Mycobacterium abscessus is a highly drug-resistant nontuberculous mycobacterium (NTM). Efforts to discover new treatments for M. abscessus infections are accelerating, with a focus on cell wall synthesis proteins (M. abscessus l,d-transpeptidases 1 to 5 [LdtMab1 to LdtMab5] and d,d-carboxypeptidase) that are targeted by β-lactam antibiotics.
KEYWORDS: antibiotic resistance, mycobacteria, bacteria, inhibitor, antibiotics, ceftaroline, imipenem, diazabicyclooctane, Mycobacterium abscessus
ABSTRACT
Mycobacterium abscessus is a highly drug-resistant nontuberculous mycobacterium (NTM). Efforts to discover new treatments for M. abscessus infections are accelerating, with a focus on cell wall synthesis proteins (M. abscessus l,d-transpeptidases 1 to 5 [LdtMab1 to LdtMab5] and d,d-carboxypeptidase) that are targeted by β-lactam antibiotics. A challenge to this approach is the presence of chromosomally encoded β-lactamase (BlaMab). Using a mechanism-based approach, we found that a novel ceftaroline-imipenem combination effectively lowered the MICs of M. abscessus isolates (MIC50 ≤ 0.25 μg/ml; MIC90 ≤ 0.5 μg/ml). Combining ceftaroline and imipenem with a β-lactamase inhibitor, i.e., relebactam or avibactam, demonstrated only a modest effect on susceptibility compared to each of the β-lactams alone. In steady-state kinetic assays, BlaMab exhibited a lower Ki app (0.30 ± 0.03 μM for avibactam and 136 ± 14 μM for relebactam) and a higher acylation rate for avibactam (k2/K = 3.4 × 105 ± 0.4 × 105 M−1 s−1 for avibactam and 6 × 102 ± 0.6 × 102 M−1 s−1 for relebactam). The kcat/Km was nearly 10-fold lower for ceftaroline fosamil (0.007 ± 0.001 μM−1 s−1) than for imipenem (0.056 ± 0.006 μM−1 s−1). Timed mass spectrometry captured complexes of avibactam and BlaMab, LdtMab1, LdtMab2, LdtMab4, and d,d-carboxypeptidase, whereas relebactam bound only BlaMab, LdtMab1, and LdtMab2. Interestingly, LdtMab1, LdtMab2, LdtMab4, LdtMab5, and d,d-carboxypeptidase bound only to imipenem when incubated with imipenem and ceftaroline fosamil. We next determined the binding constants of imipenem and ceftaroline fosamil for LdtMab1, LdtMab2, LdtMab4, and LdtMab5 and showed that imipenem bound >100-fold more avidly than ceftaroline fosamil to LdtMab1 and LdtMab2 (e.g., Ki app or Km of LdtMab1 = 0.01 ± 0.01 μM for imipenem versus 0.73 ± 0.08 μM for ceftaroline fosamil). Molecular modeling indicates that LdtMab2 readily accommodates imipenem, but the active site must widen to ≥8 Å for ceftaroline to enter. Our analysis demonstrates that ceftaroline and imipenem binding to multiple targets (l,d-transpeptidases and d,d-carboxypeptidase) and provides a mechanistic rationale for the effectiveness of this dual β-lactam combination in M. abscessus infections.
INTRODUCTION
Mycobacterium abscessus is currently recognized as an opportunistic pathogen among immunocompromised hosts. Unfortunately, the number of occurrences of M. abscessus infections has also significantly increased across the world (1). In certain regions of the world, M. abscessus is now recognized as the second most frequently reported cause of nontuberculous mycobacterial (NTM) infection (2). Regrettably, M. abscessus infections are difficult to eradicate, with resistance not only to first-line antituberculosis drugs but also to most recently developed antibiotics (3).
The most commonly used regimen of amikacin, imipenem, and clarithromycin is associated with 40% treatment failure and poor clinical outcomes; more than half of patients with pulmonary disease remain culture positive or relapse after treatment (3). Existing treatment regimens for M. abscessus increasingly employ β-lactams without combination with a β-lactamase inhibitor, even though M. abscessus produces a broad-spectrum chromosomal β-lactamase, BlaMab. To our knowledge, the understanding of the mechanistic and biochemical basis of β-lactam efficacy and the role of β-lactamase inhibitors in improving susceptibility is still insufficient.
The inhibition of the β-lactamase of M. abscessus (BlaMab), a serine class A β-lactamase, using diazabicyclooctanes (DBO) inhibitors (avibactam, relebactam, or nacubactam) was recently reported to restore susceptibility to β-lactam antibiotics and inhibit growth (4–9). In addition to their ability to penetrate the mycobacterial cell wall, the DBOs have high “on” rates and very low “off” rates; therefore, they form long-lived stable intermediates that can lead to sustained inhibition of BlaMab. Building upon this and a previous observation that tested the ability of ceftaroline and imipenem in combination with ceftazidime to lower MICs (10), we explored the ability of a novel dual β-lactam combination (ceftaroline and imipenem [Fig. 1]) to inhibit the growth of M. abscessus in cell-based assays. We further investigated whether DBOs in current clinical use (avibactam and relebactam) would potentiate the action of either of these agents, with a focus on relebactam, as it is currently FDA approved for use in the combination imipenem-cilastatin-relebactam. To this end, we assessed the susceptibilities of a collection of M. abscessus strains representing different clinical isolates. We next determined the biochemical parameters and inactivation kinetics (Ki app, k2/K, koff, and Kd [dissociation constant]) of relebactam and avibactam. We used timed mass spectrometry to trap intermediates in the inactivation pathway of BlaMab and evaluated the different binding properties of active compounds to multiple target proteins, including BlaMab, five l,d-transpeptidases (LdtMab1 to LdtMab5), and d,d-carboxypeptidase. Molecular modeling was used to further shed light on the structural aspects that support these findings. Lastly, we advance a hypothesis explaining these molecular and microbiological findings and the functional significance of target redundancy in β-lactam therapy.
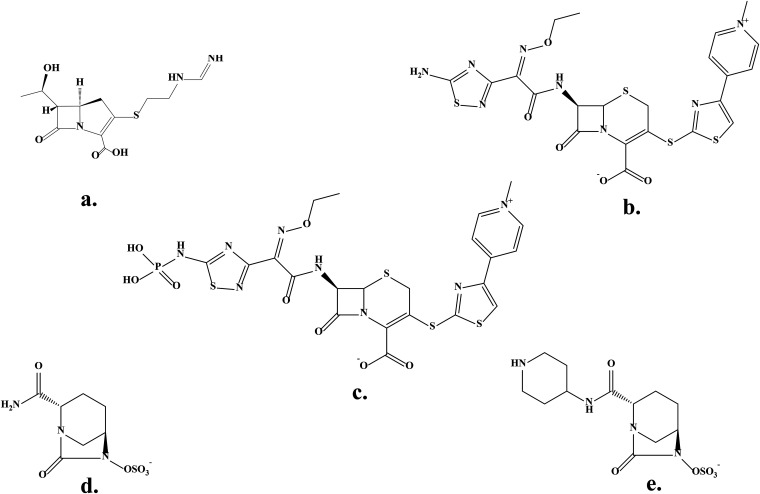
FIG 1.
Chemical structures of β-lactams and β-lactamase inhibitors. (a) Imipenem; (b) ceftaroline; (c) ceftaroline fosamil; (d) avibactam; (e) relebactam.
RESULTS
Cell-based assays determining susceptibility of M. abscessus.
Cell-based assays determining ceftaroline and imipenem susceptibility (MICs), with or without relebactam and avibactam, were performed using an initial screening collection of 20 clinical isolates of M. abscessus (Table 1).
TABLE 1.
