Abstract
Background:
Premature ejaculation (PE) is a common sexual dysfunction for which selective serotonin reuptake inhibitors (SSRIs) have been used effectively for treatment. However, compliance with therapy and predictors of long-term SSRI use in the treatment of PE are not well known.
Aim:
We sought to analyze our experience with drop-out rates with fluoxetine in the primary PE population and to identify predictors of continued use of this agent.
Methods:
Men with primary PE constituted who used fluoxetine and had at least 12 months follow-up constituted the study population. Subjects underwent a comprehensive interview to ascertain self-reported (non-stopwatch) intravaginal ejaculatory latency time (IELT), self-rated control over ejaculation, and personal and patient-reported partner distress due to PE. Patients were treated with fluoxetine 20mg daily, with the possibility of dose titration up or down based on efficacy and side effects.
Outcomes:
The PE parameters of interest included: self-reported IELT, self-rated control over ejaculation, personal and partner distress due to PE, and medication adherence.
Results:
130 men were included in the study. Dropout rates at 6 and 12 months were 56% and 72%. Self-rated ‘poor’ ejaculatory control decreased from 98% to 41% (p < 0.01), high personal distress from 47% to 11% (p < 0.01) and high partner distress rates from 72% to 27% (p < 0.01). Predictors of continued use at 12 months included high partner distress, being unpartnered, and having a post-treatment IELT ≥5 minutes (p<0.01). Overall side effects included headache (5%), dizziness 4%), nausea (5%), nervousness (5%), sleepiness (8%); however, moderate to severe side effects reported included: nausea 2%, sleepiness 2%, headache 2% and dizziness 2%.
Clinical Implications:
Compliance with SSRIs is a well-described problem in the depression literature, but data is sparse regarding continued use of SSRIs in the treatment of PE.
Strengths and Limitations:
We report on 12-month compliance with SSRIs for the treatment of PE. Our early compliance rates were more encouraging than what has been reported in the past. However, IELT was self-reported and not measured objectively and we did not use validated patient-reported outcomes but rather self-reported ejaculatory control and distress levels, which have limitations.
Conclusions:
Fluoxetine is an effective agent for the treatment of PE with significant improvement realized in IELT, ejaculatory control, and distress levels for both men and their partners. Despite its efficacy, continued use of fluoxetine, beyond six months is poor.
Keywords: premature ejaculation, SSRI, fluoxetine, compliance
INTRODUCTION
Premature ejaculation (PE) is a common sexual complaint and is associated with significant psychological distress.[1] Despite affecting 20–30% of men, PE presents a challenge for the clinician as it is without a well-defined etiology compared to other sexual disorders and is often thought of by clinicians as a non-medical issue.[2, 3]
Further compounding the difficulty understanding PE is a variety of definitions. However, in 2014, the International Society for Sexual Medicine (ISSM) assembled a panel to develop a unified definition for acquired and lifelong PE in an attempt to help clinicians recognize and treat this common problem.[4] PE is currently defined as (i) ejaculation that always or nearly always occurs prior to or within one minute of vaginal penetration (lifelong PE) or a clinically significant and bothersome reduction in latency time, often to about 3 minutes or less (acquired PE), (ii) the inability to delay ejaculation on all or nearly all vaginal penetrations, with (iii) negative personal consequences, such as distress, bother, frustration, and/or the avoidance of sexual intimacy.
