Novel antiparasitic activity was observed for the antifungal occidiofungin. It efficaciously and irreversibly inhibited the zoonotic enteric parasite Cryptosporidium parvum in vitro with limited cytotoxicity (50% effective concentration [EC50] = 120 nM versus 50% cytotoxic concentration [TC50] = 988 nM), and its application disrupted the parasite morphology. This study expands the spectrum of activity of a glycolipopeptide named occidiofungin.
KEYWORDS: protozoan parasite, Cryptosporidium parvum, occidiofungin, efficacy, cytotoxicity, in vitro
ABSTRACT
Novel antiparasitic activity was observed for the antifungal occidiofungin. It efficaciously and irreversibly inhibited the zoonotic enteric parasite Cryptosporidium parvum in vitro with limited cytotoxicity (50% effective concentration [EC50] = 120 nM versus 50% cytotoxic concentration [TC50] = 988 nM), and its application disrupted the parasite morphology. This study expands the spectrum of activity of a glycolipopeptide named occidiofungin. Occidiofungin has poor gastrointestinal tract absorption properties, supporting future investigations into its potential activities on other enteric parasites.
INTRODUCTION
The glycolipopeptide occidiofungin was originally isolated from Burkholderia contaminans for antifungal activity (Fig. 1A) (1). It has a broad spectrum of activity against fungal pathogens, including species in the genera Alternaria, Aspergillus, Fusarium, Geotrichum, Macrophomina, Microsporum, Penicillium, Pythium, Rhizoctonia, and Trichophyton, with MICs of 1 to 32 μg/ml (or MIC50s of 0.25 to 16 μg/ml), and various Candida species (MICs at 0.5 to 2.0 μg/ml) (1–3).
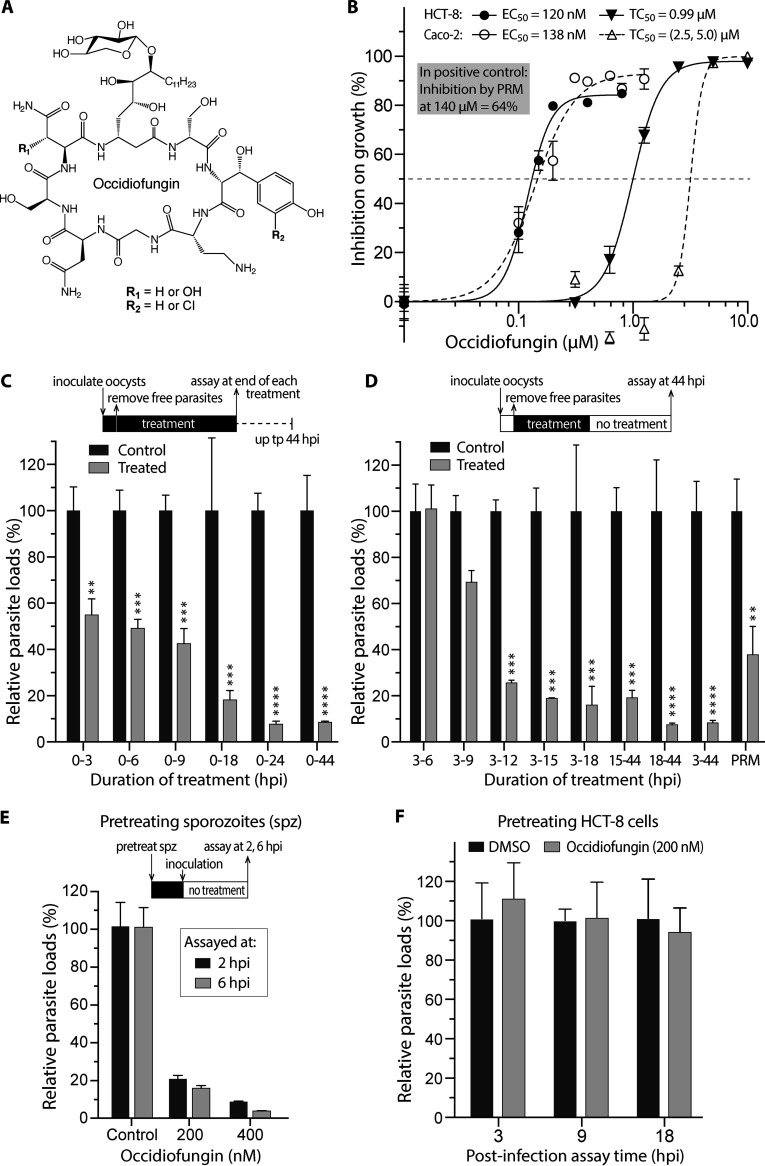
FIG 1.
