Abstract
Background
Intermediate stage hepatocellular carcinomas (HCCs) are treated by inducing ischemic cell death with transarterial embolization (TAE) or transarterial chemoembolization (TACE). A subset of HCCs harbor nuclear factor E2–related factor 2 (NRF2), a major regulator of the oxidative stress response implicated in cell survival after ischemia. NRF2-mutated HCC response to TAE and/or TACE is unknown.
Purpose
To test whether ischemia resistance is present in individuals with NRF2-mutated HCC and if this resistance can be overcome by means of NRF2 inhibition in HCC cell lines.
Materials and Methods
This was a combined retrospective review of an institutional database (from January 2011 to December 2018) and prospective study (from January 2014 to December 2018) of participants with HCC who underwent TAE and a laboratory investigation of HCC cell lines. Imaging follow-up included liver CT or MRI at 1 month after the procedure followed by 3-month interval scans. Tumor radiologic response was assessed on the basis of follow-up imaging. The time to local progression after TAE for individuals with and individuals without NRF2 pathway alterations was estimated by using competing risk analysis (Gray test). The in vitro response to ischemia in four HCC cell lines with and without NRF2 overexpression was evaluated, and the combination of ischemia with NRF2 knockdown by means of short hairpin RNA or an NRF2 inhibitor was tested. Doubling time estimates, dose response curve regression, and comparison analyses were performed.
Results
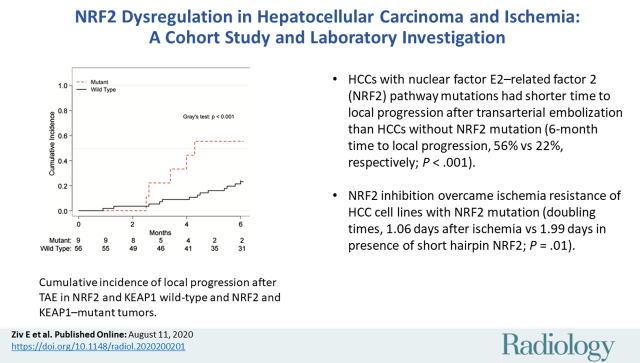
Sixty-five individuals (median age, 69 years [range, 19–84 years]; 53 men) were evaluated. HCCs with NRF2 pathway mutation had a shorter time to local progression after TAE compared to those without mutation (6-month cumulative incidence of local progression, 56% [range, 19%–91%] vs 22% [range, 12%–34%], respectively; P < .001) and confirmed ischemia resistance in NRF2-overexpressing HCC cell lines. However, ischemia and NRF2 knock-down worked synergistically to decrease proliferation of NRF2-overexpressing HCC cell lines. Dose response curves of ML385, an NRF2 inhibitor, showed that ischemia induces addiction to NRF2 in cells with NRF2 alterations.
Conclusion
Hepatocellular carcinoma with nuclear factor E2–related factor 2 (NRF2) alterations showed resistance to ischemia, but ischemia simultaneously induced sensitivity to NRF2 inhibition.
© RSNA, 2020
Online supplemental material is available for this article.
See also the editorial by Weiss and Nezami in this issue.
Summary
Hepatocellular carcinomas with nuclear factor E2–related factor 2 (NRF2) mutation had rapid progression after transarterial embolization and showed resistance to ischemia, but also became more sensitive to NRF2 inhibition in the setting of ischemia.
Key Results
■ Hepatocellular carcinomas (HCCs) with nuclear factor E2–related factor 2 (NRF2) pathway mutations had shorter time to local progression after transarterial embolization than HCCs without NRF2 mutation (6-month cumulative incidence of local progression, 56% vs 22%, respectively; P < .001).
■ NRF2 inhibition overcame ischemia resistance of HCC cell lines with NRF2 mutation (doubling times, 1.06 days after ischemia vs 1.99 days in presence of short hairpin RNA targeting NRF2; P = .01).
■ Ischemia sensitized HCC cell lines to NRF2 inhibition (estimated relative potencies of ML385 under ischemia versus normal conditions were 160 [P < .001] and 175 [P < .001] for NRF2-mutated cell lines).
Introduction
Intermediate stage hepatocellular carcinomas (HCCs) are treated with embolization by means of transarterial chemoembolization (TACE) or transarterial embolization (TAE) (1–3). Overall survival after TACE or TAE is variable, ranging from 11 to 45 months (4,5). Tumor recurrence after TACE and/or TAE is common, with a median time to progression ranging from 3.1 to 13.5 months (6). There are no preprocedure markers that enable the prediction of tumor response to TACE or TAE. It is unknown if there are subsets of patients that may benefit from alternative therapies to TACE or TAE (eg, radioembolization) or from systemic adjuvant to TACE and/or TAE.
Studied mechanisms of resistance to TACE and TAE include neoangiogenesis, epithelial-mesenchymal transition, stem-ness, and autophagy (7,8). These do not take into account the underlying biology of an individual’s tumor. Studies targeting these mechanisms with adjuvants have not shown any clinical benefit (9,10).
HCC is a heterogeneous disease defined by many distinct molecular subclasses (11). The efficacy of TACE and TAE is based on ischemia-induced cell death. Different molecular subclasses of HCC may have different responses to ischemia induced by TACE and TAE. For example, nuclear factor E2–related factor 2 (NRF2) is a known regulator of oxidative stress, and mutations in NRF2 pathway genes are present in about 15% of HCC tumors (12). Mutations in NRF2 or KEAP1 genes disrupt the high affinity binding between their proteins, allowing for stabilization, nuclear accumulation, and overexpression of NRF2. HCC tumors with NRF2 pathway mutations have a worse prognosis (13). NRF2 activation supports cell survival in the setting of hypoxia (14). Recent studies have shown some association between the molecular status of a tumor and tumor response to TACE and/or TAE (15,16). We hypothesized that NRF2-mutant tumors would be more resistant to ischemia. The aim of this study was to evaluate time to local progression after TAE in HCC tumors with and without NRF2 pathway mutations and then to test whether ischemia resistance can be overcome by NRF2 inhibition in HCC cell lines.
