The continued evolution of bacterial resistance to the β-lactam class of antibiotics has necessitated countermeasures to ensure continued effectiveness in the treatment of infections caused by bacterial pathogens. One relatively successful approach has been the development of new β-lactam analogs with advantages over prior compounds in this class. The carbapenems are an example of such β-lactam analogs possessing improved stability against β-lactamase enzymes and, therefore, a wider spectrum of activity.
KEYWORDS: UTI, carbapenem, oral antibiotic, tebipenem
ABSTRACT
The continued evolution of bacterial resistance to the β-lactam class of antibiotics has necessitated countermeasures to ensure continued effectiveness in the treatment of infections caused by bacterial pathogens. One relatively successful approach has been the development of new β-lactam analogs with advantages over prior compounds in this class. The carbapenems are an example of such β-lactam analogs possessing improved stability against β-lactamase enzymes and, therefore, a wider spectrum of activity. However, all carbapenems currently marketed for adult patients are intravenous agents, and there is an unmet need for an oral agent to treat patients that otherwise do not require hospitalization. Tebipenem pivoxil hydrobromide (tebipenem-PI-HBr or SPR994) is an orally available prodrug of tebipenem, a carbapenem with activity versus multidrug-resistant (MDR) Gram-negative pathogens, including quinolone-resistant and extended-spectrum-β-lactamase-producing Enterobacterales. Tebipenem-PI-HBr is currently in development for the treatment of complicated urinary tract infections (cUTI). Microbiological data are presented here that demonstrate equivalency of tebipenem with intravenous carbapenems such as meropenem and support its use in infections in which the potency and spectrum of a carbapenem are desired. The results from standard in vitro microbiology assays as well as efficacy in several in vivo mouse infection models suggest that tebipenem-PI-HBr could be a valuable oral agent available to physicians for the treatment of infections, particularly those caused by antibiotic-resistant Gram-negative pathogens.
INTRODUCTION
The β-lactam antibiotics have remained an important treatment option for bacterial infections for many decades, although the continued proliferation of β-lactam-inactivating enzymes, the β-lactamases, over the years has adversely affected the clinical utility of this drug class (1, 2). Development of new and improved β-lactam antibiotics along with several β-lactamase inhibitor compounds has allowed us to narrowly keep ahead of bacterial resistance to the class. At present, the carbapenems represent the most potent and broad-spectrum β-lactams and are minimally affected by most β-lactamase enzymes (3). However, all currently marketed carbapenems for adult patients, including meropenem and ertapenem, are intravenously (i.v.) administered.
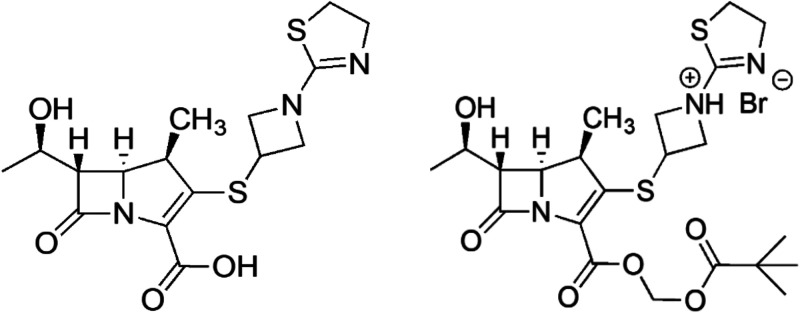
Spero Therapeutics is currently developing tebipenem pivoxil hydrobromide (tebipenem-PI-HBr [Fig. 1]) as an orally bioavailable carbapenem prodrug for complicated urinary tract infections (cUTIs) in adult patients. Tebipenem (SPR859 [Fig. 1]), the active moiety, has broad-spectrum activity with excellent potency against the Enterobacterales, including extended-spectrum-β-lactamase (ESBL)- and AmpC β-lactamase-producing organisms as well as trimethoprim-sulfamethoxazole (TMP-SMX)- and fluoroquinolone (FQ)-resistant organisms (4–6). Effective oral agents are particularly needed for cUTIs, in which Escherichia coli is the dominant causative agent and is becoming increasingly resistant to current antibiotics (7).
FIG 1.
Chemical structures of tebipenem and tebipenem-pivoxil.
Previously published studies have focused on the activity of tebipenem against pathogens responsible for respiratory tract infections (4). In the studies described here, we evaluated the in vitro microbiological activity of tebipenem in several standard assays along with the intravenous carbapenem, meropenem, and other relevant comparators against representative, contemporary UTI pathogens. We also assessed the in vivo efficacy of tebipenem-PI in infections using a murine ascending E. coli UTI model as well as mouse thigh and lung infection models. The data indicate good antibacterial potency and support the equivalency of tebipenem microbiological activity to those of intravenous carbapenems. Tebipenem-PI-HBr should add a valuable oral option for the treatment of Gram-negative bacterial infections.
RESULTS
Antimicrobial activity of tebipenem against bacterial pathogens.
Tebipenem has potent activity against current Enterobacterales clinical isolates, and production of ESBL and/or pAmpC enzymes did not adversely affect in vitro activity against E. coli, Klebsiella pneumoniae, or Proteus spp., with MIC50s of ≤0.06 μg/ml (Table 1) (6). As expected, tebipenem was less active against Enterobacteriaceae isolates displaying a carbapenem-resistant phenotype (MICs > 8 μg/ml). Tebipenem was also active against a number of Gram-positive pathogens, including Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Enterococcus faecium, Streptococcus pneumoniae, and Streptococcus pyogenes. Tebipenem possesses reduced antibacterial activity against methicillin-resistant staphylococci (MRSA) and E. faecalis but was active against most streptococci, including isolates that were penicillin nonsusceptible (Table 1) (4, 5).
TABLE 1.
