Abstract
Neoplasia and associated tissue biomarkers in benthic fishes are commonly used to characterize effects of contaminated sediments in aquatic ecosystems. However, these fish are often migratory or partially-migratory, and thus assessing the effect of location-specific contamination is challenging because the fish will have a complex exposure history. We determined liver and skin neoplasia prevalence for a benthic, partially-migratory fish, white sucker (Catostomus commersonii), and used carbon and nitrogen stable isotope ratios to determine the diet contribution associated with areas of contaminated sediments within the urbanized portion of the St. Louis River. We then tested which factors were significantly related to neoplasia prevalence, including age, sex, and the percent diet obtained from contaminated areas within the St. Louis River relative to Lake Superior, the reference area. Overall, the prevalence of contaminant-related internal and external tumors was low, <5%. For skin neoplasia prevalence, both sex and age were significant factors, whereas location-specific diet contribution based on stable isotope analysis was not a significant factor. For liver neoplasia prevalence, only age was a significant factor. Nevertheless, for all contaminants measured (polychlorinated biphenyls [PCBs], polychlorinated dibenzodioxins [PCDDs], and polychlorinated dibenzofurans [PCDFs]), there was a significant, negative correlation between liver tissue concentration and Lake Superior diet contribution, confirming that the St. Louis River is the primary source of contaminant exposure. The research highlights the complexity of exposure to location-specific contaminants and potentially infectious agents associated with neoplasia at urban, contaminated sites in the Great Lakes, and elsewhere. It also demonstrates the need to determine the full set of risk factors across life-stages, habitats, and biological endpoints.
Keywords: white sucker, migrate, neoplasia, PCBs, dioxins, furans, Area of Concern
1. Introduction
In both freshwater (Baumann, 1992; Smith et al., 1994; Pinkney et al., 2014) and marine ecosystems (Vethaak, et al. 2009; Moore et al., 2018), neoplasia and associated tissue biomarkers in benthic fishes are used to characterize effects of contaminated sediments. In the Laurentian Great Lakes, at both US and Canadian contaminated sites, neoplasia prevalence in demersal fish species has been used to assess the severity of the ecological effect, and to track improvement after remediation (Bauman et al., 1998; Rafferty et al., 2009; Mahmood et al., 2014). There are many studies that document higher skin and liver tumor rates in benthic fishes, particularly brown bullhead (Ameiurus nebulosus) and white sucker (Catostomus commersonii), at sites contaminated with industrial wastes or effluents compared to less impacted sites (Munkittrick and Dixon, 1989; Hayes et al., 1990; Premdas et al., 1995; Baumann et al., 1996; Blazer et al., 2009 a,b; Blazer et al., 2017). However, these fish are often migratory or partially-migratory, and thus assessing the effect of localized contamination is challenging because the fish will have a complex exposure history and habitat-specific bioaccumulation throughout its life (Carr et al., 2017).
In 1987, the U.S. Environmental Protection Agency designated the St. Louis River Area of Concern (AOC) owing to historical degradation, including inappropriate discharge of untreated wastewater and debris from industrial and municipal facilities, as well as poor community land-use practices (MPCA and WDNR, 1992). The AOC boundaries encompass the lower 63 km (53.5 km2) of the St. Louis River from just upstream of Cloquet, Minnesota to the river’s mouth where it enters Lake Superior and where the port cities of Duluth, Minnesota and Superior, Wisconsin are situated (Fig. 1). Contaminants of concern include polychlorinated biphenyls (PCBs), polychlorinated dibenzodioxins (PCDDs, or dioxins), polychlorinated dibenzofurans (PCDFs, or furans), polyaromatic hydrocarbons (PAHs), and heavy metals, all of which are present at multiple locations and distributed heterogeneously within the AOC (Crane et al., 2002, 2005). The combined area of all sediment remediation areas is 9.6% of the area (5.2 km2) within the St. Louis River AOC (WDNR and MPCA, 2015; Fig. 1). Generally, sediment contaminant concentration is highest in Superior Bay and St. Louis Bay. For example, PCBs are among the most spatially extensively characterized contaminants; in sediment remediation areas, surface sediment concentration varies by region, with the greatest concentrations observed in Superior Bay and the least in the lower river, between Fond du Lac dam and St. Louis Bay (Table 1, Fig. 1).
Figure 1.
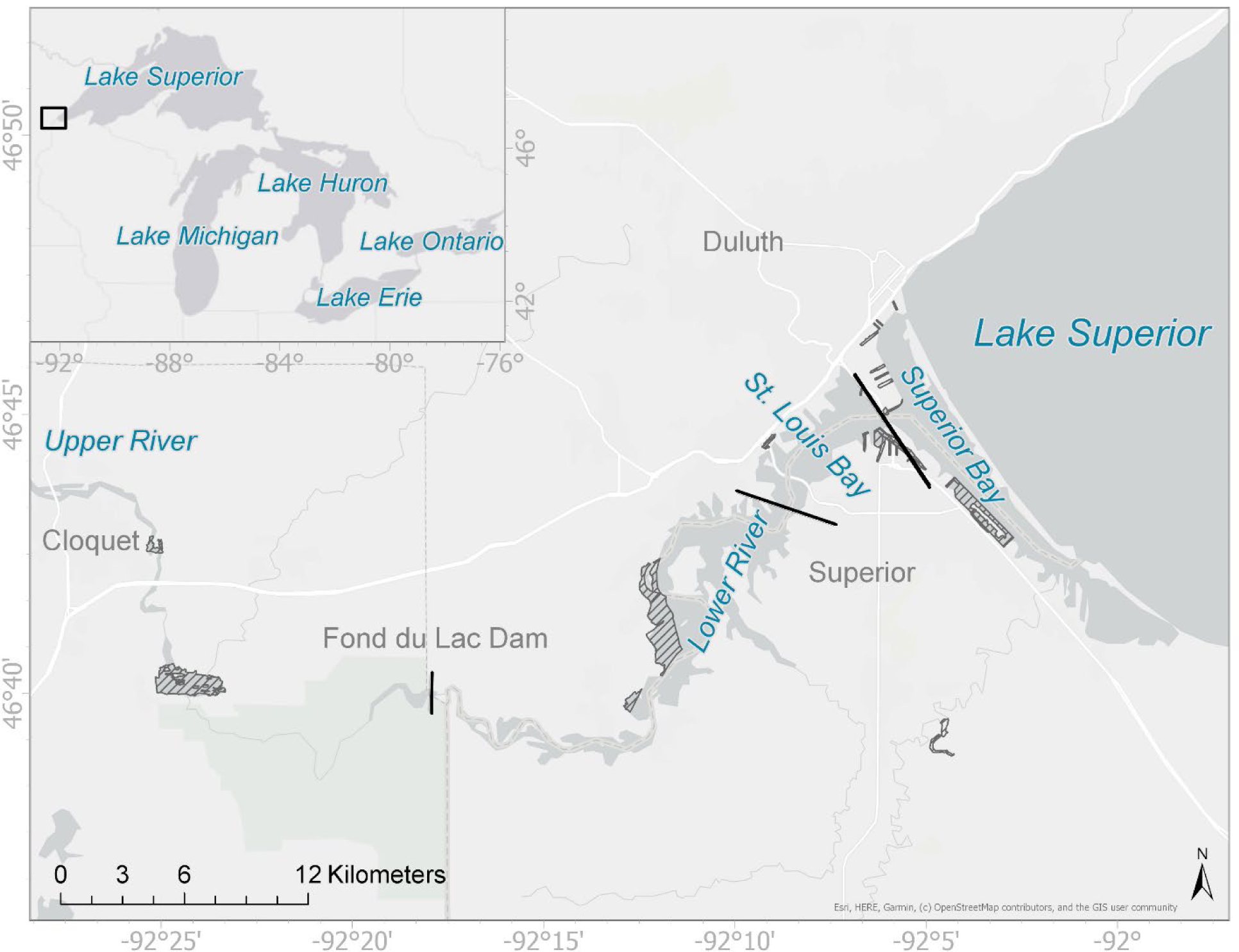
Lake Superior, Superior Bay, St. Louis Bay, Lower River, and Upper River locations. The upriver boundary of the AOC is outside the map, upriver of Cloquet, Minnesota. Hatched areas indicate boundaries of remediation sites.
Table 1.
Surface sediment (0–18 cm depth) concentrations of total polychlorinated biphenyls (PCBs) in remediation areas within the St. Louis River Area of Concern, including the number of cores (n) and the analytical method (% Aroclor data), either summed Aroclors (generally 7–9 Aroclors analyzed) or summed congeners (number of congeners study dependent). Samples were obtained between 1992–2016, and non-detects were assigned 50% of the minimum detection level, which was study dependent. All data were acquired from the DIVER database (NOAA DIVER, 2019).
| Sites | Geometric mean, range (ppb) | n | % Aroclor data |
|---|---|---|---|
| Superior Bay | 137.03, 0.02 – 17,500.00 | 332 | 86.8 |
| St. Louis Bay | 434.66, 9.00 – 4,332.42 | 283 | 63.6 |
| Lower River | 92.31, 0.71 – 1,125.00 | 217 | 100.0 |
| Upper River | 524.74, 313.00 – 1,260.00 | 71 | 100.0 |
When the AOC was designated, the Fish Tumors and Other Deformities beneficial use impairment (BUI) was listed as one of nine such impairments (or BUIs). It was identified based on observations at the time; however, no studies to document the types, severity, or prevalence of fish tumors were conducted until an assessment was initiated in 2011. The BUI removal target was established by stakeholders (MPCA, 2013) as: “Incidence rates of contaminant-related internal and external tumors and deformities in resident benthic fish species, including neoplastic or pre-neoplastic liver tumors, do not exceed incidence rates from unimpaired areas elsewhere in the Great Lakes Basin.” Based on findings from an initial assessment in 2011 (Blazer et al., 2014a), it was decided that the reference tumor prevalence would be determined by white sucker that reside in western Lake Superior owing to the relatively low concentrations of legacy contaminants, particularly PCBs and PAHs, in sediments (Marvin et al., 2004). As an indicator of effects from localized contamination, white sucker should be treated cautiously because they are migratory, sometimes undertaking movements of great distances to spawn in suitable stream habitat during the spring (Quinn and Ross, 1985; Doherty et al., 2010). The white sucker population in the St. Louis River AOC was presumed to be partially residential; that is, fish captured near the river mouth would have been able to move freely between the river and Lake Superior, and at unknown intervals. White sucker that largely reside in Lake Superior are difficult to capture while in Lake Superior, but they are readily captured in the St. Louis River during the spawning migration, when, based on the preliminary stable isotope data collected in 2011, they mix with river-resident white sucker (Blazer et al., 2014a).
