Animal infection models are invaluable in optimizing antimicrobial dosage in humans. Utilization of human-simulated regimens (HSRs) in animal models helps to evaluate antimicrobial efficacy at clinically achievable drug concentrations. To that end, pharmacokinetic studies in infected animals and confirmation of the HSR pharmacokinetic profile are essential in evaluating observed versus expected drug concentrations. We present and compare two murine meropenem-vaborbactam HSR profiles, their potential impact on bacterial killing, and clinical translatability.
KEYWORDS: β-lactams, human-simulated exposure, murine model, pharmacodynamics, pharmacokinetics
ABSTRACT
Animal infection models are invaluable in optimizing antimicrobial dosage in humans. Utilization of human-simulated regimens (HSRs) in animal models helps to evaluate antimicrobial efficacy at clinically achievable drug concentrations. To that end, pharmacokinetic studies in infected animals and confirmation of the HSR pharmacokinetic profile are essential in evaluating observed versus expected drug concentrations. We present and compare two murine meropenem-vaborbactam HSR profiles, their potential impact on bacterial killing, and clinical translatability.
TEXT
Translation of antimicrobial pharmacokinetic and pharmacodynamic (PK/PD) profiles from animal models to humans has become an invaluable tool in optimizing antimicrobial dosing strategies and predicting clinical outcomes (1–3). Murine models of human-simulated antimicrobial exposures have been utilized for >2 decades and have included agents with time-dependent bacterial killing (4, 5), agents with concentration-dependent protein binding (6, 7), and profiles humanized to exposure at the infection site (i.e., epithelial-lining fluid) (8, 9). Administration of human-simulated regimens (HSRs) in animal models ensures that antimicrobial efficacy is evaluated at clinically relevant exposures.
Recently, investigators have employed HSR to increase the translational value of their in vivo experiments; however, it is imperative that the pharmacokinetic profiles mimic human exposure in terms of biologically active free drug across the MIC distribution. With β-lactam–β-lactamase inhibitor or potentiator combinations, this is even more critical because the efficacy of the β-lactam is dependent on the humanized profile of both compounds over the dosing interval (8, 10–13). Here, we offer some considerations with regard to study design that may influence how readers interpret observations derived from murine infection models using human-simulated antimicrobial exposures.
Pharmacokinetic evaluations of human-simulated exposures in animal models may vary depending on the agent and dosing regimen, but several principles are fundamental. A significant criticism is the performance of pharmacokinetic analyses in uninfected animals and subsequent utilization in infection models (14, 15). Authors have described this as a limitation when, in fact, it renders the data suboptimal and uninterpretable from a translation standpoint. Indeed, numerous reports have demonstrated that the pharmacokinetics of antimicrobials can differ markedly in infected versus uninfected mice, as in humans (16–20). Free-drug exposures at infection sites and the volume of distribution can be altered during infection; thus, pharmacokinetic studies in uninfected mice may not reflect the true drug exposure during infection models and subsequently result in altered PK/PD relationship profiles (20, 21). Specific infection sites (e.g., epithelial lining fluid penetration in pneumonia models) also contribute to pharmacokinetic alterations between infected and uninfected animals (16). Comparative pharmacokinetic parameters and profiles should still be described in scenarios where no differences in antimicrobial exposure are observed by investigators (22, 23).
