Abstract
Context
Nonalcoholic fatty liver disease (NAFLD) prevalence is high, especially in patients with obesity and type 2 diabetes, and is expected to rise steeply in the coming decades.
Objective
We estimated NAFLD prevalence in patients with type 1 diabetes and explored associated characteristics and outcomes.
Data Sources
We reviewed PubMed and Embase for studies on NAFLD and type 1 diabetes to March 2020. We screened references of included articles.
Study Selection
Two authors independently screened titles/abstracts. One author screened full text articles. NAFLD was defined as described in the individual studies: steatosis and/or fibrosis. Studies not reporting alternative causes of hepatic steatosis or defining NAFLD only as elevated liver enzymes, were excluded. Initially, 919 articles met the selection criteria.
Data Extraction
One researcher performed data extraction and risk of bias assessment using standardized tables.
Data Synthesis
We assessed pooled prevalence rates by meta-analysis using a random-effects model, subsequently exploring heterogeneity by subgroup-, meta-regression-, and sensitivity analysis. Twenty studies between 2009 and 2019 were included (n = 3901). Pooled NAFLD prevalence was 19.3% (95% CI, 12.3%-27.5%), increasing to 22.0% (95% CI, 13.9%-31.2%) in adults only. Pooled prevalence of ultrasound studies was high (27.1%, 95% CI, 18.7%-36.3%) compared to studies using magnetic resonance imaging (8.6%, 95% CI, 2.1%-18.6%), liver biopsy (19.3%, 95% CI, 10.0%-30.7%), or transient elastography (2.3%, 95% CI, 0.6%-4.8%).
Conclusion
NAFLD prevalence in patients with type 1 diabetes is considerable and is highly dependent on the specific diagnostic modality and NAFLD definition used. These data are helpful in directing actions to standardize NAFLD diagnosis, which will help defining contributing mechanisms and outcomes.
Keywords: nonalcoholic fatty liver disease, NAFLD, NASH, fibrosis, type 1 diabetes mellitus, DM1
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum ranging from the relatively benign isolated hepatic steatosis (HS) to the more harmful problems of nonalcoholic steatohepatitis (NASH), hepatic fibrosis, and cirrhosis. NAFLD, by definition, can be diagnosed only in the absence of other causes of liver disease. For diagnosis, many different modalities are used, ranging from laboratory tests, to imaging, to the gold standard, liver biopsy (1-3).
Over the past years, the clinical and economic burden of NAFLD have become apparent and are expected to rise steeply in the coming decades as a consequence of the increased prevalence and incidence of obesity and type 2 diabetes mellitus (4, 5). Global prevalence rates of NAFLD in the general population are estimated at 25% (6). NASH prevalence in the general population is estimated at 1.5% to 6.45%, and 41% within the NASH group develop fibrosis progression (6). Since 2013, NASH cirrhosis is the second leading etiology for liver transplantation (LT) in the United States and in Europe it is anticipated to become the leading indication for LT within the next decade (7, 8). Moreover, mortality due to liver disease is increased in patients with NAFLD (6, 9). The high NAFLD burden is caused not only by these hepatic complications but also by the associated increased cardiovascular morbidity and mortality in patients with NAFLD (6, 9-12).
NAFLD and type 2 diabetes are closely associated phenomena (9, 13-15). NAFLD may be considered as a hepatic manifestation of metabolic syndrome (15-17). In contrast to the knowledge about NAFLD and type 2 diabetes, there are limited and inconsistent data on NAFLD prevalence in patients with type 1 diabetes mellitus (12). Type 1 diabetes and type 2 diabetes show major pathophysiological differences, but share certain similarities as well. Insulin resistance and systemic hyperinsulinemia are seen both in type 1 diabetes and type 2 diabetes (18, 19). Obesity, a well-known NAFLD risk factor clearly related to type 2 diabetes and insulin resistance, is becoming more prevalent in the type 1 diabetes population (6, 20, 21). Taking into account these similarities and adding the generally long lifetime exposure to type 1 diabetes, the spectrum of NAFLD and its long-term sequelae might be clinically relevant in patients with type 1 diabetes as well.
The aim of the present study is to estimate the prevalence of NAFLD in patients with type 1 diabetes. We performed a systematic review and meta-analysis on the prevalence of NAFLD and distribution of its different stages in patients with type 1 diabetes. Moreover, we explored associated characteristics and outcomes of NAFLD in the type 1 diabetes population.
Materials and Methods
This study was conducted and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (22). The research protocol was registered at the PROSPERO database (CRD42019138757).
Search strategy, selection criteria, and data extraction
We searched PubMed (MEDLINE) and Embase for studies reporting on patients with type 1 diabetes and NAFLD, including synonyms and relevant Medical Subject Headings (MeSH)/Emtree terms. Databases were searched from date of inception to March 2020. We included studies examining patients with type 1 diabetes: children, adolescents, or adults. Studies with or without a control population were both eligible. NAFLD was defined as described and diagnosed in the individual studies. Studies with a diagnosis based solely on abnormal liver tests were excluded. No other restrictions to type of diagnostic method were applied. A description of exclusion of patients with other causes of HS was mandatory. We included cross-sectional and cohort studies published in peer-reviewed journals. Case reports, case series, reviews, posters, abstracts, and descriptive studies were excluded. Language was restricted to English. No limitations to the year of publication were applied. For a detailed description of our search strategy and data extraction plan, we refer to the Supplemental materials (23).
