Pseudomonas aeruginosa bacteremia is an infection associated with a high mortality rate. Piperacillin-tazobactam is a β-lactam–β-lactamase inhibitor combination that is frequently used for the management of Pseudomonas aeruginosa infections. The pharmacokinetic-pharmacodynamic index associated with in vitro maximal bacterial killing for piperacillin-tazobactam is the percentage of the time between doses at which the free fraction concentration remains above the MIC (%fT>MIC).
ABSTRACT
Pseudomonas aeruginosa bacteremia is an infection associated with a high mortality rate. Piperacillin-tazobactam is a β-lactam–β-lactamase inhibitor combination that is frequently used for the management of Pseudomonas aeruginosa infections. The pharmacokinetic-pharmacodynamic index associated with in vitro maximal bacterial killing for piperacillin-tazobactam is the percentage of the time between doses at which the free fraction concentration remains above the MIC (%fT>MIC). However, the precise %fT>MIC target associated with improved clinical outcomes is unknown. The aim of this study was to investigate the correlation between the survival of patients with Pseudomonas aeruginosa bacteremia and the threshold of the piperacillin-tazobactam %fT>MIC. This retrospective study included all adult patients hospitalized over an 82-month period with Pseudomonas aeruginosa bacteremia and treated with piperacillin-tazobactam. Patients with a polymicrobial infection or those who died within 72 h of the time of collection of a sample for culture were excluded. The %fT>MIC of piperacillin-tazobactam associated with in-hospital survival was derived using classification and regression tree analysis. After screening 270 patients, 78 were eligible for inclusion in the study; 18% died during hospitalization. Classification and regression tree analysis identified a %fT>MIC of >60.68% to be associated with improved survival, and this remained statistically significant after controlling for clinical covariates (odds ratio = 7.74, 95% confidence interval = 1.32 to 45.2). In conclusion, the findings recommend dosing of piperacillin-tazobactam with the aim of achieving a pharmacodynamic target %fT>MIC of at least 60% in these patients.
TEXT
Pseudomonas aeruginosa bacteremia is a common hospital-acquired infection (1) associated with an increased rate of mortality ranging from 18 to 61% (2). Early appropriate antimicrobial therapy is associated with improved survival (3–8).
Piperacillin and the combination of piperacillin and tazobactam (TZP) are extensively used in the treatment of infectious diseases in critically ill patients, specifically, when P. aeruginosa is the causative pathogen (8). Protein binding for piperacillin ranges from 20 to 30%, and for tazobactam it is approximately 30% (9, 10). Various population pharmacokinetic (PK) studies have suggested that the main covariates influencing the volume of distribution and the clearance of TZP are weight and creatinine clearance, respectively (11–16). The usual dose of TZP is 4.5 g three times daily for most infections and may be increased to 4.5 g four times daily in severe health care-acquired infections (17) and in P. aeruginosa infections, as recommended by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI). Dose adjustments for renal impairment vary widely between sources (9, 17, 18). There are no sufficient data regarding dosing regimens that achieve pharmacokinetic and pharmacodynamic (PD) targets that are correlated with improved clinical outcomes.
Piperacillin, like other β-lactams, exhibits time-dependent bactericidal activity. In vitro and animal studies suggest that the PK-PD parameter for β-lactams that is most predictive of microbiological efficacy is the percentage of time between doses at which the free fraction concentration remains above the MIC (%fT>MIC) (19). For piperacillin, a PK-PD target of a %fT>MIC of 50% is often cited based on studies of other penicillins (20), for example, ticarcillin (21, 22). There is only one study that reported a relationship between bacterial killing and %fT>MIC, with significant thresholds of 27% for bacteriostasis and 75% for bactericidal activity (23).
Very few studies have tried to correlate %fT>MIC with clinical outcomes. Among them is the DALI trial, a prospective multinational study that included 361 critically ill patients who were treated with a β-lactam (24). This study concluded that for all β-lactams a %fT>MIC of >50% is associated with better outcomes. Other studies dealt specifically with meropenem and neutropenic patients (25) and cephalosporins (26, 27).
Moreover, the need for a clinically driven %fT>MIC target is augmented by the fact that β-lactams display indirect antimicrobial properties. These properties cannot be identified by standard in vivo susceptibility testing and include synergy with cationic host defense peptides and action as immune adjuvants (28). The use of an in vitro-derived PK-PD target for any antimicrobial without clinical validation is highly problematic (29). This observation is especially true in the case of β-lactams.