For migratory fish, stable isotope ratios can be used to evaluate location- or habitat-specific exposure to contaminants (Carr et al., 2017). In the St. Louis River, carbon (C) and nitrogen (N) stable isotope ratios in soft tissues (e.g., dorsal muscle) can be used to reliably distinguish among fish that reside (feed) in Lake Superior from either those that reside in the lower river (i.e., generally, above St. Louis Bay up to Fond du Lac dam) or Superior Bay and St. Louis Bay combined (Blazer et al., 2014a; Fig. 1). The carbon (C) or nitrogen (N) stable isotope composition, 13C:12C or 15N:14N (denoted as δ13C or δ15N, respectively), of soft tissues in a fish is a time-integrated biomarker that reflects its diet. The average difference between the C and N stable isotope composition of a consumer and its recent diet is +0.4 ‰ δ13C and +3.4 ‰ δ15N for whole organisms and muscle tissue (Vander Zanden and Rasmussen, 2001). Thus, where the stable isotope composition of prey differs among locations, often owing to underlying biogeochemical differences among those locations (as between Lake Superior and the St. Louis River), and where location-specific feeding occurs long enough (ca. 6–12 months) to change the fish’s isotopic composition, it can be used to characterize fish movement or site fidelity (Hoffman, 2016). We used this method because there exists isotopic mixing along the “estuarine” portion of the river from Fond du Lac dam to Lake Superior (Stortz, and Sydor, 1980); this mixing creates a persistent gradient in δ13C and δ15N values that can be used as a spatially-explicit intrinsic tag (Fig. 2; Hoffman et al., 2010). In addition, 15N-enriched nitrogen from a regional wastewater treatment plant with an outfall in St. Louis Bay provided additional ability to isotopically distinguish St. Louis Bay and Superior Bay (Hoffman et al., 2012). The assessment included acquiring residency information (specifically, location of feeding as inferred from carbon and nitrogen stable isotope ratios) to separate river residents and lake migrants captured during our study, which allowed us to address the BUI by asking whether increased feeding in the AOC (versus outside the AOC, in western Lake Superior) is associated with increased neoplasia prevalence.
Figure 2.
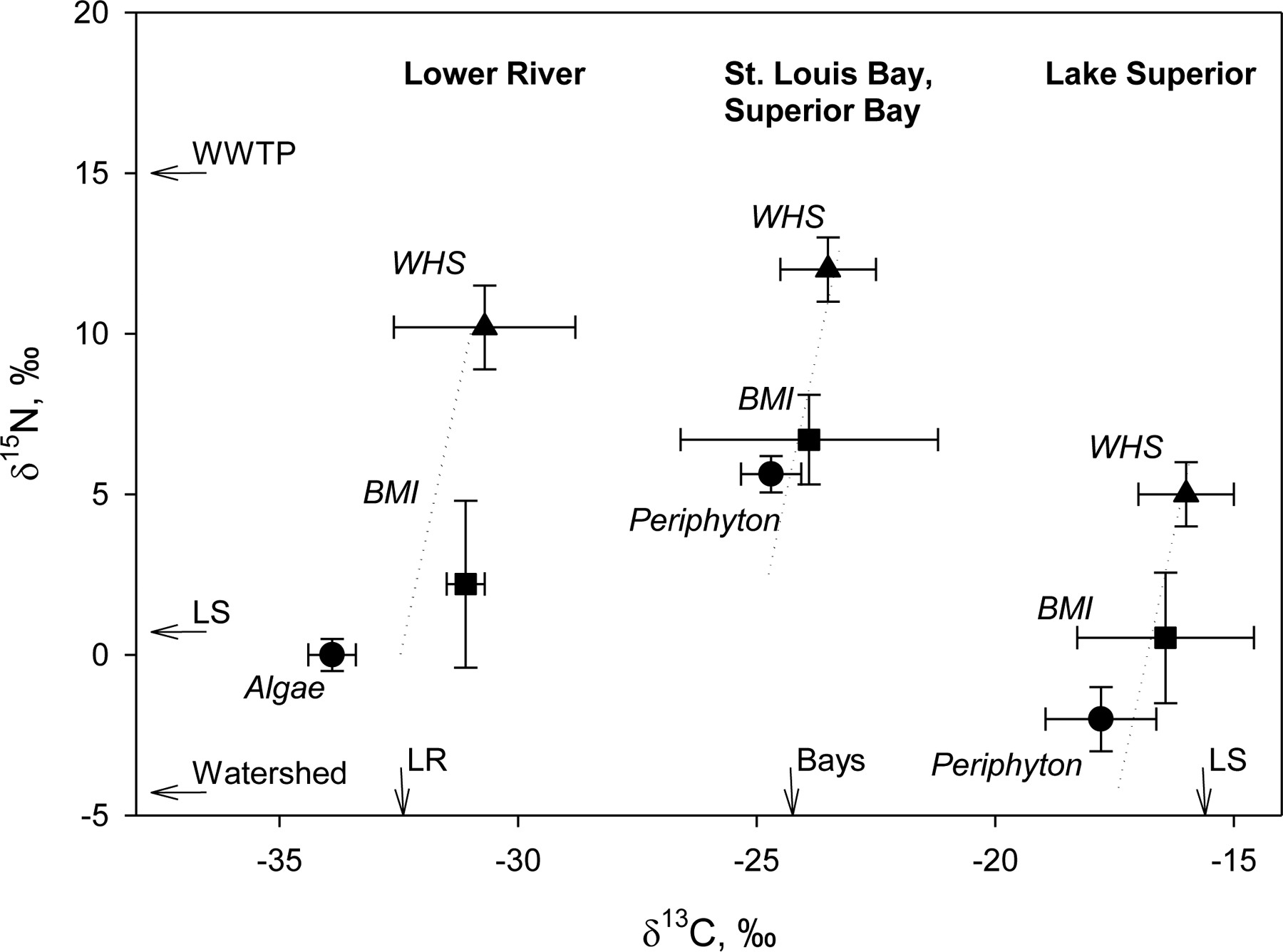
Food web δ13C and δ15N values for the Lower River, St. Louis Bay and Lake Superior Bay (Bays), and Lake Superior (LS). Food web compartments include dissolved inorganic carbon δ13C values by site (indicated by arrows, based on an isotopic fractionation of −21‰ for algae and −14‰ for periphyton; Hoffman et al., 2015a), nitrate δ15N values by source (indicated by arrows, WS: watershed; WWTP: wastewater treatment plant; Hoffman et al., 2012), primary producers (algae, periphyton; Hoffman et al., 2015a), benthic macroinvertebrates (BMI, a mix of sediment-dwelling invertebrates, including mayfly nymphs [Family: Hexagenia] and midge larvae [Family: Chironomidae]; Hoffman et al., 2012), and putative “resident” white sucker (WHS; Blazer et al., 2014a). Dashed lines indicate three trophic levels of isotopic fractionation (+0.4‰ δ13C, +3.4‰ δ15N per trophic level; Vander Zanden and Rasmussen, 2001).
To better understand exposure regimes and tumor development, we tested the hypothesis that neoplasia incidence increases with respect to the percent diet obtained from the AOC (contaminated site) relative to Lake Superior (the reference condition) using data from white sucker collected during the spawning migration in 2011, 2013, and 2015. An initial assessment based on fish captured in 2011 demonstrated a high (31%) prevalence of raised skin lesions, although only 4.5% were neoplasms (papillomas; Blazer et al. 2014a). Liver neoplasm prevalence was also low (4.5%) and only bile duct tumors were observed. Notably, the prevalence of skin tumors varied by site, with fish captured in St. Louis Bay having the highest prevalence of papillomas and other skin lesions. In the 2011 study, the approach of integrating location-specific diet tracers proved promising to address location-specific effects, but the small sample size impeded statistical analysis, and there was not contaminant data from fish tissue to demonstrate the location-specific tracers were a proxy for exposure. Thus, we also conducted contaminant analysis on a subsample of fish to test the hypothesis that concentrations of certain chemicals vary with respect to the Lake Superior diet contribution. Herein, we summarize data collection and assessment associated with the Fish Tumors and Other Deformities BUI, which includes the prevalence of white sucker fish tumors and deformities, as well as potential risk factors for liver and skin neoplasms, including exposure to location-specific contamination as indicated by carbon and nitrogen stable isotope ratios.
2. Materials and methods
2.1. Field sampling and analytical methods
We sampled adult white sucker during the spawning period: May 3–23, 2011, May 29–30, 2013 and May 6–8, 2015. A total of 200 fish were collected in 2011, 172 in 2013, and 250 in 2015. White sucker were collected at up to four sites (Fig. 1): Superior Bay, St. Louis Bay, Lower River, and Upper River. The Fond du Lac dam is located at the upriver end of the Lower River site; the dam lacks fish passage facilities, preventing upstream movement of fish. Fish were collected by seine, trap-nets, and backpack and boat electroshocking. Individuals > 250 mm total length were captured to ensure they were 3 years of age or older. Fish were euthanized with Finquel™ (Argent Chemical Laboratories, Inc., Redmond, WA), weighed (±1 gm), and measured (total length ± 1 mm).
Age was determined by reading annual rings of the lapillus otoliths, which were prepared and read using a modification of the multiple-stage process described by Koch and Quist (2007). Sections were read under transmitted light using a stereo microscope. Region-specific median ages were estimated, and regional differences were statistically analyzed for 609 white suckers (2011–2015 combined) using the Kruskal-Wallis test because the age data were not normally distributed.
A necropsy-based assessment was completed on all fish collected to document grossly visible abnormalities. Pieces of any observable abnormalities were preserved in Z-fix (Anatech LtD, Battle Creek, MI) for subsequent histological analyses. At least five sections from throughout the liver were placed in Z-fix for subsequent microscopic analyses. The remaining liver was placed in a glass vial and frozen at −80 °C. The fixed tissue samples were trimmed into cassettes, routinely processed and embedded into paraffin. Blocks were sectioned at 6 μm and routinely stained with hematoxylin and eosin (H&E). We examined microscopically tissue sections of skin for non-neoplastic (hyperplastic) proliferative, presumptive preneoplastic, and also documented neoplastic changes as previously described (Blazer et al., 2014a, 2017). We note that while some investigators have included bile duct proliferation or hyperplasia and foci of cellular alteration (including basophilic, eosinophilic, vacuolated and clear cell foci) as preneoplastic lesions, it has not been determined which, if any of these lesions are preneoplastic in white sucker. Hence, the current consensus is that these lesions should be documented but not included as “neoplastic or preneoplastic liver tumors” (Blazer et al., 2006). Thus, the data for foci of cellular alterations and bile duct proliferation are presented separately from the neoplasms. All histology slides were examined by two pathologists and a subset was reviewed by an independent reviewer for quality assurance.
A section of dorsal muscle (approximately 1 square centimeter) was removed from the mid-body area above the lateral line, dried (55° C for 24 hr), and ground for stable isotope analysis. Samples were analyzed using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (University of California-Davis Stable Isotope Facility). Stable isotope ratios were reported in δ notation in which δX: δX = (Rsample/Rstandard − 1) × 103, where X is the C or N stable isotope, R is the ratio of heavy:light stable isotopes, and Vienna Pee Dee Belemnite and air are the standards for δ13C and δ15N, respectively. The analytical error, the mean standard deviation (SD) of replicate laboratory reference material, was ±0.1 ‰ for both δ13C and δ15N. The δ13C values were corrected for lipid content because the molar C:N varied among individuals (molar ratio, range 3.1–6.7), indicating variable lipid content; we used the mass balance correction from Hoffman et al. (2015b). Of the 622 fish collected, 619 were analyzed (one sample from each of St. Louis Bay, the Lower River, and the Upper River were not successfully analyzed).
To determine whether there was a relationship between neoplasia presence and tissue contaminant concentration, in 2015 only, after samples from individual fish were diagnosed microscopically, 10 normal livers and 10 livers with abnormalities were analyzed for congener-specific concentrations of PCBs, PCDDs (dioxins), and PCDFs (furans), as well as lipid content. We focused on these three contaminants because exposure is associated with neoplasia and because they metabolize slowly relative to PAHs and bioaccumulate (e.g., Malins et al, 1987); thus, tissue concentration is an integrated proxy for lifetime exposure. The low number of individuals with neoplasms necessitated analyzing tissue from fish captured in different sites. For liver tissue contaminant analysis, the mean sample size was 2.3 gm wet weight (range 0.6 to 4.2 gm). For PCB congeners, the analytical method was in accordance with EPA Method 1668A, but modified by AXYS (AXYS, 2011a), and reported as picograms per gram wet weight for all 209 PCB congeners (78 were coelutions of more than one congener). For dioxins and furans, gas chromatography coupled with high resolution mass spectrometry (GC/HRMS) was used for sample analysis (7 PCDDs, 8 PCDFs), and reported as picograms per gram wet weight (AXYS, 2011b; AXYS, 2011c). Lipid content was determined gravimetrically (AXYS, 2011a).
