Abstract
Context
Dysregulated immune hemostasis occurs in unexplained recurrent spontaneous abortion (URSA). Synthesized by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), hydrogen sulfide (H2S) promotes regulatory T-cell differentiation and regulates immune hemostasis; yet, its role in URSA is elusive.
Objective
To determine if H2S plays a role in early pregnancy and if dysregulated H2S signaling results in recurrent spontaneous abortion.
Design
First trimester placenta villi and decidua were collected from normal and URSA pregnancies. Protein expression was examined by immunohistochemistry and immunoblotting. Human trophoblast HTR8/SVneo and JEG3 cells were treated with H2S donors; HTR8/SVneo cells were transfected with CBS ribonucleic acid interference (RNAi) or complementary deoxyribonucleic acid. Cell migration and invasion were determined by transwell assays; trophoblast transcriptomes were determined by RNA sequencing (RNA-seq). Wild-type, CBS-deficient, and CBA/J × DBA/2 mice were treated with CBS and CSE inhibitors or H2S donors to determine the role of H2S in early pregnancy in vivo.
Results
CBS and CSE proteins showed cell-specific expressions, but only CBS decreased in the villous cytotrophoblast in URSA versus normal participants. H2S donors promoted migration and invasion and MMP-2 and VEGF expression in human placenta trophoblast cells that contain SV40 viral deoxyribonucleic acid sequences (HTR8/SVneo) and human placenta trophoblast cells (JEG3 cells), similar to forced CBS expression in HTR8/SVneo cells. The CBS-responsive transcriptomes in HTR8/SVneo cells contained differentially regulated genes (ie, interleukin-1 receptor and prostaglandin-endoperoxide synthase 2) that are associated with nuclear factor-κB-mediated inflammatory response. In vivo, dysregulated CBS/H2S signaling significantly increased embryonic resorption and decidual T-helper 1/T-helper 2 imbalance in mice, which was partially rescued by H2S donors.
Conclusion
CBS/H2S signaling maintains early pregnancy, possibly via regulating maternal-fetal interface immune hemostasis, offering opportunities for H2S-based immunotherapies for URSA.
Keywords: hydrogen sulfide, cystathionine β-synthase, immune hemostasis, maternal-fetal interface, unexplained recurrent spontaneous abortion
Hydrogen sulfide (H2S), the third gaseous molecule of the gasotransmitter family discovered after nitric oxide and carbon monoxide (1), is primarily synthesized from L-cysteine (L-Cys) by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) (2-4). CBS and CSE are expressed in a variety of organs with tissue/cell-specific expression patterns (5, 6). Uterine artery H2S production is augmented during pregnancy via selectively upregulating endothelial and smooth muscle CBS expression (7, 8), contributing to pregnancy-associated uterine vasodilation in rats (7). Endogenous H2S maintains uterine quiescence via suppressing inflammation and the expression of myometrial contractile proteins in human pregnancy (9). Moreover, H2S regulates several other reproductive processes, including oviduct transportation of embryos (10) and decidualization (11) in mice. Dysregulated placental CSE/H2S signaling results in preeclampsia-like syndromes due to decreased angiogenesis and trophoblast invasion (12, 13) as well as impaired uterine spiral artery remodeling (14), leading to adverse fetal outcomes such as intrauterine growth restriction, placental abruption, and fetal demise in utero. Although these findings show that H2S signaling is clinically important in the reproductive system, it is unknown how H2S signaling elicits its function in early pregnancy.
Early pregnancy loss is rate-limiting for mammalian pregnancy. Recurrent spontaneous abortion (RSA) is a major form of early pregnancy loss in humans, which is clinically defined as 3 or more consecutive spontaneous abortions prior to 20 to 28 weeks of human pregnancy (15). RSA occurs in approximately 5% of all reproductive-age couples, representing a major fertility problem worldwide (16). RSA is identified to be a multifactorial disease involving genetic, anatomic, hormonal, and environmental factors (17), with nearly 60% cases unexplained (18). Of note, unexplained RSA (URSA) is normally associated with immunological abnormalities at the maternal-fetal interface (19), pointing to a causative role of immunological disturbance. However, the exact mechanisms as to how RSA occurs remain elusive.
The CBS-derived H2S is critical for immune homeostasis because CBS-derived H2S promotes the differentiation of regulatory T cells (Treg), and CBS deficiency leads to systemic autoimmune disease (20). Pregnant CBS-deficient mice possess significant reduced uterine natural killer cells (11) whose function is to promote stroma cell decidualization and to remodel uterine spiral arteries (21) during early pregnancy. Thus, we posited a role of CBS/H2S signaling in the maintenance of early pregnancy. This study was therefore conducted to test a hypothesis that dysregulated CBS/H2S signaling can lead to RSA via perturbations in immune homeostasis and trophoblast invasion. We report herein that CBS protein, but not CSE protein, is significantly reduced in the villous trophoblasts in women with RSA. In vitro, CBS/H2S signaling regulates trophoblast migration and invasion via regulating extracellular matrix protein, vascular endothelial growth factor (VEGF) production, and inflammatory responses. In vivo, CBS deficiency and CBS inhibition resulted in reduced litter size due to early embryonic loss and placental inflammation; all were in part restored by H2S donors. Collectively, our data support that endogenous CBS/H2S signaling is important for the maintenance of early pregnancy.
Material and Methods
Drugs and antibodies
L-Cys, phenylmethylsulphonyl fluoride, and radioimmunoprecipitation assay (RIPA) buffer were from SolarBio (Beijing, China). Antibodies for glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 10494-1-AP), CBS (14787-1-AP), CSE (12217-1-AP), and biotinylated goat anti-rabbit immunoglobulin (Ig)G (SP-9001) were from Proteintech (Rosemont, IL). Antibodies against matrix metalloproteinase-2 (MMP-2, 87809s), nuclear factor-κB (NF-κB, 8241T), and phospho-NF-κB (pNF-κB, 3033T) were from Cell Signaling Technology (Danvers, MA). Anti-VEGFA (ab52917) was purchased from Abcam (Cambridge, UK). Goat anti-rabbit IgG was from ZSBIO (Beijing, China). Amino-oxyacetic acid (AOAA, CBS inhibitor) and DL-propargylglycine (PAG, CSE inhibitor) were from Sigma (St. Louis, MI). A water-soluble slow H2S-releasing compound GYY4137 (GYY) (22) was from Cayman (Michigan, USA). Stock solutions of AOAA and PAG were dissolved in distilled water and stored at –20℃. GYY was prepared freshly before use in distilled water at a concentration of 3 mg/mL. All the drugs were diluted to working concentrations with phosphate-buffered saline (PBS; pH = 7.2)
Human participants and sample collection
Human tissues were collected with written consent from women with an approved protocol (#LL-201601005) by the Medical Ethics Committees of Shandong University. All participants were not on any pharmacological procedures prior to tissue collection. According to the anatomical structure of the first trimester human uterine-placental unit as previously described (23), the first trimester human villous and decidual tissues were obtained from removed fetuses of 10 electively terminated clinically normal pregnancies for nonmedical reasons and 10 RSA cases without any known causes. In our hospital, URSA was defined as follows: a pregnant woman with normal fetal heartbeat at the first perinatal visit (normally scheduled at gestational age [GA] of 42-49 days) who lost fetal heartbeat at least twice as detected by virginal ultrasound in follow-up visits before 12 weeks’ gestation. The average age (year, mean ± standard deviation [SD]) of normal pregnant participants was 27.30 (3.42), which was not different from that (31.3 ± 7.02) of the patients with RSA (P > 0.05). The average GA (week, mean ± SD) of URSA samples was 11.87 (4.0), which was greater (P < 0.01) than that (7.08 ± 0.96) of the normal pregnant control participants. RSA cases were further classified as URSA after the exclusion of maternal anatomic or hormonal abnormalities, or paternal and maternal chromosomal abnormalities. Tissues were washed in cold PBS immediately after collection. Placenta villi were rapidly frozen in liquid nitrogen for protein extraction. Decidua was separated from villous tissue for ribonucleic acid (RNA) extraction. The interface of placenta villi and decidua was fixed in 4% paraformaldehyde for sectioning for further analysis.
