Abstract
Trichosporon spp. are emerging opportunistic agents that cause systemic diseases and life-threatening disseminated disease in immunocompromised hosts. Trichosporon japonicum is a highly rare cause of invasive trichosporonosis. In this study, we describe 2 cases of urinary tract infection caused by Trichosporon japonicum in kidney transplant patients. Culturing of urine samples yielded bluish-green colonies of T. japonicum on Candida chromogenic fungal medium. The isolates were identified as T. japonicum by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI TOF-MS; Autof MS 1000). The identification of T. japonicum was further confirmed by 18S rRNA gene sequencing. In vitro drug susceptibility testing showed that the 2 strains of T. japonicum were resistant to 5-flucytosine, fluconazole, and caspofungin, with dose-dependent sensitivity to itraconazole and voriconazole but sensitivity to amphotericin B. The homology of the 2 T. japonicum strains, as determined by cluster analysis and principal component analysis of MALDI-TOF MS, was ~85%, suggesting a common nosocomial origin. The first 2 case reports of fluconazole-resistant T. japonicum urinary infection in kidney transplant recipients are presented.
Keywords: antifungal susceptibility testing, homology analysis, kidney transplant, Trichosporon japonicum, urinary tract infections
Trichosporon species are increasingly recognized as causative agents of superficial and invasive fungal disease after Candida species in humans. Although most species are considered commensals of the human skin, respiratory tract, gastrointestinal tract, and vagina, Trichosporon species are increasingly a cause of fungal diseases among immunocompromised hosts, and antifungal treatment is usually ineffective. However, allergic pneumonia and superficial infections caused by Trichosporon species are found predominantly in immunocompetent hosts.
Trichosporon japonicum was first isolated from the air of summer-type hypersensitivity pneumonitis (SHP) patients housed in Japan and was named in 1998 [1, 2]. Then, T. japonicum was isolated from the sputum of an acute myeloid leukemia patient; the sample was classified as T. asahii by API 20C AUX in 2000 and was identified as T. japonicum by genetic identification in 2006 [3]. T. japonicum was isolated from the nails, pleural fluid, and skin tissues of 2 patients with tinea pedis [4, 5]. Recently, T. japonicum fungemia and ventricular assist device infection in an immunocompetent patient were reported [6]. Thus, T. japonicum is a highly rare cause of invasive trichosporonosis. In this report, we describe 2 cases of T. japonicum isolated from urine in kidney transplant patients.
Case 1
A 36-year-old man with end-stage renal disease (ESRD) underwent kidney transplantation (donor father) in June 2019. He was readmitted to the hospital because of an increase in serum creatinine 2 months after kidney transplantation. B-ultrasound results showed isolation of the transplanted kidney collecting system, renal cysts, and perihepatic effusion. He underwent transurethral ureteral stent placement in August. After undergoing hormonal and immunosuppressive treatment, BK virus and cytomegalovirus were detected in the patient. Candida glabrata was detected in urinary and sputum cultures, accompanied by fever and elevated C-reactive protein. The patient was started on caspofungin and voriconazole. Then, urine culture and sputum culture turned negative. The patient continued anti-inflammatory treatment, while urinary and pelvic catheters were flushed. On November 15, he became well enough to be listed for renal ureteral replantation, 1 F4.7 double J tube was retained, and 1 latex drainage tube was placed in the pelvic cavity. Although the patient continued taking caspofungin during hospitalization, T. japonicum was detected repeatedly in urine culture after surgery, and the blood culture was negative. The patient’s overall condition was good, and the lung infection was under control. After the double J tube and the drainage tube were removed, the urinary tract infection recovered, no fungal spores were seen in the urine routine, and the subsequent review demonstrated good recovery.
