Abstract
Arrhythmogenic cardiomyopathy (ACM) is a life-threatening cardiac disease caused by mutations in genes predominantly encoding for desmosomal proteins that lead to alterations in the molecular composition of the intercalated disc. ACM is characterized by progressive replacement of cardiomyocytes by fibrofatty tissue, ventricular dilatation, cardiac dysfunction, and heart failure but mostly dominated by the occurrence of life-threatening arrhythmias and sudden cardiac death (SCD). As SCD appears mostly in apparently healthy young individuals, there is a demand for better risk stratification of suspected ACM mutation carriers. Moreover, disease severity, progression, and outcome are highly variable in patients with ACM. In this review, we discuss the aetiology of ACM with a focus on pro-arrhythmic disease mechanisms in the early concealed phase of the disease. We summarize potential new biomarkers which might be useful for risk stratification and prediction of disease course. Finally, we explore novel therapeutic strategies to prevent arrhythmias and SCD in the early stages of ACM.
Keywords: Cardiomyopathy, Arrhythmia
This article is part of the Spotlight Issue on Inherited Conditions of Arrhythmia.
1. Introduction
Electromechanical coupling that drives contraction of the heart relies on the (most often) happy marriage between two fundamental physiological principles: formation and propagation of the electrical impulse and calcium-mediated mechanical performance. These two principles allow the heart to contract in a highly coordinated spatiotemporal fashion for about 100 000 times a day, at least in humans under baseline conditions. For a long time, scientists were mesmerized by the question of how the individual activity of numerous tiny cardiomyocytes could result in such an impressive performance of the heart. When microscopy started to feed anatomical knowledge up to the resolution of individual cells, a large step forward was made in understanding the cellular basis of excitation–contraction coupling. At the longitudinal cell edges of individual cardiomyocytes, structures were discovered that appeared to interconnect cardiomyocytes. Further progression in the fields of cell biology, physiology, and molecular biology has resulted in our current knowledge of those structures, now known as intercalated discs (IDs).
IDs consist of several components that facilitate the generation of the electrical impulse (sodium channels), intercellular impulse propagation (mediated by gap junctions), and provide mechanical integration (mediated by adherens junctions and desmosomes). Based on their initially detected subcellular separation within the ID, for long it was assumed that these components functioned independently. In the last decade, however, research has uncovered several modes of interaction between these entities which has led to the concept that the ID is actually one large macromolecular network of interacting proteins.1 The importance of proper composition of this ID protein complex has become evident from the observation that alterations in one or more components commonly occur during various forms of myocardial disease.1,2 ID abnormalities particularly occur in the setting of arrhythmogenic cardiomyopathy (ACM), a life-threatening cardiac disease caused by mutations in genes that predominantly encode for desmosomal proteins.1 The disease is a familial disorder, characterized by progressive replacement of cardiomyocytes by fibrofatty tissue and the occurrence of life-threatening arrhythmias and sudden cardiac death (SCD), primarily in young and apparently healthy individuals. However, disease severity, progression, and outcome are highly variable in patients with ACM. The disease may lead to progressive signs of heart failure (HF) in some, while in others the first sign of disease consists of life-threatening arrhythmias and SCD during the very early stages of the disease; other patients may even remain relatively unaffected.3,4 Mainly based on histological manifestations, initially the disease was described having a predominantly right ventricular (RV) involvement and a left ventricular (LV) deterioration in a later stage, which explains the classical name of arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D).2 However, by now it is recognized that the disease can also have a primary LV dominance or even a biventricular phenotype, which has led to the introduction of the name ACM.4 Despite recent progress, reliable genetic, molecular, or clinical risk stratification options has proven cumbersome, making it virtually impossible to predict who is most at risk for SCD.
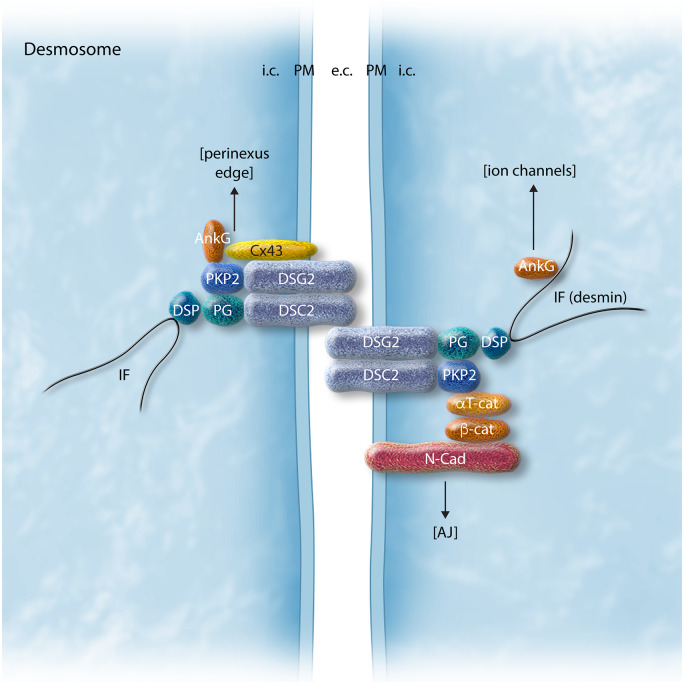
Desmosomes form intercellular junctions at the boundaries of IDs between neighbouring cardiomyocytes and consist of multiprotein complexes composed of desmosomal cadherins (desmogleins and desmocollins) and desmosomal plaque proteins (desmoplakin, plakoglobin, plakophilin) (Figure 1). Desmogleins and desmocollins mediate cell-to-cell adhesion through extracellular interaction between adjacent cardiomyocytes, and intracellular connections to desmoplakin, which is mediated by plakoglobin and plakophilin.1,5 Desmoplakin, in turn, anchors to components of the cytoskeleton such as desmin, thus forming a desmosome–intermediate filament complex, which not only allows functional communication between adjacent cells but also provides scaffolding for tissue integrity. Desmosomes have ‘hyper-adhesiveness’ properties, which refers to the fact that desmosomes can adopt a stronger adhesive state which is calcium-independent.6,7 In principle, calcium is required to form the interaction between cell adhesion molecules, however, after initial desmosome assembly, adhesion becomes calcium-independent. This hyper-adhesiveness of desmosomes is considered essential for its protective role in preventing myocyte disruption during mechanical stress.1,6 Disturbed desmosomal organization, as the result of mutations in genes that encode proteins which constitute the desmosomes, is thought to trigger myocardial fibrosis formation, fibrofatty replacement, and cardiac dilation, the classical histopathological pattern of ACM in advanced stages of the disease, setting the stage for the development of HF and arrhythmias.3,5,8 However, arrhythmias and SCD also often occur early in the disease process, prior to the development of overt cardiomyopathic alterations. Animal studies have enabled investigation of disease onset and progression in terms of electrical vs. structural changes (and their interrelation) and have provided insight into some of the underlying disease mechanisms.9,10 The clinical relevance of these early electrical alterations is still incompletely known given the fact that the experimental models most often recapitulate only some aspects of the findings in patients. Moreover, availability of patient cardiac tissue is scarce and primarily limited to end-stage remodelled hearts, while tissue from the early disease stage is generally lacking or only present in the form of tiny biopsies that might not be representative for the overall degree of maladaptive remodelling. Hence, new strategies are required for the identification of individuals who are at highest risk, as well as novel approaches for prevention and treatment of this early electrical remodelling in ACM.
Figure 1.
Schematic representation of the desmosomal protein complex at the intercalated disc and the molecular links to other associated protein complexes. AJ, adherens junction; AnkG, ankyrin G; aT-cat, alpha-catenin; b-cat, beta-catenin; Cx43, connexin43; DSC2, desmocollin-2; DSG2, desmoglein-2; DSP, desmoplakin; IF, intermediate filaments; N-cad, N-cadherin; PG, plakoglobin/gamma-catenin; PKP2, plakophilin-2.
Here, we provide a general overview of the aetiology of ACM, its clinical and histopathological characteristics, disease progression and outcome, and therapeutic considerations. We summarize insight into ACM pathogenesis obtained from studies in animal and cellular models as well as the available clinical information, focusing in particular on pro-arrhythmic disease mechanisms in the early concealed phase of the disease before the onset of structural pathology. In addition, we explore novel opportunities for risk stratification, prevention and therapy of arrhythmias and SCD in the early disease stages of ACM.
2. ACM: prevalence and genetics
The prevalence of ACM is estimated between 1:1000 and 1:50003,4,8 depending on geographical differences. Athletes with a genetic predisposition for ACM are particularly at risk for developing the disease; for instance, ACM is believed to be responsible for up to 20% of all SCD cases in athletes within the Veneto region of Italy.11 In addition, there is a male predominance of the disease, which suggests the influence of sex hormones (addressed in more detail below).8,12,13 Nearly 60% of ACM patients harbour one or even more mutations in genes encoding for major components of the cardiac desmosomes, including plakoglobin (JUP), desmoplakin (DSP), plakophilin-2 (PKP2), desmoglein-2 (DSG2), and desmocollin-2 (DSC2). Mutations in PKP2 are most commonly found in ACM patients in North America and Europe.2,4 In rare instances, homozygous JUP and DSP mutations are associated with ACM-affiliated disease like Naxos disease and Carvajal syndrome, both characterized by an additional presence of extra-cardiac abnormalities in hair and skin. In addition, mutations in genes encoding non-desmosomal proteins have been associated with ACM, including phospholamban (PLN), the cardiac ryanodine receptor (RYR2), transforming growth factor (TGF-β3), transmembrane protein-43 (TMEM43), and the cardiac sodium channel (SCN5A).8,14,15 The genetic trait of ACM inheritance is mainly an autosomal dominant, but genetic screening is hampered by the presence of incomplete disease penetrance, variable expressivity, and the presence of multiple, potentially pathogenic mutations in one or more genes in one individual suffering from ACM (i.e. compound and digenic heterozygousity). A study of Carruth et al.16 determined that the presence of ACM loss-of-function variants is approximately 1:435 in a predominantly European population without a clinical diagnosis of ACM. This finding indicates a low penetrance of pathogenic variants in a healthy population. Some of these genotype positive individuals met a part of the criteria described in the Task Force Criteria (TFC, see below) such as T-wave inversions and ventricular dysfunction, however, a comparable rate of these abnormalities was also observed in the control cohort of individuals without any known pathogenic variants.16 Nevertheless, genetic diagnosis is essential since a study by Cox et al.17 shows that asymptomatic mutation-carrying relatives have a six-fold increased risk of developing ACM compared to relatives of a proband without a genetic mutation.