Materials and Methods
Study Design and Participant Selection
Biospecimen collection and use in this study was approved by the institutional review board under the informed consent for prospective collection (cohort of patients undergoing biopsy at the time of embolization) and, respectively, under a research authorization of specifically identified banked biospecimens (cohort of patients who had tissue collected and banked before embolization). The topic of this study was not a major focus of the trial (NCT01775072). The retrospective data collection and analysis was approved under another institutional review board–approved protocol (for all patients). Both retrospective and prospective components of the study were compliant with the Health Insurance Portability and Accountability Act. Consecutive participants with available pathologic specimens were accrued from a major cancer center, including a retrospective review (database of individuals with HCC who underwent TAE between January 2011 and December 2018) and prospective accrual (participants with HCC who underwent TAE between January 2014 and December 2018). Individuals were excluded if they had a non-HCC diagnosis, if they underwent a non-TAE local-regional therapy, if they underwent TAE in combination with ablation, if they underwent TAE in combination with systemic treatment, if genetic testing was not performed, or if genetic testing was unsuccessful. Recorded clinical characteristics included age, sex, HCC origin, histopathologic findings, previous treatment, Child-Pugh score, Barcelona Clinic Liver Cancer stage (17), liver tumor burden (number of lesions), α-fetoprotein level, portal vein involvement, Eastern Cooperative Oncology Group score, presence of synchronous tumor and extrahepatic disease, complications, and time between tissue collection and embolization.
Mutation Analysis
Formalin-fixed and paraffin-embedded tissue specimens acquired from either percutaneous liver biopsies or previous liver resections were used. Genetic mutation analysis was performed by using a previously reported assay (18). Average sample coverage was ×709 (range, ×76 to ×1154). All assay versions used included the known NRF2 pathway genes NRF2 and KEAP1.
TAE Procedure
The decision to perform TAE was informed by means of Barcelona Clinic Liver Cancer classification with review in the institutional hepatobiliary tumor board. Embolization was performed with the patient under conscious sedation or general anesthesia. All procedures were performed by fellowship-trained interventional radiologists (E.Z., F.E.B., and H.Y., with 5–10 years of experience; A.M.C., C.S., L.A.B., G.G., and K.T.B., with >20 years of experience). The embolization technique used at our institution has been previously described (19). Briefly, vessels supplying the target tumor are embolized to stasis as selectively as possible with tris-acryl gelatin particles (Embosphere Microspheres; BioSphere Medical, Rockland, Mass), beginning with the smallest size (40–120 μm) and increasing in size up to 300 μm. In cases where an arterial-hepatic venous shunt is identified or in tumors measuring at least 10 cm, larger microspheres (300–500 μm) are used. Technical success was defined as successful catheterization of the desired vessel and administration of embolization into the vessel until stasis was achieved. Stasis was defined as contrast material filling the target vessel and persisting without washout for five cardiac beats. Serious adverse events (grade 3–5) were assessed and recorded according to the Common Terminology Criteria for Adverse Events, version 4.03 (20).
Imaging Response
Imaging follow-up included dedicated multiphase liver CT or liver MRI 1 month after the procedure followed by 3-month interval scans. MRI examinations were performed at either 1.5 T or 3 T (Signa HDxt, Signa EXCITE, or Optima 450w; GE Healthcare, Milwaukee, Wis) by using a torso phased-array coil. The MRI protocol included coronal T2-weighted single-shot fast spin-echo, axial dual-echo in-phase and/or out-of-phase three-dimensional gradient-recalled, axial T2-weighted fat-suppressed, axial diffusion-weighted (b value, 500 sec/mm2), and axial T1-weighted fat-suppressed three-dimensional gradient-recalled sequences for dynamic contrast material–enhanced images. All examinations were performed with gadobutrol (Gadavist; Bayer Healthcare Pharmaceuticals, Wayne, NJ), which was injected intravenously as a bolus of 0.1 mmol/kg at a standard rate of 2 mL/sec with an MRI-compatible power injector. CT examinations were performed with multidetector CT scanners (Lightspeed 16 and VCT, GE Healthcare) by using a standard triphasic or four-phase liver protocol with the following parameters: 2.5-mm slice thickness, 0.8-second rotation time, 120 kV, 220–380 milliamperes, noise index of 14.0, pitch of 0.984–1.375, non-contrast, arterial phase (35-second delay), portal venous phase (80-second delay), and, if performed, an additional delayed phase (3-minute delay). All examinations were performed with 150 mL of iohexol (Omnipaque 300, GE Healthcare), which was injected intravenously with a power injector at 4 mL/sec. Tumor radiologic response was assessed at follow-up imaging according to the modified Response Evaluation Criteria in Solid Tumors (21). Response was assessed independently by two board-certified radiologists (I.N., with 3 years postfellowship experience in abdominal MRI and oncologic imaging, and E.Z., with 5 years postfellowship training in interventional oncology) who were blinded to the mutation status of the cohort. Discordances were resolved by consensus.
Cell Lines and Media
SNU475 (RRID:CVCL_0497) and HepG2 (RRID:CVCL_0027) cell lines were obtained from American Type Culture Collection (Manassus, Va). Huh1 (RRID:CVCL_2956) and Huh7 (RRID:CVCL_0336) cell lines were obtained from Japanese Collection of Research Bioresources Cell Bank (Tokyo, Japan). Huh1 and SNU475 are KEAP1-mutated HCC cell lines, and Huh7 and HepG2 are NRF2 and KEAP1 wild type. All cells were used at less than 10 passages and tested bimonthly for mycoplasma contamination (22).