MICs of tebipenem against selected bacterial pathogensa
| Organism(s) | Phenotype | n | Tebipenem MIC (μg/ml) |
Ertapenem MIC (μg/ml) |
Meropenem MIC (μg/ml) |
|||
|---|---|---|---|---|---|---|---|---|
| Range | 50%/90% | Range | 50%/90% | Range | 50%/90% | |||
| Gram negative | ||||||||
| E. coli | Non-ESBL producing | 79 | ≤0.015 to 0.12 | ≤0.015/0.03 | ≤0.015 to 0.12 | ≤0.015/≤0.015 | NA | NA |
| ESBL producing | 21 | ≤0.015 to 0.12 | 0.03/0.06 | ≤0.015 to 0.5 | 0.03/0.5 | NA | NA | |
| AmpC producing | 6 | 0.03 to 0.12 | NA | 0.03 to 1 | NA | 0.03–0.06 | NA | |
| Klebsiella pneumoniae | Non-ESBL producing | 158 | ≤0.015 to 0.12 | 0.03/0.06 | ≤0.015 to 0.03 | ≤0.015/≤0.015 | NA | NA |
| ESBL producing | 50 | 0.03 to >32 | 0.03/>32 | ≤0.015 to >32 | 0.12/>32 | NA | NA | |
| ESBL producing, excluding CRE | 41 | 0.03 to 4 | 0.03/0.25 | ≤0.015 to >32 | 0.06/2 | NA | NA | |
| AmpC producing | 3 | 0.03 to 0.25 | NA | ≤0.015 to 0.25 | NA | 0.03 to 0.06 | NA | |
| CRE | 9 | 1 to >32 | NA | 8 to >32 | NA | NA | NA | |
| Proteus spp. | Non-ESBL producing | 94 | 0.03 to 0.5 | 0.06/0.12 | 0.03 to 0.5 | 0.12/0.25 | NA | NA |
| ESBL producing | 18 | 0.12 to 4 | 0.5/2 | ≤0.12 to 4 | 0.12/0.5 | 0.03 to 4 | 0.12/0.5 | |
| AmpC producing | 13 | 0.12 to 2 | 0.5/1 | ≤0.015 to 0.25 | 0.03/0.12 | 0.06 to 0.5 | 0.12/0.5 | |
| Pseudomonas aeruginosa | Wild type | 11 | 2 to 16 | 4/8 | 1 to 16 | 4/16 | 0.06 to 4 | 0.25/1 |
| Non-wild type | 45 | 4 to >32 | >32/>32 | 8 to >32 | >32/>32 | 0.5 to >32 | 16/>32 | |
| Gram positive | ||||||||
| Staphylococcus aureus | MSSA | 10 | ≤0.015 to 0.06 | 0.03/0.03 | 0.12 to 0.25 | 0.25/0.25 | 0.06 to 0.25 | 0.12/0.12 |
| MRSA | 10 | 0.5 to 32 | 2/16 | 2 to >32 | 4/>32 | 2 to >32 | 4/32 | |
| Staphylococcus epidermidis | MSSE | 10 | 0.004 to 0.03 | 0.03 | 0.015 to 0.25 | 0.25/0.25 | 0.015 to 0.12 | 0.06/0.12 |
| MRSE | 10 | 0.06 to 8 | 4/4 | 1 to >32 | 4/>32 | 0.25 to 32 | 4/16 | |
| Enterococcus faecalis | 10 | 1 to 2 | 2/2 | 8 to 16 | 8/16 | 4 to 8 | 8/8 | |
| Enterococcus faecium | 10 | 2 to >32 | >32/>32 | 16 to >32 | >32/>32 | 16 to >32 | >32/>32 | |
| Streptococcus pneumoniae | PSSP | 10 | 0.002 to 0.06 | 0.004/0.06 | 0.015 to 0.25 | 0.015/0.25 | 0.008 to 0.25 | 0.015/0.12 |
| PNSSP | 10 | 0.12 to 0.5 | 0.25/0.5 | 1 to 2 | 2/2 | 0.5 to 1 | 1/1 | |
| Streptococcus pyogenes | 20 | 0.002 to 0.004 | 0.004/0.004 | 0.004 to 0.015 | 0.015/0.015 | 0.002 to 0.008 | 0.008/0.008 | |
ESBL, extended-spectrum β-lactamase; CRE, carbapenem-resistant Enterobacterales; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; MSSE, methicillin-susceptible S. epidermidis; MRSE, methicillin-resistant S. epidermidis; PSSP, penicillin-susceptible S. pneumoniae; PNSSP, penicillin-nonsusceptible S. pneumoniae as defined by CLSI document M100 (29th edition) criteria for parenteral penicillin (nonmeningitis) MICs of ≥4 μg/ml (28); NA, not available.
Antibacterial activity against anaerobic bacteria.
Carbapenems are potent antibacterial agents with broad-spectrum activity. Clinical use of such antibiotics has been known to impact the anaerobic bacteria in the gut, and this dysbiosis can potentially lead to Clostridioides difficile infections (8). This can be an especially important issue for an orally administered broad-spectrum antibiotic such as tebipenem; therefore, we examined the in vitro activity against selected anaerobic bacteria. It was active against a broad collection of anaerobic bacteria, and broth MIC90 values for tebipenem against a panel of anaerobic isolates were similar to those for meropenem, ranging from ≤0.015 to 2 μg/ml (Table 2). Against C. difficile, agar MIC values were 2 μg/ml for each of 10 isolates, versus 0.25 to 0.5 μg/ml for metronidazole, while values were similar (2 to 4 μg/ml) to recently reported data for meropenem (9).
TABLE 2.
MICs of tebipenem and comparators against anaerobic clinical isolates
| Organism(s) | n | MIC50/90 (μg/ml)a |
||
|---|---|---|---|---|
| Tebipenem | Meropenem | Metronidazole | ||
| Bacteroides sp. | 25 | 0.5/2 | 1/4 | 1/1 |
| Fusobacterium spp. | 10 | ≤0.015 | ≤0.015/0.06 | ≤0.06/0.25 |
| Porphyromonas spp. | 10 | 0.03/0.06 | 0.06 | 0.25/1 |
| Prevotella spp. | 30 | 0.125/0.25 | 0.25 | 0.5/2 |
| Clostridioides difficile | 10 | 2 | 2/4 | 0.25/0.5 |
| Gram-positive rodsc | 12 | 0.03/0.25 | ≤0.03/0.25 | 16/>16 |
| Gram-positive spore-forming rodsd | 48 | 0.5/2 | 0.25/2b | 0.5/2 |
| Gram-positive non-spore-forming rodse | 26 | 0.06/1 | 0.25/8 | 2/>16 |
| Gram-positive coccif | 24 | 0.06/0.25 | 0.125/0.25 | 0.25/1 |
Agar dilution method according to procedures described in CLSI document M11-A8 (27).
n = 38.
Gram-positive rods: Actinomyces europaeus, Actinomyces israelii, Actinomyces neuii subsp. neuii, Actinomyces odontolyticus, Actinomyces turicensis, Anaerostipes caccae, Bifidobacterium adolescentis, Bifidobacterium catenulatum, and Bifidobacterium pseudocatenulatum.
Gram-positive spore-forming rods: Clostridioides aldenense, Clostridium bolteae, Clostridium butyricum, Clostridium celerecrescens, Clostridium citroniae, C. clostridioforme, Hungatella hathewayi, Clostridium novyi A, C. perfringens, Clostridium ramosum, C. difficile, Clostridium scindens, Clostridium sporogenes, Clostridium symbiosum, and Robinsoniella peoriensis.
Gram-positive non-spore-forming rods: Collinsella aerofaciens, Collinsella sp., Eggerthella lenta, Eubacterium limosum, Catabacter hongkongensis, Faecalitalea cylindroides, Lactobacillus casei, Lactobacillus fermentum, L. rhamnosus GG, L. rhamnosus, and Lactobacillus salivarius.
Anaerobic Gram-positive cocci: Finegoldia magna, Parvimonas micra, Parvimonas sp., Peptostreptococcus anaerobius, Ruminococcus gnavus, R. gnavus-like, Ruminococcus torques, Ruminococcus productus, and Blautia sp.
Time-kill assays of tebipenem and comparator antibiotics.
