Abstract
Objectives:
Chemotherapy is the standard treatment in stage IVB cervical cancer (CC). However, given that many women have a significant pelvic disease burden, whole pelvic radiation (WPR) in addition to chemotherapy for primary treatment may have utility. The aim of this study was to compare the overall survival (OS) and complication rates between women who received both WPR and chemotherapy (CT) versus CT alone in the management of stage IVB CC.
Methods:
A multi-institutional, IRB-approved, retrospective review of patients (pts) with stage IVB CC, diagnosed between 2005 and 2015, was performed. Descriptive statistics of the demographic, oncologic, and treatment characteristics were performed. OS was estimated using the Kaplan Meier method.
Results:
A total of 126 pts met inclusion criteria. Thirty one patients elected for hospice care at diagnosis and were excluded from further analysis. In the remaining population, median age was 53 yrs. The majority (72%) had squamous cell carcinoma and 82% had FIGO grade 2 or 3 tumors. Thirty four patients (35.8%) received WPR in addition to CT as a part of planned primary therapy and 64.2% (n = 61) received CT alone, with 88.2% and 80.3% receiving a cisplatin-based chemotherapy regimen, respectively. The OS was significantly longer in the WPR with CT group (41.6 vs 17.6 mo, p < 0.01). The rates of ureteral obstruction, vaginal bleeding, pelvic infection, pelvic pain, and fistula were not significantly different between the 2 groups (all p > 0.05).
Conclusion:
This study found WPR in addition to CT gives a significant OS benefit. Further study is warranted to determine which subgroups may benefit the most from this novel treatment strategy.
Keywords: Cervical cancer, Radiation, Chemotherapy, Stage IVB, Survival
1. Introduction
Cervical cancer is the third most common gynecologic malignancy in the United States. In 2019, it is estimated that 13,170 women will be diagnosed with the disease and 4250 women will die. While the majority of cases present in the early stages, approximately 5–15% of patients present with widely metastatic cancer termed Stage IVB. Unfortunately, these women have a five year survival of less than 20% and therapeutic options are limited [1].
In clinical practice, the mainstay of treatment for newly diagnosed stage IVB cervical cancer includes systemic chemotherapy with cisplatin/paclitaxel/bevacizumab, cisplatin/paclitaxel or topotecan/paclitaxel/bevacizumab (NCCN guidelines, category 1) with palliative radiation as needed. However, NCCN guidelines also indicate external beam radiation therapy may be considered in patients with isolated distant metastases and locally treatable pelvic disease [2]. A significant number of patients have bulky pelvic tumors that can cause significant morbidity and mortality, such as vaginal bleeding, ureteral obstruction, urinary or gastrointestinal tract fistulas, infections, and pain. Therefore, incorporation of radiation would theoretically aid with symptom control and reduce morbidity and mortality associated with pelvic disease.
While stage IVB cervical cancer patients are often treated with whole pelvic radiation and chemotherapy in practice, it has not been well-studied. Given the significant pelvic disease burden, in addition to distant metastases, whole pelvic radiation as a part of primary treatment may provide a benefit. The aim of the present study was to retrospectively determine if whole pelvic radiation as a planned part of primary therapy provides a reduction in pelvic morbidity or a survival benefit among patients with stage IVB cervical cancer.
2. Materials and methods
A retrospective review was conducted of patients with stage IVB cervical cancer of all histologic types treated at the University of Oklahoma Health Sciences Center, the University of Southern California, the University of Texas Southwestern, and the Phoenixbased gynecologic oncology community practice, between 2005 and 2015. All patients were staged according to the International Federation of Gynecology and Obstetrics (FIGO) 2009 guidelines [3]. This study was approved by the Institutional Review Boards (IRB) of each institution prior to initiating the study. As no data were collected prospectively, a waiver of informed consent was granted by the IRBs. Patients without adequate data in the medical record were excluded.