In normal condition, Huh1 and Huh7 cells were cultured in Dulbecco’s modified Eagle high-glucose medium plus 10% fetal bovine serum (FBS), SNU475 cells were grown in Roswell Park Memorial Institute medium plus 10% FBS, and HepG2 cells were grown in Minimal Essential Medium plus 10% FBS. Cells were incubated in humidified tissue culture incubators (ThermoFisher, Waltham, Mass) at 37°C with 5% CO2 and 21% oxygen. In ischemia condition (0.5% oxygen), Huh1, Huh7, and HepG2 cells were cultured in Dulbecco’s modified Eagle medium with 1% glucose plus 1% FBS; SNU475 cells were cultured in Roswell Park Memorial Institute medium with 1% glucose plus 1% FBS. Cells were incubated in a hypoxia chamber with an O2 controller (COY Laboratory Products, Glass Lake, Mich) at 37°C with 5% CO2 and 0.5% oxygen.
Viruses and Transduction
Doxycycline-inducible NRF2 knockdown cells were generated with short hairpin RNA targeting NRF2 (shNRF2) sequences (TTTCTTAACATCTGGCTTCTTA or TAGATCAGAAACATCAATGGGC), designed using the SplashRNA algorithm (23), and subcloned into the mire_18_LT3GEPIR_Ren713 lentiviral vector (obtained from the Gene Editing and Screening Core Facility at Sloan Kettering Institute, New York, NY). Stably transduced clones were generated by first infecting cells with lentivirus and then selecting a pool of antibiotic-resistant clones by treating cells with puromycin (Huh1: 4 µg/mL; SNU475: 1 µg/mL; Huh7: 2 µg/mL; HepG2: 2 µg/mL) after 3 days of lentivirus infection. For inductions, cells were treated with 1 µg/mL doxycycline for 3 days.
Western Blot Analysis
Protein concentrations were determined by means of BCA Protein Assay (ThermoFisher). Primary antibodies used were NRF2 (#12721; Cell Signaling, Danvers, Mass; 1:1000 ratio), NQO1 (#393700, ThermoFisher; 1:2000 ratio), hypoxia-inducible factor 1-α (#610959; BD Biosciences, Franklin Lakes, NJ; 1:1000 ratio), and actin (#A5060; Sigma, St Louis, Mo; 1:5000 ratio).
Cell Proliferation Assay
Cells were put in either normal or ischemia condition for 24 hours. Then, 104 cells were seeded and counted on the days indicated. Biologic replicates of cell culture samples (n = 6) were used for statistical testing.
Cell Viability Assay
NRF2 inhibitor ML385 (#SML1833, Sigma) was resuspended in dimethyl sulfoxide to a final concentration of 10 mmol/L (24). Drug aliquots were stored at −20°C, and each aliquot was used only once. Cells were treated with indicated doses of ML385 in either complete or ischemia medium. The ischemic cells were put in ischemia condition for 1 day and then moved to a normal culture incubator for an additional 2 days. The normoxia cells were kept in a normal incubator for 3 days. Cell viability was evaluated at day 3 after drug treatment by using the CellTiter-Glo Luminescent assay kit (Promega, Madison, Wis), and an Infinite M1000 Pro plate reader (Tecan, Männedorf, Switzerland) was used to measure the luminescence value. Biologic replicates of cell culture samples (n = 3) were used.
Flow Cytometry
CellRox deep red (#C10422, ThermoFisher) was added to the culture at a final concentration of 5 µmol/L for the last 30 minutes of treatment. Cells were harvested and run on flow cytometry with a propidium iodide co-stain. Biologic replicates of cell culture samples (n = 3) were used.
Real-time Reverse-Transcription Polymerase Chain Reaction Testing
Total RNA was extracted by using the PureLink RNA kit (ThermoFisher). The reverse-transcription polymerase chain reaction assay was performed by using a high-capacity cDNA synthesis kit (Applied Biosystems, Foster City, Calif). Quantitative reverse-transcription polymerase chain reaction analyses were performed by using SYBR Select Master Mix (ThermoFisher). Primers were synthesized by Integrated DNA Technologies, Coralville, Iowa. Primer sequences were as follows: NRF2, 5ʹ-CACATCCAGTCAGAAACCAGTGG-3ʹ and 5ʹ-GGAATGTCTGCGCCAAAAGCTG-3ʹ; NQO1, 5ʹ-CCTGCCATTCTGAAAGGCTGGT-3ʹ and 5ʹ-GTGGTGATGGAAAGCACTGCCT-3ʹ; GCLC, 5ʹ-GGAAGTGGATGTGGACACCAGA-3ʹ and 5ʹ-GCTTGTAGTCAGGATGGTTTGCG-3ʹ; GCLM, 5ʹ-AGATCACCCAGAGCAACGCCAT-3ʹ and 5ʹ-GGCTGTCCATAAACTGGTTCTCC-3ʹ; G6PD, 5ʹ-CTGTTCCGTGAGGACCAGATCT-3ʹ and 5ʹ-TGAAGGTGAGGATAACGCAGGC-3ʹ; PGD, 5ʹ-GTTCCAAGACACCGATGGCAAAC-3ʹ and 5ʹ-CACCGAGCAAAGACAGCTTCTC-3ʹ; ME1, 5ʹ-GGAGTTGCTCTTGGTGTTGTGG-3ʹ and 5ʹ-GGATAAAGCCGACCCTCTTCCA-3ʹ; TXNRD, 5ʹ-GTTACTTGGGCATCCCTGGTGA-3ʹ and 5ʹ-CGCACTCCAAAGCGACATAGGA-3ʹ; KEAP1, 5ʹ-CAACTTCGCTGAGCAGATTGGC-3ʹ and 5ʹ-TGATGAGGGTCACCAGTTGGCA-3ʹ; and RN18S1, 5ʹ-ACCCGTTGAACCCCATTCGTGA-3ʹ and 5ʹ-GCCTCACTAAACCATCCAATCGG-3ʹ. RN18S1 was used for normalization.