Activity of occidiofungin against the growth of Cryptosporidium parvum in vitro. (A) Covalent structure of occidiofungin, a glycolipopeptide. (B) In vitro efficacy of occidiofungin against C. parvum cultured in two host cell lines (HCT-8 and Caco-2) and cytotoxicity. Paromomycin (PRM) at 140 μM was used as a positive control. (C) Effect of occidiofungin on the growth of C. parvum in vitro with various lengths of treatment. Compound was added to the medium along with oocyst inoculation for the specified durations of treatment, and the parasite loads were assayed at the end of each treatment. (D) Drug withdrawal assay. Occidiofungin was added at 3 hpi after the removal of uninvaded parasites. After treatment of infected cells for specified durations, compound was removed, and the parasites were allowed to grow and recover in the absence of compound for up to 44 hpi before the parasite loads were determined. PRM at 140 μM was used as a positive control. (E) Effect of pretreating sporozoites with occidiofungin on parasite infection. Free sporozoites were treated with occidiofungin at 200 and 400 nM for 40 min in culture medium and then allowed to invade host cells for 2 and 6 h. The parasite loads were determined at 2 and 6 hpi time points. (F) Effect of pretreating host cells with occidiofungin on parasite infection. HCT-8 cells were treated with occidiofungin at 200 nM for 24 h. After removal of compound, host cells were incubated with parasite oocysts and assayed at 3, 9, and 18 hpi. Parasite loads were determined by qRT-PCR. Statistical significance was evaluated by two-way ANOVA and Sidak’s multiple-comparison test. Error bars represent standard errors of the means. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Occidiofungin is poorly absorbed by the digestive system (4) but highly efficacious in vivo against vulvovaginal Candida albicans infection in mice when administered intravaginally (3). The mode of action of occidiofungin is distinct from those of the four common classes of antifungal agents (5). Occidiofungin disrupts fungal membrane morphology and induces apoptosis (1, 6). A recent study identified actin filaments as the primary cellular target of occidiofungin in fungi (3).
The broad-spectrum antifungal activity of occidiofungin and its retention in the gastrointestinal tract (GIT) prompted us to explore its potential therapeutic value against the enteric parasite Cryptosporidium parvum, a globally important zoonotic protozoan that has a unique epicellular parasitic lifestyle (7–9).
In this study, HCT-8 and Caco-2 cells were used to grow C. parvum in vitro in 96-well plates (10–12). C. parvum oocysts (subtype IIaA17G2R1) <3 months old were used in experiments. Occidiofungin was purified from B. contaminans (3). In vitro drug efficacy was evaluated by a 44-h infection assay coupled with quantitative reverse transcription-PCR (qRT-PCR) to determine parasite loads (10, 11). Paromomycin (140 μM) was used as a positive control. In vitro cytotoxicity for host cells was evaluated by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethyoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay (13). The oral stability of occidiofungin was evaluated in BALB/c mice by oral gavage at a dosage of 50 mg/kg of body weight/day for 3 days (3 males and 3 females), together with vehicle control and positive control using fluconazole (50 mg/kg/day). Feces were collected 10 h postadministration on the third day for determining occidiofungin concentrations by chromatography-mass spectrometry. All in vitro assays included ≥3 biological replicates and were performed ≥3 times independently. Data were assessed by two-way analysis of variance (ANOVA) and Sidak’s multiple-comparison test. A more detailed description of materials and methods is provided in Supplemental Text S1.
