Key to developing a human HSV vaccine is an understanding of the virion glycoproteins involved in entry. HSV employs multiple glycoproteins for attachment, receptor interaction, and membrane fusion. Determining how these proteins function was resolved, in part, by structural biology coupled with immunological and biologic evidence. After binding, virion gD interacts with a receptor to activate the regulator gH/gL complex, triggering gB to drive fusion. Multiple questions remain, one being the physical location of each glycoprotein interaction site. Using protective antibodies with known epitopes, we documented the long-sought interaction between gD and gH/gL, detailing the region on gD important to create the gD-gH/gL triplex. Now, we have identified the corresponding gD contact sites on gH/gL. Concurrently we discovered a novel mechanism whereby gH/gL antibodies stabilize the complex and inhibit fusion progression. Our model for the gD-gH/gL triplex provides a new framework for studying fusion, which identifies targets for vaccine development.
KEYWORDS: antibodies, biosensor, cell-cell fusion, epitope mapping, glycoproteins, herpes simplex virus, protein-protein interactions, surface plasmon resonance
ABSTRACT
A cascade of protein-protein interactions between four herpes simplex virus (HSV) glycoproteins (gD, gH/gL, and gB) drive fusion between the HSV envelope and host membrane, thereby allowing for virus entry and infection. Specifically, binding of gD to one of its receptors induces a conformational change that allows gD to bind to the regulatory complex gH/gL, which then activates the fusogen gB, resulting in membrane fusion. Using surface plasmon resonance and a panel of anti-gD monoclonal antibodies (MAbs) that sterically blocked the interaction, we previously showed that gH/gL binds directly to gD at sites distinct from the gD receptor binding site. Here, using an analogous strategy, we first evaluated the ability of a panel of uncharacterized anti-gH/gL MAbs to block binding to gD and/or inhibit fusion. We found that the epitopes of four gD-gH/gL-blocking MAbs were located within flexible regions of the gH N terminus and the gL C terminus, while the fifth was placed around gL residue 77. Taken together, our data localized the gD binding region on gH/gL to a group of gH and gL residues at the membrane distal region of the heterodimer. Surprisingly, a second set of MAbs did not block gD-gH/gL binding but instead stabilized the complex by altering the kinetic binding. However, despite this prolonged gD-gH/gL interaction, “stabilizing” MAbs also inhibited cell-cell fusion, suggesting a unique mechanism by which the fusion process is halted. Our findings support targeting the gD-gH/gL interaction to prevent fusion in both therapeutic and vaccine strategies against HSV.
IMPORTANCE Key to developing a human HSV vaccine is an understanding of the virion glycoproteins involved in entry. HSV employs multiple glycoproteins for attachment, receptor interaction, and membrane fusion. Determining how these proteins function was resolved, in part, by structural biology coupled with immunological and biologic evidence. After binding, virion gD interacts with a receptor to activate the regulator gH/gL complex, triggering gB to drive fusion. Multiple questions remain, one being the physical location of each glycoprotein interaction site. Using protective antibodies with known epitopes, we documented the long-sought interaction between gD and gH/gL, detailing the region on gD important to create the gD-gH/gL triplex. Now, we have identified the corresponding gD contact sites on gH/gL. Concurrently we discovered a novel mechanism whereby gH/gL antibodies stabilize the complex and inhibit fusion progression. Our model for the gD-gH/gL triplex provides a new framework for studying fusion, which identifies targets for vaccine development.
INTRODUCTION
The entry of herpes simplex virus (HSV) into a mammalian cell requires the coordinated action of four viral glycoproteins (gD, gH/gL, gB). However, unlike many other viruses, herpesviruses encode receptor-binding and membrane fusion functions on separate proteins (gD and gB, respectively). As such, fusion is driven by a cascade of protein-protein interactions that is initiated by the binding of gD to one of its cellular receptors (HVEM or nectin-1) (1–7).
HSV gH is a type I transmembrane protein, whereas HSV gL is not membrane-anchored and associates noncovalently with the gH ectodomain. On mature virions and on the surface of HSV-infected cells, gH and gL are found together in a stable 1:1 heterodimeric complex (8). The crystal structure of a modified form of HSV-2 gH/gL revealed an extensive interaction between the two proteins that explained their interdependence for folding, transport, and function (8–11). gH/gL work together as a unit to regulate the activity of the herpesvirus fusogen, gB (1, 9, 12–14). However, the activity of gH/gL itself is regulated by another viral protein, gD (1, 13, 15).
Crystal structures of gD with and without HVEM or nectin-1 (16–19) reveal that the C terminus of the gD ectodomain normally occludes the receptor binding site and must move away before gD can interact with the receptor. We hypothesize that this conformational change in gD also promotes its physical interaction with gH/gL, thereby resulting in its functional activation and consequent promotion of the cascade of events leading to fusion (1, 12). A form of HSV gH/gL containing only the heterodimer ectodomain can trigger fusion of cells expressing gB, gD, and a gD receptor (albeit with lower efficiency than full-length gH/gL) (1), suggesting that the postulated physical interaction between gD and gH/gL occurs within the gH/gL ectodomain. Furthermore, by generating gH/gL chimeras encoding segments of HSV-1 and saimiriine herpesvirus-1 sequence, a gD interaction site on gH/gL was roughly mapped to the N-terminal half of the gH ectodomain (20).
In our previous study, we used surface plasmon resonance (SPR) to demonstrate binding between soluble forms of HSV-2 gD (gD2) and gH/gL (gH2/gL2) (21). We used a form of gD (gD2[285t]) with the C terminus removed (22), so it would already be “primed” for interaction with gH/gL (23). The soluble gD was captured to a biosensor chip via an anti-gD monoclonal antibody (MAb) that presented the gH/gL binding face of gD, and then soluble gH/gL was added. We found that gH/gL bound directly to gD with relatively fast on- and off-rates. Anti-gD MAbs were identified that blocked the binding of gH/gL and the detailed location of their epitopes outlined a potential gH/gL binding region on gD that spanned a face adjacent to, but distinct from, the nectin-1 and HVEM binding sites.
Having identified the gH/gL binding site on gD, we next set out to determine the physical location of the reciprocal binding site on gH/gL. To accomplish this, we first characterized a panel of anti-gH/gL MAbs and grouped them into antigenic communities sharing similar competitive traits. We then determined their ability to block the interaction of gD-gH/gL and assessed their function in a cell-cell fusion assay. The MAbs separated into three groups: (i) those that had no effect on either gD-gH/gL binding or cell-cell fusion; (ii) those that blocked gD-gH/gL binding and inhibited cell-cell fusion; and (iii) those that, surprisingly, stabilized the gD-gH/gL complex, a subset of which inhibited cell-cell fusion. By comparing the kinetics between gH/gL bound with IgG (dimeric binding) versus antigen-binding fragment (Fab) (monomeric binding), we determined that the stabilization effect was due to the valence of IgG. However, valence had no impact on blocking gH/gL binding to gD, as both IgG and Fabs blocked equally well. Several of the MAbs that blocked the gD-gH/gL interaction localized to regions of gH/gL that were missing in the crystal structure; therefore, to visualize this potential binding region we developed a 3D structural model for the entire gH/gL ectodomain. We concluded that the gD binding region on gH/gL includes the very N terminus of gH, the C terminus of gL, and is centered around gL residue 77.
RESULTS
Previously, we used a large panel of anti-gD MAbs (24) to screen for those that blocked gD-gH/gL binding, enabling us to determine a possible gH/gL binding region on gD (21). Here, we used a similar method to probe the corresponding side of the gD-gH/gL complex. For this, we first characterized a large panel of anti-gH/gL MAbs, and then screened them for their ability to block complex formation with gD. We hypothesized that the epitopes of anti-gH/gL MAbs that block binding to gD would locate a potential gD binding site on gH/gL. Furthermore, we screened these MAbs for inhibition of fusion, theorizing that those that blocked gD-gH/gL binding would also have a functional impact.
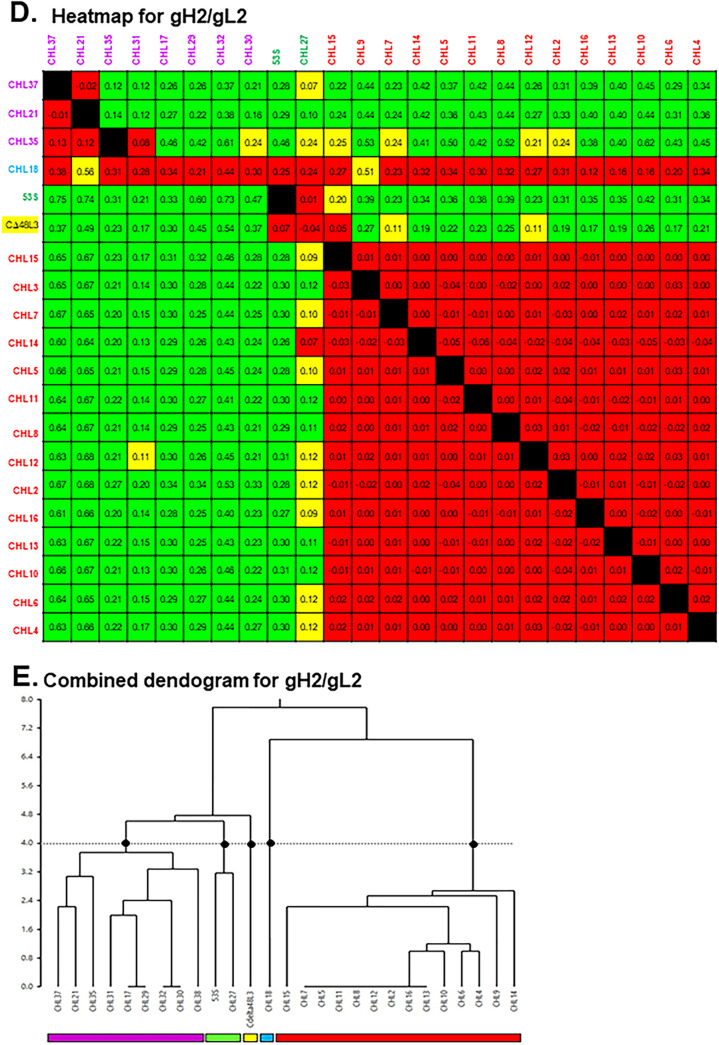
For this study, we first used a high-throughput surface plasmon resonance (SPR) technique (24–26) to determine which of a panel of 36 MAbs compete for binding to gH/gL (Fig. 1A). First, MAbs (IgG) were covalently arrayed on an SPR sensor chip. Second, soluble gH/gL was injected across the chip surface followed by each of the 36 MAbs injected in series (26). Since our panel of MAbs contained both type-specific (those that bind either HSV-1 or HSV-2) and type-common (those that bind both HSV-1 and HSV-2), all MAbs were tested against both gH1/gL1 and gH2/gL2. Sensorgrams of the binding were analyzed to generate a heat map and dendrogram for each gH/gL protein (Fig. 1B to E). MAbs were arranged into bins to reflect competition versus no competition (25–27), and grouped into a network plot, descriptive of which epitopes are engaged. The result is a graphical representation of the heat map (Fig. 1B and D). Finally, the data were depicted as MAb “communities” that reflect their competitive behavior (Fig. 2).