Statistical Analysis
Time to local progression with death without local recurrence and nonlocal progression as competing risks was used to obtain cumulative incidence function, and the Gray test was used to compare cumulative incidence function (25). Proportionality assumptions were validated on the basis of the cumulative sum of residuals and separately by checking interactions with functions of time (26). The Fisher exact test and the t test were used to calculate the association between NRF2 pathway mutation and the covariates specified earlier. Housekeeper genes for normalization of Western blot and quantitative reverse-transcription polymerase chain reaction data were checked for differences across groups by using the Kruskal-Wallis test. Data points for proliferation experiments were fit to an exponential growth equation and doubling times derived from the regression. Comparison of doubling times was performed by using the Kruskal-Wallis test across all groups followed by a pairwise Wilcoxon test with Holm correction for multiple comparison. Cellular half maximal inhibitory concentration (IC50) values were determined with nonlinear regression (log-logistic) across a broad concentration range. Two nested dose-response models (with and without grouping by ischemia) were fit to the data (27). Residuals were confirmed to be normally distributed. The F test was used to compare model fits. IC50 estimates and confidence intervals were derived from model fits, and relative potencies were evaluated with the z test (28). Change in percentage viability was compared by using the Wilcoxon test. CellRox data were log-transformed. Statistical analysis was performed by using R software (29).
Results
Participant Characteristics
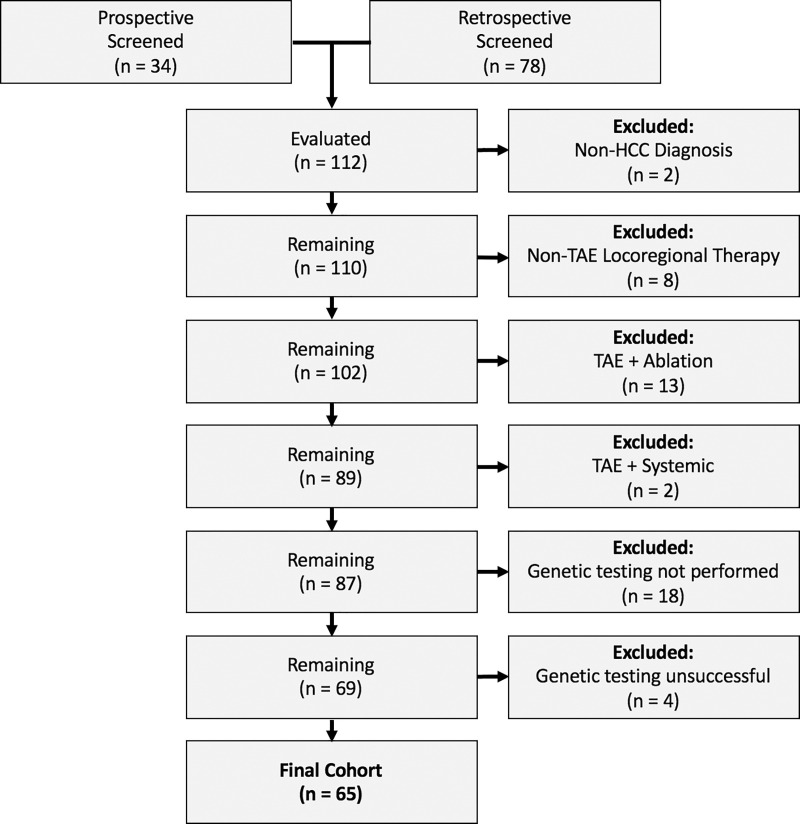
Participants were accrued through both a retrospective review of an institutional database, which identified 79 individuals with HCC who underwent TAE between January 2011 and December 2018, and a prospective study of 34 participants with HCC who underwent TAE between January 2014 and December 2018 (Fig 1). Exclusions included a non-HCC diagnosis (n = 2), a non-TAE local-regional therapy (n = 8), TAE in combination with ablation (n = 13), TAE in combination with systemic treatment (n = 2), genetic testing not performed (n = 18), and genetic testing unsuccessful (n = 4). The final cohort included 65 individuals (53 men and 12 women with a median age of 69 years [interquartile range, 14 years]) with HCC who had undergone TAE and mutation analysis of their tumor. The mean time between tissue acquisition and TAE was 2.5 months. In 37 of the 65 participants (57%), biopsy was performed at the time of embolization.
Figure 1:
Flowchart of study cohort. HCC = hepatocellular carcinoma, TAE = transarterial embolization.
Mutations were identified in NRF2 pathway genes, NRF2 and KEAP1, in nine of the 65 individuals (14%). NRF2 pathway mutations were associated with aggressive features including number of lesions (P = .03) and histopathologic findings (P = .03), but not with underlying age, Eastern Cooperative Oncology Group status, Child-Pugh class, Barcelona Clinic Liver Cancer stage, portal vein involvement, extrahepatic disease, or α-fetoprotein level (Table). A competing risk analysis showed shorter time to local progression after embolization in NRF2 pathway mutant tumors. The 3- and 6-month cumulative incidences of local progression were 22% (95% confidence interval: 0%, 52%) and 56% (95% confidence interval: 19%, 92%) for NRF2 pathway–mutant tumors compared with 7% (95% confidence interval: 0%, 14%) and 23% (95% confidence interval: 12%, 34%) in NRF2 pathway wild-type tumors (Gray test: P < .001) (Fig 2).