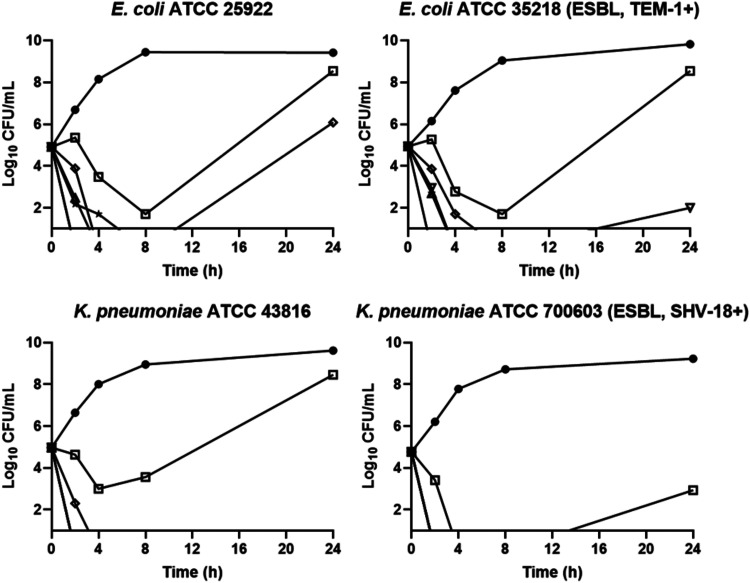
Broth time-kill studies were performed on both non-ESBL- and ESBL-producing isolates of E. coli and K. pneumoniae (Fig. 2). The non-ESBL-producing strains were E. coli ATCC 25922 and K. pneumoniae ATCC 43816. The ESBL-producing strains were E. coli ATCC 35218 and K. pneumoniae ATCC 700603. Culture viability was monitored for 24 h, and bactericidal activity was defined as a 3-log10 reduction in CFU per milliliter of the initial inoculum. By this definition, tebipenem was found to be bactericidal at 4× to 8× MIC within 4 h against both the E. coli and K. pneumoniae strains, comparable to meropenem at 4× MIC. Regrowth in tebipenem samples was observed at 1× to 2× and 8× MIC; however, in all cases colonies isolated from these samples at 24 h maintained parent-like susceptibility to tebipenem, suggesting that they were not mutants.
FIG 2.
Time-kill curves for tebipenem, meropenem, and levofloxacin versus E. coli and K. pneumoniae. Compounds were added to cultures at time zero, and samples were processed as described in Materials and Methods. Circles, growth control; squares, tebipenem at 1× MIC; open diamonds, tebipenem at 2× MIC; triangles, tebipenem at 4× MIC; inverted triangles, tebipenem at 8× MIC; stars, meropenem at 4× MIC; solid diamonds, levofloxacin at 4× MIC.
PAE assessment of tebipenem.
The in vitro postantibiotic effect (PAE) of tebipenem and comparators was established against E. coli ATCC 25922 and K. pneumoniae ATCC 43816 (10). Log-phase cultures were treated with antimicrobial agents at 4× and/or 8× MIC for 1 h and diluted into fresh cation-adjusted Mueller-Hinton broth (CAMHB), and viability was monitored for 6 h by plating for CFU per milliliter. PAE was calculated as T – C, where T and C are the times required to increase 1 log10 CFU following 1:1,000 dilution for the bacteria treated with and not treated with the agents, respectively. At 4× to 8× MIC, tebipenem produced negligible PAEs against E. coli ATCC 25922 and K. pneumoniae ATCC 43816, with values of ≤0.4 h, comparable to those of meropenem. As expected, levofloxacin at 4× MIC showed PAEs of 0.8 to 2 h against E. coli ATCC 25922 and 2.9 to 3.1 h against K. pneumoniae ATCC 43816 (11).
Impact of varied growth conditions on the in vitro activity of tebipenem.
Prior to widespread susceptibility testing of tebipenem, it was important to understand the impact of variations to the standard CLSI testing methods on in vitro activity. Standard susceptibility test parameters that can affect MIC test results when varied include growth medium pH and cation concentration, inoculum size, and inoculation duration and atmosphere. In addition, it is important to understand the effect of bodily fluids such as human urine and serum on in vitro activity of new agents. Possible effects of several growth conditions on the antibacterial properties of tebipenem and meropenem were evaluated.
Test organisms included non-ESBL- and ESBL-producing isolates of E. coli, K. pneumoniae, and Proteus mirabilis. Under standard conditions, tebipenem and meropenem had similar activities and the activity was not affected by ESBL phenotype. The antibacterial activity of both tebipenem and meropenem decreased with increasing inoculum size and was eliminated for both drugs against all strains when the starting inoculum reached 5 × 107 CFU/ml (Table 3) and for P. mirabilis isolates at acidic pH (Table 4). There were no significant changes in activity when tested in 100% pooled human urine with the exception of P. mirabilis, for which the activity of both meropenem and tebipenem decreased 4-fold (Table 4). Tebipenem displayed 8- to 32-fold less activity when tested in 50% mouse serum; however, there were no significant changes in activity when tested in 10% mouse serum or 10% or 50% human serum (Table 5). There were minimal or no changes in the activity of tebipenem or meropenem in the presence of varied divalent cations, with prolonged incubation time, or when incubated in CO2 (Spero, unpublished data). The clinical significance, if any, of decreased activity as observed with mouse serum and human urine (P. mirabilis only) remains to be determined, although protein binding was much lower in human serum (36.1 to 45.2%) than in mouse serum (97.7 to 98.4%) (12; Spero, unpublished data). The impact increased inoculum size here highlights the need for adherence to CLSI guidelines during the broth microdilution testing of both tebipenem and meropenem.
TABLE 3.
Impact of inoculum on MICs of tebipenem and meropenema
| Organism | ESBLb | Compound | MIC under standard conditions | Impact of FINAL Inoculum (CFU/ml) (fold change) |
||
|---|---|---|---|---|---|---|
| 5.0 × 104 | 5.0 × 106 | 5.0 × 107 | ||||
| E. coli ATCC 25922 | N | Tebipenem | 0.015 | 0.015 (0) | 0.06 (4) | >32 |
| Meropenem | 0.015 | 0.03 (2) | 0.06 (4) | >32 | ||
| E. coli ATCC 35218 | Y | Tebipenem | 0.015 | 0.015 (0) | 0.03 (2) | >32 |
| Meropenem | 0.015 | 0.015 (0) | 0.03 (2) | >32 | ||
| K. pneumoniae ATCC 43816 | N | Tebipenem | 0.03 | 0.03 (0) | 0.12 (4) | >32 |
| Meropenem | 0.03 | 0.03 (0) | 0.06 (2) | >32 | ||
| K. pneumoniae ATCC 700603 | Y | Tebipenem | 0.06 | 0.06 (0) | 0.25 (4) | >32 |
| Meropenem | 0.03 | 0.03 (0) | 0.25 (8) | >32 | ||
| P. mirabilis ATCC 43071 | N | Tebipenem | 0.12 | 0.03 (−4) | 8 (64) | >32 |
| Meropenem | 0.06 | 0.03 (−2) | 2 (32) | >32 | ||
| P. mirabilis MMX 6343 | Y | Tebipenem | 0.5 | 0.12 (−8) | 8 (16) | >32 |
| Meropenem | 0.12 | 0.03 (−8) | 1 (8) | >32 | ||
MICs are in micrograms per milliliter. Bold indicates >4× differences from CLSI standard inoculum (∼5 × 105 CFU/ml).