2.2. Stable isotope mixing model
To determine habitat use based on stable isotope analysis, a dual stable isotope, mass balance mixing model (Phillips and Gregg, 2001) was used to quantify the percentage of an individual white sucker’s diet that was derived from each of three areas (i.e., “sources”): Lake Superior, Superior Bay and St. Louis Bay combined, and the Lower River. Methods followed those described in Blazer et al. (2014a). In brief, we estimated the proportional source contributions for each white sucker sampled below Fond du Lac dam (n = 465; the remaining 154 fish were sampled above the Fond du Lac Dam and presumed to be land-locked). Source stable isotope ratios for putative resident white sucker were as follows: Lower River δ13C −34.0‰ (SD ±1.9%), δ15N 8.6% (SD ±1.3%); Superior Bay and St. Louis Bay δ13C −23.5% (SD ±1.0%), δ15N 12.0% (SD ±1.0%); and Lake Superior δ13C −16.0% (SD ±1.0%), δ15N 5.0% (SD ±1.0%). Source values were based on available prey data (Blazer et al., 2014a), and source error accounts for prey isotopic composition error and trophic enrichment factor error (0.4 ±1.2‰ δ13C, 3.4 ±0.2‰ δ15N; Vander Zanden and Rasmussen, 2001). Source contribution error, along with source error, was estimated using error propagation theory (Phillips and Gregg, 2001). We combined Superior Bay and St. Louis Bay because prey items have a similar isotopic composition (Hoffman et al., 2010). As required, δ13C and δ15N values were iteratively fit to constrain proportional contributions to values between 0 and 1; the mean absolute fit value for δ15N was 0.6 % (maximum = 5.9%, n = 50 individuals), and for δ13C was 1.8% (maximum = 3.1%, n = 3 individuals).
2.3. Statistical Analysis
To evaluate risk factors for tumor incidence, we used logistic regression to test relationships between prevalence of either skin or liver neoplasia and both biological variables and habitat usage (i.e., amount of diet derived from a certain area based on stable isotope signatures). The factors were sampling year, age, sex, percent Lower River diet, and percent Lake Superior diet. The latter two variables were outputs of the stable isotope mixing model. The percent Lower River diet variable was included because this is the portion of the system in which there is widespread surficial sediment contamination, including PCBs and dioxins (Crane et al., 2005). The Lake Superior diet variable was included because a lake versus estuary distinction might be significant because sediment contaminant concentrations in Lake Superior are much lower than concentrations in the river. Sampling year was not a significant factor in either model; subsequently, the data were re-analyzed excluding this variable. To determine the degree to which age and habitat use could be confounded, for fish captured below Fond du Lac dam (i.e., those that are potentially migratory), we used Spearman rank order correlation to test the correlation between age and Lake Superior diet contribution (arcsin-transformed). Finally, a logistic regression with age and site as factors was used to test whether tumor prevalence varied between the St. Louis River below (pooling Superior Bay, St. Louis Bay, and the Lower River regions; n = 455) and above Fond du Lac dam (Upper River; n = 154).
Further, using the sample of 20 fish from 2015, we tested whether fish with neoplasia had higher liver contaminant concentrations than those without liver neoplasia (lipid-normalized, log-transformed concentrations; t-test for PCBs and furans, and Mann-Whitney test for dioxins), as well as the relationship between liver contaminant concentration and Lake Superior diet contribution (Pearson correlation). We calculated total PCBs, dioxins, and furans as the sum of congener-specific concentrations; where the value was below reporting limit, similar to the blank concentration, or less than the lowest calibration equivalent, or else the detected peak was of poor quality, we did not include the value to calculate the sum. For all statistical tests, an α-level of 0.05 was used to indicate significance. Statistical analyses were conducted using SYSTAT (v. 13.00.05).
3. Results
In the three years of monitoring, a total of 622 white sucker (309 females and 313 males) were examined. Fish ranged in age from 2 to 25 years (18 cohorts spanning 24 years, 1988–2011). In all years, the female: male ratio was close to 1:1, and the mean age of females and males were similar. The mean age of fish collected in 2013 was somewhat lower than those collected in 2011 and 2015 (Table 2). We were unable to locate and capture fish in St. Louis Bay in 2013.
Table 2.
Mean (± standard deviation) total length, wet weight, and age, as well as prevalence (percentage) of select external observations and microscopic lesions for white sucker collected within the St. Louis River Area of Concern in 2011, 2013, and 2015. Data are given by sex (F – female, M – male), site, or pooled (All), with the associated sample size (n).
| External Observations | Microscopic Liver Lesions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Site or Sex | N | Age, yr | Length, mm | Weight, gm | Raised Lesions | Orocutaneous neoplasms | Bile duct proliferation | Altered foci | Hepatic neoplasms |
| 2011 | F | 94 | 7.9 ± 2.1 | 427.1 ± 46.7 | 923.4 ± 321.1 | |||||
| M | 106 | 8.5 ± 2.7 | 408.4 ± 48.5 | 736.0 ± 185.8 | ||||||
| Superior Bay | 50 | 7.3 ± 2.3 | 395.8 ± 65.1 | 678.9 ± 209.1 | 10 | 0 | 52 | 8 | 4 | |
| St. Louis Bay | 50 | 8.6 ± 2.5 | 412.2 ± 36.5 | 756.9 ± 212.8 | 44 | 12 | 40 | 6 | 4 | |
| Lower River | 50 | 8.8 ± 2.8 | 430.0 ± 42.5 | 959.6 ± 334.6 | 32 | 4 | 58 | 4 | 6 | |
| Upper River | 50 | 8.1 ± 1.9 | 430.8 ± 36.2 | 900.9 ± 231.5 | 38 | 2 | 46 | 0 | 4 | |
| All | 31 | 4.5 | 49 | 3.5 | 4.5 | |||||
| 2013 | F | 90 | 6.6 ± 2.5 | 413.2 ± 58.0 | 749.2 ± 246.7 | |||||
| M | 82 | 6.4 ± 3.2 | 379.9 ± 42.7 | 561.8 ± 177.9 | ||||||
| Superior Bay | 89 | 6.0 ± 1.9 | 380.8 ± 37.1 | 584.9 ± 189.7 | 2.2 | 1.1 | 42.7 | 4.5 | 4.5 | |
| Lower River | 30 | 8.8 ± 4.3 | 440.3 ± 40.5 | 864.2 ± 233.2 | 10 | 10 | 73.5 | 3.3 | 16.7 | |
| Upper River | 53 | 5.9 ± 2.4 | 404.6 ± 60.1 | 670.1 ± 239.5 | 11.3 | 3.8 | 45.3 | 1.9 | 1.9 | |
| All | 6.4 | 3.5 | 48.8 | 3.5 | 5.8 | |||||
| 2015 | F | 125 | 7.6 ± 2.8 | 432.4 ± 49.6 | 974.3 ± 409.0 | |||||
| M | 125 | 7.2 ± 2.5 | 399.9 ± 33.5 | 724.0 ± 188.4 | ||||||
| Superior Bay | 37 | 7.4 ± 1.8 | 397.7 ± 41.4 | 924.8 ± 484.1 | 10.8 | 5.4 | 48.6 | 2.7 | 0 | |
| St. Louis Bay | 87 | 8.1 ± 2.8 | 417.4 ± 39.4 | 874.5 ± 348.1 | 16.1 | 4.6 | 55.2 | 5.7 | 4.6 | |
| Lower River | 75 | 7.4 ± 2.6 | 422.1 ± 38.6 | 864.8 ± 267.1 | 14.7 | 8 | 64 | 0 | 6.7 | |
| Upper River | 51 | 6.2 ± 2.5 | 396.5 ± 53.6 | 728.0 ± 278.8 | 7.8 | 2 | 33.3 | 2 | 2 | |
| All | 13.2 | 5.2 | 53.2 | 2.8 | 4 | |||||
External raised lesions included raised reddened lesions, discrete white raised areas, slightly raised mucoid and papillomatous lesions on the lips and lesions on the body surface and fins (Fig. 3). Microscopically, most discrete white areas and mucoid lesions were hyperplasia of epidermal cells. Raised lip, body surface and fin papillomatous lesions were papillomas or benign neoplasms of the epidermis, as previously described (Blazer et al., 2014b; Blazer et al., 2017). Liver microscopic lesions (described in Blazer et al. 2014b; Blazer et al. 2017) included inflammation and necrosis caused by helminth (cestode) parasites within the hepatic parenchyma, foci of cellular alteration, inflammation and fibrosis of bile ducts, the presence of a myxozoan parasite within the bile ducts, bile duct proliferation and neoplasms (Fig. 4). Neoplastic lesions of the liver were bile duct neoplasms (cholangioma, cholangiocarcinoma), although one fish had both a hepatocellular adenoma and cholangiocarcinoma.
Figure 3.
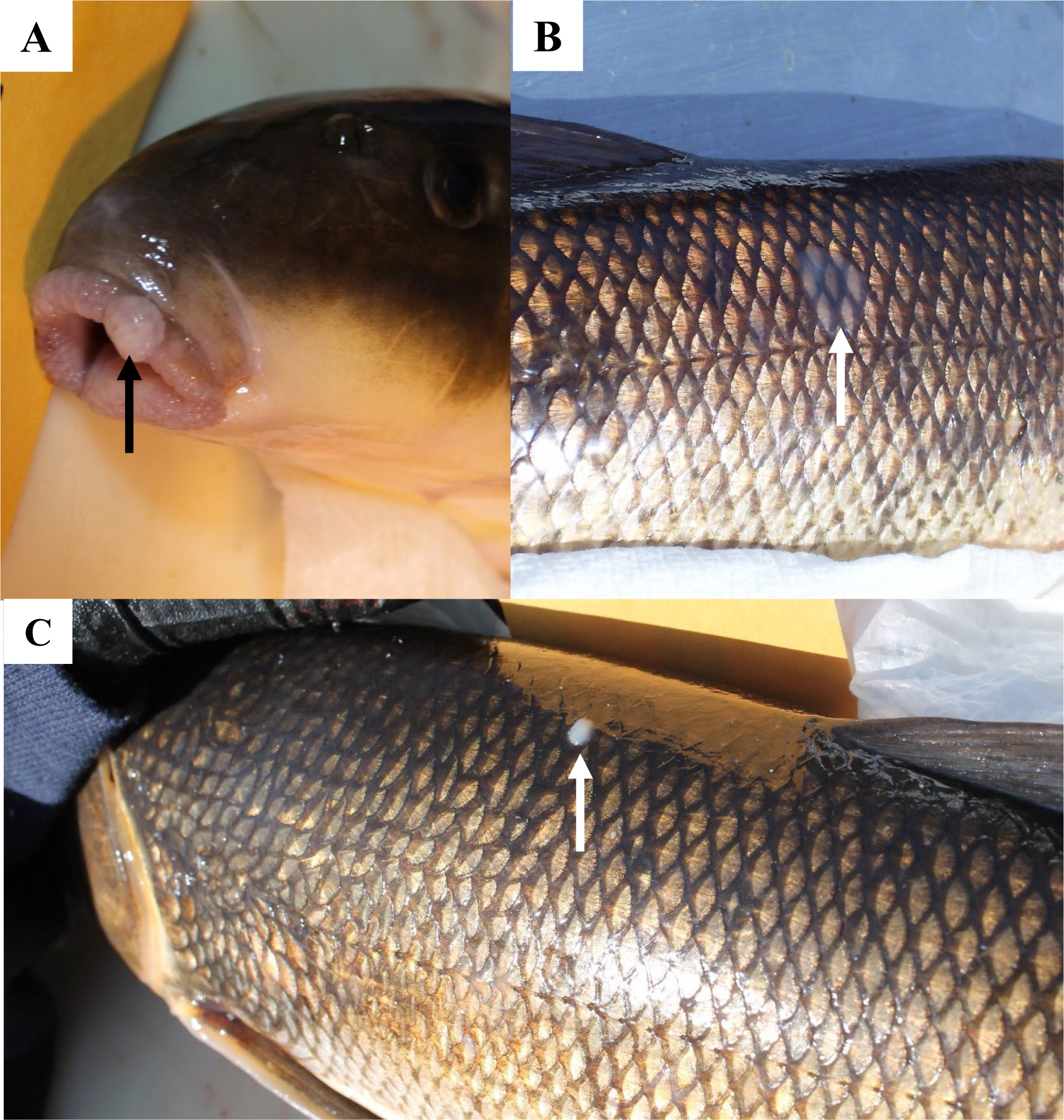
External skin and lip lesions observed on white sucker captured in the St. Louis River Area of Concern. A. Raised lip lesion (arrow). B. Slightly raised mucoid lesion (arrow) on the lateral body surface. C. Discrete white area (arrow) on dorsal body surface.