Risk of bias in individual studies
For risk of bias assessment of the individual studies, we used the risk-of-bias-assessment tool in prevalence studies developed by Hoy et al (24, 25). This was performed at the study level, judging several components of the internal and external validity and composing a summary measure, indicating a low, intermediate, or high risk of bias.
Statistical analysis
Statistical analysis was performed using SPSS Statistics version 25.0.0.2 and R version 3.5.1, metafor package. For our meta-analysis, we used double-arcsine transformed proportions for better statistical properties (26). Prevalence estimates and 95% CIs were calculated using the DerSimonian-Laird random-effects method. Pooled prevalence rates and 95% CIs were back-transformed into and reported as the original proportion scale. Statistical heterogeneity was tested by using the Cochran Q and I2 statistic (I2 values below 25% are considered low, 25% to 50% moderate, 50% to 75% substantial, above 75% high). To give an estimate of the overall NAFLD prevalence in type 1 diabetes patients, all studies were included in the primary analysis, irrespective of precise type 1 diabetes study population, definition of NAFLD, and diagnostic method used. Subsequently, to explore clinical and statistical heterogeneity, we performed subgroup and meta-regression analyses to explore moderators of heterogeneity. We estimated NAFLD prevalence of the following groups: (1) children/adolescents and adults, (2) different diagnostic modalities, (3) NAFLD subclasses. For the meta-regression analysis, we needed at least 5 studies per variable tested. We performed univariate meta-regression analysis on mean body mass index (BMI), mean diabetes duration, and mean glycated hemoglobin (HbA1c). We reported parameters of heterogeneity (I2 and adjusted R2), and parameters of association (β coefficient, P value < .05 considered significant). To alternatively explore the association between NAFLD and BMI, diabetes duration, and HbA1c, we performed a meta-analysis, pooling mean differences of these variables between patients without and with NAFLD. To adjust for potential bias within studies, a sensitivity analysis was performed excluding studies with a high risk of bias. The potential for publication bias was explored by a funnel plot and Egger regression test, using double-arcsine transformed proportions.
Results
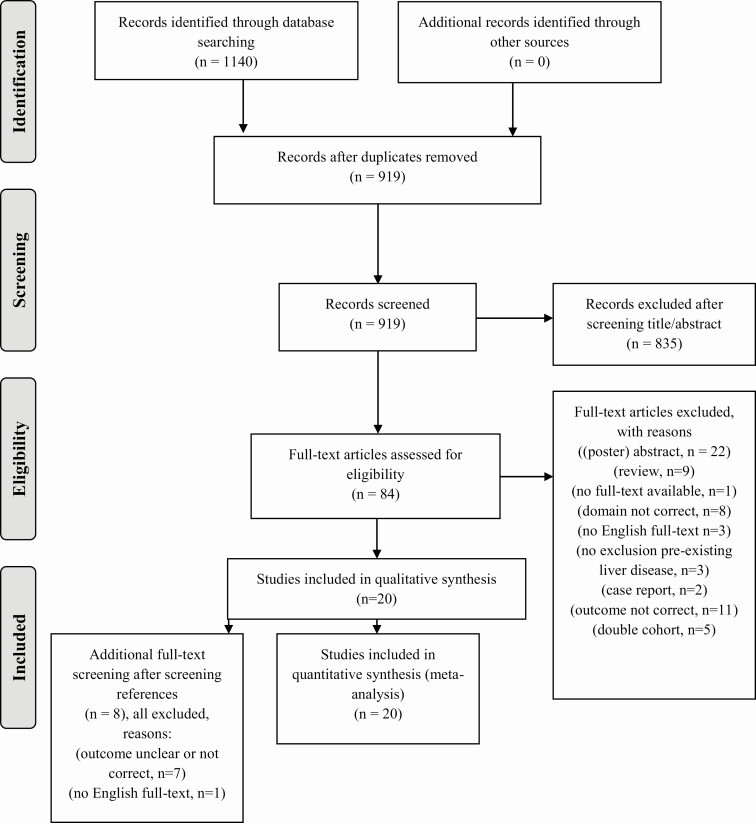
Our search yielded 1140 records. After removing duplicates, 919 records remained. After screening titles and abstracts, 835 records were excluded. Subsequently, 84 full-text articles were screened for eligibility, of which 20 studies between 2009 and 2019 were included in the qualitative and quantitative analysis (Fig. 1).
Figure 1.
Study flowchart, date of search: March 20, 2020.
The included studies involved a total of 3901 patients with type 1 diabetes. Table 1 shows the baseline characteristics and reported prevalence rates. The mean age of type 1 diabetes patients in the studies varied from 12.9 to 48.9 years. Three studies evaluated only children and adolescents. Around half of the patients were male. Mean BMI ranged from 19.3 to 28.7 kg/m2. Regulation of type 1 diabetes differed between the studies, with mean HbA1c values ranging from 60 to 115 mmol/mol. Mean diabetes duration ranged from 5.9 to 23.4 years. Only a few studies reported on waist circumference and metabolic syndrome, in most studies defined according to the National Cholesterol Education Program Adult Treatment Panel III criteria or World Health Organization criteria (48, 49).
Table 1.
