, Oliver Rosen, Rodrigo Ruiz-Soto, Suzanne George
Provision of study material or patients: Filip Janku, Albiruni R. Abdul Razak, Ping Chi, Michael C. Heinrich, Margaret von Mehren, Robin L. Jones, Kristen Ganjoo, Jonathan Trent, Hans Gelderblom, Neeta Somaiah, Michael Gordon, Suzanne George
Collection and assembly of data: Filip Janku, Albiruni R. Abdul Razak, Ping Chi, Michael C. Heinrich, Margaret von Mehren, Robin L. Jones, Kristen Ganjoo, Jonathan Trent, Hans Gelderblom, Neeta Somaiah, Oliver Rosen, Rodrigo Ruiz-Soto, Michael Gordon, Suzanne George
Data analysis and interpretation: Filip Janku, Albiruni R. Abdul Razak, Ping Chi, Michael C. Heinrich, Margaret von Mehren, Robin L. Jones, Kristen Ganjoo, Jonathan Trent, Hans Gelderblom, Simin Hu, Oliver Rosen, Ying Su, Rodrigo Ruiz-Soto, Michael Gordon, Suzanne George
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Switch Control Inhibition of KIT and PDGFRA in Patients With Advanced Gastrointestinal Stromal Tumor: A Phase I Study of Ripretinib
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Filip Janku
Stock and Other Ownership Interests: Trovagene
Consulting or Advisory Role: Deciphera, Trovagene, Novartis, Sequenom, Foundation Medicine, Guardant Health, Synlogic, Valeant/Dendreon, IFM Therapeutics, Sotio, PureTech, Jazz Pharmaceuticals, Immunomet, IDEAYA Biosciences
Research Funding: Novartis (Inst), BioMed Valley Discoveries (Inst), Roche (Inst), Agios (Inst), Astellas Pharma (Inst), Deciphera (Inst), Plexxikon (Inst), Piqur (Inst), Fujifilm (Inst), Symphogen (Inst), Bristol Myers Squibb (Inst), Asana Biosciences (Inst), Astex Pharmaceuticals (Inst), Genentech (Inst), Proximagen (Inst)
Other Relationship: Bio-Rad
Albiruni R. Abdul Razak
Honoraria: Boehringer Ingelheim
Consulting or Advisory Role: Eli Lilly, Merck, Boehringer Ingelheim, Adaptimmune
Research Funding: CASI Pharmaceuticals, Boehringer Ingelheim, Eli Lilly, Novartis, Deciphera, Karyopharm Therapeutics, Pfizer, Roche/Genentech, Boston Biomedical, Bristol Myers Squibb, MedImmune, Amgen, GlaxoSmithKline, Blueprint Medicines, Merck, AbbVie, Adaptimmune, Iterion Therapeutics
Ping Chi
Consulting or Advisory Role: Deciphera, Exelixis, Merck (I)
Research Funding: Deciphera (Inst), Array BioPharma (Inst)
Michael Heinrich
Stock and Other Ownership Interests: MolecularMD
Honoraria: Novartis
Consulting or Advisory Role: MolecularMD, Novartis, Blueprint Medicines, Deciphera
Patents, Royalties, Other Intellectual Property: Patent on treatment of GIST, licensed to Novartis (Inst)
Expert Testimony: Novartis
Margaret von Mehren
Honoraria: Pfizer
Consulting or Advisory Role: Deciphera, Exelexis, Blueprint Medicines
Research Funding: ArQule, Novartis (Inst), Blueprint Medicines (Inst), Deciphera (Inst), Gradalis (Inst), Springworks Therapeutics (Inst), Eli Lilly (Inst), Arog Pharmaceuticals (Inst), Genmab (Inst), ASCO (Inst)
Travel, Accommodations, Expenses: Deciphera, NCCN
Other Relationship: NCCN
Robin L. Jones
Consulting or Advisory Role: Eli Lilly, Immune Design, Merck Serono, Adaptimmune, Daiichi Sankyo, Eisai, Morphotek, TRACON Pharma, Immodulon Therapeutics, Deciphera, PharmaMar, Blueprint Medicines, Clinigen Group, Epizyme, Boehringer Ingelheim
Research Funding: GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: PharmaMar
Kristen Ganjoo
Consulting or Advisory Role: Daiichi Sankyo, Foundation Medicine
Jonathan Trent
Honoraria: GlaxoSmithKline
Consulting or Advisory Role: Bayer, Blueprint Medicine, Deciphera, Daiichi Sankyo, Epizyme
Hans Gelderblom
Research Funding: Novartis (Inst), Ipsen (Inst), Deciphera (Inst), Daiichi (Inst)
Neeta Somaiah
Consulting or Advisory Role: Bayer, Immune Design, Deciphera, Blueprint Medicines
Research Funding: AztraZeneca, Merck
Simin Hu
Employment: Deciphera Pharmaceuticals
Stock and Other Ownership Interests: Deciphera Pharmaceuticals
Oliver Rosen
Employment: SQZ Biotechnology, Deciphera
Leadership: Deciphera, SQZ Biotechnology
Stock and Other Ownership Interests: SQZ Biotechnology
Patents, Royalties, Other Intellectual Property: Use of 1-[4-bromo-5-[1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl]-2-fluorophenyl]-3-phenylurea and analogs for the treatment of cancers associated with genetic abnormalities in platelet derived growth factor receptor alpha.
