Abstract
Coronary microvascular dysfunction (CMD) plays a pathogenic role in cardiac and systemic conditions other than microvascular angina. In this review, we provide an overview of the pathogenic role of CMD in the setting of diabetes mellitus, obesity, hypertensive pregnancy disorders, chronic inflammatory and autoimmune rheumatic disorders, chronic kidney disease, hypertrophic cardiomyopathy, and aortic valve stenosis. In these various conditions, CMD results from different structural, functional, and/or dynamic alterations in the coronary microcirculation associated with the primary disease process. CMD is often detectable very early in the course of the primary disease, before clinical symptoms or signs of myocardial ischaemia are present, and it portrays an increased risk for cardiovascular events.
Keywords: Microvascular angina, Coronary microvascular dysfunction, Comorbidities, Endothelial dysfunction, Inflammation
Graphical Abstract
Graphical Abstract.
This article is part of the Spotlight Issue on Coronary Microvascular Dysfunction.
1. Introduction
Microvascular angina (MVA) is a form of ischaemic heart disease (IHD) characterized by signs and symptoms of cardiac ischaemia triggered by coronary microvascular dysfunction (CMD).1 CMD, however, can also occur and play a pathogenic role in cardiac conditions other than MVA, i.e. hypertrophic cardiomyopathy (HCM) and aortic valve stenosis (AoS) which are also referred to as type 2 CMD.2 Moreover, CMD can be present in systemic conditions such as diabetes mellitus (DM), obesity, hypertensive pregnancy disorders (HPD), chronic inflammatory and auto-immune rheumatic disorders, and chronic kidney disease (CKD). Importantly, although CMD may not always cause symptoms of cardiac ischaemia in the setting of the cardiac and systemic conditions mentioned above, its presence has been shown to be consistently associated with adverse clinical outcomes.
This review provides an overview of the pathogenic role of CMD in the setting of cardiac and systemic conditions other than MVA, and its clinical implications. We will describe the functional role of the coronary microcirculation in the delivery of blood for myocardial perfusion, and discuss the different conditions associated with CMD.
2. The role of the microvasculature in the coronary circulation
In the coronary circulation, the resistance of the vascular components is coordinated by different regulatory mechanisms to match blood flow with oxygen requirements.3,4 The large epicardial coronary arteries (500 µm-5 mm in diameter) as well as the capillaries and venules act mainly as conductance vessels and offer very little resistance. The coronary blood flow is mainly controlled by the pre-arterioles and arterioles, also called the microvasculature. The epicardial pre-arterioles (100-500 µm in diameter) serve to maintain pressure within narrow limits at the origin of the arterioles and respond to flow-related stimuli with endothelium-dependent vasoreactivity. The intramyocardial arterioles (<100 µm in diameter) have the highest resistance and respond either by myogenic control or metabolites, differing per size.5 Myogenic control prevails in the medium-sized arterioles (40-100 µm in diameter), where stretch receptors in vascular smooth muscle cells (VSMCs) react to changes in pressure, high intraluminal pressure leads to vasoconstriction, and vice versa. Control by metabolites prevails in the smaller arterioles (<40 µm in diameter), in which an increased metabolic activity leads to vasodilatation. This leads to a reduction in pressure in the medium-sized arterioles, stimulating myogenic dilation, and a subsequent increased flow upstream resulting in endothelium-dependent vasodilation in the pre-arterioles and epicardial coronary arteries.
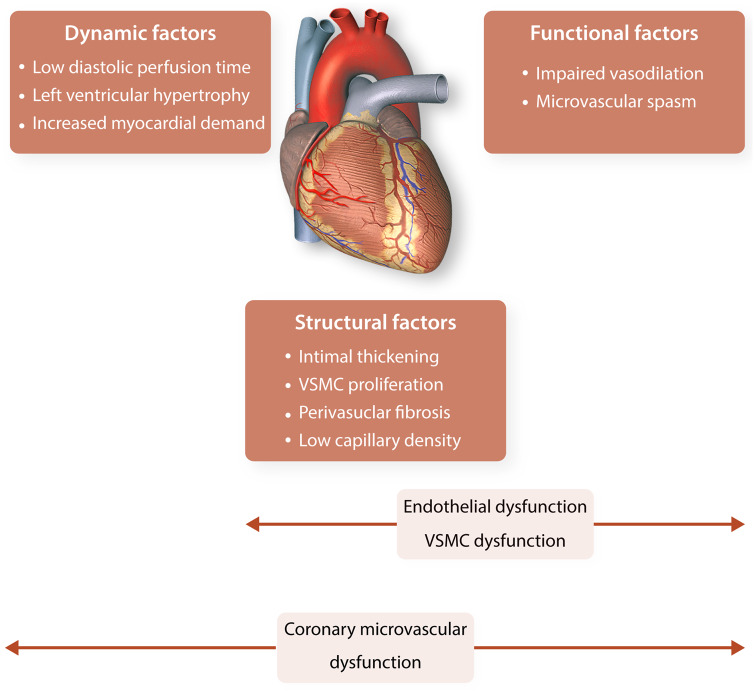
CMD can result in the inability of the coronary arteries to augment coronary blood flow (vasodilatory abnormality) and/or in a reduction in coronary blood flow (coronary microvascular spasm). CMD leading to ischaemia can occur in the absence and/or in the presence of obstructive epicardial coronary artery disease (CAD). CMD can be the consequence of an abnormal structure of the coronary microvasculature (e.g. intimal thickening, VSMC proliferation, low capillary density), a dynamic maldistribution of coronary blood flow often resulting from extracoronary causes (e.g. short diastolic perfusion time) or compressive forces generated in the myocardium, or an abnormal coronary function (e.g. impaired vasodilatation by endothelial dysfunction), as depicted in Figure 1.6 Endothelial dysfunction is defined as an imbalance between the release of vasoprotective vasorelaxant substances, such as nitric oxide (NO), prostacyclin (PGI2), endothelium-derived hyperpolarizing factors (EDHF), and pathological vasoconstricting substances, such as endothelin-1 (ET-1), superoxide, hydrogen peroxide, and thromboxanes.7 While in large epicardial coronary arteries vasorelaxation is primarily mediated by NO, in small vessels the effect of EDHF is much more pronounced.8
Figure 1.
Different factors involved in coronary microvascular dysfunction. Dynamic, structural factors, and functional factors contribute to the occurrence of coronary microvascular dysfunction the presence of other conditions. Endothelial dysfunction and vascular smooth muscle cell dysfunction are caused by (often a combination of) structural and/or functional factors. VSMC, vascular smooth muscle cell.
2.1 Assessment of coronary microvascular function
The diagnosis of CMD is established by functional assessment of the coronary arteries, which can be done by both invasive and non-invasive methods.1,9 To assess endothelium-independent microvascular function, the coronary flow reserve (CFR) can be measured, which is defined by the rate of coronary blood flow at hyperaemia compared with baseline. The cut-off for an abnormal CFR is ≤2.5 or 2.0, depending on the technique that is being used.1 Another parameter that demonstrates endothelium-dependent microvascular function is the microvascular resistance, reported as the index of microvascular resistance (IMR) or the hyperaemic microvascular resistance (hMR). Microvascular resistance is measured during a hyperaemic state with either intracoronary thermodilution (IMR) or Doppler techniques (hMR) and reflect abnormalities in the function and/or structure of the coronary microvasculature. The endothelium-dependent microvascular function can be tested with acetylcholine. In healthy endothelium, acetylcholine results in a net vasodilation because its stimulation of NO and other vasodilators exceeds its direct vasoconstrictor effects on the VSMCs. In CMD, when endothelial function and/or VSMCs function are damaged, NO resources are depleted, and the vasoconstrictor response becomes unopposed.10
3. Diabetes mellitus
Patients with diabetes have a three times higher risk of mortality compared with patients without diabetes.11,12 Microvascular and macrovascular complications are important determinants of morbidity and mortality in DM. Macrovascular complications, including IHD, occur about twice as often in patients with diabetes compared to those without, independent from other risk factors.13 Microvascular complications, including CMD, are often present before the onset of macrovascular complications. Patients with Type 1 or Type 2 diabetes have a high prevalence of CMD,14–16 which is a strong predictor of adverse cardiovascular outcome even before macrovascular complications are evident.15,17 Diabetic patients with a reduced CFR show mortality rates as least as high as those of non-diabetic patients with known obstructive CAD.18
3.1 Hyperglycaemia and insulin resistance
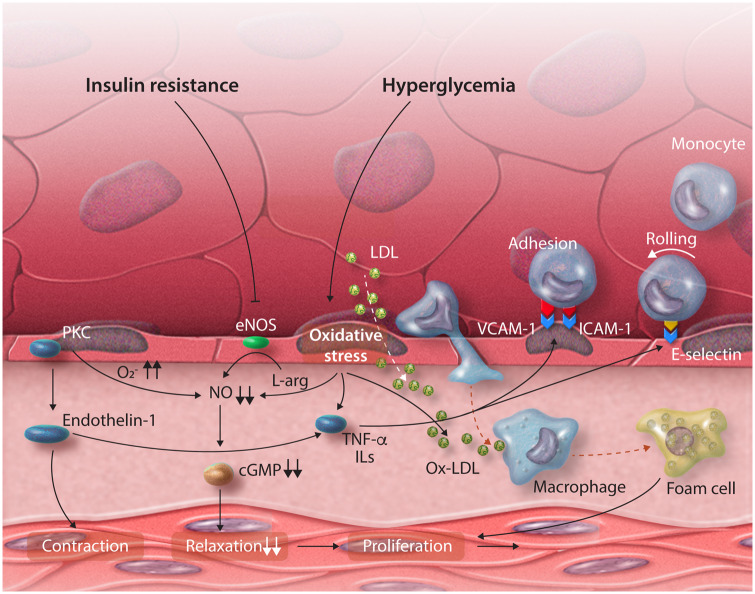
Hyperglycaemia and insulin resistance are important factors in the development of CMD in DM.19 They induce an imbalance between the bioavailability of vasoprotective NO and the accumulation of reactive oxygen species (ROS), as illustrated in Figure 2.19,20
Figure 2.