In a 44-h infection assay, occidiofungin displayed low-nanomolar anticryptosporidial activity (50% effective concentration [EC50] = 120 and 138 nM for parasites cultured in HCT-8 and Caco-2 cells, respectively) (Fig. 1B), which was more potent than its antifungal efficacy (MIC50 = 0.25 to 16 μg/ml; equivalent to ∼230 to 14,500 nM) (1, 2). The anti-Cryptosporidium potency was comparable to that of the most efficacious known lead compounds, which typically had EC50s in the low-nanomolar to single-digit-micromolar range (e.g., phosphatidylinositol 4-kinase [PI4K], histone deacetylase [HDAC], and long-chain fatty acyl coenzyme A [ACS] inhibitors at 0.1 to 0.2 μM; calcium-dependent protein kinase [CDPK], lysyl-tRNA synthetase [KRS], and methionyl-tRNA synthetase [MetRS] inhibitors at 1.3 to 6.0 μM) (13–19). Within the effective concentrations, occidiofungin was nontoxic to host cells, and it started to affect host cells only at micromolar levels (50% cytotoxic concentration [TC50] = 0.99 μM on HCT-8 cells and 2.5 to 5.0 μM on Caco-2 cells) (Fig. 1B), giving safety intervals (calculated as TC50/EC50) of 8.3 to 18.
Occidiofungin acted on C. parvum in various merogonic developmental stages (Fig. 1C to E). When present during excystation and invasion, occidiofungin (200 nM) reduced the parasite loads by 45.1% in the 0- to 3-h-postinfection (hpi) treatment group, and the anti-Cryptosporidium activity increased over the treatment time (Fig. 1C). Drug withdrawal experiments indicated that occidiofungin acted on Cryptosporidium irreversibly, as the parasite was unable to recover its growth after 9-h or longer treatments followed by removal of compound and continuous parasite growth for up to 44 hpi (Fig. 1D). In positive controls, a full course of treatment with paromomycin (140 μM) inhibited the parasite growth by 62.2% (Fig. 1D), which was comparable to previous reported values (10).
The strong inhibitory effect of occidiofungin in 0- to 3- and 0- to 6-hpi groups shown in Fig. 1C (versus no effect in the 3- to 6-hpi group [Fig. 1D]) suggested its action on sporozoites, a motile infectious stage of C. parvum. To test this hypothesis, we treated sporozoites with occidiofungin for 40 min at 37°C and removed the compound before examining their morphology and evaluating their invasion of host cells. We observed 79.2% (200 nM) and 84.1% (400 nM) reductions of parasite loads when they were assayed at 2 hpi and further reductions (91.2% and 94.1%) at 6 hpi (Fig. 1E).
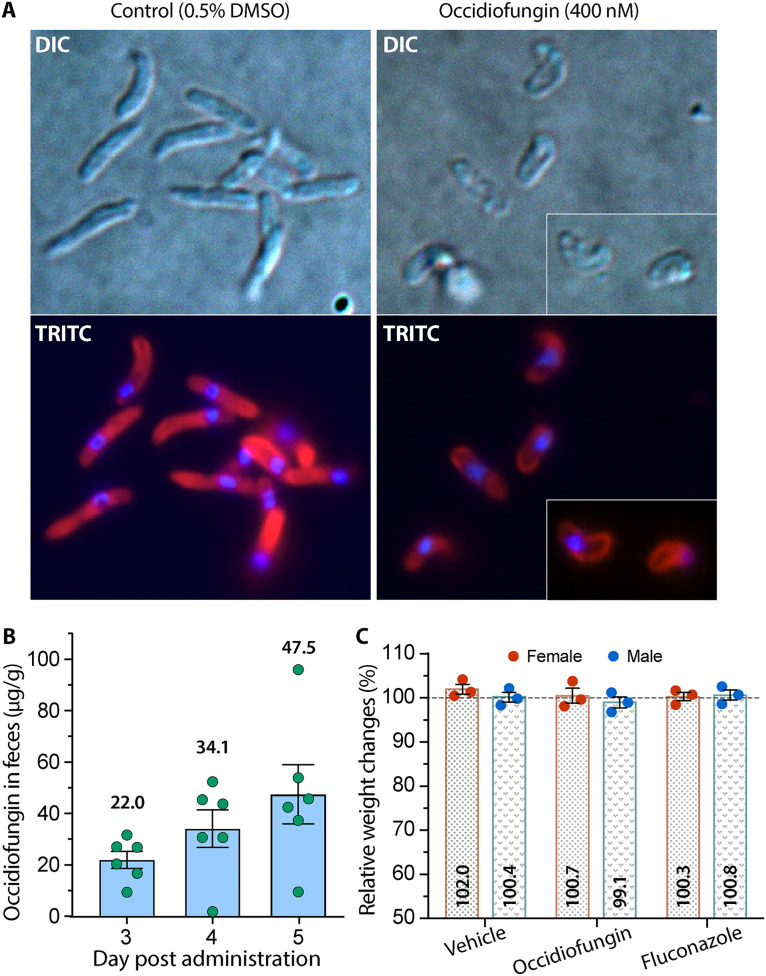
Notably, occidiofungin also significantly deformed the sporozoite morphology (Fig. 2A). In the control group treated with 0.5% dimethyl sulfoxide (DMSO), sporozoites were rod shaped, whereas occidiofungin-treated sporozoites were significantly shortened and much more transparent, indicating the loss of cytosolic contents by disruption of cytoplasm membrane integrity. However, it remains to be determined whether occidiofungin might also act on the parasite actin, as observed in fungi (3).