Figure 4.
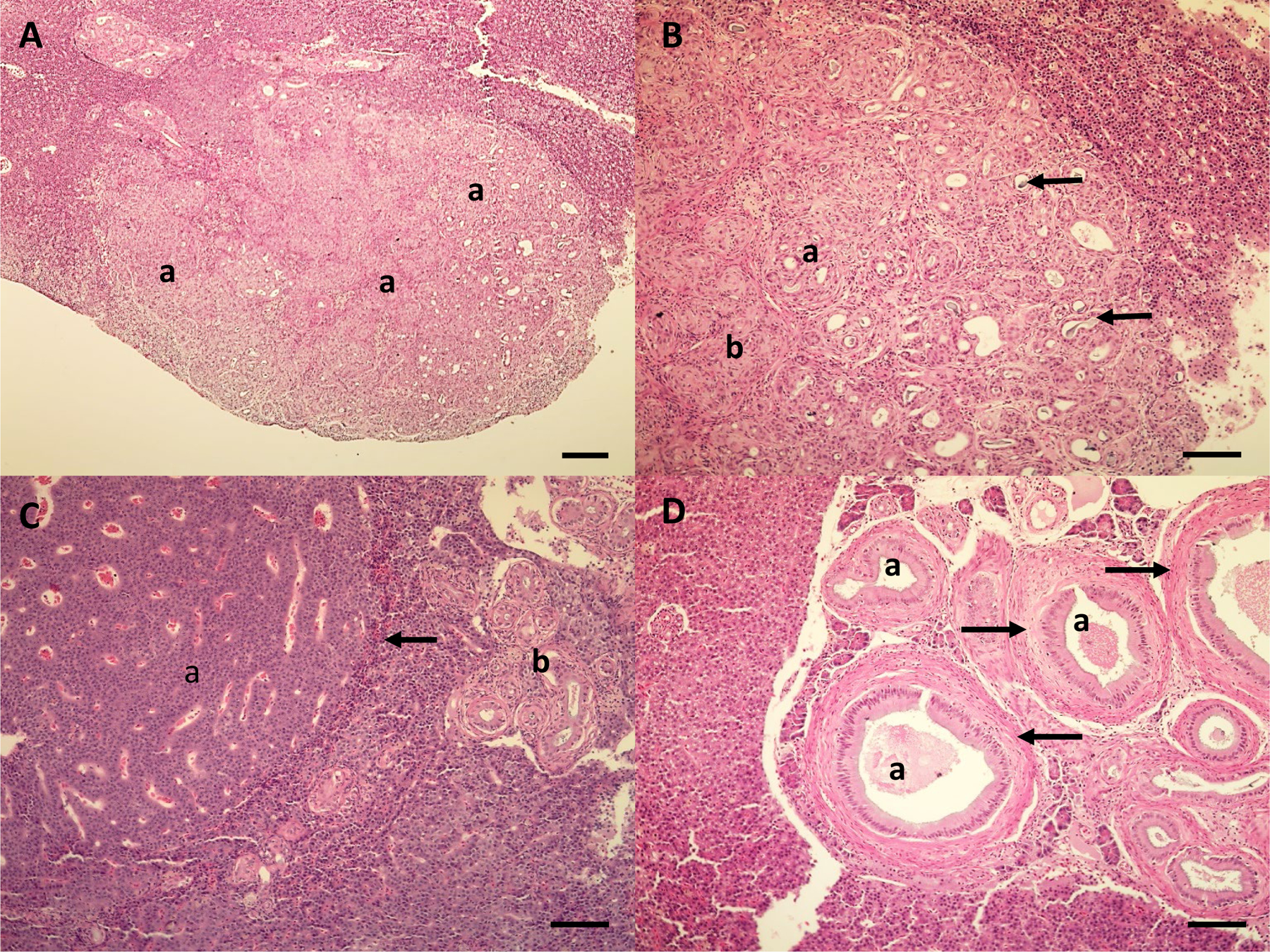
Microscopic lesions in the liver of white sucker from the St. Louis River Area of Concern. A. Cholangiocarcinoma (a) on the surface of the liver. Scale bar equals 200 μm. B. Higher magnification of cholangiocarcinoma illustrating proliferating bile ducts of varying shape and size. Some areas are well differentiated (a), while other areas (b) have increased atypia and pleomorphism. Some bile ducts have bile present in the lumen (arrows). Scale bar equals 100 μm. C. Hepatic cell adenoma (a) with compression along the periphery of the adenoma (arrow). Bile duct proliferation and fibrosis (b) is also present. Scale bar equals 100 μm. D. Proliferation of bile ducts (a) with fibrosis (arrows). Scale bar equals 100 μm. Hematoxylin and eosin stain.
In 2011, 31% of the fish had some type of raised orocutaneous lesions, however only 4.5% were neoplastic, and the prevalence varied among fish captured at the four sites, with the lowest prevalence in Superior Bay and the highest in St. Louis Bay (Table 2). All of the skin neoplasms were papillomas and were observed in fish 4 years and older. In 2013, of the 11 raised lesions observed, 6 were papillomas (3.5% of all fish sampled), and the remaining 5 were hyperplastic lesions. Neoplastic orocutaneous lesions were observed in fish age 7 or older. Similar to 2011, in 2013, the lowest prevalence was observed in white sucker collected in Superior Bay, whereas the highest was in the Lower River. In 2015, orocutaneous neoplasms were observed in 5.2% of sampled fish, and all were papillomas observed in fish 6 years of age and older. Fish collected in 2015 from the Upper River had the lowest neoplasm prevalence, while those from the Lower River had the highest. (Table 2)
The prevalence of liver neoplasms observed in 2011 was 4.5% (Table 2). All observed neoplasms were of bile duct origin and were observed in fish 6 years and older. White sucker collected in the Lower River had a slightly higher prevalence than the other sites. In 2013, liver neoplasms were observed in 5.8% of fish. One fish had both a cholangiocarcinoma and a hepatic cell adenoma, all others had only bile duct tumors. Liver neoplasms were only observed in white sucker age-7 and older, and fish collected in the Lower River had the highest prevalence. In 2015, all liver neoplasms (4.0%) observed were of bile duct origin, both cholangiomas and cholangiocarcinomas, and were observed in fish 5 years and older. Again, fish captured in the Lower River had the highest prevalence.
Pooling years, 106 (17.0%) had raised orocutaneous lesions, however only 27 (4.3%) of these were skin neoplasms, all of which were papillomas (Table 3). A total of 4.7% of white suckers had liver neoplasms. In general, liver and skin neoplasms were highest in the Lower River and lowest in the Upper River. There were significant age differences among sites (Kruskal-Wallis test statistic = 43.9, df = 3, p<0.001). The median age was 8 years in St. Louis Bay (average 8.3), 6 years in Superior Bay (average 6.7), and 7 years in both the Lower and Upper River (average 8.1 and 6.8, respectively).
Table 3.
Neoplasm prevalence in white sucker sampled at sites within the St. Louis River Area of Concern from 2011 through 2015, including sample size (n) and age (mean, ±standard deviation).
| Site | n | Age, yr | Skin Neoplasm, # observed (%) | Liver neoplasm, # observed (%) |
|---|---|---|---|---|
| Superior Bay | 176 | 6.7±2.1 | 3 (1.7%) | 6 (3.4%) |
| St. Louis Bay | 137 | 8.3±2.7 | 10 (7.3%) | 6 (4.4%) |
| Lower River | 155 | 8.1±3.2 | 11 (7.1%) | 13 (8.4%) |
| Upper River | 154 | 6.8±2.5 | 3 (1.9%) | 4 (2.6%) |
| All | 622 | 7.4±2.7 | 27 (4.3%) | 29 (4.7%) |
The δ13C and δ15N values of fish tissue samples spanned the range of values expected for fish moving between the AOC below Fond du Lac Dam and Lake Superior (Fig. 5). At each of the sites, white sucker exhibited a broad range of δ13C and δ15N values, indicating that the location where the white sucker was captured during the spawning run was not representative of the longer-term habitat from which the white sucker was feeding. We sampled a few fish that had a stable isotope composition indicating exclusive reliance on one of three habitats – Lake Superior, Superior Bay and St. Louis Bay, and the Lower River. Most white sucker had a stable isotope composition indicating they were feeding in a mix of habitats, especially a mix between Lake Superior and Superior Bay and St. Louis Bay, which is the most urbanized portion of the river (Fig. 5d).
Figure 5.
