Abstract
The objective was to study the effects of microencapsulated organic acids (OA) and essential oils (EO) on growth performance, immune system, gut barrier function, nutrient digestion and absorption, and abundance of enterotoxigenic Escherichia coli F4 (ETEC F4) in the weaned piglets challenged with ETEC F4. Twenty-four ETEC F4 susceptible weaned piglets were randomly distributed to 4 treatments including (1) sham-challenged control (SSC; piglets fed a control diet and challenged with phosphate-buffered saline (PBS)); (2) challenged control (CC; piglets fed a control diet and challenged with ETEC F4); (3) antibiotic growth promoters (AGP; CC + 55 mg·kg–1 of Aureomycin); and (4) microencapsulated OA and EO [P(OA+EO); (CC + 2 g·kg−1 of microencapsulated OA and EO]. The ETEC F4 infection significantly induced diarrhea at 8, 28, 34, and 40 hr postinoculation (hpi) (P < 0.05) in the CC piglets. At 28 d postinoculation (dpi), piglets fed P(OA+EO) had a lower (P < 0.05) diarrhea score compared with those fed CC, but the P(OA+EO) piglets had a lower (P < 0.05) diarrhea score compared with those fed the AGP diets at 40 dpi. The ETEC F4 infection tended to increase in vivo gut permeability measured by the oral gavaging fluorescein isothiocyanate-dextran 70 kDa (FITC-D70) assay in the CC piglets compared with the SCC piglets (P = 0.09). The AGP piglets had higher FITC-D70 flux than P(OA+EO) piglets (P < 0.05). The ETEC F4 infection decreased mid-jejunal VH in the CC piglets compared with the SCC piglets (P < 0.05). The P(OA+EO) piglets had higher (P < 0.05) VH in the mid-jejunum than the CC piglets. The relative mRNA abundance of Na+-glucose cotransporter and B0AT1 was reduced (P < 0.05) by ETEC F4 inoculation when compared with the SCC piglets. The AGP piglets had a greater relative mRNA abundance of B0AT1 than the CC piglets (P < 0.05). The ETEC F4 inoculation increased the protein abundance of OCLN (P < 0.05), and the AGP piglets had the lowest relative protein abundance of OCLN among the challenged groups (P < 0.05). The supplementation of microencapsulated OA and EO enhanced intestinal morphology and showed anti-diarrhea effects in weaned piglets challenged with ETEC F4. Even if more future studies can be required for further validation, this study brings evidence that microencapsulated OA and EO combination can be useful within the tools to be implemented in strategies for alternatives to antibiotics in swine production.
Keywords: Escherichia coli F4, essential oils, gut health, microencapsulation, organic acids, weaned piglets
Introduction
Postweaning diarrhea (PWD) is one of the most economically important issues in the swine industry (Yang et al., 2014), which is characterized by the frequent release of watery feces resulting in retarded growth performance, damaged gut health, increased morbidity, and mortality in young piglets (Pan et al., 2017). Enterotoxigenic Escherichia coli F4 (ETEC F4) is one of the common pathogens associated with PWD (Luise et al., 2019). The fimbriae of ETEC can attach to epithelial receptors and release toxins in the intestine of pigs (Jacobsen et al., 2011). Over the last half-century, antibiotic growth promoters (AGP), subtherapeutic doses of antibiotics in the feed or water, have been generally used to control incidences of PWD and to improve the growth rate and feed efficiency of pigs (Cromwell, 2002). However, the overuse of AGP could lead to the spread of antimicrobial-resistant pathogens in both livestock and humans, posing a significant public health threat (Yang et al., 2015). European Union prohibited the use of AGP in 2006, and worldwide authorities are also trying to decrease the use of antibiotics in the livestock industry (Bengtsson and Wierup, 2006; Murphy et al., 2017). However, still therapeutic and subtherapeutic antibiotics for swine are being used in many regions of the world (Lekagul et al., 2019). At the same time, various AGP alternatives have been developed for use in the swine industry (Heo et al., 2013). Pharmacotherapeutic concentrations (high doses) of copper and zinc, which have antimicrobial effects and can improve gut health and growth performance of weaned piglets, were considered as alternatives for AGP (Debski, 2016; Guan et al., 2017). However, oversupplementation of zinc and copper can induce the generation of antimicrobial-resistant pathogens and cause environmental issues (Ciesinski et al., 2018; Lei and Kim, 2018). Zinc and copper are also being considered to be replaced with other bioactive compounds as is the case with antibiotics. Therefore, cost-effective and eco-friendly AGP alternatives that induce less antimicrobial resistance are required in the swine industry.
Organic acids (OA), organic compounds with weak acidic properties, have been shown to improve gut health and growth performance of piglets by eliciting antimicrobial effects, increasing enzyme secretions and activities, enhancing intestinal morphology, and enhancing gut barrier integrity of piglets (De Lange et al., 2010; Upadhaya et al., 2016). Tricarboxylic acids as citric and dicarboxylic acids as fumaric and malic acids are metabolic intermediates in the Krebs cycle for energy metabolism (Tugnoli et al., 2020). Hence, di- or tricarboxylic acids are known to improve gut morphology and gut barrier integrity by providing energy for epithelial cells and by showing antimicrobial effects in pigs (Chen et al., 2018; Li et al., 2019). Sorbic acid (OA) has strong antimicrobial effects and can be an energy source by being subjected to β-oxidation in pigs (Tugnoli et al., 2020). However, most of the OA are absorbed and metabolized in the upper gut (e.g., stomach and duodenum), and dissociated form of OA shows lower positive effects (e.g., antimicrobial effects) than undissociated form of OA, which is especially of interest, in the lower intestine, where many pathogens propagate (Upadhaya et al., 2014). Microencapsulation, which provides a physical barrier for bioactive compounds from their environment until their release, can allow a slow release of OA along the pig gut, which potentially enhances the beneficial effects of OA in the lower intestine, while the effects as acidifiers in the stomach can be attenuated (Bosi et al., 1999).
Essential oils (EO) are bioactive compounds obtained from plants and are known to have antimicrobial, antioxidative, and anti-inflammatory properties, which potentially improve growth rate and gut health of weaned piglets (Puvača et al., 2013; Omonijo et al., 2018c; Yang et al., 2020). Among various EO, thymol (main EO component in oregano and thyme), eugenol (main EO component in clove and basil), and vanillin (main EO of Vanilla) have been frequently used as antimicrobial, antioxidative, and anti-inflammatory agents for human and animals (Braga et al., 2006; Si et al., 2006; Tippayatum and Chonhenchob, 2007; Tai et al., 2011). Especially, antimicrobial effects to both Gram-negative and Gram-positive bacteria of those EO are already well documented (Si et al., 2006; Ghosh et al., 2014; Chouhan et al., 2017), whereas EO have benefits of promoting the growth performance and gut health of pigs, their stability in the feed and along the gut restrains their application to pig diets (Michiels et al., 2008; Omonijo et al., 2018a). However, microencapsulation is thought to increase effectiveness and resolve stability and delivery issues of EO by protecting EO under harsh conditions (e.g., high temperature during pelleting and low pH in the stomach) (Vidhyalakshmi et al., 2009).
The supplementation of OA with EO can show the synergistic effects to improve the growth performance and gut health of animals (Yang et al., 2015; Abdelli et al., 2020). Zhou et al. (2007) reported that OA and EO showed synergistic antimicrobial effects against Salmonella typhimurium potentially because OA and EO have dissimilar modes of action for their antimicrobial effects. The supplementation of OA and EO improved nutrient digestibility and digestive enzyme activities in weaned piglets (Xu et al., 2018). In our previous study, the lipid matrix microparticles containing OA and EO (Jefo Nutrition Inc., QC, Canada) maintained the stability of EO during a feed pelleting process and storage and allowed a slow and progressive release of EO along the gastrointestinal tract of weaned piglets (Choi et al., 2020). Approximately 15% of thymol in the microparticles was released in the stomach, and 41% of thymol was delivered to the mid-jejunum section in the study (Choi et al., 2020). However, more studies are still needed to comprehensively understand the mechanisms behind the protection of microencapsulated OA and EO against pathogens in weaned piglets. Therefore, the purpose of the study was to investigate the effects of microencapsulated OA (fumaric, malic, citric, and sorbic acids) and EO (thymol, vanillin, and eugenol) on growth performance, immune system, gut barrier function, nutrient absorption, and abundance of ETEC F4 in weaned piglets challenged with ETEC F4.
Materials and Methods
The experimental and animal care protocols (F17-018, AC11280) were reviewed and approved by the Animal Care Committee of the University of Manitoba, and piglets were cared for following the Canadian Council on Animal Care guidelines (CCAC, 2009).
Virulence factors of enterotoxigenic Escherichia coli F4
The ETEC F4 strain P4 used in this study was isolated from feces of piglets with PWD by the Veterinary Diagnostic Services Laboratory—Government of Manitoba, Canada. In this study, the presence and expression of 4 virulence genes associated with adhesion including faeG (F4 fimbriae) and enterotoxins including estA (Sta, heat-stable toxin) and estB (STb, heat-stable toxin), elt (LT, heat-labile toxin) in ETEC F4 were verified by polymerase chain reaction (PCR) according to the method previously described by Zhu et al. (2011) with some modifications (Table 1). The genomic DNA from cultured ETEC F4 (1 × 109 Colony forming units [CFU]) was extracted using PureLink Genomic DNA Kits (Invitrogen, Carlsbad, CA). Total RNA was extracted using an Ambion RiboPure RNA isolation kit (Ambion Inc., Foster City, CA) and the first-strand cDNA was synthesized using oligo (dT) 20 primers and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Each PCR reaction mixture (20 μL) contained 7 μL of 0.1% diethylpyrocarbonate-treated water, 1 μL each of forward and reverse primer (10 μmol·L−1), 10 μL of DreamTaq Green PCR Master Mix (2×) (Thermo Fisher Scientific, Waltham, MA), and 1 μL genomic DNA or 1 μL cDNA. PCR thermocycler conditions were as follows: 50 °C denature for 2 min, 95 °C denature for 5 min, 40 cycles at 95 °C for 45 s, 50 °C for 45 s, and 72 °C for 30 s, and a final extension of 72 °C for 10 min. All PCR products were electrophoresed on a 3% agarose gel in a Tris-borate-EDTA buffer and visualized by staining with SYBR Green (Invitrogen). All 4 virulence genes (estA, estB, faeG, and elt) were expressed in the ETEC F4 used in this study.
Table 1.
Primers used in the study
| Genes1 | Amplicon | Sequence, 5′ to 3′ | References |
|---|---|---|---|
| estA | 158 | CAACTGAATCACTTGACTCTT | Noamani et al. (2003) |
| TTAATAACATCCAGCACAGG | |||
| estB | 113 | TGCCTATGCATCTACACAAT | Noamani et al. (2003) |
| CTCCAGCAGTACCATCTCTA | |||
| elt | 322 | TCTCTATGTGCATACGGAGC | Reischl et al. (2002) |
| CCATACTGATTGCCGCAAT | |||
| faeG | 215 | ACTGGTGATTTCAATGGTTCG | Zhu et al. (2011) |
| GTTACTGGCGTAGCAAATGC | |||
| MUC4 | 367 | GTGCCTTGGGTGAGAGGTTA | Jensen et al. (2006) |
| CACTCTGCCGTTCTCTTTCC | |||
| CycA | 160 | GCGTCTCCTTCGAGCTGTT | Farkas et al. (2015) |
| CCATTATGGCGTGTGAAGTC | |||
| ZO1 | 200 | GATCCTGACCCGGTGTCTGA | Omonijo et al. (2018a) |
| TTGGTGGGTTTGGTGGGTT | |||
| CLDN1 | 220 | CTGTGGATGTCCTGCGTGT | |
| GGTTGCTTGCAAAGTGGTGTT | |||
| CLDN3 | 123 | CTACGACCGCAAGGACTACG | Omonijo et al. (2018a) |
| TAGCATCTGGGTGGACTGGT | |||
| OCLN | 93 | CTGTGGATGTCCTGCGTGT | Lee and Kang (2017) |
| GGTTGCTTGCAAAGTGGTGTT | |||
| MUC2 | 90 | CCAGGTCGAGTACATCCTGC | |
| GTGCTGACCATGGCCCC | |||
| SGLT1 | 153 | GGCTGGACGAAGTATGGTGT | Yang et al. (2011) |
| GAGCTGGATGAGGTTCCAAA | |||
| PepT1 | 143 | ATCGCCATACCCTTCTG | Omonijo et al. (2018a) |
| TTCCCATCCATCGTGACATT | |||
| B0AT1 | 102 | AGGCCCAGTACATGCTCAC | Yang et al. (2016) |
| CATAAATGCCCCTCCACCGT | |||
| EAAC1 | 168 | CCAAGGTCCAGGTTTTGGGT | Omonijo et al. (2018a) |
| GGGCAGCAACACCTGTAATC | |||
| ASCT2 | 206 | GCCAGCAAGATTGTGGAGAT | Yang et al. (2016) |
| GAGCTGGATGAGGTTCCAAA | |||
| IL8 | 126 | CACCTGTCTGTCCACGTTGT | Omonijo et al. (2018a) |
| AGAGGTCTGCCTGGACCCCA | |||
| IL10 | 220 | CATCCACTTCCCAACCAGCC | Lee and Kang (2017) |
| CTCCCCATCACTCTCTGCCTTC | |||
| IL1β | 91 | TGGCTAACTACGGTGACAACA | |
| CCAAGGTCCAGGTTTTGGGT | |||
| TLR2 | 109 | ACATGAAGATGATGTGGGCC | Tohno et al. (2005) |
| TAGGAGTCCTGCTCACTGTA | |||
| TLR5 | 86 | GTTCTTTATCCGGGTGACTT | |
| AATAAGTCAGGATCGGGAGA | |||
| TLR7 | 107 | GCTGTTCCCACTGTTTTGCC | |
| GAGCTGGATGAGGTTCCAAA | |||
| MGA | 118 | GCCCCTTCTGCATGAGTTCT | |
| CGTCACTTTCTCTGCACCCT | |||
| SI | 113 | AGAAACTTGCCAGTGGAGCA | |
| TCCTGGCCATACCTCTCCAA | |||
| APN | 114 | GGACGATTGGGTCTTGCTGA | |
| GGGATGACCGACAGGTTTGT |
1estA, Sta, heat stable toxin A; estB, STb, heat stable toxin B; elt, LT, heat labile toxin; faeG, F4 fimbriae; MUC4, Mucin 4; CycA, cyclophilin-A; ZO1, Zonula occludens 1; CLDN1, claudin 1; CLDN3, claudin 3; OCLN, occludin; MUC2, mucin 2; IL8, interleukin 8; IL10, interleukin 10; IL1β, interleukin 1β; TLR2, toll-like receptor 2; TLR5, toll-like receptor 5; TLR7, toll-like receptor 7; SGLT1, Na+-glucose cotransporter 1; PepT1, peptide transporter 1; ASCT2, neutral amino acid transporter 2; EAAC1, excitatory amino acid transporter 1; B0AT1, neutral amino acid transporter 1; MGA, maltase-glucoamylase; SI, sucrase-isomaltose; APN, aminopeptidase N.