MICs for 20 Mycobacterium abscessus clinical strains and the reference strain ATCC 19977 of ceftaroline and imipenem with and without relebactam (REL) and avibactam (AVI)
| M. abscessus isolate | MIC (μg/ml) of: |
|||||
|---|---|---|---|---|---|---|
| Ceftaroline |
Imipenem |
|||||
| Alone | +REL (4 μg/ml) | +AVI (4 μg/ml) | Alone | +REL (4 μg/ml) | +AVI (4 μg/ml) | |
| UHCMC 1 | 8 | 4 | 1 | 1 | 1 | 1 |
| UHCMC 2 | 16 | 2 | 2 | 1 | 2 | 1 |
| UHCMC 3 | 16 | 4 | 0.5 | 2 | 2 | 0.5 |
| UHCMC 4 | 16 | 4 | 0.25 | 2 | 2 | 1 |
| UHCMC 5 | 8 | 2 | 0.5 | 1 | 1 | 1 |
| UHCMC 6 | 32 | 4 | 1 | 2 | 2 | 1 |
| UHCMC 7 | 8 | 4 | 0.5 | 1 | 1 | 1 |
| UHCMC 8 | 8 | 4 | 1 | 1 | 1 | 1 |
| UHCMC 9 | 4 | 2 | 0.5 | 0.5 | 1 | 1 |
| UHCMC 10 | 4 | 1 | 0.5 | 1 | 1 | 1 |
| Metro 1 | 4 | 2 | 1 | 1 | 1 | 1 |
| Metro 2 | 32 | 4 | 1 | 2 | 2 | 0.5 |
| Metro 3 | 4 | 2 | 0.25 | 1 | 2 | 1 |
| Metro 4 | 4 | 1 | 0.5 | 2 | 2 | 1 |
| Metro 5 | 16 | 2 | 1 | 1 | 1 | 1 |
| Metro 6 | 4 | 2 | 0.5 | 1 | 1 | 1 |
| Metro 7 | 4 | 1 | 1 | 2 | 2 | 0.5 |
| Metro xx2 | 8 | 4 | 0.5 | 1 | 1 | 1 |
| Metro xx3 | 16 | 8 | 1 | 1 | 2 | 1 |
| UHCMC RodI2 | 8 | 4 | 0.25 | 1 | 1 | 1 |
| ATCC 19977 | 16 | 4 | 1 | 1 | 1 | 1 |
As expected under these conditions (Middlebrook 7H9 broth), in vitro activity of ceftaroline alone against M. abscessus was limited, with a MIC50 of 8 μg/ml and a MIC90 of 16 μg/ml. The addition of relebactam at a concentration of 4 μg/ml lowered MICs for ceftaroline by only one to two dilutions against most isolates. Interestingly, a difference between the potencies of relebactam and avibactam was observed; adding avibactam to ceftaroline lowered MICs by four or more dilutions. In contrast to our findings with ceftaroline, adding relebactam or avibactam at concentration of 4 μg/ml did not lower MICs of imipenem; its MIC50 and MIC90 remained at 0.5 and 2 μg/ml, respectively. We concluded at this point that avibactam better enhanced susceptibility to ceftaroline than that to imipenem.
Based on the above findings, we endeavored to examine the synergistic effect of adding ceftaroline at a fixed concentration of 1 μg/ml and relebactam at 4 μg/ml to serial dilutions of imipenem against an extended set of 55 clinical isolates. As shown in Table 2 and Fig. 2 (also, see Fig. S1 in the supplemental material), the combination of ceftaroline at a fixed concentration of 1 μg/ml and imipenem lowered the MIC50 and MIC90 of clinical isolates by two dilutions or more, indicating synergy. The fractional inhibitory concentration (FIC) of the combination was 0.28. Addition of 4 μg/ml relebactam to this combination did not demonstrate a greater effect, suggesting that the in vitro impact is predominantly driven by the dual β-lactam combination. Similar MIC results were observed with the M. abscessus type strain, ATCC 19977.
TABLE 2.
MIC50s and MIC90s for 55 M. abscessus clinical strains of ceftaroline, imipenem, and ceftaroline (CEF)-imipenem with and without relebactam (REL)
| Drug | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|---|
| CEF | 2–32 | 8 | 32 |
| Imipenem | 0.5–2 | 1 | 2 |
| CEF + avibactam (4 μg/ml)a | 0.25–2 | 0.5 | 1 |
| Imipenem + CEF (1 μg/ml) | <0.001–2 | 0.25 | 0.5 |
| Imipenem + CEF (1 μg/ml) + REL (4 μg/ml) | <0.01–2 | 0.25 | 0.5 |
MICs for 21 isolates only.
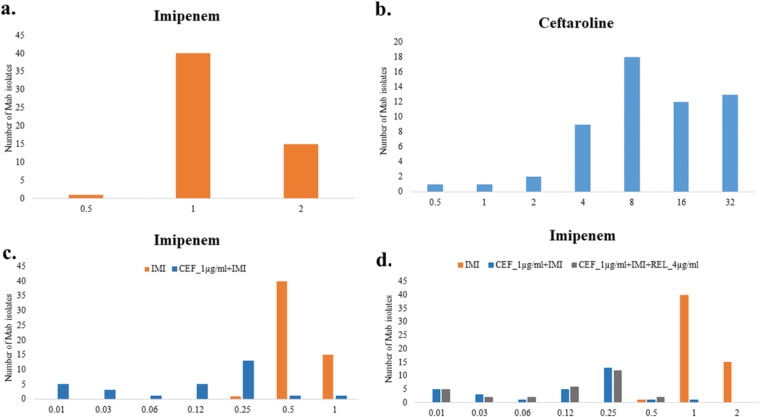
FIG 2.
MIC distributions of imipenem (IMI) (a), ceftaroline (CEF) (b), imipenem with 1 μg/ml ceftaroline (c), and imipenem with 1 μg/ml ceftaroline and 4 μg/ml relebactam (REL) (d) against 55 M. abscessus clinical strains. The MIC data are presented in Table S1.
Steady-state kinetics of BlaMab inactivation.
To correctly interpret the MICs above and to understand the biochemical basis of the effects of avibactam and relebactam on BlaMab of M. abscessus, this β-lactamase was purified to homogeneity.
Our analyses determined that BlaMab is inhibited more rapidly by avibactam than by relebactam (k2/K is 500-fold greater) (Table 3). In contrast, the koff values are similar (1 × 10−3 ± 0.1 × 10−3 s−1 and 2 × 10−3 ± 0.2 × 10−3 s−1 for relebactam and avibactam, respectively). The residence time half-life for relebactam was twice that for avibactam (11.6 ± 1.2 min versus 5.8 ± 0.6 min), but the carbamylation rate of avibactam (3.4 × 105 ± 0.4 × 105 M−1 s−1) was much greater than that of relebactam (6.0 × 102 ± 0.6 × 102 M−1 s−1), resulting in a Kd for avibactam (6.0 ± 0.6 nM) that is nearly 300 times lower than that for relebactam (1.7 × 103 ± 0.2 × 103 nM). The Ki app for relebactam (136 ± 14 μM) is appreciably greater than that for avibactam (0.30 ± 0.03 μM), and the partition ratio (kcat/kinact = 3) indicates some turnover of relebactam by BlaMab.
TABLE 3.
Kinetic parameters of BlaMab inhibition by relebactam (REL) and avibactam (AVI)
| Compound | Ki app (μM) | k2/K (M−1 s−1) | Koff (s−1) | t1/2 (min)a | Kd (nM)b | kcat/kinact |
|---|---|---|---|---|---|---|
| REL | 136 ± 14 | 6.0 × 102 ± 0.6 × 102 | 1.0 × 10−3 ± 0.1 × 10−3 | 11.6 ± 1.2 | 1.7 × 103 ± 0.2 × 103 | 3 |
| AVI | 0.30 ± 0.03 | 3.4 × 105 ± 0.4 × 105 | 2.0 × 10−3 ± 0.2 × 10−3 | 6.0 ± 0.6 | 6 ± 0.6 | 1 |
Residence time half-life (ln2/koff).