FIG 1.
Binning of anti-gH/gL MAbs. (A) Graphical representation of the Carterra continuous flowmicrospotter (CFM)/SPRi protocol. The individual anti-gH/gL MAbs are printed to distinct spots on the biosensor chip (shown as distinctly colored Ys). The antigen (gH/gL, orange/blue ovals) is then flowed across the chip surface and captured by each MAb. Next, the first anti-gH/gL MAb (analyte) is flowed across the chip surface and its binding capacity measured. Then, the chip surface is regenerated back down to the level of the printed MAbs and the cycle is repeated for each individual MAb to be tested. (B and D) Heat maps. Red boxes denote competition between the ligand MAb and the analyte MAb. Black boxes indicate self-self MAb competition. Green boxes indicate no competition between MAbs and a high level of binding of the analyte MAb onto gH/gL as bound by the ligand MAb. Yellow boxes show a lower level of binding for the analyte, but the MAbs are still scored as not competing. Excluded from analysis: ligand MAbs that bound ≤10 response units of gH/gL (gray boxes); MAbs that blocked all other MAbs. Values in each box designate the difference in the normalized y-scale values of the MAb injections compared to the antigen/buffer injections; the value is taken at the x-scale position where the measurement bar is placed. x axis, ligands; y axis, analytes. MAb names are colored according to community groupings as shown in the combined dendrograms. (C and E) Combined dendrograms. Horizontal lines at the base of the denogram indicate distinct bins of MAbs, i.e., they have identical competition profiles. MAb communities (denoted with colored rectangles) were chosen based on the dendrogram cutoff (dotted horizontal line); cutoff was chosen so as to give five different communities on each type of gH/gL (black dots).
FIG 2.
Antigenic community maps for HSV gH1/gL1 and gH2/gL2. For HSV-1 gH/gL (A) and HSV-2 gH/gL (B), the MAbs were divided into four communities based on dendrogram estimations (Fig. 1C and E) and colored accordingly. Antibody names in a circle indicate that competition was measured as both a ligand and an analyte; antibody names in a square indicate that competition was measured in one direction only, as either a ligand or an analyte. Solid connecting lines specify that competition between the two MAbs was seen as both a ligand and analyte for each, while dashed connecting lines indicate competition in one direction only. The purple community for gH2/gL2 (B) was further divided into magenta, orange, and purple subcommunities according to previous data (28). Asterisks (*) highlight MAbs that are “type-common” and bind to both gH1/gL1 and gH2/gL2.
The MAb community maps for gH1/gL1 and gH2/gL2 are shown in Fig. 2A and B, respectively. We color coded each community: purple, green, orange, blue, and yellow for gH1/gL1 (Fig. 2A) and purple, green, yellow, cyan, and red for gH2/gL2 (Fig. 2B). The purple community of gH2/gL2 was further subdivided into purple, orange, and magenta based on competition and previous mapping data (28). The arrangement of the MAbs within and between communities reflects the extent of competition. For example, the red-community MAbs for gH2/gL2 form a tight, overlapping cluster that have a high degree of competition between all members (Fig. 2B).
Here, we provided the first analysis of cross-competition between MAbs that bind specifically to either gH or gL, as our previous study examined anti-gH and anti-gL MAbs separately (28). Thus, this study allowed us to examine the relationships of MAbs in the gH/gL complex itself. Interestingly, the three MAbs against gL (28–30) were sorted into their own communities (CHL18 to the cyan community of gH2/gL2 and L4 and CΔ48L3 to the yellow communities of gH1/gL1 and gH2/gL2, respectively).
Epitope mapping MAbs that bind gH2/gL2.
Our next objective was to map the epitopes of key members of each community and position them onto the 3D structure of gH2/gL2. To account for residues that were not resolved by crystallography (9), we used a hypothetical model of the complete gH2/gL2 ectodomain (Fig. 3). Previous peptide binding data (28, 31) localizes the epitopes of yellow (CΔ48L3), cyan (CHL18), magenta (CHL17 and CHL32), and orange (CHL29, CHL30, CHL31, and CHL35) MAbs to specific stretches of amino acids (gL 173 to 183, gL 208 to 219, gH 19 to 38, gH 145 to 155 and 676 to 686, correspondingly) (Fig. 4). Likewise, monoclonal antibody resistant (mar) virus data for CHL2 localizes the red community around gH residue 116 (28). However, we had no information regarding the location of the purple (CHL21 and CHL37) and green (CHL27 and 53S) communities. Only 6 of the 36 anti-gH/gL MAbs bind type-common epitopes, i.e., bind both gH1/gL1 and gH2/gL2 (designated by asterisks in Fig. 2A and B). The type-common MAbs, which are found in purple, orange, and green communities, serve as a “bridge” between data generated on either gH1/gL1 or gH2/gL2 through the type-specific MAbs with which they compete.
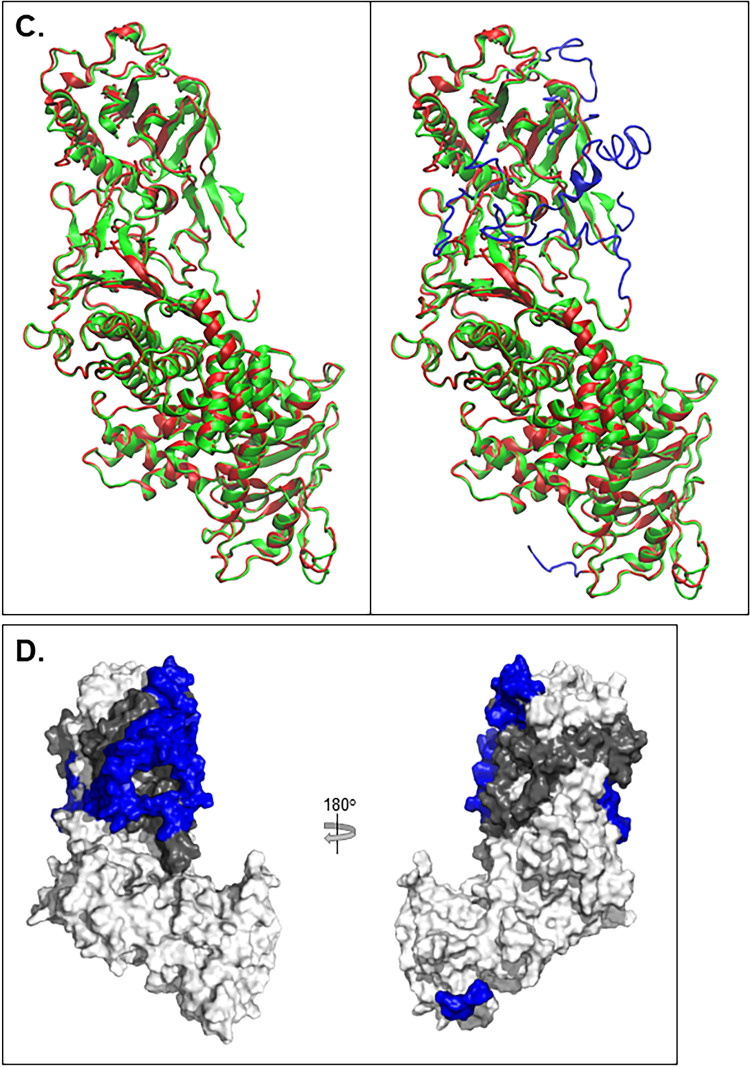
FIG 3.
gH2/gL2 3D model comparison with gH2/gL2 crystal structure. (A) The gH2 ectodomain protein sequence view. Amino acid numbers are displayed along the top. The signal peptide (residues 1 to 18) was removed for model determination. The sequence on top represents the fully constructed gH2 model while the sequence underneath represents the crystal structure with missing residues represented as “∼” (19 to 48, 116 to 136, and 798 to 803). Beta sheets are depicted with a blue arrow, and alpha-helices with a red cylinder. A similar view is shown for gL2 (B), where residues 1 to 16 encompass the signal peptide and the missing crystal structure residues span 17 to 23 and 166 to 224. (C) Superimposition of the gH2/gL2 crystal structure (PDB: 3M1C) (green) and the fully constructed ectodomain model (red). The image on the left shows only the portions of the model that overlap the crystal structure. The image on the right displays the fully constructed model with the missing residues shown in blue. (D) Surface view of the gH2/gL2 complex, with gH shown in white, gL in gray, and missing gH/gL residues shown in blue.
FIG 4.
Positioning of MAb epitope communities onto a 3D structural model of the full-length gH2/gL2 ectodomain. Surface representation of gH2 is shown in white while gL2 is shown in gray. The structure is rotated 180° between (A) and (B). Antibody-resistant mutations for individual MAbs and peptide mapping data (28, 31, 33) were used to position the colored MAb communities (red, magenta, orange, cyan, yellow, and purple) from Fig. 1C onto the structural model. These residues are similarly colored on the surface of the gH2/gL2 structure to convey points of orientation. Due to the large number of MAbs within the red community, only CHL2 is shown. MAbs CHL21 and CHL37, which strongly compete with type 1 MAbs 52S and BBH5, are positioned above the MAb-resistant mutations for 52S (gH 536) (33) and BBH5 (gH 732), shown on the structure in purple. MAb CHL27 was mapped to gH residues 37 to 47 and MAb 53S to gL residue 77, as shown in subsequent figures.