Case 2
A 50-year-old male patient was admitted to the hospital with chest tightness and wheezing for 8 days. He had received a kidney transplant 17 years before. He was initially diagnosed with a lung infection. Then, he was suspended from immunosuppressive agents and started on a full-spectrum anti-infective treatment. Ten days later, he clinically deteriorated and was transferred to the intensive care unit (ICU). Intravenous catheters, endotracheal tubes, and urinary catheters were inserted. Acinetobacter baumannii and Klebsiella pneumoniae were detected in multiple sputum cultures. T. japonicum was detected in the urine half a month after being transferred to the ICU, and voriconazole treatment was started. After 15 days of voriconazole and caspofungin treatment, T. japonicum was still cultured from urine. The patient abandoned treatment due to disseminated intravascular coagulation and multiple organ failure in the later period, with persistent trichosporonosis being observed despite posaconazole treatment.
METHODS
Isolation and Identification of Trichosporon Species
The urine specimens were cultured on Sabouraud dextrose agar (SDA), Columbia blood agar, chocolate plates, and Candida chromogenic agar. According to standard laboratory guidelines, the presence of yeast cells observed by direct microscopy of urine and total colony counts in the range of 104–105 CFU/mL or higher were considered significant, suggesting urinary tract infection. Culture plates were incubated at 28°C and were observed daily for growth for 7 days. Colonies from 48-hour SDA culture were evaluated for detection of the blastoconidia and arthroconidia by gram staining (bioMérieux, France) and fungal fluorescence staining (Delaikang, China).
MALDI-TOF MS and Homology Analysis
MALDI-TOF MS was performed using both the Autof MS1000 and Vitek MS platforms by the direct smear method according to the manufacturer’s instructions. After the acquisition of spectra, data were transferred to the analysis server, which utilized software algorithms to compare the generated spectrum with the typical spectra within the database. Meanwhile, homology analyses were performed on the obtained spectrum through cluster analysis and principal component analysis.
DNA Sequencing
The isolates were identified by DNA sequencing of internal transcribed spacer (ITS) fragments with common primers by General Biol Company. Primers ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) were used to amplify the ITS region. The polymerase chain reaction (PCR) products were sequenced with a 3739XL DNA sequence, and the sequence data were analyzed with NCBI.
In Vitro Antifungal Susceptibility Testing
In vitro antifungal susceptibilities were determined with the ATB fungus 3 system (bioMérieux, France) according to the manufacturer’s instructions and the E-test method (AutoBio, China), performed as described by Wolf et al. [7]. Minimal inhibitory concentrations (MICs) were determined at 24 hours.
RESULTS
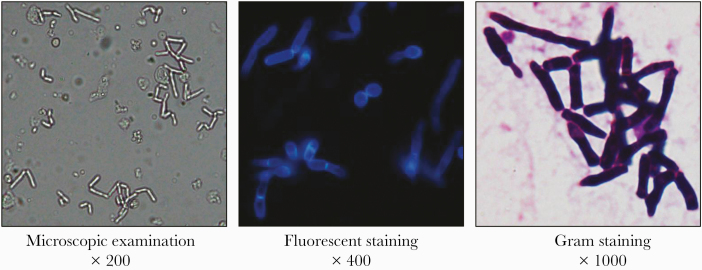
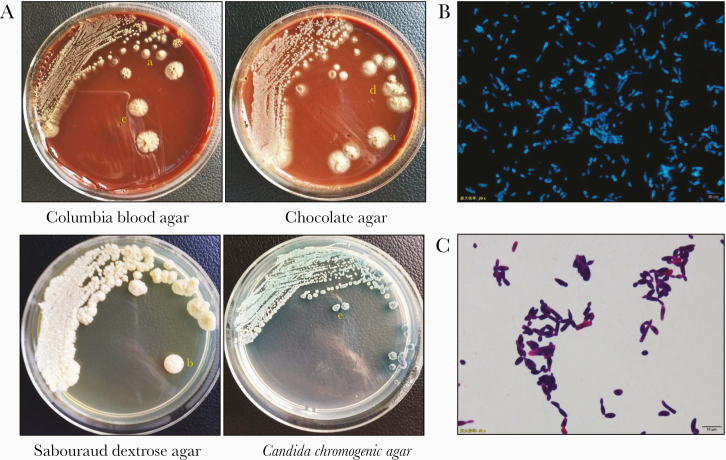
Urine was light yellow and slightly cloudy, and because of hyphal fragmentation, a large number of rounded blastoconidia and arthroconidia could be seen by direct microscopic examination, gram staining, and fungal fluorescent staining (Figure 1). Urine was inoculated onto Candida chromogenic agar and grew wrinkled bluish green yeast after 1 day of incubation. After culturing for 7 days, villous convex colonies and fissured colonies appeared on Columbia blood agar, villous convex colonies and stacked colonies appeared on chocolate plates, and large gyrus colonies appeared on Sabouraud dextrose agar; the colonies were white or light yellow (Figure 2A). Yellow-green pleated colonies appeared on Candida chromogenic agar. A single colony was taken for gram staining and fungal fluorescent staining to show the classic appearance of blastoconidia and arthroconidia of the genus Trichosporon (Figure 2B and C).