Kd = Koff/(k2/K).
The hydrolysis of imipenem and ceftaroline fosamil by BlaMab was next measured (Table 4). We determined that BlaMab kcat values for imipenem and ceftaroline fosamil were 0.95 ± 0.09 s−1 and 4.7 ± 0.5 s−1, respectively. Km values were 17 ± 2 μM for imipenem and 682 ± 70 μM for ceftaroline fosamil. Catalytic efficiencies were 0.056 ± 0.006 μM−1 s−1 (imipenem) and 0.007 ± 0.001 μM−1 s−1 (ceftaroline fosamil), which are indicative of their being rather poor substrates for hydrolysis by BlaMab.
TABLE 4.
Kinetic parameters of BlaMab hydrolysis of ceftaroline fosamil and imipenem
| Compound | kcat (s−1) | Km (μM) | kcat/Km (μM−1 s−1) |
|---|---|---|---|
| Imipenem | 0.95 ± 0.09 | 17 ± 2 | 0.056 ± 0.006 |
| Ceftaroline fosamil | 4.7 ± 0.5 | 682 ± 70 | 0.007 ± 0.001 |
Ldt binding assay.
Further interpretation of the MICs required determination of binding affinities (Ki app or Km values) of imipenem and ceftaroline fosamil for the Ldts (Table 5; also, see Table S2). Our analysis determined that (i) LdtMab1 binds imipenem with a higher affinity than ceftaroline fosamil (0.007 ± 0.001 μM versus 0.727 ± 0.080 μM), (ii) LdtMab2 binds imipenem with a higher affinity than ceftaroline fosamil (0.006 ± 0.001 μM versus 1.06 ± 0.19 μM), and (iii) LdtMab4 binds imipenem with a higher affinity than ceftaroline fosamil (14 ± 3 μM versus >100 μM). Ki app values of imipenem and ceftaroline fosamil were both >100 μM for LdtMab5(H6) (LdtMab5 with the His tag).
TABLE 5.
Ki app values of imipenem and ceftaroline fosamil for LdtMab1, LdtMab2, LdtMab4, and LdtMab5(H6)
| l,d-Transpeptidase |
Ki app (μM) of: |
|
|---|---|---|
| Imipenem | Ceftaroline fosamil | |
| LdtMab1 | 0.007 ± 0.001 | 0.727 ± 0.080 |
| LdtMab2 | 0.006 ± 0.001 | 1.06 ± 0.19 |
| LdtMab4 | 14 ± 3 | >100 |
| LdtMab5(H6) | >100 | >100 |
Timed mass spectrometry analysis. (i) BlaMab.
We next investigated the nature of the covalent binding of imipenem and ceftaroline fosamil to BlaMab. Our goal was to investigate acyl enzyme formation between ceftaroline fosamil or imipenem and BlaMab.
For imipenem, after 15 s of coincubation with BlaMab, the acyl enzyme complex was captured. In contrast, binding was not observed at 1 min, which suggests that imipenem is mostly hydrolyzed at that point. Unlike with imipenem, we were not able to detect binding of ceftaroline fosamil to BlaMab at a ratio of 1:20 for 5 s, 15 s, and 2 h or at a ratio of 1:200 for 5, 15, or 30 s. (Table 6; Fig. S4). Consistent with the nearly 600-fold-higher rate of acylation of BlaMab by avibactam, BlaMab (28,433 ± 5 Da) bound avibactam (+265 ± 5 Da) and relebactam (+349 ± 5 Da) under these conditions (Table 6; Fig. S4). BlaMab binding to avibactam was previously reported (5).
TABLE 6.
Mass spectrometry analyses of BlaMab, LdtMab1 to LdtMab5, and d,d-carboxypeptidase alone and incubated with imipenem, ceftaroline fosamil, imipenem plus ceftaroline fosamil, relebactam, and avibactam
| Protein (nominal mol wt) | Compound (nominal mol wt) | Observed mol wta (±5) | Change in mol wta |
|---|---|---|---|
| BlaMab (28,433) | None | 28,433 | |
| Avibactam (265) | 28,698 | +265 | |
| Relebactam (349) | 28,782 | +349 | |
| Ceftaroline fosamil (685) | 28,434 | +0 | |
| Imipenemb (299) | 28,728 | +299 | |
| LdtMab1 (23,059) | None | 23,059 | |
| Avibactam (265) | 23,325 | +265 | |
| Relebactam (349) | 23,407 | +348 | |
| Ceftaroline fosamil (685) | 23,744 23,664 | +685 +605 | |
| Imipenem (299) | 23,359 23,316 (minor) | +300 +257 | |
| Imipenem (299) Ceftaroline fosamil (685) | 23,359 23,664 (minor) | +300 +605 | |
| LdtMab2 (39,216) | None | 39,216 | |
| Avibactam (265) | 39,482 | +266 | |
| Relebactam (349) | 39,565 | +349 | |
| Ceftaroline fosamil (685) | 39,901 39,821 (minor) | +685 +605 | |
| Imipenem (299) | 39,515 | +299 | |
| Imipenem (299) Ceftaroline fosamil (685) | 39,516 | +300 | |
| LdtMab3 (44,795) | None | 44,795 | |
| Avibactam (265) | 44,794 | +0 | |
| Relebactam (349) | 44,795 | +0 | |
| Ceftaroline fosamil (685) | 44,795 | +0 | |
| Imipenem (299) | 44,795 | +0 | |
| Imipenem (299) Ceftaroline fosamil (685) | 44,795 | +0 | |
| LdtMab4 (32,567) | None | 32,567 | |
| Avibactam (265) | 32,566 32,831 (minor) | +0 +265 | |
| Relebactam (349) | 32,567 | +0 | |
| Ceftaroline fosamil (685) | 33,252 33,171 (minor) | +685 +604 | |
| Imipenem (299) | 32,866 32,824 (minor) | +299 +257 | |
| Imipenem (299) Ceftaroline fosamil (685) | 32,865 32,821 (minor) | +298 +254 | |
| LdtMab5(H6) (28,220) | None | 28,220 | |
| Avibactam (265) | 28,220 | +0 | |
| Relebactam (349) | 28,220 | +0 | |
| Ceftaroline fosamil (685) | 28,220 28,825 28,905 | +0 +605 +685 | |
| Imipenem (299) | 28,476 28,520 | +256 +300 | |
| Imipenem (299) Ceftaroline fosamil (685) | 28,476 28,519 | +256 +299 | |
| M. abscessus d,d-carboxypeptidase (26,791) | None | 26,791 | |
| Avibactam (265) | 26,791 27,057 | +0 +265 | |
| Relebactam (349) | 26,791 | +0 | |
| Ceftaroline fosamil (685) | 26,790 (minor) 27,396 | +0 +605 | |
| Imipenem (299) | 27,046 (minor) 27,090 | +255 +299 | |
| Imipenem (299) Ceftaroline fosamil (685) | 27,090 27,046 (minor) | +299 +255 |
Mass accuracy, ±5 Da.
Complex captured at 15 s.
(ii) l,d-Transpeptidases and d,d-carboxypeptidases.
We next examined acyl enzyme formation with the l,d-transpeptidases and the d,d-carboxypeptidase. and observed binding of ceftaroline fosamil, dephosphorylated ceftaroline fosamil (ceftaroline), imipenem, avibactam, and relebactam. LdtMab1 (28,059 ± 5 Da) and LdtMab2 (39,216 ± 5 Da) formed stable acyl enzyme complexes with all tested ligands, whereas binding to LdtMab3 was not detected for avibactam, relebactam, ceftaroline fosamil, or imipenem (Table 6; Fig. S4).