FIG 2.
Action of occidiofungin on Cryptosporidium parvum sporozoites and oral stability of occidiofungin in mice. (A) Deformation of the morphology of sporozoites after treatment with occidiofungin at 400 nM for 40 min. All treatment groups contained 0.5% DMSO, including controls. DIC, differential interference contrast microscopy; TRITC, tetramethylrhodamine isothiocyanate (sporozoites were labeled by a rabbit polyclonal antibody against C. parvum total proteins and TRITC-conjugated secondary antibody). (B) Concentrations of occidiofungin in BALB/c mouse feces 10 h after a single oral daily dose (50 mg/kg) for 3 days. (C) Mouse weight gain or loss 10 h after the third dose of occidiofungin (versus fluconazole at 50 mg/kg/day).
The enteric Cryptosporidium parasites are distinguished by their epicellular development in the GIT and the presence of extracellular stages. Therefore, the previously observed poor absorption of occidiofungin by the GIT could be advantageous in maximizing the activity of occidiofungin on the parasites (4). Indeed, the retention of occidiofungin in the mouse GIT was confirmed by an oral stability assay, in which feces collected at 10 h after oral administration of 50 mg/kg/day contained 22.0, 34.1, and 47.5 μg/g of occidiofungin following three consecutive daily doses (Fig. 2B). Pending further study on the relationship between drug absorption and retainment in the GIT, it was encouraging that the concentrations of occidiofungin in feces were theoretically ∼15 to 33 times higher than the anticryptosporidial EC50. Additionally, occidiofungin did not produce any signs of toxicity or significant weight loss in mice (Fig. 2C).
Cryptosporidiosis is a globally important zoonotic disease, and fully effective treatment is currently unavailable. There is an urgent need to develop new anticryptosporidial therapeutics. Occidiofungin has been pursued as a lead for developing antifungal therapeutics (e.g., candidiasis). The present study not only expands the activity spectrum of occidiofungin to include antiparasitic activity but also points out a potential new direction for developing anticryptosporidial therapeutics.
C. parvum is an epicellular enteric parasite separated from the host cell cytosol by an electron-dense band (7–9). It has been observed that some compounds in the circulatory system might not effectively pass the electron-dense band to act on the parasite, and accumulation in the GIT is critical to anticryptosporidial activity for some compounds (e.g., paromomycin and bumped-kinase inhibitors) (20). Therefore, poor absorption is advantageous in that it allows occidiofungin to act more effectively on enteric Cryptosporidium or other parasites (4). It would also reduce potential organ-specific toxicities. Further, occidiofungin concentrations recovered from feces were 15 to 33 times higher than anticryptosporidial EC50s, suggesting that oral administration of occidiofungin has potential for successfully treating C. parvum infections.
In summary, we report occidiofungin as a novel antiparasitic agent and a new lead for developing anticryptosporidial therapeutics. The anticryptosporidial activity of occidiofungin, together with its unique pharmacokinetic feature in the GIT, suggests that it could also be investigated for potential activity against other enteric parasites.