Cell culture
The human trophoblast-derived cell lines HTR8/SVneo (human placenta trophoblast cells that contain SV40 viral DNA sequences, lot number 70014079) and JEG3 (human placenta trophoblast cells, lot number 70000179) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in Roswell Park Memorial Institute 1640 medium (Gibco, Foster City, CA) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Invitrogen, Karlsruhe, Germany); they were used between passage 20 and 35.
Lentiviral transfection
HTR-8/SVneo cells were seeded in 12-well plates at 5 x104/well and transfected with each lentivirus: CBS complementary deoxyribonucleic acid (cDNA; LV-CBS) or its control (LV-CON238) and CBS ribonucleic acid interference (RNAi; LV-CBS-RNAi) or its control small interfering RNA (siRNA) (LV-CON077). The titer of each virus stock was 1x108 TU/mL, and the multiplicity of infection for each was 10. All the viruses were purchased from Genechem Inc. (Shanghai, China), and their target sequences were listed in the the supplemental materials (24). Puromycin (1mg/mL) was added for killing untransfected cells. Protein extracts were prepared at 24 hours after infection for immunoblotting to verify transfection.
Immunohistochemistry
The sections (4 µm) of decidua/placental tissues were incubated with rabbit polyclonal antibodies against human CBS (1:100) or CSE rabbit polyclonal antibodies (1:100) in PBS containing 1% bovine serum albumin overnight at 4°C, followed by biotinylated goat anti-rabbit IgG. After washing, enzyme horseradish peroxidase-labeled streptavidin was added to the sections. After 10 minutes of incubation, the sections were rinsed with PBS and followed by staining with diaminobenzidine working reagent for 30 to 60 seconds and then counterstained with hematoxylin. Images were acquired with a Nikon Eclipse 80i microscope (Tokyo, Japan). Image J software (National Institutes of Health) was used to analyze the immunoreactive protein signals in decidual/placental trophoblasts of the stained sections (2 sections per participant, n = 3/group). Negative controls were obtained by omitting the primary antibodies. The cell type was determined based on size, shape, and location according to the anatomical structures as previously described (23). The areas of decidua and villi were selected from the same section by using the region of interest function using Image J; signal intensity of CBS and CSE proteins was then calculated from CBS- or CSE-positive staining areas (brown) in decidua/villi regions (n = 12/section), respectively. After subtraction of negative control signals, the intensities of 3 patients per group were averaged; the data were analyzed using SPSS 25.0 and GraphPad Prism 7 software.
Enzyme-linked immunoassay
Decidua tissues free of fetal placenta were separated, weighed, and homogenized in RIPA buffer containing 1 mM phenylmethylsulphonyl fluoride. The homogenates were centrifuged at 5000 rpm for 10 minutes at 4°C. Supernatant was collected, and the concentration of total protein was adjusted to 2 to 4 mg/mL. Cytokines in the supernatants were quantified using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Dakewe, Shenzhen, China) for interferon (IFN)-γ (DKW12-2000), tumor necrosis factor (TNF)-α (DKW12-2720), interleukin (IL)-4 (DKW12-2040), IL-6 (DKW12-2060), and tumor growth factor beta 1 (TGF-β1) (DKW12-2710), according to the manufacturer’s instructions. HTR8/SVneo cells were seeded in 24-well plates (1x106 cells/well), transfected with LV-CBS-RNAi or LV-CBS or control lentiviruses as above and cultured with 5% carbon dioxide in air. After 24 hours of treatment with or without different doses of sodium hydrosulfide (NaHS) (1, 10, 100µM) or L-Cys (0.1, 0.3, 1mM), the culture medium was collected and centrifuged at 3000 rpm for 20 minutes. Cytokine secretion was quantified by using specific ELISA kits (mlbio, Shanghai; China) for thymic stromal lymphopoietin (ml060509), human chorionic gonadotropin (ml058014), indoleamine 2,3-dioxygenase (ml037385), and prostaglandin E2 (PGE2, ml03238).
Western blot
Proteins of the isolated decidua tissues free of fetal placenta or of cultured trophoblastic cells were extracted using RIPA buffer as described previously (25). Protein samples (20 μg/lane) were electrophoresed on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (0.45μm, Millipore) described previously (26). After blocking with 5% milk in tris-buffered saline, 0.1% Tween 20 detergent (Tris-buffered saline with Tween, TBST), the membrane was incubated with specific rabbit polyclonal antibodies against GAPDH (1:5000), CBS (1:1000), CSE (1:1000), MMP-2 (1:500), VEGFA (1:5000), NF-κB (1:1000), and pNF-κB (1:1000) at 4°C overnight. All the antibodies were diluted with either 5% nonfat milk or 1% bovine serum albumin in TBST as recommended by the manufacturer’s instructions. The membrane was then incubated with goat anti-rabbit IgG (1:4000) in TBST with 5% nonfat milk at 25℃ for 1 hour. Immunoreactive proteins were detected by using enhanced chemiluminescence plus reagent (Millipore, USA). The band densities were calculated using the image J. The values of blot densities were normalized to the levels of respective GAPDH or β-actin, respectively.
Trophoblast invasion and migration assays
The 24-well plate equipped with 8-mm pore size of polycarbonate membrane transwell inserts (Corning, New York, USA) were used for these assays. The bottom chamber was precoated with matrigel matrix for invasion but not for migration assays. The filter membrane of the transwell inserts was precoated with 50 μL matrigel (1 mg/mL, Becton Dickinson, Bedford, USA), which was melted at 4°C. After incubation for 4 hours at 4°C, HTR8/SVneo cells (2.0 x 105) in 200-μL serum-free medium were seeded in the upper chamber; 600 μL medium with 10% FBS was added to the bottom chamber. The plate with cells was then cultured with 5% carbon dioxide at 37°C. After NaHS (0, 1, 10, 100 µM) or L-Cys (0, 0.1, 0.3, 1 mM) was added separately, cell migration and invasion were allowed for 24 and 48 hours in culture, respectively. At the end of the experiments, the cultures were stained with methylrosanilinium chloride solution. The samples were examined under a light microscope (Olympus IX51, Japan) at a magnification of 100. The number of cells that invaded into matrigel on the bottom chamber and the number of cells that migrated to the other side of the insert were counted in 8 random fields and averaged.
Reverse transcription and real-time polymerase chain reaction
Total RNAs were extracted from cultured cells using Trizol reagent (Invitrogen, Carlsbad, CA) and quantified by OD260/280. cDNA was synthesized by reverse transcription with random primers and AMV Reverse Transcriptase (Promega, Madison, WI) and then used for quantifying mRNAs of interest by quantitative real-time polymerase chain reaction (RT-qPCR; run in triplicate) with gene-specific primers as listed in the the supplemental materials (24). Comparative CT method (ΔΔCT method) was used to calculate relative messenger RNA (mRNA) levels with GAPDH as the internal reference control.
H2S determination
Trophoblast cell lines of HTR8/SVneo and JEG3 cells were seeded in 24-well plates. Cells were divided into 5 groups that were treated with or without L-Cys, in the presence or absence of AOAA and/or PAG for 24 hours. The cells were then washed 3 times with PBS and lysed with 150 μL potassium phosphate buffer, pH 6.8, for 30 minutes on ice. The supernatants were collected and centrifuged. H2S production was determined by the methylene blue assay as described previously (27, 28).
RNA sequencing analysis
HTR-8/SVneo cells were infected with pLKO.1-short hairpin RNA against CBS or control short hairpin RNA lentiviruses and cultured for 48 hours. Transfected cells were selected for at least 2 weeks with a medium containing puromycin (2 μg/mL). Stably transfected cells were collected and washed with PBS buffer. Transfected cells (in triplicate) were prepared and each contained 1×106 cells. TRIzol was added and thoroughly reacted with the cells. The samples were stored at –80℃ until sent for RNA sequencing (RNA-seq) analysis at ANOROAD (Yiwu, Zhengjiang) in China. The differentially expressed genes were identified with DESeq2 (29). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway mapping and functional annotation clustering was performed by DAVID Tools (30).