For LdtMab4, a minor peak was detected corresponding to imipenem (missing an ethoxy group). LdtMab4 and LdtMab5(H6) binding of avibactam, imipenem, ceftaroline, and ceftaroline fosamil was captured, but complexes with relebactam were not observed under these conditions (Table 6; Fig. S4). Coincubation with imipenem and ceftaroline fosamil revealed acyl enzyme formation with imipenem only, suggesting a preference for this substrate over ceftaroline by LdtMab1, LdtMab2, LdtMab4, LdtMab5, and d,d-carboxypeptidase (Table 6).
DISCUSSION
We chose to conduct our susceptibility determinations using Middlebrook 7H9 broth with the full knowledge that (i) treatment and clinical management of patients with NTM disease (especially M. abscessus infection) are under continuous debate and (ii) that similar correlations of MICs of M. abscessus with clinical outcomes may not yet be firmly established for the antibiotics under study in this analysis. We are also aware of the stability issues present with imipenem; hence, our reading of MICs was performed at 48 h.
A combination of ceftaroline and imipenem markedly lowers MICs against M. abscessus.
The combination of imipenem and ceftaroline was very potent, with MICs equal to or less than 0.25 μg/ml for imipenem when a fixed ceftaroline concentration of 1 μg/ml was used (Table 2; Table S1). This observation was consistent for 45 of 56 different clinical isolates, including ATCC 19977, independent of ceftaroline MICs. The significance of this finding is critical to understanding the fundamental mechanisms considered here and is discussed below.
Most recently, the addition of ceftazidime at 100 μg/ml reduced the MIC50 of ceftaroline to 0.25 μg/ml and that of imipenem to 0.5 μg/ml (10). The combination of cefdinir and doripenem was previously reported to be synergistic, with a 1- to 2-fold reduction in doripenem MIC and a 4- to 16-fold reduction in cefdinir MIC (11). Several carbapenem and cephalosporin combinations also showed synergistic activity in a checkerboard assay (12); however, none of the individual compounds reduced MICs as much as the combination studied herein.
Relebactam and avibactam only modestly improved ceftaroline activity.
Recently, relebactam and vaborbactam were reported to decrease MICs of a variety of carbapenems and cephalosporins, rendering them potentially active against M. abscessus in infections (13). Similar observations were made using avibactam (6, 14). In line with these results, we found a reduction in the MIC of ceftaroline with the addition of either avibactam or relebactam, which led to a ≥2-fold reduction in MICs of avibactam for 20 strains and MICs of relebactam for 10 of 20 strains. In contrast, only a modest impact was found for the combination of either inhibitor with imipenem, leading to a 2-fold or less reduction in MICs of avibactam for 6 of 20 strains and no MIC reductions for relebactam.
In contrast to our findings, Kaushik et al. observed a modest decrease of imipenem MIC by one dilution with the addition of relebactam (13). Our kinetic assay revealed that relebactam is a less potent inhibitor of BlaMab, with rather poor affinity compared to avibactam. This contrasts with other β-lactamases, such as KPC-2, which showed high affinity for both inhibitors (15).
BlaMab hydrolyzes both imipenem and ceftaroline fosamil to various degrees (Table 4; Table S2). However, compared to other β-lactams (7), both are poor substrates for BlaMab, which is consistent with the observation that inhibition of BlaMab by β-lactamase inhibitors has only a minor effect on the efficacy of the imipenem-ceftaroline combination, as measured by MICs. Previously reported data revealed that hydrolysis parameters were a Km of 90 ± 30 μM and a kcat of 2.7 ± 3 s−1 (5) and a Km of 140 ± 30 μM and a kcat of 0.13 ± 0.02 s−1 (7) for imipenem hydrolysis and a Km of >300 μM and a kcat of >4.5 s−1 for ceftaroline (7).
Imipenem and ceftaroline bind to multiple l,d-transpeptidases and d,d-carboxypeptidase.
The biochemical rationale for β-lactam combinations is that individual compounds have different binding preferences for their molecular targets, which result in lower MICs. To illustrate, penicillins, cephalosporins, and carbapenems exhibit differential binding to the M. abscessus l,d-transpeptidases LdtMab1 and LdtMab2 (11).
We purified each of five l,d-transpeptidases, LdtMab1 to LdtMab5, and the d,d-carboxypeptidase. Due to the large amounts of enzyme required for quantitative binding kinetics, we chose an alternative approach using timed mass spectrometry to measure relative quantities of covalent enzyme-compound adduct after coincubation of the enzyme with the two compounds, while ceftaroline bound to LdtMab1, LdtMab2, and LdtMab4. With this approach, we found that imipenem bound all the tested protein targets except LdtMab3.
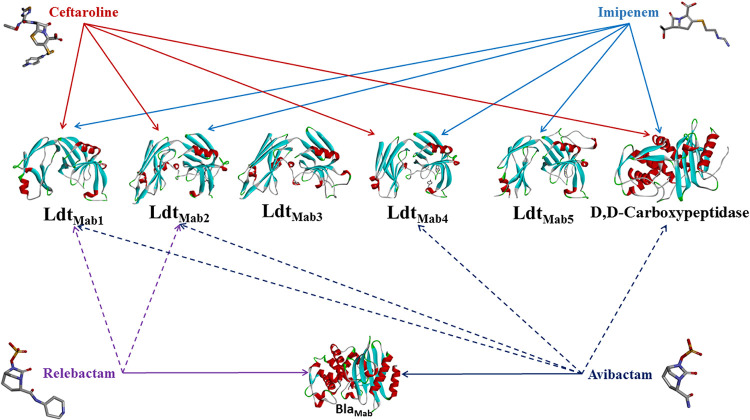
It is noteworthy that mass spectrometry results of relebactam and avibactam mixed with target proteins revealed covalent binding not only to BlaMab but also to l,d-transpeptidases and d,d-carboxypeptidase (Table 6; Fig. 3). While avibactam formed covalent adducts with BlaMab, d,d-transpeptidase, LdtMab1, LdtMab2, and LdtMab4, the DBO relebactam formed intact molecules with BlaMab, LdtMab1, and LdtMab2 but not LdtMab4 or d,d-carboxypeptidase. Figure 3 summarizes redundancy and interaction between imipenem, ceftaroline, avibactam, and relebactam and LdtMab1 to LdtMab5, d,d-carboxypeptidase, and BlaMab.
FIG 3.
Redundancy and interaction between imipenem, ceftaroline, avibactam, and relebactam and LdtMab1 to LdtMab5, d,d-carboxypeptidase, and β-lactamase (BlaMab). Both imipenem and ceftaroline are hydrolyzed by BlaMab.
Sequence analysis and catalysis—molecular simulations.
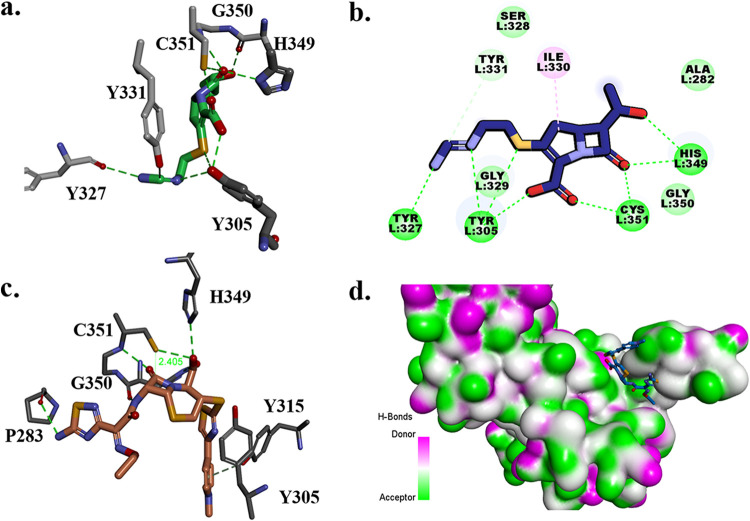
In order to understand the mechanistic basis for these observations, we first examined the primary sequences of these proteins. The sequence analysis of LdtMab1 to LdtMab5 suggests large variability in the amino acid composition among the transpeptidases (see Fig. S5c), which may result in changes in the overall secondary structure in the region of the active site. The sequence similarity among the 5 transpeptidases is 25%, and the sequence identity is 9%. However, the group including LdtMab1, LdtMab2, and LdtMab4 and the pair LdtMab3 and LdtMab5 are nearly 70% similar in the active site region (where important residues, part of the binding cavity, are located: C351, H349, H333, Y315, and M300).