N, no; Y, yes.
TABLE 4.
Effect of varying the pH on antibacterial activity of tebipenem in media and pooled human urine
| Testing condition | Compound | Tebipenem MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|---|
|
E. coli ATCC 25922, non-ESBL |
E. coli ATCC 35218, ESBL |
K. pneumoniae ATCC 43816, non-ESBL |
K. pneumoniae ATCC 700603, ESBL |
P. mirabilis ATCC 43071, non-ESBL |
P. mirabilis MMX 6343, ESBL |
||
| pH 5 | Tebipenem | 0.06 | 0.03 | 0.03 | 0.06 | 1 | 1 |
| Meropenem | 0.12 | 0.06 | 0.06 | 0.06 | 0.5 | 1 | |
| pH 6 | Tebipenem | 0.03 | 0.015 | 0.03 | 0.06 | 1 | 1 |
| Meropenem | 0.06 | 0.03 | 0.06 | 0.06 | 0.5 | 1 | |
| pH 7 | Tebipenem | 0.015 | 0.015 | 0.03 | 0.12 | 0.12 | 0.5 |
| Meropenem | 0.015–0.03 | 0.015–0.03 | 0.03–0.06 | 0.03–0.06 | 0.06 | 0.12–0.25 | |
| pH 8 | Tebipenem | 0.03 | 0.015 | 0.03 | 0.12 | 0.12 | 0.5 |
| Meropenem | 0.06 | 0.06 | 0.12 | 0.06 | 0.06 | 0.12 | |
| Human urinea | Tebipenem | 0.015 | 0.015 | 0.03 | 0.06 | 0.5 | 2 |
| Meropenem | 0.03 | 0.015 | 0.03 | 0.06 | 0.25 | 0.5 | |
Pooled human urine at pH 6.9.
TABLE 5.
Impact of human, mouse serum on tebipenem, meropenem MIC
| Organism | ESBL | Compound | CAMHB | MIC (μg/ml) (fold vs CAMHB)a |
|||
|---|---|---|---|---|---|---|---|
| 50% human serum | 10% human serum | 50% mouse serum | 10% mouse serum | ||||
| E. coli ATCC 25922 | N | Tebipenem | 0.15 | 0.03 (2) | 0.03 (2) | 0.5 (32) | 0.06 (4) |
| Meropenem | 0.015 | 0.03 (2) | 0.03 (2) | 0.03 (2) | 0.03 (2) | ||
| E. coli ATCC 35218 | Y | Tebipenem | 0.15 | 0.03 (2) | 0.03 (2) | 0.5 (32) | 0.03 (2) |
| Meropenem | 0.015 | 0.03 (2) | 0.03 (2) | 0.03 (2) | 0.015 (0) | ||
| K. pneumoniae ATCC 43816 | N | Tebipenem | 0.03 | 0.06 (2) | 0.03 (0) | 1 (32) | 0.06 (2) |
| Meropenem | 0.03 | 0.03 (0) | 0.03 (0) | 0.03 (0) | 0.03 (0) | ||
| K. pneumoniae ATCC 700603 | Y | Tebipenem | 0.06 | 0.12 (2) | 0.06 (0) | 2 (32) | 0.12 (2) |
| Meropenem | 0.03 | 0.06 (2) | 0.03 (0) | 0.12 (4) | 0.03 (0) | ||
| P. mirabilis ATCC 43071 | N | Tebipenem | 0.12 | 0.25 (2) | 0.25 (2) | 2 (16) | 0.25 (2) |
| Meropenem | 0.06 | 0.12 (2) | 0.12 (2) | 0.06 (0) | 0.06 (0) | ||
| P. mirabilis MMX 6343 | Y | Tebipenem | 0.5 | 0.5 (0) | 1 (2) | 4 (8) | 1 (2) |
| Meropenem | 0.12 | 0.12 (0) | 0.12 (0) | 0.25 (2) | 0.5 (4) | ||
Bold indicates >4× differences from standard conditions (CAMHB minus serum).
Tebipenem-PI shows efficacy in several murine infection models.
Animal infection models were used to determine the efficacy of tebipenem, administered as the oral prodrug, tebipenem-PI. Six Gram-negative bacterial strains were chosen for in vivo assessment of tebipenem-PI in three different mouse infection models: thigh, lung, and urinary tract. For the thigh infection model (13), E. coli ATCC 25922 (wild type, antibiotic susceptible) was used. MICs for these six strains are listed in Table 6. For lung studies, K. pneumoniae ATCC 43816, a broadly antibiotic-susceptible strain, and Pseudomonas aeruginosa ATCC 27853, an isolate that possesses an inducible AmpC β-lactamase (14), were used (Table 6). All isolates have tebipenem MICs of ≤0.5 μg/ml except for P. aeruginosa ATCC 27853, which has an MIC of 4 μg/ml, consistent with the reported reduced activity of this compound for Pseudomonas isolates (15). For the murine immunocompetent UTI infection model (16, 17), E. coli ATCC 700928 (CFT073 [18]) and E. coli UTI89 (19), both known uropathogenic isolates, were selected (20, 21).
TABLE 6.
MICs of tebipenem and comparators against strains used in microbiology studiesa
| Strain | Compound and MIC (μg/ml) | |||
|---|---|---|---|---|
| In vitro | Tebipenem | Meropenem | Levofloxacin | |
| E. coli ATCC 25922 | 0.015 | 0.03 | 0.015 | |
| E. coli ATCC 35218 | 0.015 | 0.015 | 0.03 | |
| K. pneumoniae ATCC 43816 | 0.03 | 0.03 | 0.03 | |
| K. pneumoniae ATCC 700603 | 0.06 | 0.03 | 0.5 | |
| Thigh study | Tebipenem | Levofloxacin | Ciprofloxacin | |
| E. coli ATCC 25922 | 0.016 | 0.12 | NA | |
| UTI study | Tebipenem | Levofloxacin | Ciprofloxacin | |
| E. coli ATCC 700928 | 0.016 | 0.03 | NA | |
| E. coli UTI89 | 0.016 | NA | 0.016 | |
| Lung study | Tebipenem | Meropenem | Tigecycline | Polymyxin B |
| K. pneumoniae ATCC 43816 | 0.06 | 0.03 | 0.5 | NA |
| P. aeruginosa ATCC 27853 | 4 | 0.5 | NA | 1 |
UTI, urinary tract infection; NA, not applicable (not used as a comparator in the study).
Thigh infection model.