The aim of this study was to investigate the correlation between the concentration-time profile of TZP and clinical outcomes in patients with P. aeruginosa bacteremia. Additionally, the study aimed to find if there is a threshold of %fT>MIC that is associated with improved survival at 30 days.
RESULTS
A total of 270 patients with P. aeruginosa bacteremia were screened for this retrospective study from January 2012 to October 2018, and 78 fulfilled the inclusion criteria for the study (Fig. 1).
FIG 1.
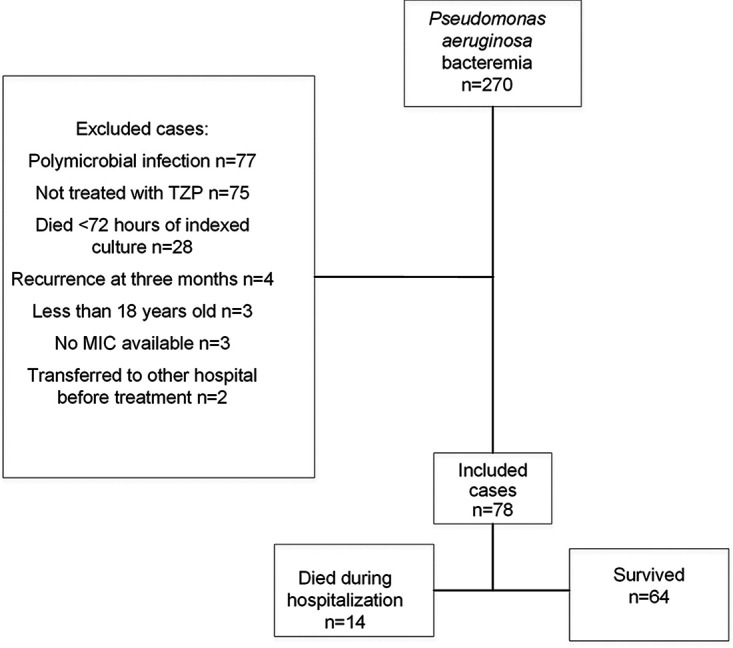
Study flow chart.
The patients’ baseline characteristics are described in Table 1. The included patients had a mean ± standard deviation (SD) age of 65 ± 17.95 years, 37.1% were female, and the mean ± SD modified acute physiology and chronic health evaluation II (APACHE II) score on the day that the specimen was collected for culture was 11.5 ± 5.46. Patients had a median creatinine clearance of 53.5 ml/min (interquartile range [IQR] = 23.25 to 97 ml/min), and 16 patients (20.5%) had an acute kidney injury (AKI). The most common source of bacteremia was respiratory (28.2%), and 7 (9%) patients were treated in the intensive care unit (ICU). The median MIC was 8 μg/ml and was similar for isolates from patients who survived and patients who died.
TABLE 1.