Supplementary Material
ACKNOWLEDGMENTS
The research was partly funded by National Institutes of Health grants R41 AI131792 and R42 AI131792 to L.S.
L.S. is a board member of Sano Chemicals, Inc. Sano Chemicals is actively developing occidiofungin for the treatment of serious fungal infections.
G.Z., L.S., F.G., and H.Z. conceived the study design. J.M., F.G., H.Z., M.J., A.R., and R.O. conducted the experiments. J.M., G.Z., H.Z., and L.S. wrote the manuscript. All authors read and approved the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lu SE, Novak J, Austin FW, Gu G, Ellis D, Kirk M, Wilson-Stanford S, Tonelli M, Smith L. 2009. Occidiofungin, a unique antifungal glycopeptide produced by a strain of Burkholderia contaminans. Biochemistry 48:8312–8321. doi: 10.1021/bi900814c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis D, Gosai J, Emrick C, Heintz R, Romans L, Gordon D, Lu SE, Austin F, Smith L. 2012. Occidiofungin’s chemical stability and in vitro potency against Candida species. Antimicrob Agents Chemother 56:765–769. doi: 10.1128/AAC.05231-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravichandran A, Geng M, Hull KG, Li J, Romo D, Lu SE, Albee A, Nutter C, Gordon DM, Ghannoum MA, Lockless SW, Smith L. 2018. A novel actin binding drug with in vivo efficacy. Antimicrob Agents Chemother 63:e01585-18. doi: 10.1128/AAC.01585-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hing SH. 2017. Mutacin 1140 and occidiofungin: natural products isolated from bacterial sources and their potential applications as therapeutics. Ph.D. thesis Texas A&M University, College Station, Texas, USA. [Google Scholar]
- 5.Tan W, Cooley J, Austin F, Lu SE, Pruett SB, Smith L. 2012. Nonclinical toxicological evaluation of occidiofungin, a unique glycolipopeptide antifungal. Int J Toxicol 31:326–336. doi: 10.1177/1091581812445185. [DOI] [PubMed] [Google Scholar]
- 6.Emrick D, Ravichandran A, Gosai J, Lu S, Gordon DM, Smith L. 2013. The antifungal occidiofungin triggers an apoptotic mechanism of cell death in yeast. J Nat Prod 76:829–838. doi: 10.1021/np300678e. [DOI] [PubMed] [Google Scholar]
- 7.Valigurová A, Jirků M, Koudela B, Gelnar M, Modrý D, Slapeta J. 2008. Cryptosporidia: epicellular parasites embraced by the host cell membrane. Int J Parasitol 38:913–922. doi: 10.1016/j.ijpara.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Lendner M, Daugschies A. 2014. Cryptosporidium infections: molecular advances. Parasitology 141:1511–1532. doi: 10.1017/S0031182014000237. [DOI] [PubMed] [Google Scholar]
- 9.Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15:85–94. doi: 10.1016/S1473-3099(14)70772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Zhu G. 2015. Quantitative RT-PCR assay for high-throughput screening (HTS) of drugs against the growth of Cryptosporidium parvum in vitro. Front Microbiol 6:991. doi: 10.3389/fmicb.2015.00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Zhu G. 2020. High-throughput screening of drugs against the growth of Cryptosporidium parvum in vitro by qRT-PCR. Methods Mol Biol 2052:319–334. doi: 10.1007/978-1-4939-9748-0_18. [DOI] [PubMed] [Google Scholar]