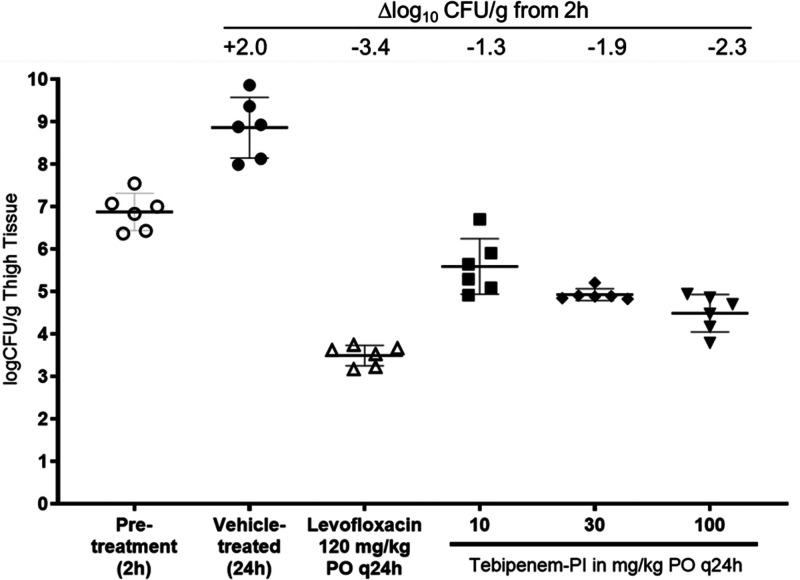
A mouse E. coli thigh infection model was established in female CD-1 mice with strains ATCC 25922 and ATCC BAA-2523. Changes in CFU with and without antibiotic treatment were measured in thigh tissue 2 h after infection and 24 h after treatment. For E. coli ATCC 25922, monotherapy of tebipenem-PI after a single dose of 10 mg/kg of body weight/day showed a 1.3-log10 CFU/g reduction compared to the 2-h pretreatment control (Fig. 3). Dose-dependent decreases in log10 CFU per gram were observed, with a 2.4-log10 CFU/g decrease observed at the highest dose of 100 mg/kg. Levofloxacin dosed at 120 mg/kg per os (p.o.) every 24 h (q24h) resulted in a 3.4-log reduction in CFU burden after treatment was initiated at 2 h.
FIG 3.
Bacterial burdens in mouse thighs infected with E. coli ATCC 25922 after 1 day of treatment with tebipenem-PI dosed at 10, 30, and 100 mg/kg p.o. q24h and levofloxacin dosed at 120 mg/kg p.o. q24h.
Lung infection model.
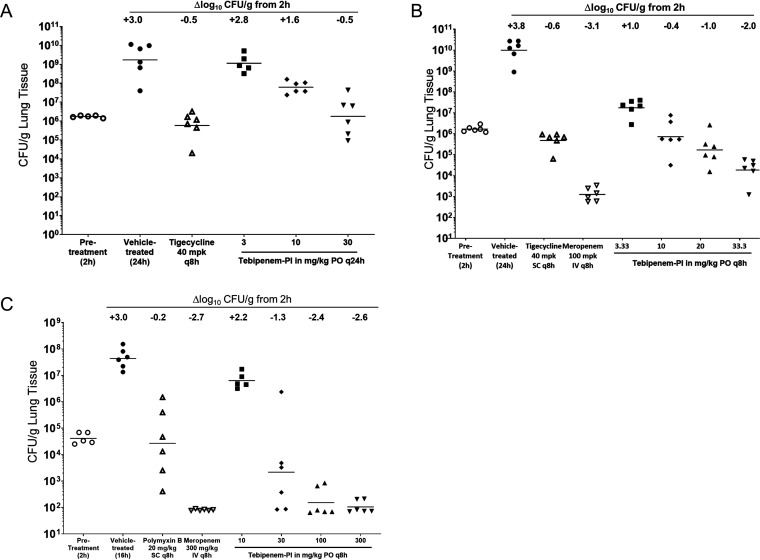
A neutropenic mouse E. coli lung infection model was established in male ICR mice with K. pneumoniae ATCC 43816 and P. aeruginosa ATCC 27853 as the infecting pathogens. For K. pneumoniae, the effects on bacterial burden in lung tissue following oral administration of tebipenem-PI were compared to those after subcutaneous (s.c.) administration of tigecycline and/or intravenous (i.v.) administration of meropenem after treatment for 24 h. Two studies were performed to compare the effects of q24h versus q8h dosing schedules. Treatment with tebipenem-PI caused a dose-dependent reduction in lung burden compared to that in vehicle-treated animals whether administered q24h (Fig. 4A) or q8h (Fig. 4B) p.o. When administered q24h, reduction of the lung burden to pretreatment levels was achieved following administration of 30 mg/kg of tebipenem-PI. When administered q8h, tebipenem-PI doses greater than 10 mg/kg reduced the bacterial burden to below pretreatment levels. Efficacy comparable to those of the tigecycline and meropenem comparator agents was observed.
FIG 4.
Bacterial burdens in mouse lungs after 1 day of treatment with tebipenem-PI and comparators (comparator antibiotics polymyxin B and tigecycline were administered intravenously, while meropenem was dosed via subcutaneous [s.c.] injection). (A and B) K. pneumoniae ATCC 43816 after 24 h of treatment with either tebipenem-PI dosed q24h (A) or tebipenem-PI dosed q8h p.o. (B); (C) P. aeruginosa ATCC 27853 after 14 h of treatment with tebipenem-PI dosed q8h p.o.
The effects on P. aeruginosa ATCC 27853 burden in lung tissue following administration of tebipenem-PI (p.o.) were compared to those of polymyxin B (s.c.) and meropenem (i.v.) at 15 h. For the control antibiotics, the burden of P. aeruginosa ATCC 27853 in the lung was reduced below pretreatment levels following 20 mg/kg q8h for polymyxin B and was reduced greater than 2.71 log10 CFU/g of lung tissue at 300 mg/kg q8h for meropenem. Despite the higher MIC of 4 μg/ml, bacterial burden was reduced to pretreatment levels by tebipenem-PI at 30 mg/kg or greater q8h, with a maximum reduction of 2.6 log10 CFU/g achieved following 300 mg/kg q8h (Fig. 4C). Efficacy comparable to that of meropenem was observed, and tebipenem-PI was superior to polymyxin B in this model.
Urinary tract infection model.