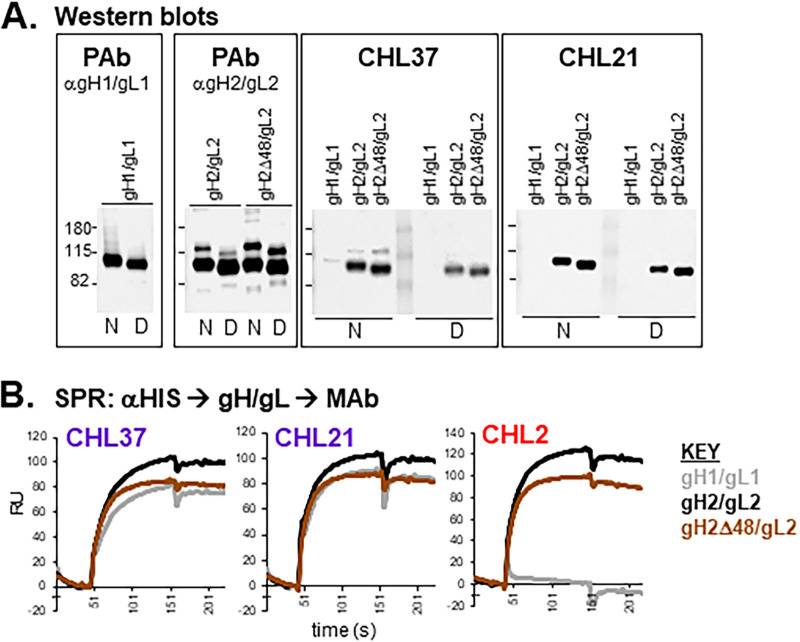
(i) Epitope mapping of CHL21 and CHL37. CHL21 and CHL37 (purple community) recognized type-common epitopes, but also competed with type 1-specific MAbs 46S, 52S, and BBH5 (Fig. 2A). CHL37 was previously characterized via Western blot analysis as a linear, type 2-specific anti-gH MAb (28). Here, we show that newly characterized MAb CHL21 behaves in a similar fashion by Western blotting (Fig. 5A). In contrast to the Western data, both CHL37 and CHL21 bind to gH1/gL1 via SPR (Fig. 5B), suggesting that the epitopes of CHL21 and CHL37 in gH1/gL1 are more sensitive to the detergent used in both denaturing and “native” Western blot analyses (32). We previously used a panel of overlapping peptides to localize CHL37 to the N terminus of gH (28). However, we found that it (and CHL21) can bind to gH2Δ48/gL2 (Fig. 5A and B), which contains an N-terminal gH deletion, suggesting that its epitope lies further downstream. This discrepancy may be due to amino acid similarity between the N terminus of gH and the CHL37 epitope. Based on competition with 52S (33) and BBH5 (see the Materials and Methods) (Fig. 2A), we placed the epitopes of CHL21 and CHL37 within the C-terminal half of gH, where the 52S epitope has been located (Fig. 4).
FIG 5.
Epitope mapping: characterization of MAbs CHL21 and CHL37. (A) Western blot analysis. Soluble gH/gL proteins were run under either native (N) or denaturing (D) conditions. Blots were probed with the indicated antibodies. R137 was used as the PAb against gH1/gL1, and R176 as the PAb against gH2/gL2 and gH2Δ48/gL2. Molecular weight markers are designated with lines and shown to the left in kDa. (B) SPR analysis of gH/gL binding. Anti-HIS IgG was coupled to a biosensor chip. Next, soluble, purified gH1/gL1 (gray), gH2/gL2 (black), or gH2Δ48/gL2 (brown) was captured via C-terminal 6-HIS tags. Curves show binding of the indicated MAb to the immobilized proteins. Each experiment was done at least twice and a representative experiment shown.
(ii) Epitope mapping of CHL27. To determine the location of the CHL27 epitope, we used SPR to screen for binding to several forms of gH/gL (Fig. 6A). CHL27 bound to both gH1/gL1 and gH2/gL2 but was unable to bind to gH2Δ48/gL2 (Fig. 6A), indicating that its epitope lies within the gH2 N terminus. Interestingly, MAb 53S, which competes with CHL27 for binding to gH/gL (Fig. 2A and B), bound to gH2Δ48/gL2 (Fig. 6A), effectively separating the location of these two MAb epitopes.
FIG 6.
Epitope mapping: characterization of MAb CHL27. (A) SPR analysis of MAb binding to gH/gL. Procotol is as outlined in Fig. 5B. (B) Peptide ELISA using MAb CHL27. gH2 peptides were biotinylated at the N terminus, which facilitated binding to a 96-well streptavidin-coated plate. Each well was probed with CHL27 and visualized with goat anti-mouse IgG-horseradish peroxidase. (C) N-terminal amino acid sequence of gH2, with amino acid numbers shown below. The signal sequence (residues 1 to 18) and the CHL27 binding region (residues 37 to 47) are indicated with underlines. The sequence deleted in the gH2Δ48 construct is indicated with a bracket above the sequence. gH residues highlighted in green are important for CHL27 binding, as shown in (D). (D) Western blotting. Cells were transfected with the desired plasmids and cell lysates were separated by electrophoresis via SDS-PAGE. Lysates from cells transfected with empty plasmid (vector) and gL only served as negative controls. Antibodies used during Western blotting are shown to the right of each blot (anti-gH/gL PAb R176 and MAb CHL27).
We next screened CHL27 for binding to gH peptides, particularly those within the N-terminal region. CHL27 bound to two gH2 peptides, one comprising residues 28 to 47 and the other comprising residues 37 to 56 (Fig. 6B), thus defining its epitope to within amino acids 37 to 47 (Fig. 4; Fig. 6C). We used Western blot analysis of five full-length gH point mutants (previously characterized in Cairns et al. [34]) to confirm the peptide data. Four mutations (R39A, Y41A, R43A, and D44A) abrogated CHL27 binding (Fig. 6D). Therefore, we conclude that the epitope for CHL27 encompasses at least these four residues within the gH N terminus (Fig. 6C, green).
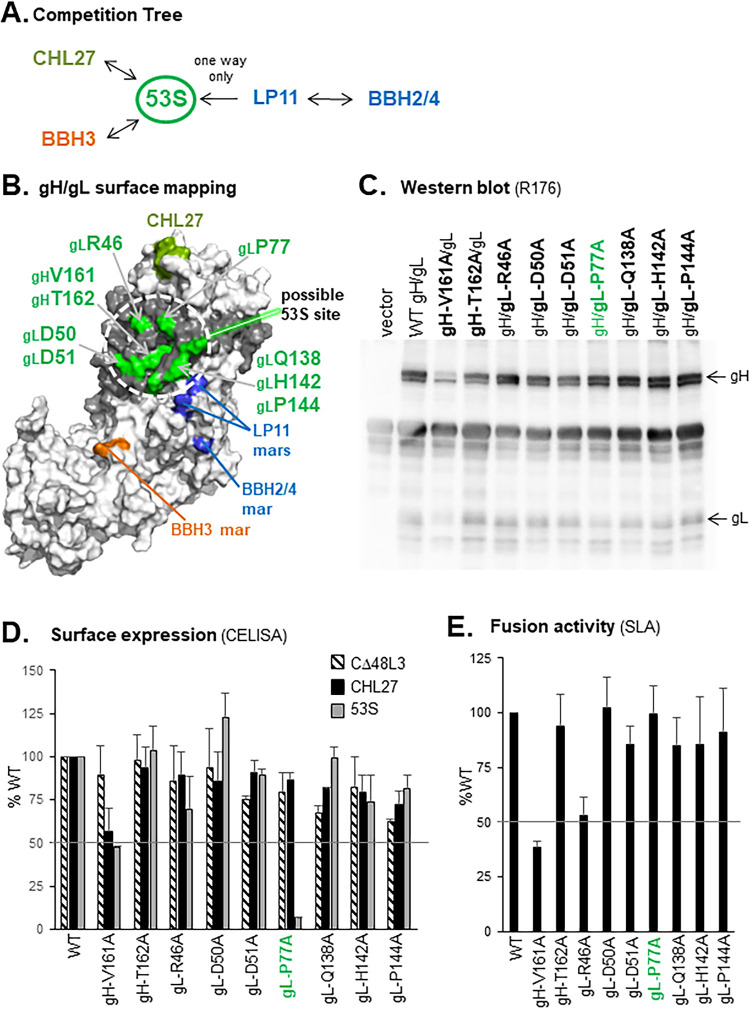
(iii) Epitope mapping of 53S. Prior studies suggested that the conformation-dependent epitope of 53S is located in the N-terminal half of gH/gL and may contain gL residues (8, 35–39). MAb 53S competes with CHL27 for gH/gL binding (Fig. 2A and B), yet these two MAbs bind to distinct regions of gH/gL, as removal of the gH N terminus abrogates CHL27 binding but not 53S binding (Fig. 6A). To localize the 53S epitope, we combined our Carterra (Fig. 1) and BIACORE (data not shown) SPR competition data to develop a 53S “competition tree” showing its relationships with other MAbs (Fig. 7A). MAb 53S competes for binding with CHL27, BBH3, and LP11, but not with BBH2 or BBH4. Interestingly, 53S and LP11 exhibited unidirectional competition, as inhibition was dependent on which MAb bound to gH/gL first; if LP11 bound first, binding of 53S was blocked, but not vice versa. This result suggests that either a conformational change occurs upon binding of LP11 that prevents 53S from binding, or that binding of LP11 prevents a conformational change required for 53S to bind. BBH2 and BBH4, which compete with LP11, do not compete with 53S or CHL27 (Fig. 2A). Using point mutations in gH1 known to abrogate the binding of these five MAbs (LP11, CHL27, BBH3, and BBH2/BBH4) as a guide, we localized a potential region for the 53S epitope on gH2 as follows: “north” of LP11, “east” of BBH3, and “west” of CHL27 in the 3D model (Fig. 7B, dotted circle). Two previously characterized gL insertion mutants which show a decreased reactivity to 53S (P48 and R55) also lie in this region (39).
