Abstract
PURPOSE
Nelarabine is effective in inducing remission in patients with relapsed and refractory T-cell acute lymphoblastic leukemia (T-ALL) but has not been fully evaluated in those with newly diagnosed disease.
PATIENTS AND METHODS
From 2007 to 2014, Children’s Oncology Group trial AALL0434 (ClinicalTrials.gov identifier: NCT00408005) enrolled 1,562 evaluable patients with T-ALL age 1-31 years who received the augmented Berlin-Frankfurt-Muenster (ABFM) regimen with a 2 × 2 pseudo-factorial randomization to receive escalating-dose methotrexate (MTX) without leucovorin rescue plus pegaspargase (C-MTX) or high-dose MTX (HDMTX) with leucovorin rescue. Intermediate- and high-risk patients were also randomly assigned after induction to receive or not receive six 5-day courses of nelarabine that was incorporated into ABFM. Patients who experienced induction failure were nonrandomly assigned to HDMTX plus nelarabine. Patients with overt CNS disease (CNS3; ≥ 5 WBCs/μL with blasts) received HDMTX and were randomly assigned to receive or not receive nelarabine. All patients, except those with low-risk disease, received cranial irradiation.
RESULTS
The 5-year event-free and overall survival rates were 83.7% ± 1.1% and 89.5% ± 0.9%, respectively. The 5-year disease-free survival (DFS) rates for patients with T-ALL randomly assigned to nelarabine (n = 323) and no nelarabine (n = 336) were 88.2% ± 2.4% and 82.1% ± 2.7%, respectively (P = .029). Differences between DFS in a four-arm comparison were significant (P = .01), with no interactions between the MTX and nelarabine randomizations (P = .41). Patients treated with the best-performing arm, C-MTX plus nelarabine, had a 5-year DFS of 91% (n = 147). Patients who received nelarabine had significantly fewer isolated and combined CNS relapses compared with patients who did not receive nelarabine (1.3% ± 0.63% v 6.9% ± 1.4%, respectively; P = .0001). Toxicities, including neurotoxicity, were acceptable and similar between all four arms.
CONCLUSION
The addition of nelarabine to ABFM therapy improved DFS for children and young adults with newly diagnosed T-ALL without increased toxicity.
INTRODUCTION
Dramatic increases in survival for pediatric acute lymphoblastic leukemia (ALL) have been achieved, from survival rates 50% in the 1980s to 90% today, and have been accomplished largely without using new agents.1-13 T-cell ALL (T-ALL) is composed of 15% of pediatric and 25% of adult ALLs.14 Compared with children and adolescents with B-lineage ALL (B-ALL), event-free survival (EFS) and overall survival (OS) rates were historically inferior for those with T-ALL, even with more intensified therapies.4,5,10,13,15 Recurring genomic lesions, age, and presenting leukocyte count do not predict relapse for T-ALL, but minimal residual disease (MRD) is highly prognostic and is used for risk stratification.1,11,16,17 Early T-precursor (ETP) T-ALL was initially reported to be prognostic of a poor outcome.18 Relapse frequently occurs during active treatment and often involves extramedullary sites, and salvage therapies typically fail.4,10,16,19,20
CONTEXT
Key Objective
Is Nelarabine safe and effective in the treatment of newly diagnosed T-cell acute lymphoblastic leukemia?
Knowledge Generated
The addition of nelarabine to ABFM therapy improved DFS for children and young adults with newly diagnosed T-ALL without increased toxicity. Nelarabine decreased CNS relapses.
Relevance
Nelarabine is safe and effective in the treatment of newly diagnosed T-ALL in children and young adults with excellent disease free survival.
Nelarabine is a DNA-terminating nucleoside prodrug for araguanosine metabolized into arabinosylguanine nucleotide triphosphate, preferentially accumulating in T lymphoblasts secondary to slowed degradation kinetics.21 Nelarabine was first studied in a single-agent phase I trial for relapsed or refractory T-ALL and produced a 42% complete and partial remission rate.22 These results were confirmed in a phase II trial in relapsed or refractory T-ALL, with an observed complete and partial remission rate of 55% in first relapse (650 mg/m2 daily for 5 days).23 Although active, nelarabine was associated with significant risk of central and peripheral neuropathies, occasionally fatal, in patients with relapsed disease that were not clearly related to dose.22-25 The Children’s Oncology Group (COG) AALL00P2 pilot study added nelarabine (six 5-day courses of 400-650 mg/m2/d) to intensive chemotherapy in patients with newly diagnosed T-ALL and found it to be safe and feasible, with a 5-year EFS rate of 73% in high-risk patients.26 The rate of grade ≥ 3 peripheral neurotoxicity was 15%. COG AALL0434 was a phase III trial with a 2 × 2 pseudo-factorial randomization testing nelarabine in patients with newly diagnosed T-ALL. Patients were randomly assigned to receive escalating-dose methotrexate (MTX) plus pegaspargase (C-MTX) or high-dose MTX (HDMTX), and patients with intermediate-risk (IR) and high-risk (HR) disease were randomly assigned to receive or not receive nelarabine.1 We previously reported on patient characteristics of the entire cohort, on the safety of integrating nelarabine into this regimen, and that C-MTX was associated with significantly better disease-free survival (DFS) and OS as compared with HDMTX.1,27 We now report the results of the nelarabine randomization.
PATIENTS AND METHODS
Trial Oversight