Ying Su
Stock and Other Ownership Interests: Deciphera Pharmaceuticals, Immunogen, Bristol Myers Squibb
Rodrigo Ruiz-Soto
Employment: Deciphera
Stock and Other Ownership Interests: Deciphera, Immunogen
Patents, Royalties, Other Intellectual Property: I am an inventor in 3 patents with Immunogen; I transferred the rights to Immunogen. I have not received (and I will not receive) any royalties. I am an inventor in pending patents at Deciphera. I have transferred the rights to Deciphera. I have not received (and will not receive) any royalties.
Michael Gordon
Stock and Other Ownership Interests: Caremission, WCCT Global, Beckon Call
Consulting or Advisory Role: Castle Biosciences, Deciphera, RedHill Biopharma, TRACON Pharma, Agenus, Daiichi Sankyo, Neon Therapeutics, Salarius Pharmaceuticals, Imaging Endpoints
Research Funding: Genentech/Roche (Inst), GlaxoSmithKline (Inst), AbbVie (Inst), Merck Serono (Inst), MedImmune (Inst), Incyte (Inst), OncoMed (Inst), Pfizer (Inst), Array BioPharma (Inst), Amgen (Inst), Eli Lilly (Inst), Eli Lilly/ImClone (Inst), Millennium (Inst), Gilead Sciences (Inst), Sirtex Medical (Inst), Calithera Biosciences (Inst), ESSA (Inst), Endocyte (Inst), Seattle Genetics (Inst), Plexxikon (Inst), Celldex (Inst), TRACON Pharma (Inst), Deciphera (Inst), Tokai Pharmaceuticals (Inst), Aduro Biotech (Inst), Advaxis (Inst), BeiGene (Inst), BiolineRx (Inst), CanBas (Inst), Celgene (Inst), CytomX Therapeutics (Inst), Driver Group (Inst), Fujifilm (Inst), Five Prime Therapeutics (Inst), Halozyme (Inst), MabVax (Inst), Macrogenics (Inst), Merrimack (Inst), Minneamrita Therapeutics (Inst), Nektar (Inst), Novita Pharmaceuticals (Inst), Phoenix Biotech (Inst), Corcept Therapeutics (Inst), Inanovate (Inst), Novartis (Inst), Syndax (Inst), Proderm IQ (Inst), Samumed (Inst), Strategia Therapeutics (Inst), SynDevRx (Inst), Tesaro (Inst), Toray Industries (Inst), Tolero Pharmaceuticals (Inst), Verastem (Inst), Hengrui Therapeutics (Inst), Trovagene (Inst), Acetylon (Inst), ImaginAb (Inst), Neon Therapeutics (Inst), Arcus Biosciences (Inst), Genzada Pharmaceuticals (Inst), Salarius Pharmaceuticals (Inst), Agenus (Inst), Inhibrx (Inst), FibroGen (Inst), Caris Centers of Excellence (Inst), TTC Oncology (Inst), AADi (Inst), Molecular Partners (Inst), Revolution Medicines (Inst), Syros Pharmaceuticals (Inst), Ayala Pharmaceuticals (Inst), Blueprint Medicines (Inst), Amal Kisima (Inst), Astellas Pharma (Inst), Athenex (Inst), BioNTech AG (Inst), Cantex (Inst), Genocea Biosciences (Inst), Helix BioPharma (Inst), IgM Biosciences (Inst), ImmuneSensor (Inst)
Patents, Royalties, Other Intellectual Property: Patent on patient selection for Clinical Trials
Suzanne George
Stock and Other Ownership Interests: Abbott Laboratories, Allergan (I)
Consulting or Advisory Role: Blueprint Medicines, Deciphera, Bayer, Eli Lilly, UpToDate, Research to Practice, MORE Health, Daiichi
Research Funding: Pfizer (Inst), Novartis (Inst), Bayer (Inst), ARIAD (Inst), Blueprint Medicines (Inst), Deciphera (Inst)
Patents, Royalties, Other Intellectual Property: UptoDate
Expert Testimony: Bayer
Other Relationship: Research to Practice
No other potential conflicts of interest were reported.
