Abstract
Context
A comprehensive characterization of racial/ethnic variations in vitamin D metabolism markers may improve our understanding of differences in bone and mineral homeostasis and the risk of vitamin D–related diseases.
Objective
Describe racial/ethnic differences in vitamin D metabolism markers and their associations with genetic ancestry.
Design, Setting, Participants
In a cross-sectional study within the Multi-Ethnic Study of Atherosclerosis (MESA), we compared a comprehensive panel of vitamin D metabolism markers across self-reported racial/ethnic groups of Black (N = 1759), White (N = 2507), Chinese (N = 788), and Hispanic (N = 1411). We evaluated associations of proportion African and European ancestry with this panel of markers in Black and Hispanic participants using ancestry informative markers. Latent class analysis evaluated associations between patterns of vitamin D measurements with race/ethnicity.
Results
Compared with Black participants, White participants had significantly higher serum concentrations of 25-hydroxyvitamin D and fibroblast growth factor-23; lower concentrations of parathyroid hormone and 1,25-dihydroxyvitamin D; circulating vitamin D metabolite ratios suggesting lower CYP27B1 and higher CYP24A1 activity; higher urinary concentrations of calcium and phosphorus with higher urinary fractional excretion of phosphorus; and differences in vitamin D binding globulin haplotypes. Higher percent European ancestry was associated with higher 25-hydroxyvitamin D and lower parathyroid hormone concentrations among Black and Hispanic participants. Latent classes defined by vitamin D measurements reflected these patterns and differed significantly by race/ethnicity and ancestry.
Conclusions
Markers of vitamin D metabolism vary significantly by race/ethnicity, may serve to maintain bone and mineral homeostasis across ranges of 25-hydroxyvitamin D production, and be attributable, at least partly, to genetic ancestry.
Keywords: vitamin d, mineral metabolism, parathyroid hormone, ancestry, race
Vitamin D and its metabolites are vital to maintaining bone and mineral homeostasis and for human health. They are closely regulated through complex pathways influenced by biologic and environmental factors, and disturbances in their metabolism have been implicated in bone disease, kidney stones, cardiovascular disease, and cancer (1-10). Vitamin D metabolism and the risk of vitamin D–related diseases vary by race/ethnicity (11-17). For instance, associations of low 25-hydroxyvitamin D (25(OH)D) concentration with bone disease, diabetes, stroke, and cardiovascular events observed in White populations are attenuated or absent in Black and Hispanic populations, despite lower 25(OH)D and higher parathyroid hormone (PTH) concentrations in these groups (13-22). Despite heterogeneity in associations of vitamin D–related diseases by race, differences in patterns of vitamin D metabolism markers by race/ethnicity remain incompletely characterized.
The majority (>99%) of circulating vitamin D and its metabolites are bound to vitamin D binding globulin (VDBG) or albumin (23, 24), and must pass through a series of steps for biologic activity. Low concentrations of 25(OH)D, the principal circulating vitamin D metabolite, result in compensatory secondary hyperparathyroidism (25). PTH promotes the production of the enzyme CYP27B1 (1-α hydroxylase), which mediates the conversion of 25(OH)D into 1,25-dihydroxyvitamin D (1,25(OH)2D), the most active vitamin D metabolite. PTH also inhibits the CYP24A1-mediated conversion of 25(OH)D to 24,25-dihydroxyvitamin D (24,25(OH)2D), the major product of vitamin D clearance (26). Ultimately, 1,25(OH)2D binds to vitamin D receptors (VDRs) throughout the body to regulate a broad range of gene expression, with end-organ effects that include intestinal calcium and phosphorus absorption (27-29). Through classic negative feedback, 1,25(OH)2D suppresses PTH release and promotes CYP24A1-mediated 25(OH)D clearance. Another key regulatory hormone, fibroblast growth factor-23 (FGF-23), is secreted in response to excess phosphorus and 1,25(OH)2D. In a feedback loop, FGF-23 inhibits 1,25(OH)2D synthesis in the kidney, suppresses CYP27B1, and induces CYP24A1 (30-33).
Separating the influence of biologic from environmental factors on observed racial/ethnic differences in vitamin D metabolism is challenging. A more complete picture may be obtained by examining differences across multiple vitamin D measures and by using ancestry informative markers (AIMs) for genetic admixture analysis (34). AIMs are single-nucleotide polymorphisms (SNPs) with distinct allele frequency differences across populations which can be used to quantify an individual’s genetic ancestry. Few studies have examined the relationship between genetic ancestry with vitamin D metabolites and their targets in the general population (35-37).
In a multi-ethnic cohort of community-dwelling adults, we comprehensively characterized racial/ethnic/ancestral heterogeneity in serum and urinary measures of the complex biological network of vitamin D metabolism.
Materials and Methods
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a community-based prospective cohort study of clinical and subclinical cardiovascular disease (38). Between 2000 and 2002, 6814 adults without overt cardiovascular disease between the ages of 45 and 84 years were recruited from 6 US communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; northern Manhattan and the Bronx, New York; and St. Paul, Minnesota (38). Institutional review boards at all participating centers approved the study, and all participants gave written informed consent.
For this study, we excluded 348 participants who were missing vitamin D metabolism measures at the baseline examination and one participant with 25(OH)D concentrations >100 ng/mL (suggestive of high-dose supplementation), leaving a sample size of 6465.
Measurement of covariates
Covariates were ascertained at the baseline MESA examination, concurrent with the vitamin D metabolite measurements, where participants completed self-administered questionnaires, interviewer-administered standardized interviews, and extensive in-person examinations, yielding demographic and lifestyle characteristics, medical history, anthropometric measurements, and laboratory data. Diabetes status was defined by the use of an oral hypoglycemic medication or insulin, fasting blood glucose ≥126 mg/dL, nonfasting blood glucose ≥200 mg/dL, or hemoglobin A1c ≥6.5% (39). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Glomerular filtration rate was estimated from the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation (40). General health was self-reported on a questionnaire as excellent, very good, good, fair, or poor. Leisure time physical activity was estimated as the total amount of intentional exercise performed in a usual week and measured in metabolic equivalent task—minutes. Participants were asked to report the average frequency of consumption of specific food items over the previous year using a 120-item food frequency questionnaire. Dietary vitamin D intake was estimated by multiplying the frequency and serving size for each food consumed by the vitamin D content of that food (Nutritional Data Systems for Research). Data on vitamin D supplement use were not available.
Race and ancestry estimation
Race and ethnicity were self-reported as White, Chinese, Black, or Hispanic using questions modeled from the year 2000 US Census. Participants who consented for genetic analysis and provided deoxyribonucleic acid samples were genotyped using the Affymetrix 6.0 SNP array at the Broad Institute Center for Genotyping and Analysis. Ancestry proportion estimates were computed assuming 2 ancestral populations (central European and West African Yoruban) using 300 AIMs, selected to be distributed as evenly across the genome as possible, and to maximize differential allele frequency between the racial/ethnic groups (41-43). Analyses of genetic ancestry were restricted to 6040 individuals with nonmissing AIM data.