There exists no regulatory agency-approved pharmacotherapy for PE in the United States and a single agent elsewhere in the form of dapoxetine. As early as the 1940s, topical anesthetics were employed in the treatment of PE.[5] Since then, a variety of pharmacotherapeutic agents have been explored with the most commonly used treatment being the off-label use of selective serotonin reuptake inhibitors (SSRIs).[6–8]
SSRIs, initially developed for the treatment of depression and anxiety in the 1970s, have been successfully used for PE. A number of studies have demonstrated that compared with placebo, daily use of SSRIs can significantly increase intravaginal ejaculatory latency time (IELT).[9, 10] Even on-demand use of the most recently developed SSRI dapoxetine, has been shown to result in a 1–3 fold increase in IELT when compared to placebo.[11, 12] In a large meta-analysis, Waldinger found that on-demand SSRIs use generally exerts less ejaculation delay than daily SSRIs, but the benefit of daily use may be at the expense of greater adverse effects.[8] There is a concern that SSRI medications may increase the odds of suicide attempts by those treated with this class of drugs. [13, 14]
Discontinuation of SSRI use is well recognized in the depression literature with rates varying between 18–42% within the first 30 days of therapy.[15–18] Unfortunately, the literature on SSRI compliance in the treatment of PE is not nearly as robust. McMahon and colleagues found a discontinuation rate of 31% after two months for subjects treated with either on-demand or daily dapoxetine.[19] Another study focusing solely on on-demand dapoxetine use found discontinuation rates of up to 47%.[11]
We sought to analyze our experience with drop-out rates with fluoxetine in the primary PE population and to identify predictors of continued use of this agent.
METHODS
Patient Population:
We retrospectively analyzed a prospectively constructed database of men who were prescribed fluoxetine for the management of PE. The database was registered with our institutional review board. The study group included (i) patients with primary (lifelong) PE (ii) having an IELT of less than one minute (ISSM definition) (iii) who used fluoxetine and (iv) were followed for at least 12 months. All subjects underwent a comprehensive baseline (pre-therapy) face-to-face interview with an experienced sexual medicine physician. Patient demographics, comorbidities and PE factors were recorded. The PE parameters of interest included: self-reported IELT, self-rated control over ejaculation (poor, fair, good), and personal and partner distress (mild, moderate, severe) due to PE.
Fluoxetine Use:
All subjects were started on fluoxetine 20mg daily. Fluoxetine was selected given our extensive clinical experience with this agent and its acceptable efficacy/adverse event profile. Each subject had the dose titrated up or down after six weeks (to either 40mg or 10mg, respectively) by the physician based on efficacy and adverse effects. Subjects were advised to follow-up at six weeks, 3, 6 and 12 months. Subjects lost to follow-up at twelve months received a telephone call to ascertain fluoxetine use and self-rated PE parameters.
Statistical Analysis:
A forward stepwise model selection was used to identify independent predictors of continued use of fluoxetine 12 months after commencement using a logistic regression model. The variables explored included: patient age, partner status, duration of PE, patient-rated self-control over ejaculation, degree of distress for patient, degree of distress for partner (patient-rated) and end of treatment IELT and presence of self-reported moderate-severe medication side effects.
RESULTS
Baseline Parameters:
A total of 130 patients were included in the study. In our final study cohort, the mean age was 31±14 (range 26–52) years. 92% of participating men were partnered at the initial visit. 94% reported poor ejaculatory control. 47% complained of severe personal distress because of their problems with PE, and 72% reported that their partners had moderate or severe distress.
Treatment Outcomes:
At 3 months, 76% of men reported an improvement in self-reported IELT of ≥1 minute. At 3 months, IELT ≥ 2 minutes, ≥ 3 minutes, and ≥ 5 minutes was reported by 64%, 38% and 11% of patients, respectively. Of those men continuing to use fluoxetine at 6 and 12 months, 72% and 69%, respectively, continued to report improvement in self-reported IELT of ≥1 minute. Of those reporting significant improvement, 10% were using fluoxetine 10mg, 60% were taking a 20mg dose, and 30% were on the maximum 40 mg. Dropout rates at 3, 6 and 12 months were 11%, 56%, and 72% respectively. At 3 months, self-rated ‘poor’ ejaculatory control decreased from 94% to 42% (p < 0.01) and severe personal distress and severe partner distress rates also decreased significantly from 45% to 11% and 70% to 27%, respectively (p < 0.01) (Figure 1). Overall side effects included headache (5%), dizziness 4%), nausea (5%), nervousness (5%), sleepiness (8%), however, moderate to severe side effects reported included: nausea 2%, sleepiness 2%, headache 2% and dizziness 2%.