The catalytic site residues C351 to H339 (a covalent bond was shown to form between LdtMab C352:SH and the opened carbapenem beta-lactam ring, and the H349:HN backbone acts as a nucleophile) (16) are conserved for all M. abscessus l,d-transpeptidases except LdtMab3, which has histidine replaced by asparagine. The residues shown to be part of the active site (10) and highlighted in Fig. 4b and Supplemental Fig. S5 are conserved in the group consisting of LdtMab1, LdtMab2, and LdtMab4 as well (M300, T304, Y305, Y315, Y331, and H333). The exception is Y331, replaced by F331 in LdtMab4. Do these comparisons help us understand the differences in the binding of relebactam, avibactam, ceftaroline, and imipenem?
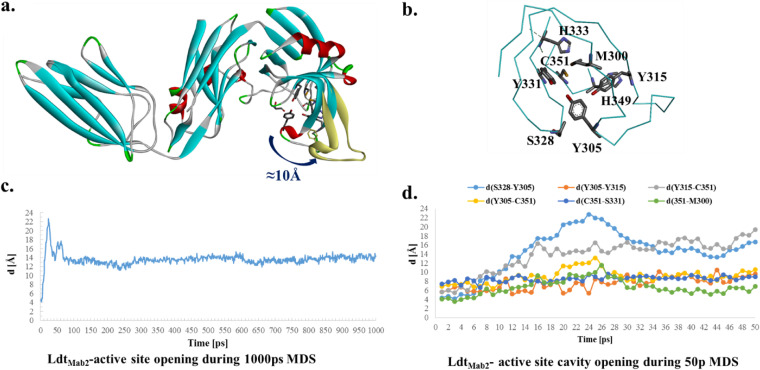
FIG 4.
The homology model and molecular dynamic simulation of LdtMab2 (a) suggest that the active site (b) is open to more than 10 Å during the 1-ns simulations. The 50-ps molecular dynamic simulation (MDS) shows the movement (distances) among the important active-site residues C351, S328, Y305, M300, and Y315 (d), which suggest a high flexibility of the loops. During the 1-ns MDS (c), the structure is equilibrated at a 12- to 14-Å active-site opening (see also Movie S1).
We advance the idea that the hydrophobic amino acid phenylalanine, which is present in LdtMab3 and LdtMab5 as well, may impede relebactam binding. Another notable exception in this group is Tyr305 (entrance of the active site), replaced by Ile in LdtMab1. The functional significance of this is unknown.
The other two transpeptidases, LdtMab3 and LdtMab5, are more similar. The catalytic cysteine is conserved, but LdtMab3 has an asparagine and LdtMab5 has a histidine as part of the proposed oxyanion hole. Met300 and His333 are preserved, but not the other conserved residues (e.g., Y331 is replaced by F331). The larger variability is in LdtMab3, with a 7-residue deletion, which contributes to a different, “floppier” secondary structure in the active-site loops (Fig. 4). This large sequence variability, which generates structural, hydrophobic, and electrostatic changes, may contribute to the differential binding.
Molecular simulation and docking of imipenem and ceftaroline in the active site of LdtMab2.
The LdtMab2 transpeptidase model, based on the crystal structure of PDB 5UWV, was created to enhance our understanding of the above observations. The active site is part of the YkuD domain (Fig. S5), with the important residues C351, H349, Y331, H333, Y315, S328, M300, and Y305. In the reported crystal structure of LdtMab2, part of the active site (D301 to D313) was missing. To restore the integrity of the active site, a homology model was generated and refined (Fig. S5a). The results generated by 1-ns NAMD (scalable molecular dynamics) were consistent with the crystal structures of LdtMtb2, in showing that the active site loops are very flexible (Fig. 4a, c, and d). During the dynamics, the LdtMtb2 active site entrance (Fig. 4a and d) opens from 4 Å to up to 20 Å in the first 50 ps, followed by equilibration around 12 to 14 Å, which is preserved for the remaining 1,000 ps (Fig. 4; Movie S1). This analysis reveals that the initial active site cavity (t = 0 ps; distance between S238 and Y305 ≈ 4 Å) is too small to accommodate the β-lactams. In this simulation, docking of imipenem and ceftaroline was not possible. We postulate that once the LdtMab2 cavity expands more than 8 Å, the active site can readily accommodate intact imipenem, which adopts a favorable conformation for acylation (Fig. 5a).
FIG 5.
Molecular docking of imipenem (a), proposed mechanism of acylation of imipenem by LdtMab2 (b), and docking of ceftaroline (c and d) into the active site of LdtMab2.
The previous studies suggest that the mechanism of the β-lactam ring acylation by LdtMab2 would be similar to the ring opening of carbapenems and penems by LdtMt2 of M. tuberculosis (16). In this mechanism, the thiol group of catalytic C354 (C351 in LdtMab2) would be reactivated (deprotonated) by the protonated NH of H336 (H333 in LdtMab2), with H352 (H349) and G353 (G350) residues being part of the oxyanion hole. The nucleophilic thiolate of reactive cysteine would attack the carbonyl carbon of β-lactam and acylation, and ring opening would follow. The crystal structures of LdtMt2 show that transpeptidases are inactivated by covalently binding with carbapenems and formation of an acyl enzyme thioester bond.
The generated model of LdtMab2 with imipenem docked in the active site (t =11 ps; active-site opening at >8 Å) suggests that this proposed mechanism would be possible (Fig. 5a and b). The C351 interacting with H349 can constitute part of the oxyanion hole and facilitates acylation. When the LdtMt2 model is analyzed, H349 side chain rotamers can take multiple positions, with one of the conformations close enough to facilitate the thiol group protonation. The Connolly representation of LdtMab2 (Fig. 5d) shows that the active-site cavity is large enough to accommodate ceftaroline (Fig. 5c and d). For ceftaroline to fit into the active site, it is essential that the cavity be flexible, which would not be possible if the initial conformation of the active site was preserved.
Based upon this in silico analysis, we advance the hypothesis that the preferential binding of imipenem to the target proteins may alter the subsequent binding properties for ceftaroline, by attaining a conformational change in the enzyme (ligand-induced conformational changes). Given that binding occurs in intact cells with a division half-life of hours, it is conceivable that turnover of imipenem can occur in intact cells to permit ceftaroline binding.