Genetic susceptibility screening and piglet selection
The ETEC F4 susceptible piglets were selected as described by Jensen et al. (2006). DNA was extracted from tails obtained on 3d after farrowing using a method described by Truett et al. (2000). The PCR of MUC4 gene was performed using DreamTaq DNA polymerase (Thermo Fisher Scientific) with 2 mmol·L−−1 MgCl2, 200 µmol·L−1 of each dNTP, 400 µmol·L−1 of each primer in a total volume of 25 µL. Thermocycling was performed using 5 min initial denaturation at 95 °C, subsequently 95 °C for 30 s, annealing at 65 °C for 30 s and extension at 72 °C for 1 min for 35 cycles. The size of the PCR product obtained from pig genomic DNA was 367 bp and 5 µL of the PCR products were digested with FastDigest XbaI (Thermo Fisher Scientific) at 37 °C for 5 min following the supplier’s instructions. All digested PCR products were electrophoresed on a 2% agarose gel in a Tris-borate-EDTA buffer and visualized by staining with SYBR Green (Invitrogen). The resistant allele (R) was indigestible by XbaI, whereas the susceptible allele (S) was digested into 151 bp and 216 bp fragments. The piglets with susceptible alleles and similar body weight (BW) were selected.
Preparation of enterotoxigenic Escherichia coli F4
The ETEC F4 was streaked on tryptic soy agar from frozen stock and grown anaerobically at 37 °C overnight. Afterward, 10 mL of tryptic soy broth (sterile) was inoculated with a single ETEC F4 colony from the streak plate and aerobically grown overnight at 37 °C in an orbital shaker (MaxQ SHKE4000; Thermo Fisher Scientific) set at 150 rpm. The culture was inclined at 45 °C to promote enough aeration. Thereafter, 300 µL of the overnight culture was used as an inoculant for a fresh 300 mL of tryptic soy broth (sterile), again incubating at 37 °C and shaking at 150 rpm by using the orbital shaker. The culture was grown for 2.5 hr. Necessary preliminary experiments such as a growth curve and standard curve were generated first before preparing the final ETEC F4 inoculum. After incubation, a small sample was taken for OD measurement at 600 nm (tryptic soy broth as blank) to check the bacterial density using a Pharmacia Ultrospec 2000 spectrophotometer (Pharmacia Biotech, Cambridge, UK) according to the standard curve generated earlier. Phosphate buffered saline (PBS; pH 7.4) was used as the diluent to achieve the targeted ETEC F4 concentration (1×107 CFU·mL−1). The culture was transported with ice packs to the site for inoculation.
Animals and experimental design
Twenty-four ETEC F4 susceptible weaned piglets (TN Tempo × TN70; 12 female and 12 castrated male piglets with average BW of 8.52 ± 0.11 kg) at the age of 28 d were obtained from the Glenlea Swine Research Unit at the University of Manitoba and housed individually in a temperature-controlled room within the T.K. Cheung Centre for Animal Science Research at the University of Manitoba. Room temperature was maintained at 29 ± 1 °C during the first week and then reduced by 1.5 °C for the rest of the experimental period (8 to 12 d). Piglets were randomly distributed to 4 treatments to give 6 replicates per each treatment. A corn–soybean meal basal diet was formulated to meet or exceed the NRC (2012) recommendations for 6 to10 kg pigs (Table 2) and fed in a mash form. The 4 treatments were: (1) sham-challenged control (SCC; piglets fed the basal diet and inoculated with PBS); (2) challenged control (CC; piglets fed the basal diet and challenged with ETEC F4; (3) AGP (CC + 55 mg·kg−1 of Aureomycin (Zoetis Canada Inc., Kirkland, QC, Canada)); and (4) microencapsulated OA and EO [P(OA+EO); (CC + 2 g·kg−1 of microencapsulated OA [fumaric, citric, malic, and sorbic acids] and EO [thymol, vanillin, and eugenol] microencapsulated in a matrix of triglycerides (Jefo Nutrition Inc.)]. Piglets were housed in individual pens and allowed free access to feed and water during the whole experimental period. On day 7 (0 d postinoculation; dpi) and day 11 (4 dpi), individual pig BW and pen feed disappearance were recorded. On day 7, 5 mL of 1 × 107 CFU·mL−1 ETEC F4 was administered to challenged piglets with a syringe attached to polyethylene tube held into the upper esophagus (Koo et al., 2017, 2020a). Core body temperature was measured by inserting a digital thermometer into the rectum before inoculation and at 3, 24, and 48 hpi (hours postinoculation). Fecal consistency score (0 = normal; well-formed solid feces, 1 = soft feces; formed soft feces, 2 = mild diarrhea; fluid feces with yellowish color, and 3 = severe diarrhea; watery, and projectile feces) was recorded at 0, 3, 8, 16, 24, 28, 34, 40, 48, and 54 hpi as described previously (Marquardt et al., 1999).
Table 2.
The ingredient composition of the basal diet, kg·ton−1, as-fed basis1
| Ingredients, kg, as-fed basis | Basal diet |
|---|---|
| Corn | 483.84 |
| Soybean meal (480 g crude protein·kg−1) | 160 |
| Whey permeate | 124.2 |
| X-SOY6002 (600 g crude protein·kg−1) | 110 |
| Fish meal | 65.73 |
| Soybean oil | 15 |
| Limestone | 14.32 |
| Monocalcium phosphate3 | 5.73 |
| Salt—bulk fine | 5 |
| Vitamin–mineral premix4 (1%) | 10 |
| l-lysine 78% | 2.83 |
| dl-methionine 99% | 1.52 |
| l-threonine | 1.32 |
| l-tryptophan | 0.51 |
| Total | 1,000.00 |
| Calculated net energy and nutrient content | |
| Metabolizable energy (MJ·kg−1) | 14.18 |
| Net energy (MJ·kg−1) | 10.35 |
| Crude protein (%) | 22.35 |
| SID5 lysine | 1.34 |
| SID5 methionine | 0.5 |
| SID5 threonine | 0.87 |
| SID5 tryptophan | 0.27 |
1The diet for AGP was prepared by adding 55 mg·kg−1 of Aureomycin 220G (Zoetis Canada Inc., Kirkland, QC, Canada) into the basal diet. The diet for P(OA+EO) was prepared by adding 2 g·kg−1 of a selected formula of (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides (Jefo Nutrition Inc., QC, Canada).
2Soy protein concentration (CJ Selecta, Goiania, State of Goiás, Brazil).
3Monocalcium phosphate containing Ca, 21% and P, 17% (The Mosaic Co., Plymouth, MN).
4Supplied the following per kilogram of diet: 2,200 IU vitamin A, 220 IU vitamin D3, 16 IU vitamin E, 0.5 mg vitamin K, 1.5 mg vitamin B1, 4 mg vitamin B2, 12 mg calcium pantothenate, 600 mg choline chloride, 30 mg niacin, 7 mg pyridoxine, 0.02 mg vitamin B12, 0.2 mg biotin, 0.3 mg folic acid, 0.14 mg calcium iodate, 6 mg Cu (copper sulfate), 100 mg Fe (ferrous sulfate), 4 mg manganese oxide, 0.3 mg sodium selenite, and 100 mg zinc oxide.
5Standardized ileal digestible amino acids.
In vivo gut permeability
On day 11 (4 dpi), oral gavage of fluorescein isothiocyanate-dextran 70 kDa (FITC-D70; 10 mg/pig), molecular weight 70 kDa (Sigma-Aldrich Co., St. Louis, MO) in 5 mL PBS was conducted. Pigs were allowed to eat and drink for 4 hr after which blood samples (serum) were collected from each piglet through jugular vein into heparin-free vacutainer tubes (Becton Dickinson, Rutherford, NJ) wrapped in aluminum foil to block the light and kept at room temperature for 3 hr to allow clotting. The blood samples were centrifuged at 750 × g for 15 min to recover serum. The serum (100 µL) was transferred to 96-well plates, and the fluorescence was measured at an excitation wavelength of 485 nm, and an emission wavelength of 528 nm using a Bio-Tek PowerWave HT Microplate Scanning Spectrophotometer (BIO-TEK Instruments, Inc., Winooski, VT). The concentrations of FITC-D70 in the serum samples (ng·mL−1) were calculated based on a standard curve (R2 = 0.99).
Tissue and digesta sample collection
At the end of the experiment (day 12; 5 dpi), all piglets were anesthetized by an intramuscular injection of ketamine:xylazine (20:2 mg·kg−1 BW) and euthanized with a captive bolt gun. The abdomen was immediately opened to expose the whole gastrointestinal tract. A 10-cm segment was taken from the mid-jejunum (400 cm from the stomach–duodenum junction) and put in ice-cold Krebs ringer buffer (KRB; in mmol·L−1: 154 Na+, 6.3 K+, 137 Cl−, 0.3 H2PO4, 1.2 Ca2+, 0.7 Mg2+, 24 HCO3− – pH 7.4 with 1 μmol·L−1 of indomethacin) with glucose (10 mmol·L−1) and immediately delivered to the laboratory for the Ussing chamber analysis. Another 10 cm segment of the mid-jejunum was removed and immediately snap-frozen in liquid nitrogen, and then stored at –80 °C until further analyses. For measurement of gut morphology measurement, a 2-cm segment of the mid jejunum was collected and fixed in a 10% formaldehyde solution. Digesta from the spiral colon (20 cm from the ileum–cecum junction) was collected and immediately frozen in liquid nitrogen before being stored at –80 °C until further analyses.
Ussing chamber
The electrophysiological properties including short-circuit current and transepithelial electrical resistance (TEER) were determined using modified Ussing chambers (VCC-MC8; Physiologic Instruments Inc., San Diego, CA) containing pairs of current (Ag wire) and voltage (Ag/AgCl pellet) electrodes housed in 3% agar bridges and filled with KRB buffer without glucose. Five milliliters of the KRB buffer solution with 10 mmol·L−1d-glucose was added to the serosal chambers, and 5 mL of KRB buffer solution enriched with 10 mmol·L−1d-mannitol instead of d-glucose was added to the mucosal chambers. Both the mucosal and serosal chambers were continuously gassed with a mixture of 95% O2 and 5% CO2. The temperature of the chambers was maintained at 37 °C by using a water-jacketed reservoir. The possible potential difference existing between the mucosal and serosal chambers was offset before tissue mounting. After gently stripping off serosal and longitudinal muscle layers using microforceps, the tissue was mounted in Ussing chambers employing a tissue slider with an aperture of 1 cm2. The tissue was left to equilibrate for 10 min followed by the recording of the short-circuit current and TEER for 10 min after mounting. Afterward, 10 mmol·L−1d-glucose was added to the mucosal chamber to measure the sodium-dependent glucose transportation and 10 mmol·L−1 mannitol was added to the serosal chamber to maintain osmotic balance across the tissue (Mrabti et al., 2019). The difference of short-circuit current generated by Na+-glucose cotransporter 1 (SGLT1) was determined by subtracting the short-circuit current value before stimulation from the peak after stimulation. When d-glucose was added, 0.1 mg·mL−1 of FITC-D4 (molecular weight 4 kDa; Sigma-Aldrich Co.) was added to the mucosal side, and after 1 hr, the sample (1 mL) was obtained from the serosal side to measure intestinal permeability. The KRB samples (100 µL) from the serosal side were transferred to 96-well plates. The fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 528 nm using a Bio-Tek PowerWave HT Microplate Scanning Spectrophotometer (BIO-TEK Instruments, Inc.). The concentrations of FITC-D4 in the KRB buffer (ng·mL−1) were calculated based on a standard curve (R2 = 0.99). The FITC-D4 flux was measured for 1 hr using a slide that has 1 cm2 of well surface area and was expressed as μg·cm−2·hr−1·mL−1.