This study was conducted by COG under an Investigational New Drug (IND) application for nelarabine (compound 506U78, IND 52611) held by the National Cancer Institute. AALL0434 was approved by the Cancer Therapy and Evaluation Program, the US Food and Drug Administration, the Pediatric Central Institutional Review Board, and institutional review boards at each participating center. In accordance with the Declaration of Helsinki, informed consent or assent was obtained before study entry.
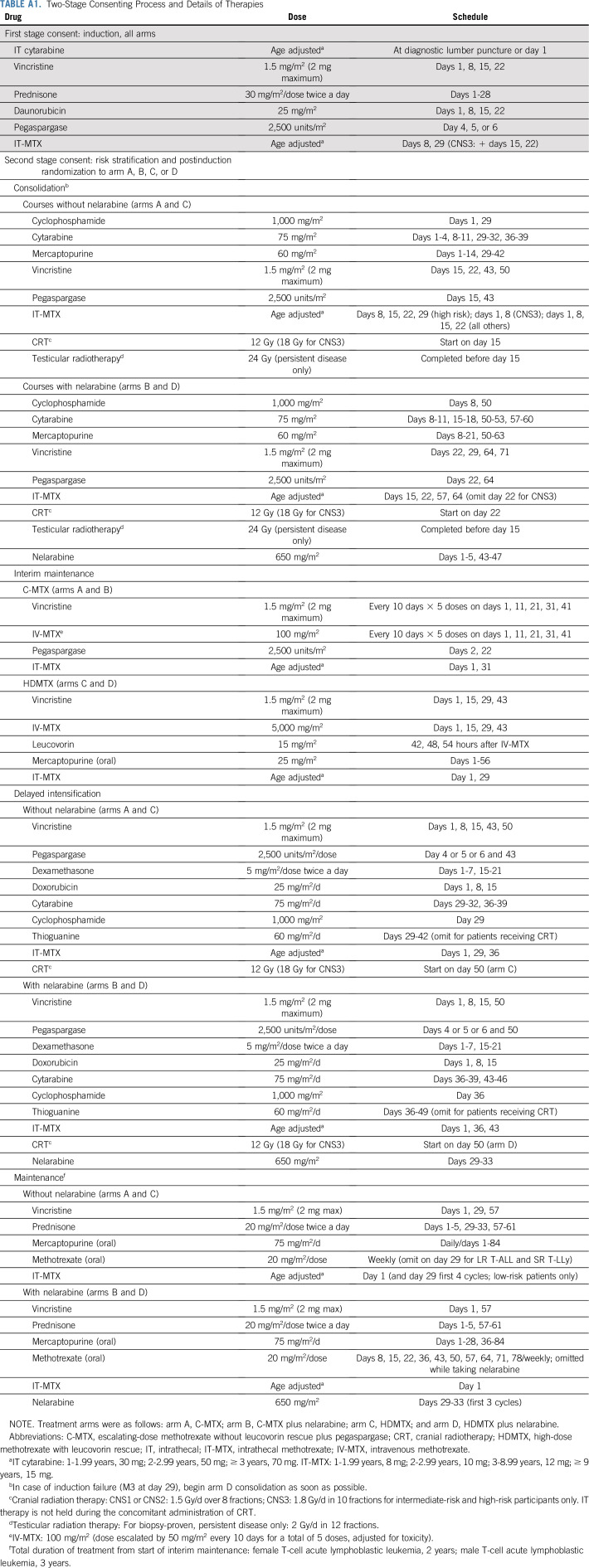
Patients
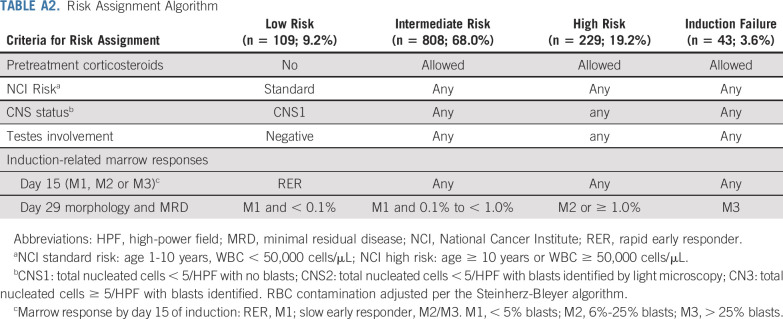
COG AALL0434 enrolled 1,596 patients with T-ALL from January 2007 to July 2014 (CONSORT diagram provided in Fig 1). Eligible patients included newly diagnosed, untreated (except corticosteroids) patients age 1-31 years.1 AALL0434 was amended in 2010 to include patients with T-cell lymphoblastic lymphoma, with a plan to analyze and report those patients separately. All participants underwent a 28-day, prednisone-based, four-drug induction (Appendix Table A1, online only). After a second-stage postinduction consent, participants underwent risk stratification as low risk (LR), IR, HR, or induction failure (IF; M3 marrow with > 25% blasts; Appendix Table A2, online only). Appendix Tables A3 and A4 (online only) summarize ineligibility reasons and reasons for going off therapy after induction for patients with T-ALL. AALL0434 used a sequential design to evaluate nelarabine during the safety and efficacy phases.27 In the safety phase, only HR patients participated in the nelarabine randomization. During the efficacy phase, HR and IR patients participated in the nelarabine randomization. Low-risk patients did not participate in this randomization. Six hundred fifty-nine IR or HR patients were randomly assigned to receive or not receive six 5-day courses of nelarabine 650 mg/m2/d (arm A: C-MTX; arm B: C-MTX plus nelarabine; arm C: HDMTX; arm D: HDMTX plus nelarabine; Appendix Table A1). Two courses were given in consolidation, on days 1-5 and 43-47; one course was given in delayed intensification, on days 29-33; and three courses were given on days 29-33 of the first three maintenance cycles. All IR and HR patients received 12 Gy of prophylactic cranial radiation therapy (CRT). Participants with overt CNS involvement (CNS3; ≥ 5 WBCs/μL with blasts or clinical signs of CNS involvement) or persistent, postinduction testicular leukemia were nonrandomly assigned to receive HDMTX on arms C or D with 18 Gy of CRT during delayed intensification for those with CNS3 or 24 Gy of testicular radiation during consolidation. Treatment duration was the same for all arms and was 2 years from the start of interim maintenance for females and 3 years for males. Patients with IF were nonrandomly assigned to arm D and could remain on study if an M1 or M2 marrow (≤ 25% blasts) was achieved by the end of consolidation. Participants with a preexisting, medication-dependent seizure disorder were ineligible for the nelarabine randomization.1 No participants were assigned risk based on cytogenetics, immunophenotype, or genomic alterations.4,28-34 Centrally determined ETP status was available for 1,125 patients (81%) and categorized as ETP, near ETP (ETP but with high CD5 expression), or not ETP.35 Although COG AALL0434 did not include an allogeneic hematopoietic stem-cell transplantation (alloHSCT) option, approximately 4% of patients were taken off protocol therapy for transplantation by investigator choice. Retrospectively collected data were available on 1,390 patients (89%) to identify who was taken off therapy, either during induction or after induction therapy, and subsequently underwent alloHSCT in first remission.
FIG 1.
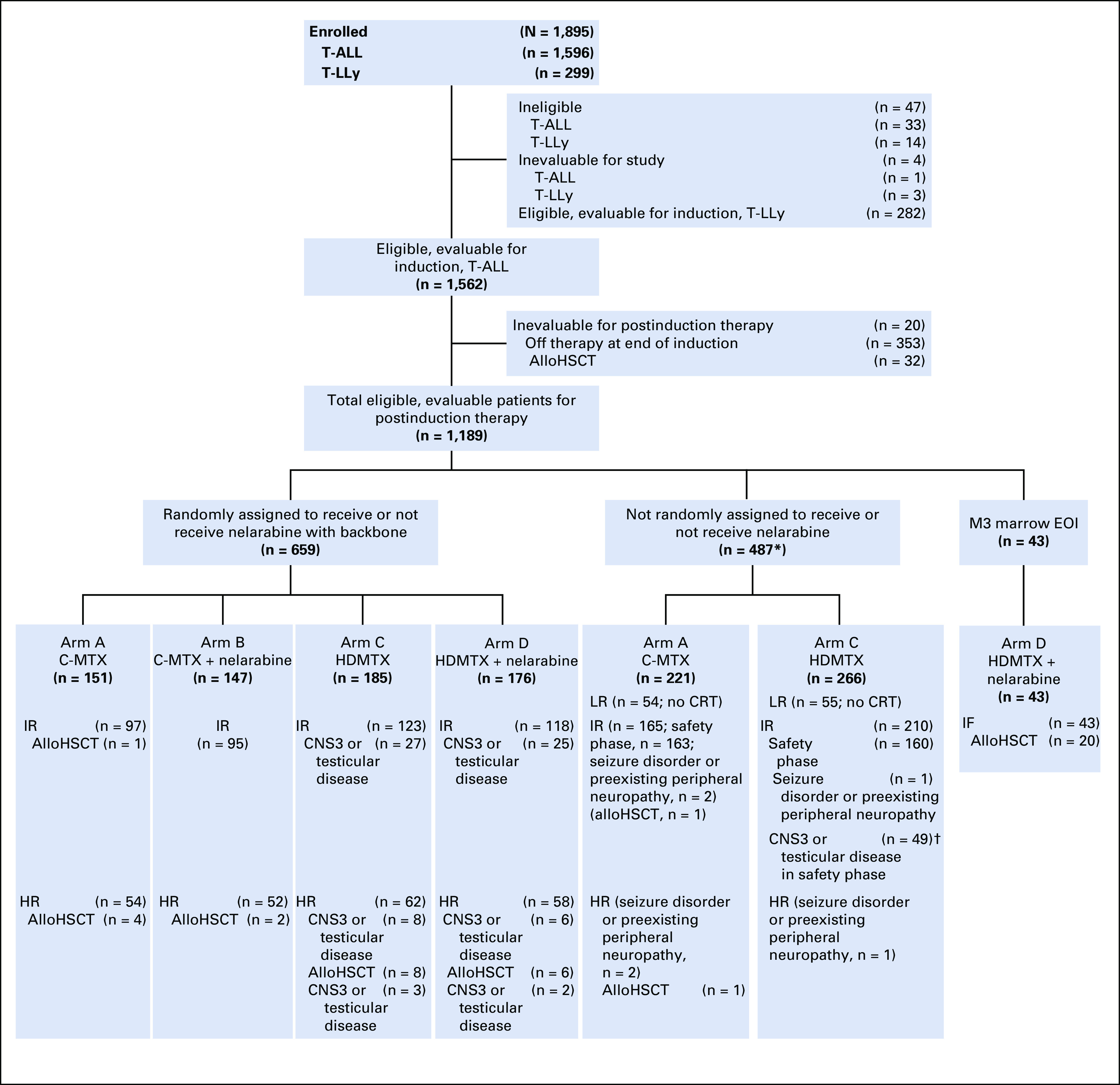
CONSORT diagram for study. (*) Includes patients who were not eligible to receive nelarabine (randomized to arms A and C only) either during the safety phase (intermediate risk [IR]) or efficacy phase (seizure disorder or preexisting peripheral neuropathy). (†) IR patients with CNS3 and testicular disease were assigned to arm C during the safety phase. The most commons reasons why patients came off study between the first and second stages of randomization were because the physician determined it was in the best interests of the patient and the participant declined to participate in the randomization. AlloHSCT, allogeneic hematopoietic stem-cell transplantation; C-MTX, escalating-dose methotrexate without leucovorin rescue plus pegaspargase; CRT, cranial radiation therapy; EOI, end of induction; HDMTX, high-dose methotrexate with leucovorin rescue; HR, high risk; IF, induction failure; LR, low risk; T-ALL, T-cell acute lymphoblastic leukemia; T-LLy, T-Cell lymphoblastic lymphoma.
Outcome and Statistical Analyses
Treatment-related adverse events were graded using Common Terminology Criteria for Adverse Events version 4. EFS was defined as time from study enrollment to first event (IF, induction death, relapse, second malignant neoplasm, or remission death) or date of last contact. The primary outcome for the randomized question was DFS, which was defined as the time from postinduction randomization to first event (relapse, second malignant neoplasm, or remission death) or date of last contact. OS was defined as the time from study enrollment or, for the randomized cohorts, from postinduction randomization to death or date of last contact. With a one-sided α of 5%, there was 80% power to detect an improvement in 4-year DFS from 82% to 89% (93 events) between the nelarabine and no nelarabine regimens for a total of 659 patients, with a minimum follow-up time of 3 years.