Schematic overview of the pathophysiological mechanisms of coronary microvascular dysfunction in diabetes. Both hyperglycaemia and insulin resistance contribute to oxidative stress, the release of proinflammatory cytokines and the decrease of the nitric oxide availability. cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; ICAM-1, intercellular adhesion molecule-1; ILs, interleukins; LDL, low-density lipoprotein; NO, nitric oxide; Ox-LDL, oxidized LDL; PKC, protein kinase C; TNF-α, tumour necrosis factor alpha; VCAM-1, vascular cell adhesion molecule-1.
Hyperglycaemia induces several events—including the activation of protein kinase C (PKC)21—that lead to the generation of ROS (e.g. superoxide anion) and oxidative stress.22 ROS leads to uncoupling of the endothelial NO synthase (eNOS) and to the production of superoxide anion via increased lipid peroxidation products. Superoxide anion reacts with NO to form peroxynitrite, which not only reduces the bioavailability of NO but also reduces the NO production and decreases the responsiveness of tissue to NO.23,24 Superoxide anion also increases the production of ROS via advanced glycation end products (AGEs) and activation of the receptor for AGE on vascular cells.21 These processes likely recruit xanthine oxidase, leading to a further increase in ROS levels and augmenting oxidative stress.25
Insulin resistance contributes to this detrimental process by decreasing the activity of eNOS and reducing the production of NO, resulting in less available vasoprotective NO.22 The mainly NO-driven endothelium-dependent vasoreactivity is related to insulin resistance and has been shown to improve when insulin resistance improves using metformin treatment.26 The hyperinsulinaemia in DM is also associated with elevated levels of free fatty acids27,28 that contribute to oxidative stress and a proinflammatory state by activating PKC, increasing the production of ROS, and exacerbating dyslipidaemia.29,30
3.2 Proinflammatory state
Many of the aforementioned processes contribute to the activation of the endothelium to a proinflammatory state, resulting in the enhanced endothelial expression of adhesion molecules such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and E-selectin, the release of proinflammatory cytokines, the migration and proliferation of VSMCs, and an increased synthesis of endothelin. Progressive arterial stiffness and a higher prothrombotic state further contribute to the development of CMD and macrovascular complications in patients with DM.22,31–33 These proinflammatory cytokines are not only implicated in the pathogenesis of DM but may also contribute to the development of CMD and IHD.33
The cycle of inflammation and oxidative stress, does not only affect cardiac cells but also induces cell damage in pancreatic beta-cells, further enhancing DM. Treatment should therefore be aimed at managing all factors that contribute to this vicious cycle. Glycaemic control is key in diabetics and even little variations in glycaemic state are important in maintaining a healthy vascular state.34,35 Long-term aggressive management of co-existing traditional cardiovascular risk factors reduces the risk of cardiovascular events in patients with Type 2 DM by about 50% and should be implemented in the treatment of patients in an early stage.36–38 Although we await studies to confirm this, we believe that patients with both diabetes and a reduced CFR could benefit even more from aggressive preventive and treatment strategies.
4. Obesity and CMD
Obesity, defined as body mass index (BMI) 30 kg/m2 or higher, is one of the ongoing epidemics in industrialized countries. Some obese individuals are at an increased risk of developing cardiovascular events.39 The mechanisms responsible for increased cardiovascular risk in obesity, however, are complex and may vary in different individuals. Increased oxidative stress, low-grade systemic inflammation, and increased sympathetic nervous system activity have been postulated as risk factors, as they can lead to CMD and reduced CFR40, which is, in turn, associated with impaired clinical outcomes including increased mortality.41,42 Positron emission tomography (PET) flow studies43 carried out in ‘metabolically healthy’ obese individuals (i.e. obesity without systemic hypertension, dyslipidaemia or diabetes) have shown CFR abnormalities to be present in these persons. Recently, Bajaj et al.44 assessed the relationship between BMI and CMD, and their possible link with adverse cardiovascular events in patients with and without obesity. They found that in obese patients, CFR decreased linearly with increasing BMI and was independently associated with cardiovascular events. In obese patients, individuals with impaired CFR showed a higher adjusted rate of cardiovascular events (5.7% vs. 2.6%; P = 0.002).44 CMD was independently associated with elevated BMI and adverse clinical outcomes. Moreover, CFR was a better marker of risk than both BMI and conventional cardiovascular risk factors. This was a retrospective study involving 827 subjects undergoing rest and stress myocardial perfusion testing with 13N-ammonia or 82rubidium PET. Clinical endpoints defined as a composite of death or non-fatal myocardial infarction or heart failure were assessed during follow-up (median follow-up 5.6 years). In the Bajaj study,44 BMI and CFR both were good prognostic markers, but only CFR was independently associated with events. Of interest, only obese patients with reduced CFR—particularly those with a very high BMI (30–39 kg/m2)—had increased cardiovascular risk, i.e. ≥2.5-fold increased rate of events. An impairment of the vasodilatory capacity of the coronary circulation has been shown to precede the development of obstructive CAD.45
In obese patients, there is growing evidence of the association among increased BMI, metabolic abnormalities, and systemic inflammation, probably as a result of the actions of adipocytokines such as leptin, adiponectin, interleukin-6 (IL-6), and tumour necrosis factor alpha (TNF-α) on microvascular function40 suggesting a pathogenic link between obesity and CMD. Current and previous research43 suggest that an imbalance among obesity-related metabolic abnormalities, endocannabinoids, and adipocytokines may be key determinants of CMD in obesity.43 The notion that a reduced CFR due to CMD rather than just ‘obesity’, is associated with impaired outcomes in obese patients has pathophysiological and therapeutic importance. Cardiovascular risk may vary in different obese individuals and not all may benefit from the same preventative or therapeutic measures. Individuals with severe CMD may benefit from treatments addressing the many pathogenic mechanisms that lead to a reduced CFR in this patient group. However, future research on this subject is much awaited.
5. Hypertensive pregnancy disorders
HPD complicate 5–15% of pregnancies. The most severe form of HPD is pre-eclampsia, occurring in 3–5% of all pregnancies.46,47 The International Society for the Study of Hypertension (ISSHP) defines pre-eclampsia as new-onset hypertension after 20 weeks gestation in combination with either proteinuria (≥300 mg/day) or other maternal dysfunctions, such as renal insufficiency, liver involvement, neurological or haematological complications, or uteroplacental dysfunction.48 Unlike previous beliefs that HPD are self-limiting conditions that resolve after delivery of the placenta, we now know that women with HPD have an up to eight-fold increased risk of cardiovascular disease (CVD) later in life.47,49 HPD should be regarded as a window into future maternal cardiovascular health; evidence points towards a partially shared pathophysiology in HPD and CVD.50
A combination of maternal and placental factors is considered to be responsible for the development of HPD, in which an increased inflammatory response and maternal (systemic) endothelial dysfunction are key features.51 In many women with HPD, there is a pre-existing (genetically) increased risk for CVD. Signs of an abnormal systemic endothelial function are present even before the onset of pre-eclampsia, possibly related to an already enhanced inflammatory state.52 The placenta itself is also important in the pathogenesis of HPD. An abnormal placentation in early pregnancy (e.g. abnormal invasion of trophoblasts and inadequate maternal spiral artery remodelling) causes placental malperfusion and hypoxia.53,54 This results in oxidative stress, a generalized hyperinflammatory state and an exaggerated endothelial activation.55 During pre-eclampsia, several markers of inflammation, such as TNF-α, IL-6, IL-17, and vasoconstrictor ET-1, are substantially increased in the maternal circulation and the placenta.56 The hyperinflammatory state and the systemic endothelial dysfunction that occur in HPD, seem to persist postpartum, which may underlie the development of CVD later in life. Months to years after delivery, affected women remain to have increased plasma concentrations of inflammatory markers compared with women who had a normal pregnancy, i.e. higher baseline levels of C-reactive protein (CRP), IL-6 and fibrinogen,57 and alterations in TNF-α, IL-6, leptin, adiponectin, homocysteine, soluble E-selectin, and pregnancy-associated plasma protein-A.58–62 An increase in the CRP response to vaccination and a consistent pattern of increased acute-phase responses to vaccination for all inflammatory markers were also found among women after pre-eclampsia compared with controls, indicating that vascular responses are altered afterwards.63
5.1 CMD and IHD after pre-eclampsia
These altered vascular responses are also observed in the coronary circulation: an impaired CFR and other signs of CMD have been shown in women up to several years after pre-eclampsia compared with women who had a healthy pregnancy and delivery (2.39 ± 0.48 vs. 2.90 ± 0.49; P < 0.001).64,65 Although prospective data are lacking, many of these women mention MVA in their fifth and sixth decade. An important trigger for MVA in these patients is premature hypertension. Premature signs of subclinical CAD have been demonstrated by carotid intima-media thickness measurements and coronary artery calcium scores in middle-aged women after pre-eclampsia.64,66–68 This reflects their two-fold higher risk to develop IHD.69 At an older age, these women may develop heart failure with preserved ejection fraction, in relation to their long-standing hypertension and enhanced inflammatory state. More prospective data are needed to better identify the life-course of women after pre-eclampsia and to determine most optimal strategies for prevention. The primary prevention guidelines are currently used for the follow-up of these high-risk women, but secondary prevention guidelines may be more appropriate.70 In this perspective, it would be interesting to study the benefit of preventive strategies in women with early signs of cardiovascular abnormalities, such as the presence of CMD.