Figure 1.
Direct microscopic examination, fungal fluorescence, and gram stain detection of urine. The magnification is 200, 400, and 1000 times, and a large number of blastoconidia and arthroconidia can be seen.
Figure 2.
Morphology of colony in agar and fungal spore of Trichosporon japonicum. A, The colony morphology was cultivated for 7 days at 28°C in Columbia blood agar, chocolate agar, Sabouraud dextrose agar, and Candida chromogenic agar. a. Villous raised colonies. b. Gyrus colony. c. Fissured colony. d. Stacked colonies. e. Pleated colony. T. japonicum cultured on Sabouraud dextrose agar with fungal fluorescence (B) and gram staining (C). Note the rounded blastoconidia, arthroconidia, and true hyphae.
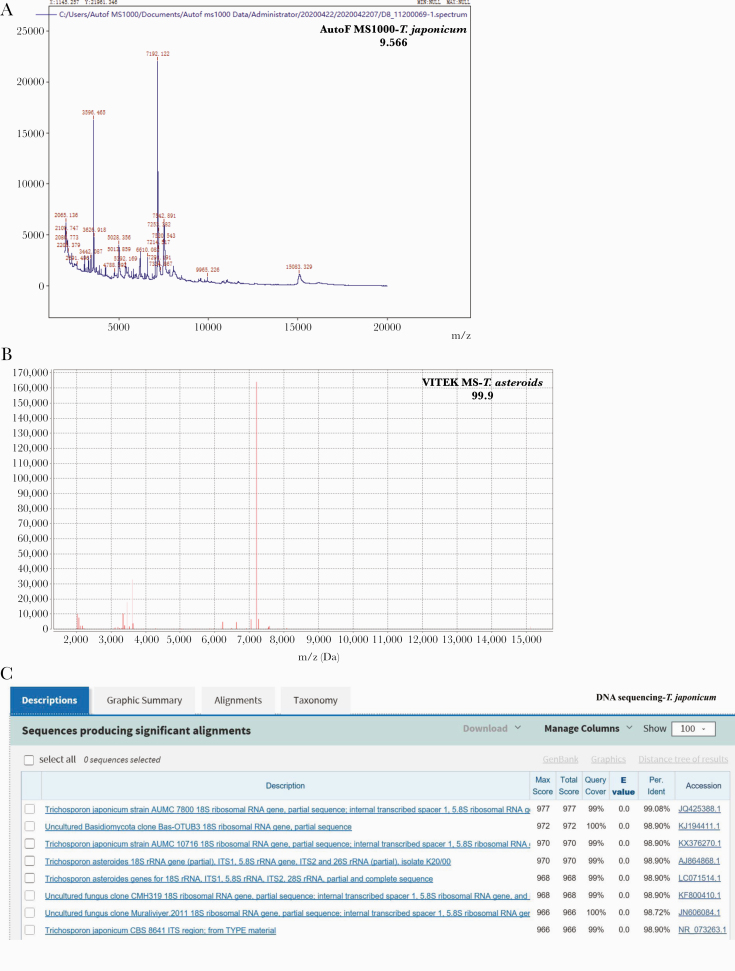
T. japonicum was identified by Autof MS 1000 with a score value of 9.566 (Figure 3A). However, VITEK-MS identified the yeast as T. asteroides (Figure 3B). The identification of T. japonicum was further confirmed, and the sequences matched the 18S ribosomal RNA gene of T. japonicum (GenBank accession No. JQ425388) (Figure 3C).