Intestinal morphology analysis
The Alcian blue/the periodic acid–Schiff (AB/PAS) staining for measuring villus height (VH), crypt depth (CD), and VH:CD and counting the number of goblet cells was conducted as described by Koo et al. (2020b). After fixation in 10% neutral-buffered formalin, samples were embedded in paraffin and a 5-µm section was sliced and subsequently mounted on glass slides. Dewaxed sections were immersed in xylene, 100% ethanol and 95% ethanol for 5 min, 2 cycles in each solution. The samples were immersed in Alcian blue solution (pH 2.5) for 15 min at room temperature and washed with water for 2 min, and placed in the Schiff reagent for 10 min and washed with water for 10 min. Finally, samples were counterstained in hematoxylin for 10 s and washed and dehydrated. For the quantification of AB/PAS staining, each sample was visualized and photographed using an Axio Scope A1 microscope (Carl Zeiss Micro-Imaging GmbH, Göttingen, Germany) coupled with an Infinity 2 digital camera (Lumenera Corporation, Ottawa, ON, Canada). VH, CD, and VH:CD were measured, and the number of goblet cells per 100 μm VH and 100 μm CD was counted using Infinity Analyze software (version 6.5.4; Lumenera Corporation, Ottawa, ON, Canada). For each sample, 50 to 150 villi and crypts were measured
Total antioxidant capacity, total GSH, and GSH/GSSG assays
Total antioxidant capacity (TAC) of the mid-jejunal tissue samples was measured in duplicate by using the Colorimetric Microplate Assay Kits for Total Antioxidant Capacity (TA02, Oxford Biomedical Research, Oxford, MI; Yang, 2011). Briefly, 200 mg of liquid nitrogen pulverized mid-jejunal tissue samples were weighed out with a 1.5-mL Eppendorf tube, homogenized with 1 mL of ice-cold PBS on ice for 30 s, and then centrifuged at 3,600 × g for 12 min at 4 °C. Aliquot of supernatant was taken for the analysis of their protein content using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The TAC in the supernatant was measured as the capacity to convert Cu2+ to Cu+ by all antioxidants according to the manufacturer’s protocol. Cu+ ion forms a stable complex with bathocuproine that is detected by measuring the absorbance at 450 nm with a 96-well plate reader (Bio-Tek PowerWave HT Microplate Scanning Spectrophotometer, BIO-TEK Instruments, Inc.). The values were compared with a standard curve obtained using uric acid as a reductant and were expressed as mM·mg protein−1.
Total glutathione (GSH) and oxidized glutathione (GSSG) in the mid-jejunal tissues were measured in duplicate by using the Glutathione Colorimetric Detection Kit (Invitrogen). Briefly, 30 mg of liquid nitrogen pulverized mid-jejunal tissue samples were weighed out with a 1.5-mL Eppendorf tube, homogenized with 750 µL of ice-cold PBS on ice for 30 s, and then centrifuged at 3,600 × g for 10 min at 4 °C. Aliquot of supernatant was taken for the analyses of protein content using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Afterward, 5-sulfosalicylic acid dihydrate was added to the obtained supernatant to precipitate protein, and then centrifuged at 3,600 × g for 10 min at 4 °C. After deproteinization, total GSH and GSSG levels in the resulting supernatant were measured according to the manufacturer’s protocol. Reduced GSH was calculated by the equation: Reduced GSH = total GSH – 2 × GSSG.
Digestive enzyme activity assays
The maximal enzyme activity (Vmax) of intestinal digestive enzymes including aminopeptidase N (APN), intestinal alkaline phosphatase (IAP), maltase, glucoamylase, and sucrase was determined. Specifically, about 200 mg of liquid nitrogen pulverized and frozen mid-jejunal tissue samples were thawed in an ice-cold homogenizing buffer (50 mmol·L−1d-mannitol and 0.1 mmol·L−1 phenylmethylsulfonyl fluoride at pH 7.4) and homogenized on ice using a polytron homogenizer. The protein content of the resulting homogenate samples was determined using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The APN (EC. 3.4.11.2) activity was measured according to the method of Maroux et al. (1973), and IAP (EC 3.1.3.1) activity was measured according to Hübscher and West (1965). The activities of disaccharidases including sucrase (EC 3.2.1.48) and maltase (EC 3.2.1.20) were determined using the procedure of Dahlqvist (1964). The glucoamylase (EC 3.2. 1.20) activity was analyzed according to the method of Lackeyram et al. (2012). The Vmax was expressed in nmol·mg−1·min−1.
RNA extraction and real-time PCR analysis
Total RNA was isolated from 50 mg of liquid nitrogen pulverized mid-jejunal tissue samples using an RNAqueous total RNA isolation kit (Ambion Inc.). The concentration and OD260:OD280 ratio of extracted RNA samples were measured using a Nanodrop UV-Vis spectrophotometer 2000 (Thermo Fisher Scientific Inc., Ottawa, ON, Canada) and the OD260:OD280 ratios of all RNA samples were between 1.9 and 2.1. The RNA samples were stored at −80 °C for further analyses. A total of 1 µg RNA was used to synthesize the first-strand cDNA using an iScript cDNA Synthesis Kit (Bio-Rad, Mississauga, ON, Canada) according to the manufacturer’s instructions. All primers were designed with Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and are shown in Table 1. The primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). Real-time PCR was carried out using an SYBR Green Supermix (Bio-Rad) on a CFX Connect Real-Time PCR Detection System (Bio-Rad; Omonijo et al., 2018b). A total of 1 μL cDNA was added to a total volume of 20 μL, containing 10 μL SYBR Green supermix and 300 nmol·L−1 of each forward and reverse primers. Thermal condition for all reactions was: denaturation for 3 min at 95 °C, then 40 cycles of 20 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C. Cyclophilin-A (CycA) was used as the internal control to normalize the amount of RNA used in the real-time PCR for all the samples. A melting curve program was conducted to confirm the specificity of each PCR product. The target mRNA abundance was normalized with that of a selected reference gene and relative mRNA abundance was determined by using the 2−ΔΔCT method (Livak and Schmittgen, 2001). Threshold cycle (Ct) values were obtained at the cycle number at which the gene is amplified beyond the threshold of 30 fluorescence units. Real-time PCR efficiencies were acquired by amplification of the dilution series of DNase-treated RNA according to formula 10(−1/slope) (Pfaffl, 2001). The efficiencies of all primers used in this study were between 96% and 105%. Negative controls without cDNA were conducted along with each run, and each sample was analyzed in duplicate for each gene.
Western blotting
Relative protein abundance of Zonula occludens 1 (ZO1), Occludin (OCLN), and neutral amino acid transporter 1 (B0AT1) in the jejunum were detected by western blotting. Briefly, an aliquot of about 50 mg of liquid nitrogen pulverized mid-jejunal tissue samples were homogenized in a radioimmunoprecipitation assay buffer (RIPA lysis buffer; Sigma-Aldrich Co.) containing a complete cocktail of proteinase inhibitors, and protein concentration was analyzed by a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. Protein samples were then denatured in 1× Laemmli buffer with mercaptoethanol at 95 °C for 5 min and loaded into the wells of 4% to 12% gradient premade SDS-PAGE gel (Bio-Rad) for electrophoresis. After electrophoresis, the proteins were transferred onto the polyvinylidene fluoride or polyvinylidene difluoride membrane using a Trans-Blot Turbo transfer system (Bio-Rad). For immunoblotting, the membranes were first blocked with 5% nonfat dry milk in tris-buffered saline with 0.1% of Tween-20 (TBST) at room temperature for 1 hr and then incubated with primary antibodies rabbit anti-ZO1 (1:1,000 dilution; Thermo Fisher Scientific), rabbit anti-OCLN (1:500 dilution; Thermo Fisher Scientific), rabbit anti-B0AT1 (1:2,000 dilution, provide by Dr. François Verrey at University of Zurich, Switzerland; Romeo et al., 2006), and rabbit anti-β actin (1:5,000 dilution; Thermo Fisher Scientific) at 4 °C overnight. Afterward, the membranes were washed 5 times with TBST and incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000 dilution; Thermo Fisher Scientific) at room temperature for 1 hr, then washed 5 times with TBST. The chemiluminescent signals were achieved by applying ClarityMax Western ECL Substrate (Bio-Rad) to the membranes and images were captured using a ChemiDoc MP imaging system (Bio-Rad). The intensity of the bands was quantified using Image Lab 6.0 software (Bio-Rad). β-Actin was used as an internal reference. The relative abundance of these proteins was semi-quantified by calculating the ratio of the band intensity of target and reference proteins. Data were presented as mean ± SEM (n = 4).
Measuring ETEC F4 abundance by droplet digital PCR (ddPCR)
DNA from the colon digesta was extracted using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The ETEC F4 abundance in the colon digesta was quantified by measuring the gene copy number of F4-specific fimbriae gene (faeG) using the droplet digital PCR (ddPCR) system (Bio-Rad). Briefly, 25 µL of PCR reaction mixture containing 1 ng (ETEC F4 challenged samples) or 100 ng (control samples without ETEC F4 challenge) of DNA templates, 100 nmol·L−1 of each faeG primer and 1× Evagreen Supermix (Bio-Rad) was prepared, and 20 µL of the mixture was transferred into a sample well of the droplet generator cartridge (DG8 cartridges; Bio-Rad). Droplet Generation Oil (70 μl) (Bio-Rad) was added to the oil wells of DG8 cartridges. Droplets were generated using a droplet generator (Bio-Rad) and were gently transferred onto the 96-well PCR plate (Bio-Rad). The faeG gene in the droplets was amplified on the C1000 Touch thermal cycler (Bio-Rad) using the following thermal cycling protocol: 95 °C for 5 min, 40 cycles of 95 °C for 30 s, and 57 °C for 1 min, and followed by 4 °C for 5 min, 90 °C for 5 min, and 4 °C for 10 min. After thermal cycling, the PCR end products were read by a QX200 droplet reader (Bio-Rad), and data were analyzed by QuantaSoft (Bio-Rad). Data were presented as log10(faeG gene copies·μg DNA−1).
Statistical analyses
Statistical analyses were conducted with SAS (version 9.4; SAS Inst. Inc., Cary, NC) with each individual animal as the experimental unit. The normality of data was confirmed using the PROC UNIVARIATE except for the data of diarrhea score. The effects of ETEC F4 inoculation (SCC vs. SC) were evaluated by unpaired t-test. The ETEC F4-challenged group (CC, AGP, and P(OA+EO)) were compared using the PROC MIXED in a completely randomized design. The statistical model included dietary treatments as the main effect with no random effects. The LSMEANS statement with the Tukey-adjusted PDIFF option was employed to calculate and compared differences among treatment mean. Results in tables were shown as least-square means and pooled standard errors of the means, and results in figures shown as mean ± SEM. Diarrhea score was analyzed using the Mann–Whitney U-test to compare SCC vs. SC and the Kruskal–Wallis test to compare the ETEC F4-challenged group (CC, AGP, and P(OA+EO)) with Dunn’s multiple comparison test (Pant et al., 2011). Differences were considered significant at P < 0.05, and trends (0.05 ≤ P ≤ 0.10) were also presented.
Results
Growth performance, rectal temperature, and diarrhea score
As shown in Table 3, there were no differences in the BW, average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) observed among treatment groups during the prechallenge period (P > 0.10). During the postchallenge period (7 to 11 d), ETEC F4 infection tended to decrease ADG of the CC piglets when compared with the SCC piglets (P = 0.05). The supplementation of Aureomycin and microencapsulated OA and EO did not affect ADG during the postchallenge period (P > 0.10), and there were no differences in the BW and ADFI among treatment groups during the postchallenge period (P > 0.10). During the whole period (0 to 11 d), there were no differences in ADG, ADFI, and FCR among treatment groups (P > 0.10).
Table 3.
Effects of microencapsulated OA and EO on the growth performance of weaned piglets during the prechallenge period (0 to 7 d), postchallenge period (7 to 11 d), and whole period (0 to 11 d)
| ETEC F4-challenged1 | ||||||
|---|---|---|---|---|---|---|
| Items | SCC2 | CC | AGP | P(OA+EO) | SEM | P-value |
| Initial BW, kg | 8.55 | 8.46 | 8.49 | 8.48 | 0.25 | 0.99 |
| Prechallenge | ||||||
| BW, kg | 9.97 | 10.25 | 10.02 | 10.20 | 0.35 | 0.88 |
| ADG, g·d−1 | 202 | 257 | 219 | 243 | 29 | 0.48 |
| ADFI, g·d−1 | 298 | 362 | 317 | 360 | 29 | 0.66 |
| FCR, g·g−1 | 1.73 | 1.42 | 1.55 | 1.55 | 0.13 | 0.75 |
| Postchallenge3 | ||||||
| BW, kg | 11.75 | 11.07 | 10.69 | 11.7 | 0.55 | 0.57 |
| ADG, g·d−1 | 446* | 240 | 183 | 354 | 61 | 0.24 |
| ADFI, g·d−1 | 635 | 538 | 477 | 584 | 43 | 0.25 |
| Whole period | ||||||
| ADG, g·d−1 | 291 | 251 | 195 | 284 | 36 | 0.28 |
| ADFI, g·d−1 | 420 | 422 | 364 | 441 | 33 | 0.28 |
| FCR, g·g−1 | 1.50 | 1.82 | 2.08 | 1.62 | 0.24 | 0.19 |
1CC, control challenged pigs; AGP, CC + 55 mg/kg Aureomycin; P(OA+EO), CC + 2 g/kg of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides. ETEC F4 challenged groups were compared by PROC MIXED followed by the Tukey’s multiple comparison test.