6. Chronic inflammatory and autoimmune rheumatic disorders
In recent years, it has become apparent that cardiovascular risk is particularly increased in patients with inflammatory disorders.71,72 In rheumatoid arthritis (RA), a meta-analysis of total of 41 490 cases showed that cardiovascular risk was increased by 48%, when compared with individuals without RA [pooled relative risk 1.48 (95% confidence interval 1.36–1.62)].73 Similar observations of increased risk have been made for other autoimmune or inflammatory diseases including ankylosing spondylitis (AS),74 psoriatic arthritis (PA),75 as well as systemic lupus erythematosus (SLE).76 Experimental studies, which allow to better control study conditions show that this increase is in part linked to common cardiovascular risk factors between these comorbidities,77 but to the large extent depend on the role of inflammation as a risk factor of CVD.78 Similarly, endothelial dysfunction is a key mechanism for both obstructive and non-obstructive forms of CAD,79 linked to both classic cardiovascular risk factors and to inflammation.80 In large vessels, endothelial dysfunction and stiffening has been widely described in a wide spectrum of inflammatory conditions. The examples include psoriasis,81 periodontitis,82,83 or inflammatory bowel disease.84 Microvascular dysfunction has also been widely described in patients with inflammatory joint diseases.85,86 A recent meta-analysis in 709 patients with rheumatic disease and 650 controls,87 showed a significantly reduced CFR in patients with various forms of arthritis. Patients with autoimmune disease such as SLE had significantly lower CFR than subjects with mixed autoinflammatory/autoimmune disorders, such as RA or PA.87 Indeed, recent 5-year follow-up study in SLE patients showed significant non-obstructive impairment of myocardial perfusion in more than half of the patients with SLE.88 However, coronary as well as peripheral microvascular dysfunction have been observed already in early RA even after 6 months since initial diagnosis.89 Several studies have also shown systemic microvascular dysfunction as measured in peripheral vascular beds in patients with RA and AS.72,90 Interesting insight into the CMD can be gained from the analysis of skin microvasculature, which has been shown to offer a useful model to study arteriole function and capillary morphology.91 Findings from these studies are in line with earlier invasive observations that NO-mediated, acetylcholine-induced vasorelaxation in microvessels is impaired in AS and may improve with anti‐TNF-α therapy.86,90 In similarity to large vessel endothelial dysfunction, microvascular dysfunction has been identified in a number of inflammatory conditions, such as severe chronic periodontitis,92 or inflammatory bowel disease,93 where impairment correlates with CRP. Thus, inflammation may provide a mechanistic link between these comorbidities and cardiovascular events.
6.1 Mechanisms contributing to CMD in rheumatoid disorders
Clinical meta-analyses of CFR in rheumatic disease do not seem to provide sufficient hints regarding the mechanisms that cause CMD. Across over 20 studies in several rheumatic diseases, reductions of CFR were not linked with either inflammatory burden, lipids, BMI, age, or even blood pressure.87 It is particularly surprising that some large studies, such as a longitudinal Dudley Rheumatoid Arthritis Comorbidity Cohort (DRACCO) study, did not show any association between cumulative inflammatory burden as measured with CRP or erythrocyte sedimentation rate (ESR), and endothelial function after 6 years follow-up.94 However, the observational nature of this study and the use of solely CRP and ESR to measure inflammation leave the main question of the relationship between inflammation and CMD open. In fact, other studies have shown that clinical inflammatory burden in patients with AS is associated with microvascular flow impairment.72 Notably, some interventional evidence shows that anti-inflammatory biological therapies such as anti-TNF treatments lead to improvement of coronary and peripheral microvascular dysfunction,90 although results are often conflicting and not emerging from randomized or placebo-controlled studies.72
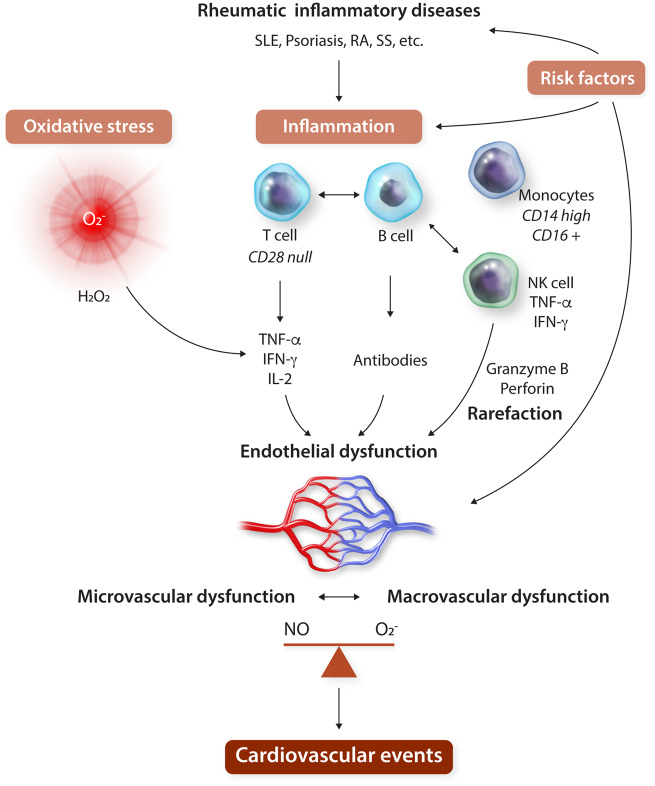
Most preclinical and observational studies point to an important mechanistic importance of systemic endothelial dysfunction in rheumatic disease, which seems to coincide with or precede both macro- and microvascular disease/dysfunction.95 A schematic overview is provided in Figure 3. Systemic endothelial dysfunction is linked with increased oxidative stress, possibly up-regulation of NADPH oxidases (Nox) as well as vascular mitochondrial dysfunction.7 Increased ROS production is part of the pathogenesis of arthritis as it is induced in endothelial and VSMCs by a number of inflammatory mediators including IL-17, interferon-γ (IFN-γ), and TNF-α.7 In fact, these proinflammatory cytokines are known to induce and activate Nox enzymes.80,96–98 Oxidative stress is reported both locally and systemically in mouse models of RA.99 Interestingly, there is a two way interaction between vascular renin–angiotensin aldosterone system (RAAS) activation, which is closely linked to oxidative stress, and disease activity in RA or SLE. Angiotensin receptor blockers inhibit Nox expression and activation and have been shown to improve endothelial function in animal models of arthritis.100 Together, this evidence suggests that oxidative stress may be intrinsically involved in establishing and potentiating RA-associated vascular damage both locally and systemically.
Figure 3.
Schematic overview of the mechanisms involved in the systemic endothelial dysfunction that is present in patients with rheumatic inflammatory diseases, in relation to the occurrence of cardiovascular events. IFN, interferon; IL-2, interleukin-2; PA, psoriatic arthritis; RA, rheumatic arthritis; RFs, risk factors; SLE, systemic lupus erythematosus; SS, systemic sclerosis; TNF, tumour necrosis factor.
Mechanistically, the L-arginine analogue asymmetric dimethylarginine (ADMA) has been suggested to play a role in with CMD in RA.72 ADMA reduces NO production and promotes endothelial dysfunction by a competitive inhibition of NOS. However, in further observational studies, neither coronary nor skin microvascular endothelial function correlated with ADMA.101 This might indicate that microvascular dysfunction in rheumatic diseases may be less dependent on this mechanism.