Figure 3.
Identification of Trichosporon japonicum isolates. The isolates were identified by Autof mass spectrometry (MS) 1000 (A) and VITEK-MS (B). C, T. japonicum was identified by DNA sequencing of internal transcribed spacer fragments.
Antifungal susceptibilities were determined with the E-test system and ATB Fungus 3 system. The results of the ATB Fungus 3 system revealed a high MIC for 5-flucytosine, fluconazole, voriconazole, and itraconazole. The in vitro susceptibility results of the E-test system revealed that fluconazole and caspofungin have poor antifungal activity. Two strains of T. japonicum were dose-dependently sensitive to itraconazole and voriconazole. The differing results obtained by these 2 methods may be due to the tailing growth of azoles in the ATB fungus 3 experiment, resulting in high resistance to azoles. Both in vitro drug susceptibility methods show that amphotericin B had good activity against T. japonicum (Table 1). At the same time, we summarized the clinically reported drug resistance trends of T. japonicum [3–6, 8, 9]. The results in Table 1 show that 10 cases reported in the literature were sensitive to triazoles and resistant to echinocandins, and the reactivity of amphotericin B was uncertain. However, the 2 cases of T. japonicum reported in this article exhibited differences in drug resistance. The cases were sensitive to amphotericin B but resistant to fluconazole.
Table 1.
Minimal Inhibitory Concentrations of Various Antifungal Agents Against 12 Clinical Trichosporon japonicum Isolates
| Trichosporon japonicum Strains Tested | MIC, mg/L | Method | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AmB | 5-FC | FCZ | ITZ | VCZ | CPF | MCF | |||
| 2 | 0.5/0.5 | >16/>16 | >128/>128 | >4/>4 | >8/>8 | ND | ND | ATB | Our study, 2020 |
| 0.094/0.19 | ND | >256/>256 | 0.38/0.75 | 2/2 | >32/>32 | ND | E-test | ||
| 2 | 0.25/1 | 4/32 | 0.12/1 | 0.5/0.5 | 0.015/0.03 | ND | ND | EUCAST/CLSI | Juan L. Rodriguez-Tudela, 2005 |
| 1 | 1 | ND | 0.032 | 0.75 | 0.032 | ND | ND | E-test | Handan Agirbasli, 2008 |
| 2 | 2/12 | ND | 2/6 | 0.094/0.125 | 0.047/0.064 | >32/>32 | ND | E-test | Lina Guo, 2009 |
| 2 | 4/16 | ND | 4 | 0.125 | 0.063/0.125 | ND | ND | CLSI | Saad J. Taj-Aldeen, 2009 |
| 1 | 1 | 8 | 2 | 0.5 | 0.06 | ND | 0.5 | ASTY colorimetric | Ayse Kalkanci, 2010 |
| 1 | 1 | 8 | 1 | 0.125 | 0.06 | ND | >4 | EUCAST | Felix Bongomin, 2019 |
| 1 | 4 | ND | 2 | 1 | ND | ND | ND | CLSI | E. P. T. do Espírito Santo, 2020 |
Abbreviations: 5-FC, flucytosine; AmB, amphotericin B; CLSI, Clinical & Laboratory Standards Institute; ASTY, a colorimetric antifungal susceptibility testing kit (Kyokuto Pharmaceutical Industry, Tokyo, Japan); ATB, ATB Fungus 3 system; CPF, caspofungin; EUCAST, European Committee on Antimicrobial Susceptibility Testing; FCZ, fluconazole; ITZ, itraconazole; MCF, micafungin; ND, no data; VCZ, voriconazole.