Figure 1.
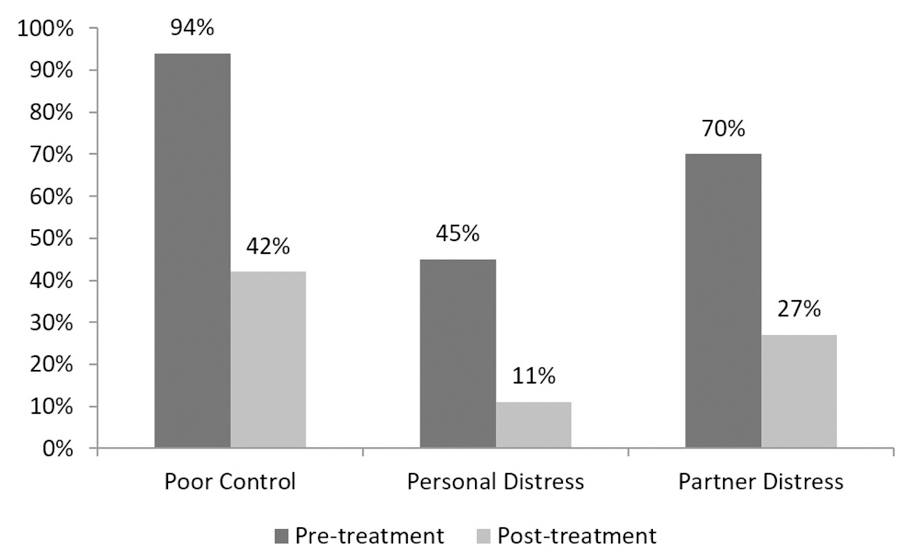
Impact of fluoxetine on patient-reported outcomes (at 3 months).
Predictors of Continued Use (Table 1):
Table 1:
Multivariable Analysis of Predictors of Continued Fluoxetine Use at 12 months after Commencement
| Variable | OR | 95% CI | p |
|---|---|---|---|
| High partner distress | 6.8 | 3.3–11.7 | < 0.01 |
| Post-treatment IELT ≥5 minutes | 2.9 | 1.6–5.9 | < 0.01 |
| No partner | 2.8 | 1.6–3.8 | < 0.01 |
On logistic regression analysis, several factors predicted continued use of fluoxetine (any dose) at one year. Men who reported not having a partner were more likely to continue using fluoxetine (OR 2.8, 95% CI 1.6–3.8, p < 0.01), as were those who reported high partner distress (OR 6.8, 95% CI 3.3–11.7, p < 0.01). High personal stress did not significantly predict continued fluoxetine use. Those who reported a post-treatment IELT ≥5 minutes (OR 2.9, 95% CI 1.6–5.9, p < 0.01) were also more likely to report continued fluoxetine use at one year. Of note, the self-reporting of fluoxetine-related moderate-severe side effects was not a predictor, although the incidence of moderate-severe side effects was low.
DISCUSSION
Premature ejaculation is a common complaint seen by urologists. Rates of PE have become better understood over the last decade because of several large epidemiological studies. One of the largest prospective studies regarding PE prevalence was the National Health and Social Life Survey conducted in the 1990s.[2] The interview-based study involved nearly 3,500 men, aged 19–59 years, who were questioned about “climaxing too early” during the preceding 12 months. The authors reported a rate of PE of 29% but made no attempt to differentiate between lifelong and acquired premature ejaculation. This high rate is in contrast to the much lower reported prevalence of 13% in the Johnson & Johnson sponsored observational study conducted by Patrick et al. in 2005.[20] Unlike the former, Patrick et al. used a more stringent definition of PE taken from the DSM-IV, which may account for their lower frequency. Additionally, they used more objective measures such as stopwatch-measured IELT and patient-reported outcome measures.