Baseline characteristics of 78 patients with P. aeruginosa bacteremiaa
| Characteristic | Value for the following group: |
P value | ||
|---|---|---|---|---|
| Total cohort | Patients who survived in hospital | Patients who died | ||
| Total no. (%) of patients | 78 | 64 (82) | 14 (18) | |
| Mean (SD) age (yr) | 65 (17.95) | 65 (18.27) | 68 (16.82) | 0.568 |
| No. (%) of female patients | 29 (37.1) | 24 (37.5) | 5 (35.7) | 0.900 |
| Median (IQR) wt (kg) | 73.9 (80.75, 62.25) | 74 (84.25, 62.75) | 71 (74, 61.75) | 0.108 |
| Mean (SD) modified APACHE II score on culture day | 11.5 (5.46) | 10.8 (5.61) | 14.5 (3.48) | 0.022 |
| No. (%) of patients with the following sources of infection: | ||||
| Respiratory | 22 (28.2) | 14 (21.8) | 8 (57) | 0.011 |
| Intra-abdominal | 7 (8.9) | 7 (10.9) | 0 (0) | 0.991 |
| Urinary | 14 (17.9) | 13 (20.3) | 1 (7.1) | 0.269 |
| Skin and wound | 11 (14.1) | 10 (15.6) | 1 (7.1) | 0.442 |
| Central line | 9 (11.5) | 9 (14) | 0 (0) | 0.994 |
| Unknown | 15 (19.2) | 11 (17.2) | 4 (28.6) | 0.333 |
| No. (%) of patients with the following medical history: | ||||
| Hypertension | 39 (50) | 32 (50) | 7 (50) | 1 |
| Type 2 diabetes | 29 (37.2) | 23 (35.9) | 6 (42.8) | 0.627 |
| IHD | 12 (15.4) | 10 (15.6) | 2 (14.3) | 0.900 |
| Heart failure | 17 (21.8) | 11 (17.2) | 6 (42.8) | 0.035 |
| Hyperlipidemia | 28 (35.9) | 23 (35.9) | 5 (35.7) | 0.987 |
| Dementia | 9 (11.5) | 8 (12.5) | 1 (7.1) | 0.57 |
| CKD | 25 (32) | 18 (28.1) | 7 (50) | 0.112 |
| COPD | 9 (11.5) | 5 (7.8) | 4 (28.5) | 0.025 |
| Receipt of immunosuppression (90 days) | 12 (15.4) | 7 (10.9) | 5 (35.7) | 0.02 |
| Median (IQR) creatinine clearance (ml/min) | 53.5 (97, 23.25) | 59.5 (98.5, 26.95) | 26.5 (78.25, 13.525) | 0.214 |
| No. (%) of patients with AKI | 16 (20.5) | 9 (14) | 7 (50) | 0.003 |
| No. (%) of patients with: | ||||
| Solid tumors | 18 (23) | 13 (20.3) | 5 (35.7) | 0.22 |
| Hematological malignancies | 4 (5.1) | 2 (3.1) | 2 (14.1) | 0.117 |
| Median (IQR) duration of hospitalization (days) until collection of index culture sample | 1 (7, 0) | 1 (7, 0) | 0 (9.5, 0) | 0.952 |
| Median (IQR) time (days) until appropriate antipseudomonal therapy | 1 (2, 0) | 1 (2, 0) | 1 (2.75, 1) | 0.603 |
| No. (%) of patients hospitalized in ICU | 7 (9) | 3 (4.7) | 4 (28.6) | 0.005 |
IQR, interquartile range; IHD, ischemic heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; AKI, acute kidney injury; ICU, intensive care unit.
The primary outcome of in-hospital survival occurred in 64 (82%) of the included patients. Patients who survived had a lower APACHE II score, had fewer cases of AKI, and were less frequently hospitalized in the ICU than patients who died (10.8 versus 14.5 [P = 0.022], 14% versus 50% [P = 0.003], and 4.7% versus 28.6% [P = 0.005], respectively).
The estimated median volume of distribution of piperacillin was 23.19 liters (IQR = 18.47 to 30.19 liters), and the estimated median elimination rate constant was 0.56 h−1 (IQR = 0.36 to 0.86 h−1). The mean %fT>MIC calculated from the pharmacokinetic model and estimated parameters was 63% (IQR = 47 to 85%). Table 2 presents the median %fT>MIC by the different creatinine clearance groups. Patients with a creatinine clearance of less than 20 ml/min had a higher median %fT>MIC than patients with a creatinine clearance above 20 ml/min (82% and 59%, respectively).
TABLE 2.