Demographic variables including age, race, tobacco use, histology, and grade were collected. Disease information including primary chemotherapy regimen, use of whole pelvic radiation in primary therapy, use of bevacizumab during primary therapy, use of palliative radiation and location, locations of disease at diagnosis and disease-related complications was also collected.
Progression free survival (PFS) was defined as the time from date of diagnosis to the first recorded evidence of progression. Without progression, survivors were censored at last follow-up and non-survivors were censored at the date of death. Overall survival (OS) was defined as the time from date of diagnosis to date of death (all causes).
For the analysis, patients were divided into two groups: the chemotherapy alone group and the radiation and chemotherapy group. Women in the radiation and chemotherapy group must have received whole pelvic radiation (WPR) as part of the primary treatment for their malignancy. Women in the chemotherapy group must not have received WPR, but may have received palliative radiation during their primary therapy. Descriptive statistics were used to summarize patient demographics and clinical characteristics. Wilcoxon rank-sum tests were used to compare skewed continuous variables between groups. Chi-square or Fisher's exact tests were used to compare categorical data between those that received radiation and chemotherapy versus chemotherapy alone. Survival curves were estimated using the Kaplan-Meier method and the difference between the curves was compared using log-rank tests. All statistical evaluation was performed using the SAS software version 9.2 (SAS Institute, Cary, NC). All reported p-values were 2-sided, and p < 0.05 was considered statistically significant.
3. Results
Of the 126 patients identified, 31 received no treatment and thus were excluded from further analysis, and the remaining 95 patients who received treatment represented the study population. The median age of the cohort was 53 years (range 23–83). Caucasian patients comprised 34.7% (n = 33) of the group and 39.0% were Hispanic (n = 37). The majority of patients had squamous cell carcinoma (71.6%, n = 68) and FIGO grade 2 or 3 tumors (81.9%, n = 77). The median follow-up for these women is 9 months (range 0–117 months).
Thirty-four patients (35.8%) received WPR and systemic chemotherapy as planned primary therapy and 61 (64.2%) received chemotherapy alone. In the chemotherapy alone group, 80.3% (n = 49) received a cisplatin-based regimen and 19.7% (n = 12) received carboplatin and paclitaxel. This is in comparison to the radiation and chemotherapy group, where 88.2% (n = 30) received a cisplatin-based regimen and 11.8% (n = 4) received carboplatin and paclitaxel. There was no significant difference in the distribution of chemotherapy regimens between the two groups (p = 0.3235).
As outlined in Table 1, there were no statistically significant demographic or disease characteristic differences between the two groups. In terms of disease location, there were no significant differences between groups. The lung was the most common site of metastasis at the time of diagnosis (48.5% for the radiation and chemotherapy group vs. 37.9% for the chemotherapy alone group, p = 0.3264). This was followed by supraclavicular lymph nodes (24.2% vs. 17.2%, p = 0.4202), abdominal parenchyma (21.2% vs. 12.1%, p = 0.2452), and intraperitoneal disease (21.2% vs 17.2%, p = 0.6403). Twenty-five patients in the chemotherapy alone group (40.1%) and 14 patients in the radiation and chemotherapy group (41.2%) had one site of metastasis. For patients with a single site of metastasis in the chemotherapy alone group, the most common site of disease was the lung parenchyma (n = 9), followed by intraperitoneal disease (n = 4), mediastinal lymph nodes (n = 4), other (n = 4), bony pelvic disease (n = 2), supraclavicular lymph nodes (n = 1), and brain metastases (n = 1). For patients with a single site of metastasis in the radiation and chemotherapy group, the most common site of metastasis was other (n = 7), followed by the lung parenchyma (n = 5), mediastinal lymph nodes (n = 1), and supraclavicular lymph nodes (n = 1).
Table 1.