Confirmatory concentration-time profiles are essential in providing readers with the opportunity to evaluate whether the developed HSR profile is similar to simulated exposures derived from single-dose pharmacokinetic analysis (24). The following data illustrate the importance of confirmatory pharmacokinetic analysis. Before conducting the meropenem-vaborbactam murine study, we reproduced the pharmacokinetic profile of the meropenem-vaborbactam murine HSR (originally developed in uninfected mice) of Sabet et al. (14) in the neutropenic thigh infection model, in addition to simulating relevant human exposures (25). All murine studies were IACUC approved. Notably, this regimen (PK1, Sabet et al. regimen [14] [Fig. 1]) consists of 4 intraperitoneal doses of meropenem 300 mg/kg and vaborbactam 50 mg/kg coadministered every 2 h (q2h) over 8 h. We subsequently developed a meropenem-vaborbactam HSR (65/10.8 mg/kg at 0 and 1.25 h, 55/6 mg/kg at 3.5 h, and 50/4 mg/kg at 6 h) administered via subcutaneous injection in a neutropenic thigh infection model (CD-1 female mice; average weight, 20 to 22 g) to yield exposures similar to those achieved in humans after administration of meropenem-vaborbactam 2/2 g q8h as a 3-h infusion (26) (PK2, this study regimen [Fig. 2]) to compare drug exposure between the two dosing regimens. Human profiles were simulated for comparison using the human pharmacokinetic parameters from published data using Phoenix (version 8.1; Certara, Princeton, NJ) (25, 26). In PK1 and PK2, the murine thigh infection model was prepared as previously described (4), except that 150 mg/kg of cyclophosphamide was administered on days −4 and −1 (14). In PK2 only, animals were pretreated with uranyl nitrate as previously described (4, 5). Groups of six mice were sacrificed by CO2 asphyxiation at prespecified time points (n = 7 and 5 for PK1 and PK2, respectively) of the 8-h dosing interval. Confirmatory plasma sampling time points were selected to compare murine drug concentrations with simulated murine concentrations. These time points captured peak and trough concentrations on the murine-simulated profile, as well as murine-simulated concentrations that were expected to be similar to human drug concentrations. Plasma sampling was collected via cardiac puncture as previously described (4), and free-drug concentrations were estimated (14). Murine-simulated regimens were calculated using Phoenix. For PK1, the simulation was constructed using the meropenem-vaborbactam 300/50-mg/kg q2h regimen and matching the simulated output to the free-drug area under the concentration-time curve (fAUC) and percentage of the dosing interval during which free drug concentrations are above the MIC of 8 mg/liter (%fT>MIC 8 mg/liter) to what was reported for both meropenem and vaborbactam to estimate how the proposed profile may look over the 8-h interval (14). Of note, the simulated exposures were based on pharmacokinetics from uninfected mice. The PK2 simulation was based on parameters of previous meropenem pharmacokinetic experiments in infected animals and in the presence of uranyl nitrate (4). Our experience with the PK2 meropenem regimen from previous publications allowed for a robust assessment with five sampling time points (4, 27). Based on equivalent human exposures (i.e., overlapping profiles) of meropenem and vaborbactam, the goal of PK2 was to attain similar meropenem and vaborbactam exposures in the mouse model. The murine-simulated meropenem concentration-time profile therefore served as a target vaborbactam profile. Drug concentration determination was performed using a validated high-performance liquid chromatography assay on murine plasma. The murine plasma assay was linear over the range 0.25 to 50 μg/ml (R2 ≥ 0.998). The coefficients of variation of the quality control samples for meropenem and vaborbactam were ≤6.1%. Interday coefficients of variation were ≤4.1% for meropenem and ≤4.0% for vaborbactam. The accuracy for interday and intraday quality control samples for both compounds was ≥95%.
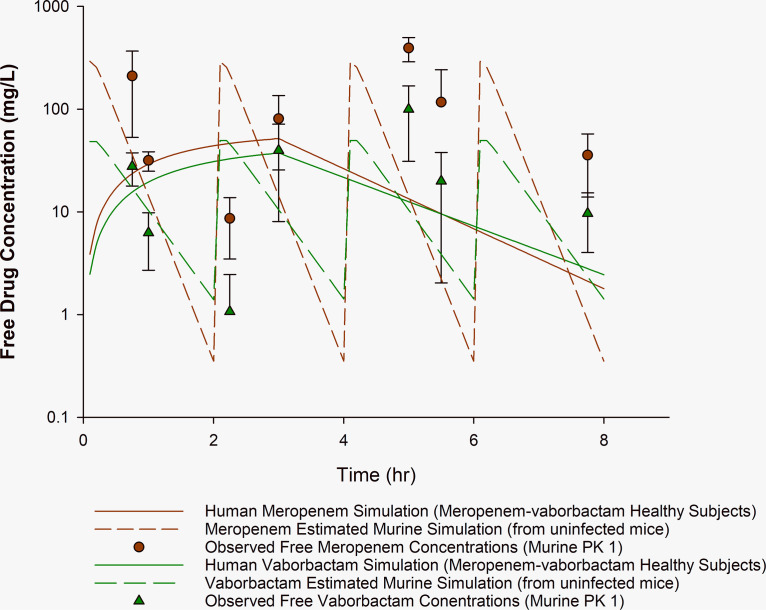
FIG 1.