%fT>MIC by the different creatinine clearance groups
| Creatinine clearance (ml/min) | %fT>MIC |
|
|---|---|---|
| Median | IQR | |
| 0–20 (n = 19) | 82 | 69–89 |
| 21–40 (n = 19) | 65 | 45–85 |
| 41–60 (n = 5) | 47 | 43–90 |
| 61–80 (n = 5) | 69 | 55–99 |
| 81–100 (n = 11) | 53 | 40–70 |
| 101–120 (n = 6) | 54 | 50–60 |
| >120 (n = 13) | 56 | 35–75 |
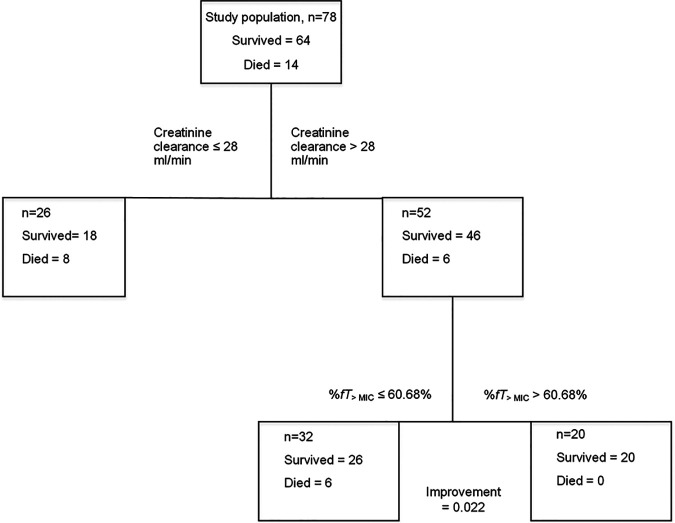
Classification and regression tree analysis (CART) identified a %fT>MIC threshold of 60.68% to be associated with improved in-hospital survival, after adjusting for creatinine clearance (Fig. 2).
FIG 2.
CART-derived %fT>MIC threshold.
The final logistic regression model included AKI, a modified APACHE II score of ≥14, and an interaction term between them as independent variables, in addition to the CART-derived %fT>MIC threshold. A %fT>MIC of >60.68% was entered in the final model as a categorical variable. The adjusted odds ratio (OR) of achieving the threshold of a %fT>MIC of >60.68% was 7.74 (95% confidence interval [CI] = 1.32 to 45.2) (Table 3). A comparison of the final model and the competing models is summarized in Table S1 in the supplemental material.
TABLE 3.
Logistic regression model of in-hospital survival
| Parameter | Adjusted OR for in-hospital survival | P value | 95% CI |
|---|---|---|---|
| %fT>MIC > 60% | 7.74 | 0.023 | 1.32–45.2 |
| AKI | 0.14 | 0.003 | 0.001–0.234 |
| Modified APACHE II score ≥ 14 | 0.113 | 0.018 | 0.019–0.685 |
| AKI · modified APACHE II score ≥ 14 | 20.65 | 0.05 | 1.99–420.8 |
The goodness of fit and regression diagnostics of the final model are summarized in Tables S2 to S4. The results of an internal validation with bootstrap analysis are summarized in Tables S5 and S6. Moreover, other %fT>MIC thresholds were tested by adjusting for the same covariates, as shown in Table 4. A %fT>MIC of >40% and a %fT>MIC of >50%, as well as a %fT>MIC of >70% and a %fT>MIC of >80%, were not significant predictors of in-hospital survival.
TABLE 4.
Effect of different %fT>MIC thresholds on in-hospital survival
| %fT>MIC | Adjusted OR for in-hospital survival | P value | 95% CI |
|---|---|---|---|
| >40 | 3.70 | 0.151 | 0.62–22 |
| >50 | 3.76 | 0.100 | 0.77–18.18 |
| >60 | 7.74 | 0.023 | 1.32–45.2 |
| >70 | 2.55 | 0.199 | 0.61–10.65 |
| >80 | 2.25 | 0.280 | 0.52–9.82 |
Eleven patients received concomitant treatment with other antimicrobial agents: ciprofloxacin (n = 9), levofloxacin (n = 1), and gentamicin (n = 1). In a univariable analysis for 30-day survival, the OR of concomitant treatment was 0.3 (P = 0.0984). In the multivariable logistic regression, adding this variable did not increase the explanatory power of the model (Akaike information criterion = 64.41, −2 log likelihood = 52.41) compared to that of the final model.
DISCUSSION
The key finding of this study is the fact that the CART-derived threshold of a %fT>MIC of TZP of >60.68% was found to be a significant predictor of in-hospital survival in patients with P. aeruginosa bacteremia, adjusting for covariates. Lower thresholds (%fT>MIC > 40% and %fT>MIC > 50%), as well as higher thresholds (%fT>MIC > 70% and %fT>MIC > 80%), were not significant predictors of in-hospital survival. To the best of our knowledge, this is the first study to report a %fT>MIC threshold of TZP that is associated with improved survival.