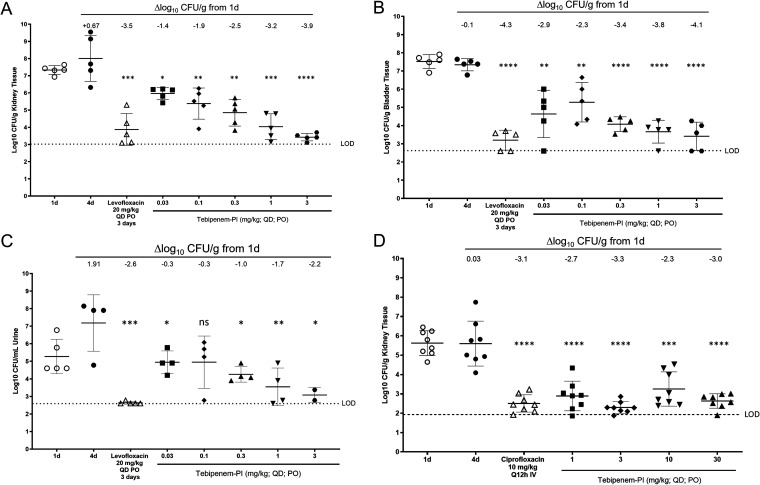
A mouse E. coli upper urinary tract infection model was established in female C3H/HeN mice with uropathogenic strains UTI89 and ATCC 700928 (CFT073) as the infecting pathogens. Changes in CFU with and without antibiotic treatment were measured in kidneys, bladders, and urine for ATCC 700928 (Fig. 5A to C) and in kidneys for UTI89 (Fig. 5D). Dosages of tebipenem-PI ranged from 0.03 to 3 mg/kg/day for 3 days starting 24 h postinoculation, while the levofloxacin control was dosed at 20 mg/kg/day for 3 days starting 24 h postinoculation. Results showed that the expected growth of the E. coli strains was observed in untreated animals between 1 and 4 days after infection in each of the analyzed body sites. For E. coli ATCC 700928, administration of levofloxacin resulted in 3.4-log10 CFU/g, 4.3-log10 CFU/g, and 2.7-log10 CFU/ml decreases in bacterial burdens in kidneys, bladder, and urine, respectively, compared to the 1-day pretreatment control. Administration of tebipenem-PI dosed at 0.03, 0.1, 0.3, 1, and 3 mg/kg showed significant dose-dependent reductions in bacterial burdens across all body sites (kidney, −1.4, −1.9, −2.5, −3.2, and −3.9 log10 CFU/g, respectively; bladder, −2.9, −2.3, −3.4, −3.8, and −4.1 log10 CFU/g, respectively; urine, −0.3, −0.3, −1.0, −1.7, and −2.2 log10 CFU/ml, respectively), comparable to levofloxacin at the highest dosage of 3 mg/kg/day. For E. coli UTI89, administration of ciprofloxacin (CIP) at 10 mg/kg/day for 3 days resulted in a 3.1-log10 CFU/g decrease in bacterial burden in the kidney compared to the 1-day pretreatment control. Administration of tebipenem-PI at 1, 3, 10, and 30 mg/kg showed significant dose-dependent reductions in the kidney, −2.7, −3.3, −2.3, −3.0 log10 CFU/g, comparable to the ciprofloxacin control.
FIG 5.
Bacterial burdens in mouse urinary tract infections after treatment with tebipenem-PI or comparators (levofloxacin or ciprofloxacin). Shown are burdens of E. coli ATCC 700928 in kidney (A) bladder (B), and urine (C) and E. coli UTI89 in kidney (D). Paired t tests of treated versus untreated group results on day 4 are shown as follows: ns, not significant; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; and ****, P ≤ 0.0001.
DISCUSSION
Tebipenem (SPR859) is an oral carbapenem antibiotic with a relatively broad spectrum of activity against bacterial pathogens, particularly key Gram-negative organisms, including Escherichia coli and Klebsiella pneumoniae, similar to that of current intravenously administered carbapenems (4, 6). Among the Enterobacterales, tebipenem retains antibacterial activity against many extended-spectrum β-lactamase (ESBL)- and AmpC β-lactamase-producing isolates as well as trimethoprim-sulfamethoxazole (TMP-SMX)- and fluoroquinolone (FQ)-nonsusceptible organisms that are increasingly prevalent, particularly among uropathogens (6). Tebipenem is bactericidal and a potent inhibitor of multiple penicillin-binding proteins (PBPs), typical of other β-lactam antibiotics, with the primary target PBP2, similar to other carbapenems (22). Microbiological evaluation of tebipenem in several in vitro and in vivo studies is reported here.
As might be expected, the microbiological activity was very similar to that of the intravenous carbapenems, typified by meropenem. This finding held true even under nonstandard conditions, including in vitro in pooled human urine and ex vivo against ESBL-producing E. coli in urine collected from subjects dosed with 300 mg of tebipenem every 8 h (q8h) (23). Addition of human serum, either 10 or 50% final concentration, did not affect MIC values, suggesting that protein binding is not a major issue. Protein binding is much higher in mouse plasma than in human plasma as determined by ultrafiltration (≥98% versus 45%) (12; Spero, unpublished data) and needs to be considered in order to accurately determine levels of free drug, particularly when interpreting results from mouse infection model studies.
As expected and in common with other carbapenems, tebipenem was relatively inactive against carbapenemase-producing organisms, such oxacillinase 48 (OXA-48)-, K. pneumoniae carbapenemase (KPC)-, and metallo-β-lactamase-producing organisms, which demonstrated MICs of ≥8 μg/ml. Tebipenem, similar to ertapenem, possesses limited antibacterial activity against Pseudomonas aeruginosa, which is not being considered as a target pathogen for this drug. These results support the development indication of tebipenem as an oral option for treating cUTIs caused by ESBL-/AmpC-producing Enterobacteriaceae with coverage of fluoroquinolone-nonsusceptible isolates. Although tebipenem exhibited broad-spectrum antibacterial activity against anaerobes, the observed activity against vegetative C. difficile may minimize overgrowth of this organism in the gastrointestinal (GI) tract. Future clinical trial data analyses will determine if oral dosing would result in significant concentrations in the gut and subsequent impact on intestinal microflora.
In vivo, tebipenem administered as oral prodrug tebipenem-PI has demonstrated proof-of-concept efficacy against wild-type and ESBL-producing organisms in the murine lung and neutropenic thigh infection models and wild-type organisms in the murine ascending UTI infection model. For the latter, E. coli and K. pneumoniae are key pathogens implicated in UTI and acute pyelonephritis, and tebipenem has demonstrated good in vitro and in vivo antibacterial activity against these organisms (6). History has indicated that success in mouse infection models is often predictive of clinical efficacy in humans, and it is hoped that this will be the case here (24). Like most β-lactams, tebipenem was shown to exhibit time-dependent pharmacodynamics, with better efficacy observed with increased dosing frequency (12).
In summary, the activity of tebipenem against ESBL-/AmpC-producing cUTI pathogens shows robust proof-of-concept efficacy in multiple in vitro and in vivo models. Based on pharmacokinetic/pharmacodynamic (PK/PD) target attainment analyses, approximately 90% of patients are predicted to achieve the nonclinical PK/PD stasis targets for cUTI isolates of E. coli and K. pneumoniae with tebipenem MICs of 0.06 μg/ml (12). The microbiological data presented here support further evaluation of tebipenem-SPR994 in the clinical setting as an oral option for cUTIs, including those caused by resistant pathogens, particularly those that are fluoroquinolone resistant and/or ESBL producers, with future potential for treatment of other types of infections.
MATERIALS AND METHODS
Bacterial strains and media.