3. Clinical presentation, diagnosis, disease progression, and management
3.1. Clinical presentation and disease progression
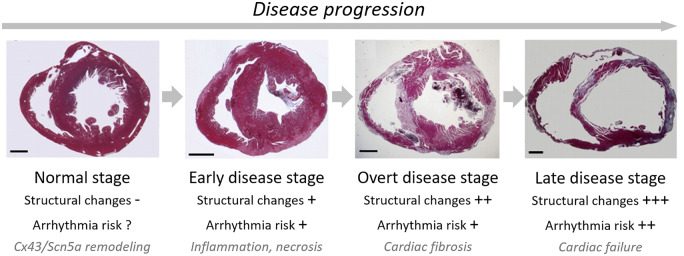
Affected ACM patients typically present between the ages of 13 and 40 with palpitations, pre-syncope, syncope, or SCD. Three phases have been described in ACM progression: (i) an early, pre-clinical phase, in which patients are asymptomatic and subtle ventricular abnormalities [electrocardiogram (ECG) changes including T-wave inversions] may go unnoticed if there is not a high index of suspicion; (ii) an overt phase, in which symptoms of ventricular arrhythmias (VA) develop; and (iii) a progressive phase, leading to biventricular HF with fluid overload and symptoms of congestion (ascites, oedema, and dyspnoea).18Figure 2 provides an overview of the characteristics during those different phases when recapitulated in an experimental mouse model of ACM.9 Electrical disturbances with regard to impulse generation and propagation may lead to SCD at any time during the disease course, with some reports stating that up to 50% of cases present with SCD.19 Importantly, this also includes the pre-clinical phase in which arrhythmias and/or SCD unfortunately are most often the first expressions of the disease.20 As a result, many studies have attempted to define clinical features that determine an early arrhythmic risk. This has resulted in a large body of literature with potential arrhythmic markers, which include (but are not limited to) depolarization delay, cardiac syncope, premature ventricular complex (PVC) frequency, feature tracking, deformation imaging, and biomarkers.21 Of note, arrhythmic presentation is not uniform: a study by Bhonsale et al.4 has shown that presentation with ventricular fibrillation (VF) or SCD occurs at a significantly younger age than presentation with haemodynamically stable ventricular tachycardia (VT). This was confirmed by a study from Te Riele et al.14,22 showing that paediatric ACM patients more often present with SCD than adult ACM subjects. These insights have led to the current hypothesis that arrhythmias in the early stage of ACM are mostly the consequence of remodelling of the ID and myocyte death/inflammation, whereas arrhythmogenesis in late-staged ACM is additionally promoted by the presence of tissue scarring and fibrofatty replacement, which sets the stage for re-entrant arrhythmias (discussed in more detail below).23
Figure 2.
Overview of the different stages in disease progression with emphasis on structural remodelling, the involved processes, and the consequences for arrhythmogenic risk. Morphological illustrations represent the different stages in cardiac remodelling as observed in an experimental mouse model of ACM.9 Permissions received.
3.2. Diagnosis of ACM
As the name ACM implies, the phenotype is determined by the presence of both an abnormal electrical (arrhythmogenic) and structural (cardiomyopathy) substrate. Unfortunately, no single test is sufficiently sensitive and specific to serve as the gold standard test for ACM evaluation. Therefore, a subset of specific criteria have been developed for ACM diagnosis.24 Definite ACM diagnosis is based on the consensus-based 2010 TFC, which include major and minor criteria in six categories (depolarization and repolarization abnormalities, arrhythmia, imaging, histology, and family history/genetics).25 Among these, repolarization abnormalities (T-wave inversion in the precordial leads) constitute the most commonly observed TFC, followed by frequent PVCs. With regards to imaging techniques, both the presence of wall motion abnormalities and an abnormal ventricular volume or function are required for TFC fulfilment. Definite ACM consists of two major criteria or one major and two minor criteria or four minor criteria from different categories. Borderline ACM consists of one major and one minor criterion or three minor criteria from different categories. Diagnosis of possible ACM consists of one major or two minor criteria from different categories. Since a comprehensive diagnostic evaluation for ACM is complex and time-consuming, several reports have called for a revision of the TFC.26 Until then, the 2010 TFC remain relevant.
3.3. Histopathological features of ACM
Histologically, ACM is classically reported as characterized by the replacement of the ventricular myocardium by fibrofatty tissue. However, clinical–pathological correlations in probands or family members with a positive genotype led to the concept of a myocardial disease with a dynamic phenotype and a variable penetrance, which is not only age-related but also influenced by external triggers or other modifiers (Figure 2). Thus, the fibrofatty tissue in the ventricular myocardium can be considered the typical substrate of late or ‘overt’ stages of the disease phenotype. A second histopathological observation is the presence of inflammatory cells, which have been reported in 60–80% of post-mortem/transplant cardiac specimens and mostly consist of T lymphocytes in the areas of fibrofatty replacement, but include also polymorphous cells in the acute stages.27 Further histological abnormalities include cardiac myocyte hypertrophy, vacuolization, and dysmetric and dysmorphic nuclei (the so-called ‘cardiomyopathic changes’).27,28 These histopathological changes usually start from the epicardium and extend towards the endocardium. Eventually changes become transmural, particularly in the RV free wall, often but not necessarily with wall thinning and aneurysm formation. The presence of a transmural involvement in the RV free wall is a common finding but it is not required to reach the final diagnosis of ACM. Aneurysms, whether single or multiple, are predominantly located in the inflow tract and outflow tract of the RV.28 Marcus et al.25 initially described a ‘triangle of dysplasia’ which involved the inflow tract, the outflow tract, and the RV apex. It has been shown (Te Riele et al.29) that the apex is only involved in ACM in the late stage and the correct third leg of the triangle is the posterolateral LV. An LV involvement has been reported in series addressing the typical RV variants of ACM, with up to 50–76% of the ACM hearts studied at post-mortem disclosing alterations in the LV.27,30 In recent years, genotype–phenotype correlation studies led to the awareness that left dominant or even isolated LV forms of ACM do exist. When the LV is affected, the fibrofatty (mostly fibrous) infiltration is usually limited to the subepicardium or mid-mural layers of the free wall, with the region most often involved being the posterolateral one.29,30 As compared to the RV, transmurality and wall thinning in the LV (with or without aneurysm formation) are exceptional. The ventricular septum is rarely involved but if so, usually this occurs on the right side. In the advanced stages of the disease phenotype, the pathological spectrum of ACM ranges from grossly normal hearts, in whom only a careful histopathological investigation can reveal the structural alterations of fibrofatty replacement, to the most frequent feature of massive biventricular disease with aneurysms and chamber dilatation. Interestingly, histological evaluation of hearts of ACM patients upon transplantation or autopsy revealed distinct patterns of fibrosis and fat in patients with a desmosomal or PLN mutation compared to patients with mutations in Lamin A/C (LMNA), and in genes encoding proteins in the desmin filament network and sarcomeres, leading to dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM). Fibrofatty replacement in ACM hearts was predominantly observed in the RV epicardium and in the posterolateral region of the LV. In addition, PLN patients showed more fibrosis in LV than patients with a desmosomal mutation with exception of those with a mutation in DSP.31
3.4. Management strategies
To date, there is no curative treatment for ACM. Therefore, current management strategies focus on attenuating symptoms, slowing disease progression, and preventing SCD.24 Affected patients in all stages of disease should be managed by lifestyle interventions (i.e. exercise restriction). In the overt disease stage, drug treatment (beta-blockers and Class III antiarrhythmics) is often added to the management regimen. In late-stage disease, treatment for HF (most commonly diuretics) and ultimately heart transplantation are among the treatment options. Given the complexity with tailoring disease management, it is recommended that treatment takes place in tertiary care centres with extensive experience managing ACM patients. While the use of antiarrhythmic medication has shown to reduce arrhythmia burden, studies have failed to show a survival benefit.32,33 In contrast, the only intervention that has been proven to improve survival is administration of an implantable cardioverter-defibrillator (ICD).20 For that, accurate patient selection is important, as ICD implantation is invasive and not free of complications. A recent transatlantic initiative aimed to improve ICD patient selection criteria by developing a prediction model for VA in ACM,34 which is available online at www.arvcrisk.com. In this model, seven pre-specified predictors (age, sex, prior non-sustained VT, syncope, PVC count, number of leads with T-wave inversion, and RV ejection fraction) are integrated to predict sustained VA with good accuracy (C-statistic 0.77, calibration slope 0.93). While these results are encouraging, true advances and potentially curative treatment strategies will only be possible by the development of strategies that limit electrical and structural remodelling. In particular, the identification of disease pathways involved in the early pre-clinical phase will enable future development of novel strategies for the identification of affected individuals at high risk for SCD, as well as improved therapeutic approaches to prevent disease progression.
4. Determinants and modifiers of ACM disease severity and SCD risk
4.1. Differential impact of desmosomal genes/proteins on disease severity and outcome
The impact of mutations in different desmosomal genes on disease severity and outcome is difficult to determine. Nevertheless, this has partly been evaluated in a large cohort from the John Hopkins/Dutch registry.14 Overall, SCD occurred in 3% of all patients within the registry in a mean follow-up of 6 ± 7 years, and SCD risk was highest in patients with a DSP mutation (11%). DSP mutations were also more frequently associated with LV dysfunction (40%) and HF (13%) compared to PKP2 mutations.14 Patients with a mutation in PLN and a diagnosis of ACM were significantly older at the time of their first symptoms (40 years) and first episode of sustained VT/VF (42 years) as compared to PKP2 patients (30 and 35 years, respectively). However, PLN patients had a worse clinical course, more often developing LV dysfunction and HF. Ageing affects the arrhythmic event-free survival of the disease, as only 42% were arrhythmia-free at the age of 60, while this was 66% at 40 years of age. Missense mutations were less common to give rise to SCD, although missense mutation carriers had similar death/transplant-free survival and VT/VF penetrance as compared to those with truncating or splice-site mutations. As expected, mutations in more than one gene accelerated both the onset of symptoms (23 years) and the first sustained VT/VF (25 years). Only 4% (n = 22) of the studied group presented with multiple mutations and had a significantly earlier occurrence of sustained VT/VF (mean age 28 ± 12 years), lower VT/VF-free survival (P = 0.037), more frequent LV dysfunction (29%), HF (19%), and cardiac transplantation (9%) as compared to those with only one mutation.14
4.2. Impact of exercise on disease progression
While pathogenic mutations predispose for ACM development, environmental factors modify disease onset and progression, as demonstrated by comparing monozygotic twins. A case has been reported where only one twin brother had rhythm disturbances, while the other brother was asymptomatic despite the fact that both brothers were raised under almost identical conditions. However, the symptomatic brother with rhythm disturbances had a history of intensive exercise, while his twin brother did not participate in sports.35 In another case report of monozygotic twin brothers (age 12), only one suffered from palpitations and syncope, while the other brother was asymptomatic, indicating a role for environmental factors in ACM.36 The modulatory impact of exercise has been confirmed in studies on genetically predisposed ACM patients. Again, endurance sports activities were found to increase the risk of SCD and to stimulate earlier expression of the disease, as competitive athletics with a genetic predisposition for ACM had a five-fold increased risk of SCD compared to non-athletes.13,15 Thus, for ACM patients exercise is an important risk factor for earlier presentation of symptoms and promotion of disease progression, enhancing the propensity for VA and HF.11 As such, discontinuation of sports activities after diagnosis of ACM is considered an important intervention to modify the clinical course of disease.15 Mechanistically, exercise is suggested to augment ACM disease expression by further weakening the already stressed intercellular connections (resulting from the mutation) between adjacent cardiomyocytes. However, experimental proof for this hypothesis is still scarce as exercise training in mouse models only sparely recapitulates the findings in patients.37 Moreover, this interpretation was recently questioned in data obtained from an experimental mouse model which even showed beneficial effects of treadmill exercise in conditional heterozygous cardiac Dsp knockout mice. In this model, heterozygousity of Dsp resulted in dysregulation of 800 genes, including genes involved in endothelial mesenchymal transition (EMT) activation, inflammation, and suppression of oxidative phosphorylation of cardiomyocytes. Although fibrosis formation and cardiac systolic dysfunction were not rescued by daily treadmill exercise for 3 months, this restored roughly two-third of the dysregulated genes.38 Again this study examples the difficulty in extrapolating data from experimental models to findings in patients.