A number of immune cells, specifically those involved in the pathogenesis of arthritis and rheumatic disorders, have been implicated in the pathogenesis of endothelial dysfunction and CMD in rheumatic disorders as well.102 Cell type indicated by clinical studies include T-cells, natural killer (NK) cells and monocytes. Notably, immune deficient mice lacking T-cells, B-cells, and NK-cells or mice lacking only T- and B-cells present smaller diameters of microvasculature (third-order cremaster arterioles). Vasoconstriction of these vessels is particularly promoted by NK-cells103 as well as potentially dysregulated CD28null (CD4+ and CD8+) that produce proinflammatory cytokines known to induce oxidative stress and endothelial dysfunction (IFN-γ, TNF-α, and IL-2) and may also cause arteriolar rarefaction.104,105 These cells are also a hallmark of other chronic inflammatory conditions such as periodontitis, and they decrease upon successful intensive therapy of periodontitis.83 Lymphocyte involvement in the vascular pathology in patients with inflammatory disease is closely linked to oxidative stress. For example, Nox2 is expressed by T-cells and antigen presenting cells and mediates their activation and ability to serve homeostatic immune functions.106,107 We have also identified that in particular proinflammatory monocytes, CD14(high)CD16+ are related to endothelial dysfunction in arthritis patients,108 while in general CAD population a different subset of monocytes (CD14dimCD16+) were primarily correlated.109 While a number of cell types may be involved, final effectors of this response appear to be linked to overexpressed cytokines. An elegant study by Ahmed et al.110 has shown that in dysfunctional vasculature of patients with RA, a particular overexpression of IL-18, IL-33, and TNF is observed which may play a role in the inflammatory process and the development of endothelial dysfunction.
6.2 Implications
Understanding the unique mechanisms of CMD in chronic inflammatory and rheumatic diseases may allow for a more specific diagnosis and prevention. In particular understanding the relationship between clinical disease severity, inflammatory burden, and the development of CVD is essential. Understanding the role of individual cell types and cytokines in this process may allow more direct targeting in the future. Statins may represent a simple and unspecific approach to reduce systemic inflammation while at the same time statins target other mechanisms of microvascular dysfunction. Recently, specific trials of immune-targeted therapies in CVD, namely the Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) trial and the Cardiovascular Inflammation Reduction Trial (CIRT) have tested the utility of IL-1β and methotrexate targeting in the prevention of major cardiovascular events showing that modes of targeting are essential for outcomes, as discussed by us extensively elsewhere.111 However, while they provide proof-of-concept, lack of focus on CMD was present in either of these trials, which makes it difficult to extrapolate their results. It is clear that we should diagnose and actively search for CMD in patients with rheumatic inflammatory and autoimmune disorders as this population is of particularly high risk.
7. Chronic kidney disease
CKD is a known risk factor for CVD. For a long time, this risk was assumed to be the result of the high prevalence of traditional cardiovascular risk factors. Indeed, hypertension and diabetes are the main causes of CKD. Also, CKD promotes the development of hypertension by activation of the RAAS system, sodium retention and increased concentrations of catecholamines. However, meta-analyses have clearly shown that an impaired kidney function is a cardiovascular risk factor independently of the presence of other traditional cardiovascular risk factors.112 Patients with an estimated glomerular filtration rate (eGFR) below 15 mL/min/1.73 m2 still have a three times higher risk of cardiovascular death compared to those with a normal kidney function, even when adjusted for other risk factors.113 The number of deaths from CVD increases as eGFR rate decreases.112 Besides cardiovascular death, patients with CKD are also at risk for developing CVD, e.g. diastolic dysfunction, left ventricular hypertrophy (LVH), or IHD, referred to as Type 4 cardiorenal syndrome.114 CMD is one of the key features in the pathophysiology of this syndrome. CMD, measured as a reduced CFR, is present in many patients with CKD compared with healthy controls.115,116 And, concordant with what we have discussed in the other sections of this article, the CFR is independently associated with adverse cardiovascular events.117,118
7.1 The link between CMD and cardiorenal syndrome
It is assumed that uraemia-specific mechanisms contribute to CMD and CVD in patients with CKD.119 It has been shown that uraemia promotes microvascular rarefraction.120 In post-mortem samples, patients with CKD have an almost 50% decrease in capillary density in the heart.121,122 Moreover, uraemia induces a state of oxidative stress and an increased inflammatory state by several mechanisms,123 an enhanced activity of NAD(P)H oxidase,124 a reduced bioavailability of NO caused by an increase in the NO-synthase inhibitor ADMA,122 and increased levels of inflammatory markers (i.e. CRP, TNF-α, IL-1β and IL-6).125 As we have discussed before, oxidative stress and inflammation link to CMD.
The most common phenotype of the Type 4 cardiorenal syndrome is LVH: nearly 75% of adults with end-stage renal disease have signs of LVH126 and the severity of LVH is an independent predictor for mortality.127 Both the abovementioned uraemia-specific mechanisms as well as other CKD-specific mechanisms contribute to this cardiomyopathy, including hypertension, increased vascular stiffness, increased levels of steroid hormones, and activation of the RAAS system.128,129
It is evident that CKD and CVD enhance each other. Future research should focus on unravelling the causality between uraemia-specific mechanisms, CMD and the development of CVD in these patients. These insights could aid preventive strategies and treatment regimens.
8. Hypertrophic cardiomyopathy
HCM is the most common genetic heart disease, with a prevalence of 1:500 in the general population and is defined by the presence of primary LVH that is not explained by abnormal loading conditions.130,131 Myocardial ischaemia often occurs in patients with HCM, even in the absence of clinical symptoms.132–135 The substrate for myocardial ischaemia are perfusion defects, as represented by a reduced CFR in patients with HCM.136 These perfusion defects are often subendocardial and are most pronounced in the most hypertrophied (septal) segments.137,138 Perfusion abnormalities are associated with the presence of myocardial fibrosis,138 and fibrosis contributes to life-threatening electrical instability in HCM.133,135,139
Even though the assessment of CFR in HCM is not yet incorporated in HCM management guidelines or risk algorithms, the presence of CMD can identify patients at risk and those with no signs of CMD seem to have a relative good prognosis.140 The degree of CMD is a strong and independent predictor of clinical deterioration and death.135,141 CMD also predicts long-term adverse left ventricular (LV) remodelling and systolic dysfunction, even in patients with no or mild symptoms and normal LV function,140,141 making it a potential target for the prevention of disease progression in HCM.
8.1 Different mechanisms contribute to CMD in HCM
CMD is more than just a supply/demand mismatch caused by the overall increase in metabolic demand of the increased myocardial mass in patients with HCM. Both structural and functional alterations are important factors in CMD and subsequent myocardial ischaemia. Already decades ago, histopathological studies showed that HCM patients have markedly abnormal coronary microvasculature structure: the luminal areas of the arterioles are severely reduced due to intimal hyperplasia or medial hypertrophy,133,142,143 and HCM patients have a lower number of capillaries and lower capillary density compared with normal controls.144 These changes are observed in both hypertrophic obstructive cardiomyopathy and end-stage HCM, but myocardial fibrosis is more severe in end-stage HCM.145 The more functional and dynamic factors associated with CMD in HCM are perfusion abnormalities that result from a deranged coronary blood flow throughout both systole and diastole.146 In the hypertrophied hearts, compression of the intramyocardial arterioles during ventricular systole results in less coronary flow, as shown by wave intensity analysis.146 This compression causes elevated pressures in the microcirculation that can stop or even reverse flow in the epicardial coronary arteries, a phenomenon that worsens during hyperaemia. In patients with transient LV outflow tract obstruction, blood flow is even further decreased during systole. In addition to these derangements during systole, there is a decrease in coronary flow during diastole as well, related to an impaired ventricular relaxation and increase in passive stiffness.
8.2 Clinical implications
It is unlikely that treatment will reverse the structural changes in the microvasculature of HCM once they are present. However, the presumably preceding dynamic changes, marked by a decreased CFR, could be influenced by medical interventions. Septal ablation has been shown to improve CFR and blood flow dynamics.147 The use of beta-blockers and calcium channel antagonists are interesting in this regard as well, for they theoretically increase diastole and decrease the contractile forces that reduce CFR. Whether these interventions decrease or prevent subendocardial ischaemia and subsequent fibrosis needs to be studied in more detail in prospective trials.
9. Aortic valve stenosis
Another condition that is associated with LVH is AoS, in which the LVH develops in response to pressure overload. Men and women with similar degrees of AoS have different LV adaptations to this pressure overload. Women more frequently have a greater degree of LVH, higher relative wall thickness, smaller end-systolic and end-diastolic chamber size.148 The development of LVH is accompanied by the development of CMD in AoS.149,150 As we have learned in the previous paragraph of this manuscript, LVH is associated with a functionally deranged coronary blood flow, while oxygen demands increase,151 causing cardiac ischaemia. Related to the pressure drop across the aortic valve, there is less systolic acceleration of coronary blood flow in patients with AoS compared to healthy controls.152 In addition to this, coronary arteries are compressed to a larger extent in the hypertrophied and pressure-overloaded left ventricle during isovolumetric contraction when the aortic valve is still closed, causing a decrease of coronary blood flow in this period of the heart cycle.153 A reduced diastolic perfusion time during exercise and a high diastolic wall stress add to this blood flow maldistribution during exercise or hyperaemia, resulting in a decreased CFR and subendocardial myocardial ischaemia during stress149 related more to the severity of AS (valve effective orifice area), haemodynamic load, and reduced diastolic perfusion time rather than to the increase in LV mass.