2SCC, sham-challenged control; SCC vs. CC (unpaired t test), *0.05 < P < 0.10.
3The FCR during the postchallenge period was unable to be calculated become some pigs lost weight (negative ADG).
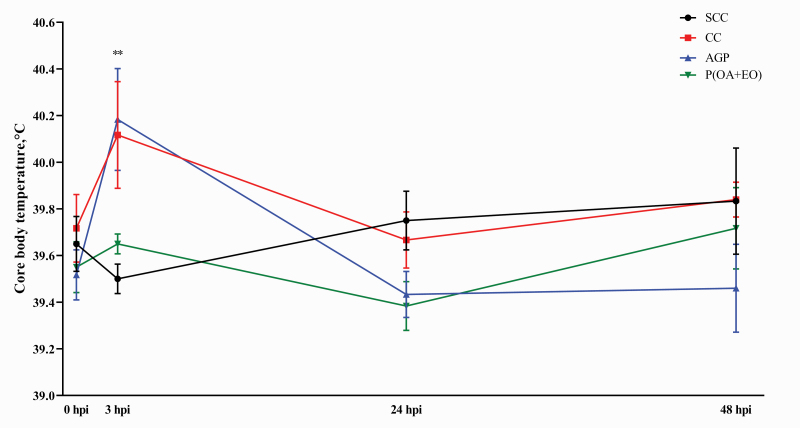
As shown in Figure 1, core body temperature was increased in the CC piglets when compared with the SCC piglets at 3 hpi (40.12 vs. 39.5 °C, P < 0.05). However, the piglets fed AGP and P(OA+EO) had statistically similar core body temperature with the piglet fed the CC diet at 3 hpi. No differences were observed among all treatments at 24 and 48 hpi (P > 0.10).
Figure 1.
Effects of microencapsulated OA and EO on core body temperature in weaned piglets. Core body temperature of weaned piglets was measured in the SCC: pigs fed a control diet and challenged with PBS; CC: pigs fed a control diet and challenged with enterotoxigenic E. coli F4; AGP: CC + 55 mg·kg−1 of Aureomycin; and P(OA+EO) (microencapsulated OA and EO): CC + 2 g·kg−1 of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides groups during 48 hpi. Each value represents the mean ± SEM. At the same time, SCC was compared with SC (unpaired t-test), and the comparison was presented as *0.05 < P < 0.10, **P < 0.05, ***P < 0.01. The ETEC F4 challenged groups (CC, AGP, and P(OA+EO)) was compared by PROC MIXED followed by the Tukey’s multiple comparison test.
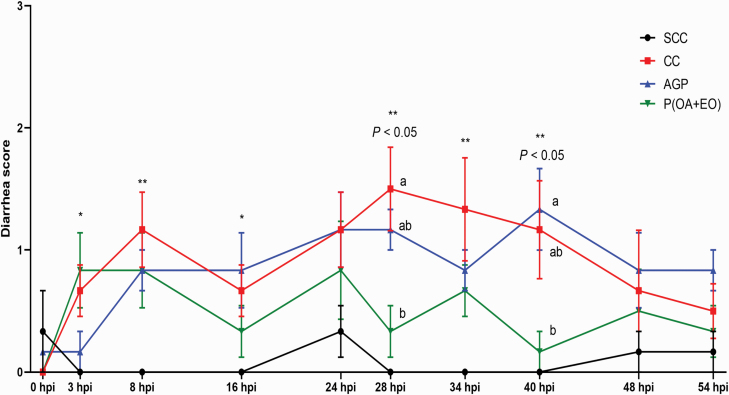
As shown in Figure 2, inoculation of ETEC F4 induced diarrhea at 8, 28, 34, and 40 hpi (P < 0.05) and tended to increase the incidence of diarrhea at the 3 hpi (P = 0.06) and 16 hpi (P = 0.06) in the CC piglets when compared with the SCC piglets. At 28 dpi, piglets fed P(OA+EO) had a lower (P < 0.05) diarrhea score compared with the piglets fed CC. However, the P(OA+EO) piglets had a lower (P < 0.05) diarrhea score compared with the AGP piglets at 40 dpi.
Figure 2.
Effects of microencapsulated OA and EO on diarrhea score in weaned piglets. Diarrhea score of weaned piglets was measured in the SCC: pigs fed a control diet and challenged with PBS; CC: pigs fed a control diet and challenged with enterotoxigenic E. coli F4; AGP: CC + 55 mg·kg−1 of Aureomycin; and P(OA+EO) (microencapsulated OA, and EO): NC + 2 g·kg−1 of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides groups during 54 hpi. Diarrhea score = 0, normal feces; 1, soft feces; 2, mild diarrhea; and 3, severe diarrhea. Each value represents the mean ± SEM. Bars with different letters at the same time point are significantly different (P < 0.05) by PROC MIXED followed by the Tukey’s multiple comparison test among SCC, SC, and P(OA+EO). At the same time point, SCC was compared with SC using Mann–Whitney U-test, and the comparison was presented as * 0.05 < P < 0.10, ** P < 0.05, and *** P < 0.01. Bars with different letters at the same time point are significantly different (P < 0.05) compared by the Kruskal–Wallis test followed by the Dunn’s multiple comparison test among SCC, SC, and P(OA+EO).
Gut permeability and glucose transport
As shown in Table 4, there were no differences in TEER, SGLT1-dependent short-circuit current and FITC-D4 as measured using the Ussing chamber (P > 0.10). Inoculation of ETEC F4 tended to increase in vivo gut permeability in the CC piglets compared with the SCC piglets (P = 0.09). The AGP piglets had higher FITC-D70 flux than P(OA+EO) piglets (P < 0.05).
Table 4.
Effects of microencapsulated OA and EO on electrophysiological properties including TEER and SGLT1-dependent short-circuit current and flux of FITC-D4 of weaned piglets’ jejunum mounted in Ussing chambers (ex vivo) and flux of FITC-D70 in weaned piglets (in vivo)
| ETEC F4-challenge1 | ||||||
|---|---|---|---|---|---|---|
| Items | SCC2 | CC | AGP | P(OA+EO) | SEM | P-value |
| Ex vivo | ||||||
| TEER, Ω·cm2 | 41.77 | 50.00 | 42.19 | 54.70 | 14.92 | 0.88 |
| SGLT1-dependent short-circuit current, μA·cm−2 | 80.85 | 48.84 | 42.10 | 54.57 | 8.08 | 0.53 |
| FITC-D4 flux, μg·cm−2·hr−1·mL−1 | 45.11 | 55.20 | 46.88 | 31.82 | 9.29 | 0.20 |
| In vivo | ||||||
| FITC-D70 flux, μg·mL−1 | 1,032* | 1,357ab | 1,682a | 1,006b | 184.8 | 0.05 |
1CC, control challenged pigs; AGP, CC + 55 mg/kg Aureomycin; P(OA+EO), CC + 2 g/kg of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides.
2SCC, sham-challenged control; SCC vs. CC (unpaired t test), * 0.05 < P < 0.10.
a,bETEC F4 challenged groups were compared by PROC MIXED followed by the Tukey’s multiple comparison test. Values within a row with different superscripts differ at P < 0.05.
Intestinal morphology and goblet cells
As shown in Table 5, ETEC F4 inoculation decreased mid-jejunal VH in the CC piglets when compared with the SCC piglets (P < 0.05). The piglets supplemented with microencapsulated OA and EO had increased (P < 0.05) VH in the mid-jejunum when compared with the CC piglets. However, no differences were found in the CD, VH:CD, the number of goblet cells per 100 μm VH and CD among all treatment groups (P > 0.10).
Table 5.
Effects of microencapsulated OA and EO on morphology including VH, CD, VH:CD, and the number of goblet cells per 100 μm VH and 100 μm CD in the mid-jejunum of weaned piglets
| ETEC F4-challenged1 | ||||||
|---|---|---|---|---|---|---|
| Items | SCC2 | CC | AGP | P(OA+EO) | SEM | P-value |
| VH, μm | 478** | 364b | 441ab | 512a | 38.75 | 0.04 |
| CD, μm | 278 | 250 | 250 | 270 | 17.02 | 0.62 |
| VH:CD | 1.96 | 1.90 | 1.94 | 2.11 | 0.23 | 0.79 |
| Number of goblet cells per 100 μm VH | 2.58 | 3.64 | 2.46 | 2.47 | 0.48 | 0.17 |
| Number of goblet cell per 100 μm CD | 5.71 | 6.04 | 5.62 | 5.91 | 6.03 | 0.75 |
1CC, control challenged pigs; AGP, CC + 55 mg/kg Aureomycin; P(OA+EO), CC + 2 g/kg of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides.
2SCC, sham-challenged control; SCC vs. CC (unpaired t test), ** P < 0.05.
a,bETEC F4 challenged groups were compared by PROC MIXED followed by the Tukey’s multiple comparison test. Values within a row with different superscripts differ at P < 0.05.
Digestive enzyme maximal activities
As shown in Table 6, no differences were found in the Vmax of APN, IAP, glucoamylase, and sucrase among all treatment groups (P > 0.10). The ETEC F4 inoculation tended to decrease Vmax of maltase (P = 0.05) when the CC piglets were compared with the SCC piglets.
Table 6.
Effects of microencapsulated OA and EO on the activities (nmol·L−1·mg protein−1·min−1) of brush border digestive enzymes in the mid-jejunum of weaned piglets
| ETEC F4-challenged1 | ||||||
|---|---|---|---|---|---|---|
| Items | SCC2 | CC | AGP | P(OA+EO) | SEM | P-value |
| Aminopeptidase N | 0.13 | 0.10 | 0.11 | 0.14 | 0.10 | 0.32 |
| Intestinal alkaline phosphatase | 0.45 | 0.40 | 0.52 | 0.46 | 0.04 | 0.16 |
| Maltase | 78.63* | 54.04 | 70.64 | 76.11 | 10.78 | 0.33 |
| Glucoamylase | 4.36 | 3.95 | 4.02 | 4.49 | 0.67 | 0.78 |
| Sucrase | 14.43 | 11.57 | 12.90 | 15.93 | 4.12 | 0.69 |
1CC, control challenged pigs; AGP, CC + 55 mg/kg Aureomycin; P(OA+EO), CC + 2 g/kg of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides. ETEC F4 challenged groups were compared by PROC MIXED followed by the Tukey’s multiple comparison test.
2SCC, sham-challenged control; SCC vs. CC (unpaired t test), *0.05 < P < 0.10.
Total antioxidant capacity, total GSH, and GSH/GSSG
As shown in Table 7, there were no differences in TAC, total GSH, GSSG, reduced GSH, and reduced GSH:GSSG observed among treatment groups (P > 0.10).
Table 7.
Effects of microencapsulated OA and EO on the total antioxidant capacity (TAC), total glutathione (GSH), oxidized glutathione (GSSG), and reduced GSH:GSSG in the mid-jejunum of weaned piglets
| ETEC F4-challenged1 | ||||||
|---|---|---|---|---|---|---|
| Items | SCC2 | CC | AGP | P(OA+EO) | SEM | P-value |
| TAC, mmol·L−1·mg protein−1 | 84.88 | 79.80 | 82.18 | 85.51 | 4.27 | 0.61 |
| Total GSH, nmol·L−1·mg protein−1 | 3.26 | 3.09 | 2.98 | 2.95 | 0.02 | 0.32 |
| GSSG, nmol·L−1·mg protein−1 | 0.44 | 0.42 | 0.50 | 0.55 | 0.19 | 0.87 |
| Reduced GSH3, nmol·L−1·mg protein−1 | 2.37 | 2.24 | 1.99 | 1.85 | 0.05 | 0.33 |
| Reduced GSH:GSSG | 5.46 | 5.69 | 4.06 | 3.81 | 0.21 | 0.44 |
1CC, control challenged pigs; AGP, CC + 55 mg/kg Aureomycin; P(OA+EO), CC + 2 g/kg of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides. ETEC F4 challenged groups were compared by PROC MIXED followed by the Tukey’s multiple comparison test.
2SCC, sham-challenged control. SCC compared to CC using unpaired t test.
3Reduced GSH = total GSH – (2 × GSSG).