Study accrual duration was driven by the time needed to meet accrual targets for the nelarabine randomization. Interim analyses for efficacy and futility were scheduled when approximately 20%, 40%, 60%, 80%, and 100% of the expected events were observed. An αt2 spending function with truncation at three standard deviations was used for interim monitoring.
Survival rates were estimated using the Kaplan-Meier method36 and standard errors of Peto et al.37 Survival rates are presented as rates ± SEs. The power calculation for the randomized nelarabine comparison was based on a one-sided log-rank test (α = .05) because the objective was to determine whether the addition of nelarabine to a standard backbone improved outcomes. Unless otherwise specified, two-sided log-rank tests were used for comparison of survival curves. Multivariable analyses used Cox regression analyses, adjusting for treatment arm and risk group, and time-dependent covariates for time to alloHSCT. Per-protocol subgroup analyses of overall outcomes, including by race and sex, were also performed. Post hoc analyses used the log-rank test to compare DFS by age group within the nelarabine and no nelarabine randomized cohorts. Proportions were compared between groups using a χ2 test or Fisher’s exact test. Cumulative incidence rates were computed using the cumulative incidence function for competing risks, with comparisons between groups made using the K-sample test.38 A P < .05 was considered significant for all comparisons. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary NC), and graphics were generated using R Version 2.13.1 (http://www.r-project.org). This report includes data current as of June 30, 2018.
RESULTS
AALL0434 cohort risk stratification and randomization are presented in the CONSORT diagram (Fig 1). There were no unexpected variances in patient characteristics (Table 1). For the 1,562 eligible and evaluable patients with T-ALL, 5-year EFS was 83.7% ± 1.1% and 5-year OS was 89.5% ± 0.9% (Fig 2A). There was no difference in overall EFS by race or ethnicity and sex (Figs 2B and 2C).
TABLE 1.
Patient Characteristics for the Nelarabine Randomized Cohort
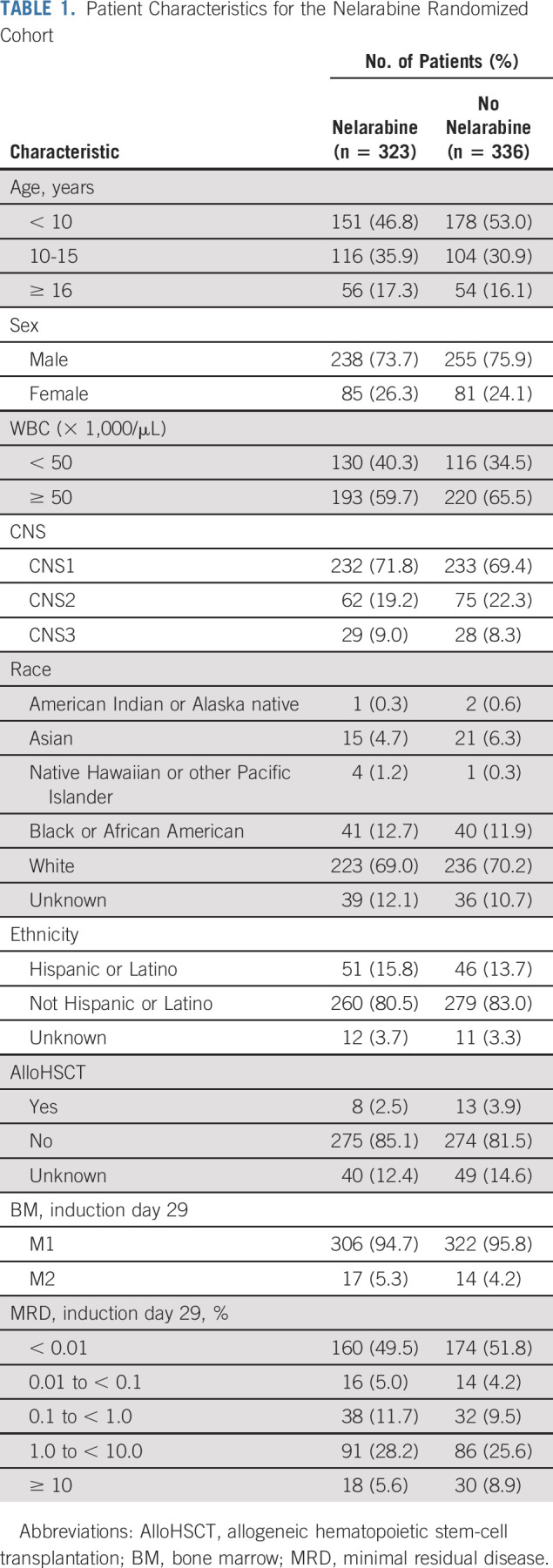
FIG 2.
(A) Event-free survival (EFS) and overall survival (OS) curves for all patients with T-cell acute lymphoblastic leukemia; 5-year EFS and OS rates were 83.7% ± 1.1% and 89.5% ± 0.9%, respectively. (B) EFS by race and ethnicity; 5-year EFS rates were 83.7% ± 1.3% for White patients, 81.8% ± 3.4% for Black patients, and 85.1% ± 2.7% for other patients (P = .702). (C) EFS by sex; 5-year EFS rates were 82.6% ± 2.3% for females and 84.1% ± 1.3% for males (P = .289).
Nelarabine Randomization
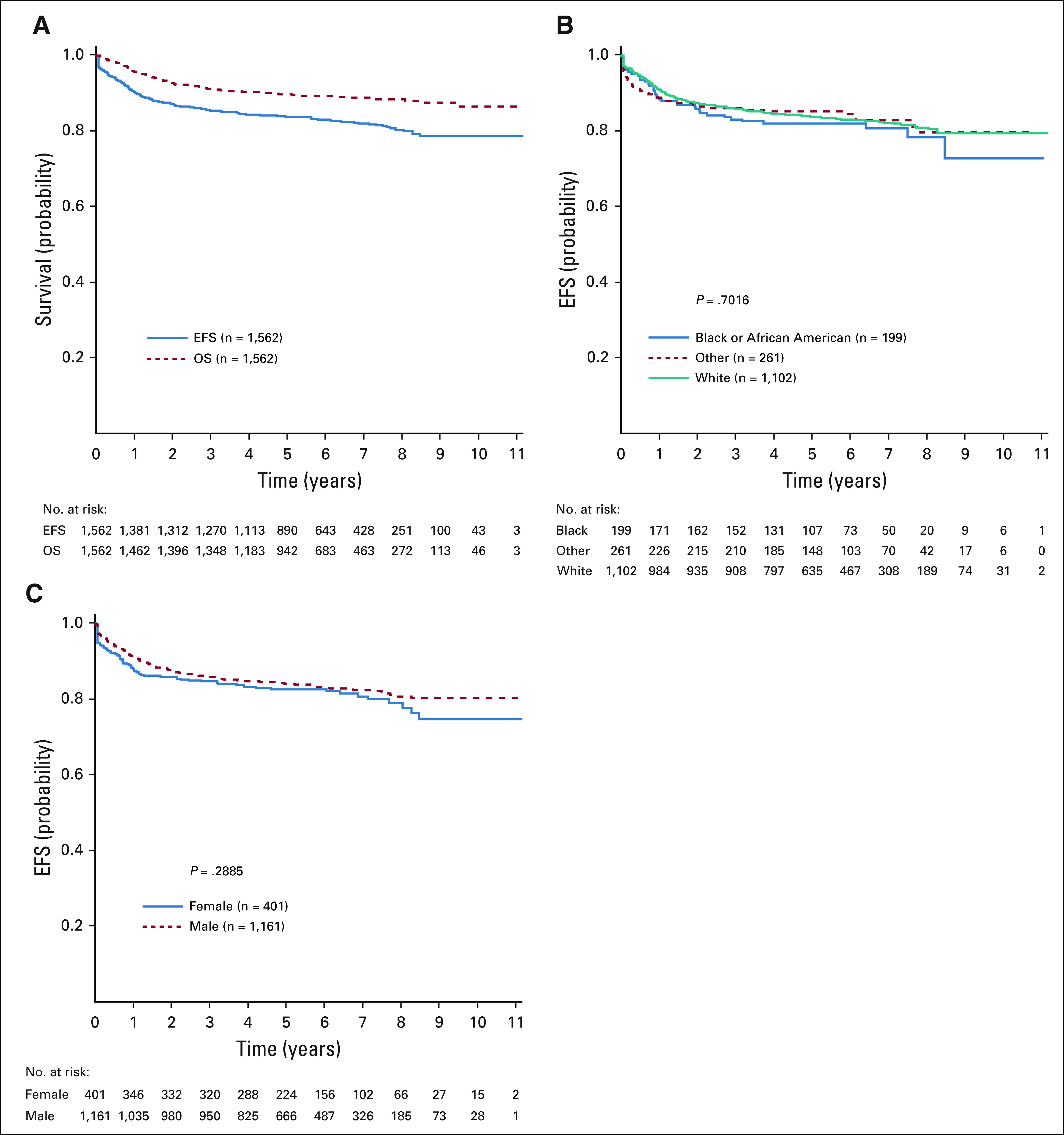
Patients randomly assigned to receive nelarabine (n = 323) had superior 5-year DFS compared with those not assigned to nelarabine (n = 336; 88.2% ± 2.4% v 82.1% ± 2.7%, respectively; P = .029; Fig 3A). The 5-year OS for patients randomly assigned to receive nelarabine was 90.3% ± 2.2%, compared with 87.9% ± 2.3% for those not receiving nelarabine (P = .168; Fig 3B). There was no significant interaction between the MTX and nelarabine randomizations (P = .41). Analysis by treatment arm revealed that C-MTX with nelarabine had the best 5-year DFS at 91.4% ± 3.1% (n = 147), followed by C-MTX without nelarabine (87.2% ± 3.5%; n = 151), HDMTX with nelarabine (85.5% ± 3.6%; n = 176), and HDMTX without nelarabine (78.1% ± 4.0%; n = 185; P = .01; Fig 3C). DFS of the HDMTX without nelarabine arm was significantly lower than that of the other arms with no increase in higher risk patient characteristics, such as higher WBC (P = .07) or higher day 29 MRD (P = .66), compared with the other arms. No differences in DFS were observed across all age groups in those randomly assigned to receive (Fig 3D) or not receive (Fig 3E) nelarabine. There was no difference in 5-year EFS between patients eligible for the nelarabine randomization who did not participate and those who were randomly assigned (85.5% ± 3.2% v 85.1% ± 1.8%, respectively; P = .53).