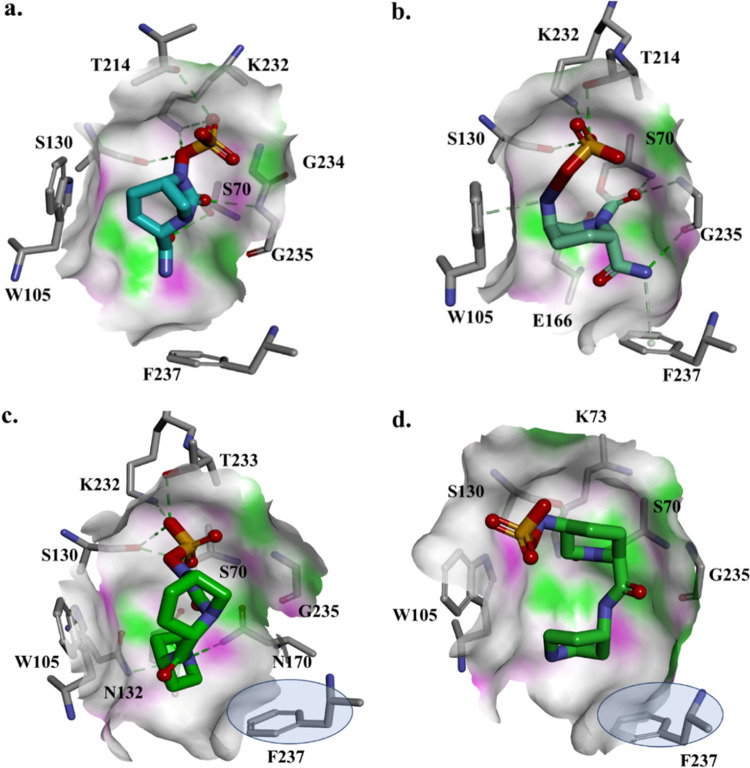
Molecular docking and simulation of avibactam and relebactam into the active site of BlaMab.
In the majority of Ambler class A enzymes, position 237, located downstream of the canonic KTG motif (Ambler positions 234 to 236), is occupied by alanine or serine. The backbone nitrogen of this residue participates in the formation of the catalytically important oxyanion hole. In the carbapenemase KPC-2 and in BlaC, the principal β-lactamase of Mycobacterium tuberculosis, this residue is occupied by threonine (KTG/T) (Fig. S6) and has been shown to interact with the carboxylate binding region of the inhibitor. The conserved arginine at position 220 in KPC-2 is replaced by a serine in BlaC. When BlaMab is compared with KPC-2 β-lactamases, F237 in BlaMab has the same role as V240 in KPC-2. The changes from KTGTCGV (KPC-2) to KTC*GGF (BlaMab) seems to make the active site of BlaMab more hydrophobic, with an obstructed active-site entrance and possibly larger inside cavity. Energetically, the active-site cavity of BlaMab is more electronegative than the KPC-2 cavity.
To attempt to understand the kinetic differences observed between relebactam and avibactam, the docking of each DBO into BlaMab was modeled. The Michaelis-Menten complex of BlaMab with avibactam shows hydrogen bond interactions between avibactam and the active-site residues (S70, S130, K232, G234, G235, and T214) (Fig. 6a). In this model, avibactam is positioned favorably for acylation, with its carbamoyl resting in the oxyanion hole and catalytic S70 positioned at ∼3 Å from C-6 (Fig. 6a). The carbamylated complex in this model preserves the H-bond networking with S130, K232, G235, and T214 and steric interactions with W105 and F237, with the carbamoyl positioned toward catalytic S70:NH and G235:NH (Fig. 6b).
FIG 6.
Molecular docking of avibactam (a and b) and relebactam (c and d) as Michaelis-Menten complexes of the β-lactamase (BlaMab) (a and c) and as acyl enzymes (b and d). Phenylalanine at position 237 creates steric hindrances with relebactam.
In contrast, docking of relebactam into the active site of BlaMab showed that the positioning of F237 at the entrance of the active site may limit the conformations of relebactam in the catalytic cavity (Fig. 6c). The larger aromatic piperidine group of relebactam was found to be in proximity to the phenylalanine residue F237 of BlaMab, which creates steric hindrance, thus impeding the placement of the inhibitor in the active site of the enzyme. As a result, the interactions with S130 and K232 are preserved, new possible interactions are formed with T233, N170, and N132 and the aromatic rings of W105 and F237, which seems to restrict the relebactam movement into and conformations in the BlaMab active site. The carbamoyl is positioned toward the oxyanion hole, but at a distance of 4 Å from the catalytic serine. When the acyl enzyme is formed, relebactam is positioned in an unfavorable conformation, with the carbamoyl located outside the oxyanion hole, facing S130 (Fig. 6d). This suggests less potent bonding interactions of relebactam, compared to avibactam, with BlaMab.
Conclusion.
In conclusion, our data advance the notion that double β-lactam therapy (ceftaroline and imipenem) may be a promising future strategy in the treatment of serious M. abscessus infections. Our results also suggest that the addition of ceftaroline and imipenem resulted in MICs which may be readily reachable using current clinical dosing regimens, with little or no apparent benefit observed with the addition of relebactam to this combination. As importantly, we demonstrated that imipenem, ceftaroline, and DBO inhibitors bind preferentially to cell wall-synthesizing enzymes. An unanticipated result was the redundancy in the number of targets and the biochemical complexity of the l,d-transpeptidases and the d,d-carboxypeptidase. How do these findings explain our observations?
Two hypotheses can be advanced. First, the binding of imipenem (and ceftaroline) to multiple cell wall-synthesizing enzymes likely alters the composition of peptidoglycan. This effect may be directly attributable to the number and sequence of cell wall-synthesizing enzymes inactivated. This raises the question of what the required concentration threshold of imipenem to achieve this would be. Once imipenem is deacylated from the l,d-transpeptidases or d,d-carboxypeptidase, does a subsequent reaction with ceftaroline occur? If so, does this add further to the permeability of the cell? Second, the l,d-transpeptidase likely undergoes large changes in conformational structure upon binding different ligands. These changes permit secondary binding of β-lactams that normally cannot be accommodated.
It is our hope that these findings will help to elucidate the surprisingly complex interplay between β-lactams, β-lactamase inhibitors, β-lactamase, and target cell wall-synthesizing enzymes. It is also hoped that this very low MIC will translate into clinically attainable pharmacokinetic and pharmacodynamic indices with a twice-daily dosing regimen, but this warrants further investigation. A critical need exists to further investigate this dual drug combination and others in animal and clinical trials to explore dosing strategies that optimize overall exposure needed to achieve bacterial killing and suppress the emergence of resistance.
MATERIALS AND METHODS
Clinical strains, chemical reagents, and antibiotics.
The screening collection consisted of 20 clinical isolates mainly isolated from respiratory samples that were received at University Hospitals Cleveland Medical Center and MetroHealth Hospital in 2018. Thirty-five isolates were part of the strain collection from the National Jewish Hospital, Denver, CO.
The M. abscessus type strain, ATCC 19977, was obtained from the American Type Culture Collection (ATCC). Custom-made Sensititre frozen MIC panels were purchased from Thermo Fisher Scientific and were used for susceptibility testing. Both avibactam and relebactam were obtained from AchemBlocks.
In vitro susceptibility testing.
Fifty-five clinical isolates were subjected to susceptibility testing against imipenem and ceftaroline each alone and in combination with relebactam in Middlebrook 7H9 broth supplement to achieve rapid growth of the isolates. MICs of ceftaroline with or without imipenem or relebactam were determined using the microdilution method. Approximately 5 × 105 CFU/ml was inoculated into Middlebrook 7H9 broth supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC), 0.05% (vol/vol) Tween 80 (Sigma), and 0.5% glycerol (vol/vol). Twofold dilutions of ceftaroline or imipenem were prepared in final concentrations ranging from 0.0.01 to 32 μg/ml. Avibactam and relebactam were added in a fixed concentration of 4 μg/ml. Ceftaroline was added at a fixed concentration of 1 μg/ml to graded imipenem dilutions. Custom-made Sensititre frozen microplates were incubated at 30°C for 48 h, and the MIC was defined as the lowest antibiotic concentration that prevented visible bacterial growth.
Cloning and purification of BlaMab.