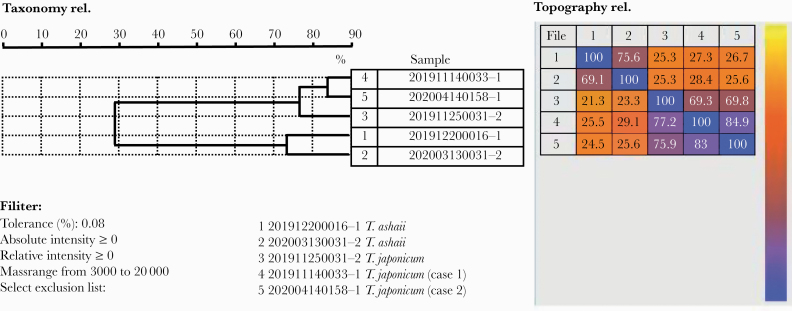
In view of the fact that both patients were admitted to the 4 urological wards, although the hospitalization time did not overlap, we performed homology analysis by cluster analysis and principal component analysis of MALDI-TOF MS and found that T. japonicum from these 2 patients was closely related (~85%), suggesting a common nosocomial origin (Figure 4). Meanwhile, we observed that T. japonicum and T. asahii showed a low degree of similarity (~30%). An investigation of medical staff, medical equipment, and ward environment revealed that both patients had catheters inserted during hospitalization, but the exact source of the pathogen was not found.
Figure 4.
The homology of 2 Trichosporon japonicum by cluster analysis and principal component analysis of time-of-flight mass spectrometry.
DISCUSSION
To the best of our knowledge, there are no published data in the literature concerning urine infection of Trichosporon japonicum. In this report, we describe 2 cases of urine infection due to a rare yeast pathogen, T. japonicum, in kidney transplant recipients. Here, we hypothesize that the portal of entry of the fungus passes through the drainage tube or urinary catheter. It is possible that the ability of Trichosporon strains to adhere to and from biofilms on implanted devices may account for the progress of invasive trichosporonosis, as this ability promotes escape from drugs and host immune responses [10].
Although most Trichosporon strains isolated in clinical laboratories are related to episodes of colonization or superficial infections, this fungus has been recognized as an opportunistic agent causing emergent, invasive infections in tertiary care hospitals worldwide. Unfortunately, invasive trichosporonosis is life-threatening with a high mortality rate, and its prognosis in the patient is notably poor [11–14]. Therefore, accurate interspecies identification and treatment are highly important for trichosporonosis patients.
Trichosporon species identification is based on the characterization of morphological aspects of colonies and biochemical properties. Performing a slide microculture to search for arthroconidia and testing for urease production are useful tools for the screening of Trichosporon spp. [15]. However, morphological aspects and biochemical tests do not allow for complete identification of Trichosporon isolates at the species level. DNA-based molecular sequencing, especially internal transcribed spacer (ITS) and intergenic spacer 1 (IGS1) sequencing, can provide useful methods for the characterization and identification of Trichosporon spp. However, this sequencing method has high requirements for the yield and purity of PCR-amplified fragments, and it needs to be completed by a special gene sequencing analysis system. Therefore, it is difficult to achieve routine application in a clinical setting.
In general microbiology laboratories, protein fingerprints have been used as genus and species signatures to discriminate among isolates and identify pathogens over the past several years. The commercially available MALDI-TOF MS systems in the world include MALDI Biotyper, VITEK MS, the AXIMA @ SARAMIS database, and Andromas. Autof MS 1000 (Anto, China) was launched in 2017 and has the advantages of being a fast, efficient, large, and constantly updated database. Biotyper 2.0 software possesses 11 related to Trichosporon spp., and VITEK MS 3.2 software can identify 9 species of Trichosporon. Autof MS 1000 can distinguish 20 kinds of Trichosporon, including Trichosporon japonicum. MALDI-TOF MS, a reliable and fast method for the identification of fungal strains, may contribute to powerful typing strains and their putative origins, with application in epidemiological studies and the control of nosocomial infections in the future [16]. Here, using MALDI-TOF MS, we found that T. japonicum from these 2 patients was highly similar (~85%), suggesting a common nosocomial origin.