FIG 3.
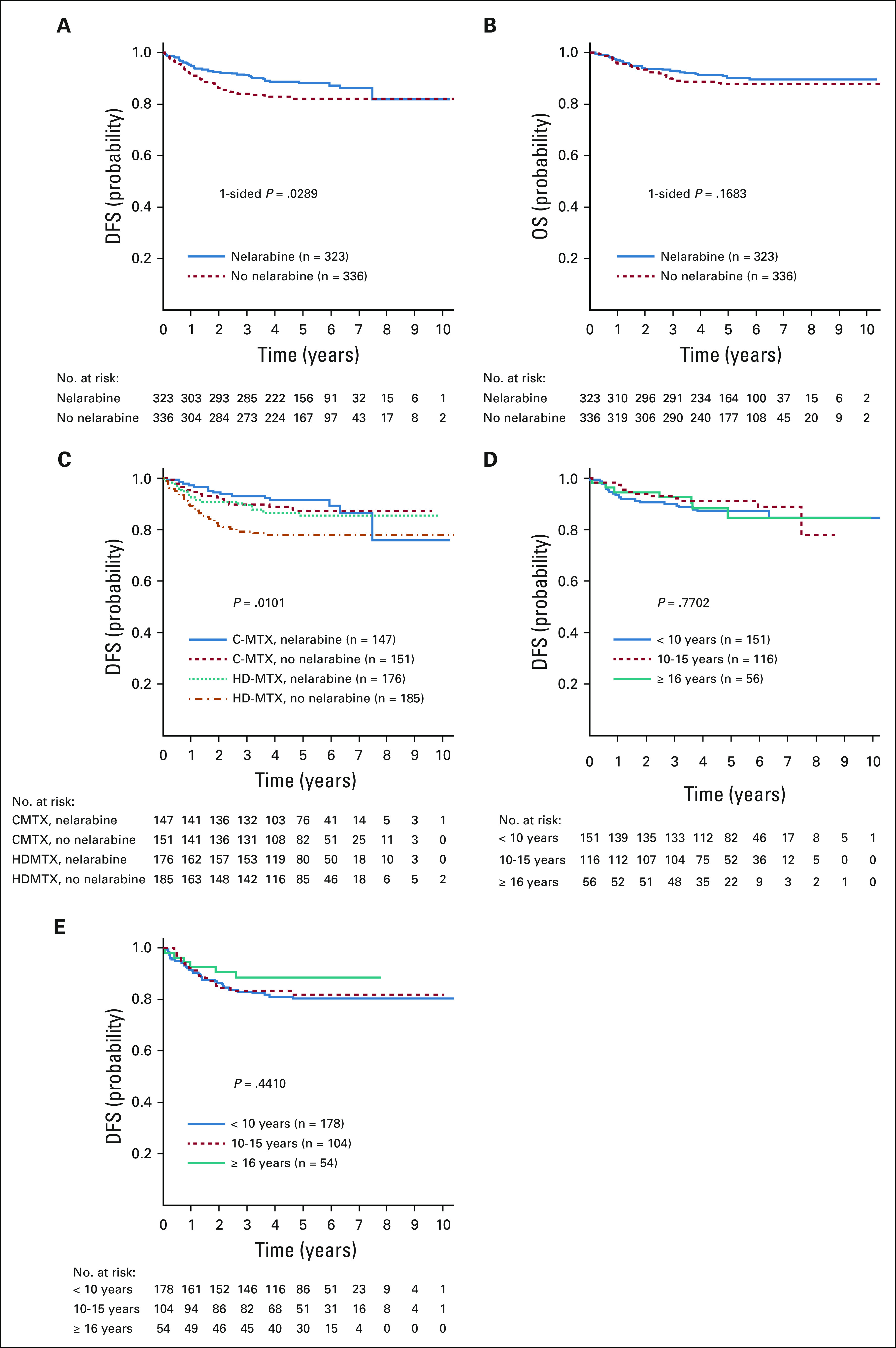
Disease-free survival (DFS) and overall survival (OS) comparisons for nelarabine versus no nelarabine in the randomized cohorts. (A) Five-year DFS rates were 88.2% ± 2.4% with nelarabine compared with 82.1% ± 2.7% without nelarabine (P = .029). (B) Five-year OS rates were 90.3% ± 2.2% with nelarabine compared with 87.9% ±without nelarabine (P = .168). (C) Five-year DFS rates for the 4 randomized arms were as follows: escalating-dose methotrexate without leucovorin rescue plus pegaspargase (C-MTX) with nelarabine, 91.4% ± 3.1% (n = 147); C-MTX without nelarabine, 87.2% ± 3.5% (n = 151); high-dose methotrexate with leucovorin rescue (HDMTX) with nelarabine, 85.5% ± 3.6% (n = 176); and HDMTX without nelarabine, 78.1% ± 4.0% (n = 185; P = .01). (D) DFS by age group in patients randomly assigned to nelarabine; 5-year DFS rates were as follows: age < 10 years, 87.1% ± 3.5%; age 10-15 years, 91.3% ± 3.7%; and age ≥ 16 years, 84.8% ± 7.1% (P = .77). (E) DFS by age group in patients not randomly assigned to nelarabine; 5-year DFS rates were as follows: age < 10 years, 80.3% ± 3.8%; age 10-15 years, 81.9% ± 4.9%; and age ≥ 16 years, 88.6% ± 5.5% (P = .441).