Studies have even shown that the reduced CFR in AoS is more related to the severity of AoS (i.e. the valve effective orifice area),154 haemodynamic load, and reduced diastolic perfusion time than it is to the increase in LV mass.149 In line with this observation is the direct improvement of the CFR after successfully treatment of AS is with a transcatheter aortic valve replacement (TAVR): immediately after TAVR baseline haemodynamics remain unchanged, whereas hyperaemic parameters are improved, if there is no important aortic regurgitation.155 Unlike in HCM, the coronary microvasculature of patients with severe AoS show no signs of intramural medial hypertrophy, making it unlikely that structural changes of the microvasculature contribute significantly to CMD in patients with AoS.156 The presence of CMD is associated with angina but not all patients with AoS and angina show signs of CMD.157,158 CMD could however play a significant pathophysiological role in the natural history of AoS contributing to the development of cardiac fibrosis and LV dysfunction. CFR was found to be an independent predictor for future cardiovascular events in AoS patients in one small study.159
In the treatment of patients with AoS, it has been shown beneficial to focus on the mechanisms involved in the dynamic alterations in coronary flow that are associated with CMD and myocardial ischaemia, such as the short diastolic time. A propensity-matched post hoc analysis showed that betablocker use reduces all-cause mortality (hazard ratio 0.5, 95% confidence interval 0.3–0.7; P < 0.001), cardiovascular death (hazard ratio 0.4, 95% confidence interval 0.2–0.7; P < 0.001), and sudden cardiac death (hazard ratio 0.2, 95% confidence interval 0.1–0.6; P = 0.004) in 1873 asymptomatic patients with mild to moderate AoS and preserved LV ejection fraction.160 The ultimate treatment of severe symptomatic AS is aortic valve replacement. It would be interesting to study if CMD could aid in choosing the optimal timing of this intervention before extensive myocardial fibrosis is present.
10. Summary and future perspectives
CMD can occur in the setting of a wide variety of cardiac and systemic clinical conditions, and often results from changes in microvascular structure, microvascular function, and/or a maldistribution of coronary blood flow. Despite the various mechanisms involved in the presence of CMD in the discussed clinical conditions, CMD is consistently associated with myocardial ischaemia and portrays an increased risk for cardiovascular events. CMD is often detectable very early in the course of the primary disease, before clinical symptoms or signs of myocardial ischaemia are present. These observations support the potential use of CMD in strategies for risk stratification, which should be explored further. Novel agents that target-specific pathways that lead to endothelial damage and a proinflammatory state are an active area of research at present and could provide novel insights regarding the management of both the primary disorders and the associated CMD.
Conflict of interest: none declared.
This paper was handled by Guest Editor, Colin Berry
References
- 1. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 2. Crea F, Camici PG, Bairey Merz CN.. Coronary microvascular dysfunction: an update. Eur Heart J 2014;35:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camici PG, Crea F.. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 4. Herrmann J, Kaski JC, Lerman A.. Coronary microvascular dysfunction in the clinical setting: from mystery to reality. Eur Heart J 2012;33:2771–2782b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuo L, Chilian WM, Davis MJ.. Coronary arteriolar myogenic response is independent of endothelium. Circ Res 1990;66:860–866. [DOI] [PubMed] [Google Scholar]
- 6. Taqueti VR, Di Carli MF.. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Small HY, Migliarino S, Czesnikiewicz-Guzik M, Guzik TJ.. Hypertension: focus on autoimmunity and oxidative stress. Free Radic Biol Med 2018;125:104–115. [DOI] [PubMed] [Google Scholar]
- 8. Kang KT. Endothelium-derived relaxing factors of small resistance arteries in hypertension. Toxicol Res 2014;30:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77. [DOI] [PubMed] [Google Scholar]
- 10. Gutierrez E, Flammer AJ, Lerman LO, Elizaga J, Lerman A, Fernandez-Aviles F.. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J 2013;34:3175–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engelen SE, van der Graaf Y, Stam-Slob MC, Grobbee DE, Cramer MJ, Kappelle LJ, de Borst GJ, Visseren FLJ, Westerink J; SMART study group. Incidence of cardiovascular events and vascular interventions in patients with type 2 diabetes. Int J Cardiol 2017;248:301–307. [DOI] [PubMed] [Google Scholar]
- 12. Rawshani A, Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjornsdottir S.. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med 2017;376:1407–1418. [DOI] [PubMed] [Google Scholar]
- 13.Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J.. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Carli MF, Janisse J, Grunberger G, Ager J.. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol 2003;41:1387–1393. [DOI] [PubMed] [Google Scholar]
- 15. Nitenberg A, Valensi P, Sachs R, Cosson E, Attali JR, Antony I.. Prognostic value of epicardial coronary artery constriction to the cold pressor test in type 2 diabetic patients with angiographically normal coronary arteries and no other major coronary risk factors. Diabetes Care 2004;27:208–215. [DOI] [PubMed] [Google Scholar]
- 16. von Scholten BJ, Hasbak P, Christensen TE, Ghotbi AA, Kjaer A, Rossing P, Hansen TW.. Cardiac (82)Rb PET/CT for fast and non-invasive assessment of microvascular function and structure in asymptomatic patients with type 2 diabetes. Diabetologia 2016;59:371–378. [DOI] [PubMed] [Google Scholar]
- 17. Cortigiani L, Rigo F, Gherardi S, Galderisi M, Bovenzi F, Sicari R.. Prognostic meaning of coronary microvascular disease in type 2 diabetes mellitus: a transthoracic Doppler echocardiographic study. J Am Soc Echocardiogr 2014;27:742–748. [DOI] [PubMed] [Google Scholar]
- 18. Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF.. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LüScher TF, Creager MA, Beckman JA, Cosentino F.. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003;108:1527–1532. [DOI] [PubMed] [Google Scholar]
- 20. Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM, Tsao PS.. Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci 2015;16:25234–25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M.. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787–790. [DOI] [PubMed] [Google Scholar]
- 22. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H.. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000;49:1939–1945. [DOI] [PubMed] [Google Scholar]
- 23. Pacher P, Beckman JS, Liaudet L.. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem 2006;387:1521–1533. [DOI] [PubMed] [Google Scholar]
- 25. Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J.. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 2002;51:1118–1124. [DOI] [PubMed] [Google Scholar]
- 26. Mather KJ, Verma S, Anderson TJ.. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol 2001;37:1344–1350. [DOI] [PubMed] [Google Scholar]
- 27. Kibel A, Selthofer-Relatic K, Drenjancevic I, Bacun T, Bosnjak I, Kibel D, Gros M.. Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res 2017;45:1901–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujimoto WY. The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. Am J Med 2000;108(Suppl. 6a):9S–14S. [DOI] [PubMed] [Google Scholar]
- 29. Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD.. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 1997;100:1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P.. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 2003;52:2882–2887. [DOI] [PubMed] [Google Scholar]
- 31. Suzuki LA, Poot M, Gerrity RG, Bornfeldt KE.. Diabetes accelerates smooth muscle accumulation in lesions of atherosclerosis: lack of direct growth-promoting effects of high glucose levels. Diabetes 2001;50:851–860. [DOI] [PubMed] [Google Scholar]
- 32. Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL.. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001;24:1476–1485. [DOI] [PubMed] [Google Scholar]
- 33. Tabit CE, Chung WB, Hamburg NM, Vita JA.. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord 2010;11:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yokoyama I, Momomura S, Ohtake T, Yonekura K, Nishikawa J, Sasaki Y, Omata M.. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 1997;30:1472–1477. [DOI] [PubMed] [Google Scholar]
- 35. Costantino S, Paneni F, Battista R, Castello L, Capretti G, Chiandotto S, Tanese L, Russo G, Pitocco D, Lanza GA, Volpe M, Luscher TF, Cosentino F.. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes 2017;66:2472–2482. [DOI] [PubMed] [Google Scholar]
- 36. Gæde P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O.. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–393. [DOI] [PubMed] [Google Scholar]
- 37. Prior JO, Quiñones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, Hsueh WA, Schelbert HR.. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation 2005;111:2291–2298. [DOI] [PubMed] [Google Scholar]