Characteristics from Analyzed Cohort of 65 Individuals
Figure 2:
Graph shows cumulative incidence of local progression after transarterial embolization in NRF2 and KEAP1 wild-type and NRF2 and KEAP1–mutant tumors. The 3- and 6-month cumulative incidences of local progression were 7% and 23%, respectively, for wild-type tumors and 22% and 56% for mutant tumors (P < .001).
Degree of NRF2 Activation Varied between Different HCC Cell Lines
Baseline NRF2 expression and activation in four HCC cell lines are summarized in Figure 3. KEAP1-mutant cell lines, Huh1 and SNU475, showed higher NRF2 levels compared with wild-type KEAP1 and NRF2 cell lines, Huh7 and HepG2 (Fig 3, A, B). Similarly, the NRF2 transcriptional target, NQO1, showed higher protein expression levels in Huh1 and SNU475 cells compared with Huh7 and HepG2 cells (Fig 3, A).
Figure 3:
A, Western blot of nuclear factor E2–related factor 2 (NRF2) and downstream target NQO1 in four hepatocellular carcinoma cell lines. B, Bar chart shows relative NRF2 protein levels. C, Western blot of NRF2 and downstream target NQO1 with doxycycline-inducible short hairpin RNA targeting NRF2 (shNRF2) in normoxia and ischemia conditions. Ischemia conditions were verified with hypoxia-inducible factor-1α (HIF1A) Western blot. MUT = mutant, WT = wild type.
NRF2 Knockdown Resulted in Accumulation of Reactive Oxygen Species in HCC Cells
Figure 3, C, shows that the NRF2–short hairpin RNA knocked down NRF2 expression and activation in all four HCC cell lines. Ischemia conditions induced hypoxia-inducible factor 1-α expression (Fig 3, C) with increased expression of hypoxia-inducible factor 1-α as demonstrated in the Western blot. NRF2–short hairpin RNA resulted in increased accumulation of reactive oxygen species in normoxia and after ischemia in Huh1 (mean log fluorescent intensity [MFI] in normoxia [±standard deviation]: 5.00 ± 0.02 without doxycycline vs 5.13 ± 0.02 with doxycycline, P < .001; mean log MFI in ischemia: 5.93 ± 0.04 without doxycycline vs 6.18 ± 0.08 with doxycycline, P = .003), SNU475 (mean log MFI in normoxia: 6.97 ± 0.05 without doxycycline vs 7.43 ± 0.04 with doxycycline, P < .001; mean log MFI in ischemia: 7.98 ± 0.09 without doxycycline vs 8.76 ± 0.02 with doxycycline, P = .003), and HepG2 (mean log MFI in normoxia: 6.32 ± 0.15 without doxycycline vs 6.82 ± 0.05 with doxycycline, P = .02; mean log MFI in ischemia: 6.20 ± 0.23 without doxycycline vs 7.39 ± 0.08 with doxycycline, P = .007) but not in Huh7 (mean log MFI in normoxia: 7.35 ± 0.09 without doxycycline vs 7.38 ± 0.07 with doxycycline, P = .7; mean log MFI in ischemia: 7.84 ± 0.21 without doxycycline vs 7.83 ± 0.17 with doxycycline, P > .99) cells (Fig E1 [online]).
NRF2 Knockdown Inhibits Postischemia Recovery in HCC Cells with NRF2 Overexpression
Mean estimated doubling times for the Huh1 cell line were 1.06 days ± 0.04 for normal conditions, 1.06 days ± 0.04 for postischemia conditions, 1.22 days ± 0.08 in the presence of shNRF2, and 1.99 days ± 0.23 in the presence of shNRF2 and postischemia (P = .9 for normal vs ischemia conditions and P = .01 for normal vs short hairpin NRF2 and ischemia). Growth curves for all four HCC cell lines are summarized in Figure 4.
Figure 4:
Growth curves for, A, Huh1, B, SNU475, C, Huh7, and, D, HepG2 cell lines in normal and postischemia (recovery) conditions with (+dox) and without NRF2 knockdown by means of short hairpin RNA targeting nuclear factor E2–related factor RNA (shNRF2). Ctrl = control.
Ischemia Sensitizes HCC Cells with NRF2 Overexpression to ML385 Treatment
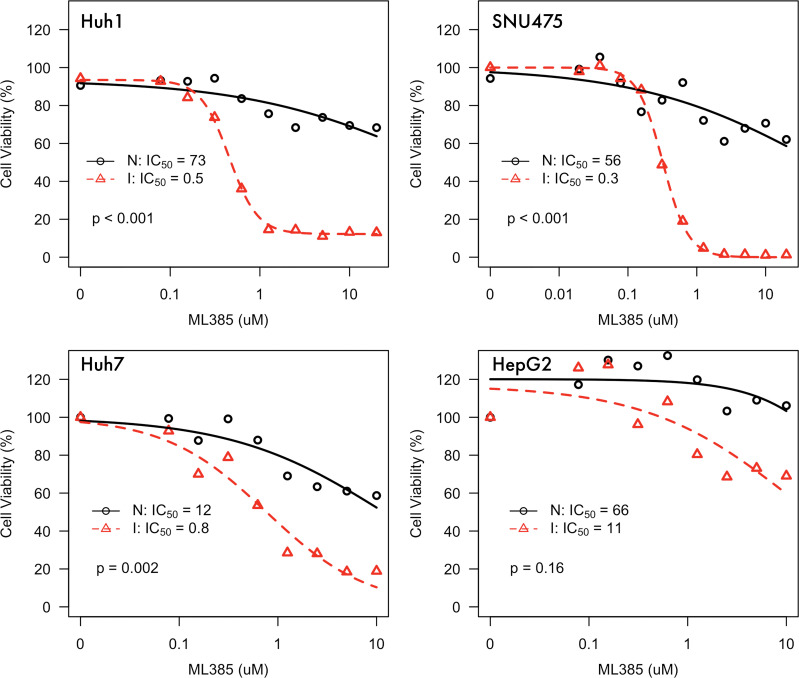
We found that ML385 inhibits NRF2 and its targets, specifically in the setting of ischemia (Fig E2 [online]). Under normal conditions, we estimated IC50 for ML385 as 73 μmol/L (range, 278–189 μmol/L) in Huh1 cells, 56 μmol/L (range, 21–150 μmol/L) in SNU475 cells, 12 μmol/L (range, 7–18 μmol/L) in Huh7 cells, and 67 μmol/L (range, 7–566 μmol/L) in HepG2 cells. Under ischemia conditions, we estimated IC50 for ML385 as 0.45 μmol/L (range, 0.40–0.51 μmol/L) in Huh1 cells, 0.32 μmol/L (range, 0.28–0.36 μmol/L) in SNU475 cells, 0.8 μmol/L (range, 0.64–0.91 μmol/L) in Huh7 cells, and 11 μmol/L (range, 5–21 μmol/L) in HepG2 cells. The estimated relative potencies of ML385 under ischemia versus normal conditions were 160 (range, 62–415; P < .001) for Huh1 cells, 175 (range, 65–471; P < .001) for SNU475 cells, 15 (range, 10–25; P = .002) for Huh7 cells, and 6 (range, 0.6–59; P = .16) for HepG2 cells. Dose response data are summarized in Figure 5. We observed greater increase in cell death after ML385 with the NRF2-mutated and over-expressing Huh1 and SNU475 cells (mean, 53% ± 3) compared with NRF2 wild-type cells Huh7 and HepG2 (mean, 13% ± 3) (P = .002) (Fig 6).