Events in Randomized Cohorts
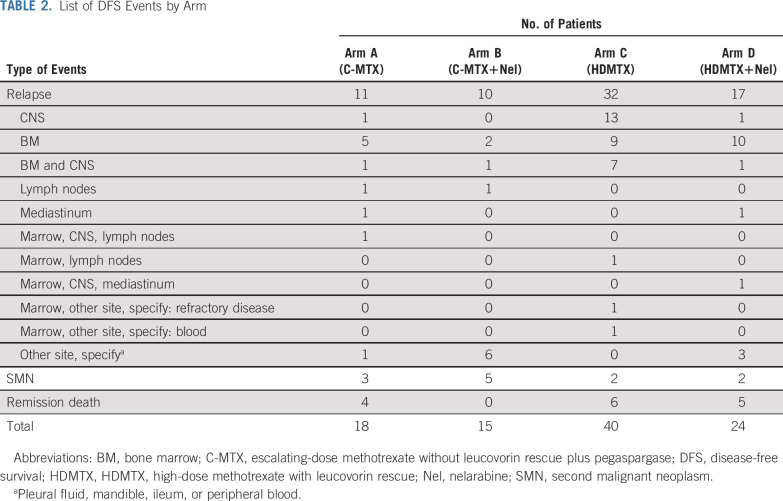
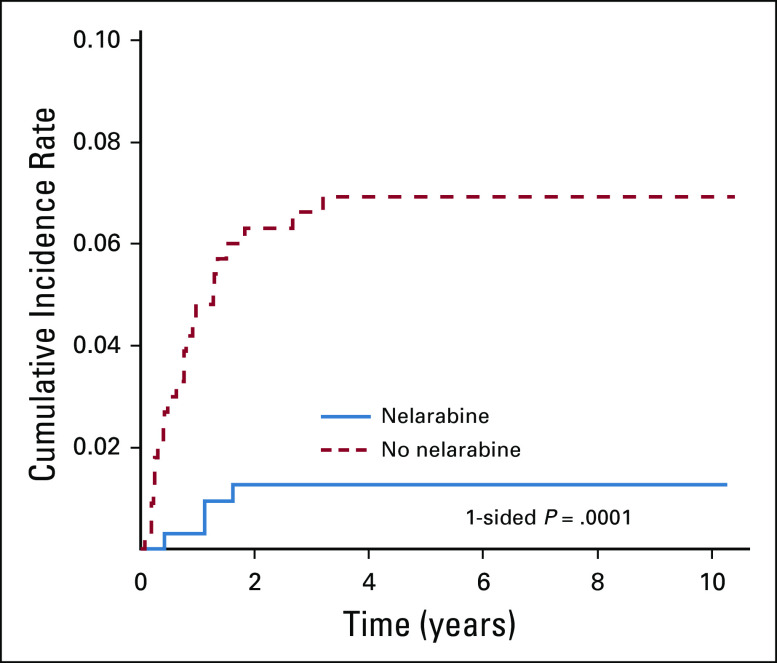
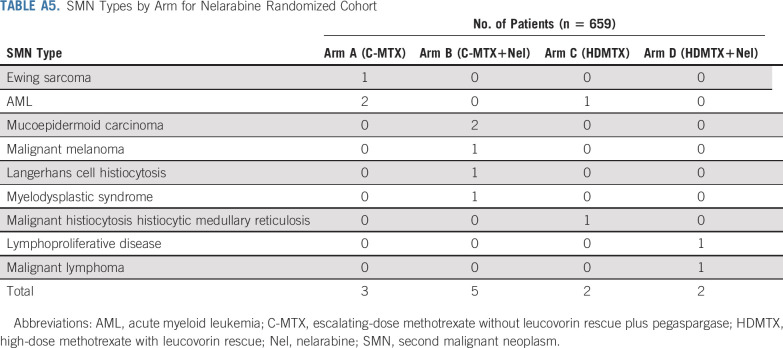
Among patients who underwent randomization, 39 of 323 patients receiving nelarabine had an event, compared with 58 of 336 patients treated without nelarabine (Table 2); relapse was the most common event (72.2%). Nelarabine was associated with a striking decrease in CNS relapses, with 5-year cumulative incidence rates of CNS relapse (isolated and combined) of 1.3% ± 0.63% in patients who received nelarabine compared with 6.9% ± 1.4% in patients who did not receive nelarabine (P = .0001; nelarabine: one CNS relapse; two CNS and marrow relapses; and one CNS, marrow, and other relapse; no nelarabine: 14 CNS relapses; eight CNS and marrow relapses; and one CNS, marrow, and other relapse; Fig 4). CNS3 patients were nonrandomly assigned to HDMTX, and six (21.4%) of 28 patients randomly assigned to HDMTX without nelarabine had a CNS relapse compared with only one (3.4%) of 29 patients randomly assigned to HDMTX with nelarabine (P = .052). Second malignancy rates were low and similar between groups (Appendix Table A5, online only).
TABLE 2.
List of DFS Events by Arm
FIG 4.
The 5-year cumulative incidence rates of CNS relapse (isolated and combined) in the nelarabine versus no nelarabine arms were 1.3% ± 0.63% and 6.9% ± 1.4%, respectively (P = .0001).
Risk-Stratified Outcomes
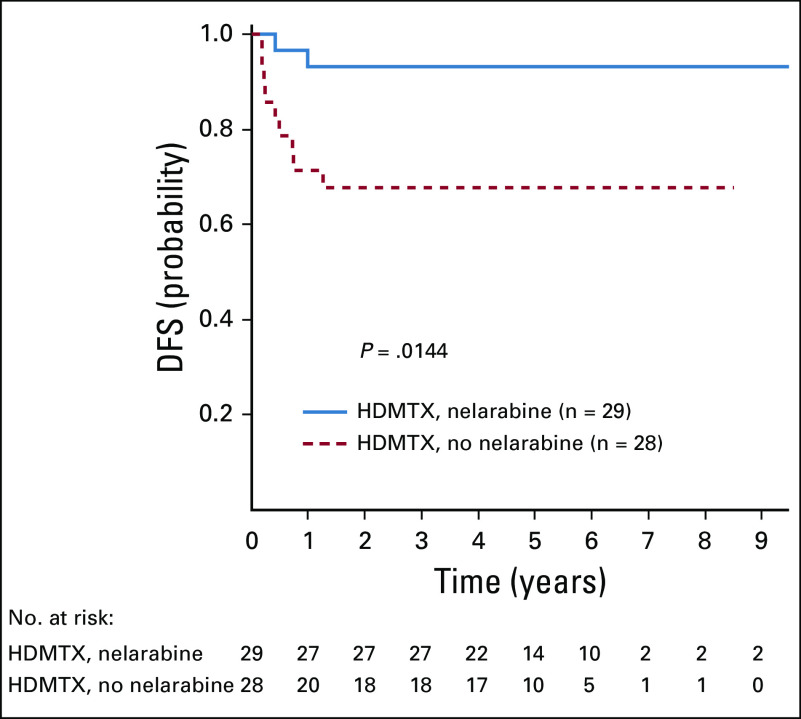
Among the 1,189 eligible and evaluable patients with T-ALL with postinduction risk stratification data, 109 (9.2%) were LR, 808 (70.0%) were IR, 229 (19.3%) were HR, and 43 (3.6%) had IF. The 5-year DFS and OS rates for patients randomly assigned to receive versus not receive nelarabine were 90.8% ± 2.8% and 91.3% ± 2.7%, respectively, versus 86.3% ± 3.1% and 92.4% ± 2.4%, respectively (P = .077 and P = .617, respectively) for IR patients, and 83.5% ± 4.4% and 88.5% ± 3.8%, respectively, versus 74.1% ± 4.8% and 79.2% ± 4.6%, respectively (P = .106 and P = .051, respectively) for HR patients. For the 43 patients who experienced IF who were nonrandomly assigned to HDMTX with nelarabine, the 5-year EFS was 53.1% ± 9.4%. The 5-year DFS rates for CNS3 patients randomly assigned between the HDMTX-containing arms were 93.1% ± 6.5% for HDMTX with nelarabine and 67.9% ± 12.2% for HDMTX without nelarabine (P = .014; Appendix Fig A1, online only). Day 29 MRD was prognostically significant in both cohorts (Appendix Figs A2A and A2B, online only). For patients who received nelarabine, 5-year DFS was 92.3% ± 2.9% for MRD < 0.1% compared with 83.5% ± 3.9% with MRD ≥ 0.1% (P = .01); without nelarabine, DFS was 89.0% ± 3.1% for MRD < 0.1% compared with 73.4% ± 4.3% with MRD ≥ 0.1% (P = .0003).
Treatment-Related Adverse Events
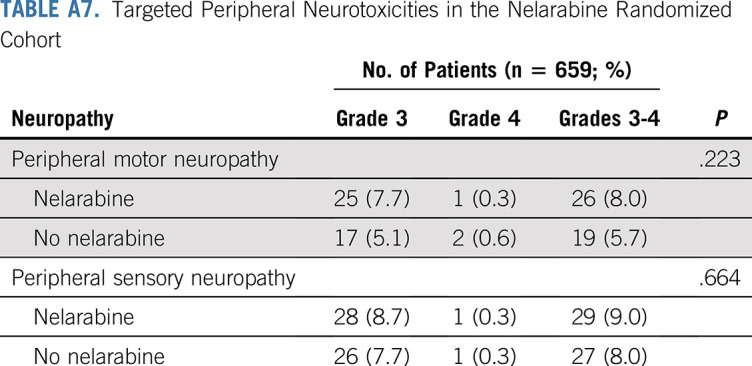
Overall toxicity and neurotoxicity were acceptable and similar between all four randomized arms. Grade ≥ 3 nontargeted toxicity rates were similar on both nelarabine arms (41.2% with nelarabine v 46.1% without nelarabine; P = .2). For the targeted neuropathy toxicities, grade 3 or 4 peripheral motor (P = .223) and sensory (P = .664) neuropathy rates were similar in the nelarabine and no nelarabine arms (Appendix Tables A6 and A7, online only). There was no significant difference in rates of grade ≥ 3 central neurotoxicity (leukoencephalopathy, encephalopathy, reversible posterior leukoencephalopathy syndrome, CNS necrosis, cerebral edema, Guillain-Barre syndrome, and pyramidal tract syndrome) in arms with versus without nelarabine (3.4% v 2.1%, respectively; P = .298; Appendix Tables A6 and A7). However, two patients with IF who were nonrandomly assigned to receive nelarabine on arm D developed symptoms of central neurocognitive decompensation either during or immediately after a nelarabine cycle during consolidation therapy and eventually died.