Measurement of vitamin D metabolism markers
We measured a panel of vitamin D metabolism markers at the University of Washington Nutrition Obesity Research Center from baseline fasting serum samples collected during 2000–2002: 25(OH)D2, 25(OH)D3, 1,25(OH)2D2, 1,25(OH)2D3 and 24,25(OH)2D3 were measured using immunoaffinity extraction and liquid chromatography tandem mass spectrometry (44-47). Total 25(OH)D and 1,25(OH)D were calculated by the sum of their respective D2 and D3 concentrations. Calibration of 25(OH)D was confirmed with National Institute of Standards and Technology standard reference material 972a (48). Interassay coefficients of variation calculated using repeat measurements of quality control specimens were 25(OH)D2: 11.8% at 7.0 ng/mL; 25(OH)D3: 8.5% at 24.8 ng/mL; 1,25(OH)D2: 10.8% to 11.9% across a range of concentrations; 1,25(OH)D3: 3.7% to 10.2% across a range of concentrations; 24,25(OH)2D3: 14.7% at 2.7 ng/mL. As there was no spectroscopic evidence of 24,25(OH)2D2, the ratio of 24,25(OH)2D3 to 25(OH)D3 in serum was used as a calculated functional estimate of CYP24A1-mediated 25(OH)D clearance (47, 49). The ratio of total 1,25(OH)2D to total 25(OH)D in serum was used as a calculated functional estimate of CYP27B1-mediated 1,25(OH)2D production (49). PTH was measured with the Beckman-Coulter DxI automated 2-site immunoassay (Beckman-Coulter Inc, Brea, CA) and intact FGF-23 via the Kainos immunoassay (Kainos Laboratories, Tokyo, Japan) using previously unthawed serum (50, 51).
A random sample of MESA participants (n = 999) was selected for measurement of albumin, VDBG, and its isoforms. Of these, 7 participants had insufficient serum and 64 samples were excluded due to assay error, for a total of 928 participants included in analyses. VDBG concentration and isoform were measured simultaneously via an liquid chromatography tandem mass spectrometry assay previously described and validated with genetic data (52-54). Albumin was measured via the modified Doumas and Rodkey procedures on the Beckman-Coulter DxC. Bioavailable 25(OH)D concentrations were calculated via published equations allowing for 6 affinity coefficients based on VDBG isoforms (55-57). Analyses were repeated with bioavailable 25(OH)D calculated from the estimation equation with a single binding coefficient (58).
Serum and urine calcium were measured on a Beckman-Coulter DxC autoanalyzer (Beckman-Coulter Inc, Brea, CA) by indirect potentiometry. Serum and urine phosphorus were measured using the same instrument by a timed-rate colorimetric reaction method (59). Urine creatinine was measured with the Array 3600 CE Protein Analyzer (Beckman-Coulter Inc, Brea, CA) by nephelometry. Fractional excretion of phosphorus (FEphos) was calculated from spot urine collections using the following equation: FEphos = (urine phosphorus × serum creatinine × 100)/(serum phosphorus × urine creatinine). Urine albumin excretion was quantified as the ratio of albumin to creatinine in a single-voided urine sample.
Statistical analysis
We evaluated associations of self-reported race/ethnicity and proportion of African ancestry with circulating concentrations of vitamin D metabolites using linear regression. Analyses of genetic ancestry were restricted to Black and Hispanic participants. Regression models were fitted with race/ethnicity as a categorical predictor, or African or European ancestry as a continuous predictor of vitamin D metabolism traits. The adjustment model included age, sex, study site, and the following covariates, selected a priori based on suspicion that they may be in the causal pathway or may act as precision variables: BMI, diabetes status, estimated glomerular filtration rate, season of blood draw, educational attainment (high school, some college, completed college), total gross family income in the past 12 months, and self-reported general health.
Approximately 5% or less of the study participants were missing data on education and income. For the regression analyses, these participants’ values were multiply imputed using chained equations (60). The multiple analyses over the imputations were combined using Rubin rules to account for the variability in the imputation procedure (61). Interactions were tested by comparing models including only main effects of race/ethnicity/genetic ancestry and the metabolites to another model including the main effects and interaction term, with a likelihood ratio test.
Functional forms of the race-specific associations of 25(OH)D and PTH with proportion African and European ancestry were examined graphically using adjusted penalized smoothing splines with evenly spaced knots, among the inner 95% of annualized 25(OH)D, PTH concentrations and proportion of ancestry.
Latent class analysis (LCA) is a statistical method used to define groups of participants (latent classes) based on observed measures that are similar for participants within each class. First, we used LCA to identify 2 classes of participants across a panel of vitamin D metabolism markers, to compare relative differences in these markers between the 2 classes. Then, we evaluated associations between class membership, each representing a collective pattern of vitamin D metabolism markers, and race/ethnicity. In order to maximize the sample size available for this analysis, we omitted vitamin D metabolites measured in only the subcohort. We included serum calcium, phosphorus, 25(OH)D, 24,25(OH)2D3, the ratio of 24,25(OH)2D3 to 25(OH)D3, fractional excretion of phosphorus, urine phosphorus clearance, log-transformed urine calcium clearance, log-transformed FGF-23 and log-transformed PTH as inputs in the LCA model. We fitted a Gaussian model with 2 classes using the Generalized Structural Equation Model estimation (gsem) command in Stata 16.0 (StataCorp, College Station, TX). To understand how the biomarkers distinguished each class, participants were assigned to their most likely class and the mean values of the metabolites compared with class assignment. Basic 2 group comparisons between the 2 cohorts were conducted using the t-test or Wilcoxon rank sum test, as appropriate. The association between class assignment and race/ethnicity, after full adjustment as per the regression models described above, was tested by the likelihood ratio test.
We used the Wald test with robust standard errors to calculate 2-sided P values and 95% confidence intervals for all model covariates. The nominal level of significance was defined as P < .05 and all analyses were conducted using Stata 16.0 (StataCorp, College Station, TX) and R version 3.4.1.
Results
Study population
Overall, mean (standard deviation [SD]) participant age was 62 (10) years and 53% of the cohort was female. White participants had higher educational attainment and family income and better self-reported general health than other racial/ethnic groups (Table 1). BMI was highest in Black and Hispanic participants, who were also more likely to have diabetes.
Table 1.