Animals and sample collection
The animal care and use protocol was approved by the animal ethics committee of Shandong University, Jinan, China. Global CBS-knockout mice (CBS+/-) were donated by Dr Fang Yi of Shandong University. ♀CBA/J, ♂DBA/2, and C57BL/6 mice (6-8 weeks, 20-22 × g) were obtained from Weitong-Lihua Experimental Animal Center (Beijing, China). All mice were fed with standard rodent chow and water ad libitum at approximately 20 to 26°C, humidity approximately 30% to 70%, and 12 hours:12 hours light/dark cycle. Virgin mice were mated; pregnancy was confirmed by the presence of a vaginal plug 12 hours later at gestational day 0.5 (GD0.5). Dams were randomly allocated to receive once daily intraperitoneal (ip) injection of AOAA (50 mg kg–1), PAG (100 mg kg–1), GYY (10 mg kg–1), NaHS (0.5 mg kg–1), or vehicle (PBS, pH = 7.2). AOAA and PAG were applied from GD3.5 to GD5.5 for implantation analysis or from GD6.5 to GD12.5 for fetal resorption rate analysis in wild-type (WT) mice, C57BL/6 mice; GYY and NaHS were administrated from GD6.5 to GD12.5 in ♀CBS+/-, ♀CBA/J mice. Blood was obtained by retro-orbital puncture under anesthesia with isoflurane. Immediately after scarifying the animals by cervical dislocation, each fetal-placental unit of dams was separated from the implantation site; the decidua was collected by scraping approximately 1-mm thick endometrium where the placenta was located. The decidua tissues from each resorbed fetus were isolated carefully for analyzing cytokine production. Samples were stored immediately at –80⁰C until analyzed.
Embryo implantation and embryo loss in mice
Implantation sites were determined by intravenous injection of Chicago Blue on GD5.5, and pregnancy outcomes were evaluated by recording total number of implantation and resorption sites on GD12.5, as described previously (31).
Statistical analysis
Data are expressed as mean ± standard error of the mean and statistically analyzed by unpaired or paired t tests where appropriate by using SPSS 15.0 software (SPSS Inc., Chicago, IL). Analysis of variance followed by Bonferroni post hoc tests were used to compare differences among multiple groups. P < 0.05 was considered significant.
Results
Cytotrophoblast CBS protein decreases in first trimester placental villi in women with URSA
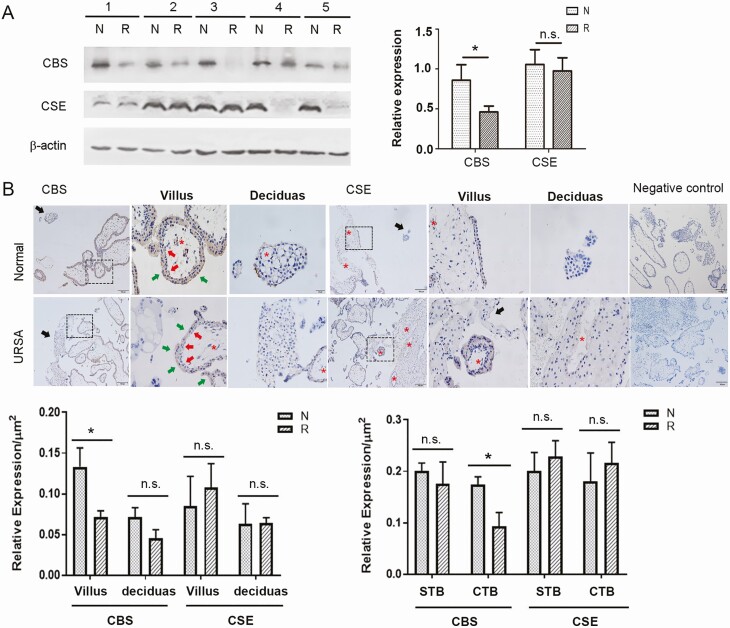
CBS and CSE proteins were detected in the first trimester placenta villi in women with normal pregnancy and in women with URSA; the villous CBS protein was significantly lower (P < 0.05), while CSE levels did not differ, in patients with URSA versus patients with normal pregnancies (Fig. 1A). CBS and CSE proteins were immunochemically localized in the first trimester placenta villi and decidua in women with URSA and women with normal pregnancies, while negative controls showed background staining. Total placental villi CBS and CSE protein levels were significantly higher in the placental villi versus the decidua. CBS and CSE proteins showed cell-specific expression patterns in first trimester placenta. CBS and CSE proteins were detectable but low in normal first trimester placental villous endothelial and stroma cells, and the protein levels in these cells were unchanged in women with URSA. Levels of CBS protein, but not CSE protein, were significantly lower in the villous cytotrophoblast (CTB), but not syntiotrophoblast, in women with URSA versus women with normal pregnancies (P < 0.05) (Fig. 1B).
Figure 1.
CBS and CSE expressions at the first trimester maternal-fetal interface in women with or without URSA. Total placental CBS and CSE protein expressions (A). First trimester placental villous tissues from normal elective abortions and patients with URSA were analyzed by Western blot, with β-actin as a control. Bar graph summarized data (mean ± SEM) from normal patients and patients with URSA patients (n = 5/group). Immunohistochemical localization of CBS and CSE proteins (B). Sections of placental villi (indicated by dashed square) and decidual tissues (indicated by black arrow heads) were stained with CBS and CSE antibodies. Images were taken, and specific staining in each cell type was quantified by imaging analysis. CTB: cytotrophoblast (red arrow); STB: syntiotrophoblast (green arrow), blood vessels (red stars). Scale bar = 100 μm. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; N, normal; n.s., not significant; R, recurrent spontaneous abortion; SEM, standard error of the mean; URSA, unexplained recurrent spontaneous abortion. * P < 0.05.
Effects of H2S on trophoblast migration, invasion, and gene expression
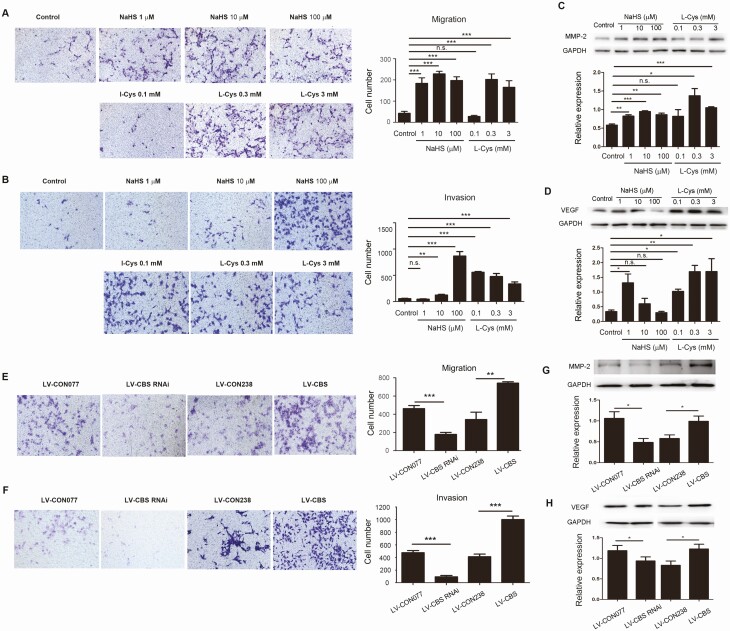
Treatment with a H2S donor NaHS and the substrate of CBS/CSE L-Cys promoted migration and invasion of HTR8/SVneo cells in different concentration-dependent and cell-specific manners. As little as 1 μM NaHS reached maximal stimulation of HTR8/SVneo cell migration as did 10 and 100 μM NaHS; however, cell invasion was stimulated with 10 μM NaHS, which was further enhanced at 100 μM. Treatment with 0.1 mM L-Cys was ineffective in cell migration but reached maximal induction in invasion; higher concentrations (0.3 and 3 mM) of L-Cys further stimulated cell migration but not invasion (Fig. 2A and 2B). Treatment with NaHS and L-Cys stimulated different concentration-dependent JEG3 cell invasion; cell invasion was significantly enhanced by 10 μM NaHS, while only slightly increased by1 µM and 100 μM NaHS with no statistical significance versus control; cell invasion was significantly enhanced by 0.3 mM L-Cys while was unaffected by 0.1 and 3 mM L-Cys [the supplemental materials (24)]. Treatment with either NaHS or L-Cys stimulated concentration-dependent upregulation of MMP-2 and VEGF proteins (P < 0.01) in HTR8/SVneo (Fig. 2C and 2D) and JEG3 [the supplemental materials (24)] cells.