Baseline characteristics and reported nonalcoholic fatty liver disease prevalence rates of included studies
| Study | Population | Diagnostic strategy | Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author country | Year | Designb | T1DM pts, No. | Age, y | Male sex, n (%) | BMI, kg/ m2 | Waist circumference, cm | Metabolic syndromec n (%) | HbA1c, mmol/mol % | Diabetes duration, y | Definition of NAFLD | NAFLD prevalence, n (%) | |
| Cipponeri (27) Italy | 2019 | Cross-sectional, prospective | 220 | 41 (31-49) | 88 (40.0) | 24.5 (21.9- 26.9) | 86 (77-95) | 38 (17.3) | 61 (53-68) | 20.0 (13.0- 30.0) | Ultrasound | Steatosis | 57 (25.9) |
| 7.7 (7.0-8.4) | |||||||||||||
| Cusi (28) 9 countriesd | 2017 | Cross-sectional, retrospective | 204 | 39.3 ± 12.2 | 117 (57.4) | 26.5 ± 3.7 | – | – | 64 ± 13 | 16.8 ± 11.5 | MRI LFC | Steatosis | 18 (8.8) |
| 8.0 ± 1.2 | |||||||||||||
| de Ledinghen (29) France | 2012 | Cross-sectional, prospective | 145 | 47.8 ± 15.5 | 80 (55.2) | 23.5 ± 3.9 | – | 35 (24.1) | 69 (61-80) | 18.0 (12.0- 26.0) | (1) FibroTest | (1) and (2) severe fibrosis (≥ F3) | (1) 4 (2.8) |
| 8.5 (7.7-9.5) | (2) 3 (2.1) | ||||||||||||
| (2) FibroScan, M probe | (1) or (2) 6 (4.1) | ||||||||||||
| Elkabanny (30) Egypt | 2017 | Cross-sectional, prospective | 100 | 13.8 ± 1.9 | 39 (39.0) | – | – | – | – | – | (1) Ultrasound | (1) Steatosis | (1) 12 (12.0) |
| (2) NR | |||||||||||||
| (2) FibroScan M probe | (2) Fibrosis, mild < F2, significant ≥ F2 | ||||||||||||
| Gaiani (31) Italy | 2009 | Cross-sectional, prospective | 17 | – | – | – | – | – | – | – | Ultrasound | Steatosis | 4 (24.0) |
| Harman (32) USA | 2014 | Cross-sectional, retrospective | 57 | 43.7 ± 17.8 | 32 (56.1) | 25.2 ± 5.4 | – | 10 (17.5) | – | 10.0 (2.1-25.3) | Liver biopsy | Histopathologically confirmed NAFLD | 11 (19.3) |
| Leeds (33) UK | 2009 | Cross-sectional, prospective | 911 | 42.7 ± NR | 515 (56.5) | – | – | – | – | – | (1) ALT preselection, (2) Ultrasound | (1) ALT 2× > 50 IU/L | (1) 17 (1.9) |
| (2) Steatosis or fibrosis on US | (2) 9/17 (52.9) HS | ||||||||||||
| 2/17 (11.8) Fib | |||||||||||||
| ➔ 11/17 (64.7) | |||||||||||||
| Li (34) China | 2018 | Cross-sectional, prospective | 39 | 48 ± 19 | 19 (48.7) | 22.6 ± 4.7 | – | – | 115 ± 25 | – | Ultrasound | Steatosis | 6 (15.4) |
| 12.7 ± 2.3 | |||||||||||||
| Mantovani (35) Italy | 2016 | Cross-sectional, retrospective | 286 | 43.4 ± 13.7a | 121 (42.3) | 24.5 ± 4.1a | – | – | 64 ± 12a | 18.8 ± 12.5a | Ultrasound | Steatosis | 150 (52.4) |
| 8.0 ± 1.1a | |||||||||||||
| Marjot (36) UK | 2018 | Cross-sectional, prospective | 251 | 40.6 ± 16.7 | 140 (55.8) | - | – | – | 68 ± 18 | 21.0 ± 15.1 | (1) Fib-4 score preselection | (1)Intermediate/high score | (1) 41 (16.3) |
| 8.4 ± 1.9 | |||||||||||||
| (2) 2/18 (11.0) Fib | |||||||||||||
| (2) Significant fibrosis (≥ F2) | |||||||||||||
| (2) FibroScan, probe NR | |||||||||||||
| ➔ 5/251 (1.8)e | |||||||||||||
| Petit (37) France | 2015 | Cross-sectional, prospective | 128 | 40.4 (29-51) | 65 (50.7) | 25.0 ± 4.7 | – | – | 69 (61-81) | 20.0 (11.0- 30.0) | MRI LFC | Steatosis | 6 (4.6) |
| 8.5 (7.7-9.6) | |||||||||||||
| Regnell (38) Sweden | 2015 | Cross-sectional, prospective | 22 | 13.5 (9-17) | 12 (54.5) | – | – | – | 62.5 (46-98) | 5.9 (0.0-13.0) | MRI LFC | Steatosis | 0 (0.0) |
| 7.9 (6.4-11.1) | |||||||||||||
| Serra-Planas (39) Spain | 2017 | Cross-sectional, prospective | 100 | 39.4 ± 7.8a | 54 (54.0) | 24.9 ± 3.2a | – | – | 64 ± 8a | 21.7 ± 2.5a | Ultrasound | Steatosis | 12 (12.0) |
| 8.0 ± 1.0a | |||||||||||||
| Şiraz (40) Turkey | 2017 | Cross-sectional, prospective | 80 | 12.9 ± 2.4a | 40 (50.0) | 20.1 ± 2.3a | 67.6 ± 6.1a | – | 63 ± 7a | 7.9 ± 1.9a | Ultrasound | Steatosis | 8 (10.0) |
| 7.9 ± 0.7a | |||||||||||||