- 38. Paulus WJ, Tschope C.. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 39. Heymsfield SB, Wadden TA.. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017;376:254–266. [DOI] [PubMed] [Google Scholar]
- 40. Tona F, Serra R, Di Ascenzo L, Osto E, Scarda A, Fabris R, Montisci R, Famoso G, Tellatin S, Foletto M, Giovagnoni A, Iliceto S, Vettor R.. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis 2014;24:447–453. [DOI] [PubMed] [Google Scholar]
- 41. Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF.. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS.. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–748. [DOI] [PubMed] [Google Scholar]
- 43. Quercioli A, Pataky Z, Vincenti G, Makoundou V, Di Marzo V, Montecucco F, Carballo S, Thomas A, Staub C, Steffens S, Seimbille Y, Golay A, Ratib O, Harsch E, Mach F, Schindler TH.. Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity. Eur Heart J 2011;32:1369–1378. [DOI] [PubMed] [Google Scholar]
- 44. Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, Bhatt DL, Di Carli MF, Taqueti VR.. Coronary microvascular dysfunction and cardiovascular risk in obese patients. J Am Coll Cardiol 2018;72:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schindler TH, Schelbert HR, Quercioli A, Dilsizian V.. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging 2010;3:623–640. [DOI] [PubMed] [Google Scholar]
- 46. Hutcheon JA, Lisonkova S, Joseph KS.. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391–403. [DOI] [PubMed] [Google Scholar]
- 47. Bellamy L, Casas JP, Hingorani AD, Williams DJ.. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA.. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97–104. [DOI] [PubMed] [Google Scholar]
- 49. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA.. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet 2005;366:1797–1803. [DOI] [PubMed] [Google Scholar]
- 50. Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ.. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol 2019;73:2106–2116. [DOI] [PubMed] [Google Scholar]
- 51. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ.. Pre-eclampsia. Lancet 2016;387:999–1011. [DOI] [PubMed] [Google Scholar]
- 52. Savvidou MD, Hingorani AD, Tsikas D, Frolich JC, Vallance P, Nicolaides KH.. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet 2003;361:1511–1517. [DOI] [PubMed] [Google Scholar]
- 53. Zhou Y, Damsky CH, Fisher SJ.. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest 1997;99:2152–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol 2015;213:S115–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Redman CW, Sacks GP, Sargent IL.. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol 1999;180:499–506. [DOI] [PubMed] [Google Scholar]
- 56. Cornelius DC. Preeclampsia: from Inflammation to Immunoregulation. Clin Med Insights Blood Disord 2018;11:1179545X17752325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Rijn BB, Franx A, Steegers EA, de Groot CJ, Bertina RM, Pasterkamp G, Voorbij HA, Bruinse HW, Roest M.. Maternal TLR4 and NOD2 gene variants, pro-inflammatory phenotype and susceptibility to early-onset preeclampsia and HELLP syndrome. PLoS One 2008;3:e1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS.. Association of maternal endothelial dysfunction with preeclampsia. JAMA 2001;285:1607–1612. [DOI] [PubMed] [Google Scholar]
- 59. Mutter WP, Karumanchi SA.. Molecular mechanisms of preeclampsia. Microvasc Res 2008;75:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Drost JT, Maas AH, Holewijn S, Joosten LA, van Eyck J, van der Schouw YT, de Graaf J.. Novel cardiovascular biomarkers in women with a history of early preeclampsia. Atherosclerosis 2014;237:117–122. [DOI] [PubMed] [Google Scholar]
- 61. Sandvik MK, Leirgul E, Nygard O, Ueland PM, Berg A, Svarstad E, Vikse BE.. Preeclampsia in healthy women and endothelial dysfunction 10 years later. Am J Obstet Gynecol 2013;209:569.e1–569.e10. [DOI] [PubMed] [Google Scholar]
- 62. Girouard JL, Giguère Y, Moutquin J-M, Forest J-C.. Previous hypertensive disease of pregnancy is associated with alterations of markers of insulin resistance. Hypertension 2007;49:1056–1062. [DOI] [PubMed] [Google Scholar]
- 63. van Rijn BB, Bruinse HW, Veerbeek JH, Post Uiterweer ED, Koenen SV, van der Bom JG, Rijkers GT, Roest M, Franx A.. Postpartum circulating markers of inflammation and the systemic acute-phase response after early-onset preeclampsia. Hypertension 2016;67:404–414. [DOI] [PubMed] [Google Scholar]
- 64. Ciftci FC, Caliskan M, Ciftci O, Gullu H, Uckuyu A, Toprak E, Yanik F.. Impaired coronary microvascular function and increased intima-media thickness in preeclampsia. J Am Soc Hypertens 2014;8:820–826. [DOI] [PubMed] [Google Scholar]
- 65. Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, Price K, Karumanchi SA, Valdés G.. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 2007;49:90–95. [DOI] [PubMed] [Google Scholar]
- 66. Milic NM, Milin-Lazovic J, Weissgerber TL, Trajkovic G, White WM, Garovic VD.. Preclinical atherosclerosis at the time of pre-eclamptic pregnancy and up to 10 years postpartum: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;49:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Grand’Maison S, Pilote L, Okano M, Landry T, Dayan N.. Markers of vascular dysfunction after hypertensive disorders of pregnancy: a systematic review and meta-analysis. Hypertension 2016;68:1447–1458. [DOI] [PubMed] [Google Scholar]
- 68. Zoet GA, Benschop L, Boersma E, Budde RPJ, Fauser BCJM, van der Graaf Y, de Groot CJM, Maas AHEM, Roeters van Lennep JE, Steegers EAP, Visseren FL, van Rijn BB, Velthuis BK, Franx A; CREW Consortium. Prevalence of subclinical coronary artery disease assessed by coronary computed tomography angiography in 45- to 55-year-old women with a history of preeclampsia. Circulation 2018;137:877–879. [DOI] [PubMed] [Google Scholar]
- 69. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA.. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- 70. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Karpouzas GA, Malpeso J, Choi TY, Li D, Munoz S, Budoff MJ.. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann Rheum Dis 2014;73:1797–1804. [DOI] [PubMed] [Google Scholar]
- 72. Bordy R, Totoson P, Prati C, Marie C, Wendling D, Demougeot C.. Microvascular endothelial dysfunction in rheumatoid arthritis. Nat Rev Rheumatol 2018;14:404–420. [DOI] [PubMed] [Google Scholar]
- 73. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D.. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–1529. [DOI] [PubMed] [Google Scholar]
- 74. Eriksson JK, Jacobsson L, Bengtsson K, Askling J.. Is ankylosing spondylitis a risk factor for cardiovascular disease, and how do these risks compare with those in rheumatoid arthritis? Ann Rheum Dis 2017;76:364–370. [DOI] [PubMed] [Google Scholar]
- 75. Kibari A, Cohen AD, Gazitt T, Bitterman H, Lavi I, Feldhamer I, Shalom G, Greenberg-Dotan S, Zisman D.. Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study. Clin Rheumatol 2019;38:2069–2075. [DOI] [PubMed] [Google Scholar]
- 76. Kuo CF, Chou IJ, Rees F, Grainge MJ, Lanyon P, Davenport G, Mallen CD, Chung TT, Chen JS, Zhang W, Doherty M.. Temporal relationships between systemic lupus erythematosus and comorbidities. Rheumatology (Oxford) 2019;58:840–848. [DOI] [PubMed] [Google Scholar]
- 77. Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen ITN, Octavia Y, van Duin RWB, Stam K, van Geuns RJ, Wielopolski PA, Krestin GP, van den Meiracker AH, Verjans R, van Bilsen M, Danser AHJ, Paulus WJ, Cheng C, Linke WA, Joles JA, Verhaar MC, van der Velden J, Merkus D, Duncker DJ.. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res 2018;114:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ketelhuth DFJ, Lutgens E, Back M, Binder CJ, Van den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O’Neill L, Weber C, Evans PC.. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc Res 2019;115:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM.. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res 2000;86:E85–E90. [DOI] [PubMed] [Google Scholar]
- 80. Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D, Sagan A, Wu J, Vinh A, Marvar PJ, Guzik B, Podolec J, Drummond G, Lob HE, Harrison DG, Guzik TJ.. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J 2016;30:1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ikonomidis I, Makavos G, Papadavid E, Varoudi M, Andreadou I, Gravanis K, Theodoropoulos K, Pavlidis G, Triantafyllidi H, Parissis J, Paraskevaidis I, Rigopoulos D, Lekakis J.. Similarities in coronary function and myocardial deformation between psoriasis and coronary artery disease: the role of oxidative stress and inflammation. Can J Cardiol 2015;31:287–295. [DOI] [PubMed] [Google Scholar]
- 82. Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, Hidaka T, Takemoto H, Nakamura S, Soga J, Chayama K, Yoshizumi M, Taguchi A.. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension 2008;51:446–453. [DOI] [PubMed] [Google Scholar]