It is not well understood exactly how SSRIs work in men with PE, though their effect on ejaculation latency was first noted with their earliest use in the treatment of depression. SSRIs are known to increase synaptic levels of serotonin via inhibition of pre-synaptic serotonin transporters. This results in an immediate increase in synaptic levels of serotonin followed by desensitization of the 5-HT1A receptor. Desensitization results in consistently elevated synaptic levels of serotonin and tonic stimulation of post-synaptic receptors. It is thought that this tonic stimulation is responsible for the increased ejaculatory latency seen in men taking SSRIs.
In our study, we used fluoxetine due to its efficacy but mainly its low reported incidence of side effects to maximize compliance. Fluoxetine proved to be effective in improving IELT and decreasing self-rated poor ejaculatory control. This is consistent with previously published data on the benefits of fluoxetine.[10, 21, 22] Giuliano et al. reported that perceived control over ejaculation had a significant effect upon both sexual satisfaction and ejaculation-related personal distress.[23] In our study, we found a similar relationship between ejaculatory control and psychological distress. In addition to improved IELT and perceived ejaculatory control, our subjects reported a significant decrease in both personal and partner distress with daily fluoxetine use. This is again consistent with the literature on the daily use of other SSRI agents (paroxetine, sertraline).[8]
Compliance with SSRIs is a well-described problem in the depression literature, but data is sparse regarding continued use of SSRIs in the treatment of PE. Vlahiotis et al. reported an overall discontinuation rate of 45% within 180 days of initiation of such therapy for patients with diagnosed major depressive disorder, with other studies demonstrating equally high rates among both privately insured and Medicaid patients.[24–26] Our compliance rate was more encouraging after three months, with only 11% of men reporting dropout from fluoxetine use. Compliance among our subjects was also much higher than the 69% compliance rate McMahon et al. reported for dapoxetine.[19] In the McMahon study, men receiving both on-demand and daily formulations of dapoxetine were included in the analysis. Interestingly, they found no significant difference in discontinuation rates with placebo, dapoxetine 30mg, and dapoxetine 60mg, and contributed discontinuation to the burden of serial evaluations in the trial. In our study, dropout rates increased dramatically over subsequent months, with 56% and 72% discontinuing fluoxetine at 6 and 12 months, respectively. Except for one study looking at paroxetine that found no change in discontinuation (nearly 31%) from 3–6 months, our dropout rates are on par with the limited data available for SSRI use in PE.[11, 27]
Factors leading to SSRI discontinuation are not well reported in the current literature. One would expect that those men who experience the greatest improvements in IELT would be more compliant. This proved to be true among our cohort with patients experiencing an on-treatment IELT greater than 5 minutes being more likely to continue using fluoxetine. Additionally, men who reported no partner were more likely to remain compliant, but so were those who reported partners with high levels of distress. Neither of the latter are surprising as the man who has PE but does not have a consistent partner is likely to be interested in controlling his PE pending establishing a new relationship. Likewise, men whose partners are distressed or at least perceive them as distressed are intuitively more likely to continue PE therapy. Perhaps the most interesting finding was that men with high levels of personal distress were not necessarily more likely to remain compliant, proving that continued use is not solely contingent on subjective concern about PE. It is possible that a man’s distress level may be dwarfed by the level of (perceived) partner distress.
Our report on fluoxetine compliance for PE has several strengths. To our knowledge, this is the first report on long-term compliance with SSRIs for the treatment of PE, with 12-month follow-up. Our early compliance rates were also more encouraging than what has been reported in the past. Finally, our data demonstrates an improvement not only in personal but also partner distress with the use of daily fluoxetine, which has not been well reported previously.