Demographics of Chemotherapy alone group (CT) vs Chemotherapy with Whole Pelvic Radiation (CT + WPR).
| CT (N = 61) | CT + WPR (N = 34) | p-value | |
|---|---|---|---|
| Age in years Median (Range) | 51 (28–83) | 55 (23–76) | 0.6094 |
| Race N (%) | 0.1631 | ||
| White | 25 (41.%) | 8 (23.5%) | |
| African American | 6 (9.8%) | 7 (20.6%) | |
| Hispanic | 21 (34.4%) | 16 (47.1%) | |
| Other/Unknown | 9 (14.80%) | 3 (8.8%) | |
| Histology N (%) | 0.3032 | ||
| Squamous Cell | 45 (73.8%) | 23 (67.7%) | |
| Adenocarcinoma | 7 (11.5%) | 8 (23.5%) | |
| Adenosquamous | 6 (9.8%) | 3 (8.8%) | |
| Other/Unknown | 3 (4.9%) | 0 (0.0%) | |
| Grade N (%) | 0.3227 | ||
| Grade 1 | 2 (3.3%) | 3 (8.8%) | |
| Grade 2 | 14 (3.0%) | 12 (35.3%) | |
| Grade 3 | 36 (59.0%) | 15 (44.1%) | |
| Unknown | 9 (14.8%) | 4 (11.8%) | |
| Primary Chemotherapy N (%) | 0.63 | ||
| Cisplatin/Paclitaxel | 12 (19.7%) | 8 (23.5%) | |
| Cisplatin alone or with Other Chemo | 37 (60.7%) | 22 (64.7%) | |
| Carboplatin/Paclitaxel | 12 (19.7%) | 4 (11.8%) | |
| Bevacizumab during Primary Therapy N (%) | 0.1041 | ||
| Yes | 16 (26.2%) | 4 (11.8%) | |
| No | 42 (68.9%) | 30 (88.2%) | |
| Unknown | 3 (4.9%) | 0 (0.0%) | |
| Palliative Pelvic Radiation | 0.0134 | ||
| Yes | 19 (31.2%) | 3 (8.8%) | |
| No | 37 (60.7%) | 30 (88.2%) | |
| Unknown | 5 (8.2%) | 1 (3.0%) | |
| Sites at Diagnosisa | |||
| Intraperitoneal Metastases | 10 (17.2%) | 7 (21.2%) | 0.6403 |
| Abdominal Parenchymal (liver, spleen) Metastases | 7 (12.1%) | 7 (21.2%) | 0.2452 |
| Lung Parenchymal Metastases | 22 (37.9%) | 16 (48.5%) | 0.3264 |
| Brain Metastases | 4 (6.09%) | 0 (0.0%) | 0.2925 |
| Bony Metastases in Pelvis | 5 (8.6%) | 2 (6.1%) | 1.0000 |
| Extrapelvic Bony Metastases | 4 (6.9%) | 3 (9.1%) | 0.7011 |
| Mediastinal Lymph Node | 15 (25.9%) | 5 (15.2%) | 0.2355 |
| Supraclavicalar Lymph Node | 10 (17.2%) | 8 (24.2%) | 0.4202 |
| Other | 23 (39.7%) | 14 (42.4%) | 0.7960 |
| Number of Sites at Diagnosis | 0.3855 | ||
| 1 | 12 (20.7%) | 2 (6.1%) | |
| 2 | 17 (29.3%) | 14 (42.2%) | |
| 3 | 19 (32.8%) | 11 (33.3%) | |
| 4 | 9 (15.5%) | 5 (15.2%) | |
| 5 | 1 (1.7%) | 1 (3.0%) |
Site is missing for 3 patients in CT group and 1 patient in CT + WPR group. Patients could have multiple sites of disease. “Other” includes any disease site not listed.
During the study period, the standard of care for metastatic cervical cancer changed to include bevacizumab with systemic chemotherapy. In our population, 16 patients (26.2%) in the chemotherapy alone group received bevacizumab with their chemotherapy as compared to only 4 (11.8%) in the radiation and chemotherapy group (p = 0.1041). 70.6% of patients in the whole pelvic radiation (WPR) and chemotherapy and 63.9% of chemotherapy alone group were diagnosed prior to 2014, or prior to the publication of GOG 240.