The results of this study are consistent with those of other clinical studies concerning the effects of the %fT>MIC of β-lactams on clinical outcomes. In the DALI study, achieving a %fT>MIC of >50% and %fT>MIC of >100% was associated with improved clinical outcomes (24). This cohort had a relatively lower mean modified APACHE II score compared to that for the cohort in the DALI study (11.5 and 18, respectively). Interestingly, in the DALI study, the adjusted odds ratios of a %fT>MIC of >50% and a %fT>MIC of >100% for an improved clinical outcome were similar among patients who did not receive renal replacement therapy (1.03 [95% CI = 1.01 to 1.04] and 1.02 [95% CI = 1.01 to 1.05], respectively). The latter finding is consistent with the threshold of a %fT>MIC of >60% reported in this study.
Ariano et al. studied the influence of the %fT>MIC of meropenem in neutropenic patients with bacteremia and found an average %fT>MIC of 83% among 42 clinical responders and an average %fT>MIC of 59% among 18 nonresponders (P = 0.04), but in their study, no adjustment for severity of illness was performed (25). Rhodes et al. reported two %fT>MIC thresholds for cefepime (68% and 74%) that were associated with improved survival (adjusted OR = 7.12 [95% CI = 1.9 to 26.7] and 6.48 [95% CI = 1.9 to 22.1], respectively) (27), similar to our results, although our patient population had a lower mean modified APACHE II score than the population in the study by Rhodes et al. (27) (11.5 and 14.6, respectively) and a lower median creatinine clearance (59.5 ml/min among patients who survived and 53.5 ml/min among patients who died in our study and 74.9 ml/min and 83 ml/min, respectively, in the study by Rhodes et al. [27]).
The EUCAST rationale document for TZP states that a piperacillin %fT>MIC of 30 to 35% is needed for stasis against P. aeruginosa and that a %fT>MIC of 40% is required for a 2-log drop in the viable organism count in animal models. This statement is based on limited data (30). Yet a higher dose of 4.5 g four times daily is recommended. This higher dose, according to EUCAST, renders all wild-type P. aeruginosa isolates susceptible to TZP and allows a clinical MIC breakpoint of 16 mg/liter. The former conclusion was based on a Monte Carlo simulation of various dosing regimens. It did not consider special populations, such as critically ill patients, who usually have a higher piperacillin volume of distribution, and patients with augmented renal clearance, whose piperacillin clearance is significantly increased. Indeed, Udy et al. demonstrated that patients with augmented renal clearance had increased clearance of piperacillin (31). They concluded that, when considering the MIC distribution of P. aeruginosa, the regimen of 4.5 g four times daily administered as a 30-min infusion is not expected to achieve a %fT>MIC of 50% in a significant portion of critically ill patients (31). The cohort in this study was too small to analyze the optimal dosing regimen.
There are some limitations to this study. First, this was a retrospective, single-center study with the limitations resulting from the study design. Still, the results contribute to evidence in an area in which there is limited literature available. Second, the small sample size may have affected the estimation of the adjusted odds ratio of the %fT>MIC of TZP of >60% on in-hospital survival, as reflected by large confidence intervals in some of the results. Third, TZP concentrations were not measured in any patient, and former studies have shown substantial variability in TZP concentrations in hospitalized patients (24). Nevertheless, the piperacillin volume of distribution and elimination rate constant were estimated for each patient using a highly qualified population model. Moreover, across various population pharmacokinetics studies, weight and creatinine clearance were identified as the main covariates affecting piperacillin`s volume of distribution and clearance, respectively. Concentrations were predicted for each patient, controlling for weight and creatinine clearance. Therefore, concentration prediction and imputation represented the best available strategy to study the influence of %fT>MIC on in-hospital survival in our patient cohort.
This study has several strengths. First, the data were extracted and reviewed by two health care professionals reviewing all medical records. Second, exact dosing times were used to calculate %fT>MIC, and symmetric dosing intervals were not assumed, an assumption that is mostly inaccurate in the hospital setting (31). Third, creatinine clearance was estimated using three different methods, to account for patients with unstable serum creatinine levels and patients whose weight was 30% higher than their ideal body weight. Fourth, the logistic regression model utilized purposeful variable selection as a model-building strategy that included testing for interactions between selected variables. Fifth, the final logistic model was tested for goodness of fit, rigorous regression diagnostics were performed, and the final model was internally validated by bootstrap analysis.