Participant characteristics by race/ethnicity
| Black | White | Chinese | Hispanic | |
|---|---|---|---|---|
| Number of participants | 1759 | 2507 | 788 | 1411 |
| Age, years | 62.1 (+10.1) | 62.6 (+10.3) | 62.4 (+10.3) | 61.3 (+10.4) |
| Female sex | 985 (56.0) | 1313 (52.4) | 408 (51.8) | 739 (52.4) |
| Study site | ||||
| Forsyth County, NC | 449 (25.5) | 518 (20.7) | 0 (0.0) | 3 (0.2) |
| New York and Bronx Counties, NY | 347 (19.7) | 210 (8.4) | 2 (0.3) | 454 (32.2) |
| Baltimore and Baltimore County, MD | 520 (29.6) | 516 (20.6) | 0 (0.0) | 0 (0.0) |
| St Paul, MN | 0 (0.0) | 584 (23.3) | 0 (0.0) | 445 (31.5) |
| Chicago, IL | 291 (16.5) | 552 (22.0) | 299 (37.9) | 0 (0.0) |
| Los Angeles, CA | 152 (8.6) | 127 (5.1) | 487 (61.8) | 509 (36.1) |
| Educational attainment | ||||
| High school completed | 529 (30.6) | 534 (21.5) | 319 (40.7) | 916 (65.4) |
| Some college | 500 (28.9) | 593 (23.8) | 110 (14.0) | 298 (21.3) |
| Completed college | 702 (40.6) | 1362 (54.7) | 355 (45.3) | 186 (13.3) |
| Gross Annual Family Income, $ | ||||
| <25 000 | 501 (31.0) | 392 (16.1) | 388 (49.6) | 677 (49.1) |
| 25 000-49 000 | 522 (32.3) | 648 (26.6) | 170 (21.7) | 453 (33.0) |
| 50 000-99 000 | 463 (28.6) | 797 (32.7) | 146 (18.6) | 219 (15.9) |
| >100 000 | 131 (8.1) | 602 (24.7) | 79 (10.1) | 31 (2.2) |
| Intentional exercise, MET-min/week | 840 (105, 2130) | 1050 (315, 2205) | 735 (0, 1470) | 630 (0, 1620) |
| Self-reported health | ||||
| Poor–Fair | 238 (12.9) | 91 (3.7) | 18 (2.2) | 235 (15.5) |
| Good | 775 (44.4) | 628 (25.2) | 522 (66.8) | 727 (52.0) |
| Very Good | 537 (30.8) | 1099 (44.1) | 211 (27.0) | 314 (22.5) |
| Excellent | 194 (11.1) | 677 (27.1) | 30 (3.8) | 122 (8.7) |
| Nutritional vitamin D, μg/day | 3.4 (2.0, 5.2) | 3.7 (2.3, 5.8) | 2.6 (1.5, 4.1) | 3.8 (2.2, 5.6) |
| BMI, kg/m2 | 30.1 (+5.8) | 27.7 (+5.0) | 24.0 (+3.3) | 29.5 (+5.1) |
| eGFR, mL/min/1.73m2 | 86.3 (+18.6) | 80.8 (+16.2) | 87.6 (+18.0) | 85.8 (+17.9) |
| Diabetes | 307 (17.5) | 145 (5.8) | 103 (13.1) | 246 (17.4) |
Continuous variables are presented as mean (±SD) or median (IQR) and categorical variables as n (%).
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; MET, metabolic equivalent of task.
Race/ethnicity and vitamin D metabolite concentrations
All examined vitamin D metabolites except serum phosphorus concentration, VDBG, and albumin exhibited statistically significant unadjusted and adjusted heterogeneity by race/ethnicity (P < .01) (Table 2). White participants had the highest mean circulating 25(OH)D concentration and lowest mean 1,25(OH)2D concentration (adjusted differences in 25(OH)D and 1,25(OH)2D concentrations comparing White with Black participants: 8.8 ng/mL, 95% CI (8.2, 9.5) and –4.9 pg/mL, 95% CI (–7.3, –2.5), respectively). PTH concentration was highest among Black participants and lowest among Chinese participants (adjusted difference in PTH concentration comparing Chinese with Black participants: –7.4 pg/mL, 95% CI: (–9.5, –5.3), P < .01). FGF-23 concentration was highest among White participants and lowest among Hispanic participants (39.2 pg/mL vs 36.0 pg/mL).
Table 2.
Vitamin D metabolism measures by race/ethnicity
| Black | White | Chinese | Hispanic | |
|---|---|---|---|---|
| Total 25(OH)D, ng/mL | 19.3 (+9.7) | 30.3 (+11.1) | 26.5 (+8.7) | 24.8 (+10.1) |
| Adjusteda difference | 0.0 (ref) | 8.8 (8.2, 9.5) | 3.8 (2.8, 4.7) | 4.3 (3.5, 5.1) |
| 25(OH)D3, ng/mL | 16.9 (+8.6) | 27.0 (+11.3) | 22.5 (+8.4) | 22.5 (+9.5) |
| Adjusteda difference | 0.0 (ref) | 8.1 (7.4, 8.8) | 3.1 (2.0, 4.1) | 4.4 (3.6, 5.3) |
| Bioavailable 25(OH)Db, ng/mL | 2.5 (+1.4) | 5.6 (+2.5) | 4.5 (+1.9) | 4.1 (+1.9) |
| Adjusteda difference | 0.0 (ref) | 2.6 (2.3, 3.0) | 1.0 (0.5, 1.6) | 1.4 (1.0, 1.8) |
| Bioavailable 25(OH)Dc, ng/mL | 2.4 (+2.0) | 5.3 (+2.9) | 4.8 (+2.1) | 3.9 (+2.3) |
| Adjusteda difference | 0.0 (ref) | 2.9 (2.4, 3.4) | 2.4 (1.7, 3.1) | 1.5 (0.9, 2.1) |
| Total 1,25(OH)2D, pg/mL | 53.2 (+15.6) | 45.5 (+14.4) | 49.8 (+15.3) | 51.3 (+16.3) |
| Adjusteda difference | 0.0 (ref) | –4.9 (–7.3, –2.5) | –2.6 (–6.2, 1.0) | –1.9 (–4.8, 0.9) |
| Bioavailable 1,25(OH)2D, pg/mL | 12.0 (+5.6) | 15.8 (+6.3) | 14.5 (+7.7) | 15.7 (+7.2) |
| Adjusteda difference | 0.0 (ref) | 2.0 (0.4, 3.5) | 4.6 (3.6, 5.7) | 3.5 (2.2, 4.7) |
| VDBG, μg/mL | 248.8 (+37.1) | 251.8 (+40.3) | 236.3 (+29.9) | 253.0 (+37.6) |
| Adjusteda difference | 0.0 (ref) | –1.1 (–7.6, 5.3) | –9.7 (–19.2, –0.2) | 2.1 (–5.5, 9.6) |
| 24,25(OH)2D, mg/dL | 1.0 (0.6, 1.6) | 2.1 (1.3, 3) | 1.7 (1.1, 2.3) | 1.6 (1.0, 2.5) |