There were no statistical differences in any of the pelvic-related morbidities between the two groups (Table 2). The most common pelvic-related morbidity was vaginal or rectal bleeding requiring hospitalization or transfusion. This occurred in 66.7% (n = 22) of the radiation and chemotherapy group as compared to 58.0% (n = 29) of the chemotherapy alone group (p = 0.4272). Pelvic pain requiring hospitalization occurred in 45.2% (n = 14) of the radiation and chemotherapy group as compared with 30.4% (n = 14) of the chemotherapy alone group (p = 0.1877). Ureteral obstruction was also fairly common in both groups, occurring in 25% (n = 8) of the WPR and chemotherapy group and 36.7% (n = 19) of the chemotherapy alone group (p = 0.2688). Less common morbidities were fistula formation (6.3% vs. 8.5%, p = 1.0000) and pelvic infection (0% vs. 4.3%, p = 0.5118).
Table 2.
Pelvic-Related Morbidities in Chemotherapy Alone (CT) vs Chemotherapy and Whole Pelvic Radiation (CT + WPR).
| Rate of Pelvic Morbidities | |||
|---|---|---|---|
| Morbidity | CT | CT + WPR | p |
| Ureteral Obstruction | 18/49 (36.7%) | 8/32 (25.0%) | 0.2688 |
| Vaginal/Rectal Bleeding | 29/50 (58.0%) | 22/33 (66.7%) | 0.4272 |
| Pelvic Infection | 2/47 (4.3%) | 0/32 (0.0%) | 0.5118 |
| Pelvic Pain | 14/46 (30.4%) | 14/31 (45.2%) | 0.1877 |
| Fistula | 4/47 (8.5%) | 2/32 (6.3%) | 1.0000 |
| Number of Pelvic Morbidities | |||
| 0.9711 | |||
| 0 | 10/46 (21.7%) | 6/31 (19.4%) | |
| 1 | 15/46 (31.6%) | 9/31 (29.0%) | |
| 2 | 17/46 (37.0%) | 13/31 (41.9% | |
| 3 | 4/46 (837%) | 3/31 (9.7%) | |
Of note, significantly more patients in the chemotherapy alone group received palliative radiation (all sites) at some point during their disease course (31.2% vs 8.8%, p = 0.0134).
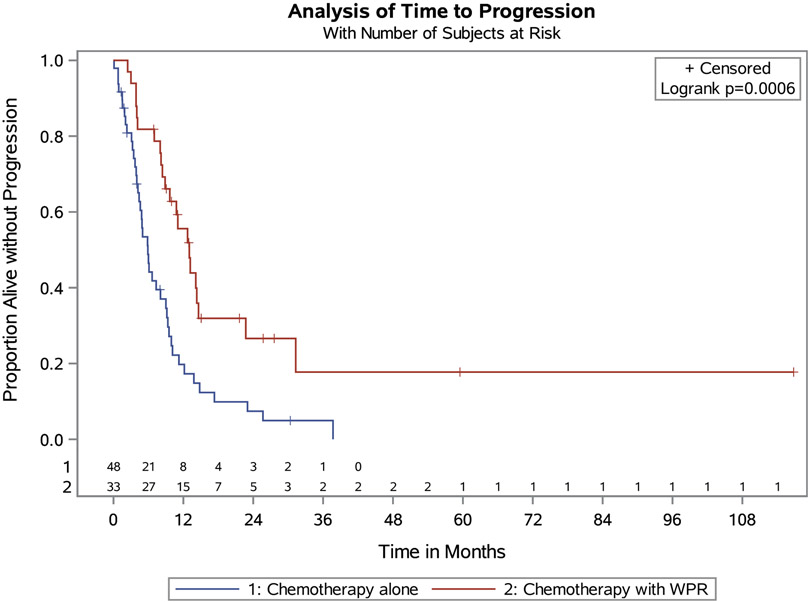
Fourteen patients were missing data on PFS (1 in radiation and chemotherapy group and 13 in chemotherapy only group). The radiation and chemotherapy group had a median PFS of 13.0 months (95% CI 8.9–14.6 months). This is compared with 5.9 months (95% CI 4.2–9.0 months) for the chemotherapy alone group (p = 0.0006, Fig. 1). When evaluated at the time of median follow-up (9 months), the PFS for the radiation and chemotherapy group was 66.1% and 34.6% for the chemotherapy alone group. In terms of OS, the radiation plus chemotherapy group had a 41.6 month median OS (95% CI 26.8 – not calculable). This is compared with the chemotherapy alone group who were found to have a median OS of 17.6 months (95% CI 8.5–37.9, p =.0055, Fig. 2). At the time of median follow-up, the OS was 87.8% for the radiation and chemotherapy group and 64.4% for the chemotherapy alone group.