Figure 5:
Dose response curves for nuclear factor E2–related factor 2 inhibitor ML385 in normal (N) and ischemia (I) conditions for Huh1, SNU475, Huh7, and HepG2 cell lines. Huh1 and SNU475 harbor KEAP1 mutations and demonstrate NRF2 overexpression and Huh7 and HepG2 are NRF2 and KEAP1 wild type. IC50 = half-maximal inhibatory concentration.
Figure 6:
Whisker plot of increase in percentage cell death after ischemia with addition of nuclear factor E2–related factor 2 (NRF2) inhibitor ML385 in NRF2-mutated and NRF2 wild-type cells (P = .002).
Discussion
Transarterial chemoembolization and transarterial embolization (TAE) kill tumor cells by inducing ischemia. Clinical responses are variable and difficult to predict, and recurrences are common. Molecular subclasses of hepatocellular carcinoma (HCC), such as nuclear factor E2–related factor 2 (NRF2) pathway–mutated HCC tumors, may be resistant to ischemia and thus determine response to TAE. In this study, we showed that NRF2 pathway–mutated HCC tumors have rapid progression after TAE (6-month cumulative incidence of local progression, 56% vs 23%; P < .001), suggesting that these tumors may be resistant to ischemia. But we also showed that ischemia sensitizes NRF2 over-expressing HCC cells to NRF2 inhibition by blocking proliferation (mean doubling time, 1.06 days ± 0.04 in normal and postischemia and 1.99 days ± 0.23 after short hairpin RNA with ischemia; P = .01) and increasing cell death (relative potency, 160 [range, 62–415] in Huh1 and 175 [range, 65–471] in SNU475 cells after ML385 in ischemia; P < .001). We note that NRF2 inhibition in normal conditions is not lethal to these cells; rather, ischemia induces NRF2 addiction. We observed the potentiation of ischemia with NRF2 inhibition in the KEAP1-mutated Huh1 and SNU475 cell lines but less or not at all in KEAP1 and/or NRF2 wild-type Huh7 and HepG2 cells. This suggests a role for a precision medicine adjuvant for NRF2 inhibition in the setting of NRF2 dysregulated tumors.
NRF2 pathway molecular alterations occur in HCC. We observed that approximately 15% of tumors in our cohort had NRF2 or KEAP1 mutations, similar to previous reports (12). There are likely more mutations and/or epigenetic mechanisms that could give rise to NRF2 overexpression, estimated to be approximately 40% of HCC tumors (30). Recently, Goldstein et al (31) reported a KEAP1 mutation score to categorize cell lines as NRF2 dysregulated or not. Huh1 and SNU475 scored high in the KEAP1 mutation score, consistent with NRF2 dysregulation, and Huh7 and HepG2 scored low. NRF2 pathway mutations are present in a broad range of tumors, including neuroendocrine carcinomas (approximately 32%), head and neck cancers (approximately 30%), lung cancers (approximately 28%), uterine cancers (approximately 21%), esophageal cancers (approximately 25%), and bladder cancers (approximately 15%) (12). The therapeutic strategy of inducing NRF2 addiction with ischemia may be relevant to a broad range of NRF2 over-expressing tumors.
Metabolic reprogramming is a hallmark of cancer (32), and NRF2 appears to play a central role in this process (33). NRF2-mediated metabolic reprogramming leads to malignant transformation (34), increased proliferation (35), and chemotherapy resistance (36) in diverse tumors. Implicated key pathways for this reprogramming in central carbon metabolism include glutamine metabolism, glutathione synthesis, the tricarboxylic acid cycle, and the pentose phosphate pathway (37). NRF2 metabolic reprogramming may also expose tumors to metabolic liabilities (38). NRF2 pathway activation enhanced glutamine dependence in a lung adenocarcinoma model (39). A recent study showed that NRF2 activation depends on deglycation (40). The low glucose in our ischemic conditions and after TAE may mimic deglycation, driving susceptible cells to NRF2 dependency (Fig E3 [online]). It is possible that these novel metabolic modulators (41) in combination with catheter-directed therapy may reduce toxicities and improve outcomes.