FIG 7.
Characterization of gH and gL mutants used to identify the 53S epitope. (A) Competition tree. Arrows indicate competition for binding to gH/gL, while no arrows between MAbs signify no competition. Data from SPR analysis (Carterra, Fig. 1, and BIACORE 3000, data not shown) were used to make the tree. MAbs are colored according to their community groups in Fig. 2. (B) Selection of gH2 and gL2 point mutations to test for 53S reactivity. Residues are mapped onto our 3D model of the gH2/gL2 ectodomain (Fig. 3). Amino acids indicated in green were mutated to alanine. The locations of monoclonal antibody-resistant mutations for MAbs CHL27 (gH residues 39, 41, 43, and 44; olive), LP11 (gH1 residues 168 and 329; blue), BBH2/4 (gH1 residue 175; blue), and BBH3 (gH1 residues 370 and 371; orange) are also shown. gH2 is shown in white and gL2 in gray. (C) Cells transfected with WT or mutant gH2 or gL2 constructs were analyzed by Western blotting under denaturing conditions of SDS-PAGE, using the anti-gHgL PAb R176. Total extracts from cells transfected with empty plasmid (vector) served as a negative control. Position of gH and gL on the blot is indicated on the right. (D) Cell-surface ELISA (CELISA). C10 cells were transfected with WT or mutant constructs and tested for their reactivity to MAb 53S. Total levels of expression were determined with MAbs CΔ48L3 (anti-gL) and CHL27 (anti-gH). (E) Cell-cell fusion activity as measured by split luciferase assay (SLA). B78 effector cells were transfected with gB2, gH2, gL2, and Rluc8(1–7) plasmids, while C10 target cells were transfected with Rluc8(8–11) plasmid. At 24 h posttransfection, effector cells were incubated with substrate for 1 h. Target cells were then transferred to effector cells, with soluble gD2(306t) added at the same time to trigger fusion. Luciferase production was measured for 2 h. For CELISA and SLA, all mutants were tested in duplicate. Error bars represent standard error. Mutant gL-P77A, a potential 53S mar, is highlighted in (C) to (E) in green.
To further define the 53S epitope, we chose nine surface-accessible amino acids (two gH residues [V161 and T162] and seven gL residues [R46, D50, D51, P77, Q138, H142, and P144]) to mutate to alanine and test for 53S binding (Fig. 7B, green). Each mutation was placed into a mammalian expression plasmid containing full-length gH2. The two mutant forms of gH2 and seven of gL2 were cotransfected into C10 cells with WT-gL2 or gH2, respectively. Total cell lysates were tested for protein expression by reactivity to anti-gH2/gL2 polyclonal antibody (PAb) R176 using Western blotting (Fig. 7C). Although all proteins were expressed, mutant gH-V161A was present at reduced levels and contained a lower level of glycosylated gH (the top band of the gH doublet, Fig. 7C), indicative of altered protein processing and insufficient trafficking to the cell surface (10, 11, 34). However, with the exception of gH-V161A, all mutants had near-WT levels of cell-surface expression as detected by MAbs CHL27 (anti-gH) and CΔ48L3 (anti-gL) in a cell-surface enzyme-linked immunosorbent assay (CELISA) (Fig. 7D).
Due to 53S binding a conformational epitope (and being unreactive in Western blotting due to the presence of detergent), 53S reactivity was examined by CELISA. Only mutant gL-P77A was severely deficient in 53S binding (Fig. 7C). This reduction in 53S binding was not indicative of poor processing or lack of cell-surface protein expression (Fig. 7C and D).
We next used the split luciferase (cell-cell) fusion assay (SLA) (29, 40) to test each mutant for function. In the SLA, reconstitution of functional luciferase from its two “split” domains, Rluc8(1–7) and Rluc8(8-11), individually expressed in effector and target cells, reflects the level of fusion. Effector B78H1 cells were transfected with gB2, gH2, gL2 and Rluc8(1–7) plasmids, while target C10 cells expressing the gD receptor nectin-1 were transfected with Rluc8(8–11) plasmid (41). Effector cells were incubated with the SLA substrate for 1 h before target cells were added. Soluble gD2(306t) was added when effector and target cells were mixed to trigger fusion and activity was measured for 2 h. As expected due to its reduced expression, mutant gH-V161A exhibited a 60% decrease in fusion activity compared to WT gH (Fig. 7E). However, mutant gL-P77A was fully functional in cell-cell fusion (Fig. 7E). Thus, with its loss of 53S binding but retention of fusion function, mutant gL-P77A appeared to resemble a 53S MAb-resistant (mar) mutant protein (42).
Anti-gH/gL MAbs block or stabilize gD-gH/gL binding.
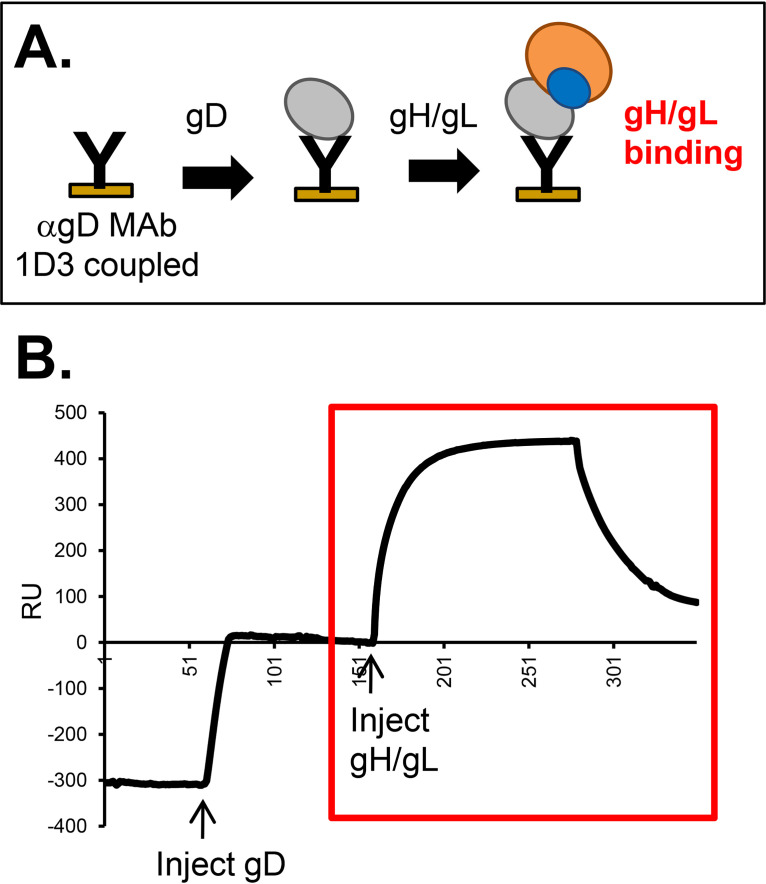
In our previously described SPR assay (Fig. 8A), we detected an interaction between gD2 and gH2/gL2 when gD was captured by certain anti-gD MAbs and presented on a biosensor chip and gH/gL was flowed (i.e., gD is the ligand and gH/gL is the analyte) (Fig. 8B), but not in the reverse orientation (21). This interaction was prevented by the binding of a subset of anti-gD MAbs bound to gD prior to addition of gH/gL, enabling us to identify the gH/gL binding “face” on gD (21). Having defined the epitopes of our anti-gH/gL MAb panel, our next goal was to exploit this technique to define the site on gH/gL that interacts with gD.
FIG 8.
Demonstration of gH2/gL2 binding to gD2, as detected by SPR. (A) Schematic diagram of the SPR protocol. Anti-gD MAb 1D3, which is permissive for gH/gL binding (21), was coupled to a CM5 biosensor chip. Sequential injections of soluble gD2(285t) and soluble gH2/gL2 were performed. (B) An increase in resonance units (RU) after the gH/gL injection indicated gH/gL binding (red box). The RU from a control flow cell where only gH/gL was injected (no gD) was subtracted.
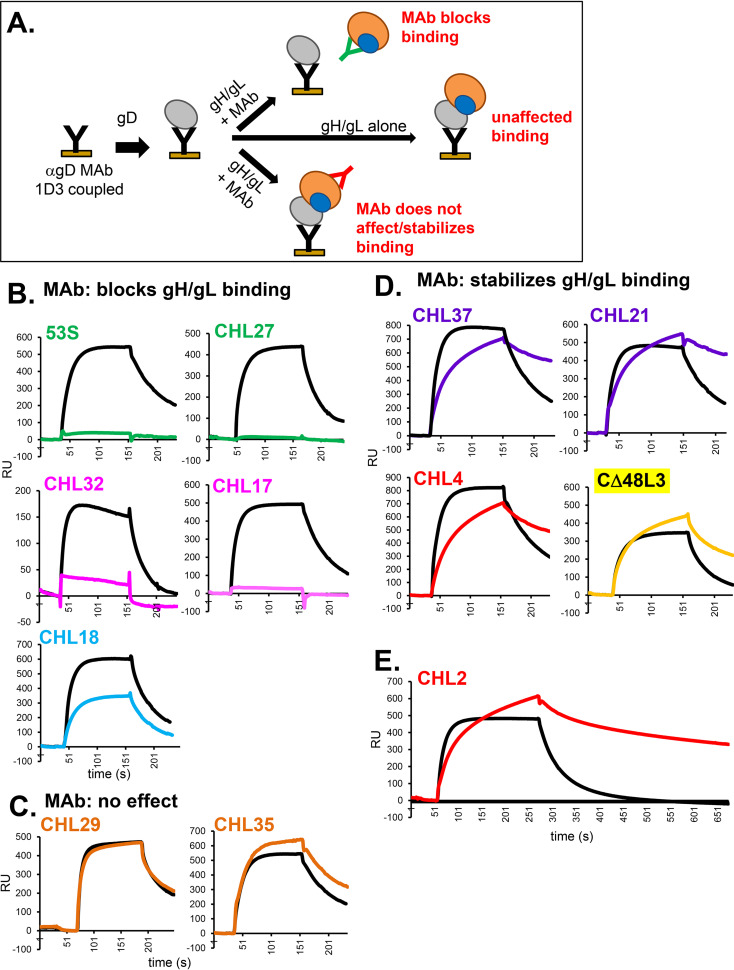
We adapted this experimental design to map the gD binding site on gH/gL (Fig. 9A). In this approach, we first incubated gH/gL with anti-gH/gL MAbs for 10 min prior to injection across a chip containing gD captured by 1D3. The control was to flow gH/gL alone (Fig. 9, black curves). The colored curves show gH2/gL2 binding when preincubated with the indicated MAb, with each color signifying its community membership (Fig. 2B). Importantly, we found that a subset of anti-gH/gL MAbs blocked gD-gH/gL binding, while others did not (Fig. 9B to E). MAbs in the green (53S and CHL27) and magenta (CHL17 and CHL32) communities blocked the interaction with gD by 85 to 100%, and MAb CHL18 decreased gH/gL binding to gD by 50% (Fig. 9B). In contrast, MAbs in the orange community had no effect on gD-gH/gL binding (Fig. 9C).