Figure 2.
CBS/H 2 S pathway on migration and invasion and gene expression in HTR-8/SVneo cells. HTR-8/SVneo cells were treated with increasing concentrations of L-Cys or NaHS for cell migration (A) and invasion (B) assays using a transwell system. Cells were treated with L-Cys or NaHS for 24 hours for determining the expression of MMP-2 (C) and VEGF (D) by Western blot with GAPDH as a control. The TR-8/SVneo cells were transfected with either CBS RNAi (LV-CBS-RNAi; LV-CON077 was the control) or CBS cDNA (LV-CBS; LV-CON238 was used as the control). After verification of CBS knockdown or upregulation, the cells were used for determining migration (E) and invasion (F) by using a transwell system. Protein levels of MMP-2 (G) and VEGF (H) were determined by Western blot, and GAPDH was used as a control. Data were calculated as means ± SD from at least 3 independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001; CBS, cystathionine β-synthase; cDNA, complementary deoxyribonucleic acid; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; H2S, hydrogen sulfide; L-Cys, L-cysteine; MMP-2, matrix metalloproteinase-2; NaHS, sodium hydrosulfide; n.s., not significant; RNAi, ribonucleic acid interference; VEGF, vascular endothelial growth factor.
CBS and CSE proteins were detected in both HTR8/SVneo and JEG-3 cells; both cell lines produced H2S in the presence of L-Cys, which was significantly inhibited by AOAA and PAG [the supplemental materials (24)], similar to a previous report (32). To determine the role of endogenous H2S derived from CBS in trophoblast migration and invasion, “gain and loss of function” studies with CBS overexpression and downregulation were performed in HTR8/SVneo cells. CBS protein was significantly decreased by transfection of CBS siRNA and increased with transfection of CBS cDNA [P < 0.01, the supplemental materials (24)]. CBS knockdown significantly inhibited cell migration and invasion (P < 0.001), whereas CBS overexpression enhanced cell migration and invasion (P < 0.01) (Fig. 2E and 2F). In addition, levels of MMP-2 and VEGF were downregulated by CBS knockdown but upregulated by CBS overexpression (P < 0.05) (Fig. 2G and 2H).
CBS/H2S targets NF-κB signal pathway
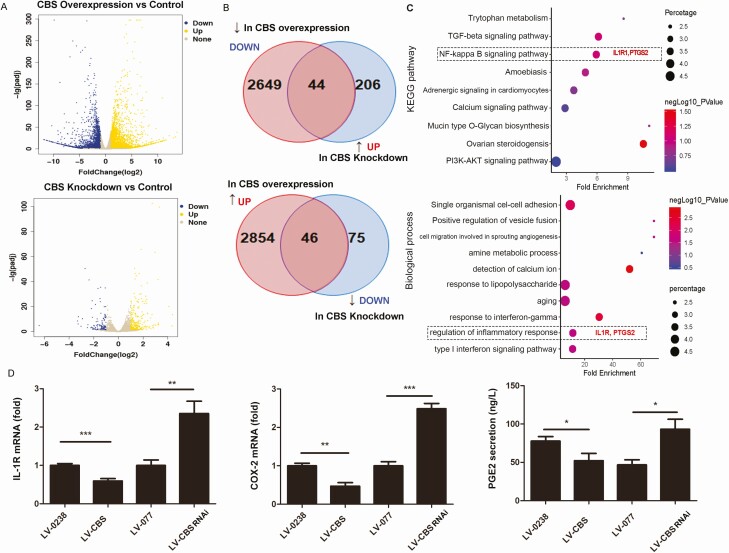
To investigate the target genes of CBS/H2S signaling in trophoblasts, we profiled the transcriptomes of HTR8/SVneo cells that were treated with CBS siRNA or cDNA by RNA-seq analysis. Compared with controls, CBS overexpression and downregulation upregulated approximately 2900 and 250 differentially regulated genes (DEGs) and downregulated 2693 and 121 DEGs, respectively (Fig. 3A and B, data were deposited as GEO datasets, PRJNA625571). Overlay analysis showed 90 common genes that were altered by both CBS overexpression and knockdown, with 44 negatively and 46 positively correlating to CBS expression. This was consistent with the KEGG pathways and biological processes associated with the overlapped genes listed in the supplemental materials (24). Of note, interleukin-1 receptor 1 (IL-1R1) and prostaglandin synthase 2/cyclooxygenase-2 (PTGS2/COX2) were enriched in the NF-κB signaling pathway and inflammatory response-related biological process (Fig. 3C). These findings were confirmed by real-time PCR and ELISA, showing that IL-1R1 and COX2 mRNAs and PGE2 secretion were downregulated by CBS overexpression but upregulated by CBS downregulation in HTR8/SVneo cells (P < 0.05) (Fig. 3D).
Figure 3.
Differentially expressed genes in HTR8/SVneo cells transfected with CBS siRNA or CBS cDNA.The transcriptomes of cells with CBS downregulation or upregulation were analyzed by RNA-seq analysis. Differentially expressed genes (DEGs) between CBS downregulation and upregulation versus control were presented in volcano plots (A) and Venn diagrams (B) and gene ontology (GO) analyses of top 9 KEGG pathways (C, upper panel) and top 10 biological processes (C, lower panel). GO analysis identified IL-1R and PTGS2 to be significantly associated with regulation of inflammatory response. IL-1R and PTGS2 (COX2) were further confirmed by qPCR (D), and PGE2 secretion was determined by ELISA (D). Data were calculated as means ± SD from at least 3 independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001. CBS, cystathionine β-synthase; cDNA, complementary deoxyribonucleic acid; COX2, cyclooxygenase-2; ELISA, enzyme-linked immunosorbent assay; IL-1R, interleukin-1 receptor 1; PGE2, prostaglandin E2; PTGS2, prostaglandin synthase 2; qPCR, quantitative polymerase chain reaction; RNA-seq, ribonucleic acid sequencing; siRNA, small interfering RNA.