- 83. Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, Nosalski R, Pelka P, Nowakowski D, Wilk G, Mikolajczyk TP, Schramm-Luc A, Furtak A, Matusik P, Koziol J, Drozdz M, Munoz-Aguilera E, Tomaszewski M, Evangelou E, Caulfield M, Grodzicki T, D'Aiuto F, Guzik TJ.. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J 2019;40:3459–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roifman I, Sun YC, Fedwick JP, Panaccione R, Buret AG, Liu H, Rostom A, Anderson TJ, Beck PL.. Evidence of endothelial dysfunction in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2009;7:175–182. [DOI] [PubMed] [Google Scholar]
- 85. Klimek E, Sulicka J, Gryglewska B, Skalska A, Kwaśny-Krochin B, Korkosz M, Grodzicki TK.. Alterations in skin microvascular function in patients with rheumatoid arthritis and ankylosing spondylitis. Clin Hemorheol Microcirc 2017;65:77–91. [DOI] [PubMed] [Google Scholar]
- 86. van Eijk IC, Peters MJ, Serne EH, van der Horst-Bruinsma IE, Dijkmans BA, Smulders YM, Nurmohamed MT.. Microvascular function is impaired in ankylosing spondylitis and improves after tumour necrosis factor alpha blockade. Ann Rheum Dis 2009;68:362–366. [DOI] [PubMed] [Google Scholar]
- 87. Erre GL, Buscetta G, Paliogiannis P, Mangoni AA, Carru C, Passiu G, Zinellu A.. Coronary flow reserve in systemic rheumatic diseases: a systematic review and meta-analysis. Rheumatol Int 2018;38:1179–1190. [DOI] [PubMed] [Google Scholar]
- 88. Sandhu VK, Wei J, Thomson LEJ, Berman DS, Schapira J, Wallace D, Weisman MH, Bairey Merz CN, Ishimori ML.. A five-year follow up of coronary microvascular dysfunction and coronary artery disease in SLE: results from a community-based lupus cohort. Arthritis Care Res (Hoboken) 2019;doi:10.1002/acr.23920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Turiel M, Atzeni F, Tomasoni L, de Portu S, Delfino L, Bodini BD, Longhi M, Sitia S, Bianchi M, Ferrario P, Doria A, De Gennaro Colonna V, Sarzi-Puttini P.. Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford) 2009;48:834–839. [DOI] [PubMed] [Google Scholar]
- 90. Batko B, Maga P, Urbanski K, Ryszawa-Mrozek N, Schramm-Luc A, Koziej M, Mikolajczyk T, McGinnigle E, Czesnikiewicz-Guzik M, Ceranowicz P, Guzik TJ.. Microvascular dysfunction in ankylosing spondylitis is associated with disease activity and is improved by anti-TNF treatment. Sci Rep 2018;8:13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Roustit M, Cracowski JL.. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 2012;19:47–64. [DOI] [PubMed] [Google Scholar]
- 92. Lira-Junior R, Figueredo CM, Bouskela E, Fischer RG.. Severe chronic periodontitis is associated with endothelial and microvascular dysfunctions: a pilot study. J Periodontol 2014;85:1648–1657. [DOI] [PubMed] [Google Scholar]
- 93. Caliskan Z, Gokturk HS, Caliskan M, Gullu H, Ciftci O, Ozgur GT, Guven A, Selcuk H.. Impaired coronary microvascular and left ventricular diastolic function in patients with inflammatory bowel disease. Microvasc Res 2015;97:25–30. [DOI] [PubMed] [Google Scholar]
- 94. Sandoo A, Chanchlani N, Hodson J, Smith JP, Douglas KM, Kitas GD.. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: a six-year prospective study. Arthritis Res Ther 2013;15:R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Faccini A, Kaski JC, Camici PG.. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J 2016;37:1799–1806. [DOI] [PubMed] [Google Scholar]
- 96. Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM.. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol 2008;28:1347–1354. [DOI] [PubMed] [Google Scholar]
- 97. Basuroy S, Bhattacharya S, Leffler CW, Parfenova H.. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 2009;296:C422–C432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Manea A, Manea SA, Florea IC, Luca CM, Raicu M.. Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic Biol Med 2012;52:1497–1507. [DOI] [PubMed] [Google Scholar]
- 99. Jeon CH, Ahn JK, Chai JY, Kim HJ, Bae EK, Park SH, Cho EY, Cha HS, Ahn KS, Koh EM.. Hypoxia appears at pre-arthritic stage and shows co-localization with early synovial inflammation in collagen induced arthritis. Clin Exp Rheumatol 2008;26:646–648. [PubMed] [Google Scholar]
- 100. Haruna Y, Morita Y, Komai N, Yada T, Sakuta T, Tomita N, Fox DA, Kashihara N.. Endothelial dysfunction in rat adjuvant-induced arthritis: vascular superoxide production by NAD(P)H oxidase and uncoupled endothelial nitric oxide synthase. Arthritis Rheum 2006;54:1847–1855. [DOI] [PubMed] [Google Scholar]
- 101. Sandoo A, Dimitroulas T, Veldhuijzen van Zanten JJ, Smith JP, Metsios GS, Nightingale P, Stavropoulos-Kalinoglou A, Kitas GD.. Lack of association between asymmetric dimethylarginine and in vivo microvascular and macrovascular endothelial function in patients with rheumatoid arthritis. Clin Exp Rheumatol 2012;30:388–396. [PubMed] [Google Scholar]
- 102. Guzik TJ, Skiba DS, Touyz RM, Harrison DG.. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res 2017;113:1009–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Leonard S, Croy BA, Murrant CL.. Arteriolar reactivity in lymphocyte-deficient mice. Am J Physiol Heart Circ Physiol 2011;301:H1276–H1285. [DOI] [PubMed] [Google Scholar]
- 104. Weyand CM, Bryl E, Goronzy JJ.. The role of T cells in rheumatoid arthritis. Arch Immunol Ther Exp (Warsz) 2000;48:429–435. [PubMed] [Google Scholar]
- 105. Maga P, Mikolajczyk TP, Partyka L, Siedlinski M, Maga M, Krzanowski M, Malinowski K, Luc K, Nizankowski R, Bhatt DL, Guzik TJ.. Involvement of CD8+ T cell subsets in early response to vascular injury in patients with peripheral artery disease in vivo. Clin Immunol 2018;194:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wen Z, Shimojima Y, Shirai T, Li Y, Ju J, Yang Z, Tian L, Goronzy JJ, Weyand CM.. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J Clin Invest 2016;126:1953–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Cachat J, Deffert C, Hugues S, Krause KH.. Phagocyte NADPH oxidase and specific immunity. Clin Sci (Lond) 2015;128:635–648. [DOI] [PubMed] [Google Scholar]
- 108. Mikolajczyk TP, Osmenda G, Batko B, Wilk G, Krezelok M, Skiba D, Sliwa T, Pryjma JR, Guzik TJ.. Heterogeneity of peripheral blood monocytes, endothelial dysfunction and subclinical atherosclerosis in patients with systemic lupus erythematosus. Lupus 2016;25:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Urbanski K, Ludew D, Filip G, Filip M, Sagan A, Szczepaniak P, Grudzien G, Sadowski J, Jasiewicz-Honkisz B, Sliwa T, Kapelak B, McGinnigle E, Mikolajczyk T, Guzik TJ.. CD14(+)CD16(++) “nonclassical” monocytes are associated with endothelial dysfunction in patients with coronary artery disease. Thromb Haemost 2017;117:971–980. [DOI] [PubMed] [Google Scholar]
- 110. Ahmed A, Hollan I, Curran SA, Kitson SM, Riggio MP, Mikkelsen K, Almdahl SM, Aukrust P, McInnes IB, Goodyear CS.. Brief report: proatherogenic cytokine microenvironment in the aortic adventitia of patients with rheumatoid arthritis. Arthritis Rheumatol 2016;68:1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Maffia P, Guzik TJ.. When, where, and how to target vascular inflammation in the post-CANTOS era? Eur Heart J 2019;40:2492–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP.. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–352. [DOI] [PubMed] [Google Scholar]
- 113. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RTChronic Kidney Disease Prognosis Consortiumvan der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T.. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341–1352. [DOI] [PubMed] [Google Scholar]
- 114. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R.. Cardiorenal syndrome. J Am Coll Cardiol 2008;52:1527–1539. [DOI] [PubMed] [Google Scholar]
- 115. Nelson AJ, Dundon BK, Worthley SG, Richardson JD, Puri R, Wong DTL, Coates PT, Faull RJ, Worthley MI.. End-stage renal failure is associated with impaired coronary microvascular function. Coron Artery Dis 2019;30:520–527. [DOI] [PubMed] [Google Scholar]
- 116. Caliskan Y, Demirturk M, Ozkok A, Yelken B, Sakaci T, Oflaz H, Unsal A, Yildiz A.. Coronary artery calcification and coronary flow velocity in haemodialysis patients. Nephrol Dial Transplant 2010;25:2685–2690. [DOI] [PubMed] [Google Scholar]
- 117. Charytan DM, Skali H, Shah NR, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Bibbo CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF.. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int 2018;93:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shah NR, Charytan DM, Murthy VL, Skali Lami H, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Foster CR, Hainer J, Gaber M, Klein J, Dorbala S, Blankstein R, Di Carli MF.. Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol 2016;27:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Fliser D, Wiecek A, Suleymanlar G, Ortiz A, Massy Z, Lindholm B, Martinez-Castelao A, Agarwal R, Jager KJ, Dekker FW, Blankestijn PJ, Goldsmith D, Covic A, London G, Zoccali C; for EUropean REnal and Cardiovascular Medicine working group of the European Renal Association–European Dialysis and Transplant Association (ERA–EDTA). The dysfunctional endothelium in CKD and in cardiovascular disease: mapping the origin(s) of cardiovascular problems in CKD and of kidney disease in cardiovascular conditions for a research agenda. Kidney Int Suppl (2011) 2011;1:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Prommer HU, Maurer J, von Websky K, Freise C, Sommer K, Nasser H, Samapati R, Reglin B, Guimaraes P, Pries AR, Querfeld U.. Chronic kidney disease induces a systemic microangiopathy, tissue hypoxia and dysfunctional angiogenesis. Sci Rep 2018;8:5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Amann K, Breitbach M, Ritz E, Mall G.. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol 1998;9:1018–1022. [DOI] [PubMed] [Google Scholar]