FIG 9.
Effect of anti-gH/gL MAbs on gD-gH/gL binding. (A) Schematic diagram of the SPR protocol. Anti-gD MAb 1D3 was coupled to a CM5 biosensor chip and then soluble gD2(285t) was injected across the flow cell. Next, either gH2/gL2 alone or gH2/gL2 that was preincubated with 0.4 mg/ml of the indicated MAb (IgG) was injected across the flow cell. The RU from a control flow cell where only gH/gL or gH/gL + IgG was injected (no gD) was subtracted from each. (B) MAbs that block gH/gL binding to gD. (C) MAbs that do not affect gD-gH/gL binding. (D) MAbs that stabilize the gD-gH/gL binding. Only the gH/gL binding portion of the curves (Fig. 8B, red box) are shown in parts (B) to (E). Black curves show gH/gL binding to gD when no MAb is added (positive control). Curves where gH/gL was preincubated with MAbs are colored according to their MAb community in Fig. 2B. Each experiment was done at least twice and a representative experiment is shown.
We also found a third, unexpected, outcome. When MAbs from the purple (CHL21 and CHL37), red (CHL2 and CHL4) or yellow (CΔ48L3) communities were preincubated with gH/gL, binding to gD was not blocked. Yet, the gH/gL binding kinetics were markedly different from that observed under preincubation with either orange-community MAbs or no MAb (compare Fig. 9D to C). Whereas gH/gL binding normally exhibits a fast on- and off-rate (black curves) (21), its off-rate slowed considerably when it was premixed with purple, red, or yellow MAbs (Fig. 9D and E). These results suggest that the red, purple, and yellow MAbs stabilize the gD-gH/gL binding complex.
Effects of anti-gH/gL MAbs on gD-gH/gL binding are dose-dependent.
Next, we tested if the effects of the anti-gH/gL MAbs on gD-gH/gL binding were dose-dependent. As in the previous figure, the level of gH/gL binding to gD in the absence of antibody is shown in each graph as a black curve. We used anti-gB MAb A22 (43), which does not bind to gD or gH/gL, as a control; as expected, it did not inhibit the binding of gH/gL to gD at any concentration tested (Fig. 10A).
FIG 10.
Blocking of gH2/gL2 binding by anti-gH/gL MAbs is dose-responsive. The SPR protocol was as outlined in Fig. 9A. Soluble gH2/gL2 was preincubated with a range of IgG concentrations as listed to the right of each set of curves: control IgG (anti-gB MAb A22) (A); 53S (B); CHL17 (C); CHL18 (D); CHL2 (E); and CD48L3 (F). The RU from a control flow cell where only gH/gL or gH/gL + IgG was injected (no gD) was subtracted from each. Only the gH/gL binding portions of the curves are shown. Black curves show gH/gL binding to gD when no MAb is added (positive control). Colored curves represent the same IgG concentration as shown in Fig. 9 (0.4 mg/ml).
In these studies, we found that MAb 53S inhibited gH/gL binding to gD in a dose-dependent manner, with binding partially reduced at the lowest concentration of IgG tested and binding was completely blocked by 0.2 mg/ml (Fig. 10B). MAb CHL17 blocked gD-gH/gL binding in a similar dose-dependent manner, with complete blocking by 0.4 mg/ml IgG (Fig. 10C). In contrast, blocking by MAb CHL18 was dose-dependent but not complete, even at the highest concentration tested (1 mg/ml IgG) (Fig. 10D). One interpretation of these data is that the epitope of CHL18, which is at the C terminus of gL (28), is only partially exposed (31) and therefore the MAb cannot fully access the epitope to prevent binding to gD.
MAb CHL2, which appeared to stabilize the gD-gH/gL interaction (Fig. 9E), did not block the interaction at any concentration tested (Fig. 10E). However, effects on the kinetics of gH/gL binding to gD were dose-dependent, with the off-rate appearing to be slower at higher concentrations of IgG (Fig. 10E). This effect was also observed with MAb CΔ48L3 (Fig. 10F).
Stabilization of the gD-gH/gL complex requires bivalent IgG.
The subset of anti-gH/gL MAbs that stabilized the gD-gH/gL complex were found in three communities (purple, red, and yellow) whose epitopes are spread across the gH/gL molecule (Fig. 4), therefore this “function” cannot be localized to a single domain on the heterodimer. As each individual MAb (IgG) can bind to two antigens simultaneously, we asked whether the gD-gH/gL complex was stabilized through cross-linking of two gH/gL molecules. To address this question, we generated antigen-binding fragments (Fabs) that are capable of binding only one antigenic molecule. Hence, if cross-linking was a factor, preincubation of gH/gL with Fab would not stabilize gD-gH/gL complexes.
We generated Fabs for six of the anti-gH/gL MAbs tested in Fig. 9, representing five of the six MAb communities (CHL32, magenta; CHL27 and 53S, green; CΔ48L3, yellow; CHL2, red; CHL37, purple) (Fig. 2B). All Fabs were tested using SPR for gH/gL binding; unfortunately, the Fab generated from CHL18 (cyan community) did not bind to gH/gL and was omitted from this study (data not shown). We found that MAbs CHL2, CHL37, and CΔ48L3, which stabilized the complex as IgG, failed to do so as Fabs, there was no change in the off-rates of gH/gL/Fab on gD, and the binding curves overlapped that of gH/gL alone on gD (Fig. 11). For comparison, MAbs that blocked the gD-gH/gL interaction (CHL32, CHL27, and 53S) did so as both Fabs (Fig. 11) and IgG (Fig. 9). We can draw two conclusions from these data. First, the ability of MAbs to stabilize gH/gL binding to gD is due to the cross-linking of two separate gH molecules. Second, the ability of certain MAbs to block the gD-gH/gL interaction is not due to cross-linking, but more likely is due to interfering with the interaction itself. As such, we suggest that the epitopes of these MAbs overlap the gD binding site on gH/gL.
FIG 11.
Effect of anti-gH/gL Fabs on gH2/gL2 binding to gD2. Experiments were set up as outlined in Fig. 9A. Anti-gD MAb 1D3 was coupled to a CM5 biosensor chip and then soluble gD2(285t) was injected across the flow cell. Next, either gH2/gL2 alone or gH2/gL2 that was preincubated with 0.6 mg/ml of the indicated Fab was injected across the flow cell. Fab concentration was chosen so it was in excess. The RU from a control flow cell where only gH/gL or gH/gL + Fab was injected (no gD) was subtracted from each. Only gH/gL binding curves are shown. Black curves show gH/gL binding to gD when no Fab is added (positive control). Curves where gH/gL was preincubated with Fabs are colored according to their MAb community in Fig. 2B.
Effect of anti-gH/gL MAbs on cell-cell fusion.
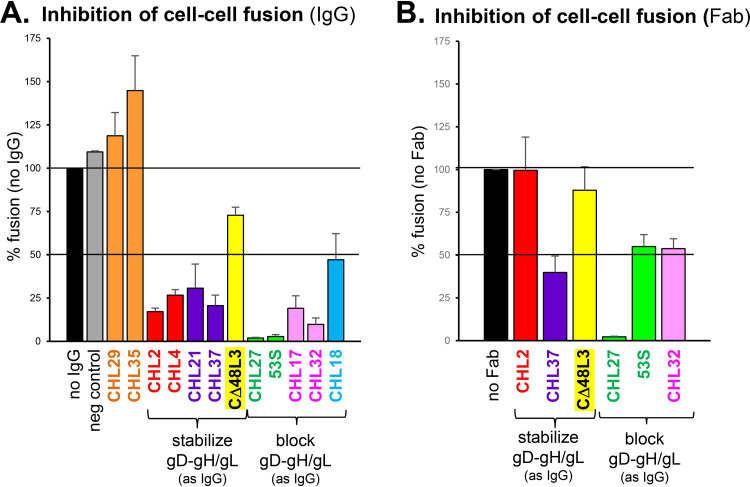
Having characterized our panel of anti-gH2/gL2 MAbs, our goal was to use this information to determine the functional site on gH/gL that interacts with gD, thereby enabling gH/gL to be triggered to activate the fusion protein gB to drive fusion. To monitor fusion, we again used the SLA (Fig. 7E) (29, 40). Specifically, effector cells were transfected with gB2, gH2, gL2, and Rluc8(1–7) plasmids, while target cells expressing the gD receptor nectin-1 were transfected with Rluc8(8–11) plasmid. Effector cells were incubated with the luciferase substrate, incubated with the indicated IgG for 1 h before target cells were added, and fusion was triggered by the addition of soluble gD2(306t) and target cells. Luciferase activity was measured for 2 h and data were normalized to the 2-h reading of the no antibody sample (Fig. 12A, black bar).
FIG 12.