Additionally, the findings of the present study support individualization of the TZP dose with the aim of achieving a threshold of a %fT>MIC of 60%. In settings where therapeutic drug monitoring of TZP is available, concentration monitoring is recommended for critically ill patients with P. aeruginosa bacteremia in order to achieve the pharmacodynamic target of a %fT>MIC of 60%.
In conclusion, we have found that achieving a %fT>MIC of TZP of 60% was associated with improved in-hospital survival in patients with P. aeruginosa bacteremia. Until more data become available, it is prudent to recommend dosing TZP with the aim of achieving the pharmacodynamic target of a %fT>MIC of at least 60% in patients with P. aeruginosa bacteremia.
MATERIALS AND METHODS
This retrospective study was conducted at a secondary university-affiliated hospital with 495 beds. The study methods were approved by the ethics committees at Hillel Yaffe Medical Center, Hadera, Israel, and Robert Gordon University, Aberdeen, Scotland. Patients above 18 years old who had a blood culture positive for P. aeruginosa and who were hospitalized in the Hillel Yaffe Medical Center between January 2012 and October 2018 were reviewed for inclusion. Patients not treated with TZP, for whom treatment was delayed by >96 h from the time of collection of blood for the index blood culture, who had polymicrobial blood culture findings, or for whose isolates the MIC of TZP was not reported were excluded. Moreover, patients who died less than 72 h after a blood sample for culture was obtained were excluded. If a patient had two episodes of P. aeruginosa-positive blood cultures in less than 3 months, only the data for the first episode were included.
Information was extracted from paper-based and electronic patient records. Data for patients with P. aeruginosa-positive blood cultures were extracted from the microbiology laboratory database, as were the MICs for TZP. The extracted data included age, gender, comorbidities, modified APACHE II score (32, 33), Glasgow coma scale, absolute neutrophil count, weight, serum creatinine concentration, TZP dose, and dosing interval. For patients with stable serum creatinine concentrations (defined as a difference of less than 0.3 mg/dl between two consecutive serum creatinine levels), creatinine clearance was estimated using the Cockcroft and Gault equation for patients whose actual body weight was no more than 30% greater than their ideal body weight; otherwise, the Salazar-Corcoran equation was used. In patients with unstable serum creatinine levels, the Jelliffe equation was used.
Pharmacokinetic analysis.
To estimate the %fT>MIC of piperacillin, we utilized the population pharmacokinetics 1-compartment model published by Chen et al. (15). This model was selected because it best fitted the population in our study, reported intersubject variability, was qualified by visual predictive checks, and was validated using nonparametric bootstrap analysis. Using the NONMEM (version 7.4) program, the volume of distribution and clearance were estimated for each patient. Subsequently, for each patient, the free fraction piperacillin concentration was generated every 15 min for the first 48 h of TZP treatment, assuming a mean protein binding of 25%. The cumulative time above the MIC was calculated and divided by 48, giving the estimated %fT>MIC of piperacillin. MICs were determined using a Vitek 2 system (Vitek 2; bioMérieux). The primary outcome was in-hospital survival.
Statistical analysis.
Statistical analysis was performed with SPSS (version 25) and R software. The %fT>MIC threshold associated with improved survival was derived by using the classification and regression tree (CART) analysis function in SPSS (version 25) software, using %fT>MIC and creatinine clearance as continuous independent variables.
To test the influence of the CART-derived %fT>MIC threshold on in-hospital survival, adjusting for significant covariates, a logistic regression model was utilized. Variable selection was performed using purposeful variable selection (34). In brief, candidate variables at a univariate level of significance of a P value of <0.25 were assessed as possible predictors of in-hospital survival. The importance of each variable was tested using the likelihood ratio test with 1 degree of freedom. Variables were retained in the model if their deletion resulted in a change in the likelihood ratio of >3.84. Moreover, the presence of interactions among the retained variables was explored, and significant interactions were added into the final model (34). Goodness of fit was assessed using the Hosmer and Lemeshow test in SPSS (version 25) software, and regression diagnostics were performed using the car package in R software (35) (see the supplemental material). Internal validation of the final model was performed with bootstrap analysis using the boot package in R software (20,000 replications; confidence intervals were calculated using the percentile method; see the supplemental material).