A truncated sequence of M. abscessus blaMab (Δ1–30 blaMab) was generated by Celtek Biosciences (Franklin, TN), cloned into the pET28(a)+ vector, and electroporated into Escherichia coli BL21(DE3). Cells were grown to an optical density at 600 nm (OD600) of 0.8, and protein expression was induced with 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG). After incubation for 18 h at 16°C, cells were harvested and lysed using a QIAexpress nickel-nitrilotriacetic acid (Ni-NTA) fast-start kit, followed by nickel column purification of the His-tagged protein according to the manufacturer’s protocol (Qiagen, Inc., Valencia, CA). Briefly, the His tag was removed from the protein by adding thrombin (Novagen, Madison, WI) overnight at 4°C (1.6 U/mg protein). The cleaved protein was separated from the His-tagged peptides by size exclusion chromatography using a HiLoad 16/60 Superdex 75 column (GE Healthcare Life Science, Uppsala, Sweden). The predicted mass of the BlaMab enzyme (28,433 Da) was confirmed by mass spectrometry.
Steady-state kinetics.
Avibactam and relebactam inhibition kinetics were performed with purified BlaMab enzyme as previously described (15). In brief, the reaction scheme is represented in equation 1. Kinetic parameters were determined using an Agilent 8453 diode array spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA), and reactions were conducted in 100 mM morpholineethanesulfonic acid (MES) (pH 6.4) at room temperature.
| (1) |
where E is the enzyme and I is the inhibitor.
A direct competition assay was performed to approximate the relative Michaelis constant (Ki app) of the inhibitor, the concentration leading to reduction of the reaction velocity by 50% as measured by nitrocefin (NCF) hydrolysis. A final concentration of 5× Km of nitrocefin (KmNCF = 22 μM) was used as the indicator substrate in the presence of nanomolar concentrations of BlaMab. The data were corrected to account for the affinity of nitrocefin (KmNCF) for the BlaMab according to equation 2:
| (2) |
where [S] is the concentration of nitrocefin.
To determine the acylation rate (k2/K), which is the second-order rate constant for enzyme and inhibitor complex inactivation with K = k1/k−1, assays were performed using fixed concentrations of enzyme and nitrocefin and increasing concentrations of avibactam or relebactam. The progress curves to obtain kobs values for inactivation were fit graphically using equation 3:
| (3) |
where Vf is the final velocity, V0 is the initial velocity, and A0 is the initial absorbance at 482 nm. The values for kobs versus avibactam and relebactam concentrations were plotted. The acylation rate was determined by correcting the slope of the line to account for the affinity of nitrocefin (KmNCF) using equation 4:
| (4) |
Off rates (koff) were determined by incubating BlaMab with avibactam and relebactam at 10× Ki app for 5 min. The mixtures were diluted 1:1,000, and 100 μM NCF was added. Progress curves of nitrocefin hydrolysis were measured and fitted to a single exponential decay equation to obtain koff. Reaction mixtures containing the β-lactamase alone and inhibitor alone were used as controls. The residence half-life (t1/2) of the drug-enzyme complex was determined as a reciprocal of the dissociation constant (ln2/koff). Partition ratios (kcat/kinact) were obtained by incubating BlaMab with increasing concentrations of avibactam or relebactam for 24 h. The ratio of inhibitor to enzyme required to inhibit hydrolysis of nitrocefin by >90% is kcat/kinact.
Ldt binding assays.
Steady-state kinetic parameters were determined with purified Ldts (LdtMab1, LdtMab2, LdtMab4, and LdtMab5(H6)) using a BioTek Synergy2 multimode reader and Gen5 analysis software. Assays were performed at 30°C using 50 mM Tris-HCl, pH 7.5, and 300 mM sodium chloride.
Increasing concentrations of nitrocefin (Δɛ482 = 17,400 M−1 cm−1) and fixed concentrations of Ldt were monitored for change in absorbance at 482 nm for 0 to 6 min. For velocity determinations, a 0.25-cm path length was employed. A nonlinear least-square fit of the data (Henri-Michaelis-Menten equation) using Origin 8.1 (OriginLab, Northampton, MA) was employed to obtain the steady-state kinetic parameters Vmax, kcat, and Km according to equation 5 and equation 6, with v being the reaction rate at substrate concentration [S]:
| (5) |
| (6) |
For determination of Ki app, concentrations of nitrocefin (5× Km) were used with fixed concentrations of Ldts and increasing concentrations of imipenem and ceftaroline fosamil. Changes in absorbance at 482 nm for 0 to 30 min were determined for each concentration of imipenem or ceftaroline fosamil. Data were linearized using a Dixon plot of inverse changes in absorbance (1/ΔA) versus imipenem or ceftaroline fosamil concentration. The observed Ki app was determined by dividing the value for the y axis intercept by the slope of the line. The data were corrected to account for the substrate concentration and affinity for the Ldt using equation 2.
Cloning and purification of LdtMab1-5 and M. abscessus d,d-carboxypeptidase.
Cloning and purification of LdtMab1 and LdtMab2 were based on established protocols (11). Truncated sequences of LdtMab1 (Δ1–31) and LdtMab2 (Δ1–41) were generated by Celtek Biosciences and cloned into the pET28(a)+ vector with a TEV (tobacco etch virus) protease cleavage site prior to the start codon of the LdtMab sequences. Clones were electroporated into E. coli BL2(DE3) and grown at an OD600 of 0.8, and protein expression was induced with 0.25 mM IPTG. After incubation for 18 h at 18°C, cells were harvested and stored at −20°C overnight. LdtMab2 cell pellets were resuspended in buffer containing 50 mM Tris (pH 8.0), 400 mM sodium chloride, and 1 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), followed by sonication and centrifugation. The supernatant was passed through a His Prep FF 16/10 column (GE Healthcare) and washed with 25 column volumes of buffer, and bound protein was eluted with a gradient of 1 to 500 mM imidazole. Eluted protein was subjected to dialysis overnight at 4°C in buffer containing 50 mM Tris (pH 8.0), 150 mM sodium chloride, and 0.5 mM TCEP in the presence of His-tagged TEV protease (ratio of TEV protease to LdtMab2, 1:30). To remove the His tag, uncleaved fusion protein, and His-tagged TEV protease, passage over the His Prep FF 16/10 column was performed. Fractions containing LdtMab2 were pooled, concentrated, and stored in 20% glycerol at −20°C. LdtMab1 was purified similarly to LdtMab2 except that the QIAexpress Ni-NTA fast-start kit instead of His Prep FF 16/10 column purification was used.
A BLAST search of M. abscessus l,d-transpeptidases resulted in three additional sequences (MAB_4061c, MAB_4537c, and MAB_4775c), which are referred to here as LdtMab3, LdtMab4, and LdtMab5, respectively. Transmembrane regions were determined using online prediction sites (TMHMM server, MEMSAT2, and HMMTOP). Truncated sequences of LdtMab3 (Δ1–36), LdtMab4 (Δ1–31), LdtMab5 (Δ1–31), and M. abscessus d,d-carboxypeptidase (Δ1–30) were cloned, and protein was expressed and purified in the same manner as LdtMab1. Due to difficulties in purification, mass spectrometry and binding studies were performed on LdtMab5 with the His tag (referred to here as LdtMab5(H6)).
Mass spectrometry analyses.
Ten micrograms of BlaMab, LdtMab1 to LdtMab5, and M. abscessus d,d-carboxypeptidase was incubated at room temperature with substrate (ceftaroline fosamil or imipenem) or inhibitor (avibactam or relebactam) at a molar ratio of 1:20 for 2 h in 50 mM Tris-HCl (pH 7.5) and 300 mM sodium chloride for a total reaction volume of 20 μl. Reactions were quenched with 10 μl acetonitrile, and the mixtures were added to 1 ml 0.1% formic acid in water. Samples were analyzed using a quadrupole time-of-flight (Q-TOF) Waters Synapt-G2-Si electrospray ionization mass spectrometer (ESI-MS) and Waters Acquity H class ultraperformance liquid chromatography (UPLC) with a BEH C18 1.7-μm column (2.1 by 50 mm). The Synapt G2-Si spectrometer was calibrated with sodium iodide with a 50-to-2,000 m/z mass range. MassLynx V4.1 was used to deconvolute protein peaks. The tune settings for each sample were as follows: capillary voltage at 3 kV, sampling cone at 35 V, source offset at 35, source temperature of 100°C, desolvation temperature of 500°C, cone gas at 100 liters/h, desolvation gas at 800 liters/h, and 6.0 nebulizer. Mobile phase A was 0.1% formic acid (FA) in water. Mobile phase B was 0.1% FA in acetonitrile. The mass accuracy for this system is ±5 Da.