Despite the increasing relevance of the genus Trichosporon in contemporary medicine, treating patients with trichosporonosis remains a challenge, given that we have few data available on the in vitro and in vivo activities of antifungal drugs against clinically relevant species of the genus [17]. Increasing data suggest that Trichosporon spp. are resistant to amphotericin B and fluconazole. Although itraconazole does not have a high MIC in vitro for Trichosporon, the clinical efficacy of itraconazole is not good, which may be related to its higher bactericidal concentration. Voriconazole exhibited excellent in vitro and in vivo activity against Trichosporon spp., which is recommended for trichosporosis treatment. 5-flucytosine (5-FC) has limited in vitro activity against Trichosporon spp., while combination therapy with 5-FC and amphotericin B in the treatment of trichosporonosis has good results. Finally, echinocandins alone have little to no activity against Trichosporon spp. and are not recommended for the treatment of trichosporonosis. The drug resistance trend of T. japonicum is similar to that of Trichosporon spp., with good sensitivity to triazoles and natural resistance to echinocandins. In the 2 patients reported in this article, urinary tract infections caused by T. japonicum occurred during the prevention of infection with caspofungin; therefore, caspofungin is not recommended for the treatment of T. japonicum. The in vitro drug susceptibility results show that T. japonicum is highly resistant to 5-FC and fluconazole, while it is more sensitive to amphotericin B.
Recent studies have found that Trichosporon spp. tend to form biofilms in medical devices, which are related to antifungal resistance and therapeutic failure. Therefore, in addition to conventional antifungal drugs, the HIV aspartyl protease inhibitors ritonavir (RIT), sodium butyrate (NaBut), and farnesol inhibit planktonic growth, biofilm formation, and proteolytic activity in Trichosporon spp. The present studies reinforce the importance of these new antifungals with broader antibiofilm activity as promising antifungal agents [18–20]. The patient in case 1 who underwent urinary catheter and renal pelvis catheter irrigation treatment was eventually cured and discharged.
In conclusion, this article reports 2 cases of urinary tract infection caused by fluconazole-resistant T. japonicum in kidney transplant patients. T. japonicum is a highly rare cause of invasive trichosporonosis in urinary tract infections, even in patients with immunodeficiency. Urinary obstruction, vesical catheterization, and antibiotic treatment may contribute to infection with this pathogen. In addition, removal of catheters and control of underlying conditions to improve immunity should be considered to optimize clinical outcomes. This study represents the rare cases of T. japonicum infection in patients with kidney transplant.
Acknowledgments
Abbreviations: 5-FC, flucytosine; AmB, amphotericin B; CLSI, Clinical & Laboratory Standards Institute; ASTY, ; ATB, ; CPF, caspofungin; EUCAST, European Committee on Antimicrobial Susceptibility Testing; FCZ, fluconazole; ITZ, itraconazole; MCF, micafungin; ND, no data; VCZ, voriconazole.
Author contributions. T.L., Y.H., Z.W., and Y.X. conceived and designed the study. Y.H. and Z.W. were responsible for data interpretation. T.L. wrote the first draft of the paper. Y.H., Z.W., and Y.X. revised subsequent versions. T.L. carried out the experimental works in the clinical microbiology laboratory. X.C. reviewed clinical metadata. All authors have read and approved the final manuscript.
Financial support. This work was supported by the Anhui Provincial Key Research and Development Plan Project (201904a07020049), the University Natural Science Research Project of Anhui Province (KJ2015A337), and the Natural Science Foundation Youth Fund Cultivation Plan of the First Affiliated Hospital of Anhui Medical University (2017kj10).