4.3. Gender and pregnancy
The male predominance in disease onset and severity indicates a potential role for sex hormones in ACM.12,39 Akdis et al.12 found correlations of high testosterone with the development of major arrhythmic cardiovascular events in males, whereas low oestradiol levels increased the risk of a major arrhythmic cardiovascular event in female ACM patients. In human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) obtained from an ACM patient, testosterone increased cardiomyocyte apoptosis and lipogenesis, while addition of oestradiol reduced this.12 One would think that pregnancy has an important influence on ACM development: not only through the effect of sex hormones but also through the haemodynamic stresses that are placed on the pregnant body. Hodes et al.40 studied 39 singleton pregnancies of whom five were complicated by VA and two by new-onset HF. Reassuringly, no detrimental (long-term) effects on foetuses/newborns were observed, and no difference was observed in disease course and outcomes when comparing this cohort to a similar cohort of women of childbearing age. Castrini et al.41 similarly found that pregnancy had no effect on LV or RV function or VA in 43 definite ACM patients and 34 mutation carriers. A retrospective study of Gandjbakhch et al.42 revealed that in DCM and HCM patients, the risk of experiencing an adverse cardiac event during pregnancy was higher than in ACM patients. In addition, they reported no compromised haemodynamics or HF in ACM patients during pregnancy. In summation, these results suggest that pregnancy in ACM is generally tolerated well and does not lead to adverse long-term outcome.
5. Pathogenesis of ACM
The sequence of events in ACM, and underlying disease mechanisms and pathways, from initial desmosomal dysorganization in the early disease stage to the ultimate full-blown ACM phenotype in advanced stages has partly been unravelled through studies in animal, and in vitro human disease models.
5.1. Remodelling at the ID: desmosomes, gap junctions, and Nav1.5
Desmosomal rearrangement, with disruption of the interaction between DSP and intermediate filaments, may lead to reduced cell adhesiveness and ultimately result in cardiomyocyte damage secondary to the continuous mechanical strain afforded by the contracting myocardium. Although, as summarized below, an accumulating amount of morphological, immunohistological, and cell culture data support the idea of reduced cell adhesiveness,43 direct proof in terms of strain measurements is still lacking. In mice overexpressing the patient-specific Dsg2-N271S mutation, one of the earliest pathological changes was a widening of the ID intercellular space at the level of the desmosome/adherens junction with focal lysis of the myofilaments, similar to observations made in tissue from ACM patients.9,10 These findings and similar observations in other Dsg2 and Pkp2 mouse models10 indicated that the ID widening was likely the result of decreased adhesive intercellular interactions of junctional cadherins. Another commonly observed feature in ACM patients is reduced presence of plakoglobin at the ID, which indicates that preserved localization of proteins within the ID depends on each other.44–47 For other desmosomal proteins, such as PKP2 and DSP, this reduction was only observed in some but not all patients.45 Interestingly, one study showed that plakoglobin was decreased only in ACM patients, while in patients with DCM, HCM or ischaemic heart disease plakoglobin levels at the ID were similar to controls.45 Therefore, redistribution of plakoglobin may be an early molecular event specifically seen in ACM, and this has been linked to alterations in intracellular signalling pathways (discussed in more detail below). Importantly, in order to distinguish control from ACM patients, testing a broad dilution range of the plakoglobin antibody is required.48 Beyond the high sensitivity of plakoglobin remodelling as documented in ACM, later studies in patients with affiliated diseases like sarcoidosis and giant cell myocarditis also showed reduced signals for plakoglobin.49 Therefore, investigation of plakoglobin labelling in cardiac tissue may be useful as an additional but not conclusive test for ACM.
In addition to ID widening and alterations in desmosomal protein distribution, remodelling of gap junctions has been shown to occur in cardiomyocytes of ACM patients, in coherence with a reduced presence of the cardiac sodium channel. In ACM patients, immunohistology revealed that when plakoglobin protein was reduced at the ID, sodium channel (Nav1.5), connexin43 gap junction protein (Cx43), or both were also affected.50 Abnormal desmosomal composition, distribution, or function directly interferes with gap junctions, leading to a depressed level of Cx43 and consequently impaired electrical conduction.45–47,50 Furthermore, cell adhesion is essential for gap junction formation,51 and PKP2 expression for normal intercellular communication through gap junctions.52 Accordingly, a loss of gap junction plaques has been observed in cardiac tissue from ACM patients.47 Cardiac sodium channels also form part of this ID macromolecular complex, and its structural link with the desmosomal protein complex may contribute to conduction disturbances and arrhythmias in ACM. Indeed, Nav1.5 remodelling has been observed in mouse, zebrafish, and hiPSC-CM models of ACM.10,53–55 This remodelling may be the consequence of intercellular space widening leading to local disruption of the Nav1.5 macromolecular protein complex, or alternatively due to a dysfunctional interaction between the desmosomal complex and Nav1.5 independent of ID widening. The observation by Sato et al.56 that loss of PKP2 expression decreased sodium current density in isolated rat ventricular cardiomyocytes (which lack intercellular connections), is in favour of the latter, i.e. a direct impact of desmosomal proteins on Nav1.5. Independent of the exact mechanisms, reduced Nav1.5 levels lead to conduction abnormalities thereby setting the stage for arrhythmias (see Section 6). Importantly, this Nav1.5 remodelling occurs already very early in the disease process, prior to the development of cardiomyopathic changes and hence could theoretically contribute to VA and SCD in the concealed stage of ACM.53 This, however, should be taken with some caution since no clinical case has been reported so far of an individual carrying a pathogenic desmosomal gene mutation suffering SCD in the absence of cardiac structural abnormalities. Notably, not every case of SCD is routinely subjected to genetic screening in combination with autopsy.
5.2. Cardiomyocyte loss, necrosis, and apoptosis
Cardiomyocyte loss is another relatively early feature in the ACM disease process, which may be caused by a number of mechanisms including apoptosis which has been described in ACM and may be the consequence of disruption of intercellular cardiomyocyte adhesion.27,57 Apoptosis was a prominent feature reported in some ACM mouse models,58–60 whereas in contrast, studies in Dsg2-N271S transgenic mice demonstrated that necrotic myocyte death and not apoptosis is the key initiator of myocardial injury, subsequently triggering an inflammatory response followed by fibrosis formation, myocardial atrophy, and dilation.9 Features of cardiomyocyte necrosis, i.e. sarcolemmal disruption, disaggregation of myofilaments, and mitochondrial swelling, preceded the development of cardiac structural abnormalities, with myocyte necrosis originating in the subepicardial myocardium. Apoptosis did not appear to trigger disease onset since apoptotic cells were only observed in advanced stages of the disease, and particularly in fibrotic areas. While these findings were in accordance with observations in cardiac tissue from a patient carrying the DSG2-N266S mutation,9 myocyte necrotic cell death is not often reported in autopsy or endomyocardial biopsy studies of human ACM. This may be due to the focal distribution and potential episodic nature of necrosis during the various stages of disease; alternatively, myocyte necrosis may not be a prominent pathogenic feature in ACM. Nevertheless, cardiomyocyte loss in ACM invariably contributes to reduced cardiac function and ventricular dilatation, irrespective of the underlying initiating event.
5.3. Adiposis and fibrofatty replacement
As the ACM disease process progresses, necrotic and apoptotic cardiomyocytes are replaced by fibrosis due to the wound healing response in an attempt to preserve structural integrity of the myocardium.61 Activation of both the canonical TGF-β/SMAD262 and non-canonical TGF-β–Mitogen-activated protein kinase (MAPK) signalling pathways63 is significantly relevant for fibrosis formation in mouse and cardiomyocyte models of ACM. At difference with other conditions associated with cardiomyocyte injury, fibrosis formation in ACM is typically accompanied by fatty infiltration. The mechanisms underlying this fibrofatty replacement are not well established, in part due to the fact that mouse ACM models typically do not recapitulate the clear fatty infiltrations seen in human. Nevertheless, genetic lineage tracing in mouse models suggests that cardiac progenitor cells derived from the second heart field and a subset of mesenchymal progenitor-like cells, referred to as fibroadipocyte progenitors, may differentiate into adipocytes during the ACM disease process.64 In addition, several signalling pathways have been implicated in ACM-related adipogenesis, including Wnt, Hippo–Yes-associated protein (YAP), and peroxisome proliferator-activated receptor-γ (PPARγ) signalling.
Within the Wnt/β-catenin signalling pathway, cytosolic β-catenin is stabilized by Wnt ligand and able to translocate to the nucleus to modify transcription, where it binds T cell factor/lymphoid enhancer factor (TCF/LEF).1,63 Cytoplasmic β-catenin, a protein homologous to plakoglobin (γ-catenin), is regulated by glycogen synthase kinase 3α/β (GSK3α/β), axin, and the adenomatous polyposis coli (APC) degradation complex to restrict activation of target genes. Loss of plakoglobin in mice increased β-catenin expression both at the desmosome and in the cytoplasm,63 whereas GSK3β is redistributed to the ID in human and mouse ACM hearts.65 Moreover, plakoglobin not only serves as an important component of the desmosome but also competes upon nuclear translocation with β-catenin to bind TCF/LEF, as was demonstrated in Dsp-deficient mice. This resulted in suppression of Wnt signalling and expression of pro-adipogenic genes.62,66 When plakoglobin is mutated at the ID, enhanced binding of β-catenin to LEF modulates transcription, such as enhanced expression of c-MYC to establish hypertrophy of the heart.1,63 Moreover, stabilization of β-catenin is a result of protein kinase B (PKB/AKT) activation, affecting the activity of GSK3β and further supporting expression of target genes of β-catenin.63 Overall, these findings demonstrate a close interaction between desmosome (dys)function and transcriptional regulation contributing to fibrosis and adipogenesis in the setting of ACM. This was independently underscored in a recent study employing RNASeq analysis in mice with inducible, cardiac-specific Pkp2 deficiency, where a large number of cardiac transcripts were found to be differentially expressed.67 Interestingly, a number of genes involved in intracellular calcium homeostasis were downregulated in Pkp2 deficient hearts, which may be of particular relevance for subsequent development of early arrhythmogenesis and structural derangements (as further discussed below).