Relative mRNA abundance of genes related to gut barrier function, immune system, nutrient transport, and digestive enzymes
There were no differences in the relative mRNA abundance of ZO1 and OCLN among all treatment groups (P > 0.10) (Table 8). The ETEC F4 inoculation decreased the relative mRNA abundance of claudin 1 (CLDN1), CLDN3, and mucin 2 (MUC2) in the NC piglets when compared with the SCC piglets (P < 0.05). There were no differences in the relative mRNA abundance of peptide transporter 1 (PepT1), excitatory amino acid carrier 1 (EAAC1), and neutral amino acid transporter 2 (ASCT2) observed among treatment groups (P > 0.10). The relative mRNA abundance of SGLT1 and B0AT1 was decreased due to ETEC F4 inoculation when compared with the SCC piglets (P < 0.05). The AGP piglets had a higher relative mRNA abundance of B0AT1 when compared with the CC piglets (P < 0.05). Among the genes related to the immune system including interleukin 8 (IL8), IL10, IL1β, toll-like receptor 2 (TLR2), TLR5, and TLR7, only the relative mRNA abundance of IL8 tended to increase due to ETEC F4 inoculation (P = 0.10) in the CC piglets when compared with the SCC piglets. The ETEC F4 inoculation decreased relative mRNA abundance of maltase-glucoamylase (MGA) and APN (P < 0.05) and tended to decrease relative mRNA abundance of SI (P = 0.05) in the CC piglets compared with the SCC piglets. However, the relative mRNA abundance of MGA, SI, and APN among CC, AGP, and P(OA+EO) piglets was similar (P > 0.10).
Table 8.
Effects of microencapsulated OA and EO on the relative mRNA abundance of genes associated with gut barrier integrity, nutrient transporters, immune system, and digestive enzymes in the mid-jejunum of weaned piglets
| ETEC F4-challenge1 | ||||||
|---|---|---|---|---|---|---|
| Items | SCC2 | CC | AGP | P(OA+EO) | P-value | SEM |
| Gut barrier integrity | ||||||
| ZO1 | 1.02 | 0.78 | 0.87 | 0.72 | 0.71 | 0.14 |
| CLDN1 | 1.04** | 0.61 | 0.50 | 0.41 | 0.22 | 0.08 |
| CLDN3 | 1.01** | 0.75 | 1.15 | 0.92 | 0.24 | 0.15 |
| OCLN | 1.03 | 0.89 | 0.86 | 0.81 | 0.91 | 0.15 |
| MUC2 | 1.01*** | 0.40 | 0.49 | 0.33 | 0.71 | 0.18 |
| Nutrient transporters | ||||||
| SGLT1 | 1.01*** | 0.50 | 0.69 | 0.52 | 0.49 | 0.12 |
| PepT1 | 1.05 | 0.64 | 0.80 | 0.73 | 0.82 | 0.18 |
| B0AT1 | 1.02*** | 0.36b | 0.84a | 0.49ab | 0.03 | 0.09 |
| EAAC1 | 1.06 | 0.76 | 0.81 | 0.96 | 0.73 | 0.18 |
| ASCT2 | 1.03 | 1.18 | 1.11 | 1.02 | 0.97 | 0.46 |
| Immune system | ||||||
| IL8 | 1.02* | 1.92 | 1.03 | 1.02 | 0.08 | 0.30 |
| IL10 | 1.09 | 1.04 | 0.73 | 1.01 | 0.50 | 0.20 |
| IL1β | 1.02 | 0.99 | 0.78 | 0.72 | 0.44 | 0.15 |
| TLR2 | 1.04 | 1.35 | 0.82 | 1.12 | 0.32 | 0.24 |
| TLR5 | 1.02 | 0.86 | 0.52 | 0.55 | 0.43 | 0.20 |
| TLR7 | 1.05 | 0.75 | 0.70 | 0.65 | 0.81 | 0.11 |
| Digestive enzymes | ||||||
| MGA | 1.01*** | 0.41 | 0.61 | 0.53 | 0.70 | 0.14 |
| SI | 1.04* | 0.56 | 0.65 | 0.63 | 0.18 | 0.13 |
| APN | 1.00** | 0.49 | 0.64 | 0.57 | 0.39 | 0.48 |
1CC, control challenged pigs; AGP, CC + 55 mg/kg Aureomycin; P(OA+EO), CC + 2 g/kg of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides.
2SCC, sham-challenged control; SCC vs. CC (unpaired t test), *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
a,bETEC F4 challenged groups were compared by PROC MIXED followed by the Tukey’s multiple comparison test. Values within a row with different superscripts differ at P < 0.05.
Relative protein abundance of tight junction proteins and nutrient transporter
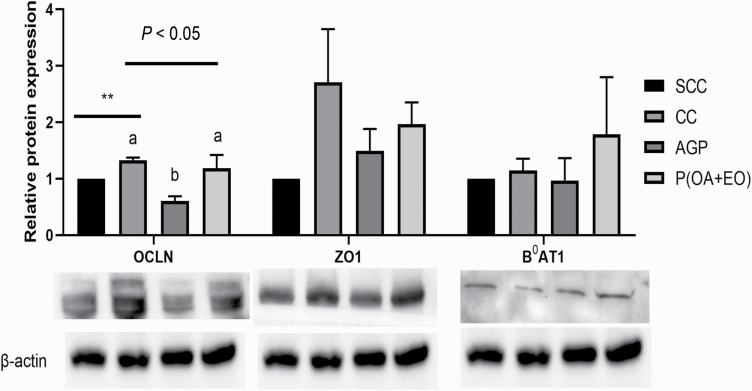
As shown in Figure 3, ETEC F4 inoculation increased the protein abundance of OCLN (P < 0.05), and the AGP piglets had the lowest relative protein abundance of OCLN (P < 0.05) when compared with the CC and P(OA+EO) piglets (P < 0.05). However, the relative protein abundance of ZO1 and B0AT1 was similar among all treatment groups (P > 0.10).
Figure 3.
Effects of microencapsulated OA and EO on the relative abundance of proteins associated with gut barrier integrity and nutrient transporters in weaned piglets. Mid-jejunal relative protein abundance of zonula occludens 1 (ZO1), occludin (OCLN), and neutral amino acid transporter 1 (B0AT1) was measured in the SCC: pigs fed a control diet and challenged with PBS; CC: pigs fed a control diet and challenged with ETEC F4; AGP: CC + 55 mg·kg−1 of Aureomycin; and P(OA+EO) (microencapsulated OA and EO): CC + 2 g·kg−1 of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides groups. Each value represents the mean ± SEM. Bars with different letters are significantly different (P < 0.05) by PROC MIXED followed by the Tukey’s multiple comparison test among SCC, SC, and P(OA+EO). At each time point, SCC was compared with SC (unpaired t-test), and the comparison was presented as * 0.05 < P < 0.10, ** P < 0.05, *** P < 0.01. The ETEC F4 challenged groups (CC, AGP, and P(OA+EO)) was compared by PROC MIXED followed by the Tukey’s multiple comparison test.
ETEC F4 abundance in the colon digesta
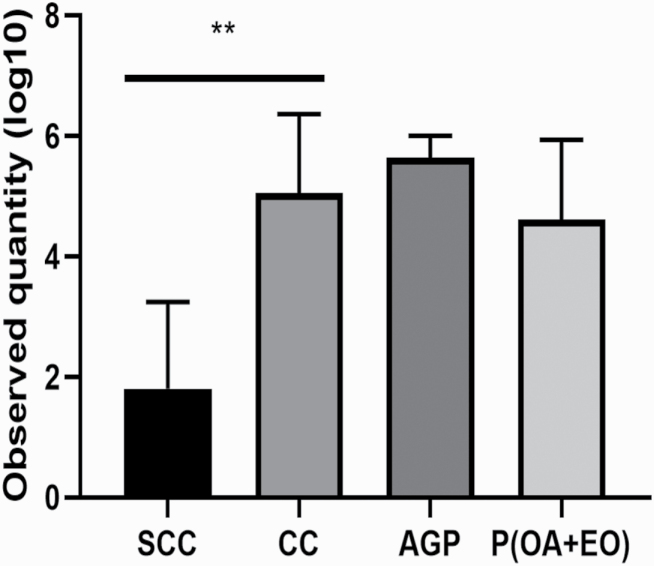
As shown in Figure 4, ETEC F4 inoculation increased the ETEC F4 gene (faeG) (P < 0.05) in the colon digesta in the CC piglets when compared with the SCC piglets. However, there were no differences in the copy number of faeG observed among the piglets challenged with ETEC F4 (P > 0.10).
Figure 4.
Effects of microencapsulated OA and EO on DNA abundance of faeG (F4 fimbriae) in the colon digesta in weaned piglets. DNA abundance of faeG (F4 fimbriae) in the colon digesta (20 cm from the ileum–cecum junction) was measured in the SCC: pigs fed a control diet and challenged with PBS; CC: pigs fed a control diet and challenged with ETEC F4; AGP: CC + 55 mg·kg−1 of Aureomycin; P(OA+EO) (microencapsulated OA and EO): CC + 2 g·kg−1 of a selected formula of OA (fumaric, citric, malic, and sorbic acids) and EO (thymol, vanillin, and eugenol) microencapsulated in a matrix of triglycerides groups. Data were presented as log10(faeG gene copies·μg DNA−1). Each value represents the mean ± SEM. At each time point, SCC was compared with SC (unpaired t test), and the comparison was presented as * 0.05 < P < 0.10, ** P < 0.05, and *** P < 0.01. Bars with different letters at the same time point are significantly different (P < 0.05) by PROC MIXED followed by the Tukey’s multiple comparison test among SCC, SC, and P(OA+EO).
Discussion
This study was to investigate whether the supplementation of microencapsulated OA and EO could alleviate the response to ETEC infection (e.g., diarrhea, inflammation, and compromised gut health) in weaned piglets. A model for inducing bacterial infection in weaned piglets was established by inoculating ETEC F4 (Opapeju et al., 2015). The pathogenesis of ETEC F4 in pigs depends on 2 major factors: ETEC F4 virulence and F4 fimbriae receptors in piglets (Kim et al., 2019). The F4 fimbriae (faeG) attach to the F4 receptors (MUC4) on the intestinal brush borders and induce ETEC F4 colonization in the intestine and then release toxins (estA, estB, and elt; Moonens et al., 2015). The toxins of ETEC F4, including estA, estB, elt, and lipopolysaccharides (LPS), can cause the disorders of electrolytes and fluid secretion in the intestine, which results in watery feces (Koo et al., 2020a). The presence and expression of virulence factors in ETEC F4 strain P4’s were verified in this experiment, and 4 virulence genes (faeG, estA, estB, and elt) were expressed in the ETEC F4 used in the current study.
The ETEC F4 susceptible piglets were selected by verifying the susceptible alleles of MUC4 according to a previous publication (Jensen et al., 2006). Gibbons et al. (1977) showed that the susceptibility to ETEC F4 was inherited as an autosomal dominant Mendelian trait with the 2 alleles: S (adhesion and dominant) and R (nonadhesion and recessive). The ETEC F4 induces more clinical symptoms if piglets have susceptible alleles of the MUC4 gene (Fairbrother et al., 2005). Therefore, it is necessary to choose susceptible piglets for this challenge study to successfully induce diarrhea and minimize variations among piglets (Trevisi et al., 2015; Sterndale et al., 2019). The ultimate purpose of this study was to evaluate dietary OA and EO, microencapsulated in this case, to replace AGP in the swine industry. The AGP (low dosage of antibiotics) are mostly expected to show subtherapeutic effects rather than the therapeutic effects that may alleviate clinical diarrhea (Diarrhea score 2 or 3) or mortality (Adewole et al., 2016). Thus, for implementing the ETEC F4 challenge model, the ETEC F4 dosage that could induce mild diarrhea was inoculated to the piglets in current the study. In our pilot studies (data not published), piglets inoculated with 10 mL of 1 × 109 CFU·mL−1 and 5 mL of 3 × 108 CFU·mL−1 of ETEC F4 showed 75% (15 dead piglets out of 20 piglets) and 65% (13 dead piglets out of 20 piglets) of mortality in all treatment groups, respectively. A pilot study showed that the oral gavage of 5 mL of 1 × 107 CFU·mL−1 ETEC F4 induced mild diarrhea, and thus 5 mL of 1 × 107 CFU·mL−1 was chosen in this study. In the current study, the symptoms of ETEC F4 infection were successfully achieved, which can be indicated by increased diarrhea score at 3 to 40 hpi, core body temperature at 3 hpi and compromised gut health in the CC piglets compared with the SCC piglets.
The OA and EO microparticles used in this study contained fumaric acid, citric acid, malic acid, sorbic acid as OA, and thymol, vanillin and eugenol as EO, both microencapsulated within a matrix-based of hydrogenated vegetable oil for slow release purpose. In our previous study, the microparticles could maintain their stability during a pelleting process and storage and were slowly released along the pig gut (Choi et al., 2020). Within the active ingredients (OA + EO), OA were the most representative bioactive compounds by comparison to EO with a minimum guarantee of 18% for fumaric acid, the ingredient with the highest proportion in those microparticles. Based on the composition and inclusion levels of microparticles, the dose of OA supplied in the feed was in line with minimum inhibitory concentration (MIC) levels reported to inhibit the growth of pathogens (He et al., 2011; Gao et al., 2012). The EO supplied at low or even sub-MIC levels proved to be beneficial for sustainable swine production since sub-MIC levels of EO still can disrupt quorum sensing of bacteria, which is responsible for regulating pathogenicity and antibiotic resistance (Szabó et al., 2010). Omonijo et al. (2018b) also reported that thymol even at sub-MIC level could improve barrier integrity and antioxidative capacity and attenuated inflammatory responses in the intestinal porcine epithelial cell line-J2 (IPEC-J2) challenged with lipopolysaccharides (LPS). Similar effects were also recently reported with eugenol by Hui et al. (2020). In addition, the use of a blend of diverse OA and EO can show synergistic effects in antimicrobial effects and may reduce the possibility of the development of resistant microorganisms because microorganisms are hampered to develop resistant systems against numerous targets at the same time (Yap et al., 2014; Yang et al., 2019).