| Adjusteda difference | 0.0 (ref) | 0.8 (0.7, 0.9) | 0.3 (0.1, 0.4) | 0.5 (0.4, 0.5) |
| Calcium, mg/dL | 9.7 (+0.4) | 9.7 (+0.4) | 9.5 (+0.3) | 9.7 (+0.4) |
| Adjusteda difference | 0.0 (ref) | –0.03 (–0.06, 0.00) | –0.22 (–0.26, –0.18) | –0.09 (–0.13, –0.06) |
| Phosphorus, mg/dL | 3.6 (+0.5) | 3.7 (+0.5) | 3.7 (+0.5) | 3.7 (+0.5) |
| Adjusteda difference | 0.0 (ref) | 0.02 (–0.01, 0.05) | 0.02 (–0.03,0.07) | 0.01 (–0.03, 0.05) |
| Albumin, g/dL | 4.1 (+0.3) | 4.1 (+0.3) | 4.2 (+0.2) | 4.2 (+0.2) |
| Adjusteda difference | 0.0 (ref) | 0.02 (–0.02, 0.06) | 0.07 (0.01, 0.14) | 0.04 (–0.01,0.09) |
| Fibroblast growth factor-23, pg/mL | 37.0 (29.6, 45.3) | 39.2 (31.7, 48.4) | 37.4 (30.7, 46.1) | 36.0 (29.1, 44.0) |
| Adjusteda difference | 0.0 (ref) | 1.1 (–0.2, 2.3) | 2.2 (0.4, 4.0) | –1.4 (–2.9, 0.1) |
| Parathyroid hormone, pg/mL | 46.8 (35.4, 60.8) | 37.5 (29.4, 48.6) | 35.8 (28.2, 45.4) | 42.5 (32.9, 56.2) |
| Adjusteda difference | 0.0 (ref) | –8.4 (–9.8, –7.0) | –7.4 (–9.5, –5.3) | –2.9 (–4.6, –1.2) |
| 24,25(OH)2D3/25(OH)D3 | 7.0 (+2.2) | 8.1 (+2.1) | 7.7 (+2.0) | 7.6 (+2.1) |
| Adjusteda difference | 0.0 (ref) | 0.9 (0.8, 1.1) | 0.4 (0.2, 0.6) | 0.6 (0.5, 0.8) |
| 1,25(OH)2D/25(OH)D | 4.1 (2.5, 5.7) | 2.0 (1.5, 2.8) | 2.6 (1.8, 3.7) | 2.7 (2.1, 4.1) |
| Adjusteda difference | 0.0 (ref) | –1.5 (–1.7, –1.2) | –1.1 (–1.5, –0.7) | –0.9 (–1.3, –0.6) |
| Urine calcium, mg/day | 0.06 (0.03, 0.09) | 0.08 (0.05, 0.13) | 0.11 (0.07, 0.16) | 0.08 (0.05, 0.13) |
| Adjusteda difference | 0.0 (ref) | 0.03 (0.03, 0.04) | 0.05 (0.04, 0.06) | 0.03 (0.02, 0.04) |
| Urine phosphorus, mg/day | 0.42 (+0.18) | 0.48 (+0.19) | 0.50 (+0.19) | 0.46 (+0.19) |
| Adjusteda difference | 0.0 (ref) | 0.08 (0.06, 0.09) | 0.10 (0.08, 0.12) | 0.05 (0.03, 0.06) |
| Fractional excretion of phosphorus | 11.9 (+5.2) | 12.8 (+5.4) | 12.1 (+4.5) | 11.4 (+5.3) |
| Adjusteda difference | 0.0 (ref) | 0.3 (0.0, 0.7) | 0.9 (0.4,1.4) | –0.2 (–0.7, 0.2) |
| VDBG haplotype | ||||
| Gc1f/Gc1f | 82 (56.6) | 14 (3.8) | 26 (32.5) | 31 (18.3) |
| Gc1f/Gc1s | 33 (22.8) | 70 (19.2) | 19 (23.8) | 39 (23.1) |
| Gc1s/Gc1s | 7 (4.8) | 153 (41.9) | 6 (7.5) | 52 (30.8) |
| Gc2/Gc1f | 10 (6.9) | 28 (7.7) | 12 (15.0) | 17 (10.1) |
| Gc2/Gc1s | 9 (6.2) | 76 (20.8) | 10 (12.5) | 23 (13.6) |
| Gc2/Gc2 | 4 (2.8) | 24 (6.6) | 7 (8.8) | 7 (4.1) |
Continuous variables are presented as mean (±SD) or median (25th, 50th percentile) and categorical variables as n (%). P for heterogeneity <.01 for all variables except phosphorus, (P = .36), vitamin D binding globulin (P = .08) and albumin (P = .11).
Abbreviation: VDBG, vitamin D binding globulin
a Adjusted difference in circulating metabolite concentration, relative to level in Black participants estimated from linear regression model which included the following baseline covariates: age, sex, educational attainment, family income, study site, blood draw season, self-reported general health, intentional exercise, body mass index, diabetes status, estimated glomerular filtration rate, and nutritional vitamin D intake.
b Bioavailable 25(OH)D estimated from published equations with 6 affinity coefficients based on VDBG isoforms (55-57).
c Bioavailable 25(OH)D estimated equation with a single binding coefficient.
Circulating bioavailable 25(OH)D, 1,25(OH)2D, and 24,25(OH)2D3 concentrations were higher in White than in Black participants, with intermediate concentrations observed in Chinese and Hispanic participants. The ratio of serum 24,25(OH)2D3 to 25(OH)D3) was highest and the ratio of 1,25(OH)2D to 25(OH)D was lowest in White participants.
While VDBG and albumin concentrations did not vary materially by race or ethnicity, the measured VDBG isoform was significantly different. The most common haplotype among White and Hispanic participants was Gc1s/Gc1s (47% and 33%, respectively) and among black and Chinese participants was Gc1f/Gc1f (57% and 33%, respectively).
Genetic ancestry and vitamin D metabolite concentrations
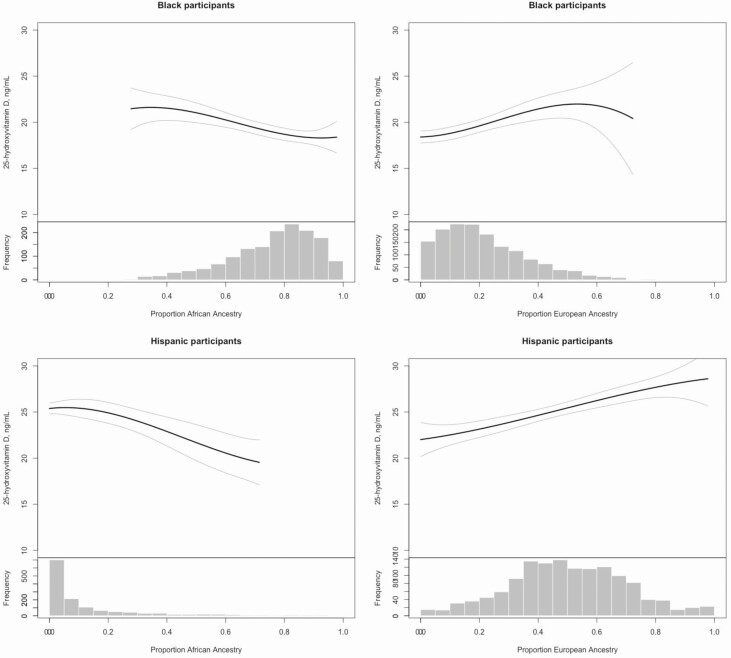
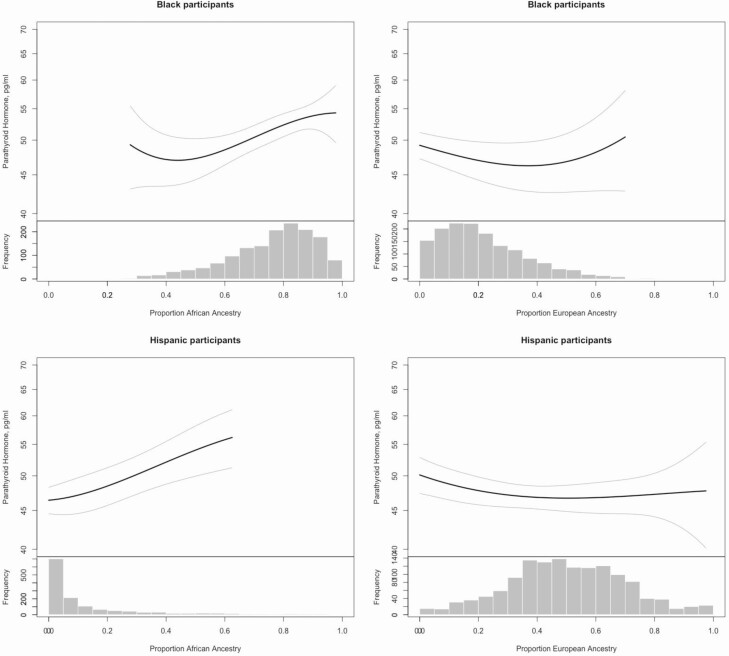
Among Black participants, higher percent African ancestry was associated with lower bioavailable and circulating 25(OH)D concentrations (Fig. 1), lower 24,25(OH)2D3 concentrations and urine calcium clearance (Table 3), and higher PTH concentration (Fig. 2). VDBG concentration was not associated with percent African ancestry, but VDBG haplotype was, with a higher prevalence of the Gc1f/Gc1f isoform among Black participants with higher proportion of African ancestry.