| Sviklāne (41) Latvia | 2018 | Cross-sectional, prospective | 40 | 44.5 ± 13.2a | 17 (42.5) | 28.7 ± 4.3a | 91.6 ± 13.9a | 21 (52.5) | 62 ± 27a | 23.4 ± 10.9a | (1) HSI | (1), (2), and (3) Steatosis | (1) 12 (30.0) |
| 7.8 ± 0.3a | |||||||||||||
| (2) 14 (35.0) | |||||||||||||
| (3) 12 (30.0) | |||||||||||||
| (2) FLI | |||||||||||||
| (3) MRI | |||||||||||||
| Targherf (42) Italy | 2010 | Cross-sectional, retrospective | 250 | 42.5 ± 12.0a | 106 (52.5) | 24.8 ± 4.1a | – | 79 (39.1) | 66 ± 13a | 18.4 ± 11.6a | Ultrasound | Steatosis | 111 (44.4) |
| 8.2 ± 1.2a | |||||||||||||
| Targher (43) Italy | 2012 | Cross-sectional, retrospective | 343 | 44.3 ± 14.1a | 156 (45.5) | 24.6 ± 4.5a | 88.5 ± 15.1a | 157 (45.8) | 78 ± 25a | 15.7 ± 14.7a | Ultrasound | Steatosis | 182 (53.0) |
| 9.3 ± 2.3a | |||||||||||||
| Vendhan (44) India | 2014 | Cross-sectional, retrospective | 736 | 19.4 ± 11.0a | 384 (52.2) | 19.3 ± 4.3a | 69.0 ± 13.2a | – | 88 ± 28a | 7.6 ± 8.3a | Ultrasound | Steatosis | 204 (27.7) |
| 10.2 ± 2.6a | |||||||||||||
| Yoneda (45) Japan | 2012 | Cross-sectional, prospective | 144 | 48.9 ± 20.4a | 62 (43.1) | 22.7 ± 3.6a | – | – | 60 ± 24a | 14.9 ± 13.2a | Ultrasound | Steatosis | 25 (17.4) |
| 7.6 ± 2.4a | |||||||||||||
| Zhang (46) China | 2016 | Cross-sectional, retrospective | 722 | 46.2 ± 13.1a | 371 (51.4) | 21.8 ± 3.8a | 79.1 ± 9.8 | 274 (38.0) | 77 ± 28a | 7.6 ± 4.1a | Ultrasound | Steatosis | 123 (17.0) |
| 9.2 ± 2.6a | |||||||||||||
Data are mean ± SD, median (IQR), or n (%).
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; Fib, fibrosis; FLI, fatty liver index; HbA1c, glycated hemoglobin; HS, hepatic steatosis; HSI, hepatic steatosis index; IQR, interquartile range; MRI LFC, magnetic resonance imaging–liver fat content; NAFLD, nonalcoholic fatty liver disease; NR, not reported; pts, patients; T1DM, type 1 diabetes mellitus; UK, United Kingdom; ULN, upper limit of normal; USA, United States of America.
a Calculated mean ± SD from median (IQR) using formula of Wan (47), and calculated weighted mean from subgroups patients with NAFLD and patients without NAFLD.
b Cross-sectional means a cross-sectional design for the assessment of NAFLD. Prospective means that methods to diagnose NAFLD were performed prospectively. Retrospective means that methods to diagnose NAFLD were performed retrospectively.
c Metabolic syndrome as defined by the different studies.
d Nine countries: Austria, France, Germany, Italy, Japan (approximately 20% of cohort), Mexico, Poland, Russia, and United States.
e Prevalence of NAFLD in total T1DM population after extrapolation.
f Baseline characteristics are described for the patient group without alcoholic fatty liver disease (n = 202). Prevalence rate of NAFLD is calculated from the complete group of patients with T1DM (n = 250).
Different diagnostic strategies were used to evaluate NAFLD: Thirteen studies (65%) used ultrasound, 3 (15%) assessed magnetic resonance imaging liver fat content (MRI LFC), 1 (5%) used a different MRI technique to assess steatosis (comparing brightness of the liver to brightness of the spleen), 2 studies (10%) used a combination of a noninvasive risk score and transient elastography (TE), and 1 (5%) diagnosed NAFLD by liver biopsy. Two studies applied imaging after preselection in the type 1 diabetes population: (1) Leeds et al performed ultrasound only in 17 patients with persistently elevated alanine aminotransferase (ALT) levels higher than 50 IU/L; (2) Marjot et al performed TE only in 11 patients with an intermediate or high risk Fib-4 score and subsequently calculated an extrapolated prevalence rate in their total type 1 diabetes population (33, 36). Most studies defined NAFLD as hepatic steatosis. Two studies looked at significant or severe fibrosis.