CBS deficiency leads to embryonic resorption in mice
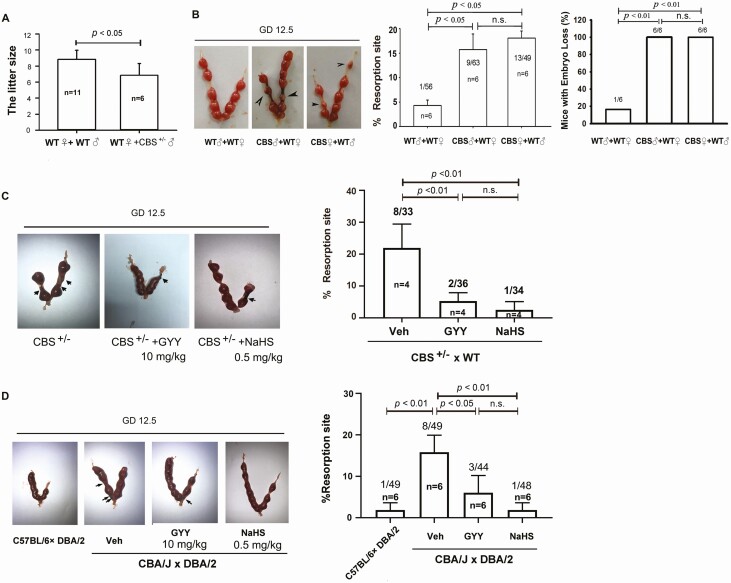
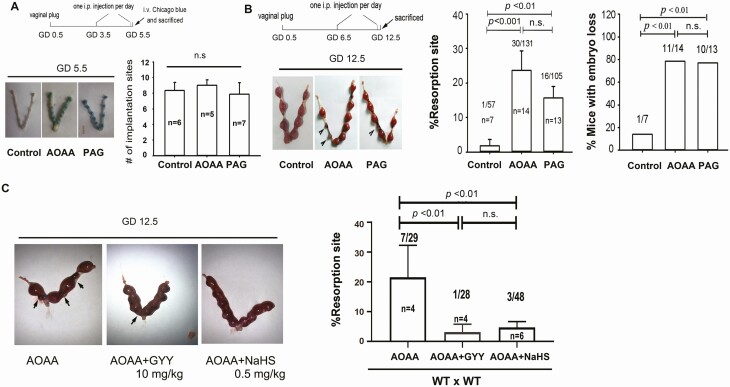
WT females mated with CBS-deficient males normally, and the dams (♀WT x ♂CBS+/–) formed vaginal plugs; however, the litter size of dams (♀WT x ♂CBS+/–) was markedly lower (P < 0.05) than that of WT dams (Fig. 4A), suggesting an important role of CBS in murine pregnancy. We then performed cross-breeding experiments among WT and CBS-deficient female and male mice to determine the importance of maternal or fetal/placental CBS-derived H2S in pregnancy. On GD12.5, the resorbed implantation sites and the percentage of embryo loss in dams of WT (♀WT) females mated with ♂CBS+/– males and ♀CBS+/– males mated with ♂WT males were significantly greater than that in WT dams (P < 0.05 for resorption; P < 0.01 for mice with embryo loss); however, both measurements in GD12.5 dams did not differ between dams with paternal (♂CBS+/–+ ♀WT) and maternal (♀CBS+/–+ ♂WT) CBS-deficiencies (Fig. 4B). These data suggest that dysregulated H2S signaling due to fetal/placental but not maternal CBS deficiency leads to early pregnancy loss in mice, independent of fetal sex. We then tested whether administration of exogenous H2S with H2S donors GYY or NaHS could rescue early pregnancy loss in CBS-deficient mice. On GD6.5, ♀CBS+/–dams were given GYY(10 mg kg-1) or NaHS (0.5 mg kg–1) via ip injection and then sacrificed on GD12.5. Treatment with either GYY or NaHS significantly alleviated CBS deficiency-induced embryo resorption (P < 0.01) (Fig. 4C). We then used the CAB/J × DBA/2 mouse model of RSA (33, 34) to further clarify whether H2S signaling plays a role in spontaneous abortion. On GD12.5, ♀CBA/J x ♂DBA/2 dams, as expected, displayed significantly (P < 0.01) increased embryo resorption compared with that of ♀C57BL/6 dams mated with ♂DBA/2 males. Administration of exogenous H2S by ip injection of GYY or NaHS significantly (P < 0.05 for GYY and P < 0.01 for NaHS) rescued embryonic resorption on GD12.5 in ♀CBA/J x ♂DBA/2 dams (Fig. 4D). We treated WT C57BL/6 dams by ip injection of either vehicle, the CBS inhibitor AOAA (50 mg kg–1), or the CSE inhibitor PAG (100 mg kg–1); the mice were used to further clarify when embryonic loss takes place due to dysregulated H2S signaling during gestation. The implantation sites on GD5.5 in all 3 groups did not differ statistically (P > 0.05) (Fig. 5A); however, on GD12.5, the embryonic resorption rates and the percentage of dams with embryonic loss in AOAA- and PAG-treated dams were significantly (P < 0.01 or greater) different from that of the vehicle controls [Fig. 5B, the supplemental materials (24)]. Consistently, treatment with either GYY or NaHS was able to effectively alleviate AOAA-induced embryonic resorption (Fig. 5C).
Figure 4.
Effects of H 2 S donors on pregnancy in CBS-deficiency mice and a spontaneous abortion mouse model. (A) Litter size in WT x ♂CBS+/– and WT x WT dams. Embryonic resorption and representative uteri with fetuses on gestational day 12.5 in CBS+/– cross-breading (♀WT/♂CBS+/– and ♂ WT/♀CBS+/–) and wild-type (WT) dams (B). The H2S donor (GYY or NaHS)-treated CBS+/– dams (C, WT as control) and CBA/J x DBA/2 spontaneous abortion dams (D, C57BL/6 x DBA/2 dams as control). Black arrowheads denote the resorption sites. The numbers within bars indicate the number of dams examined; the numbers above bars indicate the number of resorption events divided by the number of implantation sites. Differences between groups were indicated. CBS, cystathionine β-synthase; GYY, GYY4137; H2S, hydrogen sulfide; NaHS, sodium hydrosulfide; WT, wild-type.
Figure 5.
Effect of H 2 S donors on pregnancy in wild-type mice receiving CBS and CSE inhibitors. (A) WT dams were treated with daily AOAA (50 mg/kg) or PAG (100 mg/kg) starting on gestational day (GD) 3.5; implantation sites on GD 5.5 were determined and averaged to be presented in the bar graph. n.s, not significant. (B) WT dams were treated with daily AOAA (50 mg/kg) or PAG (100 mg/kg) starting on GD 6.5. (C) WT dams were treated with daily AOAA (50 mg/kg) or PAG (100 mg/kg) starting on GD 6.5 for 6 days, followed by daily treatment with either GYY (10 mg/kg) or NaHS (0.5 mg/kg) starting on GD 6.5. Dams were killed on GD12.5 for examining the number of resorbed fetuses. Resorption rate and mice with embryo loss were then calculated as means ± SD and plotted. Black arrowheads denote the resorption sites. The numbers within bars indicate the number of pregnant mice, and the numbers above bars indicate the number of resorption events divided by the total number of implantation sites. AOAA, amino-oxyacetic acid; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; GYY, GYY4137; H2S, hydrogen sulfide; n.s., not significant; PAG, propargylglycine; SD, standard deviation; WT, wild-type.
Decidual cytokine production during post-implantation period in mice
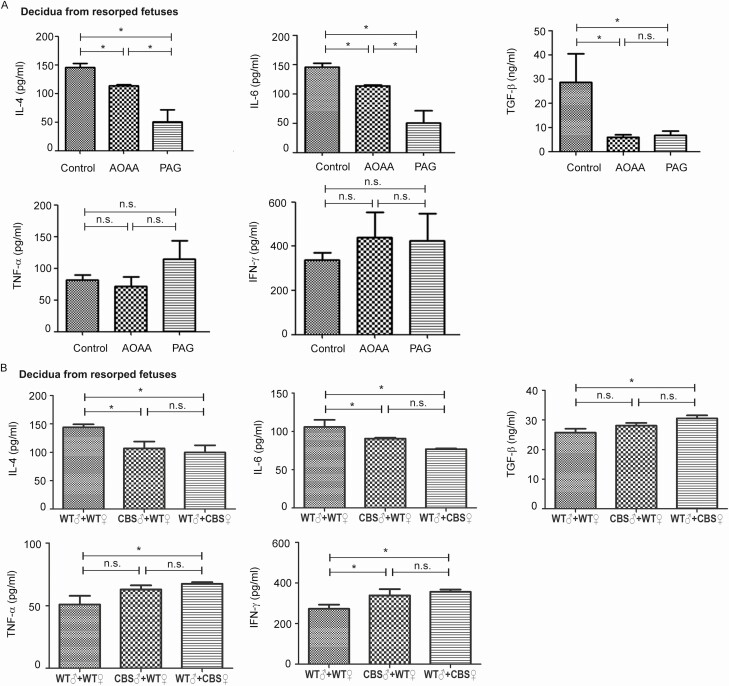
We determined whether dysregulated H2S signaling induces decidua T-helper 1 (Th1)/Th2 imbalance since imbalanced cytokine production by Th1 and Th2 cells has been shown to be causative for RSA (35). Treatment with AOAA (50 mg kg–1) or PAG (100 mg kg–1) via ip injection on GD5.5 daily for 6 days resulted in significantly (P < 0.05) lower levels of Th2 cytokines (IL-4 and IL-6) in the decidua from resorbed fetuses, without significantly altering Th1 cytokines (IFN-γ and TNFα) (Fig. 6A). Consistently, in cross-breading experiments using WT and CBS-deficient mice, both paternal and maternal CBS-deficiencies resulted in decreased Th2 cytokines (IL-4 and IL-6) and increased Th1 cytokines (IFN-γ and TNFα) in the decidua from resorbed fetuses (Fig. 6B). In addition, H2S inhibition by AOAA and PAG significantly decreased decidual TGF-β production (Fig. 6A), an immunosuppressive cytokine important for implantation (36). Consistently, the decidual TGF-β level was significantly lower in the pregnant mice model of RSA (CBA/J × DBA/2 dams), which was effectively restored by treatment with GYY and NaHS [the supplemental materials (24)].