AlloHSCT at Investigator Discretion
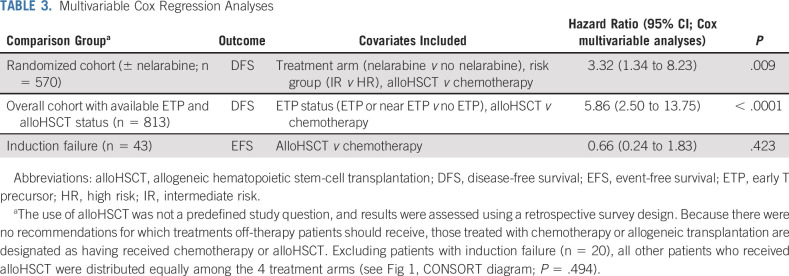
Among the 1,390 eligible and evaluable patients with T-ALL for whom survey information was available, 333 (24%) were taken off protocol therapy during induction before randomization. Thirty-two of these patients (9.6%) underwent alloHSCT. Of 917 patients with T-ALL randomized or assigned after induction to one of the four treatment arms, 23 (2.5%) were taken off protocol therapy and underwent alloHSCT. Multivariable Cox regression analyses on this cohort (adjusting for treatment arm and risk group and using time-dependent covariates for time to alloHSCT) showed worse DFS for those who received alloHSCT compared with patients who received protocol chemotherapy (hazard ratio, 3.32; 95% CI, 1.34 to 8.23; P = .009; Table 3). For patients who experienced IF, there was no difference in outcome for those receiving HDMTX plus nelarabine (n = 23) compared with those receiving alloHSCT (n = 20; hazard ratio, 0.66; 95% CI, 0.24 to 1.83; P = .423). Centrally determined ETP status was known for 1,125 patients (81%), including 279 patients taken off therapy before randomization, with 92 patients having ETP or near ETP phenotype.38 Of the 279 patients, 28 received alloHSCT (21 with ETP or near ETP [75%]) and 251 received chemotherapy alone (71 with ETP or near ETP [28%]; Fisher’s exact test, P < .001). In multivariable analyses including ETP status, alloHSCT (n = 21) was associated with inferior DFS compared with chemotherapy (n = 792; hazard ratio, 5.86; 95% CI, 2.50 to 13.75; P < .0001), and ETP status did not have a statistically significant impact on DFS (hazard ratio, 0.99; 95% CI, 0.59 to 1.67; P = .981).
TABLE 3.
Multivariable Cox Regression Analyses
DISCUSSION
Typically, children and young adults with T-ALL have had EFS and OS rates that are inferior to patients with B-ALL.4,5,10 In AALL0434, the 1,562 patients with T-ALL had a 5-year EFS rate of 83.7% and 5-year OS rate of 89.5%, which are superior to the outcomes in the companion COG high-risk B-ALL trial AALL0232 (5-year EFS, 75.2%; 5-year OS, 85.0%).2 Other investigators have reported a 5-year EFS of 81.2% and 5-year OS of 86.4% in 388 patients with T-ALL in the UKALL 2003 study, 4-year EFS of 83% and 4-year OS of 89% in 97 patients with T-ALL in the DFCI 05-001 study, and 7-year EFS of 76.3% and 7-year OS of 81.2% in 464 patients with T-ALL in the AIEOP-BFM 2000 study.6-9,11 Children and young adults with T-ALL treated on AALL0434 showed superior 5-year DFS with nelarabine (88%) versus without nelarabine (82%). The best results were obtained among patients who were randomly assigned to receive nelarabine plus C-MTX. The addition of nelarabine to the HDMTX regimen also improved DFS compared with HDMTX alone and decreased CNS relapse in both patients with and without CNS disease at diagnosis. Remarkably, there was only one isolated CNS relapse and four total relapses involving the CNS among the 323 patients randomly assigned to nelarabine versus 14 isolated CNS relapses and 23 total relapses involving CNS among the 336 patients randomly assigned to not receive nelarabine. We did not observe a difference in OS between the nelarabine and no nelarabine arms, although the OS for patients with HR T-ALL trended toward statistical significance.
Unlike in other studies, the nelarabine DFS advantage was not restricted by age or race.7,9-11 The UKALL 2003 study showed improvement in T-ALL survival using dexamethasone instead of prednisone, but only for patients younger than age 10 years, and the AIEOP-BFM 2000 trial showed improvement in T-ALL survival using dexamethasone only for those who were prednisone good responders.3,7,9 The lack of age restriction on DFS is particularly important and novel because T-ALL tends to affect older children and approximately 25% of adults with ALL, but survival for older children and young adults was equivalent to that of younger patients on AALL0434.3,7,39
The improvement in outcome may be explained by the activity of nelarabine in patients with higher-risk disease (high MRD and IF) and enhanced CNS prophylaxis. Higher postinduction MRD (≥ 0.1%) was highly prognostic on AALL0434, but patients with high MRD who received nelarabine had a 5-year DFS rate of 83.5%, compared with 73.4% for those who did not receive nelarabine. The excellent outcome for older patients might also be affected by this because older patients had increased higher-risk features. Nelarabine has good penetration into the CNS,23 and T-ALL has a higher likelihood of CNS relapse as compared with B-ALL. The incidence of CNS relapse was significantly lower for patients assigned to the nelarabine arms (P = .0001), even though all patients received CRT. The decrease in incidence of CNS relapse was seen both in patients who were CNS3 at diagnosis and those who were not. The advantage in CNS protection did not come at the cost of increased toxicity. This study and COG AALL00P2 showed that nelarabine was not associated with an overall increased risk of neurotoxicity in newly diagnosed patients, although there were rare serious neurotoxicities (three of 366 patients; 0.8%), similar to those described for patients with heavily pretreated relapsed T-ALL.22-26 Notably, two of the three patients with severe neurotoxicity had induction failure and developed fatal neurotoxicity during consolidation. Although AALL0434 did not address whether nelarabine would improve outcome in patients treated without CRT, the significant CNS-protective effect of nelarabine supports its use in future clinical trials.
Patients with T-ALL and IF typically die of disease, but the 5-year EFS was 53% for such patients in AALL0434. A study from 14 cooperative study groups in the United States and the European Union found that patients with postinduction M3 marrow had a 10-year EFS of only 19%.40 Approximately 4% of AALL0434 participants received alloHSCT, including approximately half of patients with IF. There was no EFS advantage for patients with IF who subsequently received alloHSCT even when standard risk factors were taken into consideration in a multivariable analysis. However, we do not have information regarding whether there were other clinical factors that resulted in the patient undergoing HSCT. Schrappe et al40 reported that patients who received chemotherapy alone for IF had a 10-year EFS of 26%, whereas those who received matched alloHSCT or other donor types had 10-year EFS rates of 40% and 45%, respectively. Given the excellent results for patients who received C-MTX with nelarabine in AALL0434, this regimen deserves consideration for patients with IF.
In summary, the addition of nelarabine decreased CNS relapses and improved overall DFS, especially for patients with higher-risk disease, independent of age or race. Although many centers have used alloHSCT in first CR for HR T-ALL, this study showed a survival advantage for chemotherapy alone for patients with IR, HR, and IF. Patients with relapsed T-ALL have dismal survival with salvage therapy, and unlike B-ALL, there are no excellent salvage options such as chimeric antigen receptor T-cell immunotherapy, supporting the use of nelarabine in patients with newly diagnosed disease.
ACKNOWLEDGMENT
We thank all of the care providers in Children’s Oncology Group institutions who enrolled and treated patients on the study. Most importantly, we thank the children and families who supported the study through their participation.
APPENDIX
FIG A1.
Disease-free survival (DFS) for patients with CNS3 randomly assigned to high-dose methotrexate with leucovorin rescue (HDMTX) with or without nelarabine; 5-year DFS rates were 93.1% ± 6.5% for HDMTX with nelarabine and 67.9% ± 12.2% for HDMTX without nelarabine (P = .014).
FIG A2.