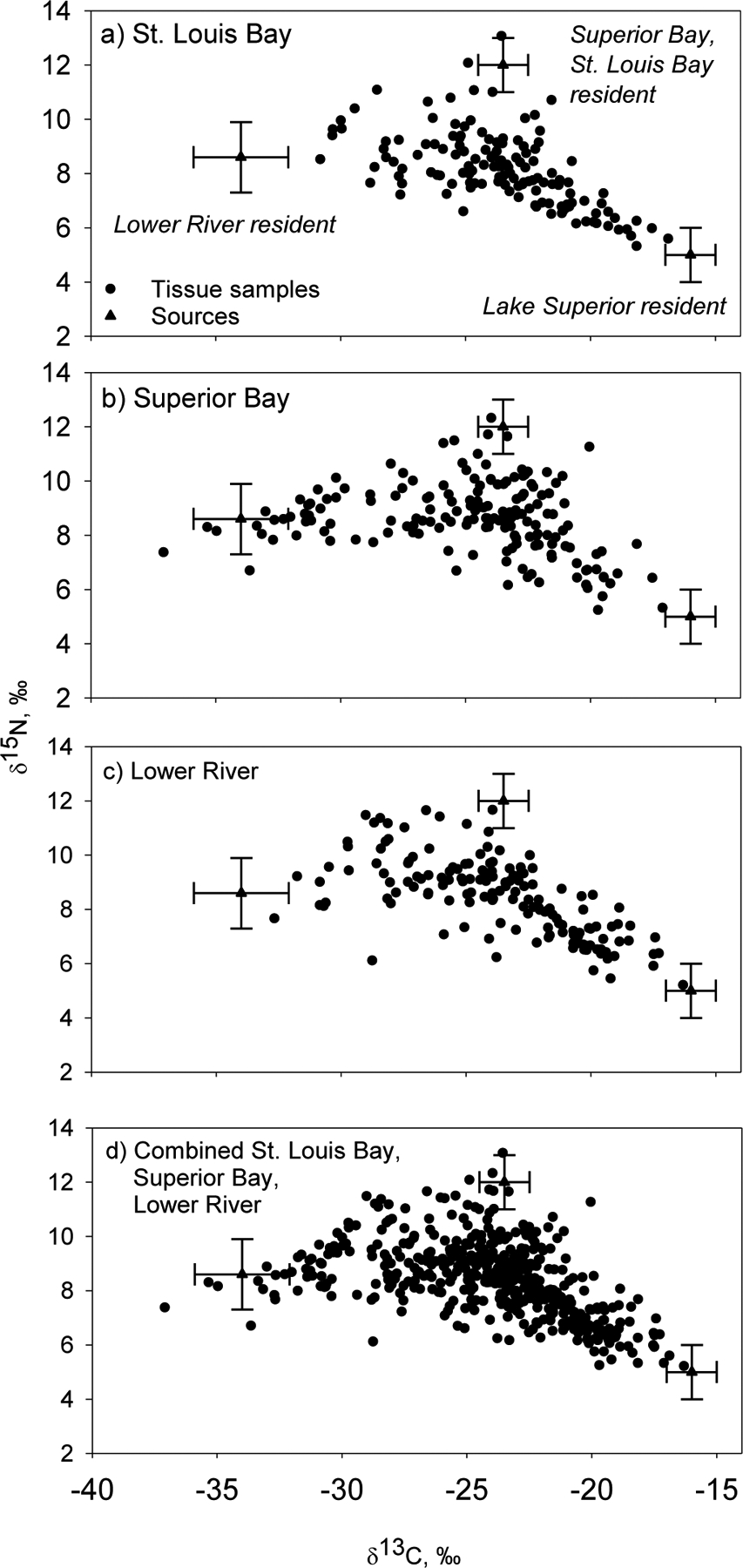
Distribution of δ13C and δ15N values in muscle samples of white sucker collected at the sampling sites below Fond du Lac dam, including the three sites combined, from 2011 through 2015. The source mean stable isotope ratios with error bars (± 1 standard deviation) for putative resident white sucker are shown.
Based on the mixing model results for fish captured below Fond du Lac Dam from 2011 through 2015, 17.2% of white sucker obtained >50% of their diet from the Lower River, 18.9% of white sucker obtained >50% of their diet from Superior Bay and St. Louis Bay, and 26.2% of white sucker obtained >50% of their diet from Lake Superior (Fig. 6). Thus, nearly three-quarters of white sucker sampled below Fond du Lac Dam had a diet that was at least 50% dependent on habitats within the Area of Concern (i.e., Superior Bay and St. Louis Bay, Lower River), implying recent exposure to AOC sediment contaminants. Notably, habitat use and age were confounded; white sucker age and diet contribution from Lake Superior were significantly, positively correlated (coefficient = 0.26, p<0.001; Fig.7).
Figure 6.
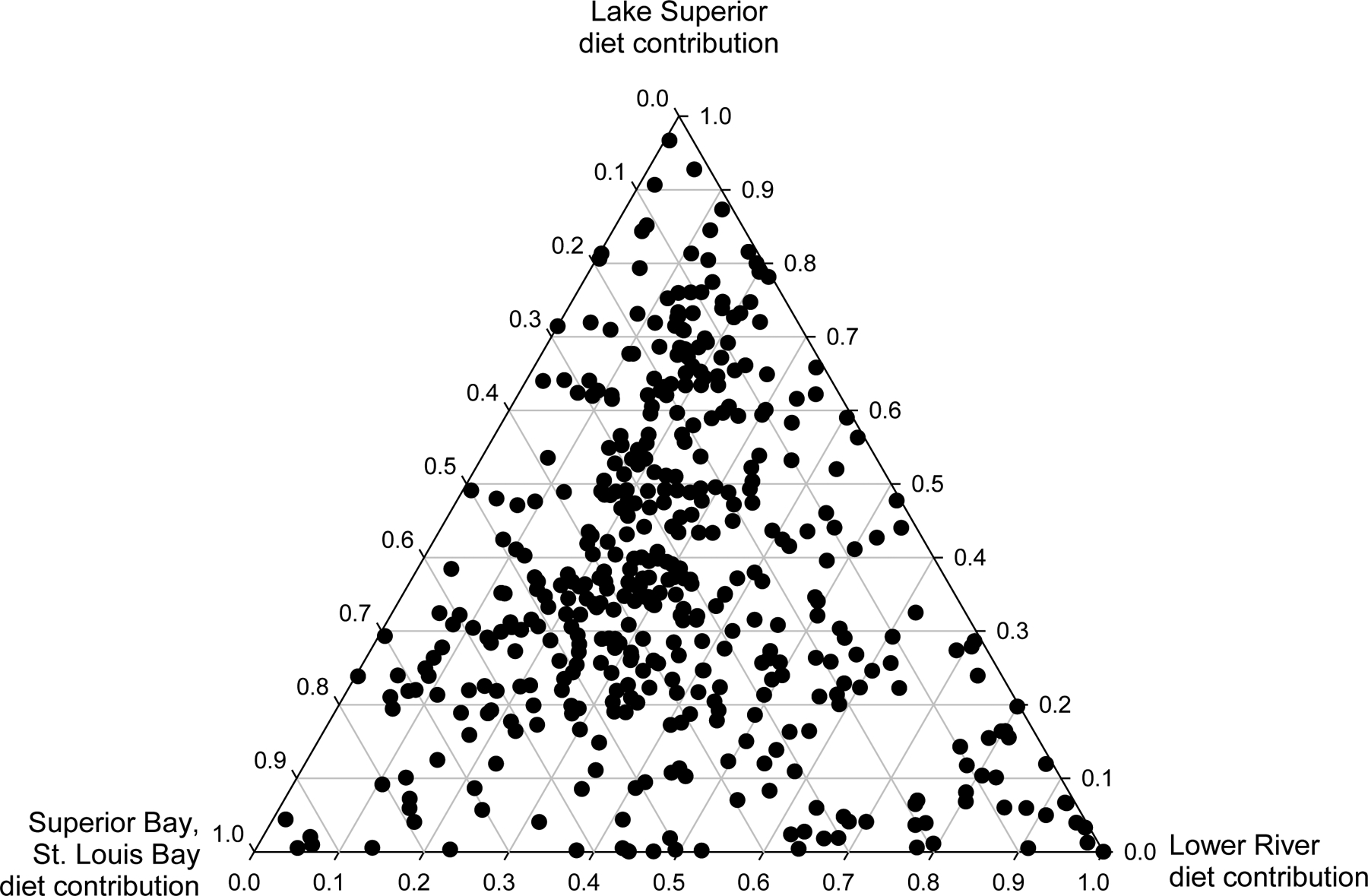
Diet contribution estimates from the stable isotope mixing model for white sucker captured below Fond du Lac Dam from 2011 through 2015. Each point represents the estimate for an individual fish (n = 619); white suckers in the upper triangle have a 100% diet contribution from Lake Superior (i.e., Lake Superior diet contribution = 1.0), those in the lower left triangle a 100% diet contribution from Superior Bay and St. Louis Bay, and those in the lower right triangle a 100% diet contribution from the Lower river.
Figure 7.
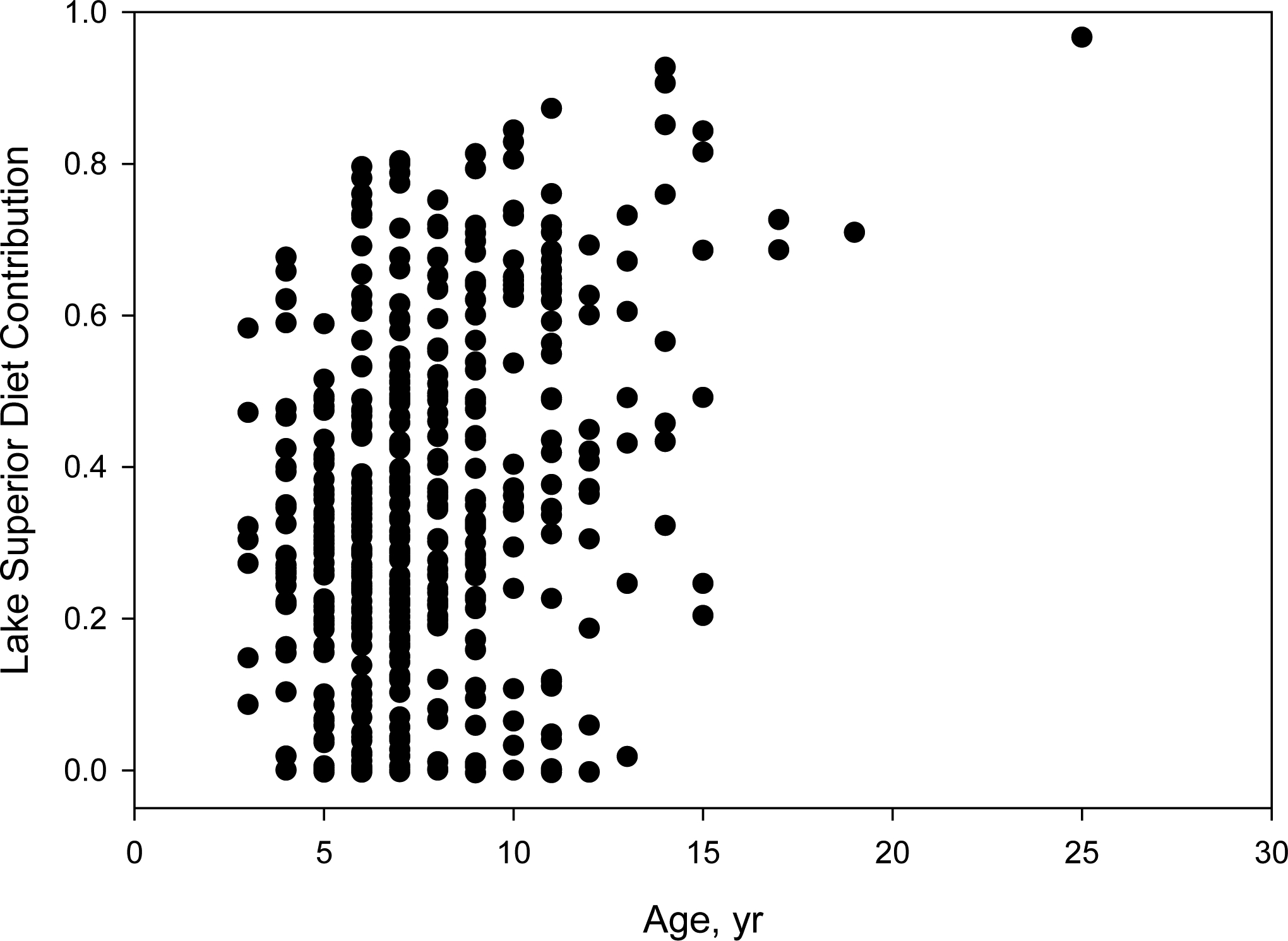
Lake Superior diet contribution with respect to age for individual white sucker captured below Fond du Lac dam from 2011 through 2015 (n = 619).
For skin neoplasia prevalence, both sex and age were significant factors, whereas neither habitat use variables were significant factors (Table 4). With increasing age, white sucker were significantly more likely to have skin neoplasia, and female white sucker less likely than males. Because the Lake Superior diet contribution increased with age (Fig. 7), suggesting that exposure and age are confounded, we calculated mean diet contributions for white sucker ages 5–11 to compare fish with and without skin neoplasia. We found diet contribution was similar among white sucker with and without skin neoplasia, indicating there was not an apparent habitat effect. For fish with skin neoplasia (n = 15), the average diet fractions were 0.28 for the Upper River, 0.38 for the Lower River, and 0.35 for Lake Superior, whereas they were 0.31, 0.33 and 0.35, respectively, for white sucker without skin neoplasia (n = 365).