Figure 6.
Dysregulated H 2 S signaling on Th1 and Th2 cytokine production in the decidua of resorbed fetuses. Decidua tissues were isolated from gestational day (GD)12.5 dams with resorbed fetuses; the tissue samples were homogenized for determining IL-4, IL-6, TNF-ɑ, IFN-γ, and TGF-β levels by ELISA. (A) Wild-type (WT) dams receiving AOAA (50 mg/kg) or PAG (100 mg/kg) or PBS (indicated as control, pH = 7.2) as indicated in Figure 5. (B) CBS+/– cross-breading (♀WT/♂CBS+/– and ♂ WT/♀CBS+/–) and WT dams. Data were presented as means ± SD. * P < 0.05. AOAA, amino-oxyacetic acid; CBS, cystathionine β-synthase; ELISA, enzyme-linked immunosorbent assay; H2S, hydrogen sulfide; IL, interleukin; n.s., not significant; PAG, propargylglycine; PBS, phosphate buffered saline; SD, standard deviation; TGF, tumor growth factor; Th, T-helper; TNF, tumor necrosis factor.
Discussion
Our current study demonstrates for the first time that RSA is associated with downregulated CTB CBS expression and presumptively dysregulated placental H2S signaling as CBS protein, but not CSE protein, is specifically downregulated in the CTBs in URSA fetus, whereas these H2S-producing enzymes are not significantly altered in other types of placental and decidua cells at the maternal interface. These data therefore suggest a role of endogenous placental CBS-derived H2S in the maintenance of early human pregnancy. This notion is supported by the cross-breading studies using WT and CBS-deficient (CBS+/–) mice because on GD12.5, fetal resorption occurs in fetuses from both ♂CBS+/– x ♀WT and ♀CBS+/– x ♂WT dams; the effects of dysregulated fetal/placental H2S signaling due to CBS deficiency in early pregnancy loss is independent of fetal sex because there are no differences in the fetal resorption rate and the percentage rate of pregnant mice with embryo loss on GD12.5 between ♂CBS+/– + ♀WT and ♀CBS+/– + ♂WT dams. Moreover, H2S donors can partially rescue early pregnancy loss in CBS+/–dams and CBA/J × DBA/2 RSA dams. Thus, these in vivo studies collectively demonstrate that placenta/fetal CBS-derived H2S plays a crucial role in the maintenance of early pregnancy.
Endogenous H2S is primarily synthesized by CBS and CSE that are expressed in a tissue/cell-specific manner (5). Human placenta CBS and CSE mRNAs and proteins were initially reported to be highly expressed in the fetal-placental endothelia, and CBS protein was also immunolocalized to the Hofbauer cells (12). Follow-up studies have further clarified that both CBS and CSE are expressed in the trophoblasts (14, 37). Dysregulated placental H2S signaling due to decreased trophoblast CBS and CSE promotes placental release of soluble fms-like tyrosine kinase 1 in preeclampsia (38). Trophoblast CSE protein, but not CBS protein, is downregulated in growth-restricted infants and preeclampsia with abnormal umbilical artery Doppler findings (37). In our current study, CBS is specifically downregulated in the CTB of the first trimester RSA placentas. Thus, human placenta CBS and CSE are differentially dysregulated in a cell-specific manner in different pregnancy complications. Of note, the normal samples were collected earlier in gestation than the URSA samples (GD, 7.08 ± 0.96 vs, 11.87 ± 4.0; P < 0.05), respectively, in our current study. However, the gestational age difference may be as short as 1 week and may not be a decisive factor to change our conclusion because the fetus in the URSA cases might have stopped development shortly after the first perinatal visit on GD42-49, but confirmation of fetal death takes 2 more vaginal sonography detections of fetal heartbeat, which delayed the GA of the URSA decidua/placentas to a much latter time.
How does CTB CBS-derived H2S signaling affect early pregnancy? Our data suggest that H2S signaling has little or no effect on preimplantation embryo development and implantation because inhibition of endogenous H2S production with pharmacological CBS and CSE inhibitors does not affect implantation sites on GD5.5 in mice. Following implantation, trophoblast differentiation and invasion are fundamental processes for placentation, which must be finely tuned via various pathways (39). The villous CTB differentiate into extravillous trophoblasts (EVTs); EVTs migrate and invade maternal decidua where they remodel the high-resistance, low-flow spiral artery into low-resistance, high-flow vessels (40), which is crucial for the bidirectional maternal-fetal exchanges to support fetal development and survival (41). Angiogenesis is also a key mechanism for controlling maternal-fetal interface blood flow; human trophoblast-derived endogenous H2S stimulates placental angiogenesis in vitro (32). Inadequate trophoblast invasion is detrimental to pregnancy, causing various pregnancy complications including early pregnancy loss (39). Indeed, our gain and loss of function studies with human trophoblast cell lines have shown a role of CBS-H2S signaling in regulating trophoblast function because forced CBS expression regulates cell migration and invasion, in association with altered MMP-2 and VEGF that have been previously shown to be important in trophoblast migration and invasion (42, 43). In addition, among the 46 DEGs regulated by CBS, TIMP3 (44), ITGA1 (45), and PDGFD (46) (Fig.S4) also have been proved to regulate cell migration.
Invasive EVTs also express human leukocyte antigen G and other immune regulatory molecules that protect the allograft fetus/placenta from immune rejection (47, 48). Crosstalk among decidual stromal cells, trophoblasts, and immune cells is crucial for pregnancy (49, 50). Implantation destroys luminal epithelium and initializes an inflammatory response that must be well controlled for the establishment of pregnancy (51); the maternal adaptive immune system is reshaped from Th1 to Th2 status to facilitate immune tolerance in pregnancy (52). In humans, spontaneous preterm birth is associated with elevated Th1 cytokines, including IL-1β (53) and TNF-α (54). H2S induces Th1/Th2 imbalance in broiler pneumonia (55). IL-1 is a Th1 cytokine whose production rises the earliest during early pregnancy (56). IL-1 binds its receptor IL-1R1 to activate NF-κB signaling that augments the production of leukemia inhibitory factor, IL-6, and COX2 in endometrial epithelium (57). H2S is an antioxidant that blocks the toll-like receptor 4/NF-κB pathway to inhibit inflammation (58-63). Endogenous CBS-derived H2S maintains uterine quiescence during pregnancy via inhibiting NF-κB-mediated production of IL-1β, IL-6, and TNFα (9). Aberrant COX2 and PGE2 production is causative for URSA (64, 65). Our RNA-seq analyses show that a set of NF-κB-responsive genes in HTR8/SVneo cells are altered by over- and downregulation of CBS. Moreover, CBS deficiency and inhibition result in increased IL-6 and TNF-α in the decidua of resorbed fetuses. Thus, our data demonstrate a role of CBS/H2S signaling in the Th1/Th2 balance at the maternal-fetal interface.
The immune-suppressive Treg cells are also essential for early pregnancy, and Treg deficiency is associated with unexplained infertility, miscarriage, and preeclampsia (66). CBS-/– mice develop systemic autoimmune diseases in association with dysregulated differentiation and function of Foxp3+ Treg cells; exogenous H2S can rescue Treg cell deficiency and the systemic autoimmune phenotype in CBS-/– mice (20). TGF-β is indispensable in Treg cell development, differentiation, and cell identity (67) and thus plays an important role in maintaining Treg cell homeostasis. Our data show that CBS deficiency and H2S inhibition result in decreased decidual TGF-β production, suggesting a role of CBS/H2S signaling in Treg cell homeostasis during pregnancy. This idea is further supported by our data showing that H2S regulates the other 3 immune tolerance-associated proteins [the supplemental materials (24)], including human chorionic gonadotropin and indoleamine 2,3-dioxygenase that are involved in regulating immune tolerance (68-72) and thymic stromal lymphopoietin that favors the Th2 status in the decidua (73) during early human pregnancy. Altogether, our current study provides novel evidence to demonstrate that endogenous placental CBS/H2S signaling plays a crucial role in early pregnancy by modulating immune balance at the maternal-fetal interface. Because immune imbalance is casual for URSA (35), our findings provide a novel pathway for the understanding of URSA.