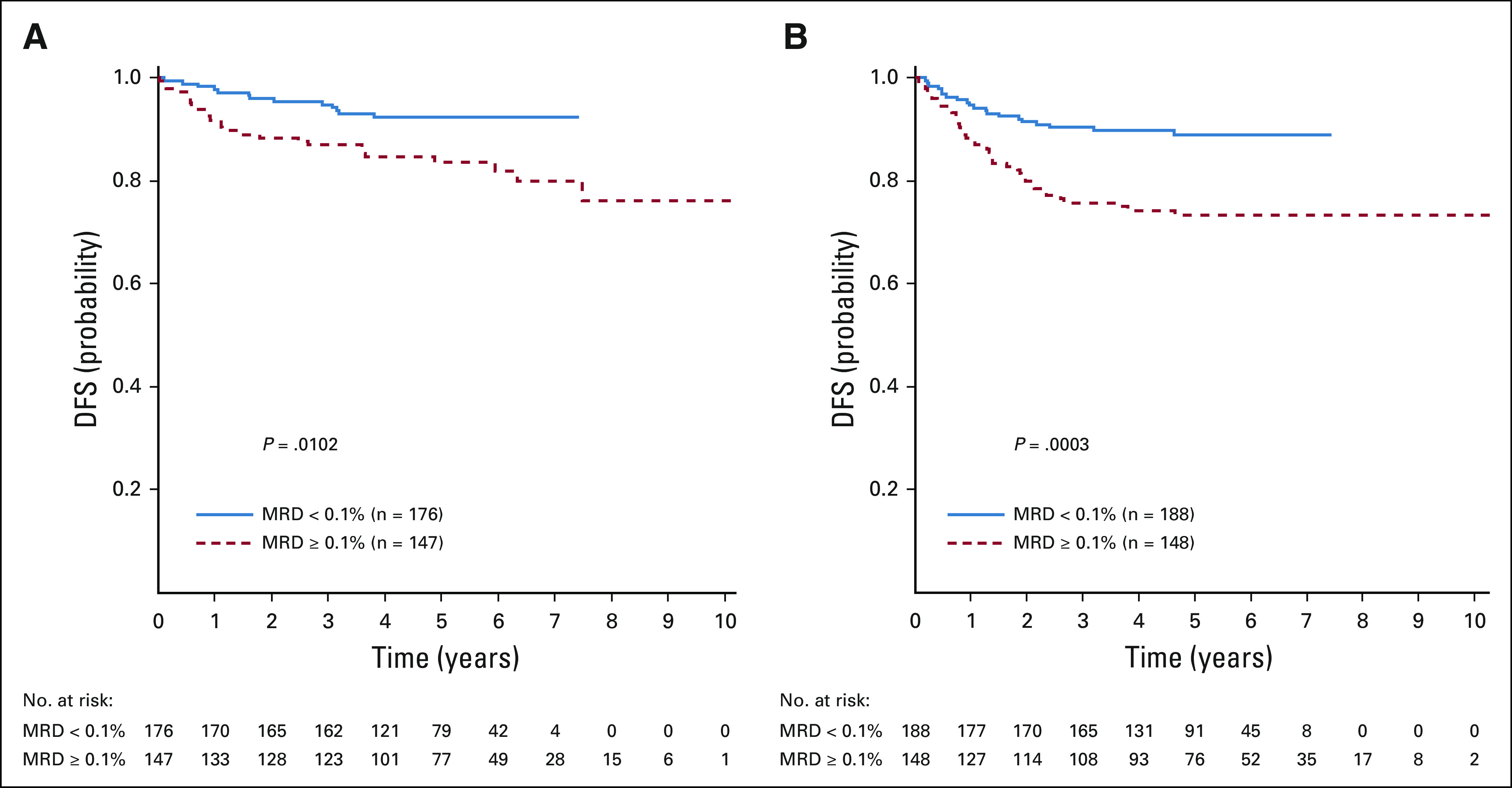
Disease-free survival (DFS) by day 29 minimal residual disease (MRD) status. (A) Five-year DFS rates in the nelarabine group were 92.3% ± 2.9% in those with MRD < 0.1% and 83.5% ± 3.9% for those with MRD ≥ 0.1% (P = .0102). (B) Five-year DFS rates in the no nelarabine group were 89.0% ± 3.1% in those with MRD < 0.1% and 73.4% ± 4.3% in those with MRD ≥ 0.1% (P = .0003).
TABLE A1.
Two-Stage Consenting Process and Details of Therapies
TABLE A2.
Risk Assignment Algorithm
TABLE A3.
Reasons for Study Ineligibility of Patients With T-Cell Acute Lymphoblastic Leukemia
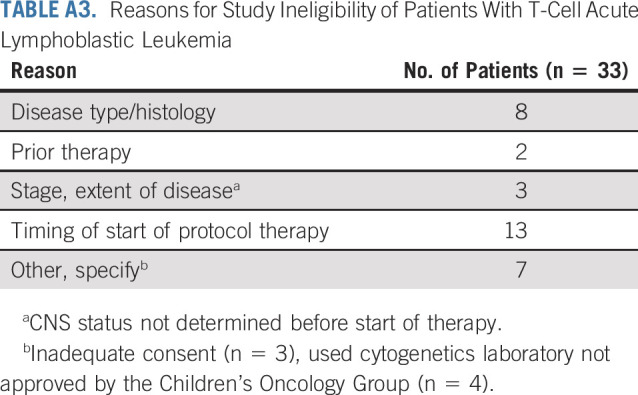
TABLE A4.
Reason for Receiving Off-Protocol Therapy at the End of Induction
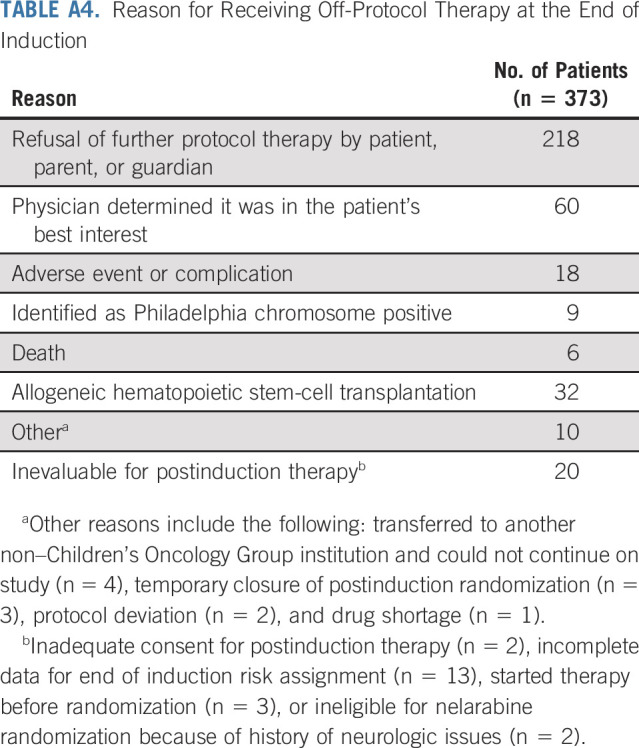
TABLE A5.
SMN Types by Arm for Nelarabine Randomized Cohort
TABLE A6.
Target Central Neurotoxicities in the Nelarabine Randomized Cohort
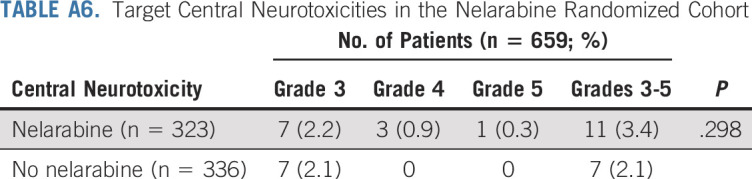
TABLE A7.
Targeted Peripheral Neurotoxicities in the Nelarabine Randomized Cohort
PRIOR PRESENTATION
Presented at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 5-8, 2009; 52nd Annual Meeting of the American Society of Hematology, Orlando FL, December 4-7, 2010; 2011 European Working Group for Acute Lymphocytic Leukemia Nelarabine Expert Meeting Frankfurt Germany, November 2011; 57th Annual Meeting of the American Society of Hematology, Orlando, FL, December 5-8, 2015; 60th Annual Meeting of the American Society of Hematology, San Diego, CA, December 1-4, 2018; 54th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018; 50th Congress of the International Society of Pediatric Oncology, Kyoto, Japan, November 16-19, 2018; 2019 American Society of Pediatric Hematology/Oncology Conference, New Orleans, LA, May 1-4, 2019; and 51st Congress of the International Society of Pediatric Oncology, Lyon, France, October 23-26, 2019.