Observed meropenem and vaborbactam murine concentration-time profile compared with human simulation PK1. Observed concentrations of free meropenem and vaborbactam in the neutropenic murine thigh infection model after meropenem-vaborbactam 300/50 mg/kg administered intraperitoneally q2h. Plasma sampling occurred at 0.75, 1, 2.25, 3, 5, 5.5, and 7.75 h. Solid lines, human simulations derived from healthy volunteers for meropenem and vaborbactam. Dashed lines, estimated simulated murine exposure based on indices reported by Sabet et al. (14) from uninfected mice. Circles (red) and triangles (green), observed murine meropenem and vaborbactam concentrations (means ± SD), respectively.
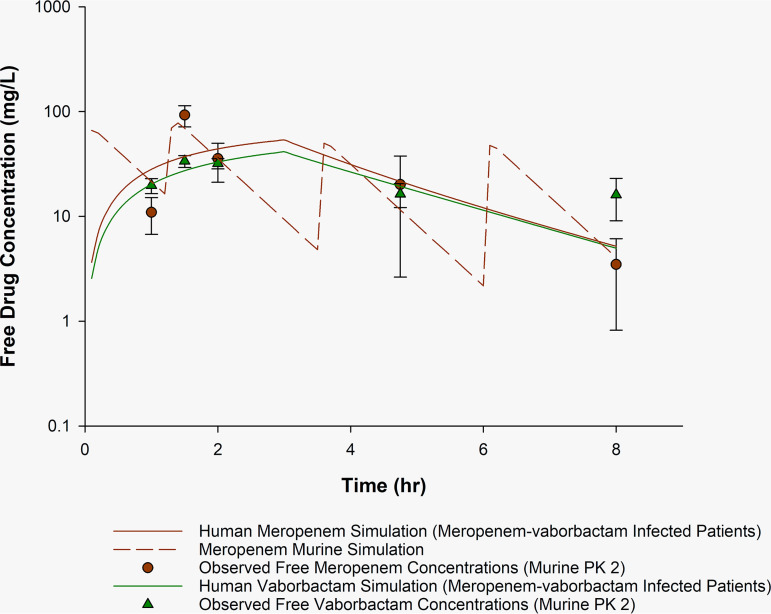
FIG 2.
Observed meropenem and vaborbactam murine concentration-time profile compared with human simulation PK2. Observed concentrations of free meropenem and vaborbactam in the neutropenic murine thigh infection model receiving meropenem-vaborbactam 65/10.8 mg/kg at 0 and 1.25 h, 55/6 mg/kg at 3.5 h, and 50/4 mg/kg at 6 h. Plasma sampling occurred at 1, 1.5, 2, 4.75, and 8 h. Solid lines, simulated human meropenem (red) and vaborbactam (green) concentrations derived from infected patients in the phase 3 meropenem-vaborbactam trials. Dashed line (red), murine-simulated exposure of meropenem and vaborbactam. Circles (red) and triangles (green), observed murine meropenem and vaborbactam concentrations (means ± SD), respectively.