Several cross-regulations have been described between the Wnt/β-catenin and Hippo–YAP signalling pathway; the latter is known to be involved in cellular proliferation, apoptosis, and differentiation. In human and mouse ACM hearts as well as PKP2-deficient HL-1 myocytes, activation of the Hippo pathway has been observed, including phosphorylation and cytoplasmic retention of YAP.68 YAP interacts with β-catenin, thereby suppressing its nuclear translocation and subsequent Wnt-target gene expression.69 Thus, elevated levels of phosphorylated YAP, as a result of Hippo pathway activation, lead to sequestration of β-catenin and consequent inhibition of Wnt signalling, potentially contributing to adipogenesis.68,69 Similar to Hippo–YAP, a functional relationship has been demonstrated between Wnt/β-catenin and PPARγ signalling.70 PPARγ plays an essential role in adipocyte differentiation and metabolism, and mice overexpressing PPARγ1 developed DCM associated with increased lipid and glycogen stores and mitochondrial alterations.71,72 In cardiac tissue from ACM patients, activation of the PPARγ pathway has also been demonstrated, most notably in the RV.73 Further evidence for a role for abnormal PPARγ pathway activation was obtained in studies employing hiPSC-CMs from PKP2 mutation carriers.74 Interestingly, the TMEM43 gene in which mutations have been identified in some ACM patients contains a response element for PPARγ, but its functional relevance remains to be determined.75
5.4. Inflammation
Explanted hearts and myocardial biopsies from ACM patients frequently show patchy inflammatory infiltrates, indicating a potential etiopathological role for inflammation and/or myocarditis.27 It is, however, unclear whether myocarditis triggers fibrofatty replacement, or if the inflammatory reaction is merely secondary to ongoing myocyte death. Neonatal rat cardiomyocytes overexpressing a truncated form of plakoglobin were found to secrete pro-inflammatory cytokines such as interleukin (IL)-6 and tumour necrosis factor (TNF)-α,55 suggesting a direct causal relation. A recent study in murine and (human) cellular ACM models demonstrated activation of the pro-inflammatory nuclear factor-κB (NFκB) signalling pathway.59 Inflammation plays a role in disease progression as lymphocytes infiltrate into necrotic areas of the myocardium of ACM patients.66,76 These infiltrates appear to be correlated to a higher risk of VA.77 In addition, higher levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α were detected in ACM patients, in contrast to the anti-inflammatory cytokine IL-10, which was not differently expressed between ACM patients and controls.78 The pro-inflammatory cytokines TNF-α and IL-1β can promote apoptosis via upregulation of inducible nitric oxide synthase expression.78 Also, acute myocyte loss itself triggers inflammation, which is accompanied by clinical symptoms such as chest pain and palpitations.76,79 Inflammation is not a continuous process, but occurs in so-called ‘hot phases’ of the disease.66,76 These active phases, which may be triggered by exercise, appear to be associated with acceleration of the disease as apoptosis and inflammation lead to fibrosis formation and remodelling of the heart.79 During these hot phases, increased troponin levels (up to four times the normal limit) are measured in ACM patients, while there is no viral infection detected,80 hence, troponin levels might be useful as a marker to detect ongoing hot phases.66,76,79,81
6. Pro-arrhythmic mechanisms and pathways in ACM
6.1. Re-entrant-based arrhythmias in advanced disease stages
In advanced stages of ACM, widespread structural alterations including fibrosis and dilatation form a clear pro-arrhythmic substrate, with focal scars causing electrical isolation of cardiomyocytes within non-conducting fibrous tissue. The residual heterogeneous electrical conduction through the surviving myocytes results in slow conduction, heterogeneity, and delayed activation, providing the ideal setting for re-entrant circuits and hence arrhythmias. Lessons learned from experimental mouse models revealed that haploinsufficiency for Cx43 can cause a decrease in conduction velocity,82 though other studies were unable to support this.83 A sole slowing of conduction is not necessarily pro-arrhythmic.84 However, the safety factor for conduction becomes critical if also excitability is hampered (by a concomitant decrease in sodium current) or when the development of fibrosis combines with slow conduction and a reduced excitability.83–86 These combinations increase the incidence of VA in experimental mouse models and show that alterations in one determinant for conduction affect others too. This is exemplified by the observation that a reduction in Cx43 has been associated with both a decrease in Nav1.585 and an enhancement of fibrosis formation.86 This combination of heterogeneous (fibro)fatty replacement of cardiomyocytes, together with a reduction in intercellular impulse propagation and excitability, likely generates a dangerous combination of factors promoting the development of VA in ACM patients.
6.2. Sodium channel remodelling in the early ACM disease stage
The functional consequences of Nav1.5 remodelling have been studied in ACM mouse models, demonstrating reduced sodium current at young age, when there is merely (sub)acute damage consisting of myocyte necrosis and inflammation but no widespread fibrotic lesions.53,67 Consequently, increased arrhythmia susceptibility was observed in early disease stages in these mice.53 Similarly, monolayers of neonatal rat ventricular myocytes lacking PKP2 demonstrated decreased Cx43 expression and sodium current, which affected cell–cell coupling and sodium current kinetics leading to a decreased conduction velocity and increased re-entrant activity in the absence of structural alterations.87 These findings are in line with clinical observations in ACM patients, in whom arrhythmias and SCD can occur in different stages of the disease,14 and they support the hypothesis that life-threatening VA could already occur in hearts that appear structurally unaffected. Sodium current reduction and consequent conduction disturbances may also enhance arrhythmia susceptibility in later diseases stages, by further increasing the risk of re-entrant arrhythmias in the presence of structural alterations.
6.3. Early pro-arrhythmic alterations in Ca2+-handling preceding structural remodelling
Recently, an additional aspect related to arrhythmogenesis in the setting of ACM has been proposed based on findings in a genetically engineered mouse model of induced cardiomyocyte-specific Pkp2 deletion, and in a model of Pkp2 haploinsufficiency.88,89 In these models, downregulation has been observed of several proteins related to calcium handling, such as Cav1.2, RyR2, and Ankyrin B, which contributed to the observed decreased L-type Ca2+ current density and slower rate of inactivation of the channel. The summation of these alterations in calcium handling proteins functionally resulted in an increased sarcoplasmic reticulum (SR) calcium load, a higher diastolic [Ca2+], and a high propensity for early or late after-depolarization.88 Moreover, flecainide treatment reduced the occurrence of arrhythmias; in addition to its sodium channel blocking activity, flecainide also blocks RYR2 thereby inhibiting spontaneous Ca2+ outflow of the overloaded SR.88 Although similar data on alterations in calcium homeostasis are still lacking in ACM patients (particularly due to the absence of material to study these early events), these observations may clearly explain why some ACM patients benefit from flecainide (Pilot Randomized Trial With Flecainide in ARVC Patients, NCT03685149, currently ongoing) and how these alterations link to the polymorphic nature of arrhythmias seen in the early phase of the disease.
7. Early detection of pathological remodelling: towards improved risk stratification
7.1. Biomarkers
Biomarkers are defined by the National Institutes of Health Biomarkers Definitions Working Group as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention’.90,91 Biomarkers can be used to identify risk of disease, for screening, to support diagnosis, to describe disease severity, or to predict disease course and treatment efficacy in the patient. Biomarkers are not only found in blood of patients but also in all tissues, secretions, and cells.92 For example, the detection of a pathogenic mutation in a family member related to an index patient is a predictive biomarker for the development of the disease. Different techniques are applied to detect differences in biomarker expression in patients, such as enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, or polymerase chain reaction (PCR). Several well-known biomarkers are already used in the clinic, such as B-type natriuretic peptides to diagnose patients with HF or elevated Troponin I and T (cTnI and cTnT) levels, which are associated with myocardial infarction (MI). While biomarkers are also used as one of the tools to diagnose a patient, it is difficult to link a single biomarker to a complex disease such as ACM.15 Therefore, multiple biomarkers should be used together for optimal outcomes.
7.2. Assessment of protein remodelling at the ID
Changes in expression of Cx43, Nav1.5, or desmosomal proteins, with plakoglobin being the most sensitive one, may be determined already during the early phase of ACM. Early detection of such pro-arrhythmic remodelling could provide a method for identification of patients at risk, but assessment of these maladaptive alterations in the human myocardium is cumbersome. Cardiac biopsies may provide this information, but they are not easily obtained, are only scarcely available and provide only local information on disease status at one single moment or in end-stage diseased hearts after transplantation or death.44 Hence, new biomarkers are required that allow a frequent assessment of disease onset and/or progression through less invasive or ethically sensitive procedures. With regard to ID remodelling, a tentatively promising approach comprises the use of buccal mucosa cells to study ACM pathogenicity and disease progression. Collection of buccal mucosa specimen is easy, cost-effective, and non-invasive, hence allowing frequent repetition.44 The idea to use such specimen is based on the fact that patients with Carvajal syndrome or Naxos disease (having mutations in DSP and JUP, respectively) not only present with cardiomyopathies reminiscent of ACM but also with extra-cardiac features in tissues that also express these desmosomal proteins. Following this line of thinking, Asimaki et al.44 demonstrated that expression of desmosomal proteins is altered in buccal mucosa cells from ACM patients. Hence, a mutation in DSP may be associated with a reduction in expression of DSP protein in the patients’ buccal mucosa. In addition, expression of Cx43 and plakoglobin may be reduced in buccal mucosa cells, likewise observed in the myocardium of ACM patients.44,50 While interesting as a source, it still remains to be examined if, and if so to what extent, the changes in buccal mucosa smears reflect desmosomal changes in the myocardium.
7.3. Non-invasive/biomarker assessment of fibrosis
Different types of fibrosis exist, such as patchy, diffuse, interstitial, and compact fibrosis, where especially, patchy and diffuse fibrosis can reduce and disperse conduction velocity, making the heart susceptible for VA.61 Collagen Type I (85%) and collagen Type III (11%) are the most common types of collagen found in the heart. The turnover of collagen is 90–120 days, so there is continuous synthesis and degradation of collagen.61,93,94 Cardiac fibroblasts synthesize preprocollagen fibres, which are modified to procollagen in the endoplasmatic reticulum. In the extracellular matrix (ECM), the amino (N)-propeptide and carboxy (C)-propeptide are cleaved off by proteinases. These procollagen Type I amino-terminal propeptide and procollagen Type III amino-terminal propeptide (PIIINP) and procollagen Type I carboxy-terminal propeptide and procollagen Type III carboxy-terminal propeptide (PIIICP) are released into the circulation. After cleavage of propeptides, large collagen fibres are formed together with other collagen chains. Breakdown of collagen is mediated by metalloproteinases (MMPs) which cleave collagen into two fragments.93,95 These MMPs are synthesized by fibroblasts, and other cell types such as leucocytes and cardiac myocytes, and controlled in their activity by tissue inhibitor of metalloproteinase-1 (TIMP-1).93,95,96 Further degradation is primarily mediated by MMP-2 and probably MMP-9 which, for example, generate C-terminal telopeptide of collagen Type I (ICTP) which is also released into the circulation.93,95 Some of those procollagen peptides are already used as biomarker for other cardiac diseases such as HF, DCM, or HCM, where upregulation of PIIINP is correlated to collagen Type III synthesis.93 However, for ACM there are no circulating fibrotic biomarkers available yet.97 Given the importance of fibrosis formation in patients with ACM, determination of procollagen peptide levels in their sera may prove of benefit.