MICs were determined using tebipenem by broth microdilution in accordance with CLSI document M7-A10 (25). Bacterial strains used in the microbiology experiments were E. coli ATCC 25922 (wild type, antibiotic susceptible), E. coli ATCC 35218 (expresses ESBL), K. pneumoniae ATCC 43816 (broadly antibiotic susceptible), and K. pneumoniae ATCC 700603 (expresses SHV-18). Bacterial strains used in the mouse infection studies were E. coli ATCC 25922, E. coli ATCC BAA-2523 (expresses OXA-48 carbapenemase), E. coli ATCC 700928 (uropathogenic clinical isolate), E. coli UTI89 (uropathogenic clinical isolate), K. pneumoniae ATCC 43816 (broadly antibiotic susceptible), and P. aeruginosa ATCC 27853 (inducible AmpC β-lactamase) (16). Bactericidal activity was determined by NCCLS (now the CLSI) standard methods (26). MICs of bacterial strains used in in vitro and in vivo studies are listed in Table 6.
The clinical isolates listed in Table 1 were recovered from a diverse range of human clinical specimens in patients examined or hospitalized in medical institutions in the United States, Europe (including Russia and Turkey), Latin America, and the Asia-Western Pacific region. Contemporary isolates were preferentially used, and the majority were collected during the SENTRY Antimicrobial Surveillance Program for 2013 to 2016 (6). Some isolates exhibiting key resistance phenotypes originated from older collections (2005 to 2012). The ESBL phenotype was defined for E. coli, K. pneumoniae, and P. mirabilis as isolates that displayed MIC values of ≥2 μg/ml for ceftriaxone, ceftazidime, and/or aztreonam but were not typed by molecular methods. This study was intended to characterize the microbiology profile of tebipenem rather than provide a representative sampling of any particular set of clinical isolates.
For anaerobic organism MIC assays, isolates were recovered from clinical infections of patients and stored as pure cultures in 20% skim milk at –70°C. Prior to testing, isolates were transferred to brucella agar supplemented with sheep blood, vitamin K, and hemin to ensure purity and good growth. Agar dilution testing was performed according to procedures described in CLSI document M11-A8 (27). Meropenem and metronidazole were included for quality control (QC) and comparison.
Antibiotics.
Tebipenem and tebipenem-PI were synthesized by Spero Therapeutics. Comparator antibiotics (meropenem, tigecycline, levofloxacin, ciprofloxacin, polymyxin B, and metronidazole) were purchased from commercial sources.
Time-kill assays.
Time-kill assays were performed by the broth macrodilution method, according to NCCLS guideline M26-A (26). Briefly, log-phase cultures at ∼1.0 × 105 CFU/ml in cation-adjusted Mueller-Hinton broth (CAMHB) were treated with antimicrobial agents at 2× to 8× MIC, viability was monitored for 24 h, and bactericidal activity was defined as a 3-log10 reduction in CFU per milliliter of the initial inoculum.
PAE.
The in vitro postantibiotic effect (PAE) of tebipenem and comparators was established using a standard method (10). Log-phase cultures were treated with antimicrobial agents at 4× and/or 8× MIC for 1 h and diluted 1:1,000 into fresh CAMHB, and viability was monitored for 6 h by plating for CFU per milliliter. PAE = T – C, where T and C are the times required to increase 1-log10 CFU following 1:1,000 dilution for the bacteria treated with and not treated with the agents, respectively.
Impact of varied growth conditions on in vitro antibacterial activity.
CAMHB, which contains divalent cation concentrations of 10 to 12.5 μg/ml of Ca2+ for calcium and 20 to 25 μg/ml of Mg2+ for magnesium, was used as the growth medium unless otherwise indicated. MICs were determined by broth microdilution in accordance with CLSI document M7-A10 (25) under standard and nonstandard conditions in parallel. Testing conditions were modified as follows. Medium pH was adjusted to pH 5, 6, or 7 from the standard pH 7.2 to 7.4. Standard inoculum was ∼5 × 105 CFU/ml, with low inoculum (∼5 × 104 CFU/ml) and high inoculum (∼5 × 106-7) used where indicated. Altered atmosphere was 6.5% CO2. For assays with MHB plus serum, pooled heat-inactivated human or mouse serum (10% and 50% wt/vol) was used. For assays containing urine, 100% pooled human urine was used, with pH adjusted to 7.2 to 7.4 to match CAMHB.
Animal welfare.
All studies were approved by the Spero institutional Animal Care and Use Committee (IACUC).
Mouse thigh infection.
Neutropenia was induced in CD-1 mice by administering cyclophosphamide by intraperitoneal (i.p.) injection on days −4 and −1 (150 and 100 mg/kg, respectively). Female mice were infected by intramuscular (i.m.) injection into the lateral thigh muscles with E. coli ATCC 25922. Tebipenem-PI was dosed p.o. at various concentrations and intervals as shown in Fig. 2. Mice were euthanized 24 h postinfection, the thigh muscle was quantitatively cultured, serially diluted, and plated on appropriate media, and CFU were counted after overnight incubation. CFU/thigh were calculated.
Mouse lung infection.
Male ICR mice were rendered neutropenic using cyclophosphamide on days −4 and −1 (200 and 150 mg/kg, respectively). Mice were infected intranasally with either K. pneumoniae ATCC 43816 (∼2.5 × 105 CFU) or P. aeruginosa ATCC 27853 (4 × 104 CFU). Treatment was initiated 2 h postinfection. For K. pneumoniae models (26-h duration), tebipenem-PI was administered either as a single oral dose (p.o.) of 3, 10, or 30 mg/kg or as three doses of 3.33, 10, 20, or 33.3 mg/kg/dose p.o. given q8h. For the P. aeruginosa model (15-h duration), tebipenem-PI was administered as two doses of 10, 30, 100, or 300 mg/kg p.o. at 2 and 10 h postinfection. Comparator antibiotics polymyxin B and tigecycline were dosed via i.v. administration, while meropenem was dosed via subcutaneous (s.c.) administration. Lung tissue was quantitatively cultured, serially diluted, and plated on appropriate media, and CFU were quantified following overnight incubation.
Mouse urinary tract infection.
All mice were placed on 5% glucose solution ad libitum 5 days prior to infection. Mice were infected transurethrally with either E. coli ATCC 700928 or E. coli UTI89 following published methods (20, 21). Tebipenem-PI prodrug was dosed orally at various concentrations once per day (QD) for 3 days starting 24 h postinoculation. Mice were euthanized 24 h after the final dose, the kidneys, bladder, and urine were collected and quantitatively cultured, serially diluted, and plated on appropriate media, and CFU were counted after overnight incubation.
Statistical analyses.
A one-way analysis of variance (ANOVA) was performed followed by post hoc unpaired t test using GraphPad Prism version 8.3.0 for Windows, GraphPad Software, San Diego, CA. Differences were deemed statistically significant when a P value of ≤0.05 was obtained.
ACKNOWLEDGMENTS
We acknowledge Akash Jain, Kate Sulham, and Tom Parr for helpful discussions and critical review of the manuscript. We acknowledge Lena Grosser and Katherine Heang, formerly of Spero Therapeutics, and David Corbett and colleagues at Evotec (UK) Ltd. for performing murine efficacy studies. We acknowledge Yanming Zou and colleagues at HD Biosciences Company for conducting the time-kill and postantibiotic effect studies. We acknowledge Diane Citron and colleagues at R. M. Alden Research Laboratory for providing anaerobe MIC data. We acknowledge Debora Sweeney at Micromyx for performing the conditional-dependence MIC study.