We recognize that our study is not without limitations. Perhaps the greatest is that in our study IELT was self-reported and not measured objectively as has been done in recent, especially industry-sponsored studies. We also did not use validated patient-reported outcomes but rather self-reported ejaculatory control and patient/partner distress levels, which of course have limitations. To our knowledge, validated distress instruments exist but have been used far more frequently in female sexual medicine research and have not been validated in the PE population. Lastly, the absence of standardized outcome measures can be seen as a limitation; however, our study focused on patient dropout rates rather than discrete measures on ejaculation control.
CONCLUSION
Fluoxetine is an effective agent for the treatment of PE with significant improvement realized in IELT, ejaculatory control, and distress levels for both men and their partners. Despite the potential for improvement, continued use of fluoxetine, especially beyond 3 months, is low.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Rowland DL, Patrick DL, Rothman M, Gagnon DD. The Psychological Burden of Premature Ejaculation. The Journal of urology. 2007;177: 1065–70. [DOI] [PubMed] [Google Scholar]
- [2].Laumann EO, Paik A, Rosen RC. Sexual Dysfunction in the United States: Prevalence and Predictors. JAMA : the journal of the American Medical Association. 1999;281: 537–44. [DOI] [PubMed] [Google Scholar]
- [3].Jannini EA, Lenzi A. Epidemiology of Premature Ejaculation. Current opinion in urology. 2005;15: 399–403. [DOI] [PubMed] [Google Scholar]
- [4].Serefoglu EC, McMahon CG, Waldinger MD, et al. An Evidence-Based Unified Definition of Lifelong and Acquired Premature Ejaculation: Report of the Second International Society for Sexual Medicine Ad Hoc Committee for the Definition of Premature Ejaculation. The journal of sexual medicine. 2014;11: 1423–41. [DOI] [PubMed] [Google Scholar]
- [5].Semans JH. Premature Ejaculation: A New Approach. Southern medical journal. 1956;49: 353–8. [DOI] [PubMed] [Google Scholar]
- [6].Giuliano F, Hellstrom WJ. The Pharmacological Treatment of Premature Ejaculation. BJU international. 2008;102: 668–75. [DOI] [PubMed] [Google Scholar]
- [7].McMahon CG, Porst H. Oral Agents for the Treatment of Premature Ejaculation: Review of Efficacy and Safety in the Context of the Recent International Society for Sexual Medicine Criteria for Lifelong Premature Ejaculation. The journal of sexual medicine. 2011;8: 2707–25. [DOI] [PubMed] [Google Scholar]
- [8].Waldinger MD, Zwinderman AH, Schweitzer DH, Olivier B. Relevance of Methodological Design for the Interpretation of Efficacy of Drug Treatment of Premature Ejaculation: A Systematic Review and Meta-Analysis. International journal of impotence research. 2004;16: 369–81. [DOI] [PubMed] [Google Scholar]
- [9].Mattos RM, Marmo Lucon A, Srougi M. Tadalafil and Fluoxetine in Premature Ejaculation: Prospective, Randomized, Double-Blind, Placebo-Controlled Study. Urologia internationalis. 2008;80: 162–5. [DOI] [PubMed] [Google Scholar]
- [10].Kara H, Aydin S, Yucel M, et al. The Efficacy of Fluoxetine in the Treatment of Premature Ejaculation: A Double-Blind Placebo Controlled Study. The Journal of urology. 1996;156: 1631–2. [PubMed] [Google Scholar]
- [11].Buvat J, Tesfaye F, Rothman M, Rivas DA, Giuliano F. Dapoxetine for the Treatment of Premature Ejaculation: Results from a Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial in 22 Countries. European urology. 2009;55: 957–67. [DOI] [PubMed] [Google Scholar]