In addition to collagen peptides, there is an increased interest in the evaluation of circulating miRNAs, as several miRNAs appear to be involved in profibrotic remodelling due to their role in post-transcription regulation of genes related to fibrosis formation. miRNA profiling in cardiac tissue of end-stage ACM patients revealed 21 differential expressed miRNAs compared to controls.98 Among them was miR-21, which is linked to increased myocardial fibrosis when it is expressed by cardiac fibroblasts.99,100 miR-21 targets sprouty homologue 1, which results in prolonged fibroblast survival and secretion of fibroblast growth factor 2 thereby stimulating the development of interstitial fibrosis. In contrast to the effect of miR-21 on fibroblasts, it apparently does not modify cardiomyocyte function or morphology.101 A second miRNA involved in regulation of profibrotic mRNA expression is miR-29. miR-29 is downregulated by TGF-β, which enhances the expression of collagen and fibrillin-1.99,100 Recently, miR-320a has also been proposed as a potential biomarker in ACM.97 In ACM patients, miR-320a is significantly lower expressed in serum compared to healthy individuals, while it does not seem to be affected by sports activities. Thus, miR-320a is not correlated to severity of ACM, but it might be useful to discriminate between ACM and patients with a related but slightly different disease such as idiopathic ventricular tachycardia (IVT). As treatment options differ upon diagnosis, it is important to exclude IVT from ACM.97
Non-invasive detection of myocardial fibrosis can be obtained by contrast-enhanced cardiac magnetic resonance using gadolinium (‘late gadolinium enhancement’, LGE-CMR).8 LGE-CMR is based on qualitative evaluation of differences in signal intensity between regions with fibrosis and normal myocardium, which is very suitable to detect replacement fibrosis. However, reactive or diffuse fibrosis may be missed on LGE-magnetic resonance imaging (MRI), so novel techniques including T1 mapping and CMR feature tracking provide a possible solution.8,93,102 Measurement of myocardial relaxation times (T1 mapping) with native or gadolinium-enhanced inversion recovery sequences provides quantitative measures of the extracellular volume fraction, reflecting interstitial myocardial fibrosis.103 In contrast to LGE, T1 mapping provides a continuous measure of interstitial myocardial fibrosis which has been shown to provide a good correlation with ex vivo measures of fibrosis analyses.104,105 In ACM, T1 mapping was recently shown to discriminate affected patients and unaffected mutation carriers from controls.106 In addition, fibrotic alteration of the myocardium results in abnormal regional wall motion that is present prior to development of global ventricular dysfunction. CMR feature tracking is able to reliably track ventricular motion throughout the cardiac cycle, thereby obtaining quantitative data on regional peak strain (maximum contraction) and strain rate (velocity of contraction).105,107 The feature-tracking algorithm is scanner- and vendor-independent, has a favourable signal-to-noise ratio, and can retrospectively be used in available cine CMRs. It has been shown that feature tracking is a feasible and reliable tool to distinguish overt ACM from controls.107,108 In a future perspective it will be important to correlate plasma biomarker indices of fibrosis to those obtained with MRI-based techniques, with the aim to find correlations similar to those between T1 mapping and ex vivo analysis in explanted cardiac specimen.
7.4. Autoantibody desmoglein-2 as a marker for ACM
Next to the identification of biomarkers associated with fibrosis, also other circulating biomarkers associated with inflammation are investigated. One of such promising new diagnostic tools might be the use of autoantibodies to identify ACM.109 This idea is adapted from pemphigus vulgaris, a desmosomal skin disease caused by autoantibodies against desmoglein 1 and 3.110,111 In ACM patients, the presence of autoantibodies against desmoglein 2 (anti-DSG2) have been detected by western blot independently of the underlying genetic cause. In contrast, anti-DSG2 autoantibodies were not detected in patients with DCM or HCM. A striking, but useful finding was the correlation between autoantibody level, disease severity, and arrhythmia incidence. Mechanistically, anti-DSG2 autoantibodies reduced gap junction functionality which was demonstrated by reduced dye transfer in an experimental cell model.109 Taken together, anti-DSG2 plasma levels may prove useful as a biomarker in ACM. Nevertheless, only studies in small cohorts have so far demonstrated the presence of anti-DSG2, and therefore additional studies should be performed in a larger cohort to assess the usefulness of anti-DSG2 as a diagnostic and/or prognostic marker. In particular, anti-DSG2 seems to have high sensitivity and specificity, potentially facilitating its use as biomarker in the early, pre-symptomatic phase of ACM, and its potency to uncover changes in disease status associated with an increased risk for arrhythmias.
7.5. Early detection of disturbed conduction and excitability
Electrical uncoupling and fibrofatty myocardial replacement that lead to load mismatch, anisotropic conduction, and conduction delay, remain hidden on conventional 12-lead ECG in early disease. Cardiac activation imaging (CAI) enables non-invasive estimation of epicardial and endocardial conduction delay using 64-lead recordings of body surface potentials and hence may be useful for early risk stratification.112 Furthermore, Nav1.5 remodelling and associated sodium current alterations may effectively be unmasked by a sodium channel blocker challenge such as ajmaline, similar to that used for identification of patients suffering from Brugada syndrome.113 Recent data have shown ST-segment elevation upon ajmaline challenge in 16% of ACM patients, indicating the potential usefulness of this approach.114 Echocardiographic deformation imaging can be used to reliably quantify regional wall motion, but its high temporal resolution also enables assessment of electromechanical dyssynchrony as a result of conduction delay. Studies have indeed shown that this assessment may distinguish PKP2 mutation carriers from controls,115,116 and may identify those at high risk of arrhythmias.117 Currently available expression systems (such as for instance HEK293 cells) are limitedly suitable for studying pathogenicity of ACM mutations in vitro. iPSC-CMs obtained from patients allow for robust quantification of sodium current magnitude in addition to assessment of conduction characteristics in iPSC-CM monolayers.118 Hence, electrophysiological characterization of patient-specific iPSC-CM may provide a tempting in vitro tool for personalized prediction of disease severity and arrhythmia risk in patients, and to test the effects of pharmacological intervention strategies.
8. Novel therapeutic strategies
Available therapeutic strategies are often not sufficient in ACM patients, as progression of ACM still leads to HF.8 Improved anti-fibrotic and anti-inflammatory strategies might be beneficial to improve cardiac function and suppress further deterioration of the disease. As explained above, current research focuses on both the Wnt/β signalling pathway and the NFκB pathway.59,119 Using plakoglobin mutant zebrafish, Asimaki et al.55 identified SB216763, an inhibitor of GSK3β and activator of the Wnt/β-catenin pathway, as a pharmacological modulator capable of preventing and rescuing the ACM phenotype in zebrafish. In addition, in ACM fish treated with SB216763, maladaptive remodelling of ion channels underlying action potential formation was reversed.55 Comparable results were obtained in a mouse model which expressed patient-specific mutations in Ank2,119 in cultured buccal mucosa cells,44 in neonatal rat ventricular myocytes transfected with the same 2057-del2-plakoglobin mutation and in iPSC-CMs of ACM patients with a PKP2 mutation.55 Upon treatment with SB216763, ACM features like fibrosis formation, downregulation of Cx43 and plakoglobin, and contractile dysfunction were almost completely reversed back to control conditions.55,119 These results indicate the importance of the Wnt/β-catenin pathway in progression of ACM. However, continuous activation of the Wnt/β-catenin pathway increases the risk of developing cancer, limiting the clinical applicability of this therapeutic strategy.59 Importantly, inhibition of NFκB, a downstream target of GSK3β which regulates the inflammatory response, with Bay 11-7082 in a Dsg2mut/mut mouse model prevented the development of ACM features, such as loss of cell-surface immunoreactive signal for desmosomal proteins and Cx43, and apoptosis. In addition, less inflammatory cytokines were produced by the cardiac myocytes which attenuated ACM disease features.59 These findings not only demonstrated a pivotal role for inflammation in ACM development but also provided evidence for a beneficial effect of anti-inflammatory therapy in inhibiting or reversing the ACM phenotype. Following these observations, pentoxifylline (PTX), an inhibitor of TNF-α production, proved to have anti-inflammatory and anti-fibrotic activity via modulation of NFκB activity.120 Importantly, this drug is already approved for clinical application and is used in patients with occlusive peripheral vascular disorders. Studies have shown the beneficial effects of PTX in animals and humans suffering from MI, HF, and idiopathic DCM. Interestingly, PTX treatment in rats with angiotensin II-dependent hypertension reduced cardiac fibrosis and improved cardiac function.120 A different potential target is IL-1, as electrophysiological studies have shown that IL-1β can change Ca2+ handling and intercellular coupling such as a reduction in Cx43 in both post-MI mice and canine cardiac myocytes.121 Indeed, inhibition of IL-1β or the NLRP3 inflammasome has been shown to prevent arrhythmias in a diabetic mouse model, and hence may potentially be beneficial in the treatment of ACM. Pharmacological targeting of IL-1β is of further interest given the fact that registered drugs such as canakinumab (antibody therapy) have shown efficacy, e.g. in the CANTOS trial.122
As the underlying genetic mutation differs between patients, a better risk stratification also might help in deciding who can be protected by ICD implantation. Current guidelines to determine the risk to experience a sustained VT or SCD include (i) previous experience of a sustained VT, (ii) degree of structural heart disease, (iii) electrical instability such as PVCs, (iv) cardiac syncope, (v) young age, (vi) male gender, (vii) mutation status, and (viii) vigorous exercise.13 This decision should be made with caution, as impropriate ICD shocks can cause physiological strain and thereby reduced quality of life. Therefore, novel research should finetune risk stratification in ACM patients to compose therapeutic strategies according to underlying genetic mutations.
9. Conclusions
ACM is a highly heterogeneous disease, both from a histopathological point of view as well as clinical observations. The disease typically progresses from an early, subclinical phase during which electrical instability may in some patients lead to fatal arrhythmias, to an overt late cardiomyopathic stage additionally characterized by HF. Given its highly variable disease expressivity and severity, identification of patients at risk for life-threatening arrhythmias remains difficult, particularly during the early, concealed phase. Recent studies in primarily experimental models have provided essential insight into (early) disease mechanisms, enabling the development and implementation of novel risk prediction strategies. The latter is also facilitated by the development of innovative and highly sensitive imaging technologies. Table 1 summarizes the new proposed methods and leads applicable for early recognition. Ultimately, successful identification and prediction of ACM patients at high risk for SCD could lead to mutation- and patient-specific treatment strategies, including for instance more stringent follow-up and/or implantation of an ICD at young age in high-risk individuals. Our advanced knowledge on ACM disease mechanisms and signalling pathways is furthermore paving the way for novel pharmatherapeutic strategies. Importantly, pro-arrhythmic mechanisms likely differ between ACM disease stages, and targeting the newly identified pathways involved in the early disease phase will hopefully prove vital in preventing SCD in mutation-positive individuals without overt cardiomyopathy.
Table 1.