Pharmacokinetic parameters of the simulated human regimens and evaluated murine regimens for PK1 and PK2 are described in Table 1. For comparison, pharmacokinetic data from healthy volunteers (25) and a population pharmacokinetic model (derived from phase 3 clinical trials) (26) were simulated. Figure 1 (PK1) depicts our reproduced meropenem-vaborbactam murine profile (14). Notably, we observed an initial meropenem concentration similar to the previously reported peak concentration (210 versus 260 mg/liter) (14). Compared with the simulated murine meropenem profiles, elevated meropenem concentrations were observed, particularly at the end of the murine dosing interval, with observed meropenem concentrations of 393 ± 104 and 36 ± 22 mg/liter at 5 and 7.75 h (trough), respectively, compared with murine-simulated exposures of 14 and 1 mg/liter, respectively. To avoid exposures that are inconsistent with those of humans during the pharmacodynamic investigations, confirmation of the proposed pharmacokinetic profile should be evaluated before initiation of these studies. Furthermore, the presence of infection may explain this supratherapeutic exposure, highlighting potential pharmacokinetic differences in infected versus uninfected animals. The observed elevated concentrations may explain the unexpected meropenem efficacy (bacterial stasis) reported by Sabet et al. (14, 28) against a range of KPC-harboring Enterobacterales, including isolates with meropenem MICs of >64 mg/liter. Similarly, using the same meropenem-vaborbactam regimen, unexpected differences in bacterial density reduction were observed between meropenem-vaborbactam and meropenem alone against Pseudomonas aeruginosa despite the same MIC (29). Confirmatory pharmacokinetic studies of the HSR are ultimately required to determine whether pharmacokinetic discrepancies, like those described above, are products of aberrant drug exposures (e.g., due to drug accumulation, murine drug-drug interactions when combination therapy is administered). In comparison, the confirmatory pharmacokinetic studies for PK2, as depicted in Fig. 2 and Table 1, show a meropenem-vaborbactam HSR that results in observed murine concentrations and pharmacokinetic indices comparable to those of the human profile. For example, at the 4.75- and 8-h time points, the observed murine meropenem concentration was 20 ± 18 and 4 ± 4 mg/liter, respectively, compared to target murine-simulated exposures of 11 and 4 mg/liter, respectively.
TABLE 1.
Estimated meropenem-vaborbactam murine pharmacodynamic parameters compared with humans for the two murine regimens
| Regimena | %fT>MIC at MIC (mg/liter) of: |
fAUC0–24 (mg · h/liter) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ||
| PK1 (Sabet et al. regimen [14]) | ||||||||||
| Meropenem (2 g i.v. q8h over 3 h), human (25),b healthy subjects | 100 | 100 | 98 | 85 | 70 | 54 | 33 | 0 | 0 | 533 |
| Meropenem (300 mg/kg i.p. q2h), murine (14), reported | 51 | 1,572 | ||||||||
| Meropenem (300 mg/kg i.p. q2h), murine (PK1),c simulation | 95 | 85 | 75 | 65 | 55 | 45 | 35 | 25 | 20 | 1,387 |
| Vaborbactam (2 g i.v. q8h over 3 h), human (25),b healthy subjects | 413e | |||||||||
| Vaborbactam (50 mg/kg i.p. q2h), murine (14), reported | 360e | |||||||||
| Vaborbactam (50 mg/kg i.p. q2h), murine (PK1),d simulation | 391e | |||||||||
| PK2 (this study regimen) | ||||||||||
| Meropenem (2 g i.v. q8h over 3 h), human (26),f infected patients | 100 | 100 | 100 | 100 | 86 | 63 | 37 | 0 | 0 | 623 |
| Meropenem (various doses s.c.), murineg | 100 | 100 | 100 | 94 | 75 | 55 | 29 | 5 | 0 | 560 |
| Vaborbactam (2 g i.v. q8h over 3 h), human (26),f infected patients | 504e | |||||||||
| Vaborbactam (various doses s.c.) murineg | 560e | |||||||||
i.v., intravenous; i.p., intraperitoneal; s.c., subcutaneous.
Parameters from Wenzler et al. (25): meropenem: elimination rate constant (ke), 0.673; volume of distribution (V), 16.3; vaborbactam: Ke, 0.55; V, 17.6.
Parameters used in simulation: central volume of distribution (V1), 0.6; absorption rate constant (k01), 17; elimination rate constant (k10), 3.7 (uninfected mice).
Parameters used in simulation: V1, 0.7; k01, 17; k10, 2 (uninfected mice).
Other described human vaborbactam fAUC0–24s included 343 mg · h/liter (14) and ∼500 mg · h/liter (33).