In this study, during the prechallenge period, the supplementation of Aureomycin and microencapsulated OA and EO did not affect the growth performance. The ETEC F4 infection decreased ADG (46.18%) in the present study, which is consistent with the results of Trevisi et al. (2009) and Rong et al. (2015). The potential reasons for decreased ADG of piglets due to ETEC F4 in the current study could be (1) decreased efficiency of nutrient digestion and absorption (Chen et al., 2018); (2) induced inflammation (Kim et al., 2016); (3) increased diarrhea, which cause loss of water in the body (Cho et al., 2012); and (4) decreased available nutrients for pigs because ETEC F4 may have competed for nutrients with the host (Richards et al., 2005). In the current study, the supplementation of microencapsulated OA and EO did not alter growth performance during the postchallenge period. In contrast, Devi et al. (2015) showed that the supplementation of a blend of EO including cinnamon, fenugreek, and clove improved ADG when compared with the control group, while the supplementation of a coated OA containing formic acid, lactic acid, fumaric acid, and citric acid could not improve growth performance of weaned piglets challenged with ETEC F4. In addition, Kwak et al. (2019) showed that microencapsulated OA and EO attenuated the decrease of ADG and ADFI in the LPS-challenged piglets. However, Ahmed et al. (2013) reported that when a mixture of ETEC KCTC 2571 and S. typhimurium was inoculated to piglets, the blend of EO including oregano (Origanum vulgare), anise (Pimpinella anisum), orange peel (Citrus sinensis), and chicory (Cichorium intybus) did not improve the growth performance when compared with the control group. These inconsistent results in piglets with physiological challenges may come from different kinds of OA and EO, challenging inoculums or experimental conditions (e.g., hygiene, experimental period, and replicates). However, Xu et al. (2020) reported that the supplementation of the same microencapsulated OA and EO combined with antibiotics improved growth performance of weaned piglets challenged with ETEC F4, which may imply that P(OA+EO) can partially replace antibiotics to improve growth performance in pigs challenged with ETEC F4.
In the current study, ETEC F4 infection increased core body temperature at 3 hpi, and the relative mRNA abundance of IL8 (pro-inflammatory cytokines) was increased when SCC piglets compared with CC piglets. Increased core body temperature and upregulated pro-inflammatory cytokines, which are produced by various cell types such as macrophages, endothelial cells, B cells, and mast cells, imply inflammatory reactions in pigs (Akira et al., 1993; Kwak et al., 2019). Potentially, colonization and toxins of ETEC F4 induced inflammatory response in the weaned piglets. Induced inflammatory response potentially was associated with damaged gut barrier integrity, the first line of defense against the hostile environment and prevents noxious antigens and pathogens from permeating into the body (Wijtten et al., 2011). In this study, ETEC F4 infection increased in vivo gut permeability measured by using FITC-D70 flux and decreased the relative mRNA expression of genes related to gut barrier integrity such as CLDN1, CLDN3, and MUC2. According to Suzuki et al. (2011) and Al-Sadi et al. (2014), pro-inflammatory cytokines could modulate the expression of tight junction proteins and gut permeability in pigs, and ETEC F4 toxins would directly modulate the expression of tight junction proteins (Dubreuil, 2017). Furthermore, damaged gut barrier integrity increased the flux of toxins of ETEC F4, which possibly caused a more severe inflammatory response in pigs in the present study. However, the relative protein expression of OCLN was overexpressed due to ETEC F4 infection, which was inconsistent with the data of relative mRNA expression in the present study. According to Wu and Su (2018), ETEC infection increased the expression of tight junction proteins via myosin light chain kinase (MLCK)-myosin II regulatory light chain (MLC20) pathways as a defensive system. However, increased protein expression of tight junction proteins did not affect in vivo and ex vivo gut permeability. The supplementation of Aureomycin and microencapsulated OA and EO did not show anti-inflammatory responses in the current study. However, the AGP piglets even had higher in vivo FITC-D70 flux in the current study compared with the P(OA+EO) piglets. In this experiment, relative protein expression of OCLN was lower in the AGP piglets compared with the CC and P(OA+EO) piglets, which is in line with an increased gut permeability of piglets as a side effect, and this could possibly explain the higher diarrhea score of AGP piglets compared with the P(OA+EO) piglets at 40 dpi.
The FITC-D70 was used to measure gut permeability from the esophagus possibly up to ileum of weaned piglets, and FITC-D4 was used to study intestinal permeability of 1 cm2 of mid-jejunum in the Ussing chamber assay in the current study. The FITC-D70 cannot permeate the epithelial barrier if the epithelial barrier is closed, however, once gut barrier integrity is damaged due to the inflammation or pathogenic infection, FITC-D70 molecule can permeate the gut barrier and enter blood circulation (Yan et al., 2009; Baxter et al., 2017). Hence, FITC-D70 was used to assess in vivo gut permeability because there could be many damaged areas from esophagus to ileum even in SCC piglets. Although the FITC-D4 can diffuse across the intestinal barrier even though the intestine barrier is closed in pigs (Weström et al., 1984), it is expected that a higher amount would permeate across the intestinal barrier if intestinal barrier integrity is damaged. Thus, FITC-D4 may be more appropriate to detect differences in intestinal permeability among the treatments in the small part of samples in the Ussing chamber rather than FITC-D70. Therefore, FITC-D70 was appropriate to measure in vivo gut permeability, and FITC-D4 was suitable to measure intestinal permeability in the Ussing chamber assay in this study. The differences in molecular size of FITC-D4 and -D70 and area of analysis could explain the different results of in vivo and ex vivo gut permeability assay in the current study.
Enterocytes play a crucial role in nutrient digestion and absorption because brush border digestive enzymes and nutrient transporters are expressed in the enterocytes, and therefore, the VH may represent intestinal digestion and absorption capacity (Kong et al., 2018). In this study, ETEC F4 infection decreased the mid-jejunal VH, which is consistent with a study by Yi et al. (2005). This observation could be due to villous atrophy caused by ETEC F4 toxins (Rong et al., 2015). Reduced mid-jejunal VH in CC piglets is potentially associated with the decreased maltase activity and downregulation of genes related to nutrient transporters including SGLT1 (main sugar transport system in the intestine of pigs) and B0AT1 (a nutrient transporter of leucine, valine, isoleucine, methionine, and proline; Hwang et al., 1991; Yang et al., 2016) and digestive enzymes (MGA, SI, and APN) observed in the current study. In this study, the piglets fed the AGP diet had significantly higher relative mRNA expression of B0AT1, which may imply that AGP can increase nutrient absorption in piglets. That P(OA+EO) piglets had the highest VH compared with CC, and AGP piglets is consistent with the results of Liu et al. (2017), indicating that the microencapsulated OA and EO also improved VH in the broiler chickens. However, increased VH did not translate into increased growth rate or increased nutrient digestion and absorption parameters in the current research.
The ddPCR assay is a novel and promising absolute quantification method in the animal science field due to its sensitivity, specificity, and speed (Sui et al., 2019). According to the ddPCR analysis in this study, ETEC F4 also existed in the SCC piglets, and ETEC F4 inoculation increased the number of ETEC F4 in the colon digesta, but the supplementation of Aureomycin or microencapsulated OA and EO did not statistically alter the number of ETEC F4 in the colon digesta of piglets. However, the supplementation of microencapsulated OA and EO reduced diarrhea score at 28 hpi compared with the CC piglets, which may imply that microencapsulated OA and EO potentially reduced the pathogenicity of ETEC F4 or modulated gut microbiota in piglets challenged with ETEC F4, while microencapsulated OA and EO could not inhibit the growth of ETEC F4.
In summary, ETEC F4 infection decreased growth performance, induced inflammatory response and diarrhea, damaged gut morphology, impaired digestive enzymes and nutrient transporters, and decreased the gut barrier integrity of weaned piglets. While the supplementation of Aureomycin increased mRNA expression of an amino acid transporter and gut permeability of weaned piglets challenged with ETEC F4. The supplementation of microencapsulated OA and EO enhanced intestinal morphology and showed anti-diarrhea effects at 1 time point in weaned piglets challenged with ETEC F4. Therefore, whereas the supplementation of AGP or microencapsulated OA and EO did not significantly attenuate the induced inflammation, the reduced digestive enzyme activities and the increased gut permeability in the piglets infected with ETEC F4 in this study, microencapsulated OA and EO combination partially enhanced gut health (e.g., diarrhea score and intestinal morphology) in the piglets infected with ETEC F4. This may imply that microencapsulated OA and EO combination can be useful within the tools to be implemented in strategies for alternatives to antibiotics in swine production.
Acknowledgements
We appreciate Robert Stuski for his help with the animal trial and Fernando Esposito for his help with enzyme activity analyses. The authors are grateful to Kyungbin Bae, who was an NSERC summer undergraduate student in the Department of Animal Science at the University of Manitoba, for her help in the manuscript preparation. Equipment used for analyses in this study was generously funded by the Canada Foundation for Innovation (CFI).
Glossary
Abbreviations
- AB/PAS
Alcian blue/the periodic acid–Schiff
- ADFI
average daily feed intake
- ADG
average daily gain
- AGP
antibiotic growth promoters
- APN
aminopeptidase N
- ASCT2
neutral amino acid transporter 2
- B0AT1
neutral amino acid transporter 1
- BW
body weight
- CC
challenged control
- CD
crypt depth
- CFU
colony forming units
- CLDN1
claudin 1
- CLDN3
claudin 3
- Ct
threshold cycle
- CycA
cyclophilin-A
- ddPCR
droplet digital polymerase chain reaction
- dpi
days postinoculation
- EAAC1
excitatory amino acid carrier 1
- EO
essential oils
- ETEC
enterotoxigenic Escherichia coli
- FCR
feed conversion ratio
- FITC-D4
fluorescein isothiocyanate-dextran 4 kDa
- FITC-D70
fluorescein isothiocyanate-dextran 70 kDa
- GSH
glutathione
- GSSG
oxidized glutathione
- hpi
hours postinoculation
- IAP
intestinal alkaline phosphatase
- IL10
interleukin 10
- IL1β
interleukin 1β
- IL8
interleukin 8
- IPEC-J2
intestinal porcine epithelial cell line-J2
- KRB
Krebs ringer buffer
- LPS
lipopolysaccharides
- MCFA
medium chain fatty acids
- MGA
maltase-glucoamylase
- MIC
minimum inhibitory concentration
- MUC2
Mucin 2
- MUC4
Mucin 4
- OA
organic acids
- OCLN
occludin
- P(OA+EO)
microencapsulated organic acids and essential oils
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PepT1
peptide transporter 1
- PWD
postweaning diarrhea
- RIPA
radioimmunoprecipitation assay
- SCC
sham-challenged control
- SCFA
short chain fatty acids
- SGLT1
Na+-glucose cotransporter 1
- SI
sucrase-isomaltase
- TAC
total antioxidant capacity
- TBST
tris-buffered saline with 0.1% Tween-20
- TEER
transepithelial electrical resistance
- TLR2
toll-like receptor 2
- TLR5
toll-like receptor 5
- TLR7
toll-like receptor 7
- TSA
tryptic soy agar
- VH
villus height
- Vmax
maximal enzyme activity
- ZO1
Zonula occludens 1
Funding
This work was financially supported by the Natural Sciences and Engineering Council of Canada (NSERC) CRD Grant (C. Yang), Manitoba Pork Council (C. Yang, 47370), Jefo Nutrition Inc. (C. Yang, 47369), and the Start-Up Grant (C. Yang, 46561) from the University of Manitoba and Manitoba Graduate Scholarship (MGS).
Conflict of interest statement
Ludovic Lahaye and Elizabeth Santin are coauthors in this manuscript. They are employees at Jefo Nutrition Inc. that provided microencapsulated organic acids and essential oils to this work.