Early recognition possibilities using biomarkers
| Biomarker | Technique | Tissue/challenge | Information |
|---|---|---|---|
| ↓ Plakoglobin | Immunohistochemistry | Buccal mucosa | Visible before structural and electrical remodelling occurs44 |
| PICP/ICTP | ELISA | Serum | Fibrosis formation correlates with VA61 |
| ↑ miR-21, ↓ miR-29 | qPCR | Serum | Linked to increased fibrosis formation98–100 |
| ↓ miR-320a | qPCR | Serum | Discrimination ACM vs. IVT → treatment optimization97 |
| ↓ Relaxation time | MRI | T1 mapping | Increased interstitial fibrosis104,105 |
| Abnormal regional wall motion | MRI | CMR feature tracking | Distinguish overt ACM patients from controls107,108 |
| Anti-DSG2 antibody | ELISA/western blot | Serum | Present in ACM patients and correlates to disease severity109 |
| ↑ Conduction delay | ECG | CAI | Early risk stratification112 |
| ↑ ST segment | ECG | Ajmaline challenge | Sodium channel blocker to detect Nav1.5 remodelling113 |
| ↓ Wall motion + desynchrony | Echocardiographic deformation imaging | Strain echo | Distinguish patients for low and high risk of arrhythmias117 |
| Conduction characteristics | iPSC-CMs | Cells | Patient-specific tool118 |
Conflict of interest: none declared.
Funding
This work was supported by a grant from the Netherlands Cardio Vascular Research Initiative (CVON): the Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences (CVON-eDETECT 2015-12, CVON-PREDICT2 2018-30 (S.M.v.d.V., C.A.R., T.A.B.v.V.) and the Registry for Cardio-Cerebro-Vascular Pathology, Veneto Region, Venice, Italy, Ministry of Health (RF 2013İ 02356762 and RF-2016-02363774), PRIN Ministry of Education, University and Research (2015ZLNETW_001 and 20173ZWACS), Rome, Italy, and the CARIPARO Foundation, Padua, Italy (C.B.).
References
- 1. Vermij SH, Abriel H, van Veen TA.. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc Res 2017;113:259–275. [DOI] [PubMed] [Google Scholar]
- 2. Saffitz JE. Desmosome mutations in arrhythmogenic right ventricular cardiomyopathy: important insight but only part of the picture. Circ Cardiovasc Genet 2009;2:415–417. [DOI] [PubMed] [Google Scholar]
- 3. Corrado D, Basso C, Judge DP.. Arrhythmogenic cardiomyopathy. Circ Res 2017;121:784–802. [DOI] [PubMed] [Google Scholar]
- 4. Groeneweg JA, van der Heijden JF, Dooijes D, van Veen TA, van Tintelen JP, Hauer RN.. Arrhythmogenic cardiomyopathy: diagnosis, genetic background, and risk management. Neth Heart J 2014;22:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corrado D, Link MS, Calkins H.. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 6. Garrod D, Tabernero L.. Hyper-adhesion: a unique property of desmosomes. Cell Commun Adhes 2014;21:249–256. [DOI] [PubMed] [Google Scholar]
- 7. Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L.. Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. J Cell Sci 2005;118:5743–5754. [DOI] [PubMed] [Google Scholar]
- 8. Haugaa KH, Haland TF, Leren IS, Saberniak J, Edvardsen T.. Arrhythmogenic right ventricular cardiomyopathy, clinical manifestations, and diagnosis. Europace 2016;18:965–972. [DOI] [PubMed] [Google Scholar]
- 9. Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P, Scicluna BP, Bauce B, van den Hoff MJ, de Bakker JM, Tan HL, Valente M, Nava A, Wilde AA, Moorman AF, Thiene G, Bezzina CR.. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med 2009;206:1787–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cerrone M, Noorman M, Lin X, Chkourko H, Liang FX, van der Nagel R, Hund T, Birchmeier W, Mohler P, van Veen TA, van Rijen HV, Delmar M.. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res 2012;95:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thiene G, Nava A, Corrado D, Rossi L, Pennelli N.. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988;318:129–133. [DOI] [PubMed] [Google Scholar]
- 12. Akdis D, Saguner AM, Shah K, Wei C, Medeiros-Domingo A, von Eckardstein A, Luscher TF, Brunckhorst C, Chen HSV, Duru F.. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: from a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur Heart J 2017;38:1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calkins H, Corrado D, Marcus F.. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation 2017;136:2068–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JD, Murray B, Te Riele AS, van den Berg MP, Bikker H, Atsma DE, de Groot NM, Houweling AC, van der Heijden JF, Russell SD, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Calkins H, Hauer RN.. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–855. [DOI] [PubMed] [Google Scholar]
- 15. James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H.. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carruth ED, Young W, Beer D, James CA, Calkins H, Jing L, Raghunath S, Hartzel DN, Leader JB, Kirchner HL, Smelser DT, Carey DJ, Kelly MA, Sturm AC, Alsaid A, Fornwalt BK, Haggerty CM.. Prevalence and electronic health record-based phenotype of loss-of-function genetic variants in arrhythmogenic right ventricular cardiomyopathy-associated genes. Circ Genom Precis Med 2019;12:e002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cox MG, van der Zwaag PA, van der Werf C, van der Smagt JJ, Noorman M, Bhuiyan ZA, Wiesfeld AC, Volders PG, van Langen IM, Atsma DE, Dooijes D, van den Wijngaard A, Houweling AC, Jongbloed JD, Jordaens L, Cramer MJ, Doevendans PA, de Bakker JM, Wilde AA, van Tintelen JP, Hauer RN.. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype-phenotype follow-up study. Circulation 2011;123:2690–2700. [DOI] [PubMed] [Google Scholar]
- 18. Basso C, Corrado D, Marcus FI, Nava A, Thiene G.. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009;373:1289–1300. [DOI] [PubMed] [Google Scholar]
- 19. Quarta G, Muir A, Pantazis A, Syrris P, Gehmlich K, Garcia-Pavia P, Ward D, Sen-Chowdhry S, Elliott PM, McKenna WJ.. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy: impact of genetics and revised task force criteria. Circulation 2011;123:2701–2709. [DOI] [PubMed] [Google Scholar]
- 20. Groeneweg JA, Bhonsale A, James CA, Te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld AC, Sawant AC, Kassamali B, Atsma DE, Volders PG, de Groot NM, de Boer K, Zimmerman SL, Kamel IR, van der Heijden JF, Russell SD, Jan Cramer M, Tedford RJ, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Hauer RN, Calkins H.. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet 2015;8:437–446. [DOI] [PubMed] [Google Scholar]
- 21. Bosman LP, Sammani A, James CA, Cadrin-Tourigny J, Calkins H, van Tintelen JP, Hauer RNW, Asselbergs FW, Te Riele ASJM.. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta-analysis. Heart Rhythm 2018;15:1097–1107. [DOI] [PubMed] [Google Scholar]
- 22. Te Riele A, James CA, Sawant AC, Bhonsale A, Groeneweg JA, Mast TP, Murray B, Tichnell C, Dooijes D, van Tintelen JP, Judge DP, van der Heijden JF, Crosson J, Hauer RNW, Calkins H, Tandri H.. Arrhythmogenic right ventricular dysplasia/cardiomyopathy in the pediatric population: clinical characterization and comparison with adult-onset disease. JACC Clin Electrophysiol 2015;1:551–560. [DOI] [PubMed] [Google Scholar]
- 23. Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K, Marcus F, Estes NA 3rd. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol 2014;64:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, Estes NAM 3rd, Hua W, Indik JH, Ingles J, James CA, John RM, Judge DP, Keegan R, Krahn AD, Link MS, Marcus FI, McLeod CJ, Mestroni L, Priori SG, Saffitz JE, Sanatani S, Shimizu W, van Tintelen JP, Wilde AAM, Zareba W.. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: executive summary. Heart Rhythm 2019;16:e373–e407. [DOI] [PubMed] [Google Scholar]
- 25. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W.. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, Basso C, Bauce B, Brunckhorst C, Bucciarelli-Ducci C, Duru F, Elliott P, Hamilton RM, Haugaa KH, James CA, Judge D, Link MS, Marchlinski FE, Mazzanti A, Mestroni L, Pantazis A, Pelliccia A, Marra MP, Pilichou K, Platonov PGA, Protonotarios A, Rampazzo A, Saffitz JE, Saguner AM, Schmied C, Sharma S, Tandri H, Te Riele A, Thiene G, Tsatsopoulou A, Zareba W, Zorzi A, Wichter T, Marcus FI, Calkins H; International Experts. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J 2020;41:1414–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M.. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation 1996;94:983–991. [DOI] [PubMed] [Google Scholar]
- 28. Rizzo S, Pilichou K, Thiene G, Basso C.. The changing spectrum of arrhythmogenic (right ventricular) cardiomyopathy. Cell Tissue Res 2012;348:319–323. [DOI] [PubMed] [Google Scholar]
- 29. Te Riele AS, James CA, Philips B, Rastegar N, Bhonsale A, Groeneweg JA, Murray B, Tichnell C, Judge DP, Van Der Heijden JF, Cramer MJ, Velthuis BK, Bluemke DA, Zimmerman SL, Kamel IR, Hauer RN, Calkins H, Tandri H.. Mutation-positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced. J Cardiovasc Electrophysiol 2013;24:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F.. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol 1997;30:1512–1520. [DOI] [PubMed] [Google Scholar]
- 31. Sepehrkhouy S, Gho J, van Es R, Harakalova M, de Jonge N, Dooijes D, van der Smagt JJ, Buijsrogge MP, Hauer RNW, Goldschmeding R, de Weger RA, Asselbergs FW, Vink A.. Distinct fibrosis pattern in desmosomal and phospholamban mutation carriers in hereditary cardiomyopathies. Heart Rhythm 2017;14:1024–1032. [DOI] [PubMed] [Google Scholar]
- 32. Marcus GM, Glidden DV, Polonsky B, Zareba W, Smith LM, Cannom DS, Estes NA 3rd, Marcus F, Scheinman MM; Multidisciplinary Study of Right Ventricular Dysplasia Investigators. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol 2009;54:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wichter T, Borggrefe M, Haverkamp W, Chen X, Breithardt G.. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation 1992;86:29–37. [DOI] [PubMed] [Google Scholar]
- 34. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, Bourfiss M, Fortier A, Lie OH, Saguner AM, Svensson A, Andorin A, Tichnell C, Murray B, Zeppenfeld K, van den Berg MP, Asselbergs FW, Wilde AAM, Krahn AD, Talajic M, Rivard L, Chelko S, Zimmerman SL, Kamel IR, Crosson JE, Judge DP, Yap SC, van der Heijden JF, Tandri H, Jongbloed JDH, Guertin MC, van Tintelen JP, Platonov PG, Duru F, Haugaa KH, Khairy P, Hauer RNW, Calkins H, Te Riele A, James CA.. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019;40:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wlodarska EK, Konka M, Zaleska T, Ploski R, Cedro K, Pucilowska B, Bekiesinska-Figatowska M, Rydlewska-Sadowska W, Ruzyllo W, Hoffman P.. Arrhythmogenic right ventricular cardiomyopathy in two pairs of monozygotic twins. Int J Cardiol 2005;105:126–133. [DOI] [PubMed] [Google Scholar]