Figure 1.
Race/ethnicity-specific associations of proportion African and European ancestry with 25-hydroxyvitamin D.
Table 3.
Associations of percent African ancestry with measures of vitamin D metabolism among Black participants in the Multi-Ethnic Study of Atherosclerosis
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P valuea | |
|---|---|---|---|---|---|
| Percent African ancestry, % | 16.6–67.6 | 67.7–79.3 | 79.4–87.5 | 87.6–98.7 | |
| Total 25(OH)D, ng/mL | 21.4 (+9.6) | 18.5 (+9.0) | 19.1 (+10.5) | 18.0 (+9.5) | <.001 |
| 25(OH)D3, ng/mL | 18.6 (+8.5) | 16.3 (+8.3) | 16.6 (+8.8) | 15.8 (+8.5) | <.001 |
| Bioavailable 25(OH)Db, ng/mL | 3.1 (+1.7) | 2.6 (+1.3) | 2.4 (+1.4) | 1.9 (+0.8) | .003 |
| Bioavailable 25(OH)Dc, ng/mL | 2.9 (+2.0) | 2.5 (+1.7) | 2.5 (+2.3) | 1.9 (+1.8) | .117 |
| Total 1,25(OH)2D, pg/mL | 50.8 (+15.8) | 50.5 (+17.8) | 56.4 (+12.6) | 53.4 (+15.2) | .184 |
| Bioavailable 1,25(OH)2D, pg/mL | 12.8 (+4.7) | 12.1 (+6.7) | 11.8 (+4.6) | 11.0 (+5.4) | .167 |
| VDBG, μg/mL | 252.8 (+38.5) | 248.8 (+35.3) | 245.7 (+40.1) | 245.5 (+36.6) | .797 |
| 24,25(OH)2D3, mg/dL | 1.2 (0.7, 1.95) | 1 (0.55, 1.6) | 1 (0.6, 1.65) | .9 (0.55, 1.45) | <.001 |
| Calcium, mg/dL | 9.7 (+0.4) | 9.7 (+0.4) | 9.7 (+0.4) | 9.7 (+0.4) | .732 |
| Phosphorus, mg/dL | 3.7 (+0.5) | 3.6 (+0.5) | 3.6 (+0.5) | 3.7 (+0.5) | .373 |
| Albumin, g/dL | 4.1 (+0.3) | 4.0 (+0.2) | 4.0 (+0.2) | 4.1 (+0.3) | .360 |
| Fibroblast growth factor-23, pg/ml | 37.7 (29.5,44.8) | 36.7 (29.3, 45) | 36.1 (29.2, 45) | 37.1 (30.2, 46.7) | .347 |
| Parathyroid hormone, pg/mL | 45.2 (33.9, 58) | 46.6 (36.4, 60.3) | 44.7 (34.5, 61.3) | 49.6 (36.5, 62.8) | .008 |
| 24,25(OH)D3/25(OH)D3 | 7.2 (+2.2) | 6.9 (+2.1) | 6.9 (+2.1) | 6.9 (+2.3) | .095 |
| 1,25(OH)D/25(OH)D | 3.2 (2, 4.9) | 3.3 (2.2, 5.2) | 4.3 (3, 6.7) | 4.2 (3.2, 6.3) | .059 |
| Urine calcium/creatinine (mg/g) | 0.07 (0.04, 0.11) | 0.06 (0.03, 0.09) | 0.06 (0.03, 0.09) | 0.05 (0.03, 0.09) | <.001 |
| Urine Phosphorus/Creatinine (mg/g) | 0.48 (+0.19) | 0.50 (+0.19) | 0.42 (+0.18) | 0.46 (+0.19) | .200 |
| Fractional excretion of phosphorus | 11.8 (+5.0) | 11.9 (+5.0) | 12.0 (+5.1) | 12.3 (+6.0) | .261 |
| VDBG haplotype | <.001 | ||||
| Gc1f/Gc1f | 12 (35.3) | 15 (51.7) | 15 (60.0) | 33 (76.7) | |
| Gc1f/Gc1s | 10 (29.4) | 5 (17.2) | 8 (32.0) | 5 (11.6) | |
| Gc1s/Gc1s | 3 (8.8) | 2 (6.9) | 0 (0.0) | 2 (4.7) | |
| Gc2/Gc1f | 3 (8.8) | 3 (10.3) | 1 (4.0) | 2 (4.7) | |
| Gc2/Gc1s | 4 (11.8) | 4 (13.8) | 1 (4.0) | 0 (0.0) | |
| Gc2/Gc2 | 2 (5.9) | 0 (0.0) | 0 (0.0) | 1 (2.3) |
Continuous variables are presented as mean (±SD) or median (25th, 50th percentile) and categorical variables as n (%).
Abbreviation: VDBG, vitamin D binding globulin.
a P value is from Wald test on percent African ancestry modeled continuously and log-transformed.
b Bioavailable 25(OH)D estimated from published equations with 6 affinity coefficients based on VDBG isoforms (55-57).
c Bioavailable 25(OH)D estimated from equation with a single binding coefficient.
Figure 2.
Race/ethnicity-specific associations of proportion African and European ancestry with parathyroid hormone.
Among Hispanic participants, higher percent African ancestry was associated with lower circulating 25(OH)D concentration (Fig. 1) and 24,25(OH)2D3 concentration, lower urine calcium and phosphorus clearance (see supplementary material located in a digital research materials repository (62)) and higher PTH concentration (Fig. 2). Again, VDBG concentration was not associated with percent African ancestry, but there was a higher prevalence of the Gc1f/Gc1f isoform among Hispanic participants with higher proportion of African ancestry.
Latent class analysis
Of 2 latent classes identified, class 1 was defined by lower concentrations of 25(OH)D, 24,25(OH)2D3, calcium, and phosphorus, and higher PTH concentration and lower urine calcium clearance (Table 4) than class 2. Self-reported Black or Hispanic race/ethnicity was more common in class 1, while White and Chinese race/ethnicity was more common in class 2. Among both Black and Hispanic participants, proportion African ancestry was higher and proportion European ancestry lower in class 1.
Table 4.