SUPPORT
Supported by Grants No. U10 CA98543, U10 CA98413, U10 CA180886, 1U24-CA196173 and U10 CA180899 from the National Institutes of Health and by St Baldrick’s Foundation. E.A.R. is a KiDS of New York University (NYU) Foundation Professor at NYU Langone Health. M.L.L. is the University of California, San Francisco Benioff Chair of Children’s Health and Deborah and Arthur Ablin Endowed Chair in Pediatric Molecular Oncology. S.P.H. is the Jeffrey E. Perelman Distinguished Chair in the Department of Pediatrics at the Children’s Hospital of Philadelphia.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Kimberly P. Dunsmore, Stuart S. Winter, Meenakshi Devidas, Brent L. Wood, Robert J. Hayashi, Julie M. Gastier-Foster, Barbara L. Asselin, Patrick A. Zweidler-Mckay, Elizabeth A. Raetz, Mignon L. Loh, Naomi J. Winick, William L. Carroll, Stephen P. Hunger
Administrative support: Kimberly P. Dunsmore, Stuart S. Winter, Zhiguo Chen, Stephen P. Hunger
Provision of study materials or patients: Nikki Briegel, Julie M. Gastier-Foster, Barbara L. Asselin, Kirk R. Schultz, William L. Carroll
Collection and assembly of data: Kimberly P. Dunsmore, Stuart S. Winter, Meenakshi Devidas, Brent L. Wood, Zhiguo Chen, Nancy Eisenberg, Nikki Briegel, Julie M. Gastier-Foster, Andrew J. Carroll, Nyla A. Heerema, Barbara L. Asselin, Karen R. Rabin, William L. Carroll
Data analysis and interpretation: Kimberly P. Dunsmore, Stuart S. Winter, Meenakshi Devidas, Brent L. Wood, Natia Esiashvili, Zhiguo Chen, Robert J. Hayashi, Barbara L. Asselin, Mignon L. Loh, Kirk R. Schultz, Naomi J. Winick, Stephen P. Hunger
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Children’s Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kimberly P. Dunsmore
Employment: Dexcom (I)
Stock and Other Ownership Interests: Dexcom (I)
Travel, Accommodations, Expenses: Dexcom (I)
Stuart S. Winter
Honoraria: Jazz Pharmaceuticals
Consulting or Advisory Role: Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Meenakshi Devidas
Honoraria: PSI, Novartis
Brent L. Wood
Honoraria: Amgen, Seattle Genetics, AbbVie, Janssen, Amgen, Astellas Pharma
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BiolineRx (Inst), Biosight (Inst), Stemline Therapeutics (Inst), Janssen Oncology (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Amgen
Robert J. Hayashi
Consulting or Advisory Role: Magenta Therapeutics
Patrick A. Zweidler-Mckay
Employment: Immunogen
Stock and Other Ownership Interests: Immunogen
Patents, Royalties, Other Intellectual Property: Patent applications submitted, no royalties
Elizabeth A. Raetz
Research Funding: Pfizer (Inst)
Other Relationship: Celgene
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
Kirk R. Schultz
Honoraria: Shire
Consulting or Advisory Role: MesoScale Discovery, Juno Therapeutics
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
William L. Carroll
Other Relationship: Amgen
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck (I)
Honoraria: Amgen
Consulting or Advisory Role: Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Winter SS, Dunsmore KP, Devidas M, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: Results from the Children’s Oncology Group AALL0434 methotrexate randomization. J Clin Oncol. 2018;36:2926–2934. doi: 10.1200/JCO.2018.77.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children’s Oncology Group study AALL0232. J Clin Oncol. 2016;34:2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127:2101–2112. doi: 10.1182/blood-2015-09-670729. [DOI] [PubMed] [Google Scholar]
- 4.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: Progress through collaboration. J Clin Oncol. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): A randomised, open-label phase 3 trial. Lancet Oncol. 2015;16:1677–1690. doi: 10.1016/S1470-2045(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 7.Patrick K, Wade R, Goulden N, et al. Improved outcome for children and young people with T-acute lymphoblastic leukemia: Results of the UKALL 2003 trial. Blood. 2014;124:3702. [Google Scholar]
- 8. Place AE, Stevenson KE, Harris MH, et al: Outcome of childhood T-cell acute lymphoblastic leukemia (T-ALL): Results from DFCI protocol 05-001. J Clin Oncol 32, 2014 (suppl 15; abstr 10015) [Google Scholar]
- 9.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): A randomised controlled trial. Lancet Oncol. 2013;14:199–209. doi: 10.1016/S1470-2045(12)70600-9. [DOI] [PubMed] [Google Scholar]
- 10.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the Children’s Oncology Group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrappe M, Valsecchi MG, Bartram CR, et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118:2077–2084. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]
- 12.Asselin BL, Devidas M, Wang C, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: A randomized study by the Children’s Oncology Group (POG 9404) Blood. 2011;118:874–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinherz PG, Gaynon PS, Breneman JC, et al. Treatment of patients with acute lymphoblastic leukemia with bulky extramedullary disease and T-cell phenotype or other poor prognostic features: Randomized controlled trial from the Children’s Cancer Group. Cancer. 1998;82:600–612. doi: 10.1002/(sici)1097-0142(19980201)82:3<600::aid-cncr24>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Vadillo E, Dorantes-Acosta E, Pelayo R, et al. T cell acute lymphoblastic leukemia (T-ALL): New insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018;32:36–51. doi: 10.1016/j.blre.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Uckun FM, Sensel MG, Sun L, et al. Biology and treatment of childhood T-lineage acute lymphoblastic leukemia. Blood. 1998;91:735–746. [PubMed] [Google Scholar]
- 16.Willemse MJ, Seriu T, Hettinger K, et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood. 2002;99:4386–4393. doi: 10.1182/blood.v99.12.4386. [DOI] [PubMed] [Google Scholar]
- 17.Heerema NA, Sather HN, Sensel MG, et al. Frequency and clinical significance of cytogenetic abnormalities in pediatric T-lineage acute lymphoblastic leukemia: A report from the Children’s Cancer Group. J Clin Oncol. 1998;16:1270–1278. doi: 10.1200/JCO.1998.16.4.1270. [DOI] [PubMed] [Google Scholar]
- 18.Coustan-Smith E, Mullighan CG, Onciu M, et al. Early T-cell precursor leukaemia: A subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10:147–156. doi: 10.1016/S1470-2045(08)70314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachman J, Sather HN, Cherlow JM, et al. Response of children with high-risk acute lymphoblastic leukemia treated with and without cranial irradiation: A report from the Children’s Cancer Group. J Clin Oncol. 1998;16:920–930. doi: 10.1200/JCO.1998.16.3.920. [DOI] [PubMed] [Google Scholar]
- 20.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buie LW, Epstein SS, Lindley CM. Nelarabine: A novel purine antimetabolite antineoplastic agent. Clin Ther. 2007;29:1887–1899. doi: 10.1016/j.clinthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzberg J, Ernst TJ, Keating MJ, et al. Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol. 2005;23:3396–3403. doi: 10.1200/JCO.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 23.Berg SL, Blaney SM, Devidas M, et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: A report from the Children’s Oncology Group. J Clin Oncol. 2005;23:3376–3382. doi: 10.1200/JCO.2005.03.426. [DOI] [PubMed] [Google Scholar]
- 24.DeAngelo DJ, Yu D, Johnson JL, et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood. 2007;109:5136–5142. doi: 10.1182/blood-2006-11-056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhlen M, Bleckmann K, Möricke A, et al. Neurotoxic side effects in children with refractory or relapsed T-cell malignancies treated with nelarabine based therapy. Br J Haematol. 2017;179:272–283. doi: 10.1111/bjh.14877. [DOI] [PubMed] [Google Scholar]
- 26.Dunsmore KP, Devidas M, Linda SB, et al. Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2753–2759. doi: 10.1200/JCO.2011.40.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winter SS, Dunsmore KP, Devidas M, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0434. Pediatr Blood Cancer. 2015;62:1176–1183. doi: 10.1002/pbc.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matlawska-Wasowska K, Kang H, Devidas M, et al. MLL rearrangements impact outcome in HOXA-deregulated T-lineage acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia. 2016;30:1909–1912. doi: 10.1038/leu.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrando AA, Look AT. Gene expression profiling in T-cell acute lymphoblastic leukemia. Semin Hematol. 2003;40:274–280. doi: 10.1016/s0037-1963(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 30.Ferrando AA, Herblot S, Palomero T, et al. Biallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemia. Blood. 2004;103:1909–1911. doi: 10.1182/blood-2003-07-2577. [DOI] [PubMed] [Google Scholar]
- 31.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 32.Palomero T, Odom DT, O’Neil J, et al. Transcriptional regulatory networks downstream of TAL1/SCL in T-cell acute lymphoblastic leukemia. Blood. 2006;108:986–992. doi: 10.1182/blood-2005-08-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. doi: 10.1200/JCO.2010.28.3390. Gutierriz A, Dahlberg SE, Neuberg DS, et al: Absence of biallelic TCR gamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol 28:3816-3823, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patrick K, Wade R, Goulden N, et al. Outcome for children and young people with early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2014;166:421–424. doi: 10.1111/bjh.12882. [DOI] [PubMed] [Google Scholar]
- 35.Wood BL, Winter SS, Dunsmore KP, et al. T-lymphoblastic leukemia (T-ALL) shows excellent outcome, lack of significance of the early thymic precursor (ETP) immunophenotype and validation of the prognostic value of end-induction minimal residual disease (MRD) in Children’s Oncology Group (COG) study AALL0434. Blood. 2014;124:21. (abstr 1) [Google Scholar]
- 36.Kaplan EL. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 37.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 39.Hough R, Rowntree C, Goulden N, et al. Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: Results from UKALL 2003. Br J Haematol. 2016;172:439–451. doi: 10.1111/bjh.13847. [DOI] [PubMed] [Google Scholar]
- 40.Schrappe M, Hunger SP, Pui CH, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–1381. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]