- 36. Buja G, Nava A, Daliento L, Scognamiglio R, Miorelli M, Canciani B, Alampi G, Thiene G.. Right ventricular cardiomyopathy in identical and nonidentical young twins. Am Heart J 1993;126:1187–1193. [DOI] [PubMed] [Google Scholar]
- 37. Fabritz L, Hoogendijk MG, Scicluna BP, van Amersfoorth SC, Fortmueller L, Wolf S, Laakmann S, Kreienkamp N, Piccini I, Breithardt G, Noppinger PR, Witt H, Ebnet K, Wichter T, Levkau B, Franke WW, Pieperhoff S, de Bakker JM, Coronel R, Kirchhof P.. Load-reducing therapy prevents development of arrhythmogenic right ventricular cardiomyopathy in plakoglobin-deficient mice. J Am Coll Cardiol 2011;57:740–750. [DOI] [PubMed] [Google Scholar]
- 38. Cheedipudi SM, Hu J, Fan S, Yuan P, Karmouch J, Czernuszewicz G, Robertson MJ, Coarfa C, Hong K, Yao Y, Moore HC, Wehrens X, Gurha P, Marian AJ.. Exercise restores dysregulated gene expression in a mouse model of arrhythmogenic cardiomyopathy. Cardiovasc Res 2019;doi:10.1093/cvr/cvz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boese AC, Kim SC, Yin KJ, Lee JP, Hamblin MH.. Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am J Physiol Heart Circ Physiol 2017;313:H524–H545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hodes AR, Tichnell C, Te Riele AS, Murray B, Groeneweg JA, Sawant AC, Russell SD, van Spaendonck-Zwarts KY, van den Berg MP, Wilde AA, Tandri H, Judge DP, Hauer RN, Calkins H, van Tintelen JP, James CA.. Pregnancy course and outcomes in women with arrhythmogenic right ventricular cardiomyopathy. Heart 2016;102:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Castrini AI, Lie ØH, Leren IS, Estensen ME, Stokke MK, Klæboe LG, Edvardsen T, Haugaa KH.. Number of pregnancies and subsequent phenotype in a cross-sectional cohort of women with arrhythmogenic cardiomyopathy. Eur Heart J Cardiovasc Imaging 2019;20:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gandjbakhch E, Varlet E, Duthoit G, Fressart V, Charron P, Himbert C, Maupain C, Bordet C, Hidden-Lucet F, Nizard J.. Pregnancy and newborn outcomes in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Int J Cardiol 2018;258:172–178. [DOI] [PubMed] [Google Scholar]
- 43. Leo-Macias A, Agullo-Pascual E, Sanchez-Alonso JL, Keegan S, Lin X, Arcos T, Feng Xia L, Korchev YE, Gorelik J, Fenyo D, Rothenberg E, Rothenberg E, Delmar M.. Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc. Nat Commun 2016;7:10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Asimaki A, Protonotarios A, James CA, Chelko SP, Tichnell C, Murray B, Tsatsopoulou A, Anastasakis A, Te Riele A, Kleber AG, Judge DP, Calkins H, Saffitz JE.. Characterizing the molecular pathology of arrhythmogenic cardiomyopathy in patient buccal mucosa cells. Circ Arrhythm Electrophysiol 2016;9:e003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE.. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2009;360:1075–1084. [DOI] [PubMed] [Google Scholar]
- 46. Fidler LM, Wilson GJ, Liu F, Cui X, Scherer SW, Taylor GP, Hamilton RM.. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med 2009;13:4219–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE.. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease). Heart Rhythm 2004;1:3–11. [DOI] [PubMed] [Google Scholar]
- 48. Noorman M, Hakim S, Asimaki A, Vreeker A, van Rijen HV, van der Heyden MA, de Jonge N, de Weger RA, Hauer RN, Saffitz JE, van Veen TA.. Reduced plakoglobin immunoreactivity in arrhythmogenic cardiomyopathy: methodological considerations. Cardiovasc Pathol 2013;22:314–318. [DOI] [PubMed] [Google Scholar]
- 49. Asimaki A, Tandri H, Duffy ER, Winterfield JR, Mackey-Bojack S, Picken MM, Cooper LT, Wilber DJ, Marcus FI, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, Stevenson WG, McKenna WJ, Gautam S, Remick DG, Calkins H, Saffitz JE.. Altered desmosomal proteins in granulomatous myocarditis and potential pathogenic links to arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Noorman M, Hakim S, Kessler E, Groeneweg JA, Cox MG, Asimaki A, van Rijen HV, van Stuijvenberg L, Chkourko H, van der Heyden MA, Vos MA, de Jonge N, van der Smagt JJ, Dooijes D, Vink A, de Weger RA, Varro A, de Bakker JM, Saffitz JE, Hund TJ, Mohler PJ, Delmar M, Hauer RN, van Veen TA.. Remodeling of the cardiac sodium channel, connexin43, and plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy. Heart Rhythm 2013;10:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Geisler SB, Green KJ, Isom LL, Meshinchi S, Martens JR, Delmar M, Russell MW.. Ordered assembly of the adhesive and electrochemical connections within newly formed intercalated disks in primary cultures of adult rat cardiomyocytes. J Biomed Biotechnol 2010;2010:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M.. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res 2007;101:703–711. [DOI] [PubMed] [Google Scholar]
- 53. Rizzo S, Lodder EM, Verkerk AO, Wolswinkel R, Beekman L, Pilichou K, Basso C, Remme CA, Thiene G, Bezzina CR.. Intercalated disc abnormalities, reduced Na(+) current density, and conduction slowing in desmoglein-2 mutant mice prior to cardiomyopathic changes. Cardiovasc Res 2012;95:409–418. [DOI] [PubMed] [Google Scholar]
- 54. Cerrone M, Lin X, Zhang M, Agullo-Pascual E, Pfenniger A, Chkourko Gusky H, Novelli V, Kim C, Tirasawadichai T, Judge DP, Rothenberg E, Chen HS, Napolitano C, Priori SG, Delmar M.. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014;129:1092–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kleber AG, Saffitz JE.. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med 2014;6:240ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patiño GA, Taffet SM, Isom LL, Delmar M.. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res 2009;105:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mallat Z, Tedgui A, Fontaliran F, Frank R, Durigon M, Fontaine G.. Evidence of apoptosis in arrhythmogenic right ventricular dysplasia. N Engl J Med 1996;335:1190–1196. [DOI] [PubMed] [Google Scholar]
- 58. Yang Z, Bowles NE, Scherer SE, Taylor MD, Kearney DL, Ge S, Nadvoretskiy VV, DeFreitas G, Carabello B, Brandon LI, Godsel LM, Green KJ, Saffitz JE, Li H, Danieli GA, Calkins H, Marcus F, Towbin JA.. Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Res 2006;99:646–655. [DOI] [PubMed] [Google Scholar]
- 59. Chelko SP, Asimaki A, Lowenthal J, Bueno-Beti C, Bedja D, Scalco A, Amat-Alarcon N, Andersen P, Judge DP, Tung L, Saffitz JE.. Therapeutic modulation of the immune response in arrhythmogenic cardiomyopathy. Circulation 2019;140:1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li D, Liu Y, Maruyama M, Zhu W, Chen H, Zhang W, Reuter S, Lin SF, Haneline LS, Field LJ, Chen PS, Shou W.. Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy. Hum Mol Genet 2011;20:4582–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Jong S, van Veen TA, van Rijen HV, de Bakker JM.. Fibrosis and cardiac arrhythmias. J Cardiovasc Pharmacol 2011;57:630–638. [DOI] [PubMed] [Google Scholar]
- 62. Lorenzon A, Calore M, Poloni G, De Windt LJ, Braghetta P, Rampazzo A.. Wnt/beta-catenin pathway in arrhythmogenic cardiomyopathy. Oncotarget 2017;8:60640–60655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li J, Swope D, Raess N, Cheng L, Muller EJ, Radice GL.. Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling. Mol Cell Biol 2011;31:1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lombardi R, Chen SN, Ruggiero A, Gurha P, Czernuszewicz GZ, Willerson JT, Marian AJ.. Cardiac fibro-adipocyte progenitors express desmosome proteins and preferentially differentiate to adipocytes upon deletion of the desmoplakin gene. Circ Res 2016;119:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chelko SP, Asimaki A, Andersen P, Bedja D, Amat-Alarcon N, DeMazumder D, Jasti R, MacRae CA, Leber R, Kleber AG, Saffitz JE, Judge DP.. Central role for GSK3beta in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 2016;1:e85923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pilichou K, Thiene G, Bauce B, Rigato I, Lazzarini E, Migliore F, Perazzolo Marra M, Rizzo S, Zorzi A, Daliento L, Corrado D, Basso C.. Arrhythmogenic cardiomyopathy. Orphanet J Rare Dis 2016;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim JC, Perez-Hernandez M, Alvarado FJ, Maurya SR, Montnach J, Yin Y, Zhang M, Lin X, Vasquez C, Heguy A, Liang FX, Woo SH, Morley GE, Rothenberg E, Lundby A, Valdivia HH, Cerrone M, Delmar M.. Disruption of Ca(2+)i homeostasis and connexin 43 hemichannel function in the right ventricle precedes overt arrhythmogenic cardiomyopathy in plakophilin-2-deficient mice. Circulation 2019;140:1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ.. The Hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res 2014;114:454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E.. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. Embo J 2012;31:1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu J, Wang H, Zuo Y, Farmer SR.. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol 2006;26:5827–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ.. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest 2007;117:2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S.. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab 2014;25:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Djouadi F, Lecarpentier Y, Hebert JL, Charron P, Bastin J, Coirault C.. A potential link between peroxisome proliferator-activated receptor signalling and the pathogenesis of arrhythmogenic right ventricular cardiomyopathy. Cardiovasc Res 2009;84:83–90. [DOI] [PubMed] [Google Scholar]
- 74. Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge DP, Chen HS.. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature 2013;494:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Merner ND, Hodgkinson KA, Haywood AF, Connors S, French VM, Drenckhahn JD, Kupprion C, Ramadanova K, Thierfelder L, McKenna W, Gallagher B, Morris-Larkin L, Bassett AS, Parfrey PS, Young TL.. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet 2008;82:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Saguner AM, Brunckhorst C, Duru F.. Arrhythmogenic ventricular cardiomyopathy: a paradigm shift from right to biventricular disease. Wjc 2014;6:154–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Campuzano O, Alcalde M, Iglesias A, Barahona-Dussault C, Sarquella-Brugada G, Benito B, Arzamendi D, Flores J, Leung TK, Talajic M, Oliva A, Brugada R.. Arrhythmogenic right ventricular cardiomyopathy: severe structural alterations are associated with inflammation. J Clin Pathol 2012;65:1077–1083. [DOI] [PubMed] [Google Scholar]
- 78. Campian ME, Verberne HJ, Hardziyenka M, de Groot EA, van Moerkerken AF, van Eck-Smit BL, Tan HL.. Assessment of inflammation in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Nucl Med Mol Imaging 2010;37:2079–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Martins D, Ovaert C, Khraiche D, Boddaert N, Bonnet D, Raimondi F.. Myocardial inflammation detected by cardiac MRI in Arrhythmogenic right ventricular cardiomyopathy: a paediatric case series. Int J Cardiol 2018;271:81–86. [DOI] [PubMed] [Google Scholar]