Supplementary Material
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M, Righi E, Viscoli C. 2008. Pseudomonas aeruginosa serious infections: mono or combination antimicrobial therapy? Curr Med Chem 15:517–522. doi: 10.2174/092986708783503186. [DOI] [PubMed] [Google Scholar]
- 3.Bennett JE, Dolin R, Blaser MJ. 2015. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases, 8th ed Saunders, Philadelphia, PA. [Google Scholar]
- 4.Hirsch EB, Cottreau JM, Chang K-T, Caeiro J-P, Johnson ML, Tam VH. 2012. A model to predict mortality following Pseudomonas aeruginosa bacteremia. Diagn Microbiol Infect Dis 72:97–102. doi: 10.1016/j.diagmicrobio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Bodey GP, Jadeja L, Elting L. 1985. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med 145:1621–1629. doi: 10.1001/archinte.145.9.1621. [DOI] [PubMed] [Google Scholar]
- 6.Vidal F, Mensa J, Almela M, Martínez JA, Marco F, Casals C, Gatell JM, Soriano E, de Jimenez Anta MT. 1996. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med 156:2121–2126. doi: 10.1001/archinte.1996.00440170139015. [DOI] [PubMed] [Google Scholar]
- 7.Mendelson MH, Gurtman A, Szabo S, Neibart E, Meyers BR, Policar M, Cheung TW, Lillienfeld D, Hammer G, Reddy S. 1994. Pseudomonas aeruginosa bacteremia in patients with AIDS. Clin Infect Dis 18:886–895. doi: 10.1093/clinids/18.6.886. [DOI] [PubMed] [Google Scholar]
- 8.Vardakas KZ, Tansarli GS, Bliziotis IA, Falagas ME. 2013. β-Lactam plus aminoglycoside or fluoroquinolone combination versus β-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int J Antimicrob Agents 41:301–310. doi: 10.1016/j.ijantimicag.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Wyeth Pharmaceuticals. 2017. Zocyn (piperacillin/tazobactam) package insert. Wyeth Pharmaceuticals, Philadelphia, PA. [Google Scholar]
- 10.Sörgel F, Kinzig M. 1993. The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J Antimicrob Chemother 31(Suppl A):39–60. doi: 10.1093/jac/31.suppl_a.39. [DOI] [PubMed] [Google Scholar]
- 11.Sime FB, Hahn U, Warner MS, Tiong IS, Roberts MS, Lipman J, Peake SL, Roberts JA. 2017. Using population pharmacokinetic modeling and Monte Carlo simulations to determine whether standard doses of piperacillin in piperacillin-tazobactam regimens are adequate for the management of febrile neutropenia. Antimicrob Agents Chemother 61:e00311-17. doi: 10.1128/AAC.00311-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulitta JB, Kinzig M, Jakob V, Holzgrabe U, Sörgel F, Holford N. 2010. Nonlinear pharmacokinetics of piperacillin in healthy volunteers—implications for optimal dosage regimens. Br J Clin Pharmacol 70:682–693. doi: 10.1111/j.1365-2125.2010.03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alobaid AS, Wallis SC, Jarrett P, Starr T, Stuart J, Lassig-Smith M, Mejia JLO, Roberts MS, Roger C, Udy AA, Lipman J, Roberts JA. 2017. Population pharmacokinetics of piperacillin in nonobese, obese, and morbidly obese critically ill patients. Antimicrob Agents Chemother 61:e01276-16. doi: 10.1128/AAC.01276-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai D, Stewart P, Goud R, Gourley S, Hewagama S, Krishnaswamy S, Wallis SC, Lipman J, Roberts JA. 2016. Pharmacokinetics of piperacillin in critically ill Australian indigenous patients with severe sepsis. Antimicrob Agents Chemother 60:7402–7406. doi: 10.1128/AAC.01657-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, Qian Q, Sun M-R, Qian C-Y, Zou S-L, Wang M-L, Wang L-Y. 2016. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with nosocomial infections. Eur J Drug Metab Pharmacokinet 41:363–372. doi: 10.1007/s13318-015-0276-3. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JA, Kirkpatrick CMJ, Roberts MS, Dalley AJ, Lipman J. 2010. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents 35:156–163. doi: 10.1016/j.ijantimicag.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Joint Formulary Committee, British National Formulary. 2017. Piperacillin with tazobactam. BMJ Group and Pharmaceutical Press, London, United Kingdom. [Google Scholar]