Molecular modeling and docking.
The crystal structure of class A β-lactamase from M. abscessus (PDB code 4YFM) was used for simulation and molecular docking. Discovery Studio Modeling (BIOVIA environment, release 2017; Dassault Systèmes, San Diego, CA) software was used. The structure was minimized using a conjugate gradient method, with a root mean square (RMS) gradient of 0.001 kcal/(mol · Å). The generalized Born with a simple switching (GBSW) solvation model was used, and long-range electrostatics were treated using a particle mesh Ewald method with periodic boundary condition. The SHAKE algorithm was applied.
The intact and acyl avibactam and relebactam were built and minimized. The CDOCKER protocol was used to dock the compounds into the active site of M. abscessus. The protocol uses a CHARMm-based molecular dynamics (MD) scheme to dock ligands into a receptor binding site. Random ligand conformations were generated using high-temperature MD. The conformations are then translated into the binding site. Candidate poses are created using random rigid-body rotations, followed by simulated annealing. A final minimization is used to refine the ligand poses. The generated poses were analyzed, and the best-ranked poses were used to create the Michaelis-Menten and acyl enzyme complexes and were further minimized.
The molecular model of l,d-transpeptidase (LdtMab2) was done using the crystal structure of PDB 5UWV. The missing loop (D301 to D313) was reconstructed using the LdtMt2 (PDB 6IYW) structure as a template and SWISS-MODEL homology-modeling server accessible via the ExPASy web server (17). The structure was future minimized, and the loop was refined. To equilibrate the structure, a medium-long molecular dynamic simulation (1 ns) was performed, using a NAMD protocol (18). The structural models of the other 4 LdtMabs were similarly generated. Imipenem and ceftaroline were built and docked into the active site of LdtMab2. The complexes between the compounds and LdtMab2 formed as Michaelis-Menten complexes and acyl enzymes were further minimized.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Roe Green Center for Travel Medicine. Additionally, research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) to R.A.B. under award numbers R01AI100560, R01AI063517, and R01AI072219. This study was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award number 1I01BX001974 to R.A.B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development, and the Geriatric Research Education and Clinical Center VISN 10. This work was also supported by the National Allergy and Infectious Disease of the National Institutes of Health (NIH) to B.N.K. under award number R01AI41805-02.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Department of Veterans Affairs.
We declare that we have no conflicts of interest related to the contents of this article.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. 2013. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg Infect Dis 19:1889–1891. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons S, van Ingen J, Hsueh PR, Van Hung N, Dekhuijzen PN, Boeree MJ, van Soolingen D. 2011. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis 17:343–349. doi: 10.3201/eid1703.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 4.Meir M, Bifani P, Barkan D. 2018. The addition of avibactam renders piperacillin an effective treatment for Mycobacterium abscessus infection in an in vivo model. Antimicrob Resist Infect Control 7:151. doi: 10.1186/s13756-018-0448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soroka D, Dubee V, Soulier-Escrihuela O, Cuinet G, Hugonnet JE, Gutmann L, Mainardi JL, Arthur M. 2014. Characterization of broad-spectrum Mycobacterium abscessus class A beta-lactamase. J Antimicrob Chemother 69:691–696. doi: 10.1093/jac/dkt410. [DOI] [PubMed] [Google Scholar]
- 6.Dubee V, Bernut A, Cortes M, Lesne T, Dorchene D, Lefebvre AL, Hugonnet JE, Gutmann L, Mainardi JL, Herrmann JL, Gaillard JL, Kremer L, Arthur M. 2015. β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother 70:1051–1058. doi: 10.1093/jac/dku510. [DOI] [PubMed] [Google Scholar]
- 7.Dubee V, Soroka D, Cortes M, Lefebvre AL, Gutmann L, Hugonnet JE, Arthur M, Mainardi JL. 2015. Impact of beta-lactamase inhibition on the activity of ceftaroline against Mycobacterium tuberculosis and Mycobacterium abscessus. Antimicrob Agents Chemother 59:2938–2941. doi: 10.1128/AAC.05080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefebvre AL, Le Moigne V, Bernut A, Veckerle C, Compain F, Herrmann JL, Kremer L, Arthur M, Mainardi JL. 2017. Inhibition of the beta-lactamase BlaMab by avibactam improves the in vitro and in vivo efficacy of imipenem against Mycobacterium abscessus. Antimicrob Agents Chemother 61:e02440-16. doi: 10.1128/AAC.02440-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Run E, Arthur M, Mainardi JL. 2019. In vitro and intracellular activity of imipenem combined with tedizolid, rifabutin, and avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother 63:e01915-18. doi: 10.1128/AAC.01915-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey R, Chen L, Manca C, Jenkins S, Glaser L, Vinnard C, Stone G, Lee J, Mathema B, Nuermberger EL, Bonomo RA, Kreiswirth BN. 2019. Dual beta-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio 10:e02895-18. doi: 10.1128/mBio.02895-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P, Chauhan V, Silva JRA, Lameira J, d'Andrea FB, Li S-G, Ginell SL, Freundlich JS, Alves CN, Bailey S, Cohen KA, Lamichhane G. 2017. Mycobacterium abscessus l,d-transpeptidases are susceptible to inactivation by carbapenems and cephalosporins but not penicillins. Antimicrob Agents Chemother 61:e00866-17. doi: 10.1128/AAC.00866-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Story-Roller E, Maggioncalda EC, Lamichhane G. 2019. Select beta-lactam combinations exhibit synergy against Mycobacterium abscessus in vitro. Antimicrob Agents Chemother 63:e02613-18. doi: 10.1128/AAC.02613-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushik A, Ammerman NC, Lee J, Martins O, Kreiswirth BN, Lamichhane G, Parrish NM, Nuermberger EL. 2019. In vitro activity of the new beta-lactamase inhibitors relebactam and vaborbactam in combination with beta-lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob Agents Chemother 63:e02623-18. doi: 10.1128/AAC.02623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushik A, Gupta C, Fisher S, Story-Roller E, Galanis C, Parrish N, Lamichhane G. 2017. Combinations of avibactam and carbapenems exhibit enhanced potencies against drug-resistant Mycobacterium abscessus. Future Microbiol 12:473–480. doi: 10.2217/fmb-2016-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papp-Wallace KM, Nguyen NQ, Jacobs MR, Bethel CR, Barnes MD, Kumar V, Bajaksouzian S, Rudin SD, Rather PN, Bhavsar S, Ravikumar T, Deshpande PK, Patil V, Yeole R, Bhagwat SS, Patel MV, van den Akker F, Bonomo RA. 2018. Strategic approaches to overcome resistance against Gram-negative pathogens using beta-lactamase inhibitors and beta-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem 61:4067–4086. doi: 10.1021/acs.jmedchem.8b00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar P, Kaushik A, Lloyd EP, Li SG, Mattoo R, Ammerman NC, Bell DT, Perryman AL, Zandi TA, Ekins S, Ginell SL, Townsend CA, Freundlich JS, Lamichhane G. 2017. Non-classical transpeptidases yield insight into new antibacterials. Nat Chem Biol 13:54–61. doi: 10.1038/nchembio.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guex N, Peitsch MC, Schwede T. 2009. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: a historical perspective. Electrophoresis 30(Suppl 1):S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- 18.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. 2005. Scalable molecular dynamics with NAMD. J Comput Chem 26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.