Literature Cited
- Abdelli N, Pérez J F, Vilarrasa E, Cabeza Luna I, Melo-Duran D, D’Angelo M, and Solà-Oriol D. . 2020. Targeted-release organic acids and essential oils improve performance and digestive function in broilers under a necrotic enteritis challenge. Animals 10(2):259–289. doi: 10.3390/ani10020259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewole D, Kim I, and Nyachoti C. . 2016. Gut health of pigs: challenge models and response criteria with a critical analysis of the effectiveness of selected feed additives—a review. Asian Australas. J. Anim. Sci. 29(7):909–924. doi: 10.5713/ajas.15.0795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S T, Hossain M E, Kim G M, Hwang J A, Ji H, and Yang C J. . 2013. Effects of resveratrol and essential oils on growth performance, immunity, digestibility and fecal microbial shedding in challenged piglets. Asian-Australas. J. Anim. Sci. 26:683–690. doi: 10.5713/ajas.2012.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Taga T, and Kishimoto T. . 1993. Interleukin-6 in biology and medicine. Advances in immunology No. 54. Amsterdam, Netherlands: Elsevier; p. 1–78. doi: 10.1016/S0065-2776(08)60532-5 [DOI] [PubMed] [Google Scholar]
- Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, and Ma T Y. . 2014. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One 9:e85345. doi: 10.1371/journal.pone.0085345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M F A, Merino-Guzman R, Latorre J D, Mahaffey B D, Yang Y, Teague K D, Graham L E, Wolfenden A D, Hernandez-Velasco X, Bielke L R, . et al. 2017. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 4:56. doi: 10.3389/fvets.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson B, and Wierup M. . 2006. Antimicrobial resistance in Scandinavia after ban of antimicrobial growth promoters. Anim. Biotechnol. 17:147–156. doi: 10.1080/10495390600956920. [DOI] [PubMed] [Google Scholar]
- Bosi P, Jung H, Han I K, Perini S, Cacciavillani J, Casini L, Creston D, Gremokolini C, and Mattuzzi S. . 1999. Effects of dietary buffering characteristics and protected or unprotected acid on piglet growth, digestibility and characteristics of gut content. Asian Australas. J. Anim. 12(7):1104–1110. doi: 10.5713/ajas.1999.1104 [DOI] [Google Scholar]
- Braga P C, Dal Sasso M, Culici M, Bianchi T, Bordoni L, and Marabini L. . 2006. Anti-inflammatory activity of thymol: inhibitory effect on the release of human neutrophil elastase. Pharmacology. 77(3):130–136. doi: 10.1159/000093790 [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care (CCAC) Guidelines 2009. CCAC guidelines on: the care and use of farm animals in research, teaching and testing. Ottawa, ON, Canada: CCAC. [Google Scholar]
- Chen J, Kang B, Jiang Q, Han M, Zhao Y, Long L, Fu C, and Yao K. . 2018. Alpha-ketoglutarate in low-protein diets for growing pigs: effects on cecal microbial communities and parameters of microbial metabolism. Front. Microbiol. 9:1057. doi: 10.3389/fmicb.2018.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Yu B, Chen D, Mao X, Zheng P, Luo J, and He J. . 2018. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 96:1108–1118. doi: 10.1093/jas/skx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J H, Zhang S, and Kim I H. . 2012. Effects of anti-diarrhoeal herbs on growth performance, nutrient digestibility, and meat quality in pigs. Asian-Australas. J. Anim. Sci. 25:1595–1604. doi: 10.5713/ajas.2012.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Wang L, Ammeter E, Lahaye L, Liu S, Nyachoti M, and Yang C. . 2020. Evaluation of lipid matrix microencapsulation for intestinal delivery of thymol in weaned pigs. Transl. Anim. Sci. 4:411–422. doi: 10.1093/tas/txz176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan S, Sharma K, and Guleria S. . 2017. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines. 4(3):58–79. doi: 10.3390/medicines4030058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesinski L, Guenther S, Pieper R, Kalisch M, Bednorz C, and Wieler L H. . 2018. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS One 13:e0191660. doi: 10.1371/journal.pone.0191660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell G L. 2002. Why and how antibiotics are used in swine production. Anim. Biotechnol. 13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. 1964. Method for assay of intestinal disaccharidases. Anal. Biochem. 7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- De Lange C, Pluske J, Gong J, and Nyachoti C. . 2010. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 134 (1–3):124–134. doi: 10.1016/j.livsci.2010.06.117 [DOI] [Google Scholar]
- Debski B. 2016. Supplementation of pigs diet with zinc and copper as alternative to conventional antimicrobials. Pol. J. Vet. Sci. 19(4):917–924. doi: 10.1515/pjvs-2016-0113 [DOI] [PubMed] [Google Scholar]
- Devi S M, Lee S I, and Kim I H. . 2015. Effect of phytogenics on growth performance, fecal score, blood profiles, fecal noxious gas emission, digestibility, and intestinal morphology of weanling pigs challenged with Escherichia coli K88. Pol. J. Vet. Sci. 18:557–564. doi: 10.1515/pjvs-2015-0072. [DOI] [PubMed] [Google Scholar]
- Dubreuil J D. 2017. Enterotoxigenic Escherichia coli targeting intestinal epithelial tight junctions: an effective way to alter the barrier integrity. Microb. Pathog. 113:129–134. doi: 10.1016/j.micpath.2017.10.037. [DOI] [PubMed] [Google Scholar]
- Fairbrother J M, Nadeau É, and Gyles C L. . 2005. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Health. Res. Rev. 6(1):17–39. doi: 10.1016/j.livsci.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Farkas O, Palócz O, Pászti-Gere E, and Gálfi P. . 2015. Polymethoxyflavone apigenin-trimethylether suppresses LPS-induced inflammatory response in nontransformed porcine intestinal cell line IPEC-J2. Oxid. Med. Cell. Longev. 2015:673847. doi: 10.1155/2015/673847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Shao J, Sun H, Zhong W, Zhuang W, and Zhang Z. . 2012. Evaluation of different kinds of organic acids and their antibacterial activity in Japanese Apricot fruits. Afr. J. Agric. Res. 7(35):4911–4918. doi: 10.5897/AJAR12.1347 [DOI] [Google Scholar]
- Ghosh V, Mukherjee A, and Chandrasekaran N. . 2014. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B. Biointerfaces 114:392–397. doi: 10.1016/j.colsurfb.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Gibbons R A, Sellwood R, Burrows M, and Hunter P A. . 1977. Inheritance of resistance to neonatal E. coli diarrhoea in the pig: examination of the genetic system. Theor. Appl. Genet. 51:65–70. doi: 10.1007/BF00299479. [DOI] [PubMed] [Google Scholar]
- Guan X, Bass B, van der Aar P, Piñeiro C, Frank J, Morales J, Molist F, and Cadogan D. . 2017. Lactobacillus acidophilus fermentation product as an alternative to therapeutic zinc oxide in weaned pig diets on performance and response to Escherichia coli challenge. Anim. Prod. Sci. 57(12):2497. doi: 10.1071/ANv57n12Ab058 [DOI] [Google Scholar]
- He C-L, Fu B-D, Shen H-Q, Jiang X-L, and Wei X-B. . 2011. Fumaric acid, an antibacterial component of Aloe vera L. Afr. J. Biotechnol. 10(15):2973–2977. doi: 10.5897/AJB10.1497 [DOI] [Google Scholar]
- Heo J, Opapeju F, Pluske J, Kim J, Hampson D, and Nyachoti C. . 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 97(2):207–237. Doi: 10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Hübscher G, and West G. . 1965. Specific assays of some phosphatases in subcellular fractions of small intestinal mucosa. Nature. 205(4973):799–800. doi: 10.1038/205799a0 [DOI] [PubMed] [Google Scholar]
- Hui Q, Ammeter E, Liu S, Yang R, Lu P, Lahaye L, and Yang C. . 2020. Eugenol attenuates inflammatory response and enhances barrier function during lipopolysaccharide (LPS)-induced inflammation in the porcine intestinal epithelial (IPEC-J2) cells. J. Anim. Sci. doi: 10.1093/jas/skaa245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E S, Hirayama B A, and Wright E M. . 1991. Distribution of the SGLT1 Na+/glucose cotransporter and mRNA along the crypt-villus axis of rabbit small intestine. Biochem. Biophys. Res. Commun. 181:1208–1217. doi: 10.1016/0006-291x(91)92067-t. [DOI] [PubMed] [Google Scholar]
- Jacobsen M, Cirera S, Joller D, Esteso G, Kracht S S, Edfors I, Bendixen C, Archibald A L, Vogeli P, Neuenschwander S, . et al. 2011. Characterisation of five candidate genes within the ETEC F4ab/ac candidate region in pigs. BMC Res. Notes 4:225. doi: 10.1186/1756-0500-4-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen G M, Frydendahl K, Svendsen O, Jørgensen C B, Cirera S, Fredholm M, Nielsen J P, and Møller K. . 2006. Experimental infection with Escherichia coli O149:F4ac in weaned piglets. Vet. Microbiol. 115:243–249. doi: 10.1016/j.vetmic.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kim K, Ehrlich A, Perng V, Chase J A, Raybould H, Li X, Atwill E R, Whelan R, Sokale A, and Liu Y. . 2019. Algae-derived β-glucan enhanced gut health and immune responses of weaned pigs experimentally infected with a pathogenic E. coli. Anim. Feed. Sci. Tech. 248:114–125. doi: 10.1016/j.anifeedsci.2018.12.004 [DOI] [Google Scholar]
- Kim J C, Mullan B P, Black J L, Hewitt R J, van Barneveld R J, and Pluske J R. . 2016. Acetylsalicylic acid supplementation improves protein utilization efficiency while vitamin E supplementation reduces markers of the inflammatory response in weaned pigs challenged with enterotoxigenic E. coli. J. Anim. Sci. Biotechnol. 7:58. doi: 10.1186/s40104-016-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S, Zhang Y H, and Zhang W. . 2018. Regulation of intestinal epithelial cells properties and functions by amino acids. Biomed Res. Int. 2018:2819154. doi: 10.1155/2018/2819154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B, Bustamante-García D, Kim J, and Nyachoti C. . 2020a. Health-promoting effects of Lactobacillus-fermented barley in weaned pigs challenged with Escherichia coli K88+. Animal 14(1):39–49. doi: 10.1017/S1751731119001939 [DOI] [PubMed] [Google Scholar]
- Koo B, Choi J, Yang C, and Nyachoti C M. . 2020b. Diet complexity and l-threonine supplementation: effects on growth performance, immune response, intestinal barrier function, and microbial metabolites in nursery pigs. J. Anim. Sci. 98(5):skaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B, Hossain M, and Nyachoti C. . 2017. Effect of dietary wheat bran inclusion on nutrient and energy digestibility and microbial metabolites in weaned pigs. Livest. Sci. 203:110–113. doi: 10.1016/j.livsci.2017.07.011 [DOI] [Google Scholar]
- Kwak W, Song M, Lee D H, Yun W, Lee J, Lee C, Oh H J, Liu S, An J S, and Kim H B. . 2019. The effects of microencapsulate compounds supplementation on growth performance, immune cells, rectal temperature in weaned pigs by lipopolysaccharides. Can. J. Anim. Sci. 99(3): 505–513. doi: 10.1139/cjas-2018-0166 [DOI] [Google Scholar]
- Lackeyram D, Mine Y, Widowski T, Archbold T, and Fan M. . 2012. The in vivo infusion of hydrogen peroxide induces oxidative stress and differentially affects the activities of small intestinal carbohydrate digestive enzymes in the neonatal pig. J. Anim. Sci. 90(suppl_4):418–420. doi: 10.2527/jas.54011 [DOI] [PubMed] [Google Scholar]
- Lee S I, and Kang K S. . 2017. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 7:16530. doi: 10.1038/s41598-017-16561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X J, and Kim I H. . 2018. Low dose of coated zinc oxide is as effective as pharmacological zinc oxide in promoting growth performance, reducing fecal scores, and improving nutrient digestibility and intestinal morphology in weaned pigs. Anim. Feed Sci. Technol. 245:117–125. doi: 10.1016/j.anifeedsci.2018.06.011 [DOI] [Google Scholar]
- Lekagul A, Tangcharoensathien V, and Yeung S. . 2019. Patterns of antibiotic use in global pig production: a systematic review. Vet. Anim. Sci. 7:100058. doi: 10.1016/j.vas.2019.100058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Burrough E R, Gabler N K, Loving C L, Sahin O, Gould S A, and Patience J F. . 2019. A soluble and highly fermentable dietary fiber with carbohydrases improved gut barrier integrity markers and growth performance in F18 ETEC challenged pigs. J. Anim. Sci. 97:2139–2153. doi: 10.1093/jas/skz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mao M, Zhang Y, Yu K, and Zhu W. . 2019. Succinate modulates intestinal barrier function and inflammation response in pigs. Biomolecules. 9(9):486. doi: 10.3390/biom9090486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang X, Xin H, Chen S, Yang C, Duan Y, and Yang X. . 2017. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- Livak K J, and Schmittgen T D. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luise D, Lauridsen C, Bosi P, and Trevisi P. . 2019. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J. Anim. Sci. Biotechnol. 10:53. doi: 10.1186/s40104-019-0352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroux S, Louvard D, and Baratti J. . 1973. The aminopeptidase from hog intestinal brush border. Biochim. Biophys. Acta 321:282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Marquardt R R, Jin L Z, Kim J W, Fang L, Frohlich A A, and Baidoo S K. . 1999. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol. Med. Microbiol. 23:283–288. doi: 10.1111/j.1574-695X.1999.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Michiels J, Missotten J, Dierick N, Fremaut D, Maene P, and De Smet S. . 2008. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 88(13):2371–2381. doi: 10.1002/jsfa.3358 [DOI] [Google Scholar]