Table 4.
Logistic regression model of skin and liver neoplasia prevalence excluding sampling year as a factor, including parameter estimates, Z scores, and associated p-values (p <0.05 in bold). For skin neoplasia, the full model p = 0.001. For liver neoplasia, the full model p = 0.027.
| Tissue | Parameter | Estimate (±SE) | Z | p-value |
|---|---|---|---|---|
| skin | Constant | 4.37 (0.92) | 4.77 | <0.001 |
| Sex (F) | 1.34 (0.57) | 2.34 | 0.019 | |
| Age | −0.24 (0.07) | −3.4 | <0.001 | |
| % Lake | 0.66 (1.20) | 0.55 | 0.581 | |
| % Lower River | −0.04 (1.37) | −0.03 | 0.975 | |
| liver | Constant | 4.44 (0.90) | 4.92 | <0.001 |
| Sex (F) | 0.40 (0.46) | 0.87 | 0.385 | |
| Age | −0.20 (0.07) | −2.98 | <0.001 | |
| % Lake | −0.05 (1.17) | −0.04 | 0.969 | |
| % Lower River | −0.09 (1.35) | −0.07 | 0.946 |
For liver neoplasia prevalence, only age was a significant factor (Table 4). As with skin neoplasia, older white sucker were significantly more likely to have liver neoplasia than younger white sucker. Again, we calculated mean diet contributions for white sucker ages 5–11 with and without liver neoplasia, and found diet fraction was similar among fish with and without liver neoplasia. For fish with neoplasia (n = 17), the average diet fractions were 0.29 for the Upper River, 0.34 for the Lower River, and 0.37 for Lake Superior, whereas they were 0.31, 0.33 and 0.35, respectively, for white sucker without liver neoplasia (n = 363).
Because habitat use (e.g., % diet contribution Lake Superior) was not a significant factor for neoplasia prevalence in fish captured below Fond du Lac Dam, neoplasia prevalence was compared between fish captured at sites below the dam (Lower River, St. Louis Bay, Superior Bay sites) to those captured from above the dam (Upper River site). Although both areas are within the Area of Concern boundaries, sediment contaminants are confined to the two reservoirs above Fond du Lac Dam and not as widespread as below. We found that age was a significant factor for both skin and liver neoplasia prevalence, but sampling site was not (Table 5), indicating there was no significant difference in neoplasia prevalence between fish sampled above and below the dam.
Table 5.
Logistic regression model of skin and liver neoplasia prevalence with both fish age and sampling site (Superior Bay, St. Louis Bay, and Lower River combined versus Upper River) as factors, with parameter estimates, Z scores, and associated p-values. For skin neoplasia, the full model p = 0.002. For liver neoplasia, the full model p <0.001.
| Tissue | Parameter | Estimate (±SE) | Z | p-value |
|---|---|---|---|---|
| skin | Constant | −5.01 (0.68) | −7.36 | <0.001 |
| Age | 0.19 (0.06) | −3.31 | <0.001 | |
| Site (Combined) | 0.54 (0.55) | 0.98 | 0.33 | |
| liver | Constant | −5.29 (0.70) | −7.58 | <0.001 |
| Age | 0.22 (0.06) | 3.83 | <0.001 | |
| Site (Combined) | 0.45 (0.56) | 0.81 | 0.417 |
For the 20 fish sampled in 2015, liver concentrations of total PCBs (range: 680.8 to 147,329.7 pg/g wet weight lipid−1) were higher than those for total dioxins (range: 0 to 0.91 pg/g wet weight lipid−1) and total furans (range: 0 to 2.24 pg/g wet weight lipid−1). The number of flagged results made statistical comparison difficult (Table A.1). Fish with tumors had a higher liver PCBs concentration than those without tumors (p = 0.035), but not for dioxins (p =0.614) or furans (p = 0.069). For those fish captured below Fond du Lac dam (n = 15), there was a significant negative correlation between liver tissue concentration and Lake Superior diet contribution (PCBs: coefficient −0.53, p = 0.040; dioxins: coefficient = −0.65, p = 0.008, furans: coefficient −0.73, p = 0.002; Fig. 8). The result confirms that the river is the primary source of contaminant exposure for those fish that freely move between the St. Louis River and Lake Superior, and that feeding in Lake Superior causes a decline in contaminant bioaccumulation.
Figure 8.
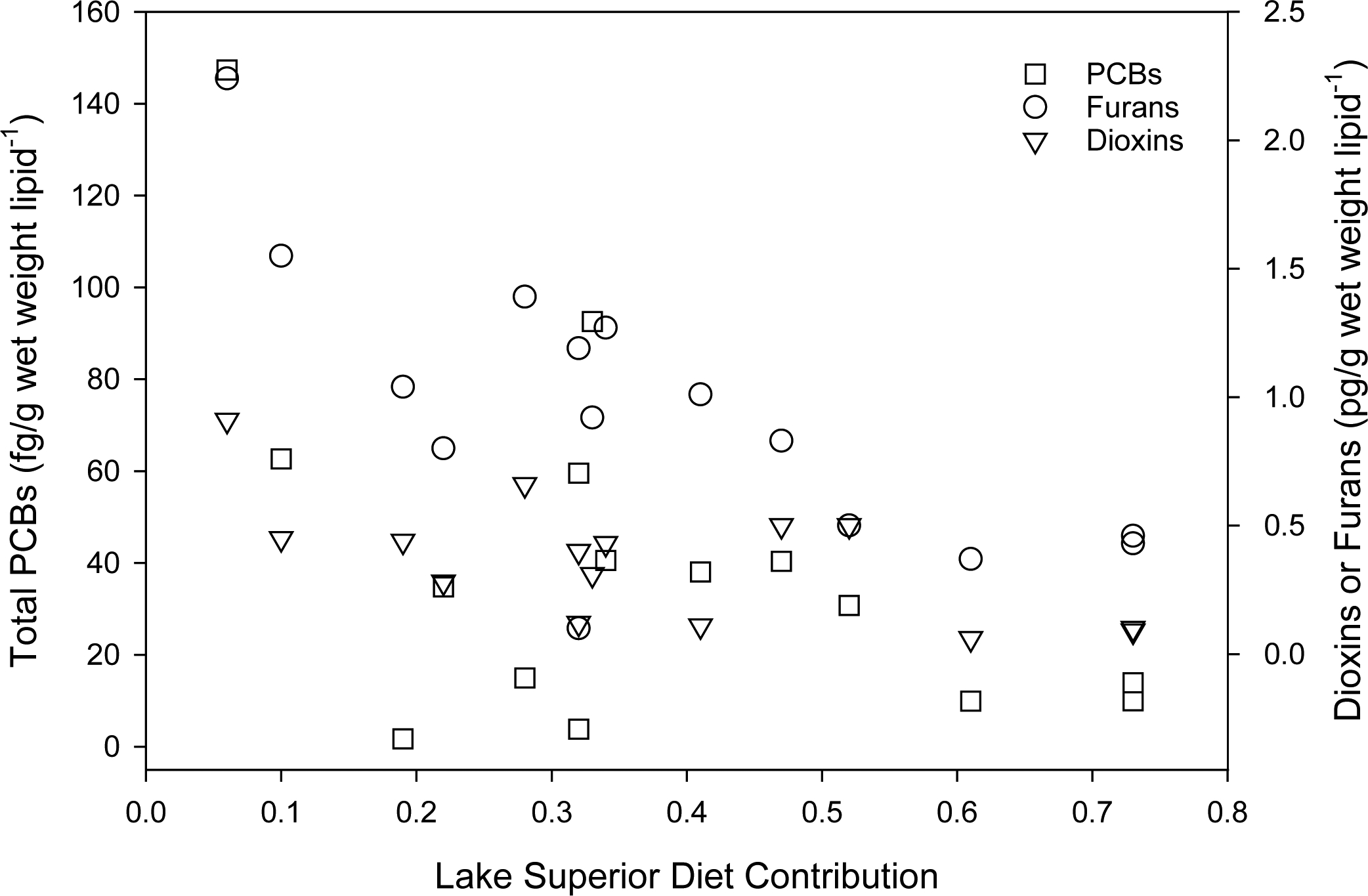
Total PCBs, dioxins, or furans concentration in liver tissue with respect to the Lake Superior diet contribution for select fish sampled in 2015 (n = 15).
4. Discussion
The prevalence of contaminant-related internal and external tumors (<5%) and deformities within the St. Louis River AOC was similar to or less than observed at non-AOC areas elsewhere in the Great Lakes Basin (Mahmond, et al. 2014; Blazer et al., 2017). Using identical methods to this study, white sucker from two other Wisconsin AOCs (Sheboygan River and Milwaukee Estuary) and the Kewaunee River as a “least impacted site” were sampled in 2011 through 2013. The prevalence of skin tumors was higher (p < 0.0001; Fisher’s exact test) at the Kewaunee River (21.0%) than at the St. Louis River AOC (4.3%), whereas, with respect to liver neoplasm prevalence, there was no significant difference (p = 0.5570) between the St. Louis River and Kewaunee River (3.5%). Both Kewaunee and St. Louis River AOC had significantly lower liver tumor prevalence when compared to the Sheboygan and Milwaukee AOCs (8.3% and 15.0%, respectively). Interestingly, at both the Milwaukee and Sheboygan numerous large external body surface tumors were observed and many of these were squamous cell carcinomas (malignant) which were not observed at St. Louis River or Kewaunee. Liver neoplasms at Milwaukee and Sheboygan included hepatocellular carcinomas not observed in white suckers from the St. Louis River AOC (Blazer et al., 2017).
We found differences in tumor prevalence among the sites sampled, which is notable given that the δ13C and δ15N data revealed that capture site and feeding habitat were not related. Tumor prevalence for white sucker captured in Superior Bay and the Upper River was lower than for white sucker captured in St. Louis Bay and the Lower River. Given the logistic regression model results, these regional differences in tumor prevalence are affected by the underlying age distribution, such that in those regions in which we captured slightly older white sucker, we would expect to find a higher tumor prevalence. An age-effect notwithstanding, the higher neoplasm rates together with the higher PCBs concentrations in tissues of white sucker captured in these two areas of the AOC do suggest a role for contaminants in carcinogenesis. While PCBs may play a role, other co-occurring compounds may also be important as either initiators or promoters, and thus it is important to comprehensively characterize contaminants at every site. The 2015 fish for which we found a positive relationship between liver neoplasia prevalence and liver concentration of PCBs are indicative of this potential affect, recognizing that contaminants of concern are co-located at the same sites in the AOC (Crane et al., 2005).
There are some factors which make it difficult to utilize this data to better understand the causal relationships among ontogenetic development, age, contaminant exposure, and the presence of other initiators or promoters. The low sample size of white suckers with tumors made comparisons of affected and non-affected individuals from specific sites impossible. Additionally, it is possible white sucker are exposed to initiators of proliferative responses early in life, and either annual sporadic exposure during migrations to spawning habitat or continued exposure in feeding habitats may eventually induce actual neoplasia. The stable isotope ratios were limited in this aspect, because the isotopic turnover time does not allow us to investigate habitat use during early life. Stable isotope ratios in slow-growing fish are a mid-term biomarker; given the allometric relationship between fish size and isotopic turnover (Vander Zanden et al., 2015), the sampled fish (ca. 500 g to 1000 g wet weight) have an isotopic turnover half-life of 2 to 2.5 months, suggesting the stable isotope ratios represent feeding over the previous 6 months to 1 year. While we found that older fish, which have higher neoplasia prevalence than younger fish, are also more likely to feed in Lake Superior and thereby reduce contaminant exposure, this pertains only to recent life history. Early life exposures to both chemical carcinogens (Birnbaum and Fenton, 2003; Bailey et al., 2016) and infectious agents (Vedham et al., 2015) can be risk factors for cancer development later in life. Hence, where white suckers spend the first year or so of their life may be important with respect to risk factors.