- [12].Pryor JL, Althof SE, Steidle C, et al. Efficacy and Tolerability of Dapoxetine in Treatment of Premature Ejaculation: An Integrated Analysis of Two Double-Blind, Randomised Controlled Trials. Lancet. 2006;368: 929–37. [DOI] [PubMed] [Google Scholar]
- [13].Gunnell D, Saperia J, Ashby D. Selective Serotonin Reuptake Inhibitors (Ssris) and Suicide in Adults: Meta-Analysis of Drug Company Data from Placebo Controlled, Randomised Controlled Trials Submitted to the Mhra’s Safety Review. Bmj. 2005;330: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fergusson D, Doucette S, Glass KC, et al. Association between Suicide Attempts and Selective Serotonin Reuptake Inhibitors: Systematic Review of Randomised Controlled Trials. Bmj. 2005;330: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Andersson S Early Discontinuation of Antidepressant Treatment in Adults Is Very Common in the USA. Evid Based Ment Health. 2006;9: 65. [DOI] [PubMed] [Google Scholar]
- [16].Anderson IM. Ssris Versus Tricyclic Antidepressants in Depressed Inpatients: A Meta-Analysis of Efficacy and Tolerability. Depress Anxiety. 1998;7 Suppl 1: 11–7. [PubMed] [Google Scholar]
- [17].Anderson IM, Tomenson BM. Treatment Discontinuation with Selective Serotonin Reuptake Inhibitors Compared with Tricyclic Antidepressants: A Meta-Analysis. BMJ. 1995;310: 1433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vanelli M, Coca-Perraillon M. Role of Patient Experience in Antidepressant Adherence: A Retrospective Data Analysis. Clin Ther. 2008;30: 1737–45. [DOI] [PubMed] [Google Scholar]
- [19].McMahon CG, Althof SE, Kaufman JM, et al. Efficacy and Safety of Dapoxetine for the Treatment of Premature Ejaculation: Integrated Analysis of Results from Five Phase 3 Trials. The journal of sexual medicine. 2011;8: 524–39. [DOI] [PubMed] [Google Scholar]
- [20].Patrick DL, Althof SE, Pryor JL, et al. Premature Ejaculation: An Observational Study of Men and Their Partners. The journal of sexual medicine. 2005;2: 358–67. [DOI] [PubMed] [Google Scholar]
- [21].Yilmaz U, Tatlisen A, Turan H, Arman F, Ekmekcioglu O. The Effects of Fluoxetine on Several Neurophysiological Variables in Patients with Premature Ejaculation. The Journal of urology. 1999;161: 107–11. [PubMed] [Google Scholar]
- [22].Wang WF, Chang L, Minhas S, Ralph DJ. Selective Serotonin Reuptake Inhibitors in the Treatment of Premature Ejaculation. Chinese medical journal. 2007;120: 1000–6. [PubMed] [Google Scholar]
- [23].Giuliano F, Patrick DL, Porst H, et al. Premature Ejaculation: Results from a Five-Country European Observational Study. European urology. 2008;53: 1048–57. [DOI] [PubMed] [Google Scholar]
- [24].Melfi CA, Chawla AJ, Croghan TW, et al. The Effects of Adherence to Antidepressant Treatment Guidelines on Relapse and Recurrence of Depression. Archives of general psychiatry. 1998;55: 1128–32. [DOI] [PubMed] [Google Scholar]
- [25].Kobak KA, Taylor L, Katzelnick DJ, et al. Antidepressant Medication Management and Health Plan Employer Data Information Set (Hedis) Criteria: Reasons for Nonadherence. The Journal of clinical psychiatry. 2002;63: 727–32. [DOI] [PubMed] [Google Scholar]
- [26].Vlahiotis A, Devine ST, Eichholz J, Kautzner A. Discontinuation Rates and Health Care Costs in Adult Patients Starting Generic Versus Brand Ssri or Snri Antidepressants in Commercial Health Plans. J Manag Care Pharm. 2011;17: 123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Salonia A, Rocchini L, Sacca A, et al. Acceptance of and Discontinuation Rate from Paroxetine Treatment in Patients with Lifelong Premature Ejaculation. The journal of sexual medicine. 2009;6: 2868–77. [DOI] [PubMed] [Google Scholar]