REFERENCES
- 1.Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: A prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108:2366–2372. doi: 10.1182/blood-2006-04-015545. [DOI] [PubMed] [Google Scholar]
- 4.Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: Expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–3219. [PubMed] [Google Scholar]
- 5.Casali PG, Zalcberg J, Le Cesne A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic GI stromal tumors: Long-term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group intergroup phase III randomized trial on imatinib at two dose levels. J Clin Oncol. 2017;35:1713–1720. doi: 10.1200/JCO.2016.71.0228. [DOI] [PubMed] [Google Scholar]
- 6.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 7.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: Randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardelmann E, Merkelbach-Bruse S, Pauls K, et al. Polyclonal evolution of multiple secondary KIT mutations in gastrointestinal stromal tumors under treatment with imatinib mesylate. Clin Cancer Res. 2006;12:1743–1749. doi: 10.1158/1078-0432.CCR-05-1211. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich MC, Patterson J, Beadling C, et al. Genomic aberrations in cell cycle genes predict progression of KIT-mutant gastrointestinal stromal tumors (GISTs) Clin Sarcoma Res. 2019;9:3. doi: 10.1186/s13569-019-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemming ML, Heinrich MC, Bauer S, et al. Translational insights into gastrointestinal stromal tumor and current clinical advances. Ann Oncol. 2018;29:2037–2045. doi: 10.1093/annonc/mdy309. [DOI] [PubMed] [Google Scholar]
- 14.2017. Stivarga [package insert]. Whippany, NJ, Bayer HealthCare Pharmaceuticals,
- 15.2017. Sutent [package insert]. New York, NY, Pfizer Labs,
- 16.Serrano C, Mariño-Enríquez A, Tao DL, et al. Correction: Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2019;121:281. doi: 10.1038/s41416-019-0487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2020. Ayvakit [package insert]. Cambridge, MA, Blueprint Medicines,
- 18.Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23:5357–5364. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- 19.Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell. 2019;35:738–751.e9. doi: 10.1016/j.ccell.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Piraccini BM, Patrizi A, Fanti PA, et al. RASopathic alopecia: Hair changes associated with vemurafenib therapy. J Am Acad Dermatol. 2015;72:738–741. doi: 10.1016/j.jaad.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 21.González R, Moffatt G, Hagner A, et al. Platelet-derived growth factor signaling modulates adult hair follicle dermal stem cell maintenance and self-renewal. NPJ Regen Med. 2017;2:11. doi: 10.1038/s41536-017-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.2018. Gleevec [package insert]. East Hanover, NJ, Novartis Pharmaceuticals,
- 23.Blay J-Y, Serrano C, Heinrich MC, et al. Ripretinib in patients with advanced gastrointestinal stromal tumors (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:923–934. doi: 10.1016/S1470-2045(20)30168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemunaitis J, Bauer S, Blay JY, et al. Intrigue: Phase III study of ripretinib versus sunitinib in advanced gastrointestinal stromal tumor after imatinib. Future Oncol. 2020;16:4251–4264. doi: 10.2217/fon-2019-0633. [DOI] [PubMed] [Google Scholar]
- 25.2020. QINLOCK [package insert]. Waltham, MA, Deciphera Pharmaceuticals, LLC.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deciphera will share the redacted Phase I study protocol in the publication supplement. Qualified scientific and medical researchers may make requests for individual participant data that underlie the results (text, tables, figures, and appendices) reported in this article, after de-identification, at info@deciphera.com.
Methodologically sound proposals for such data will be evaluated and approved by Deciphera in its sole discretion. All approved researchers must sign a data access agreement prior to accessing the data. Data will be available as soon as possible but no later than within 1 year of the acceptance of the article for publication, and for 3 years following article publication. Deciphera will not share identified participant data or a data dictionary.