Neoplasia is most often a multifactorial disease which may involve initiators and promoters of abnormal cell proliferation. Historically, polyaromatic hydrocarbons (PAHs) have been the risk factor most correlated with liver neoplasia in fishes (Malins, et al 1987; Vogelbein et al., 1990; Myers et al., 1994; Baumann and Harshbarger, 1998). However, elevated PCBs concentrations in walleye Stizostedium vitreum vitreum from the Lower Fox River and Green Bay AOC were associated with an increased prevalence of neoplastic liver lesions, although there was no association between PCBs concentrations and presence or absence of liver tumors in individual fish (Barron et al., 2000). Multiple PCBs can act as tumor promoters (Glauert et al., 2001) as can numerous other chemicals including endocrine disruptors (Cooke and Hinton, 1999; Soto and Sonnenschein, 2010). In other animals, including humans, viruses and parasites have been recognized as risk factors for carcinogenesis. Chronic infections of Hepatitis B (Asia and developing countries) and C (United States) viruses are one of the most common risk factors for hepatocellular carcinoma. During liver transcriptome analyses of fishes at Great Lakes AOCs, including the St. Louis River (Hahn et al., 2016), a novel Hepatitis B virus was identified in white sucker (Hahn et al., 2015). Although the presence of the virus was not correlated with observed liver tumors or other lesions, more research is necessary to determine any possible role it may play in carcinogenesis in fish. A major risk factor for cholangiocarcinoma in humans are the trematodes Opisthorchis and Clonorchis, primarily in East Asia and Eastern Europe where uncooked fish are part of the diet. The adult worms reside in the bile ducts and mechanical damage, oxidative DNA damage and excretory or secretory products of the parasites that induce cell proliferation have all been linked with the carcinogenesis (Sripa et al., 2012).
Multiple stressors, both ecological and toxicological, exist in urban aquatic ecosystems, and these stressors could play a role in carcinogenesis. In this instance, known stressors recognized in the St. Louis River include poor water quality and waste water effluent (Bellinger et al., 2016; Martinovìc et al., 2008), degraded habitat (Angradi et al., 2017), invasive species introduction (Peterson et al., 2011), and chemicals of emerging concern (Christensen et al., 2012). The research highlights the complexity of exposure to contaminants and infectious agents associated with neoplasia in fishes, not only at urban, contaminated sites in the Great Lakes but worldwide. Many fishes used as environmental sentinels can freely move among habitats with varying levels of environmental contaminants or other stressors. This study demonstrates the need to determine the full set of risk factors across life-stages, habitats, and biological endpoints.
Acknowledgements
This project was jointly funded by the Great Lakes Restoration Initiative (through the Fish and Wildlife Service Contaminants program), the U.S. Geological Survey’s Contaminant Biology (Environmental Health), Fisheries (Ecosystems) and Cooperative Fish and Wildlife programs and the U.S. Environmental Protection Agency. Histology slide reviews were provided by John Fournie. Assistance from Wisconsin DNR, Minnesota DNR, U.S. Fish and Wildlife Service, the Minnesota Land Trust, and the Fond du Lac Band of Lake Superior Chippewa is gratefully acknowledged. We thank Brittany Story, Tracey Ledder, Pat Collins, Rick Gitar, Zachary Jorgenson, Glenn Miller, Rich Davis, Sara Werner, Brian Borkholder, John Lindgren, Daryl Peterson, Greg Peterson, Anett Trebitz, George Grant, Will Bartsch, Elon O’Malia, Darlene Bowling, Kathy Spring, and Megan McGovern for assistance with field collections and laboratory analysis, and Jonathon Launspach for GIS support. The views expressed herein are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency or U.S. Fish and Wildlife Service. Use of trade names is for identification purposes only and does not imply endorsement by the U.S. Government.
Appendix 1
Table 1.
Total PCBs, dioxins, and furans concentrations (pg/g wet weight) in liver of selected white sucker collected within the St. Louis River Area of Concern 2015. For liver data, CO = cholangioma; CC = cholangiocarcinoma; bd prol = bile duct proliferation. For concentration values, BD = below detection or any flagged values.
| Fish ID | Age, yr | Liver | Site | Total PCBs | Total Tetra-dioxins | Total Penta-dioxins | Total Hexa-dioxins | Total Hepta-dioxins | Total Tetra-furans | Total Penta-furans | Total Hexa-furans | Total Hepta-furans |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 257 | 4 | Normal | St. Louis Bay | 225,204 | BD | BD | 0.65 | 1.14 | 1.64 | 1.66 | 1.87 | BD |
| 263 | 4 | Normal | St. Louis Bay | 27,191 | BD | 0.37 | BD | 0.5 | 0.74 | BD | BD | BD |
| 271 | 5 | Normal | St. Louis Bay | 329,296 | BD | 1.07 | 1.18 | 3.12 | 3.96 | BD | BD | 1.44 |
| 289 | 6 | Normal | St. Louis Bay | 35,144 | BD | BD | 0.2 | BD | BD | 0.58 | 0.72 | BD |
| 210 | 6 | Normal | Superior Bay | 8,008 | BD | BD | BD | 2.06 | 1.62 | BD | 2.05 | 1.21 |
| 212 | 4 | Normal | Superior Bay | 43,735 | BD | BD | 0.28 | 1.66 | 0.77 | BD | 2.27 | 1.03 |
| 230 | 6 | Normal | Superior Bay | 256,257 | 0.65 | BD | 1.04 | 1.49 | 3.39 | 0.75 | BD | 1.1 |
| 360 | 4 | Normal | Upper River | 2,345 | BD | BD | BD | BD | 0.16 | 0.16 | BD | BD |
| 361 | 4 | Normal | Upper River | 3,717 | BD | BD | BD | BD | BD | BD | BD | BD |
| 369 | 4 | Normal | Upper River | 7,443 | BD | BD | BD | BD | BD | 0.34 | BD | BD |
| 240 | 12 | CO | St. Louis Bay | 111,131 | BD | 0.32 | BD | BD | 0.94 | 0.35 | 1.19 | 0.47 |
| 283 | 7 | CC | St. Louis Bay | 316,501 | 1.06 | BD | BD | BD | 1.5 | BD | 1.33 | 0.31 |
| 405 | 5 | CC | St. Louis Bay | 263,907 | 0.56 | 0.62 | 0.61 | BD | 2.01 | 1.03 | 1.36 | 0.86 |
| 324 | 11 | bd prol | Lower River | 149,703 | 0.47 | 0.71 | 0.41 | BD | 1.42 | 1.8 | 1.11 | 0.34 |
| 438 | 5 | CC | Lower River | 338,520 | 0.78 | 0.55 | 1.08 | BD | 1.97 | 1.67 | 4.32 | 0.39 |
| 442 | 12 | CO | Lower River | 1,187,477 | 1.25 | 2.14 | 3.94 | BD | 3.83 | 3.19 | 8.23 | 2.8 |
| 445 | 6 | CC | Lower River | 67,077 | BD | 0.7 | BD | BD | 2.01 | 1.05 | BD | BD |
| 449 | 6 | CC | Lower River | 91,922 | BD | 0.4 | 0.22 | BD | 1.75 | 0.25 | 0.6 | 0.25 |
| 353 | 4 | CC | Upper River | 7,144 | BD | BD | BD | BD | BD | BD | BD | BD |
| 368 | 9 | bd prol | Upper River | 6,576 | 0.14 | 0.49 | 0.22 | BD | 0.56 | 0.81 | 0.15 | BD |
References
- Angradi TR, Bartsch WM, Trebitz AS, Brady VJ, Launspach JJ 2017. A depth-adjusted ambient distribution approach for setting numeric removal targets for a Great Lakes Area of Concern beneficial use impairment: Degraded benthos. J. Great Lakes Res 43, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AXYS. October 2011a. Analytical Method for the Determination of 209 PCB Congeners by EPA Method 1668A, EPA Method 1668C or EPA Method CBC01.2”. AXYS Analytical Services Ltd., Sidney, BC: AXYS Method MLA-010, Rev 11.06. [Google Scholar]
- AXYS. January 2011b. AXYS Method MLA-017: Analytical Method for the Determination of Polychlorinated Dibenzodioxins and Dibenzofurans”. AXYS Analytical Services Ltd., Sidney, BC, AXYS Method MLA-017, Rev 20.09. [Google Scholar]
- AXYS. June 2011c. Analytical Method for the Determination of: Polybrominated Diphenyl Ethers, Polychlorinated Biphenyl Congeners, Chlorinated Pesticides, Technical Toxaphene, Toxaphene Congeners/Parlars and Polychlorinated Dibenzodioxins and Dibenzofurans Using Co-extraction Techniques”. AXYS Analytical Services Ltd., Sidney, BC: AXYS Method MLA-013, Rev 09.05. [Google Scholar]
- Bailey KA, Smith AH, Tokar EJ, Graziano JH, Kim K-W, Navasumrit P, Ruchirawat M, Thiantanawat A, Suk WA, Fry RC 2016. Mechanisms underlying latent disease risk associated with early-life arsenic exposure: current research trend and scientific gaps. Environ. Health Perspect 124, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MG, Anderson MJ, Cacela D, Lipton J, The SJ, Hinton DE, Zelikoff JT, Dikkeboom AL, Tillitt DE, Holey M, Denslow N 2000. PCBs, liver lesions, and biomarker responses in adult walleye (Stizostedium vitreum vitreum) collected from Green Bay, Wisconsin. J. Great Lakes Res 26, 250–271. [Google Scholar]
- Baumann PC 1992. The use of tumors in wild populations of fish to assess ecosystem health. J. Aquat. Ecosyst. Health 1, 135–146. [Google Scholar]
- Baumann PC, Harshbarger JC 1998. Long term trends in liver neoplasm epizootics of brown bullhead in the Black River, Ohio. Environ. Monit. Assess 53, 213–223. [Google Scholar]
- Baumann PC, Smith IR, Metcalfe CD 1996. Linkages between chemical contaminants and tumors in benthic Great Lakes fish. J. Great Lakes Res 22, 131–152. [Google Scholar]
- Bellinger BJ, Hoffman JC, Angradi TR, Bolgrien DW, Starry M, Elonen C, Jicha T, Lehto L, Monson L, Pearson MS, Anderson L, Hill BH 2016. Water quality in the St. Louis River Area of Concern, Lake Superior: historical and current conditions and delisting implications. J. Great Lakes Res 42, 28–38. [Google Scholar]
- Birnbaum LS, Fenton SE 2003. Cancer and developmental exposure to endocrine disruptors. Environ. Health Perspect 111, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer VS, Fournie JW, Wolf JC, Wolfe MJ 2006. Diagnostic criteria for proliferative liver lesions in the brown bullhead (Ameiurus nebulosus). Dis. Aquat. Organ 72, 19–30. [DOI] [PubMed] [Google Scholar]
- Blazer VS, Hoffman JC, Walsh HL, Braham RP, Hahn C, Collins P, Jorgenson Z, Ledder T 2014a. Health of white sucker within the St. Louis river Area of Concern associated with habitat usage as assessed using stable isotopes. Ecotoxicology 23, 236–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer VS, Mazik PM, Iwanowicz LR, Braham R, Hahn C, Walsh HL, Sperry A 2014b. Monitoring of wild fish health at selected sites in the Great Lakes basin – methods and preliminary results. U.S. Geological Survey Open-File Report 2014–1027. [Google Scholar]
- Blazer VS, Rafferty SD, Baumann PC, Smith SB, Obert EC 2009a. Assessment of the “tumors or other deformities” beneficial use impairment in brown bullhead: I. Orocutaneous tumors. J. Great Lakes Res 35, 517–526. [Google Scholar]