- 12.Jin Z, Ma J, Zhu G, Zhang H. 2019. Discovery of novel anti-cryptosporidial activities from natural products by in vitro high-throughput phenotypic screening. Front Microbiol 10:1999. doi: 10.3389/fmicb.2019.01999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo F, Zhang H, McNair NN, Mead JR, Zhu G. 2018. The existing drug vorinostat as a new lead against cryptosporidiosis by targeting the parasite histone deacetylases. J Infect Dis 217:1110–1117. doi: 10.1093/infdis/jix689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang W, Hulverson MA, Choi R, Arnold SLM, Zhang Z, McCloskey MC, Whitman GR, Hackman RC, Rivas KL, Barrett LK, Ojo KK, Van Voorhis WC, Fan E. 2019. Development of 5-aminopyrazole-4-carboxamide-based bumped-kinase onhibitors for cryptosporidiosis therapy. J Med Chem 62:3135–3146. doi: 10.1021/acs.jmedchem.9b00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckner FS, Ranade RM, Gillespie JR, Shibata S, Hulverson MA, Zhang Z, Huang W, Choi R, Verlinde C, Hol WGJ, Ochida A, Akao Y, Choy RKM, Van Voorhis WC, Arnold SLM, Jumani RS, Huston CD, Fan E. 2019. Optimization of methionyl tRNA-synthetase inhibitors for treatment of Cryptosporidium infection. Antimicrob Agents Chemother 63:e02061-18. doi: 10.1128/AAC.02061-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baragaña B, Forte B, Choi R, Nakazawa Hewitt S, Bueren-Calabuig JA, Pisco JP, Peet C, Dranow DM, Robinson DA, Jansen C, Norcross NR, Vinayak S, Anderson M, Brooks CF, Cooper CA, Damerow S, Delves M, Dowers K, Duffy J, Edwards TE, Hallyburton I, Horst BG, Hulverson MA, Ferguson L, Jiménez-Díaz MB, Jumani RS, Lorimer DD, Love MS, Maher S, Matthews H, McNamara CW, Miller P, O'Neill S, Ojo KK, Osuna-Cabello M, Pinto E, Post J, Riley J, Rottmann M, Sanz LM, Scullion P, Sharma A, Shepherd SM, Shishikura Y, Simeons FRC, Stebbins EE, Stojanovski L, Straschil U, Tamaki FK, Tamjar J, Torrie LS, Vantaux A, Witkowski B, Wittlin S, Yogavel M, Zuccotto F, Angulo-Barturen I, Sinden R, Baum J, Gamo F-J, Mäser P, Kyle DE, Winzeler EA, Myler PJ, Wyatt PG, Floyd D, Matthews D, Sharma A, Striepen B, Huston CD, Gray DW, Fairlamb AH, Pisliakov AV, Walpole C, Read KD, Van Voorhis WC, Gilbert IH. 2019. Lysyl-tRNA synthetase as a drug target in malaria and cryptosporidiosis. Proc Natl Acad Sci U S A 116:7015–7020. doi: 10.1073/pnas.1814685116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manjunatha UH, Vinayak S, Zambriski JA, Chao AT, Sy T, Noble CG, Bonamy GMC, Kondreddi RR, Zou B, Gedeck P, Brooks CF, Herbert GT, Sateriale A, Tandel J, Noh S, Lakshminarayana SB, Lim SH, Goodman LB, Bodenreider C, Feng G, Zhang L, Blasco F, Wagner J, Leong FJ, Striepen B, Diagana TT. 2017. A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature 546:376–380. doi: 10.1038/nature22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulverson MA, Vinayak S, Choi R, Schaefer DA, Castellanos-Gonzalez A, Vidadala RSR, Brooks CF, Herbert GT, Betzer DP, Whitman GR, Sparks HN, Arnold SLM, Rivas KL, Barrett LK, White AC Jr, Maly DJ, Riggs MW, Striepen B, Van Voorhis WC, Ojo KK. 2017. Bumped-kinase inhibitors for cryptosporidiosis therapy. J Infect Dis 215:1275–1284. doi: 10.1093/infdis/jix120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo F, Zhang H, Fritzler JM, Rider SD Jr, Xiang L, McNair NN, Mead JR, Zhu G. 2014. Amelioration of Cryptosporidium parvum infection in vitro and in vivo by targeting parasite fatty acyl-coenzyme A synthetases. J Infect Dis 209:1279–1287. doi: 10.1093/infdis/jit645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold SLM, Choi R, Hulverson MA, Schaefer DA, Vinayak S, Vidadala RSR, McCloskey MC, Whitman GR, Huang W, Barrett LK, Ojo KK, Fan E, Maly DJ, Riggs MW, Striepen B, Van Voorhis WC. 2017. Necessity of bumped kinase inhibitor gastrointestinal exposure in treating Cryptosporidium infection. J Infect Dis 216:55–63. doi: 10.1093/infdis/jix247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.