Patient consent and ethics approval. This study mainly used medical records and biological specimens obtained in previous clinical diagnosis and treatment, so patient informed consent was not required. All clinical data of the patients were collected in accordance with the Local Research Ethics committee of the First Affiliated Hospital of Anhui Medical University (Quick2020-10-07).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sugita T, Nakase T. Trichosporon japonicum sp. nov. isolated from the air. Int J Syst Bacteriol 1998; 48(Pt 4):1425–9. [DOI] [PubMed] [Google Scholar]
- 2. Sugita T, Ikeda R, Nishikawa A. Analysis of Trichosporon isolates obtained from the houses of patients with summer-type hypersensitivity pneumonitis. J Clin Microbiol 2004; 42:5467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ağirbasli H, Bilgen H, Ozcan SK, et al. Two possible cases of Trichosporon infections in bone-marrow-transplanted children: the first case of T. japonicum isolated from clinical specimens. Jpn J Infect Dis 2008; 61:130–2. [PubMed] [Google Scholar]
- 4. Taj-Aldeen SJ, Al-Ansari N, El Shafei S, et al. Molecular identification and susceptibility of Trichosporon species isolated from clinical specimens in Qatar: isolation of Trichosporon dohaense Taj-Aldeen, Meis & Boekhout sp. nov. J Clin Microbiol 2009; 47:1791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodriguez-Tudela JL, Diaz-Guerra TM, Mellado E, et al. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob Agents Chemother 2005; 49:4026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bongomin F, Otu A, Calisti G, et al. Trichosporon japonicum fungemia and ventricular assist device infection in an immunocompetent patient. Open Forum Infect Dis 2019; 6:ofz343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolf DG, Falk R, Hacham M, et al. Multidrug-resistant Trichosporon asahii infection of nongranulocytopenic patients in three intensive care units. J Clin Microbiol 2001; 39:4420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kalkanci A, Sugita T, Arikan S, et al. Molecular identification, genotyping, and drug susceptibility of the basidiomycetous yeast pathogen Trichosporon isolated from Turkish patients. Med Mycol 2010; 48:141–6. [DOI] [PubMed] [Google Scholar]
- 9. do Espírito Santo EPT, Monteiro RC, da Costa ARF, Marques-da-Silva SH. Molecular identification, genotyping, phenotyping, and antifungal susceptibilities of medically important Trichosporon, Apiotrichum, and Cutaneotrichosporon species. Mycopathologia 2020; 185:307–17. [DOI] [PubMed] [Google Scholar]
- 10. Duarte-Oliveira C, Rodrigues F, Gonçalves SM, et al. The cell biology of the Trichosporon-host interaction. Front Cell Infect Microbiol 2017; 7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Padovan ACB, Rocha WPDS, Toti ACM, et al. Exploring the resistance mechanisms in Trichosporon asahii: triazoles as the last defense for invasive trichosporonosis. Fungal Genet Biol 2019; 133:103267. [DOI] [PubMed] [Google Scholar]
- 12. Ramírez I, Moncada D. Fatal disseminated infection by Trichosporon asahii under voriconazole therapy in a patient with acute myeloid leukemia: a review of breakthrough infections by Trichosporon spp. Mycopathologia 2020; 185:377–88. [DOI] [PubMed] [Google Scholar]
- 13. Feugray G, Krzisch D, Dehais M, et al. Successful treatment of Trichosporon asahii fungemia with isavuconazole in a patient with hematologic malignancies. Infect Drug Resist 2019; 12:2015–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milan EP, Silva-Rocha WP, de Almeida JJS, et al. Trichosporon inkin meningitis in Northeast Brazil: first case report and review of the literature. BMC Infect Dis 2018; 18:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colombo AL, Padovan AC, Chaves GM. Current knowledge of Trichosporon spp. and trichosporonosis. Clin Microbiol Rev 2011; 24:682–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pulcrano G, Roscetto E, Iula VD, et al. MALDI-TOF mass spectrometry and microsatellite markers to evaluate Candida parapsilosis transmission in neonatal intensive care units. Eur J Clin Microbiol Infect Dis 2012; 31:2919–28. [DOI] [PubMed] [Google Scholar]
- 17. de Almeida Júnior JN, Hennequin C. Invasive Trichosporon infection: a systematic review on a re-emerging fungal pathogen. Front Microbiol 2016; 7:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cordeiro RA, Aguiar ALR, Pereira VS, et al. Sodium butyrate inhibits planktonic cells and biofilms of Trichosporon spp. Microb Pathog 2019; 130:219–25. [DOI] [PubMed] [Google Scholar]
- 19. Cordeiro RA, Pereira LMG, de Sousa JK, et al. Farnesol inhibits planktonic cells and antifungal-tolerant biofilms of Trichosporon asahii and Trichosporon inkin. Med Mycol 2019; 57:1038–45. [DOI] [PubMed] [Google Scholar]
- 20. Cordeiro RA, Serpa R, Mendes PBL, et al. The HIV aspartyl protease inhibitor ritonavir impairs planktonic growth, biofilm formation and proteolytic activity in Trichosporon spp. Biofouling 2017; 33:640–50. [DOI] [PubMed] [Google Scholar]