Association between mineral metabolite latent class, race/ethnicity, and African ancestry
| Characteristic | Class 1 | Class 2 | P valueb |
|---|---|---|---|
| N | 2735 | 3730 | |
| Mineral metabolite | |||
| Total 25(OH)D, ng/mL | 16.34 (15.99, 16.70) | 32.69 (32.27, 32.95) | <.001 |
| 24,25(OH)2D3, mg/dL | 0.81 (0.79, 0.83) | 2.35 (2.30, 2.40) | <.001 |
| Calcium, mg/dL | 9.61 (9.59, 9.62) | 9.68 (9.67, 9.70) | <.001 |
| Phosphorus, mg/dL | 3.64 (3.62, 3.66) | 3.69 (3.67, 3.71) | <.001 |
| Fibroblast growth factor-23, pg/mL | 36.3 (35.8, 36.8) | 38.7 (38.3, 39.2) | <.001 |
| Parathyroid hormone, pg/mL | 49.23 (48.5, 50.1) | 35.7 (35.2, 36.2) | <.001 |
| Fractional excretion of phosphorus | 12.1 (11.8, 12.3) | 12.2 (12.1, 12.4) | .276 |
| Urine phosphorus/creatinine (mg/g) | 0.45 (0.44, 0.46) | 0.47 (0.46, 0.48) | <.001 |
| Urine calcium/creatinine (mg/g) | 0.06 (0.06, 0.07) | 0.08 (0.08, 0.09) | <.001 |
| Additional vitamin D metabolites a | |||
| Bioavailable 25(OH)Dc, ng/mL | 2.83 (1.44) | 5.70 (2.30) | <.001 |
| Bioavailable 25(OH)Dd, ng/mL | 2.02 (1.44) | 5.94 (2.35) | <.001 |
| Total 1,25(OH)2D, pg/mL | 50.5 (15.9) | 47.5 (15.1) | .158 |
| Bioavailable 1,25(OH)2D, pg/mL | 14.8 (6.9) | 15.0 (6.6) | .160 |
| Vitamin D binding globulin, μg/mL | 243.2 (34.6) | 254.3 (40.1) | <.001 |
| 24,25(OH)D3/25(OH)D3 | 6.29 (1.59) | 8.57 (1.84) | <.001 |
| 1,25(OH)D/25(OH)D | 4.2 (3.0, 5.7) | 2.0 (1.4, 2.5) | <.001 |
| Albumin, g/dL | 4.1 (0.27) | 4.1 (0.26) | .935 |
| Race/ethnicity | <.001 | ||
| White | 644 (23.5) | 1863 (49.9) | |
| Chinese | 252 (9.2) | 536 (14.4) | |
| Black | 1215 (44.4) | 544 (14.6) | |
| Hispanic | 624 (22.8) | 787 (21.1) | |
| Proportion African ancestry | |||
| Among Black participants | 77.2 (14.3) | 73.3 (15.5) | <.001 |
| Among Hispanic participants | 15.0 (21.2) | 11.5 (16.4) | .023 |
| Proportion European ancestry | |||
| Among Black participants | 20.4 (14.0) | 24.3 (15.4) | <.001 |
| Among Hispanic participants | 47.8 (19.9) | 52.1 (19.6) | .013 |
Variables included in the latent class analysis are presented as mean (95% CI). Other continuous variables are presented as mean (SD) or median (25th, 50th percentile) and categorical variables as n (%).
Abbreviation: VDBG, vitamin D binding globulin.
a These metabolites were not included in the latent class analysis model as they were only available in a subcohort of participants.
b P value is from Wald test of characteristic from logistic regression on class, adjusted for age, sex, educational attainment, family income, self-reported general health, study site, blood draw season, intentional exercise, body mass index, diabetes status, estimated glomerular filtration rate, and nutritional vitamin D intake.
c Bioavailable 25(OH)D estimated from published equations with six affinity coefficients based on VDBG isoforms (55-57).
d Bioavailable 25(OH)D estimated from equation with a single binding coefficient.
Discussion
In this large, community-based, multi-ethnic cohort of adults, we comprehensively evaluated differences in vitamin D metabolism markers across self-described racial/ethnic groups and genetic ancestry. Compared with Black participants, White participants had higher circulating 25(OH)D concentration with lower PTH and 1,25(OH)2D concentrations; circulating vitamin D metabolite ratios suggesting lower CYP27B1 and higher CYP24A1 activity; higher urinary concentrations of calcium and phosphorus with higher urinary fractional excretion of phosphorus; differences in VDBG haplotypes but not concentrations; and higher FGF-23 concentrations. Empirically derived subgroups of vitamin D metabolism markers differed significantly by race/ethnicity. Associations of ancestry with 25(OH)D and PTH concentrations among Black and Hispanic participants suggested a genetic component to differences by self-reported race/ethnicity. Chinese and Hispanic participants had higher 25(OH)D and lower PTH concentrations than Black participants, among other differences.
Compared with Black individuals, White individuals have higher 25(OH)D and lower PTH concentrations, a phenotype that maintains similar serum calcium concentrations but may contribute to higher risks of kidney stones and osteoporosis. Support for this concept can be found as far back as 1985, when Bell et al. reported lower PTH and 1,25(OH)2D concentrations with higher urinary calcium excretion in White relative to Black participants (63). Our results confirm and extend those of similar studies (64, 65) by showing a comparable pattern of racial differences using reliable mass spectroscopic measurements of vitamin D metabolites, expanded to include calculated functional estimates of CYP activity, ancestral analysis, and VDBG. Latent class analysis supports the concept that this broad pattern of vitamin D metabolism differs both by self-reported race/ethnicity and genetic ancestry.
PTH induces CYP27B1 and inhibits CYP24A1 activity, while FGF-23 mediates the opposite effects. Lower PTH and higher FGF-23 concentrations, in combination with lower 1,25(OH)2D to 25(OH)D and higher 24,25(OH)2D3 to 25(OH)D3 ratios suggest reduced CYP27B1 and increased CYP24A1 activities in White relative to Black participants. Higher urinary calcium and phosphorous concentrations, and higher urinary fractional excretion of phosphorus in White relative to Black participants in the face of similar serum calcium and phosphorous concentrations support the concept of vitamin D metabolism phenotypes that are different yet maintain mineral homeostasis.
Only a few previous studies have explored associations of African ancestry with vitamin D measures. Two reported an inverse association between 25(OH)D concentration and African ancestry, a finding we confirm (35, 36). In another, greater African ancestry was independently associated with lower urinary fractional excretion of phosphorus, which we observed among Hispanic participants (37).
As previously reported, VDBG concentrations measured by mass spectrometry did not vary by self-reported race (54). Here, we extend this finding by confirming that VDBG concentrations are not associated with percent African ancestry and hence do not underlie racial or ethnic heterogeneity in circulating 25(OH)D. On the other hand, bioavailable 25(OH)D was strongly associated with race and percent African ancestry. These concentrations were calculated using published equations which incorporate the VDBG isoforms, which vary substantially by race and genetic ancestry (55-57). Whether measured free circulating 25(OH)D varies by race/ethnicity and genetic ancestry remains to be determined.
Our findings may partly explain observed racial/ethnic differences with respect to health outcomes. First, lower circulating PTH concentration and higher urinary calcium excretion relative to Black individuals likely contribute to a higher incidence of kidney stones in White individuals, as the majority of kidney stones contain calcium and hypercalciuria is an established risk factor (11). Second, despite lower concentrations of 25(OH)D, Black individuals have higher bone mineral density, favorable bone microarchitecture in histomorphometry studies, and lower fracture rates than White populations (12, 66, 67). Higher CYP27B1 activity leading to higher 1,25(OH)2D concentration and lower urinary calcium excretion are likely physiologic differences that allow Black individuals to maintain calcium homeostasis and contribute to stronger bones relative to White individuals for a given 25(OH)D concentration. Though speculative, differences in vitamin D metabolism reflected through these markers may be sufficient for optimal bone health among healthy Black individuals, and may explain why fracture rates do not increase with lower 25(OH)D concentrations, nor improve with vitamin D supplementation in this population (13, 68, 69). Our findings also highlight the limitations of using a universal serum 25(OH)D threshold to define vitamin D deficiency (70), which many clinicians currently use to guide vitamin D treatment.