REFERENCES
- 1.Bush K, Bradford PA. 2016. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonomo RA. 2017. β-Lactamases: a focus on current challenges. Cold Spring Harb Perspect Med 7:a025239. doi: 10.1101/cshperspect.a025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Gamal MI, Brahim I, Hisham N, Aladdin R, Mohammed H, Bahaaeldin A. 2017. Recent updates of carbapenem antibiotics. Eur J Med Chem 131:185–195. doi: 10.1016/j.ejmech.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Jain A, Utley L, Parr TR, Zabawa T, Pucci MJ. 2018. Tebipenem, the first oral carbapenem antibiotic. Expert Rev Anti Infect Ther 16:513–522. doi: 10.1080/14787210.2018.1496821. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki S, Hosoyama T, Furuya N, Ishii Y, Matsumoto T, Ohno A, Tateda K, Yamaguchi K. 2001. In vitro and in vivo antibacterial activities of L-084, a novel oral carbapenem, against causative organisms of respiratory tract infections. Antimicrob Agents Chemother 45:203–207. doi: 10.1128/AAC.45.1.203-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arends SJR, Rhomberg PR, Cotroneo N, Rubio A, Flamm RK, Mendes RE. 2019. Antimicrobial activity evaluation of tebipenem (SPR859), an orally available carbapenem, against a global set of Enterobacteriaceae collected from urinary tract infections. Antimicrob Agents Chemother 63:e02618-18. doi: 10.1128/AAC.02618-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiger SN, Comito RR, Nicolau DP. 2017. Clinical and economic implications of urinary tract infections. Expert Rev Pharmacoecon Outcomes Res 17:377–383. doi: 10.1080/14737167.2017.1358618. [DOI] [PubMed] [Google Scholar]
- 8.Dicks LMT, Mikkelsen LS, Brandsborg E, Marcotte H. 2019. Clostridium difficile, the difficult “Kloster” fueled by antibiotics. Curr Microbiol 76:774–782. doi: 10.1007/s00284-018-1543-8. [DOI] [PubMed] [Google Scholar]
- 9.Putsathit P, Maneerattanaporn M, Piewngam P, Knight DR, Kiratisin P, Riley TV. 2017. Antimicrobial susceptibility of Clostridium difficile isolated in Thailand. Antimicrob Resist Infect Control 6:58. doi: 10.1186/s13756-017-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig WA, Gudmundsson S. 1996. Postantibiotic effect, p 296–329. In Lorian V. (ed), Antibiotics in laboratory medicine. Williams and Wilkins Co, Baltimore, MD. [Google Scholar]
- 11.McCloskey L, Moore T, Niconovich N, Donald B, Broskey J, Jakielaszek C, Rittenhouse S, Coleman K. 2000. In vitro activity of gemifloxacin against a broad range of recent clinical isolates from the USA. J Antimicrob Chemother 45(Suppl 1):13–21. doi: 10.1093/jac/45.suppl_3.13. [DOI] [PubMed] [Google Scholar]
- 12.McEntee L, Johnson A, Farrington N, Unsworth J, Dane A, Jain A, Cotroneo N, Critchley I, Melnick D, Parr T, Ambrose PG, Das S, Hope W. 2019. Pharmacodynamics of tebipenem: new options for oral treatment of multi-drug resistant Gram-negative infections. Antimicrob Agents Chemother 63:e00603-19. doi: 10.1128/AAC.00603-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zak O, Sande MA. 1999. Handbook of animal models of infection. Academic Press, London, United Kingdom. [Google Scholar]
- 14.Pérez-Gallego M, Torrens G, Castillo-Vera J, Moya B, Zamorano L, Cabot G, Hultenby K, Albertí S, Mellroth P, Henriques-Normark B, Normark S, Oliver A, Juan C. 2016. Impact of AmpC derepression on fitness and virulence: the mechanism or the pathway? mBio 7:e01783-16. doi: 10.1128/mBio.01783-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Q, Wang J, Cui T, Yang Z, Su M, Zhao P, Yan H, Zhan Y, Yang H. 2016. Antibacterial properties of tebipenem pivoxil tablet, a new oral carbapenem preparation against a variety of pathogenic bacteria in vitro and in vivo. Molecules 21:62. doi: 10.3390/molecules21010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung CS, Dodson KW, Hultgren SJ. 2009. A murine model of urinary tract infection. Nat Protoc 4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conover MS, Flores-Mireles AL, Hibbing ME, Dodson K, Hultgren SJ. 2015. Establishment and characterization of UTI and CAUTI in a mouse model. J Vis Exp 2015(100):e52892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welch RA, Burland V, Plunkett G, Redford P, Roesch P, Rasko D, Buckles EL, Liou S-R, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HLT, Donnenberg MS, Blattner FR. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen SL, Hung CS, Xu J, Reigstad CS, Magrini V, Sabo A, Blasiar D, Bieri T, Meyer RR, Ozersky P, Armstrong JR, Fulton RS, Latreille JP, Spieth J, Hooton TM, Mardis ER, Hultgren SJ, Gordon JI. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A 103:5977–5982. doi: 10.1073/pnas.0600938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58:1281–1289. doi: 10.1128/IAI.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunstad DA, Justice SS, Hung CS, Lauer SR, Hultgren SJ. 2005. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect Immun 73:3999–4006. doi: 10.1128/IAI.73.7.3999-4006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacasse E, Brouillette E, Larose A, Parr TR Jr, Rubio A, Malouin F. 2019. In vitro activity of tebipenem (SPR859) against penicillin-binding proteins of Gram-negative and Gram-positive bacteria. Antimicrob Agents Chemother 63:e02181-18. doi: 10.1128/AAC.02181-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thamlikitkul V, Lorchirachoonkul N, Tiengrim S. 2014. In vitro and in vivo activity of tebipenem against ESBL-producing E. coli. J Med Assoc Thai 97:1259–1268. [PubMed] [Google Scholar]
- 24.Abdelraouf K, Linder KE, Nailor MD, Nicolau DP. 2017. Predicting and preventing antimicrobial resistance utilizing pharmacodynamics: part II Gram-negative bacteria. Expert Opin Drug Metab Toxicol 13:705–714. doi: 10.1080/17425255.2017.1329417. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. 2015. M7-A10: methods for dilution antimicrobial susceptibility tests for bacterial that grow aerobically; approved standard, 10th ed CLSI, Wayne, PA. [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 1999. M26-A: methods for determining bactericidal activity of antimicrobial agents: approved guideline. NCCLS, Wayne, PA. [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2018. M11-A8: methods for antimicrobial susceptibility testing of anaerobic bacteria, 9th ed CLSI, Wayne, PA. [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2019. M100: performance standards for antimicrobial susceptibility testing, 29th ed CLSI, Wayne, PA. [Google Scholar]