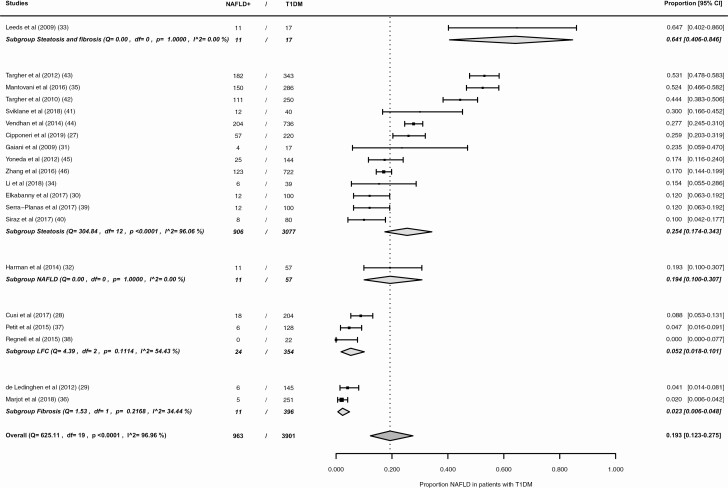
The results of the individual studies are presented in Fig. 2. The reported prevalence rates range from 0.0% (95% CI, 0.0%-7.7%) to 64.7% (95% CI, 40.2%-86.0%). The pooled NAFLD prevalence is 19.3% (95% CI 12.3–27.5%) (see Fig. 2), with an I2 statistic showing a high level of heterogeneity of effect sizes of 96.96%.
Figure 2.
Forest plot overall NAFLD prevalence in patients with type 1 diabetes. NAFLD+, patients with nonalcoholic fatty liver disease; T1DM, type 1 diabetes mellitus.
Of the total type 1 diabetes group, 3699 patients were adults and 202 patients were children/adolescents. Fig. 3 shows the results of this subgroup meta-analysis. The pooled NAFLD prevalence in adults and children/adolescents with type 1 diabetes is 22.0% (95% CI, 13.9%-31.2%) and 7.9% (95% CI, 2.6%-15.5%) respectively. The statistical heterogeneity remained high in the adult group (I2 = 97.30%). In the subgroup of children/adolescents, it was substantial (I2 = 58.46%).
Figure 3.
Forest plot NAFLD prevalence in patients with type 1 diabetes, subgroups according to age-group: (1) adults, (2) children/adolescents. NAFLD+, patients with nonalcoholic fatty liver disease; T1DM, type 1 diabetes mellitus.
The pooled NAFLD prevalence differed significantly per diagnostic strategy (Fig.4). In ultrasound studies the pooled prevalence is 27.1% (95% CI, 18.7%-36.3%, I2 = 96.18%). The prevalence is lower in MRI studies, with a pooled prevalence of 8.6% (95% CI, 2.1%-18.6%, I2 = 84.51%). Using the gold standard, liver biopsy, an NAFLD prevalence of 19.3% (95% CI, 10.0%-30.7%) was found. In the subgroup of studies using a combination of a noninvasive risk score and TE, the pooled NAFLD prevalence is 2.3% (95% CI, 0.6%-4.8%, I2 = 34.44%).
Figure 4.
Forest plot NAFLD prevalence in patients with type 1 diabetes, subgroups according to diagnostic modality: (1) ultrasound, (2) MRI, (3) biopsy, (4) combination non-invasive risk score and TE. NAFLD+, patients with nonalcoholic fatty liver disease; T1DM, type 1 diabetes mellitus; MRI, magnetic resonance imaging; TE, transient elastography.
The pooled NAFLD prevalence differed significantly per NAFLD subset used (Fig. 5). In the majority of studies the reported NAFLD definition is steatosis (n = 13), yielding a pooled prevalence of 25.4% (95% CI, 17.4%-34.3%, I2 = 96.06%). The pooled prevalence is 5.2% (95% CI, 1.8%-10.1%, I2 = 54.43%) when steatosis is quantified using MRI, defining steatosis as an LFC of greater than 5.5/6%. Pooling the 2 studies defining NAFLD as significant or advanced fibrosis yields a prevalence rate of 2.3% (95% CI, 0.6%-4.8%, I2 = 34.44%).
Figure 5.
Forest plot NAFLD prevalence in patients with type 1 diabetes, subgroups according to NAFLD definition: (1) steatosis and fibrosis, (2) steatosis, (3) NAFLD, (4) LFC, (5) fibrosis. NAFLD+, patients with nonalcoholic fatty liver disease; T1DM, type 1 diabetes mellitus; LFC, liver fat content.
Results of the meta-regression analysis are shown in Figs. S1-S3. None of the tested continuous variables explained any of the heterogeneity of NAFLD prevalence. No significant associations between the explanatory variables and NAFLD were found: mean BMI, β coefficient equal to .011, P equal to .650; mean diabetes duration, β coefficient equal to .005, P equal to .665; mean HbA1c, β coefficient equal to .001, P equal to .889. Results of the meta-analysis on mean differences are shown in Figs. S4-S6. Pooled mean differences of BMI, diabetes duration, and HbA1c between patients without and with NAFLD were all significant: BMI –3.0 kg/m2 (95% CI, –3.8 to –2.2 kg/m2), diabetes duration –5.1 years (95% CI, –9.3 to –0.9 years), HbA1c –2.7 mmol/mol (95% CI, –4.0 to –1.4 mmol/mol).
An overview of risk of bias assessment of the individual studies is given in Table S1. Overall, 8 studies had a high risk of bias, mainly because of a poor external validity in combination with an inconsequent mode of data collection for all participants or a nonoptimal exclusion of other causes of liver disease. Six studies had an intermediate risk of bias due to a moderate external validity. Another 6 studies had a low risk of bias.