Chinese and Hispanic participants had 25(OH)D concentrations higher than Black participants but lower than White participants. Interestingly, the adjusted differences in other vitamin D metabolism markers in Chinese and Hispanic relative to Black participants were largely similar in direction to that of White participants: lower PTH concentrations, lower 1,25(OH)2D to 25(OH)D ratio, and higher 24,25(OH)2D3 to 25(OH)D3 ratio. Although statistical significance varied depending on the marker, the similarity in the patterns of difference among Chinese, Hispanic, and White compared with Black participants suggest similar mechanisms that mitigate high 25(OH)D may exist across racial/ethnic groups. Our study shows associations between African ancestry with 25(OH)D, PTH and urinary fractional excretion of phosphorus in a Hispanic population, noting that additional heterogeneity may be present within Hispanic participants owing to their cultural and genetic diversity (42).
Strengths of our study include the use of a large, community-based, multi-ethnic population; comprehensive evaluation of vitamin D/mineral metabolism using precise and reliable measurements; and the investigation of genetic contributions to racial/ethnic differences using large number of AIMs. This study also has limitations. Causal inferences between race/ethnicity and vitamin D metabolism markers are limited in observational studies. Next, our use of vitamin D metabolic ratios may not reflect true enzymatic activity. In addition, it is currently not possible to directly measure bioavailable 25(OH)D. As such, we used estimation equations for the calculation of bioavailable 25(OH)D, which include terms for circulating levels of VDBG, albumin, and participant VDBG haplotypes as estimates of 25(OH)D binding affinities. These equations are based on the free-hormone hypothesis (ie, those used to calculate free testosterone) and there is debate regarding whether they are applicable to vitamin D and whether these equations should account for genotypic differences in VDBG. We repeated our analyses using the estimation equations which do not account for VDBG haplotype and found similar results (71, 72). While free 25(OH)D can be directly measured (DIAsource, Louvain-la-Neuve, Belgium) it is present at a very low concentrations, and at this time, the assay requires validation and recent evidence suggests that these may be racial differences in the correlation between directly measured and calculated free 25(OH)D (73, 74). Additionally, genetic ancestry may influence environmental determinants of vitamin D metabolism, thus weakening our conclusion that genetic variation accounts for racial/ethnic differences in vitamin D metabolism. Next, without clinical outcomes we cannot conclude whether the racial differences we describe are beneficial or harmful, or to what extent modifying the observed abnormalities affects health. Lastly, there could be regional differences in admixture between the 6 recruitment communities or interactions between region and race/ethnicity that are not adequately captured.
In summary, we describe significant differences in a comprehensive panel of vitamin D/mineral metabolism markers across racial/ethnic groups and show they may have a genetic basis. These differences likely serve to maintain bone and mineral homeostasis across ranges of 25(OH)D production and may explain racial/ethnic differences in the risk and prevalence of vitamin D–related diseases. This study underscores the importance of future admixture studies that can identify genetic contributions to outcomes such as fractures and locate causal/risk alleles.
Acknowledgments
The authors thank the MESA investigators, staff, and participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Financial Support: This research was supported by grants N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, 75N92020D00001, 75N92020D00002, 75N92020D00003, 75N92020D00004, 75N92020D00005, 75N92020D00006, 75N92020D00007, HHSN268201500003I, and R01HL096875 from the National Heart, Lung, and Blood Institute, grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS), grants R01DK088762, R01DK099199, and K01DK109019 from the National Institute of Diabetes and Digestive and Kidney Diseases, and grant 2T32DK007467-36.
Glossary
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 24,25(OH)2D
24,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- AIM
ancestry informative marker
- BMI
body mass index
- FGF
fibroblast growth factor
- LCA
latent class analysis
- PTH
parathyroid hormone
- SNP
single-nucleotide polymorphism
- VDBG
vitamin D binding globulin
- VDR
vitamin D receptor
Additional Information
Disclosure Summary: Dr. Gutierrez has received grant funding and consulting fees from Akebia Therapeutics; grant funding and consulting fees from Amgen; grant funding from GSK; and consulting fees from QED Therapeutics. Dr. Peralta is chief medical officer at Cricket Health, Inc.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available to investigators and other applicants according to established MESA policies through the MESA database/data coordinating center
References
- 1. Gunta SS, Thadhani RI, Mak RH. The effect of vitamin D status on risk factors for cardiovascular disease. Nat Rev Nephrol. 2013;9(6):337-347. [DOI] [PubMed] [Google Scholar]
- 2. Smith ER. Vascular calcification in uremia: new-age concepts about an old-age problem. Methods Mol Biol. 2016;1397:175-208. [DOI] [PubMed] [Google Scholar]
- 3. Moe SM, Duan D, Doehle BP, O’Neill KD, Chen NX. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63(3):1003-1011. [DOI] [PubMed] [Google Scholar]
- 4. Fox CS, Larson MG, Vasan RS, et al. Cross-sectional association of kidney function with valvular and annular calcification: the Framingham heart study. J Am Soc Nephrol. 2006;17(2):521-527. [DOI] [PubMed] [Google Scholar]
- 5. Bansal N, Zelnick L, Robinson-Cohen C, et al. Serum parathyroid hormone and 25-hydroxyvitamin D concentrations and risk of incident heart failure: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3(6):e001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Boer IH, Levin G, Robinson-Cohen C, et al. Serum 25-hydroxyvitamin D concentration and risk for major clinical disease events in a community-based population of older adults: a cohort study. Ann Intern Med. 2012;156(9):627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kestenbaum B, Sachs MC, Hoofnagle AN, et al. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014;7(3):409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson-Cohen C, Katz R, Hoofnagle AN, et al. Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab. 2011;96(7):2186-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Ballegooijen AJ, Robinson-Cohen C, Katz R, et al. Vitamin D metabolites and bone mineral density: the multi-ethnic study of atherosclerosis. Bone. 2015;78:186-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ebeling PR, Adler RA, Jones G, et al. Management of endocrine disease: therapeutics of vitamin D. Eur J Endocrinol. 2018;179(5):R239-R259. [DOI] [PubMed] [Google Scholar]
- 11. Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project . Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jorgetti V, dos Reis LM, Ott SM. Ethnic differences in bone and mineral metabolism in healthy people and patients with CKD. Kidney Int. 2014;85(6):1283-1289. [DOI] [PubMed] [Google Scholar]
- 13. Cauley JA, Danielson ME, Boudreau R, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women’s Health Initiative (WHI). J Bone Miner Res. 2011;26(10):2378-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuleihan GE, Gundberg CM, Gleason R, et al. Racial differences in parathyroid hormone dynamics. J Clin Endocrinol Metab. 1994;79(6):1642-1647. [DOI] [PubMed] [Google Scholar]
- 15. Laster M, Soohoo M, Streja E, et al. Racial-ethnic differences in chronic kidney disease-mineral bone disorder in youth on dialysis. Pediatr Nephrol. 2019;34(1):107-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scragg R, Sowers M, Bell C; Third National Health and Nutrition Examination Survey . Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813-2818. [DOI] [PubMed] [Google Scholar]
- 20. Michos ED, Reis JP, Post WS, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: the NHANES-III linked mortality files. Nutrition. 2012;28(4):367-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Boer IH, Gorodetskaya I, Young B, Hsu CY, Chertow GM. The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol. 2002;13(11):2762-2769. [DOI] [PubMed] [Google Scholar]