Effect of anti-gH/gL MAbs on cell-cell fusion as measured by the split luciferase assay (SLA). B78 effector cells were transfected with gB2, gH2, gL2, and Rluc8(1–7) plasmids, while C10 target cells were transfected with Rluc8(8–11) plasmid. At 24 h posttransfection, effector cells were incubated with substrate and either IgG (A) or Fab (B) for 1 h. Target cells were then transferred to effector cells, with soluble gD2(306t) added to trigger fusion. Luciferase production was measured for 2 h. All antibodies were tested a minimum of two times. Error bars represent standard error. Anti-myc MAb was used as a negative control (gray bar). MAbs are colored according to their community as shown in Fig. 2B.
We found that orange community MAbs, which did not block gH/gL binding to gD, also did not block fusion (Fig. 12A), as was reported previously (28). With one exception (CΔ48L3), all MAbs that stabilized the gD-gH/gL interaction also inhibited fusion. To test whether the fusion inhibition was due to stabilization of gD-gH/gL complex, we also tested Fabs of these MAbs. The CHL2 Fab could neither stabilize the complex (Fig. 11) nor inhibit fusion (Fig. 12B). Surprisingly, although the CHL37 Fab did not stabilize the complex (Fig. 11), it still inhibited fusion (Fig. 12B), suggesting the existence of a third, as yet undetermined, mechanism of inhibiting gH/gL activity. All MAbs that blocked the interaction of gH/gL with gD inhibited cell-cell fusion as both IgG and Fabs (Fig. 12). Therefore, the blocking of gD-gH/gL complex formation has a functional consequence (the inhibition of fusion). Interestingly, Fabs 53S and CHL32 did not inhibit fusion as well as their IgG counterparts (Fig. 12), perhaps because the smaller Fabs were less able to sterically interfere with binding to gD.
Epitopes of anti-gH/gL MAbs that block gD binding identify a potential binding site for gD on gH/gL.
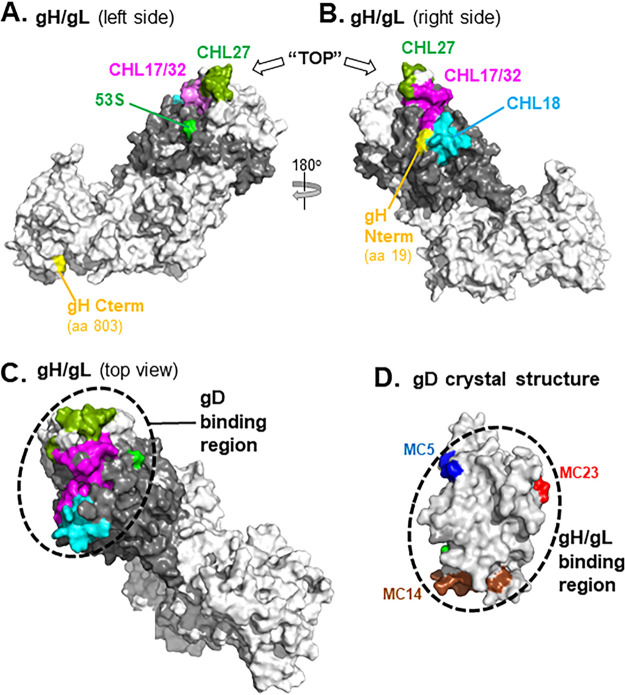
Having mapped the location of the epitopes of the anti-gH/gL MAbs that block the gD-gH/gL interaction, we used this information to identify the gD binding site on gH/gL (Fig. 13). Because the gH/gL crystal structure lacks the gH N terminus and gL C terminus, we used our modeled gH/gL structure that includes these regions (Fig. 2). When we examine the left side of gH/gL, the epitope MAb 53S (centered around gL residue 77) is in close proximity to those of MAbs CHL17/32 and CHL27 (Fig. 13A), all of which block the gD-gH/gL interaction. Turning the gH/gL molecule 180 degrees to the right side (Fig. 13B), the CHL18 epitope is now visible, along with more of the CHL17/32 and CHL27 epitopes. By looking at the gH/gL molecule from a “top-down” viewpoint, the epitopes of all five gD-gH/gL blocking MAbs are seen clustered together (Fig. 13C). We propose that this region, which contains portions of the gH N terminus and much of gL, comprises the gD binding site on gH/gL.
FIG 13.
Localization of the gD binding site on a gH/gL 3D modeled structure. The gH/gL model is rotated 180° between (A) and (B). gH is shown in white and gL in gray. Residues within each of the following MAb epitopes are highlighted by color: CHL17/32 (gH residues 19 to 38; magenta); CHL27 (gH residues 39, 41, 43, and 44; olive); 53S (gL residue 77; green); and CHL18 (gL residues 209 to 219; cyan). To orient the molecule, the gH N and C termini are highlighted in yellow. In (C), gH/gL is uniformly colored white and positioned from the “top” so the epitopes that define the gD binding region (black dotted circle) can be viewed together. (D) Surface representation of the gD crystal structure (PDB: 2C36). Antibody-resistant mutations that were used to position the epitopes of anti-gD MAbs that block gH/gL binding are colored on the structure (MC5, blue; MC23, red; MC14, brown) (21). The gH/gL binding region is marked with a dashed circle.
DISCUSSION
HSV entry and cell-cell fusion occur in a cascade of events involving the four essential glycoproteins: gD (the receptor binding protein), gH/gL (the key regulator of fusion), and gB (the actual fusogen). Although it was theorized that gD transmitted the fusion-triggering signal through direct interaction with gH/gL, the complex remained elusive (7, 20, 31, 44–47). In our previous study (21), we showed direct evidence that the gD ectodomain physically interacts with the ectodomain of gH/gL. The gD-gH/gL interaction was shown in real time using SPR and could be blocked by specific neutralizing and fusion-blocking anti-gD MAbs. By mapping the epitopes of these anti-gD MAbs that either allowed or blocked gD-gH/gL binding, we identified a potential gH/gL binding site on gD (Fig. 13D), located on the opposite face of the molecule as the receptor-binding region.
In the present study, we turned our focus on gH/gL. gH/gL sits in the middle of the fusion cascade, interacting first with gD and subsequently with gB. gH/gL is the “bridge” between the receptor-binding protein and the fusogen and has been proposed to regulate the fusion process (3, 7, 15, 31). To examine its physical interaction with gD, we used SPR and a panel of newly organized anti-gH/gL MAbs (28, 31, 33, 35, 48) in order to map those that blocked gD-gH/gL binding (Table 1). The epitopes of MAbs that blocked gH/gL binding to gD mapped to two presumably flexible regions, the N terminus of gH and the C terminus of gL. Because neither region was visible in the gH/gL crystal structure (9), we used 3D modeling to portray them (Fig. 2). The epitopes of CHL27 and CHL17/32 (gH N terminus) and CHL18 (gL C terminus), which block gD binding, created a continuous “patch” on this in silico model (Fig. 13C). These MAbs are in separate communities and do not compete for gH/gL binding, signifying that their epitopes are not overlapping (Fig. 2B). This group of epitopes neighbor that of another gD-blocking MAb, 53S, which is present on the “body” of gH/gL near gL residue 77 (Fig. 13C) and competes strongly with CHL27 (Fig. 2A and B). We conclude that the epitopes of these MAbs that block gD binding define the gD binding site on gH/gL, located at the N terminus of the heterodimer and involving both gH and gL residues. Our data are in agreement with another study that suggested that the gD binding site on gH/gL was located within the N-terminal half of gH (20). In that study, gH/gL chimeras containing segments of HSV-1 and herpesvirus saimiri-1 proteins were tested for fusion and it was only when gD and the first two domains of gH were from the same virus that the proteins were functional.
TABLE 1.
Properties of αgH/gL MAbs
| MAb | Community/subcommunity | Type | Epitope residues | Competes with | Blocks gD-gH/gL binding | Stabilizes gD-gH/gL | Blocks fusion (SLA) |
|---|---|---|---|---|---|---|---|
| CHL27 | green | TC | gH 37–47 | 53S | Yes | No | Yes |
| 53S | green | TC | gL 48a, 55a, 77 | CHL27, LP11, BBH3 | Yes | No | Yes |
| CHL17 | magenta | T2S | gH 19–38b | CHL32 | Yes | No | Yes |
| CHL32 | magenta | T2S | gH 19–38b | CHL17 | Yes | No | Yes |
| CHL21 | purple | TC | NDc | CHL37, 52S, 46S, BBH5 | No | Yes | Yes |
| CHL37 | purple | TC | ND | CHL21, 52S, 46S, BBH5 | No | Yes | Yes |
| CHL29 | orange | TC | gH 676–686b | CHL30 | No | No | No |
| CHL35 | orange | TC | gH 145–155b | CHL31 | No | No | No |
| CHL2 | red | T2S | gH 116b | CHL4-16 | No | Yes | Yes |
| CΔ48L3 | yellow | T2S | gL 173–183d | - | No | Yes | Noe |
| CHL18 | cyan | T2S | gL 209–218b | - | Yes (50%) | No | Yes (50%) |
Four of the five anti-gH/gL MAbs that block binding to gD have epitopes that are located on the flexible termini of gH (CHL17, CHL32, and CHL27 on the N terminus) or of gL (CHL18 on the C terminus) (Table 1). It had previously been postulated that these flexible regions may move in response to gD binding, as a mutant form of gH missing the N terminus (gHΔ48/gL) was partially activated for fusion in a gD-independent fashion (31). Furthermore, MAbs with epitopes on the C terminus of gL that either did not inhibit or only weakly inhibited fusion gained activity when the gH N terminus was removed. These data lead to a model where the gH N terminus may partially occlude the gL C terminus until the gH N terminus is moved through its binding to gD. Indeed, our in silico model of the complete gH/gL ectodomain places these two regions next to each other (Fig. 13B). Movement of the gH N terminus and the gL C terminus may be an important conformational step for the binding of gD and activation of gH/gL.
We previously postulated that gH/gL has distinct “sides” for interacting with gD and gB, although it is unknown if it can bind these molecules simultaneously. Current evidence suggests that the gB binding region on gH/gL may be adjacent to the LP11 epitope (9), which is just below the potential gD binding site (Fig. 7B). It is possible that gH/gL would need to disengage from gD before binding gB. Unfortunately, the ectodomain of gB used in our experiments is in the postfusion form and there is no evidence that this form can bind to gH/gL (2, 21, 49, 50) unless both soluble proteins are treated with low pH and incubated with liposomes (51). Perhaps a membrane-bound, prefusion gB (52, 53) can be used to examine gH/gL-gB binding in future experiments.