Sensitivity analysis, excluding studies with a high individual risk of bias, did not yield any other results (Fig. S7). Although the pooled prevalence estimate increased slightly to 26.1% (95% CI, 17.7%-35.4%), heterogeneity remained high (I2 = 96.64%). Another sensitivity analysis, excluding the outlier of Leeds et al (33), caused a slight decrease in prevalence, but did not change heterogeneity (pooled prevalence 17.8% (95% CI, 11.0%-25.9%, I2 = 97.06%). The funnel plot (Fig. S8) shows no asymmetry, and neither Egger regression test was significant, indicating that no publication or related biases were present.
Discussion
Our systematic review and meta-analysis shows that the overall NAFLD prevalence in patients with type 1 diabetes is substantial at 19.3%, with an even higher prevalence of 22% in adults only. The prevalence estimate is highly dependent on the diagnostic strategy and NAFLD definition used.
Because NAFLD prevalence data in age- and BMI-matched reference groups are scarce, we can only speculate on the prevalence difference between patients with type 1 diabetes and healthy controls. NAFLD prevalence in the general population has been previously estimated at 25% (6). The general population contains considerable numbers of elderly individuals with obesity and/or type 2 diabetes and NAFLD, whereas populations of people with type 1 diabetes are younger and mostly nonobese. The study by Petit et al was the only one in our meta-analysis that had a small adult age- and BMI-matched control group of patients without diabetes; NAFLD prevalence was 13.4% (37). Furthermore, a recent meta-analysis estimated NAFLD prevalence in lean/nonobese individuals at 10% to 16% (50). Therefore, we consider NAFLD prevalence in patients with type 1 diabetes at least comparable to and probably higher than in a matched general population.
NAFLD prevalence in patients with type 1 diabetes is highly dependent on the diagnostic modality used. In general, liver biopsy is considered the gold standard for diagnosing NAFLD. However, in clinical practice only patients with a high index of suspicion undergo liver biopsy. The histologically proven prevalence of 19.3% might therefore be an overestimation of true NAFLD prevalence. Ultrasound may falsely classify patients as having NAFLD because hepatic glycogenosis mimics HS seen on ultrasound (51). MRI LFC has shown to be reliable in quantifying HS compared to liver biopsy and is less prone for confounding by indication and interobserver variability, suggesting this MRI LFC subgroup might reflect the most accurate estimation of pooled NAFLD prevalence (52). The studies using a combination of a noninvasive risk score and TE found low NAFLD prevalence rates because they defined NAFLD as fibrosis, which is much less prevalent than HS in the general and type 2 diabetes population as well (6, 9). Finally, the finding that BMI, diabetes duration (ie, exposure time to insulin resistance and hyperinsulinemia), and diabetes regulation in our analysis were not significant moderators of heterogeneity, supports the idea that the heterogeneity of NAFLD prevalence is substantially explained by diagnostic modality and NAFLD definition.
NASH prevalence was assessed only in the study by Harman et al; a second opinion on 49 liver histology specimens yielded a NASH prevalence of 20.4% (34). On longitudinal follow-up NASH cirrhosis was found in one patient. HCC was reported in 2 patients with type 1 diabetes, but underlying liver disease in these patients was not specified (34). None of the studies reported on histologically confirmed NAFLD fibrosis in patients with type 1 diabetes patients. TE-defined NAFLD fibrosis prevalence was low, but this might be an underestimation of true NAFLD fibrosis prevalence. In the TE studies a substantial number of patients was excluded from further analysis because preselection was applied, no valid FibroScan measurement was obtained, only M probe was available, or patients were not willing to undergo TE. Currently, there are no longitudinal data on decompensated liver cirrhosis and LT in patients with type 1 diabetes and NAFLD, nor on liver-specific, cardiovascular, and all-cause mortality, precluding any detailed discussion on these long-term sequelae.
Our study has several strengths. We performed an extensive systematic literature search and meta-analysis including the best available data on NAFLD prevalence in patients with type 1 diabetes. To limit the amount of bias, we excluded studies using only laboratory tests for diagnosing NAFLD. These have shown to be nonreliable for diagnosing NAFLD because the majority of patients with NAFLD have normal aspartate aminotransferase and ALT values (53). Also, we performed a risk of bias assessment by using a tool especially developed for prevalence studies. Another strength is that we have shown the overall prevalence in the whole type 1 diabetes population as well as in children/adolescents and adults separately.
The most important limitation of this meta-analysis is that the study populations, diagnostic method, and outcome definitions differed so much across studies. We tried to explain heterogeneity in NAFLD prevalence to the best of our ability. Still, because of the small number of studies, performed in selected patient populations, we could perform only exploratory subgroup and meta-regression analysis, and we were not able to draw any unambiguous conclusions on associations between continuous variables and NAFLD. Moreover, we could not elaborate on characteristics associated with the more severe NAFLD stages. Finally, the studies in this review did not allow any conclusion on long-term vascular or hepatic consequences of NAFLD in patients with type 1 diabetes.
Speculating on the future NAFLD burden in patients with type 1 diabetes, we believe that the prevalence of NAFLD in patients with type 1 diabetes may increase considerably in the coming decades. The growing obesity prevalence in the type 1 diabetes population will come with an increase in insulin resistance, an important clinical factor associated with NAFLD progression (20, 54). Patients will combine features of type 1 diabetes with those of type 2 diabetes, with increased prevalence of dyslipidemia and hypertension. This is labeled as “double diabetes,” and is associated with an increased cardiovascular risk (55). Moreover, patients with type 1 diabetes usually have a lifetime exposure to diabetes mellitus. These developments will presumably lead to an increased risk for cardiovascular events as well as advanced NAFLD in patients with type 1 diabetes (9).