Population model using phase 3 study patients. Assumptions used for simulation approximate the median from the derived study population: age, 58 years; estimated glomerular filtration rate, 90 ml/min/1.73 m3; body surface area, 1.88 m3; height, 168 cm. Meropenem: k10, 0.54; rate constant to the peripheral from the central compartment (k12), 0.09; rate constant to the central from the peripheral compartment (k21), 0.61; volume of distribution in the central compartment (Vc), 17.4; volume of distribution in the peripheral compartment (Vp), 2.5; vaborbactam: k10, 0.48; k12, 0.18; k21, 2.21; Vc, 16.9; Vp, 1.41.
PK2 meropenem-vaborbactam regimen: 65/10.8 mg/kg at 0 and 1.25 h, 55/6 mg/kg at 3.5 h, and 50/4 mg/kg at 6 h. Mice pretreated with uranyl nitrate before study (day −3). Simulated murine meropenem parameters: V1, 0.04; k01, 1.34; k10, 27.13.
As described in Table 1, Sabet and colleagues (14, 28, 30) compared murine %fT>MIC for meropenem and free area under the curve from 0 to 24 h (fAUC0–24) for vaborbactam with human data, utilizing PK/PD relationships that best correlate with efficacy for each agent. Unfortunately, key pharmacokinetic parameters and indices were not reported, making interpretation of the results challenging. The comparison of the murine and human exposures was solely based on the percentage of the dosing interval during which meropenem exceeds a single MIC of 8 mg/liter (i.e., %fT>8 mg/liter) (14). The murine exposure may in fact be similar to the human profile at other MICs, but given the discordant meropenem exposures (i.e., 4-fold difference) in the murine model compared with that in humans (meropenem fAUC0–24, 1,572 versus 402 mg · h/liter, respectively) (14), the %fT above other MICs should be presented. Additionally, Table 1 (PK2) provides an example displaying the %fT>MIC for a murine meropenem regimen compared with the human profile. By providing the %fT>MIC data over a range of clinically relevant MICs, readers can assess how well the murine exposure compares with the human PK/PD relationships against all included isolates at different meropenem-vaborbactam MICs. This includes isolates with MICs around the CLSI (i.e., 4 mg/liter) and EUCAST (i.e., 8 mg/liter) breakpoints (31, 32).
In summary, consideration of the points above for future studies utilizing humanized drug exposures in mice will allow for valid inferences with respect to breakpoint determination or activity against multidrug-resistant isolates when clinical data are difficult to obtain. No model will perfectly simulate human exposure in mice; however, these considerations are imperative for translation of findings from mice to humans.
ACKNOWLEDGMENTS
We thank Kamilia Abdelraouf for review of the manuscript and critical conversations. We also thank Christina Sutherland for expertise in the development and conduction of the meropenem and vaborbactam high-performance liquid chromatography assay.
We recognize Alissa Padget, Charlie Cote, Deborah Santini, Janice Cunningham, Elias Mullane, Courtney Bouchard, Jennifer Tabor-Rennie, Rebecca Stewart, Nicole DeRosa, Kimelyn Greenwood, Lauren McLellan, Elizabeth Cyr, Elizabeth Martin, Olesya Slipchuk, Maxwell Lasko, Iris Chen, Sergio Reyes, and Wendylee Rodriguez from the Center for Anti-Infective Research and Development for vital assistance in this study.
This project was internally funded by the Center for Anti-Infective Research and Development.