- 80. Calabrese F, Basso C, Carturan E, Valente M, Thiene G.. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: is there a role for viruses? Cardiovasc Pathol 2006;15:11–17. [DOI] [PubMed] [Google Scholar]
- 81. Patrianakos AP, Protonotarios N, Nyktari E, Pagonidis K, Tsatsopoulou A, Parthenakis FI, Vardas PE.. Arrhythmogenic right ventricular cardiomyopathy/dysplasia and troponin release. Myocarditis or the “hot phase” of the disease? Int J Cardiol 2012;157:e26–e28. [DOI] [PubMed] [Google Scholar]
- 82. Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS.. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res 2001;51:681–690. [DOI] [PubMed] [Google Scholar]
- 83. Stein M, van Veen TA, Remme CA, Boulaksil M, Noorman M, van Stuijvenberg L, van der Nagel R, Bezzina CR, Hauer RN, de Bakker JM, van Rijen HV.. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction. Cardiovasc Res 2009;83:52–60. [DOI] [PubMed] [Google Scholar]
- 84. Shaw RM, Rudy Y.. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 1997;81:727–741. [DOI] [PubMed] [Google Scholar]
- 85. Jansen JA, Noorman M, Musa H, Stein M, de Jong S, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV.. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm 2012;9:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jansen JA, van Veen TA, de Jong S, van der Nagel R, van Stuijvenberg L, Driessen H, Labzowski R, Oefner CM, Bosch AA, Nguyen TQ, Goldschmeding R, Vos MA, de Bakker JM, van Rijen HV.. Reduced Cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ Arrhythm Electrophysiol 2012;5:380–390. [DOI] [PubMed] [Google Scholar]
- 87. Deo M, Sato PY, Musa H, Lin X, Pandit SV, Delmar M, Berenfeld O.. Relative contribution of changes in sodium current versus intercellular coupling on reentry initiation in 2-dimensional preparations of plakophilin-2-deficient cardiac cells. Heart Rhythm 2011;8:1740–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cerrone M, Montnach J, Lin X, Zhao YT, Zhang M, Agullo-Pascual E, Leo-Macias A, Alvarado FJ, Dolgalev I, Karathanos TV, Malkani K, Van Opbergen CJM, van Bavel JJA, Yang HQ, Vasquez C, Tester D, Fowler S, Liang F, Rothenberg E, Heguy A, Morley GE, Coetzee WA, Trayanova NA, Ackerman MJ, van Veen TAB, Valdivia HH, Delmar M.. Plakophilin-2 is required for transcription of genes that control calcium cycling and cardiac rhythm. Nat Commun 2017;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. van Opbergen CJM, Noorman M, Pfenniger A, Copier JS, Vermij SH, Li Z, van der Nagel R, Zhang M, de Bakker JMT, Glass AM, Mohler PJ, Taffet SM, Vos MA, van Rijen HVM, Delmar M, van Veen T.. Plakophilin-2 haploinsufficiency causes calcium handling deficits and modulates the cardiac response towards stress. Int J Mol Sci 2019;20:4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- 91. Strimbu K, Tavel JA.. What are biomarkers? Curr Opin HIV AIDS 2010;5:463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Coats CJ, Heywood WE, Mills K, Elliott PM.. Current applications of biomarkers in cardiomyopathies. Expert Rev Cardiovasc Ther 2015;13:825–837. [DOI] [PubMed] [Google Scholar]
- 93. de Jong S, van Veen TA, de Bakker JM, Vos MA, van Rijen HV.. Biomarkers of myocardial fibrosis. J Cardiovasc Pharmacol 2011;57:522–535. [DOI] [PubMed] [Google Scholar]
- 94. de Jong S, van Veen TA, de Bakker JM, van Rijen HV.. Monitoring cardiac fibrosis: a technical challenge. Neth Heart J 2012;20:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chalikias GK, Tziakas DN.. Biomarkers of the extracellular matrix and of collagen fragments. Clin Chim Acta 2015;443:39–47. [DOI] [PubMed] [Google Scholar]
- 96. Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res 2002;90:520–530. [DOI] [PubMed] [Google Scholar]
- 97. Sommariva E, D’Alessandra Y, Farina FM, Casella M, Cattaneo F, Catto V, Chiesa M, Stadiotti I, Brambilla S, Dello Russo A, Carbucicchio C, Vettor G, Riggio D, Sandri MT, Barbuti A, Vernillo G, Muratori M, Dal Ferro M, Sinagra G, Moimas S, Giacca M, Colombo GI, Pompilio G, Tondo C.. MiR-320a as a potential novel circulating biomarker of arrhythmogenic cardiomyopathy. Sci Rep 2017;7:4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang H, Liu S, Dong T, Yang J, Xie Y, Wu Y, Kang K, Hu S, Gou D, Wei Y.. Profiling of differentially expressed microRNAs in arrhythmogenic right ventricular cardiomyopathy. Sci Rep 2016;6:28101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Thum T, Condorelli G.. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res 2015;116:751–762. [DOI] [PubMed] [Google Scholar]
- 100. Bauersachs J. Regulation of myocardial fibrosis by MicroRNAs. J Cardiovasc Pharmacol 2010;56:454–459. [DOI] [PubMed] [Google Scholar]
- 101. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S.. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980–984. [DOI] [PubMed] [Google Scholar]
- 102. Te Riele A, Tandri H, Sanborn DM, Bluemke DA.. Noninvasive multimodality imaging in ARVD/C. JACC Cardiovasc Imaging 2015;8:597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB; Society for Cardiovascular Magnetic Resonance Imaging; Cardiovascular Magnetic Resonance Working Group of the European Society of Cardiology. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 2013;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Burt JR, Zimmerman SL, Kamel IR, Halushka M, Bluemke DA.. Myocardial T1 mapping: techniques and potential applications. Radiographics 2014;34:377–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Aus Dem Siepen F, Buss SJ, Messroghli D, Andre F, Lossnitzer D, Seitz S, Keller M, Schnabel PA, Giannitsis E, Korosoglou G, Katus HA, Steen H.. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur Heart J Cardiovasc Imaging 2015;16:210–216. [DOI] [PubMed] [Google Scholar]
- 106. Bourfiss M, Prakken NHJ, van der Heijden JF, Kamel I, Zimmerman SL, Asselbergs FW, Leiner T, Velthuis BK, Te Riele ASJM.. Diagnostic value of native T1 mapping in arrhythmogenic right ventricular cardiomyopathy. JACC Cardiovasc Imaging 2019;12:1580–1582. [DOI] [PubMed] [Google Scholar]
- 107. Heermann P, Hedderich DM, Paul M, Schülke C, Kroeger JR, Baeßler B, Wichter T, Maintz D, Waltenberger J, Heindel W, Bunck AC.. Biventricular myocardial strain analysis in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) using cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 2014;16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vigneault DM, Te Riele AS, James CA, Zimmerman SL, Selwaness M, Murray B, Tichnell C, Tee M, Noble JA, Calkins H, Tandri H, Bluemke DA.. Right ventricular strain by MR quantitatively identifies regional dysfunction in patients with arrhythmogenic right ventricular cardiomyopathy. J Magn Reson Imaging 2016;43:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chatterjee D, Fatah M, Akdis D, Spears DA, Koopmann TT, Mittal K, Rafiq MA, Cattanach BM, Zhao Q, Healey JS, Ackerman MJ, Bos JM, Sun Y, Maynes JT, Brunckhorst C, Medeiros-Domingo A, Duru F, Saguner AM, Hamilton RM.. An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. Eur Heart J 2018;39:3932–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kalantari-Dehaghi M, Anhalt GJ, Camilleri MJ, Chernyavsky AI, Chun S, Felgner PL, Jasinskas A, Leiferman KM, Liang L, Marchenko S, Nakajima-Sasaki R, Pittelkow MR, Zone JJ, Grando SA.. Pemphigus vulgaris autoantibody profiling by proteomic technique. PLoS One 2013;8:e57587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sinha AA, Sajda T.. The evolving story of autoantibodies in pemphigus vulgaris: development of the “super compensation hypothesis”. Front Med (Lausanne) 2018;5:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Oostendorp TF, van Dessel PF, Coronel R, Belterman C, Linnenbank AC, van Schie IH, van Oosterom A, Oosterhoff P, van Dam PM, de Bakker JM.. Noninvasive detection of epicardial and endocardial activity of the heart. Neth Heart J 2011;19:488–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rolf S, Bruns HJ, Wichter T, Kirchhof P, Ribbing M, Wasmer K, Paul M, Breithardt G, Haverkamp W, Eckardt L.. The ajmaline challenge in Brugada syndrome: diagnostic impact, safety, and recommended protocol. Eur Heart J 2003;24:1104–1112. [DOI] [PubMed] [Google Scholar]
- 114. Peters S. Arrhythmogenic right ventricular dysplasia-cardiomyopathy and provocable coved-type ST-segment elevation in right precordial leads: clues from long-term follow-up. Europace 2008;10:816–820. [DOI] [PubMed] [Google Scholar]
- 115. Teske AJ, Cox MG, Te Riele AS, De Boeck BW, Doevendans PA, Hauer RN, Cramer MJ.. Early detection of regional functional abnormalities in asymptomatic ARVD/C gene carriers. J Am Soc Echocardiogr 2012;25:997–1006. [DOI] [PubMed] [Google Scholar]
- 116. Teske AJ, De Boeck BW, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJ.. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound 2007;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mast TP, Teske AJ, Te Riele AS, Groeneweg JA, Van Der Heijden JF, Velthuis BK, Loh P, Doevendans PA, Van Veen TA, Dooijes D, De Bakker JM, Hauer RN, Cramer MJ.. Prolonged electromechanical interval unmasks arrhythmogenic right ventricular dysplasia/cardiomyopathy in the subclinical stage. J Cardiovasc Electrophysiol 2016;27:303–314. [DOI] [PubMed] [Google Scholar]
- 118. Casini S, Verkerk AO, Remme CA.. Human iPSC-derived cardiomyocytes for investigation of disease mechanisms and therapeutic strategies in inherited arrhythmia syndromes: strengths and limitations. Cardiovasc Drugs Ther 2017;31:325–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Roberts JD, Murphy NP, Hamilton RM, Lubbers ER, James CA, Kline CF, Gollob MH, Krahn AD, Sturm AC, Musa H, El-Refaey M, Koenig S, Aneq MA, Hoorntje ET, Graw SL, Davies RW, Rafiq MA, Koopmann TT, Aafaqi S, Fatah M, Chiasson DA, Taylor MR, Simmons SL, Han M, van Opbergen CJ, Wold LE, Sinagra G, Mittal K, Tichnell C, Murray B, Codima A, Nazer B, Nguyen DT, Marcus FI, Sobriera N, Lodder EM, van den Berg MP, Spears DA, Robinson JF, Ursell PC, Green AK, Skanes AC, Tang AS, Gardner MJ, Hegele RA, van Veen TA, Wilde AA, Healey JS, Janssen PM, Mestroni L, van Tintelen JP, Calkins H, Judge DP, Hund TJ, Scheinman MM, Mohler PJ.. Ankyrin-B dysfunction predisposes to arrhythmogenic cardiomyopathy and is amenable to therapy. J Clin Invest 2019;129:3171–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang X, Meng F, Song J, Zhang L, Wang J, Li D, Li L, Dong P, Yang B, Chen Y.. Pentoxifylline ameliorates cardiac fibrosis, pathological hypertrophy, and cardiac dysfunction in angiotensin ii-induced hypertensive rats. J Cardiovasc Pharmacol 2016;67:76–85. [DOI] [PubMed] [Google Scholar]
- 121. Szekely Y, Arbel Y.. A review of interleukin-1 in heart disease: where do we stand today? Cardiol Ther 2018;7:25–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]