Fig. 1.
PFS in Whole Pelvic Radiation + Chemotherapy vs. Chemotherapy Alone.
Fig. 2.
OS in Whole Pelvic Radiation + Chemotherapy vs. Chemotherapy Alone.
Given that patients with only supraclavicular nodal metastases may have curable disease, a sub analysis was performed excluding the two patients who met this criterion. The PFS for the remaining patients remained significantly in favor of the radiation and chemotherapy group (p = 0.015). While OS also trended in favor of the radiation and chemotherapy group, it was no longer statistically significant (p = 0.083) (Fig. 3).
Fig. 3.
PFS and OS excluding patients with only supraclavicular nodal disease.
4. Discussion
The present study demonstrates a 7 month increase in PFS and a 24 month increase in OS among patients with metastatic cervical cancer who received radiation with systemic chemotherapy for their primary treatment when compared with those who received chemotherapy alone. However for the OS outcome, only 21 of 34 patients in the radiation and chemotherapy group (5 died and 8 were censored) and 17 of 61 patients in chemotherapy alone group (9 died and 19 were censored) were still being followed at year one. For the radiation and chemotherapy group between 1 year and the median of 41.6 months, an additional 14 patients are censored and 4 died. This may have resulted in an overestimation of the median for this group. Additionally, there were no significant differences in morbidity between groups. These findings are noteworthy as the current standard for this disease is systemic chemotherapy with cisplatin, paclitaxel, and bevacizumab. The survival benefit of this regimen was demonstrated by Tewari and colleagues in GOG 240 where patients with metastatic, persistent, or recurrent cervical cancer were randomized to cisplatin/paclitaxel or topotecan/ paclitaxel with or without bevacizumab. The addition of bevacizumab to chemotherapy was found to improve PFS by 2.2 months (p = 0.0002) and OS by 3.5 months (p = 0.007). Specifically, in patients who had not previously received radiation-a subgroup more similar to the population in this study-the final OS was 24.5 vs 16.8 months with and without bevacizumab added (HR 0.64; 95% CI 0.37–1.10; p = 0.11). Survival post progression was 8.4 vs. 7.1 months with and without bevacizumab (HR 0.83; 95% CI 0.659–1.052; p = 0.06) [4].
However, many patients with widely metastatic cervical cancer also have bulky pelvic disease that can lead to significant morbidity, such as vaginal bleeding, pelvic pain, and urinary obstruction. While these issues undoubtedly adversely affect quality of life, they also may be negatively correlated with survival. For example, two retrospective studies have demonstrated poorer OS among patients with stage IIIB-IVB cervical cancer and hydronephrosis at the time of diagnosis [5,6]. Higher symptom burdens in these women may also negatively impact their disease course. In a study of 991 patients with advanced or recurrent cervical cancer, Chase and colleagues determined that better patient-reported physical wellbeing prior to starting treatment is associated with improved OS [7]. Given these findings, it stands to reason that treating pelvic disease with radiation may have a positive impact on the patient's disease course. In the present study, there appears to be similar rates of common pelvic comorbidities between groups. However, given the inherent difficulty in abstracting accurate symptomatology from the medical record, further investigation is warranted.