In conclusion, our meta-analysis shows that NAFLD prevalence in patients with type 1 diabetes is considerable, and is at least comparable to and probably higher than in an adequately matched sample of the general population. The prevalence estimate is highly dependent on the specific diagnostic modality and NAFLD definition used. From this meta-analysis, we cannot draw any conclusions on the risk of progression of disease to more harmful NAFLD stages and long-term NAFLD-related outcomes. In addition to the well-known NAFLD risk groups of patients with obesity and type 2 diabetes, patients with type 1 diabetes should—with their changing phenotype—be considered a group at risk for NAFLD.
Acknowledgments
We thank Paco Welsing for providing statistical expertise.
Financial Support: No funding was received for conducting this study.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- BMI
body mass index
- HbA1c
glycated hemoglobin
- HS
hepatic steatosis
- LFC
liver fat content
- LT
liver transplantation
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TE
transient elastography
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
The data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 2016;9(2):65-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang XJ, Malhi H. Nonalcoholic fatty liver disease. Ann Intern Med. 2018;169(9):ITC65-ITC80. [DOI] [PubMed] [Google Scholar]
- 3. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328-357. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577-1586. [DOI] [PubMed] [Google Scholar]
- 5. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73-84. [DOI] [PubMed] [Google Scholar]
- 7. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547-555. [DOI] [PubMed] [Google Scholar]
- 8. Adam R, Karam V, Cailliez V, et al. ; all the other 126 contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 annual report of the European Liver Transplant Registry (ELTR)–50-year evolution of liver transplantation. Transpl Int. 2018;31(12):1293-1317. [DOI] [PubMed] [Google Scholar]
- 9. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793-801. [DOI] [PubMed] [Google Scholar]
- 10. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330-344. [DOI] [PubMed] [Google Scholar]
- 11. Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30(5):1212-1218. [DOI] [PubMed] [Google Scholar]
- 12. Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14(2):99-114. [DOI] [PubMed] [Google Scholar]
- 13. Lee YH, Cho Y, Lee BW, et al. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J. 2019;43(1):31-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Silva NMG, Borges MC, Hingorani AD, et al. ; UCLEB Consortium . Liver function and risk of type 2 diabetes: bidirectional mendelian randomization study. Diabetes. 2019;68(8):1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936-944. [DOI] [PubMed] [Google Scholar]
- 16. Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol. 2013;19(22):3375-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(1):27-38. [DOI] [PubMed] [Google Scholar]
- 18. DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982;23(4):313-319. [DOI] [PubMed] [Google Scholar]
- 19. Stadler M, Anderwald C, Pacini G, et al. Chronic peripheral hyperinsulinemia in type 1 diabetic patients after successful combined pancreas-kidney transplantation does not affect ectopic lipid accumulation in skeletal muscle and liver. Diabetes. 2010;59(1):215-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ; Advancing Care for Type 1 Diabetes and Obesity Network (ACT1ON) . Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev. 2018;39(5):629-663. [DOI] [PubMed] [Google Scholar]
- 21. Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672-2682. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PloS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Vries M, Westerink J, Kaasjager HAH, de Valk HW. Supplementary material of “Prevalence of nonalcoholic fatty liver disease (NAFLD) in patients with type 1 diabetes mellitus: a systematic review and meta-analysis.” Dataverse NL Repository 2020. Deposited 6 September 2020. 10.34894/VEIOXM [DOI] [PMC free article] [PubMed]
- 24. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement: appendix 1: Risk of Bias Tool. J Clin Epidemiol. 2012;65(9):934-939. [DOI] [PubMed] [Google Scholar]
- 25. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9): 934-939. [DOI] [PubMed] [Google Scholar]
- 26. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974-978. [DOI] [PubMed] [Google Scholar]
- 27. Cipponeri E, Vitturi N, Mariano V, et al. Vitamin D status and non-alcoholic fatty liver disease in patients with type 1 diabetes. J Endocrinol Invest. 2019;42(9):1099-1107. [DOI] [PubMed] [Google Scholar]
- 28. Cusi K, Sanyal AJ, Zhang S, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab. 2017;19(11):1630-1634. [DOI] [PubMed] [Google Scholar]
- 29. de Lédinghen V, Vergniol J, Gonzalez C, et al. Screening for liver fibrosis by using FibroScan® and FibroTest in patients with diabetes. Dig Liver Dis. 2012;44(5):413-418. [DOI] [PubMed] [Google Scholar]
- 30. Elkabbany ZA, Elbarbary NS, Ismail EA, et al. Transient elastography as a noninvasive assessment tool for hepatopathies of different etiology in pediatric type 1 diabetes mellitus. J Diabetes Complications. 2017;31(1):186-194. [DOI] [PubMed] [Google Scholar]