- 122. Charytan DM, Padera R, Helfand AM, Zeisberg M, Xu X, Liu X, Himmelfarb J, Cinelli A, Kalluri R, Zeisberg EM.. Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol 2014;176:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J.. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl 2008;74:S4–S9. [DOI] [PubMed] [Google Scholar]
- 124. Dounousi E, Papavasiliou E, Makedou A, Ioannou K, Katopodis KP, Tselepis A, Siamopoulos KC, Tsakiris D.. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis 2006;48:752–760. [DOI] [PubMed] [Google Scholar]
- 125. Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, Stenvinkel P.. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 2003;41:1212–1218. [DOI] [PubMed] [Google Scholar]
- 126. Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O.. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999;34:125–134. [DOI] [PubMed] [Google Scholar]
- 127. Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B.. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA 2005;293:1737–1745. [DOI] [PubMed] [Google Scholar]
- 128. Herzog CA, Asinger RW, Berger AK, Charytan DM, Diez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E.. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: improving Global Outcomes (KDIGO). Kidney Int 2011;80:572–586. [DOI] [PubMed] [Google Scholar]
- 129. Pannier B, Guérin AP, Marchais SJ, Safar ME, London GM.. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension 2005;45:592–596. [DOI] [PubMed] [Google Scholar]
- 130.Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H.. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 131. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287:1308–1320. [DOI] [PubMed] [Google Scholar]
- 132. Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I, Biller L, Lesser JR, Udelson JE, Manning WJ, Appelbaum E.. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 2009;54:220–228. [DOI] [PubMed] [Google Scholar]
- 133. Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A.. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia. Hum Pathol 2000;31:988–998. [DOI] [PubMed] [Google Scholar]
- 134. Camici P, Chiriatti G, Lorenzoni R, Bellina RC, Gistri R, Italiani G, Parodi O, Salvadori PA, Nista N, Papi L, L'abbate A.. Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: a study with nitrogen-13 ammonia and positron emission tomography. J Am Coll Cardiol 1991;17:879–886. [DOI] [PubMed] [Google Scholar]
- 135. Olivotto I, Girolami F, Sciagra R, Ackerman MJ, Sotgia B, Bos JM, Nistri S, Sgalambro A, Grifoni C, Torricelli F, Camici PG, Cecchi F.. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations. J Am Coll Cardiol 2011;58:839–848. [DOI] [PubMed] [Google Scholar]
- 136. Krams R, Kofflard MJ, Duncker DJ, Von Birgelen C, Carlier S, Kliffen M, ten Cate FJ, Serruys PW.. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation 1998;97:230–233. [DOI] [PubMed] [Google Scholar]
- 137. Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H.. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation 2007;115:2418–2425. [DOI] [PubMed] [Google Scholar]
- 138. Chiribiri A, Leuzzi S, Conte MR, Bongioanni S, Bratis K, Olivotti L, De Rosa C, Lardone E, Di Donna P, Villa AD, Cesarani F, Nagel E, Gaita F, Bonamini R.. Rest perfusion abnormalities in hypertrophic cardiomyopathy: correlation with myocardial fibrosis and risk factors for sudden cardiac death. Clin Radiol 2015;70:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Maron MS, Olivotto I, Maron BJ, Prasad SK, Cecchi F, Udelson JE, Camici PG.. The case for myocardial ischemia in hypertrophic cardiomyopathy. J Am Coll Cardiol 2009;54:866–875. [DOI] [PubMed] [Google Scholar]
- 140. Olivotto I, Cecchi F, Gistri R, Lorenzoni R, Chiriatti G, Girolami F, Torricelli F, Camici PG.. Relevance of coronary microvascular flow impairment to long-term remodeling and systolic dysfunction in hypertrophic cardiomyopathy. J Am Coll Cardiol 2006;47:1043–1048. [DOI] [PubMed] [Google Scholar]
- 141. Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG.. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med 2003;349:1027–1035. [DOI] [PubMed] [Google Scholar]
- 142. Maron BJ, Wolfson JK, Epstein SE, Roberts WC.. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol 1986;8:545–557. [DOI] [PubMed] [Google Scholar]
- 143. Tanaka M, Fujiwara H, Onodera T, Wu DJ, Matsuda M, Hamashima Y, Kawai C.. Quantitative analysis of narrowings of intramyocardial small arteries in normal hearts, hypertensive hearts, and hearts with hypertrophic cardiomyopathy. Circulation 1987;75:1130–1139. [DOI] [PubMed] [Google Scholar]
- 144. Johansson B, Morner S, Waldenstrom A, Stal P.. Myocardial capillary supply is limited in hypertrophic cardiomyopathy: a morphological analysis. Int J Cardiol 2008;126:252–257. [DOI] [PubMed] [Google Scholar]
- 145. Foa A, Agostini V, Rapezzi C, Olivotto I, Corti B, Potena L, Biagini E, Martin Suarez S, Rotellini M, Cecchi F, Stefano P, Coppini R, Ferrantini C, Bacchi Reggiani ML, Leone O.. Histopathological comparison of intramural coronary artery remodeling and myocardial fibrosis in obstructive versus end-stage hypertrophic cardiomyopathy. Int J Cardiol 2019;291:77–82. [DOI] [PubMed] [Google Scholar]
- 146. Raphael CE, Cooper R, Parker KH, Collinson J, Vassiliou V, Pennell DJ, de Silva R, Hsu LY, Greve AM, Nijjer S, Broyd C, Ali A, Keegan J, Francis DP, Davies JE, Hughes AD, Arai A, Frenneaux M, Stables RH, Di Mario C, Prasad SK.. Mechanisms of myocardial ischemia in hypertrophic cardiomyopathy: insights from wave intensity analysis and magnetic resonance. J Am Coll Cardiol 2016;68:1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Soliman OI, Geleijnse ML, Michels M, Dijkmans PA, Nemes A, van Dalen BM, Vletter WB, Serruys PW, ten Cate FJ.. Effect of successful alcohol septal ablation on microvascular function in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol 2008;101:1321–1327. [DOI] [PubMed] [Google Scholar]
- 148. Bech-Hanssen O, Wallentin I, Houltz E, Suurkula MB, Larsson S, Caidahl K.. Gender differences in patients with severe aortic stenosis: impact on preoperative left ventricular geometry and function, as well as early postoperative morbidity and mortality. Eur J Cardiothorac Surg 1999;15:24–30. [DOI] [PubMed] [Google Scholar]
- 149. Rajappan K, Rimoldi OE, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, Camici PG.. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation 2002;105:470–476. [DOI] [PubMed] [Google Scholar]
- 150. Marcus ML, Doty DB, Hiratzka LF, Wright CB, Eastham CL.. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med 1982;307:1362–1366. [DOI] [PubMed] [Google Scholar]
- 151. Breisch EA, Houser SR, Carey RA, Spann JF, Bove AA.. Myocardial blood flow and capillary density in chronic pressure overload of the feline left ventricle. Cardiovasc Res 1980;14:469–475. [DOI] [PubMed] [Google Scholar]
- 152. Lumley M, Williams R, Asrress KN, Arri S, Briceno N, Ellis H, Rajani R, Siebes M, Piek JJ, Clapp B, Redwood SR, Marber MS, Chambers JB, Perera D.. Coronary physiology during exercise and vasodilation in the healthy heart and in severe aortic stenosis. J Am Coll Cardiol 2016;68:688–697. [DOI] [PubMed] [Google Scholar]
- 153. Dunn RB, Griggs DM Jr.. Ventricular filling pressure as a determinant of coronary blood flow during ischemia. Am J Physiol 1983;244:H429–H436. [DOI] [PubMed] [Google Scholar]
- 154. Garcia D, Camici PG, Durand LG, Rajappan K, Gaillard E, Rimoldi OE, Pibarot P.. Impairment of coronary flow reserve in aortic stenosis. J Appl Physiol (1985) 2009;106:113–121. [DOI] [PubMed] [Google Scholar]
- 155. Wiegerinck EM, van de Hoef TP, Rolandi MC, Yong Z, van Kesteren F, Koch KT, Vis MM, de Mol BA, Piek JJ, Baan J Jr.. Impact of aortic valve stenosis on coronary hemodynamics and the instantaneous effect of transcatheter aortic valve implantation. Circ Cardiovasc Interv 2015;8:e002443. [DOI] [PubMed] [Google Scholar]
- 156. Schwartzkopff B, Frenzel H, Dieckerhoff J, Betz P, Flasshove M, Schulte HD, Mundhenke M, Motz W, Strauer BE.. Morphometric investigation of human myocardium in arterial hypertension and valvular aortic stenosis. Eur Heart J 1992;13(Suppl. D):17–23. [DOI] [PubMed] [Google Scholar]
- 157. Julius BK, Spillmann M, Vassalli G, Villari B, Eberli FR, Hess OM.. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation 1997;95:892–898. [DOI] [PubMed] [Google Scholar]
- 158. Gould KL. Why angina pectoris in aortic stenosis. Circulation 1997;95:790–792. [DOI] [PubMed] [Google Scholar]
- 159. Nemes A, Balazs E, Csanady M, Forster T.. Long-term prognostic role of coronary flow velocity reserve in patients with aortic valve stenosis—insights from the SZEGED study. Clin Physiol Funct Imaging 2009;29:447–452. [DOI] [PubMed] [Google Scholar]
- 160. Bang CN, Greve AM, Rossebo AB, Ray S, Egstrup K, Boman K, Nienaber C, Okin PM, Devereux RB, Wachtell K.. Antihypertensive treatment with beta-blockade in patients with asymptomatic aortic stenosis and association with cardiovascular events. J Am Heart Assoc 2017;6:e0006709. [DOI] [PMC free article] [PubMed] [Google Scholar]