There is a paucity of literature assessing the role of pelvic radiation in stage IVB cervical cancer patients. A retrospective evaluation of 24 women with stage IVB cervical cancer treated with whole pelvic radiation found the OS at 5 years was 22%. The majority of patients in this study also received radio-sensitizing chemotherapy and brachytherapy [8]. Two retrospective database studies have also evaluated this question. Venigalla and colleagues reported on 2838 women from the National Cancer Database with stage IV cervical cancer who received chemotherapy. They found that women who also received “definitive local therapy,” defined as concurrent chemoradiation or surgery, had longer OS than those who did not (19.2 months vs 10.1 months, p ≤ 0.001) [9]. Similarly, in their study of more than 3100 patients in the National Cancer Database, Wang and colleagues found that receipt of radiation with chemotherapy improved survival as compared with chemotherapy alone [10]. While these findings are consistent with the present study, it should be noted that many patients in these database studies received concurrent chemoradiation and some received surgery, making comparisons difficult.
It has been reported that patients with stage IVB cervical cancer who's only site of disease metastasis is the supraclavicular lymph nodes have better survival that those with extranodal metastases [11-13]. For example, in their retrospective analysis of 52 patients with stage IV cervical cancer, Ioffe and colleagues found that women with isolated supraclavicular disease had an OS of 10.5 months while women with extranodal metastasis had an OS of 3 months (p = 0.009) [12]. Only two patients in our cohort had isolated supraclavicular disease. When they were excluded from the analysis, the PFS remained statistically significant and in favor of the radiation and chemotherapy group. The OS was also in favor of the radiation and chemotherapy group and trended towards statistical significance.
This study was subject to the inherent limitations of a retrospective study with a small sample size, including selection bias, non-standard treatment regimens, and limited documentation. Details regarding the sequence of chemotherapy and radiation and the dose and type of radiation were unable to be collected. There was incomplete data for pelvic symptomatology, unless it resulted in hospitalization or was detected via imaging, and thus was likely underreported in both groups. There is also no quality of life data available for either group. Furthermore, this study was limited by the variety of chemotherapy regimens utilized in both groups as well as changes in standard treatment practices during the span of the study. GOG 240 was published in 2014 resulting in a shift in practice patterns during the study period. Because of this, only 12% of patients in the radiation plus chemotherapy group and 26% of patients in the chemotherapy alone group received bevacizumab-the current standard of care. Performance status and comorbidities were not assessed in the study but likely impact outcome.
Importantly, the rationale behind the decision to offer pelvic radiation was not available in the medical record. Provider preference, patient symptom burden, and institutional practice patterns are likely to play a role in this decision. However, this is mere speculation and the actual reasons for including radiation with chemotherapy may be more complex.
In conclusion, the addition of pelvic radiation to chemotherapy for the treatment of stage IVB cervical cancer appears to confer improved survival for these patients. However, as a retrospective study, these data require further validation. While the current standard chemotherapy for this patient population includes bevacizumab, few patients in our study received this drug. Therefore, it remains unknown how bevacizumab might affect these results. A prospective trial that includes quality of life measures and incorporates bevacizumab is needed to further investigate this question.
HIGHLIGHTS.
WPR for pelvic disease burden in Stage IVB cervical cancer may have symptomatic utility.
The study identified a statistically significant survival benefit by adding WPR to CT in Stage IVB cervical cancer.
There is no difference in rates of pelvic disease-related morbidity between CT alone and WPR plus CT groups.