- 22. de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis. 2007;50(1):69-77. [DOI] [PubMed] [Google Scholar]
- 23. Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954-959. [DOI] [PubMed] [Google Scholar]
- 24. Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61(5):969-975. [DOI] [PubMed] [Google Scholar]
- 25. Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31-38. [DOI] [PubMed] [Google Scholar]
- 26. Bosworth C, de Boer IH. Impaired vitamin D metabolism in CKD. Semin Nephrol. 2013;33(2):158-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka Y, Deluca HF. Role of 1,25-dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc Natl Acad Sci U S A. 1974;71(4):1040-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rizzoli R, Fleisch H, Bonjour JP. Role of 1,25-dihydroxyvitamin D3 on intestinal phosphate absorption in rats with a normal vitamin D supply. J Clin Invest. 1977;60(3):639-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christakos S, Dhawan P, Porta A, Mady LJ, Seth T. Vitamin D and intestinal calcium absorption. Mol Cell Endocrinol. 2011;347(1-2):25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205-2215. [DOI] [PubMed] [Google Scholar]
- 31. Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44(2):250-256. [DOI] [PubMed] [Google Scholar]
- 33. Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272-2279. [DOI] [PubMed] [Google Scholar]
- 34. Rotimi CN, Jorde LB. Ancestry and disease in the age of genomic medicine. N Engl J Med. 2010;363(16):1551-1558. [DOI] [PubMed] [Google Scholar]
- 35. Signorello LB, Williams SM, Zheng W, et al. Blood vitamin d levels in relation to genetic estimation of African ancestry. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2325-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haddad SA, Ruiz-Narváez EA, Cozier YC, Gerlovin H, Rosenberg L, Palmer JR. Association of degree of European genetic ancestry with serum vitamin D levels in African Americans. Am J Epidemiol. 2018;187(7):1420-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gutiérrez OM, Parsa A, Isakova T, et al. Genetic African ancestry and markers of mineral metabolism in CKD. Clin J Am Soc Nephrol. 2016;11(4):653-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881. [DOI] [PubMed] [Google Scholar]
- 39. International Advertising Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Suppl 1):S37-S42. [DOI] [PubMed] [Google Scholar]
- 40. Inker LA, Schmid CH, Tighiouart H, et al. ; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Divers J, Redden DT, Rice KM, et al. Comparing self-reported ethnicity to genetic background measures in the context of the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Genet. 2011;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manichaikul A, Palmas W, Rodriguez CJ, et al. Population structure of Hispanics in the United States: the multi-ethnic study of atherosclerosis. PLoS Genet. 2012;8(4):e1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoggart CJ, Shriver MD, Kittles RA, Clayton DG, McKeigue PM. Design and analysis of admixture mapping studies. Am J Hum Genet. 2004;74(5):965-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97(6):1243-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laha TJ, Strathmann FG, Wang Z, de Boer IH, Thummel KE, Hoofnagle AN. Characterizing antibody cross-reactivity for immunoaffinity purification of analytes prior to multiplexed liquid chromatography-tandem mass spectrometry. Clin Chem. 2012;58(12):1711-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(9):1279-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Boer IH, Sachs MC, Chonchol M, et al. Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: a participant-level analysis of 5 cohort studies and clinical trials. Am J Kidney Dis. 2014;64(2):187-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88(2):511S-512S. [DOI] [PubMed] [Google Scholar]
- 49. Batacchi Z, Robinson-Cohen C, Hoofnagle AN, et al. Effects of Vitamin D2 supplementation on Vitamin D3 metabolism in health and CKD. Clin J Am Soc Nephrol. 2017;12(9):1498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bosworth C, Sachs MC, Duprez D, et al. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin Endocrinol (Oxf). 2013;79(3):429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Imel EA, Peacock M, Pitukcheewanont P, et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91(6):2055-2061. [DOI] [PubMed] [Google Scholar]
- 52. Henderson CM, Lutsey PL, Misialek JR, et al. Measurement by a Novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D-binding protein in blacks and whites. Clin Chem. 2016;62(1):179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hoofnagle AN, Eckfeldt JH, Lutsey PL. Vitamin D-binding protein concentrations quantified by mass spectrometry. N Engl J Med. 2015;373(15):1480-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robinson-Cohen C, Zelnick LR, Hoofnagle AN, et al. Associations of Vitamin D-binding globulin and bioavailable vitamin D concentrations with coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Endocrinol Metab. 2017;102(8):3075-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bhan I, Powe CE, Berg AH, et al. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82(1):84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almås B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Invest. 2014;74(3):177-183. [DOI] [PubMed] [Google Scholar]
- 58. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666-3672. [DOI] [PubMed] [Google Scholar]
- 59. Linefsky JP, O’Brien KD, Katz R, et al. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study. J Am Coll Cardiol. 2011;58(3):291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227-241. [Google Scholar]
- 61. Rubin DB Multiple Imputation for Nonresponse in Surveys. Hoboken (NJ): John Wiley & Sons; 2004. [Google Scholar]
- 62. Hsu S, Hoofnagle AN, Gupta DK, et al. Data from: Race, ancestry, and Vitamin D metabolism: the Multi-Ethnic Study of Atherosclerosis. Supplementary Table 1. https://github.com/Citizen5010/mesa_race_vitd. Deposited August 4, 2020. [DOI] [PMC free article] [PubMed]
- 63. Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76(2):470-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meier DE, Luckey MM, Wallenstein S, Clemens TL, Orwoll ES, Waslien CI. Calcium, vitamin D, and parathyroid hormone status in young white and black women: association with racial differences in bone mass. J Clin Endocrinol Metab. 1991;72(3):703-710. [DOI] [PubMed] [Google Scholar]
- 65. Bikle DD, Ettinger B, Sidney S, Tekawa IS, Tolan K. Differences in calcium metabolism between black and white men and women. Miner Electrolyte Metab. 1999;25(3):178-184. [DOI] [PubMed] [Google Scholar]
- 66. Schnitzler CM, Mesquita JM. Cortical bone histomorphometry of the iliac crest in normal black and white South African adults. Calcif Tissue Int. 2006;79(6):373-382. [DOI] [PubMed] [Google Scholar]
- 67. Schnitzler CM, Pettifor JM, Mesquita JM, Bird MD, Schnaid E, Smyth AE. Histomorphometry of iliac crest bone in 346 normal black and white South African adults. Bone Miner. 1990;10(3):183-199. [DOI] [PubMed] [Google Scholar]
- 68. Looker AC. Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res. 2013;28(5):997-1006. [DOI] [PubMed] [Google Scholar]
- 69. Aloia JF, Talwar SA, Pollack S, Yeh J. A randomized controlled trial of vitamin D3 supplementation in African American women. Arch Intern Med. 2005;165(14):1618-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National academies collection: reports funded by national institutes of health. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US). National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 71. Bouillon R. Free or total 25OHD as marker for vitamin D status? J Bone Miner Res. 2016;31(6):1124-1127. [DOI] [PubMed] [Google Scholar]
- 72. Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jukic AMZ, Hoofnagle AN, Lutsey PL. Measurement of vitamin D for epidemiologic and clinical research: shining light on a complex decision. Am J Epidemiol. 2018;187(4):879-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Denburg MR, Hoofnagle AN, Sayed S, et al. ; Chronic Renal Insufficiency Cohort study investigators . Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res. 2016;31(6):1128-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available to investigators and other applicants according to established MESA policies through the MESA database/data coordinating center