- Moonens K, Van den Broeck I, Okello E, Pardon E, De Kerpel M, Remaut H, and De Greve H. . 2015. Structural insight in the inhibition of adherence of F4 fimbriae producing enterotoxigenic Escherichia coli by llama single domain antibodies. Vet. Res. 46:14. doi: 10.1186/s13567-015-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrabti H N, Faouzi M E A, Mayuk F M, Makrane H, Limas-Nzouzi N, Dibong S D, Cherrah Y, Elombo F K, Gressier B, and Desjeux J-F. . 2019. Arbutus unedo L. (Ericaceae) inhibits intestinal glucose absorption and improves glucose tolerance in rodents. J. Ethnopharmacol. 235:385–391. doi: 10.1016/j.jep.2019.02.013 [DOI] [PubMed] [Google Scholar]
- Murphy D, Ricci A, Auce Z, Beechinor J G, Bergendahl H, Breathnach R, Bureš J, Duarte Da Silva J P, and Hederová J. . 2017. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J. 15(1):e04666. doi: 10.2903/j.efsa.2017.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noamani B N, Fairbrother J M, and Gyles C L. . 2003. Virulence genes of O149 enterotoxigenic Escherichia coli from outbreaks of postweaning diarrhea in pigs. Vet. Microbiol. 97:87–101. doi: 10.1016/j.vetmic.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Omonijo F A, Kim S, Guo T, Wang Q, Gong J, Lahaye L, Bodin J C, Nyachoti M, Liu S, and Yang C. . 2018a. Development of novel microparticles for effective delivery of thymol and lauric acid to pig intestinal tract. J. Agric. Food Chem. 66:9608–9615. doi: 10.1021/acs.jafc.8b02808. [DOI] [PubMed] [Google Scholar]
- Omonijo F A, Liu S, Hui Q, Zhang H, Lahaye L, Bodin J C, Gong J, Nyachoti M, and Yang C. . 2019. Thymol improves barrier function and attenuates inflammatory responses in porcine intestinal epithelial cells during lipopolysaccharide (LPS)-induced inflammation. J. Agric. Food Chem. 67:615–624. doi: 10.1021/acs.jafc.8b05480. [DOI] [PubMed] [Google Scholar]
- Omonijo F A, Ni L, Gong J, Wang Q, Lahaye L, and Yang C. . 2018C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 4:126–136. doi: 10.1016/j.aninu.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opapeju F, Rodriguez-Lecompte J, Rademacher M, Krause D, and Nyachoti C. . 2015. Low crude protein diets modulate intestinal responses in weaned pigs challenged with Escherichia coli K88. Can. J. Anim. Sci. 95(1):71–78. doi: 10.4141/cjas-2014-071 [DOI] [Google Scholar]
- Pan L, Zhao P, Ma X, Shang Q, Xu Y, Long S, Wu Y, Yuan F, and Piao X. . 2017. Probiotic supplementation protects weaned pigs against enterotoxigenic Escherichia coli K88 challenge and improves performance similar to antibiotics. J. Anim. Sci. 95(6):2627–2639. doi: 10.2527/jas.2016.1243 [DOI] [PubMed] [Google Scholar]
- Pant N, Marcotte H, Hermans P, Bezemer S, Frenken L, Johansen K, and Hammarström L. . 2011. Lactobacilli producing bispecific llama-derived anti-rotavirus proteins in vivo for rotavirus-induced diarrhea. Future Microbiol. 6: 583–593. doi: 10.2217/fmb.11.32. [DOI] [PubMed] [Google Scholar]
- Pfaffl M W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvača N, Stanaćev V, Glamočić D, Lević J, Perić L, Stanaćev V, and Milić D. . 2013. Beneficial effects of phytoadditives in broiler nutrition. World Poultry Sci. J. 69(1):27–34. doi: 10.1017/S0043933913000032 [DOI] [Google Scholar]
- Reischl U, Youssef M T, Kilwinski J, Lehn N, Zhang W L, Karch H, and Strockbine N A. . 2002. Real-time fluorescence PCR assays for detection and characterization of Shiga toxin, intimin, and enterohemolysin genes from Shiga toxin-producing Escherichia coli. J. Clin. Microbiol. 40(7):2555–2565. doi: 10.1128/JCM.40.7.2555-2565.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Gong J, and De Lange C. . 2005. The gastrointestinal microbiota and its role in monogastric nutrition and health with an emphasis on pigs: current understanding, possible modulations, and new technologies for ecological studies. Can. J. Anim. Sci. 85(4):421–435. doi: 10.4141/A05-049 [DOI] [Google Scholar]
- Romeo E, Dave M H, Bacic D, Ristic Z, Camargo S M, Loffing J, Wagner C A, and Verrey F. . 2006. Luminal kidney and intestine SLC6 amino acid transporters of B0AT-cluster and their tissue distribution in Mus musculus. Am. J. Physiol. Renal Physiol. 290:F376–F383. doi: 10.1152/ajprenal.00286.2005. [DOI] [PubMed] [Google Scholar]
- Rong Y, Lu Z, Zhang H, Zhang L, Song D, and Wang Y. . 2015. Effects of casein glycomacropeptide supplementation on growth performance, intestinal morphology, intestinal barrier permeability and inflammatory responses in Escherichia coli K88 challenged piglets. Anim. Nutr. 1:54–59. doi: 10.1016/j.aninu.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W, Gong J, Chanas C, Cui S, Yu H, Caballero C, and Friendship R M. . 2006. In vitro assessment of antimicrobial activity of carvacrol, thymol and cinnamaldehyde towards Salmonella serotype Typhimurium DT104: effects of pig diets and emulsification in hydrocolloids. J. Appl. Microbiol. 101:1282–1291. doi: 10.1111/j.1365-2672.2006.03045.x. [DOI] [PubMed] [Google Scholar]
- Sterndale S O, Miller D W, Mansfield J P, Kim J C, O’Dea M, and Pluske J R. . 2019. Technical note: novel delivery methods for an enterotoxigenic Escherichia coli infection model in MUC4-locus sequenced weaner pigs1. J. Anim. Sci. 97: 4503–4508. doi: 10.1093/jas/skz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Liu S, Liu S, Wang J, Xue L, Liu X, Wang B, Gu S, and Wang Y. . 2019. Evaluation of digital PCR for absolute and accurate quantification of Hepatitis A virus. 2019 International Conference on Biomedical Sciences and Information Systems (ICBSIS 2019). Shijiazhuang, China. February 23th 2019. doi: 10.25236/icbsis.2019.021 [DOI] [Google Scholar]
- Suzuki T, Yoshinaga N, and Tanabe S. . 2011. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 286(36):31263–31271. doi: 10.1074/jbc.M111.238147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó M Á, Varga G Z, Hohmann J, Schelz Z, Szegedi E, Amaral L, and Molnár J. . 2010. Inhibition of quorum-sensing signals by essential oils. Phytother. Res. 24(5):782–786. doi: 10.1002/ptr.3010 [DOI] [PubMed] [Google Scholar]
- Tai A, Sawano T, Yazama F, and Ito H. . 2011. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta 1810:170–177. doi: 10.1016/j.bbagen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Tippayatum P, and Chonhenchob V. . 2007. Antibacterial activities of thymol, eugenol and nisin against some food spoilage bacteria. Agric. Nat. Resour. 41(5):319–323. [Google Scholar]
- Tohno M, Shimosato T, Kitazawa H, Katoh S, Iliev I D, Kimura T, Kawai Y, Watanabe K, Aso H, Yamaguchi T, . et al. 2005. Toll-like receptor 2 is expressed on the intestinal M cells in swine. Biochem. Biophys. Res. Commun. 330:547–554. doi: 10.1016/j.bbrc.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Trevisi P, Corrent E, Mazzoni M, Messori S, Priori D, Gherpelli Y, Simongiovanni A, and Bosi P. . 2015. Effect of added dietary threonine on growth performance, health, immunity and gastrointestinal function of weaning pigs with differing genetic susceptibility to Escherichia coli infection and challenged with E. coli K88ac. J. Anim. Physiol. Anim. Nutr. 99(3):511–520. doi: 10.1111/jpn.12216 [DOI] [PubMed] [Google Scholar]
- Trevisi P, Melchior D, Mazzoni M, Casini L, De Filippi S, Minieri L, Lalatta-Costerbosa G, and Bosi P. . 2009. A tryptophan-enriched diet improves feed intake and growth performance of susceptible weanling pigs orally challenged with Escherichia coli K88. J. Anim. Sci. 87:148–156. doi: 10.2527/jas.2007-0732. [DOI] [PubMed] [Google Scholar]
- Truett G E, Heeger P, Mynatt R L, Truett A A, Walker J A, and Warman M L. . 2000. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29:52, 54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Tugnoli B, Giovagnoni G, Piva A, and Grilli E. . 2020. From acidifiers to intestinal health enhancers: how organic acids can improve growth efficiency of pigs. Animals. 10(1):134–152. doi: 10.3390/ani10010134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya S D, Lee K Y, and Kim I H. . 2014. Protected organic Acid blends as an alternative to antibiotics in finishing pigs. Asian-Australas. J. Anim. Sci. 27:1600–1607. doi: 10.5713/ajas.2014.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya S D, Lee K Y, and Kim I H. . 2016. Effect of protected organic acid blends on growth performance, nutrient digestibility and faecal micro flora in growing pigs. J. Appl. Anim. Res. 44(1):238–242. doi: 10.1080/09712119.2015.1031775 [DOI] [Google Scholar]
- Vidhyalakshmi R, Bhakyaraj R, and Subhasree R. . 2009. Encapsulation “the future of probiotics”—a review. Adv. Biol. Res. 3(3–4):96–103. [Google Scholar]
- Weström B, Sv.endsen J, Ohlsson B, Tagesson C, and Karlsson B. . 1984. Intestinal transmission of macromolecules (BSA and FITC-labelled dextrans) in the neonatal pig. Neonatology. 46(1):20–26. doi: 10.1159/000242028 [DOI] [PubMed] [Google Scholar]
- Wijtten P J, van der Meulen J, and Verstegen M W. . 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- Wu X, and Su D. . 2018. Enterotoxigenic Escherichia coli infection induces tight junction proteins expression in mice. Iran. J. Vet. Res. 19:35–40. [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lahaye L, He Z, Zhang J, Yang C, and Piao X. . 2020. Micro-encapsulated essential oil and organic acid combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88)-positive. Anim. Nutr. doi: 10.1016/j.aninu.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Liu L, Long S, Pan L, and Piao X. . 2018. Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Anim. Feed Sci. Technol. 235:110–119. doi: 10.1016/j.anifeedsci.2017.10.012 [DOI] [Google Scholar]
- Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman S V, and Merlin D. . 2009. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS One 4:e6073. doi: 10.1371/journal.pone.0006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. 2011. Expression of porcine intestinal nutrient transporters along crypt-villus axis and during postnatal development. Guelph, Canada:University of Guleph. [Google Scholar]
- Yang C, Albin D M, Wang Z, Stoll B, Lackeyram D, Swanson K C, Yin Y, Tappenden K A, Mine Y, Yada R Y, . et al. 2011. Apical Na+-D-glucose cotransporter 1 (SGLT1) activity and protein abundance are expressed along the jejunal crypt-villus axis in the neonatal pig. Am. J. Physiol. Gastrointest. Liver Physiol. 300:G60–G70. doi: 10.1152/ajpgi.00208.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Chowdhury M A, Huo Y, and Gong J. . 2015. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens 4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K M, Jiang Z Y, Zheng C T, Wang L, and Yang X F. . 2014. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 92: 1496–1503. doi: 10.2527/jas.2013-6619. [DOI] [PubMed] [Google Scholar]
- Yang C, Kennes Y M, Lepp D, Yin X, Wang Q, Yu H, Yang C, Gong J, and Diarra M S. . 2020. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult. Sci. 99:936–948. doi: 10.1016/j.psj.2019.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yang X, Lackeyram D, Rideout T C, Wang Z, Stoll B, Yin Y, Burrin D G, and Fan M Z. . 2016. Expression of apical Na(+)-L-glutamine co-transport activity, B(0)-system neutral amino acid co-transporter (B(0)AT1) and angiotensin-converting enzyme 2 along the jejunal crypt-villus axis in young pigs fed a liquid formula. Amino Acids 48:1491–1508. doi: 10.1007/s00726-016-2210-7. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang L, Cao G, Feng J, Yue M, Xu Y, Dai B, Han Q, and Guo X. . 2019. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. Anim. Sci. 97:133–143. doi: 10.1093/jas/sky426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap P S X, Yiap B C, Ping H C, and Lim S H E. . 2014. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open. Microbiol. J. 8:6–14. doi: 10.2174/1874285801408010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi G F, Carroll J A, Allee G L, Gaines A M, Kendall D C, Usry J L, Toride Y, and Izuru S. . 2005. Effect of glutamine and spray-dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+-challenged weaned pigs. J. Anim. Sci. 83:634–643. doi: 10.2527/2005.833634x. [DOI] [PubMed] [Google Scholar]
- Zhou F, Ji B, Zhang H, Jiang H, Yang Z, Li J, Li J, Ren Y, and Yan W. . 2007. Synergistic effect of thymol and carvacrol combined with chelators and organic acids against Salmonella Typhimurium. J. Food Prot. 70:1704–1709. doi: 10.4315/0362-028x-70.7.1704. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yin X, Yu H, Zhao L, Sabour P, and Gong J. . 2011. Involvement of quorum sensing and heat-stable enterotoxin a in cell damage caused by a porcine enterotoxigenic Escherichia coli strain. Infect. Immun. 79:1688–1695. doi: 10.1128/IAI.01281-10. [DOI] [PMC free article] [PubMed] [Google Scholar]