Acknowledgments
Grant support
SV reports research presented in this publication was supported in part by the National Cancer Institute Cancer Center Support Grant P30CA225520 awarded to the University of Oklahoma Stephenson Cancer Center and used the Biostatistics and Research Design Shared Resource. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of competing interest
VP, TTS, SC, MC, DB, SM, and LLH have no disclosures or conflicts of interest. KNM discloses advisory board participation for Astra Zeneca, Clovis, Immunogen, Merck, VBL Therapeutics, Aravive, OncoMed, Genentech/Roche, Tesaro, Janssen, Eisai and Samumed. JL discloses advisory board participation for Clovis. BM discloses consulting for Abbvie, Advaxis, Agenus, Amgen, AstraZeneca, Biodesix, Clovis, Conjupro, Genmab, Gradalis, ImmunoGen, Immunomedics, Incyte, Janssen/Johnson&Johnson, Mateon (formally Oxigene), Merck, Myriad, Perthera, Pfizer, Precision Oncology, Puma, Roche/Genentech, Samumed, Takeda, Tesaro, and VBL. KM discloses Honorarium for Chugai, textbook editorial for Springer and investigator meeting attendance expense from VBL therapeutics. LM discloses consulting for Tempus Labs. SV reports grant support as mentioned above.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, CA A Cancer J. Clin 69 (1) (2019) 7–34, 2019. [DOI] [PubMed] [Google Scholar]
- [2].National Comprehensive Cancer Network, Cervical cancer (Version 3.2019) [cited 2019 February 25]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf, 2019.
- [3].Pecorelli S, Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium, Int. J. Gynaecol. Obstet 105 (2) (2009) 103–104. [DOI] [PubMed] [Google Scholar]
- [4].Tewari KS, et al. , Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240), Lancet 390 (10103) (2017) 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goklu MR, et al. , Effect of hydronephrosis on survival in advanced stage cervical cancer, Asian Pac. J. Cancer Prev. APJCP 16 (10) (2015) 4219–4222. [DOI] [PubMed] [Google Scholar]
- [6].Pradhan TS, et al. , Hydronephrosis as a prognostic indicator of survival in advanced cervix cancer, Int. J. Gynecol. Cancer 21 (6) (2011) 1091–1096. [DOI] [PubMed] [Google Scholar]
- [7].Chase DM, et al. , Quality of life and survival in advanced cervical cancer: a Gynecologic Oncology Group study, Gynecol. Oncol 125 (2) (2012) 315–319. [DOI] [PubMed] [Google Scholar]
- [8].Zighelboim I, et al. , Outcomes in 24 selected patients with stage IVB cervical cancer and excellent performance status treated with radiotherapy and chemotherapy, Radiat. Med 24 (9) (2006) 625–630. [DOI] [PubMed] [Google Scholar]
- [9].Venigalla S, et al. , Definitive local therapy is associated with improved overall survival in metastatic cervical cancer, Pract. Radiat. Oncol 8 (6) (2018) e377–e385. [DOI] [PubMed] [Google Scholar]
- [10].Wang Y, et al. , Association of definitive pelvic radiation therapy with survival among patients with newly diagnosed metastatic cervical cancer, JAMA Oncol. 4 (9) (2018) 1288–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim JY, et al. , Curative chemoradiotherapy in patients with stage IVB cervical cancer presenting with paraortic and left supraclavicular lymph node metastases, Int. J. Radiat. Oncol. Biol. Phys 84 (3) (2012) 741–747. [DOI] [PubMed] [Google Scholar]
- [12].Ioffe YJ, et al. , Survival of cervical cancer patients presenting with occult supraclavicular metastases detected by FDG-positron emission tomography/CT: impact of disease extent and treatment, Gynecol. Obstet. Investig 83 (1) (2018) 83–89. [DOI] [PubMed] [Google Scholar]
- [13].Ning MS, et al. , Outcomes and patterns of relapse after definitive radiation therapy for oligometastatic cervical cancer, Gynecol. Oncol 148 (1) (2018) 132–138. [DOI] [PubMed] [Google Scholar]