Multiple MAbs in separate, noncompeting antigenic communities stabilize the gD-gH/gL binding complex, as evidenced by their kinetics. When these IgGs were converted to Fabs, each MAb lost the ability to stabilize the observed gD-gH/gL complex. Stabilization may be explained by avidity, the notion that IgG (but not Fab) binding creates a bivalent gH/gL exhibiting a slower off-rate because both molecules of gH/gL must disengage gD for the binding signal to disappear.
Numerous small-molecule inhibitors convey their physiological activity by stabilizing specific protein complexes (54). Stabilization of the gD-gH/gL complex does correlate with a functional MAb phenotype in some cases. When CHL2 is converted from IgG to Fab, it loses its ability to inhibit fusion (Fig. 12), suggesting that stabilization may be a mechanism of fusion inhibition. If the gD-gH/gL complex, which normally comes together and dissociates rapidly (21), is forced to stay together, this may affect the subsequent interaction of gH/gL with gB and break the fusion cascade. Interestingly, CHL37, which loses its ability to stabilize when converted to a Fab, is still able to inhibit cell-cell fusion (Fig. 12B). CHL37 may have a second mode of action beyond stabilization. MAbs against HSV glycoproteins that have multiple modes of action have been reported previously (e.g., anti-gD MAb MC23 blocks the interaction of gD with both its receptor nectin-1 and gH/gL) (21, 55). Since CHL37 Fab does not stabilize or block the gD-gH/gL interaction, what is its mechanism of action for inhibiting cell-cell fusion? One hypothesis is that it could be preventing a conformational change in gH/gL needed to allow interactions with gB. Since the epitope of CHL37 is located on the opposite side of gH/gL, distant from the proposed gH/gL-gB interaction domain (9), we do not believe it is sterically blocking this interaction. Another possibility involves a caveat of our gD-gH/gL binding and cell-cell fusion assays. Whereas our binding assay uses the shorter form of gD, gD(285t), the longer gD(306t) is used in the fusion assay (see Materials and Methods). CHL37 may disrupt a function of the C-terminal gD(306t) tail.
We previously hypothesized that the gD binding region on gH/gL centered around the 52S epitope (gH amino acid 536) due to the inability of 52S to inhibit cell-cell fusion in a gD-independent system using a mutant gH (31, 33). Here, we show that MAbs CHL21 and CHL37, which compete with 52S, did not block gD-gH/gL binding. Yet, these MAbs both have an effect on gH/gL function and inhibit cell-cell fusion. If we assume that 52S, CHL21, and CHL37 have epitopes that are overlapping or in close proximity to each other and inhibit the same function of gH/gL, it would suggest that these MAbs impede an as-yet-unknown function other than the binding of either gD or gB.
Clearly, structural information on the gD-gH/gL complex is required to define contact residues. Crystallization of the complex is not feasible due to the rapid off-rate of gH/gL on gD. One approach would be to use the stabilizing anti-gH/gL MAbs to “lock” the molecules together in order to visualize the complex. Although these MAbs lose their ability to stabilize the complex as Fabs, longer F(ab)2 fragments could be tested for this purpose. Once we enhance our understanding of the gD and gH/gL interface, this information will be useful to design targeted therapies that disrupt this interaction and inhibit the viral fusion cascade and HSV infection.
MATERIALS AND METHODS
Cells and soluble proteins.
All soluble proteins used in this study were purified from baculovirus-infected insect (Sf9) cells. HSV type-2 gD(285t) and gD2(306t) were purified using a DL6 immunosorbent column as described previously (22, 55, 56). The hexahistidine-tagged proteins gH2/gL2 and gH1/gL1 (containing full-length gH ectodomains) and the N-terminal truncation mutant gH2Δ48/gL2 were purified via nickel affinity chromatography (9, 51). B78-H1 mouse melanoma cells were grown in Dulbecco modified Eagle medium (DMEM) containing 5% fetal bovine serum (FBS) and 100 μg/ml of penicillin-streptomycin. For B78-C10 cells (stably expressing nectin-1 receptor), the medium was supplemented with 500 μg/ml of Geneticin (G418) (57).
Antibodies.
The following anti-gH/gL MAbs were used in this study: 46S, 52S, 53S (33, 35, 48); CHL2, CHL4 to 16, CHL17, CHL18, CHL29 to 31, CHL35, CHL37, CHL43 (28); LP11 (gift of A. Minson) (33, 58); H6 (8); L4 (30); and CΔ48L3 (31). CHL21 and CHL27 were generated in the same hybridoma fusion as the other CHL MAbs (28) but were previously uncharacterized. To generate anti-gH/gL MAbs BBH2, 3, 4, and 5, mice were immunized with HSV-1 strain SC16. Following fusion, hybridoma supernatants were screened by ELISA against soluble gH/gL protein (59). Monoclonal antibody resistant (mar) mutants were generated using the protocol described in Minson et al. (60). Anti-gD MAb 1D3 (55, 61, 62) was used to capture soluble gD2(285t) to the biosensor chip. Anti-gB MAb A22 (43) was used as a negative control. PAbs R137 and R176 were generated against purified gH1/gL1 and gH2/gL2, respectively (63, 64).
Plasmid DNAs.
Plasmids pTC510 (gH2-WT), pTC579 (gL2-WT), pTC642 (gH2Δ48), pTC684 (gH2Δ64), pTC673 (gH2-R39A), pTC755 (gH2-T40A), pLF681 (gH2-Y41A), pTC712 (gH2-R43A), pTC756 (gH2-D44A), and pCAGGS/MCS (a gift of P. G. Spear) were previously described (34, 64–66). The remaining plasmids were generated by GenScript (Piscataway, NJ) as follows: plasmids pTC1065 (gH2-WT) and pTC1066 (gL2-WT) were generated with optimized HSV-2 (333) codons and placed into vector pcDNA3.1 (EcoRI/HindIII); plasmids pDA1069 (gH2-V161A), pDA1070 (gH2-T162A), pDA1071 (gL2-R46A), pDA1072 (gL2-D50A), pDA1073 (gL2-D51A), pDA1074 (gL2-P77A), pDA1075 (gL2-Q138A), pDA1076 (gL2-H142A), and pDA1077 (gL2-P144A) were generated by site-directed mutagenesis of pTC1065 (for gH2) or pTC1066 (for gL2). Full-length plasmids used in the fusion assay (Rluc8(1–7), Rluc8(8–11), and WT glycoprotein constructs pTC580 [gB2], pTC510 [gH2], and pTC579 [gL2]) have all been described previously (41, 64).
Generating gH/gL MAb community maps using the continuous flow microspotter (CFM)/surface plasmon resonance imaging (SPRi).
Epitope binning experiments of 36 anti-gH/gL MAbs were performed on soluble gH2t/gL2 and gH1t/gL1 using the Carterra CFM/SPRi system. We used a method described previously (24–26). Briefly, a CFM 2 was used to create a 48-spot microarray of amine-coupled MAbs on a CDM200M sensor chip (Xantec GmbH). Upon docking the printer chip into the SPR imager (IBIS MX96), the chip was blocked with ethanolamine and the system primed with a running buffer of PBS-0.01% Tween 20. Epitope binning was performed in a classical sandwich assay format using 100 nM soluble gH/gL as antigen, 100 nM per MAb as analyte, and 1 M glycine pH 2.0 for regeneration. All MAbs were tested in the role of both analyte (in solution) and ligand (on chip). However, several MAbs were inactive as ligands so their competitive profiles were determined solely from their performance as analytes. SPRi data were processed in SPRint software and analyzed using Carterra’s Epitope Binning 2.0 software for heat map generation, sorting, and network plotting. Binary sorting routines were used to organize the heat maps and epitope bins were viewed as community network plots. All experiments were performed at room temperature.
Modeling of the full-length ectodomain of gH2/gL2.
The full-length ectodomain structure of HSV-2 gH was constructed using the Zhang lab’s ITASSER web server (67) by inputting the gH2 sequence (P89445) (68). The gH2/gL2 crystal structure (PDB ID: 3M1C) (9) was used as the template to model the missing residues. gH2 residues 19 to 48,116 to 136, and 797 to 803 were now visible in the 3D model. The same process was repeated to construct the full-length structure of gL2 using the sequence (P28278) and the same structure (PDB ID: 3M1C) as the template to fill in the missing residues 17 to 23 and 166 to 224. The superimposed images of the model and crystal structure of gH2, gL2, and the gH2/gL2 complex (Fig. 3C and D) show that all missing loops have been filled.
CELISA.
To detect glycoprotein cell surface expression, we used a modified cell-based ELISA (29, 40). B78 cells growing in clear 96-well plates (5 × 104 cells per well) were transfected overnight with plasmids expressing gH2 and gL2. Cells were fixed with 3% paraformaldehyde and rinsed with phosphate-buffered saline (PBS) containing Ca2+and Mg2+. Cells were incubated with the indicated primary antibodies diluted in 3% BSA-PBS and then incubated for 30 min with goat anti-mouse secondary antibody coupled to horseradish peroxidase. Cells were rinsed with 20 mM citrate buffer (pH 4.5) and ABTS (2,2′-Azino-di[3-ethylbenzthiazoline] sulfonic acid peroxidase substrate) substrate (Moss, Inc.) was added. The absorbance at 405 nm was recorded using a BioTek plate reader.
Split luciferase assay.
The split luciferase assay (SLA) protocol has been described previously (29, 40, 69). Briefly, 5 × 104 B78 cells (effector cells) were seeded on white, cell-culture-treated 96-well plates and 4 × 105 C10 cells (target cells) were seeded on 6-well plates. Transfection was performed the following day. A master mix containing 125 ng each of the gB2, gH2, gL2, and Rluc8(1–7) plasmids was split over three wells of effector cells. Target cells were transfected with 1 μg of Rluc8(8–11) plasmid/per well. Twenty-four hours posttransfection, effector cells were preincubated for 1 h at 37°C with both EnduRen substrate (Promega) diluted 1:1,000 in fusion medium (DMEM without phenol red supplemented with 50 mM HEPES and 5% FBS) and 20 μg/ml of MAb. Fusion was triggered by the addition of 50 μg/ml soluble gD2(306t) and target cells. Luciferase production was monitored over a 2 h period, with measurements taken every 5 min using a BioTek plate reader.