The role of NRF2 in postischemia recovery of cells has been well-studied in the context of reperfusion injuries of normal organs (42), although less studied in the context of cancer. Gade et al (8) showed that, after ischemia, HCC cell lines have a full recovery and return to baseline proliferation rates. We observed recovery in all four of our cell lines, although HepG2 did not reach back to baseline. NRF2 inhibition diminished this recovery in the Huh1 and SNU475 cell lines (24).
Our study had several limitations. First, the clinical data were limited because of the small sample size and, in particular, the very small non–wild-type population. Second, the cohort is heterogeneous, including both a retrospective and prospective cohort with variable liver disease, tumor burden, and aggressiveness. For this reason, we wanted to test our observation by modulating NRF2 activity in HCC cell lines. But we recognize that the human HCC cell lines remain a limited model of human HCC tumors. Moreover, in vitro ischemia conditions do not completely recapitulate the complexity of tumors and their microenvironment in the setting of TAE. Still, we observed consistency between the clinical data and the in vitro data. TAE is standard of care at our institution for HCC. Although the mechanism of action of TAE and TACE is thought to be the same (5,8), it is possible that our results do not generalize to TACE. Alternative strategies including transarterial radioembolization and TACE and/or TAE combined with ablation were not explored.
In summary, we reported on a clinical cohort of individuals with hepatocellular carcinoma (HCC) tumors with nuclear factor E2–related factor 2 (NRF2) pathway mutations that showed rapid progression after transarterial embolization; we further showed that survival and proliferation of NRF2 pathway–mutant HCC cell lines are dependent on NRF2 expression after ischemia. Further clinical and laboratory studies are needed to validate our findings and evaluate their generalizability in each of these treatment strategies.
SUPPLEMENTAL FIGURES
Disclosures of Conflicts of Interest: E.Z. disclosed no relevant relationships. Y.Z. disclosed no relevant relationships. L.K. disclosed no relevant relationships. I.N. disclosed no relevant relationships. F.E.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received a grant from Guerbet; received payment for lectures including service on speakers bureaus from Society of Interventional Oncology sponsored by Guerbet; has patents issued; is the cofounder of Claripacs; receives research support from GE, Bayer, and Steba Biotech; is an investor in Labdoor, Qventus, CloudMedx, Notable Labs, and Xgenomes. Other relationships: has patents pending and issued. J.P.E. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is on the board at Astra Zeneca. Other relationships: disclosed no relevant relationships. L.C. disclosed no relevant relationships. E.N.P. disclosed no relevant relationships. L.A.B. disclosed no relevant relationships. A.M.C. disclosed no relevant relationships. G.G. disclosed no relevant relationships. J.J.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Bristol Myers Squibb, Eisai, Exelexis, Eli Lilly, ImVax, and QED; has grants/grants pending from Bristol Myers Squib. Other relationships: disclosed no relevant relationships. C.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Ethicon, BTG, Sirtex, Terumo, and Varian; institution has grants/grants pending from Ethicon, Sirtex, and BTG; receives payment for lectures including service on speakers bureaus from Ethicon; receives payment for development of educational presentations from Terumo and Ethicon; receives travel/accommodations/meeting expenses unrelated to activities listed from Ethicon and Terumo. Other relationships: disclosed no relevant relationships. G.K.A.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Agios, Astra Zeneca, Autem, Bayer, Beigene, Berry Genomics, Bioline, Celgene, CytomX, Debio, Eisai, Eli Lilly, Flatiron, Genentech, Genoscience, Gilead, Incyte, Ipsen, LAM, Loxo, Merck, MINA, QED, Redhill, Roche, Silenseed, Sillajen, Sobi, Targovax, Therabionics, Twoxar, and Yiviva; institution has grants/grants pending from ActaBiologica, Agios, Array, Astra Zeneca, Bayer, Beigene, BMS, Casi, Celgene, Exelixis, Genentech, Halozyme, Incyte, Mabvax, Polaris Puma, QED, and Roche; has a patent issued. Other relationships: disclosed no relevant relationships. S.B.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid board member for Aperture Medical Technology; is a paid consultant for Varian Medical, BTG, Johnson & Johnson, Adgero, XACT Robotics, Endoways, and Innoblative; institution has grants/grants pending from GE Healthcare, AngioDynamics, Elesta, and Johnson & Johnson; has patents (planned pending, or issued) from Aperture Medical; has stock/stock options in Aspire Bariatrics, Aperture Medical, Johnson & Johnson, Immunomedics, MOTUS GI, and Progenics. Other relationships: disclosed no relevant relationships. K.T.B. disclosed no relevant relationships. H.Y. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is paid to be on the advisory boards at BD Medical and Genentech; institution has grants/grants pending from SIR Ernest Ring Academic Development Grant, Guerbet Group, and the Thompson Foundation.
All authors are supported by Cancer Center Support Grant P30 CA008748 from the National Cancer Institute. E.Z. supported in part by a Dr. F. Ernest J. Ring Academic Development Grant.