- 18.Patel N, Scheetz MH, Drusano GL, Lodise TP. 2010. Identification of optimal renal dosage adjustments for traditional and extended-infusion piperacillin-tazobactam dosing regimens in hospitalized patients. Antimicrob Agents Chemother 54:460–465. doi: 10.1128/AAC.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig WA, Ebert SC. 1992. Continuous infusion of beta-lactam antibiotics. Antimicrob Agents Chemother 36:2577–2583. doi: 10.1128/aac.36.12.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 21.Gerber AU, Craig WA, Brugger HP, Feller C, Vastola AP, Brandel J. 1983. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J Infect Dis 147:910–917. doi: 10.1093/infdis/147.5.910. [DOI] [PubMed] [Google Scholar]
- 22.Mordenti J, Nightingale C, Quintaliani R, Tilton R. 1983. Combination antibiotic therapy: in vivo and in vitro assessment of mode of administration, part 50 In Spitzy KH, Karrer K, Breyer S (ed), Proceedings of the 13th International Congress of Chemotherapy. H. Egermann, Vienna, Austria. [Google Scholar]
- 23.Zelenitsky S, Nash J, Weber Z, Iacovides H, Ariano R. 2016. Targeted benefits of prolonged-infusion piperacillin-tazobactam in an in vitro infection model of Pseudomonas aeruginosa. J Chemother 28:390–394. doi: 10.1080/1120009X.2016.1140858. [DOI] [PubMed] [Google Scholar]
- 24.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen K-M, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, Roberts JA, Lipman J, Starr T, Wallis SC, Paul SK, Margarit Ribas A, De Waele JJ, De Crop L, Spapen H, Wauters J, Dugernier T, Jorens P, Dapper I, De Backer D, Taccone FS, Rello J, Ruano L, Afonso E, Alvarez-Lerma F, Gracia-Arnillas MP, Fernandez F, Feijoo N, Bardolet N, Rovira A, Garro P, Colon D, Castillo C, Fernado J, Lopez MJ, Fernandez JL, Arribas AM, Teja JL, Ots E, Carlos Montejo J, Catalan M, et al. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 25.Ariano RE, Nyhlén A, Donnelly JP, Sitar DS, Harding GKM, Zelenitsky SA. 2005. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann Pharmacother 39:32–38. doi: 10.1345/aph.1E271. [DOI] [PubMed] [Google Scholar]
- 26.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes NJ, Kuti JL, Nicolau DP, Van Wart S, Nicasio AM, Liu J, Lee BJ, Neely MN, Scheetz MH. 2016. Defining clinical exposures of cefepime for Gram-negative bloodstream infections that are associated with improved survival. Antimicrob Agents Chemother 60:1401–1410. doi: 10.1128/AAC.01956-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakoulas G, Geriak M, Nizet V. 2019. Is a reported penicillin allergy sufficient grounds to forgo the multidimensional antimicrobial benefits of β-lactam antibiotics? Clin Infect Dis 68:157–164. doi: 10.1093/cid/ciy557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Velde F, Mouton JW, de Winter BCM, van Gelder T, Koch B. 2018. Clinical applications of population pharmacokinetic models of antibiotics: challenges and perspectives. Pharmacol Res 134:280–288. doi: 10.1016/j.phrs.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 30.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2010. Piperacillin-tazobactam rationale for the EUCAST clinical breakpoints, version 1.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Piperacillin-tazobactam_rationale_Nov2010_v_1.0.pdf.
- 31.Udy AA, Lipman J, Jarrett P, Klein K, Wallis SC, Patel K, Kirkpatrick CMJ, Kruger PS, Paterson DL, Roberts MS, Roberts JA. 2015. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit Care 19:28. doi: 10.1186/s13054-015-0750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Sunenshine RH, Wright M-O, Maragakis LL, Harris AD, Song X, Hebden J, Cosgrove SE, Anderson A, Carnell J, Jernigan DB, Kleinbaum DG, Perl TM, Standiford HC, Srinivasan A. 2007. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis 13:97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosmer DW, Lemeshow S, Sturdivant RX. 2013. Applied logistic regression, 3rd ed Wiley, Hoboken, NJ. [Google Scholar]
- 35.Fox J, Weisberg S. 2010. An R companion to applied regression, 2nd ed Sage Publications, Thousand Oaks, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.