- 31. Gaiani S, Avogaro A, Bombonato GC, et al. Nonalcoholic fatty liver disease (NAFLD) in nonobese patients with diabetes: Prevalence and relationships with hemodynamic alterations detected with Doppler sonography. J Ultrasound. 2009;12(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harman DJ, Kaye PV, Harris R, Suzuki A, Gazis A, Aithal GP. Prevalence and natural history of histologically proven chronic liver disease in a longitudinal cohort of patients with type 1 diabetes. Hepatology. 2014;60(1):158-168. [DOI] [PubMed] [Google Scholar]
- 33. Leeds JS, Forman EM, Morley S, Scott AR, Tesfaye S, Sanders DS. Abnormal liver function tests in patients with type 1 diabetes mellitus: prevalence, clinical correlations and underlying pathologies. Diabet Med. 2009;26(12):1235-1241. [DOI] [PubMed] [Google Scholar]
- 34. Li TT, Wang AP, Lu JX, et al. Prevalence and clinical characteristics of non-alcoholic fatty liver disease in newly diagnosed patients with ketosis-onset diabetes. Diabetes Metab. 2018;44(5):437-443. [DOI] [PubMed] [Google Scholar]
- 35. Mantovani A, Mingolla L, Rigolon R, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular disease in adult patients with type 1 diabetes. Int J Cardiol. 2016;225:387-391. [DOI] [PubMed] [Google Scholar]
- 36. Marjot T, Sbardella E, Moolla A, et al. Prevalence and severity of non-alcoholic fatty liver disease are underestimated in clinical practice: impact of a dedicated screening approach at a large university teaching hospital. Diabet Med. 2018;35(1):89-98. [DOI] [PubMed] [Google Scholar]
- 37. Petit JM, Pedro L, Guiu B, et al. Type 1 diabetes is not associated with an increased prevalence of hepatic steatosis. Diabet Med. 2015;32(12):1648-1651. [DOI] [PubMed] [Google Scholar]
- 38. Regnell SE, Peterson P, Trinh L, et al. Magnetic resonance imaging reveals altered distribution of hepatic fat in children with type 1 diabetes compared to controls. Metabolism. 2015;64(8):872-878. [DOI] [PubMed] [Google Scholar]
- 39. Serra-Planas E, Aguilera E, Castro L, et al. Low prevalence of non-alcoholic fatty liver disease in patients with type 1 diabetes is associated with decreased subclinical cardiovascular disease. J Diabetes. 2017;9(12):1065-1072. [DOI] [PubMed] [Google Scholar]
- 40. Şiraz ÜG, Doğan M, Hatipoğlu N, Muhtaroğlu S, Kurtoğlu S. Can fetuin-A be a marker for insulin resistance and poor glycemic control in children with type 1 diabetes mellitus? J Clin Res Pediatr Endocrinol. 2017;9(4):293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sviklāne L, Olmane E, Dzērve Z, Kupčs K, Pīrāgs V, Sokolovska J. Fatty liver index and hepatic steatosis index for prediction of non-alcoholic fatty liver disease in type 1 diabetes. J Gastroenterol Hepatol. 2018;33(1):270-276. [DOI] [PubMed] [Google Scholar]
- 42. Targher G, Bertolini L, Padovani R, et al. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53(4):713-718. [DOI] [PubMed] [Google Scholar]
- 43. Targher G, Pichiri I, Zoppini G, Trombetta M, Bonora E. Increased prevalence of cardiovascular disease in type 1 diabetic patients with non-alcoholic fatty liver disease. J Endocrinol Invest. 2012;35(5):535-540. [DOI] [PubMed] [Google Scholar]
- 44. Vendhan R, Amutha A, Anjana RM, Unnikrishnan R, Mohan V. Clinical profile of nonalcoholic Fatty liver disease among young patients with type 1 diabetes mellitus seen at a diabetes speciality center in India. Endocr Pract. 2014;20(12): 1249-1257. [DOI] [PubMed] [Google Scholar]
- 45. Yoneda C, Ogino J, Matsuura H, Haruki T, Suzuki Y, Hashimoto N. Increased prevalence of diabetic complications in Japanese patients with type 1 diabetes and nonalcoholic fatty liver disease. Diabetol Int. 2012;3(1):37-41. [Google Scholar]
- 46. Zhang L, Guo K, Lu J, et al. Nonalcoholic fatty liver disease is associated with increased carotid intima-media thickness in type 1 diabetic patients. Sci Rep. 2016;6: 26805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497. [DOI] [PubMed] [Google Scholar]
- 49. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539-553. [DOI] [PubMed] [Google Scholar]
- 50. Shi Y, Wang Q, Sun Y, et al. The prevalence of lean/nonobese nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Clin Gastroenterol. 2020;54(4):378-387. [DOI] [PubMed] [Google Scholar]
- 51. Torbenson M, Chen YY, Brunt E, et al. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. Am J Surg Pathol. 2006;30(4):508-513. [DOI] [PubMed] [Google Scholar]
- 52. Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150(3):626-637.e7. [DOI] [PubMed] [Google Scholar]
- 53. Targher G, Mantovani A, Pichiri I, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2014;37(6):1729-1736. [DOI] [PubMed] [Google Scholar]
- 54. Marjot T, Moolla A, Cobbold JF, Hodson L, Tomlinson JW. Nonalcoholic fatty liver disease in adults: current concepts in etiology, outcomes, and management. Endocr Rev. 2020;41(1):bnz009. [DOI] [PubMed] [Google Scholar]
- 55. Kietsiriroje N, Pearson S, Campbell M, Ariëns RAS, Ajjan RA. Double diabetes: a distinct high-risk group? Diabetes Obes Metab. 2019;21(12):2609-2618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.