Acknowledgements
The authors thank Dr Fang Yi of Shandong University for providing the CBS+/– mice and all the participants for donating the decidua/placental tissues for the study.
Financial Support: This work was supported by grants from the National Natural Science Foundation of China (#81771601), National Key Research and Development Program of China (2016YFC1302203), Taishan Scholars Program, the National Key Research and Development Program of China (2016YFC1000202, 2018YFC1002804), and the Open Project of National Research Center for Assisted Reproductive Technology and Reproductive Genetics, Shandong University. D.B. Chen was supported by the National Intitute of Health (NIH) grants RO1 HL70562 and R21 HD097498.
Glossary
Abbreviations
- AOAA
aminooxyacetic acid, CBS inhibitor
- CBS
cystathionine β-synthase
- CBS+/–
CBS-deficient
- cDNA
complementary deoxyribonucleic acid
- COX2
cyclooxygenase-2
- CSE
cystathionine γ-lyase
- CTB
cytotrophoblast
- DEGs
differentially regulated genes
- ELISA
enzyme-linked immunosorbent assay
- EVT
extravillous trophoblasts
- GA
gestational age
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GD
gestational day
- GEO
Gene Expression Omnibus
- GYY
GYY4137, water-soluble H2S donor
- H2S
hydrogen sulfide
- HTR8/SVneo cells
human placenta trophoblast cells contain SV40 viral DNA sequences
- IFN-γ
interferon-gamma
- IL-4/6
interleukin 4/6
- IL-1β
interleukin 1 beta
- IL-1R1
interleukin-1 receptor 1
- ip
intraperitoneal
- JEG3 cells
human placenta trophoblast cells
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- L-Cys
L-cysteine
- MMP-2
matrix metalloproteinase-2
- mRNA
messenger RNA
- NaHS
sodium hydrosulfide hydrate
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PAG
DL-propargylglycine, CSE inhibitor
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- pNF-κB
phospho-NF-κB
- PTGS2
prostaglandin synthase 2
- RIPA
radioimmunoprecipitation assay
- RNA
ribonucleic acid
- RNAi
RNA interference
- RNA-seq
RNA sequencing
- RSA
recurrent spontaneous abortion
- SD
standard deviation
- siRNA
small interfering RNA
- TBST
Tris-buffered saline with Tween
- TGF-β
transforming growth factor beta
- Th1/2
T-helper 1/2 cell
- TNFα
tumor necrosis factor alpha
- T-reg
regulatory T-cells
- URSA
unexplained recurrent spontaneous abortion
- VEGF
vascular endothelial growth factor
- WT
wild-type
Additional Information
Disclosure Summary: The authors have no other financial interests to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791-896. [DOI] [PubMed] [Google Scholar]
- 2. Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792-1798. [DOI] [PubMed] [Google Scholar]
- 3. Bukovska G, Kery V, Kraus JP. Expression of human cystathionine beta-synthase in Escherichia coli: purification and characterization. Protein Expr Purif. 1994;5(5):442-448. [DOI] [PubMed] [Google Scholar]
- 4. Erickson PF, Maxwell IH, Su LJ, Baumann M, Glode LM. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990;269(2):335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113(1):14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal. 2009;2(68):re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sheibani L, Lechuga TJ, Zhang H, et al. Augmented H2S production via cystathionine-beta-synthase upregulation plays a role in pregnancy-associated uterine vasodilation. Biol Reprod. 2017;96(3):664-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lechuga TJ, Qi QR, Magness RR, Chen DB. Ovine uterine artery hydrogen sulfide biosynthesis in vivo: effects of ovarian cycle and pregnancy†. Biol Reprod. 2019;100(6): 1630-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. You X, Chen Z, Zhao H, et al. Endogenous hydrogen sulfide contributes to uterine quiescence during pregnancy. Reproduction. 2017;153(5):535-543. [DOI] [PubMed] [Google Scholar]
- 10. Ning N, Zhu J, Du Y, Gao X, Liu C, Li J. Dysregulation of hydrogen sulphide metabolism impairs oviductal transport of embryos. Nat Commun. 2014;5:4107. [DOI] [PubMed] [Google Scholar]
- 11. Nuño-Ayala M, Guillén N, Arnal C, et al. Cystathionine β-synthase deficiency causes infertility by impairing decidualization and gene expression networks in uterus implantation sites. Physiol Genomics. 2012;44(14):702-716. [DOI] [PubMed] [Google Scholar]
- 12. Holwerda KM, Bos EM, Rajakumar A, et al. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta. 2012;33(6):518-521. [DOI] [PubMed] [Google Scholar]
- 13. Patel P, Vatish M, Heptinstall J, Wang R, Carson RJ. The endogenous production of hydrogen sulphide in intrauterine tissues. Reprod Biol Endocrinol. 2009;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang K, Ahmad S, Cai M, et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine γ-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127(25):2514-2522. [DOI] [PubMed] [Google Scholar]
- 15. Crosignani PG, Rubin BL. Recurrent spontaneous abortion. Hum Reprod. 1991;6(4):609-610. [DOI] [PubMed] [Google Scholar]
- 16. Coulam CB, Clark DA, Beer AE, et al. Current clinical options for diagnosis and treatment of recurrent spontaneous abortion. Clinical guidelines recommendation committee for diagnosis and treatment of recurrent spontaneous abortion. Am J Reprod Immunol. 1997;38(2):57-74. [DOI] [PubMed] [Google Scholar]
- 17. Garrido-Gimenez C, Alijotas-Reig J. Recurrent miscarriage: causes, evaluation and management. Postgrad Med J. 2015;91(1073):151-162. [DOI] [PubMed] [Google Scholar]
- 18. Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril. 2010;93(4):1234-1243. [DOI] [PubMed] [Google Scholar]
- 19. Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18(10):478-482. [DOI] [PubMed] [Google Scholar]
- 20. Yang R, Qu C, Zhou Y, et al. Hydrogen sulfide promotes Tet1- and Tet2-Mediated Foxp3 demethylation to drive regulatory T Cell differentiation and maintain immune homeostasis. Immunity. 2015;43(2):251-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang J, Chen Z, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth? Cell Mol Immunol. 2011;8(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li L, Whiteman M, Guan YY, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351-2360. [DOI] [PubMed] [Google Scholar]
- 23. Turco MY, Moffett A. Development of the human placenta. Development. 2019;146(22). [DOI] [PubMed] [Google Scholar]
- 24. Banqin W, Tonghui X, Yan L, et al. Supplementary data to the paper: Trophoblast H2S maintains early pregnancy via regulating maternal-fetal interface immune hemostasis. Harvard Dataverse. Deposited 5 May 2020. 10.7910/DVN/4BT9YB. [DOI] [Google Scholar]
- 25. Zhang D, Cheng D, Liu T, Zhang Y, Chen ZJ, Zhang C. Dysfunction of liver receptor homolog-1 in decidua: possible relevance to the pathogenesis of preeclampsia. PLoS One. 2015;10(12):e0145968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feng L, Zhang HH, Wang W, Zheng J, Chen DB. Compartmentalizing proximal FGFR1 signaling in ovine placental artery endothelial cell caveolae. Biol Reprod. 2012;87(2):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206(2):267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81(9):848-853. [DOI] [PubMed] [Google Scholar]
- 29. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44-57. [DOI] [PubMed] [Google Scholar]
- 31. Das SK, Wang XN, Paria BC, et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120(5):1071-1083. [DOI] [PubMed] [Google Scholar]
- 32. Chen DB, Feng L, Hodges JK, Lechuga TJ, Zhang H. Human trophoblast-derived hydrogen sulfide stimulates placental artery endothelial cell angiogenesis. Biol Reprod. 2017;97(3):478-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clark DA, Rahmati M, Gohner C, Bensussan A, Markert UR, Chaouat G. Seminal plasma peptides may determine maternal immune response that alters success or failure of pregnancy in the abortion-prone CBAxDBA/2 model. J Reprod Immunol. 2013;99(1-2):46-53. [DOI] [PubMed] [Google Scholar]