REFERENCES
- 1.Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, Drusano GL, Hoover JL. 2019. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 63:e02307-18. doi: 10.1128/AAC.02307-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19:261–268. doi: 10.1016/S0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis 44:79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 4.Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. 2017. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of Gram-negative bacteria in a murine thigh infection model. Antimicrob Agents Chemother 61:e01022-17. doi: 10.1128/AAC.01022-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolau DP, Onyeji CO, Zhong M, Tessier PR, Banevicius MA, Nightingale CH. 2000. Pharmacodynamic assessment of cefprozil-against Streptococcus pneumoniae: implications for breakpoint determinations. Antimicrob Agents Chemother 44:1291–1295. doi: 10.1128/AAC.44.5.1291-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thabit AK, Monogue ML, Nicolau DP. 2016. Eravacycline pharmacokinetics and challenges in defining humanized exposure in vivo. Antimicrob Agents Chemother 60:5072–5075. doi: 10.1128/AAC.00240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monogue ML, Thabit AK, Hamada Y, Nicolau DP. 2016. Antibacterial efficacy of eravacycline in vivo against Gram-positive and Gram-negative organisms. Antimicrob Agents Chemother 60:5001–5005. doi: 10.1128/AAC.00366-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asempa TE, Motos A, Abdelraouf K, Bissantz C, Zampaloni C, Nicolau DP. 2019. Efficacy of human-simulated epithelial lining fluid exposure of meropenem-nacubactam combination against class A serine β-lactamase-producing Enterobacteriaceae in the neutropenic murine lung infection model. Antimicrob Agents Chemother 63:e02382-18. doi: 10.1128/AAC.02382-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidd JM, Abdelraouf K, Nicolau DP. 2019. Comparative efficacy of human-simulated epithelial lining fluid exposures of tedizolid, linezolid and vancomycin in neutropenic and immunocompetent murine models of staphylococcal pneumonia. J Antimicrob Chemother 74:970–977. doi: 10.1093/jac/dky513. [DOI] [PubMed] [Google Scholar]
- 10.Monogue ML, Giovagnoli S, Bissantz C, Zampaloni C, Nicolau DP. 2018. In vivo efficacy of meropenem with a novel non-β-lactam–β-lactamase inhibitor, nacubactam, against Gram-negative organisms exhibiting various resistance mechanisms in a murine complicated urinary tract infection model. Antimicrob Agents Chemother 62:e02596-17. doi: 10.1128/AAC.02596-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. In vivo efficacy of humanized exposures of ceftazidime-avibactam in comparison with ceftazidime against contemporary Enterobacteriaceae isolates. Antimicrob Agents Chemother 58:6913–6919. doi: 10.1128/AAC.03267-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelraouf K, Stainton SM, Nicolau DP. 2019. In vivo pharmacodynamic profile of ceftibuten-clavulanate combination against extended-spectrum-β-lactamase-producing Enterobacteriaceae in the murine thigh infection model. Antimicrob Agents Chemother 63:e00145-19. doi: 10.1128/AAC.00145-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avery LM, Abdelraouf K, Nicolau DP. 2018. Assessment of the In vivo efficacy of WCK 5222 (cefepime-zidebactam) against carbapenem-resistant Acinetobacter baumannii in the neutropenic murine lung infection model. Antimicrob Agents Chemother 62:e00948-18. doi: 10.1128/AAC.00948-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabet M, Tarazi Z, Nolan T, Parkinson J, Rubio-Aparicio D, Lomovskaya O, Dudley MN, Griffith DC. 2017. Activity of meropenem-vaborbactam in mouse models of infection due to KPC-producing carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 62:e01446-17. doi: 10.1128/AAC.01446-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers HF, Basuino L, Diep BA, Steenbergen J, Zhang S, Tattevin P, Alder J. 2009. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob Agents Chemother 53:1463–1467. doi: 10.1128/AAC.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keel RA, Crandon JL, Nicolau DP. 2012. Pharmacokinetics and pulmonary disposition of tedizolid and linezolid in a murine pneumonia model under variable conditions. Antimicrob Agents Chemother 56:3420–3422. doi: 10.1128/AAC.06121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crandon JL, Kim A, Nicolau DP. 2009. Comparison of tigecycline penetration into the epithelial lining fluid of infected and uninfected murine lungs. J Antimicrob Chemother 64:837–839. doi: 10.1093/jac/dkp301. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe A, Matsumoto K, Igari H, Uesato M, Yoshida S, Nakamura Y, Morita K, Shibuya K, Matsubara H, Yoshino I, Kamei K. 2010. Comparison between concentrations of amphotericin B in infected lung lesion and in uninfected lung tissue in a patient treated with liposomal amphotericin B (AmBisome). Int J Infect Dis 14:e220–e223. doi: 10.1016/j.ijid.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Oshima K, Nakamura S, Iwanaga N, Takemoto K, Miyazaki T, Yanagihara K, Miyazaki Y, Mukae H, Kohno S, Izumikawa K. 2017. Efficacy of high-dose meropenem (six grams per day) in treatment of experimental murine pneumonia induced by meropenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 61:e02056-16. doi: 10.1128/AAC.02056-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez D, Schmidt S, Derendorf H. 2013. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin Microbiol Rev 26:274–288. doi: 10.1128/CMR.00092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong J, Zhu Q, Yang S, Zhao Y, Cui L, Zhuang F, Qiu Y, Cao J. 2019. Comparison of pharmacokinetics of tilmicosin in healthy pigs and pigs experimentally infected with Actinobacillus pleuropneumoniae. N Z Vet J 67:257–263. doi: 10.1080/00480169.2019.1633434. [DOI] [PubMed] [Google Scholar]
- 22.Housman ST, Crandon JL, Nichols WW, Nicolau DP. 2014. Efficacies of ceftazidime-avibactam and ceftazidime against Pseudomonas aeruginosa in a murine lung infection model. Antimicrob Agents Chemother 58:1365–1371. doi: 10.1128/AAC.02161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bulik CC, Okusanya ÓO, Lakota EA, Forrest A, Bhavnani SM, Hoover JL, Andes DR, Ambrose PG. 2017. Pharmacokinetic-pharmacodynamic evaluation of gepotidacin against Gram-positive organisms using data from murine infection models. Antimicrob Agents Chemother 61:e00115-16. doi: 10.1128/AAC.00115-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drusano GL, Louie A. 2019. Breakpoint determination when multiple organisms are tested for effect targets. Eur J Pharm Sci 130:196–199. doi: 10.1016/j.ejps.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Wenzler E, Gotfried MH, Loutit JS, Durso S, Griffith DC, Dudley MN, Rodvold KA. 2015. Meropenem-RPX7009 concentrations in plasma, epithelial lining fluid, and alveolar macrophages of healthy adult subjects. Antimicrob Agents Chemother 59:7232–7239. doi: 10.1128/AAC.01713-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration. 2017. Center for Drug Evaluation and Research application number 209776Orig1s000: clinical pharmacology and biopharmaceutics review(s) addendum. U.S. Food and Drug Administration, Silver Spring, MD: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209776Orig1s000ClinPharmR.pdf. [Google Scholar]
- 27.Kidd JM, Abdelraouf K, Nicolau DP. 2019. Development of neutropenic murine models of iron overload and depletion to study the efficacy of siderophore-antibiotic conjugates. Antimicrob Agents Chemother 64:1–8. doi: 10.1128/AAC.01961-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith DC, Sabet M, Tarazi Z, Lomovskaya O, Dudley MN. 2018. Pharmacokinetics/pharmacodynamics of vaborbactam, a novel beta-lactamase inhibitor, in combination with meropenem. Antimicrob Agents Chemother 63:e01659-18. doi: 10.1128/AAC.01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabet M, Tarazi Z, Griffith DC. 2018. Activity of meropenem-vaborbactam against Pseudomonas aeruginosa and Acinetobacter baumannii in a neutropenic mouse thigh infection model. Antimicrob Agents Chemother 63:e01665-18. doi: 10.1128/AAC.01665-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolau DP. 2008. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis 47:S32–S40. doi: 10.1086/590064. [DOI] [PubMed] [Google Scholar]
- 31.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing—29th ed CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 32.European Committee on Antimicrobial Susceptibility Testing. 2020. Breakpoint tables for interpretation of MICs and zone diameters. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf.
- 33.Sabet M, Tarazi Z, Rubio-Aparicio D, Nolan TG, Parkinson J, Lomovskaya O, Dudley MN, Griffith DC. 2017. Activity of simulated human dosage regimens of meropenem and vaborbactam against carbapenem-resistant enterobacteriaceae in an in vitro hollow-fiber model. Antimicrob Agents Chemother 62:e01969-17. doi: 10.1128/AAC.01969-17. [DOI] [PMC free article] [PubMed] [Google Scholar]