- Blazer VS, Rafferty SD, Baumann PC, Smith SB, Obert EC 2009b. Assessment of the “tumors and other deformities” beneficial use impairment in brown bullhead: II. Liver tumors. J. Great Lakes Res 35, 527–537. [Google Scholar]
- Blazer VS, Walsh HL, Braham RP, Hahn CM, Mazik P, McIntyre PB 2017. Tumours in white suckers from Lake Michigan tributaries: pathology and prevalence. J. Fish Dis 40, 377–393. [DOI] [PubMed] [Google Scholar]
- Carr MK, Jardine TD, Doig LE, Jones PD, Bharadwaj L, Tendler B, Chételat J, Cott P, Lindenschmidt K-E 2017. Stable sulfur isotopes identify habitat-specific foraging and mercury exposure in a highly mobile fish community. Sci. Total Environ 586, 338–346. [DOI] [PubMed] [Google Scholar]
- Christensen VG, Lee KE, Kieta KA, Elliott SM, 2012. Presence of selected chemicals of emerging concern in water and bottom sediment from the St. Louis River, St. Louis Bay, and Superior Bay, Minnesota and Wisconsin, 2010: U.S. Geological Survey Scientific Investigations Report 2012–5184, 23 p. [Google Scholar]
- Cooke JB, Hinton DE 1999. Promotion by 17β-estradiol and β-hexachlorocyclohexane of hepatocellular tumors in medaka, Oryzias latipes. Aquat. Toxicol 45, 127–145. [Google Scholar]
- Crane JL, MacDonald DD, Ingersoll CG, Smorong DE, Lindskoog RA, Severn CG, Berger TA, Field LJ 2002. Evaluation of numerical sediment quality targets for the St. Louis River Area of Concern. Arch. Environ. Contam. Toxicol 43, 1–10. [DOI] [PubMed] [Google Scholar]
- Crane JL, Richards C, Breneman D, Lozano S, Schuldt JA 2005. Evaluating methods for assessing sediment quality in a Great Lakes embayment. Aquat. Ecosyst. Health Manag 8, 323–349. [Google Scholar]
- Doherty CA, Curry RA, Munkittrick KR 2010. Spatial and temporal movements of white sucker: implications for use as a sentinel species. Trans. Am. Fish. Soc 139, 1818–1827. [Google Scholar]
- Glauert HP, Robertson LW, Silberhorn EM 2001. PCBs and tumor promotion, pp. 355–401 In: Robertson LW, Hansen LG (eds.), PCBs: Recent Advances in Environmental Toxicology and Health Effects, University Press of Kentucky, Lexington, Kentucky. [Google Scholar]
- Hahn CM, Iwanowicz LR, Cornman RS, Conway CM, Winton JR, Blazer VS 2015. Characterization of a novel Hepadnavirus in white sucker (Catostomus commersonii) from the Great Lakes region of the USA. J. Virol 89, 11801–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn CM, Iwanowicz LR, Corman RS, Mazik PM, Blazer VS 2016. Transcriptome discovery in non-model wild fish species for the development of quanitative transcript abundance assays. Comp. Biochem. Phys. D 20, 27–40. [DOI] [PubMed] [Google Scholar]
- Hayes MA, Smith IR, Rushmore TH, Crane TL, Thorn C, Kocal TE, Ferguson HW 1990. Pathogenesis of skin and liver neoplasms in white suckers from industrially polluted areas in Lake Ontario. Sci. Total Environ 94, 105–123. [DOI] [PubMed] [Google Scholar]
- Hoffman JC 2016. Tracing origin, migration and other movements of fish using stable isotopes, pp. 169–196 In Morais P, Daverat F (eds.), An Introduction to Fish Migration, CRC Press. [Google Scholar]
- Hoffman JC, Cotter AM, Peterson GS, Kelly JR 2010. Using stable isotope mixing in a Great Lakes coastal tributary to determine food web linkages in young fishes. Estuaries Coasts 33, 1391–1405. [Google Scholar]
- Hoffman JC, Kelly JR, Peterson GS, Cotter AM, Starry M, Sierszen ME 2012. Using δ15N in fish as an indicator of watershed sources of anthropogenic nitrogen: response at multiple spatial scales. Estuaries Coasts 35, 1453–1467. [Google Scholar]
- Hoffman JC, Kelly JR, Peterson GS, Cotter AM 2015. Landscape-scale food webs of fish nursery habitat along a river-coast mixing zone. Estuaries Coasts 38, 1335–1349. [Google Scholar]
- Hoffman JC, Sierszen ME, Cotter AM 2015b. Fish tissue lipid-C:N relationships for correcting δ13C values and estimating lipid content in aquatic food web studies. Rapid Commun. Mass Spectrom 29, 2069–2077. [DOI] [PubMed] [Google Scholar]
- Koch JD, Quist MC 2007. A technique for preparing fin rays and spines for age and growth analysis. N. Am. J. Fish. Manag 27, 782–784. [Google Scholar]
- Lee KE, Langer SK, Menheer MA, Foreman WT, Furlong ET, Smith SG 2012. Chemicals of emerging concern in water and bottom sediment in Great Lakes areas of concern, 2010 to 2011 – Collection methods, analyses methods, quality assurance and data. U.S. Geological Survey Data Series 723, 26 p. [Google Scholar]
- Mahmood M, Blukacz-Richards EA, Baumann PC, McMaster M, Hossain M, Arhonditsis GB 2014. A Bayesian methodological framework for setting fish tumor occurrence delisting criteria: A case study in St. Marys River Area of Concern. J. Great Lakes Res 40, 88–101. [Google Scholar]
- Malins DC, McCain BB, Myers MS, Brown DW, Krahn MM, Roubal WT, Schiewe MH, Landahl JT, Chan SL 1987. Field and laboratory studies of the etiology of liver neoplasms in marine fish from Puget Sound. Environ. Health Perspect 71, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinović D, Denny JS, Schmieder PK, Ankley GT, Sorensen PW 2008. Temporal variation in the estrogenicity of a sewage treatment plant effluent and its biological significance. Environ. Sci. Technol 42, 3421–3427. [DOI] [PubMed] [Google Scholar]
- Marvin C, Painter S, Williams D, Richardson V, Rossman R, Van Hoof P 2004. Spatial and temporal trends in surface water and sediment contamination in the Laurentian Great Lakes. Environ. Poll 129, 131–144. [DOI] [PubMed] [Google Scholar]
- Minnesota Pollution Control Agency. 2013. St. Louis River Area of Concern Implementation Framework: Roadmap to Delisting (Remedial Action Plan Update). St. Paul, Minnesota. [Google Scholar]
- Minnesota Pollution Control Agency and Wisconsin Department of Natural Resources (MPCA and WDNR). 1992. The St. Louis River System Remedial Action Plan Stage One, 263 p. St. Paul, Minnesota. [Google Scholar]
- Moore M, Pembroke A, Nestler E, Hall M, Letkovitz L, Lambert M, Keay K 2018. Toxics source reduction and sewage upgrades eliminated winter flounder liver neoplasia (1984–2017) from Boston Harbor, MA, USA. Dis. Aquat. Organ 131, 239–243. [DOI] [PubMed] [Google Scholar]
- Munkittrick KR, Dixon DG 1989. Use of white sucker (Catostomus commersoni) populations to assess the health of aquatic ecosystems exposed to low-level contaminant stress. Can. J. Fish. Aquat. Sci 46, 1455–1462. [Google Scholar]
- Myers MS, Stehr CM, Olson OP, Johnson LL, McCain BB, Chan SL, Varanasi U 1994. Relationships between toxicopathic hepatic lesions and exposure to chemical contaminants in English sole (Pleuronectes vetulus), starry flounder (Platichthys stellatus), and white croaker (Genyonemus lineatus) from selected marine sites on the Pacific Coast, USA. Environ. Health Perspect 102, 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [dataset] National Oceanic and Atmospheric Administration, St. Louis River 2019, Data Integration Visualization Exploration and Reporting Explorer (NOAA DIVER), 2019. Web address: diver.orr.noaa.gov.
- Peterson GS, Hoffman JC, Trebitz AS, West CW, Kelly JR 2011. Establishment patterns of non-native fishes: lessons from the Duluth-Superior harbor and lower St. Louis River, an invasion-prone Great Lakes coastal ecosystem. J. Great Lakes Res 37, 349–358. [Google Scholar]
- Phillips DL, Gregg JW 2001. Uncertainty in source partitioning using stable isotopes. Oecologia 127, 171–179. [DOI] [PubMed] [Google Scholar]
- Pinkney AE, Harshbarger JC, Rutter MA 2014. Temporal and spatial patterns in tumour prevalence in brown bullhead Ameiurus nebulosus (Lesueur) in the tidal Potomac River watershed (USA). J. Fish Dis 37, 863–876. [DOI] [PubMed] [Google Scholar]
- Premdas PD, Metcalfe TL, Bailey ME, Metcalfe CD 1995. The prevalence and histological appearance of lip papillomas in white sucker (Catostomus commersoni) from two sites in central Ontario, Canada. J. Great Lakes Res 21, 207–218. [Google Scholar]
- Quinn SP, Ross MR 1985. Non-annual spawning in the white sucker, Catostomus commersoni. Copeia 1985, 613–618. [Google Scholar]
- Rafferty SD, Blazer VS, Pinkney AE, Grazio JL, Obert EC, Boughton L 2009. A historic perspective on the “fish tumors or other deformities” beneficial use impairment at Great Lakes areas of concern. J. Great Lakes Res 35, 496–506. [Google Scholar]
- Smith SB, Blouin MA, Mac MJ 1994. Ecological comparisons of Lake Erie tributaries with elevated incidence of fish tumors. J. Great Lakes Res 20, 701–716. [Google Scholar]
- Soto AM, Sonnenschen C 2010. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat. Rev. Endocrinol 6, 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A 2012. The tumorigenic liver fluke Opisthorchis viverrini – multiple pathways to cancer. Trends Parasitol 28, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stortz KR, Sydor M 1980. Transports in the Duluth–Superior harbor. J. Great Lakes Res 6, 223–231. [Google Scholar]
- Vander Zanden MJ, Rasmussen JB 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol. Oceanogr 46, 2061–2066. [Google Scholar]
- Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC 2015. Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE 10(1): e0116182. doi: 10.1371/journal.pone.0116182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedham V, Verma M, Mahabi S 2015. Early-life exposures to infectious agents and later cancer development. Cancer Med. 4, 1908–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vethaak AD, Jol JG, Pieters JPF 2009. Long-term trends in the prevalence of cancer and other major diseases among flatfish in the southeastern North Sea as indicators of changing ecosystem health. Environ. Sci. Technol 43, 2151–2158. [DOI] [PubMed] [Google Scholar]
- Vogelbein WK, Fournie JW, Van Veld PA, Huggett RJ 1990. Hepatic neoplasms in the mummichog Fundulus heteroclitus from a creosote-contaminated site. Cancer Res. 50, 5978–5986. [PubMed] [Google Scholar]
- Wisconsin Department of Natural Resources and Minnesota Pollution Control Agency. 2015. St. Louis River Area of Concern Remedial Action Plan Annual Update. Madison, Wisconsin. [Google Scholar]