- 34. Ahmed A, Singh J, Khan Y, Seshan SV, Girardi G. A new mouse model to explore therapies for preeclampsia. PLoS One. 2010;5(10):e13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273(24):1933-1936. [PubMed] [Google Scholar]
- 36. Yoshinaga K. Review of factors essential for blastocyst implantation for their modulating effects on the maternal immune system. Semin Cell Dev Biol. 2008;19(2):161-169. [DOI] [PubMed] [Google Scholar]
- 37. Cindrova-Davies T, Herrera EA, Niu Y, Kingdom J, Giussani DA, Burton GJ. Reduced cystathionine γ-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: hydrogen sulfide as a placental vasodilator. Am J Pathol. 2013;182(4):1448-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu T, Wang G, Zhu Z, Huang Y, Gu H, Ni X. Increased ADAM10 expression in preeclamptic placentas is associated with decreased expression of hydrogen sulfide production enzymes. Placenta. 2015;36(8):947-950. [DOI] [PubMed] [Google Scholar]
- 39. Velicky P, Knöfler M, Pollheimer J. Function and control of human invasive trophoblast subtypes: Intrinsic vs. maternal control. Cell Adh Migr. 2016;10(1-2):154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Dijk M, Oudejans C. (Epi)genetic control of human trophoblast invasion. Front Genet. 2014;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;21(1):15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang CY, Tsai HL, Syu JS, Chen TY, Su MT. Primary cilium-regulated EG-VEGF signaling facilitates trophoblast invasion. J Cell Physiol. 2017;232(6):1467-1477. [DOI] [PubMed] [Google Scholar]
- 43. Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie D, Zhu J, Liu Q, et al. Dysregulation of HDAC9 represses trophoblast cell migration and invasion through TIMP3 activation in preeclampsia. Am J Hypertens. 2019;32(5):515-523. [DOI] [PubMed] [Google Scholar]
- 45. Li H, Wang Y, Rong SK, et al. Integrin α1 promotes tumorigenicity and progressive capacity of colorectal cancer. Int J Biol Sci. 2020;16(5):815-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jiang B, Chen J, Yuan W, et al. Platelet-derived growth factor-D promotes colorectal cancer cell migration, invasion and proliferation by regulating Notch1 and matrix metalloproteinase-9. Oncol Lett. 2018;15(2):1573-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hunt JS, Langat DL. HLA-G: a human pregnancy-related immunomodulator. Curr Opin Pharmacol. 2009;9(4): 462-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petroff MG, Chen L, Phillips TA, Hunt JS. B7 family molecules: novel immunomodulators at the maternal-fetal interface. Placenta. 2002;23(Suppl A):S95-S101. [DOI] [PubMed] [Google Scholar]
- 49. Oreshkova T, Dimitrov R, Mourdjeva M. A cross-talk of decidual stromal cells, trophoblast, and immune cells: a prerequisite for the success of pregnancy. Am J Reprod Immunol. 2012;68(5):366-373. [DOI] [PubMed] [Google Scholar]
- 50. Triggianese P, Perricone C, Chimenti MS, De Carolis C, Perricone R. Innate immune system at the maternal-fetal interface: mechanisms of disease and targets of therapy in pregnancy syndromes. Am J Reprod Immunol. 2016;76(4): 245-257. [DOI] [PubMed] [Google Scholar]
- 51. Chavan AR, Griffith OW, Wagner GP. The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr Opin Genet Dev. 2017;47:24-32. [DOI] [PubMed] [Google Scholar]
- 52. Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353-356. [DOI] [PubMed] [Google Scholar]
- 53. Romero R, Mazor M, Brandt F, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27(3-4):117-123. [DOI] [PubMed] [Google Scholar]
- 54. Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166(5):1576-1587. [DOI] [PubMed] [Google Scholar]
- 55. Wang W, Chen M, Jin X, et al. H2S induces Th1/Th2 imbalance with triggered NF-κB pathway to exacerbate LPS-induce chicken pneumonia response. Chemosphere. 2018;208:241-246. [DOI] [PubMed] [Google Scholar]
- 56. De M, Sanford TR, Wood GW. Expression of interleukin 1, interleukin 6 and tumour necrosis factor alpha in mouse uterus during the peri-implantation period of pregnancy. J Reprod Fertil. 1993;97(1):83-89. [DOI] [PubMed] [Google Scholar]
- 57. Geisert R, Fazleabas A, Lucy M, Mathew D. Interaction of the conceptus and endometrium to establish pregnancy in mammals: role of interleukin 1β. Cell Tissue Res. 2012;349(3):825-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun L, Chen L, Wang F, et al. Exogenous hydrogen sulfide prevents lipopolysaccharide-induced inflammation by blocking the TLR4/NF-κB pathway in MAC-T cells. Gene. 2019;710:114-121. [DOI] [PubMed] [Google Scholar]
- 59. Wu D, Zhong P, Wang J, Wang H. Exogenous hydrogen sulfide mitigates LPS + ATP-induced inflammation by inhibiting NLRP3 inflammasome activation and promoting autophagy in L02 cells. Mol Cell Biochem. 2019;457(1-2):145-156. [DOI] [PubMed] [Google Scholar]
- 60. Zhang GY, Lu D, Duan SF, et al. Hydrogen sulfide alleviates lipopolysaccharide-induced diaphragm dysfunction in rats by reducing apoptosis and inflammation through ROS/MAPK and TLR4/NF-κB signaling pathways. Oxid Med Cell Longev. 2018;2018:9647809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bourque C, Zhang Y, Fu M, et al. H2S protects lipopolysaccharide-induced inflammation by blocking NFκB transactivation in endothelial cells. Toxicol Appl Pharmacol. 2018;338:20-29. [DOI] [PubMed] [Google Scholar]
- 62. Huang Z, Dong X, Zhuang X, Hu X, Wang L, Liao X. Exogenous hydrogen sulfide protects against high glucose‑induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol Med Rep. 2016;14(5):4911-4917. [DOI] [PubMed] [Google Scholar]
- 63. Zimmermann KK, Spassov SG, Strosing KM, et al. Hydrogen sulfide exerts anti-oxidative and anti-inflammatory effects in acute lung injury. Inflammation. 2018;41(1):249-259. [DOI] [PubMed] [Google Scholar]
- 64. Singh N, Prasad P, Kumar P, Singh LC, Das B, Rastogi S. Does aberrant expression of cyclooxygenase-2 and prostaglandin-E2 receptor genes lead to abortion in Chlamydia trachomatis-infected women. J Matern Fetal Neonatal Med. 2016;29(6):1010-1015. [DOI] [PubMed] [Google Scholar]
- 65. Wang Y, Zhao AM, Lin QD. Role of cyclooxygenase-2 signaling pathway dysfunction in unexplained recurrent spontaneous abortion. Chin Med J (Engl). 2010;123(12):1543-1547. [PubMed] [Google Scholar]
- 66. La Rocca C, Carbone F, Longobardi S, Matarese G. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett. 2014;162(1 Pt A):41-48. [DOI] [PubMed] [Google Scholar]
- 67. Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schumacher A. Human chorionic gonadotropin as a pivotal endocrine immune regulator initiating and preserving fetal tolerance. Int J Mol Sci. 2017;18(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schumacher A, Heinze K, Witte J, et al. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J Immunol. 2013;190(6):2650-2658. [DOI] [PubMed] [Google Scholar]
- 70. Ban Y, Chang Y, Dong B, Kong B, Qu X. Indoleamine 2,3-dioxygenase levels at the normal and recurrent spontaneous abortion fetal-maternal interface. J Int Med Res. 2013;41(4):1135-1149. [DOI] [PubMed] [Google Scholar]
- 71. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191-1193. [DOI] [PubMed] [Google Scholar]
- 72. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196(4):459-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guo PF, Du MR, Wu HX, Lin Y, Jin LP, Li DJ. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood. 2010;116(12):2061-2069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.