Abbreviations:
- HCC
- hepatocellular carcinoma
- IC50
- half maximal inhibitory concentration
- NRF2
- nuclear factor E2–related factor 2
- shNRF2
- short hairpin RNA targeting NRF2
- TACE
- transarterial chemoembolization
- TAE
- transarterial embolization
References
- 1.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359(9319):1734–1739. [DOI] [PubMed] [Google Scholar]
- 2.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35(5):1164–1171. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67(1):358–380. [DOI] [PubMed] [Google Scholar]
- 4.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 5.Brown KT, Do RK, Gonen M, et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J Clin Oncol 2016;34(17):2046–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64(1):106–116. [DOI] [PubMed] [Google Scholar]
- 7.Huang GW, Yang LY, Lu WQ. Expression of hypoxia-inducible factor 1alpha and vascular endothelial growth factor in hepatocellular carcinoma: Impact on neovascularization and survival. World J Gastroenterol 2005;11(11):1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gade TPF, Tucker E, Nakazawa MS, et al. Ischemia Induces Quiescence and Autophagy Dependence in Hepatocellular Carcinoma. Radiology 2017;283(3):702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2(8):565–575. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64(5):1090–1098. [DOI] [PubMed] [Google Scholar]
- 11.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015;149(5):1226–1239.e4. [DOI] [PubMed] [Google Scholar]
- 12.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Zhang C, Zhang L, et al. Nrf2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Cancer 2015;15(1):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolamunne RT, Dias IH, Vernallis AB, Grant MM, Griffiths HR. Nrf2 activation supports cell survival during hypoxia and hypoxia/reoxygenation in cardiomyoblasts; the roles of reactive oxygen and nitrogen species. Redox Biol 2013;1(1):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaba RC, Groth JV, Parvinian A, Guzman G, Casadaban LC. Gene expression in hepatocellular carcinoma: pilot study of potential transarterial chemoembolization response biomarkers. J Vasc Interv Radiol 2015;26(5):723–732. [DOI] [PubMed] [Google Scholar]
- 16.Ziv E, Yarmohammadi H, Boas FE, et al. Gene Signature Associated with Upregulation of the Wnt/β-Catenin Signaling Pathway Predicts Tumor Response to Transarterial Embolization. J Vasc Interv Radiol 2017;28(3):349–355.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llovet JM, Fuster J, Bruix J; Barcelona-Clínic Liver Cancer Group . The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl 2004;10(2 Suppl 1):S115–S120. [DOI] [PubMed] [Google Scholar]
- 18.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borgheresi A, Covey A, Yarmohammadi H, et al. Embolization with microspheres alone for hepatocellular carcinoma with portal vein tumor: analysis of outcome and liver function at disease progression. HPB (Oxford) 2020;22(4):588–594. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute . Common Terminology Criteria For Adverse Events (CTCAE). Bethesda, Md: National Cancer Institute, 2010. [Google Scholar]
- 21.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30(1):52–60. [DOI] [PubMed] [Google Scholar]
- 22.Young L, Sung J, Stacey G, Masters JR. Detection of Mycoplasma in cell cultures. Nat Protoc 2010;5(5):929–934. [DOI] [PubMed] [Google Scholar]
- 23.Pelossof R, Fairchild L, Huang CH, et al. Prediction of potent shRNAs with a sequential classification algorithm. Nat Biotechnol 2017;35(4):350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A, Venkannagari S, Oh KH, et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem Biol 2016;11(11):3214–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray R. A class of K-sample tests for comparing the cumulative incidence of competing risk. Ann Stat 1988;16(3):1141–1154. [Google Scholar]
- 26.Li J, Scheike TH, Zhang MJ. Checking Fine and Gray subdistribution hazards model with cumulative sums of residuals. Lifetime Data Anal 2015;21(2):197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motulsky H, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. New York, NY: Oxford University Press, 2004. [Google Scholar]
- 28.Ritz C, Baty F, Streibig JC, Gerhard D. Dose-Response Analysis Using R. PLoS One 2015;10(12):e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- 30.Chen J, Yu Y, Ji T, et al. Clinical implication of Keap1 and phosphorylated Nrf2 expression in hepatocellular carcinoma. Cancer Med 2016;5(10):2678–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein LD, Lee J, Gnad F, et al. Recurrent Loss of NFE2L2 Exon 2 Is a Mechanism for Nrf2 Pathway Activation in Human Cancers. Cell Rep 2016;16(10):2605–2617. [DOI] [PubMed] [Google Scholar]
- 32.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 33.Wang YY, Chen J, Liu XM, Zhao R, Zhe H. Nrf2-Mediated Metabolic Reprogramming in Cancer. Oxid Med Cell Longev 2018;2018:9304091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalik MA, Guzzo G, Morandi A, et al. Metabolic reprogramming identifies the most aggressive lesions at early phases of hepatic carcinogenesis. Oncotarget 2016;7(22):32375–32393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitsuishi Y, Taguchi K, Kawatani Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012;22(1):66–79. [DOI] [PubMed] [Google Scholar]
- 36.Riz I, Hawley TS, Marsal JW, Hawley RG. Noncanonical SQSTM1/p62-Nrf2 pathway activation mediates proteasome inhibitor resistance in multiple myeloma cells via redox, metabolic and translational reprogramming. Oncotarget 2016;7(41):66360–66385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SB, Sellers BN, DeNicola GM. The Regulation of NRF2 by Nutrient-Responsive Signaling and Its Role in Anabolic Cancer Metabolism. Antioxid Redox Signal 2018;29(17):1774–1791. [DOI] [PubMed] [Google Scholar]
- 38.Lien EC, Lyssiotis CA, Juvekar A, et al. Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat Cell Biol 2016;18(5):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galan-Cobo A, Sitthideatphaiboon P, Qu X, et al. LKB1 and KEAP1/NRF2 Pathways Cooperatively Promote Metabolic Reprogramming with Enhanced Glutamine Dependence in KRAS-Mutant Lung Adenocarcinoma. Cancer Res 2019;79(13):3251–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanghvi VR, Leibold J, Mina M, et al. The Oncogenic Action of NRF2 Depends on De-glycation by Fructosamine-3-Kinase. Cell 2019;178(4):807–819.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meric-Bernstam F, Lee RJ, Carthon BC, et al. CB-839, a glutaminase inhibitor, in combination with cabozantinib in patients with clear cell and papillary metastatic renal cell cancer (mRCC): Results of a phase I study. J Clin Oncol 2019;37(7_suppl):549. [Google Scholar]
- 42.Shen Y, Liu X, Shi J, Wu X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol Macromol 2019;125:496–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.