Glycoprotein binding using the BIACORE 3000.
Experiments were carried out on a BIACORE 3000 optical biosensor at 25°C following previous guidelines (55, 70, 71). All injections were performed at a flow rate of 5 μl/min using HBS-EP buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20). After each experiment, the chip surface was treated with brief pulses of 0.2 M Na2CO3 (pH 10) until the response unit (RU) signal returned to baseline (regeneration). First, the gH/gL binding-permissive anti-gD MAb 1D3 (IgG) (21) was amine-coupled to a CM5 sensor chip (GE Healthcare Bio-Sciences, Pittsburgh, PA). Second, 0.05 mg/ml of soluble, purified gD2(285t) was injected across the chip surface until approximately 150 to 300 RU was captured. Lastly, purified gH2t/gL2 (0.2 mg/ml) was injected for 240 s (analyte) and the binding was recorded. To test for blocking of gD-gH/gL binding via anti-gH/gL MAbs, soluble gH2/gL2 was preincubated with 0.6 mg/ml IgG or Fab at RT for 10 min before flowing the mixture across the captured gD2(285t).
Western blotting.
B78-C10 cells were transfected with the desired plasmids according to the GenePORTER protocol (Gene Therapy Systems, Inc.). At 24 h posttransfection, cells were lysed in 10 mM Tris (pH 8), 150 mM NaCl, 10 mM EDTA, 1% NP-40, 0.5% deoxycholic acid, 1 mM phenylmethylsulfonyl fluoride. Typically, 5% of the total cell extract (from a single well of a 6-well plate) was separated by electrophoresis on a sodium dodecyl sulfate (SDS)-12% polyacrylamide gel. Proteins were detected by Western blotting on nitrocellulose and probing with the desired counterantibody.
Peptide ELISA.
Synthetic 20-mer peptides were purchased from Mimotopes Pty. Ltd. (Melbourne, Australia) and described previously (28). Fifty microliters of an approximately 1 μM concentration (in PBS) of each peptide was placed in each well of a 96-well Reacti-bind high-binding-capacity streptavidin-coated plate (Pierce) and incubated for 1 h. The plate was then blocked with 200 μl of 5% milk, PBS-T for 30 min and probed with 50 μl of 20 μg/ml MAb for 1 h in milk/PBS-T. All steps were performed at room temperature. Bound IgG was visualized with goat anti-mouse IgG-horseradish peroxidase.
ACKNOWLEDGMENTS
The authors dedicate this article to the memory of our friend Roselyn J. Eisenberg. Roz was a world-class scientist, known for her groundbreaking HSV research in the lab she co-ran for decades with Gary H. Cohen. She was integral to the development of this manuscript and will be sorely missed by all of her friends, students, and mentees. Her contribution to this paper was testimony to her love of research.
We thank Leslie King, Beth Schachter, and Jeannie Hirsch for critical readings of the manuscript.
This research was supported by AI-18289, AI-142940, AI-139618, and a grant from BIONTECH, Inc. (to G.H.C.) and NSF grants RUI-1904797/ACI-1429467 and XSEDE MCB 170088 (to C.W.) and The Wellcome Trust, UK (to H. B.).
N.T.D. is employed by Carterra, Inc.
B.D.B. is employed by Inovan, Inc.
REFERENCES
- 1.Atanasiu D, Saw WT, Cohen GH, Eisenberg RJ. 2010. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J Virol 84:12292–12299. doi: 10.1128/JVI.01700-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldwein EE, Krummenacher C. 2008. Entry of herpesviruses into mammalian cells. Cell Mol Life Sci 65:1653–1668. doi: 10.1007/s00018-008-7570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vollmer B, Grunewald K. 2020. Herpesvirus membrane fusion—a team effort. Curr Opin Struct Biol 62:112–120. doi: 10.1016/j.sbi.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg RJ, Atanasiu D, Cairns TM, Gallagher JR, Krummenacher C, Cohen GH. 2012. Herpes virus fusion and entry: a story with many characters. Viruses 4:800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krummenacher C, Carfi A, Eisenberg RJ, Cohen GH. 2013. Entry of herpesviruses into cells: the enigma variations. Adv Exp Med Biol 790:178–195. doi: 10.1007/978-1-4614-7651-1_10. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol 9:369–381. doi: 10.1038/nrmicro2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallbracht M, Backovic M, Klupp BG, Rey FA, Mettenleiter TC. 2019. Common characteristics and unique features: a comparison of the fusion machinery of the alphaherpesviruses Pseudorabies virus and Herpes simplex virus. Adv Virus Res 104:225–281. doi: 10.1016/bs.aivir.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Peng T, Ponce de Leon M, Novotny MJ, Jiang H, Lambris JD, Dubin G, Spear PG, Cohen GH, Eisenberg RJ. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J Virol 72:6092–6103. doi: 10.1128/JVI.72.7.6092-6103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdary TK, Cairns TM, Atanasiu D, Cohen GH, Eisenberg RJ, Heldwein EE. 2010. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat Struct Mol Biol 17:882–888. doi: 10.1038/nsmb.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roop C, Hutchinson L, Johnson DC. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol 67:2285–2297. doi: 10.1128/JVI.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson AC, Johnson DC. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol 66:2240–2250. doi: 10.1128/JVI.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atanasiu D, Whitbeck JC, de Leon MP, Lou H, Hannah BP, Cohen GH, Eisenberg RJ. 2010. Bimolecular complementation defines functional regions of herpes simplex virus gB that are involved with gH/gL as a necessary step leading to cell fusion. J Virol 84:3825–3834. doi: 10.1128/JVI.02687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stampfer SD, Heldwein EE. 2013. Stuck in the middle: structural insights into the role of the gH/gL heterodimer in herpesvirus entry. Curr Opin Virol 3:13–19. doi: 10.1016/j.coviro.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heldwein EE. 2016. gH/gL supercomplexes at early stages of herpesvirus entry. Curr Opin Virol 18:1–8. doi: 10.1016/j.coviro.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atanasiu D, Saw WT, Eisenberg RJ, Cohen GH. 2016. Regulation of herpes simplex virus glycoprotein-induced cascade of events governing cell-cell fusion. J Virol 90:10535–10544. doi: 10.1128/JVI.01501-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell 8:169–179. doi: 10.1016/s1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 17.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7:e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu G, Zhang N, Qi J, Li Y, Chen Z, Zheng C, Gao GF, Yan J. 2014. Crystal structure of herpes simplex virus 2 gD bound to nectin-1 reveals a conserved mode of receptor recognition. J Virol 88:13678–13688. doi: 10.1128/JVI.01906-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, Eisenberg RJ, Cohen GH, Wiley DC, Carfi A. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J 24:4144–4153. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Q, Longnecker R, Connolly SA. 2015. A functional interaction between herpes simplex virus 1 glycoprotein gH/gL domains I and II and gD is defined by using alphaherpesvirus gH and gL chimeras. J Virol 89:7159–7169. doi: 10.1128/JVI.00740-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cairns TM, Ditto NT, Atanasiu D, Lou H, Brooks BD, Saw WT, Eisenberg RJ, Cohen GH. 2019. Surface plasmon resonance reveals direct binding of herpes simplex virus glycoproteins gH/gL to gD and locates a gH/gL binding site on gD. J Virol 93:e00289-19. doi: 10.1128/JVI.00289-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rux AH, Willis SH, Nicola AV, Hou W, Peng C, Lou H, Cohen GH, Eisenberg RJ. 1998. Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to the herpesvirus entry mediator. J Virol 72:7091–7098. doi: 10.1128/JVI.72.9.7091-7098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher JR, Saw WT, Atanasiu D, Lou H, Eisenberg RJ, Cohen GH. 2013. Displacement of the C-terminus of herpes simplex virus gD is sufficient to expose the fusion activating interfaces on gD. J Virol 87:12656–12666. doi: 10.1128/JVI.01727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cairns TM, Ditto NT, Lou H, Brooks BD, Atanasiu D, Eisenberg RJ, Cohen GH. 2017. Global sensing of the antigenic structure of herpes simplex virus gD using high-throughput array-based SPR imaging. PLoS Pathog 13:e1006430. doi: 10.1371/journal.ppat.1006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdiche YN, Harriman R, Deng X, Yeung YA, Miles A, Morishige W, Boustany L, Zhu L, Izquierdo SM, Harriman W. 2016. Assessing kinetic and epitopic diversity across orthogonal monoclonal antibody generation platforms. MAbs 8:264–277. doi: 10.1080/19420862.2015.1118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdiche YN, Miles A, Eckman J, Foletti D, Van Blarcom TJ, Yeung YA, Pons J, Rajpal A. 2014. High-throughput epitope binning assays on label-free array-based biosensors can yield exquisite epitope discrimination that facilitates the selection of monoclonal antibodies with functional activity. PLoS One 9:e92451. doi: 10.1371/journal.pone.0092451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks BD, Miles AR, Abdiche YN. 2014. High-throughput epitope binning of therapeutic monoclonal antibodies: why you need to bin the fridge. Drug Discov Today 19:1040–1044. doi: 10.1016/j.drudis.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Cairns TM, Shaner MS, Zuo Y, Ponce-de-Leon M, Baribaud I, Eisenberg RJ, Cohen GH, Whitbeck JC. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J Virol 80:2596–2608. doi: 10.1128/JVI.80.6.2596-2608.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atanasiu D, Saw WT, Gallagher JR, Hannah BP, Matsuda Z, Whitbeck JC, Cohen GH, Eisenberg RJ. 2013. Dual split protein-based fusion assay reveals that mutations to herpes simplex virus (HSV) glycoprotein gB alter the kinetics of cell-cell fusion induced by HSV entry glycoproteins. J Virol 87:11332–11345. doi: 10.1128/JVI.01700-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubin G, Jiang H. 1995. Expression of herpes simplex virus type 1 glycoprotein L (gL) in transfected mammalian cells: evidence that gL is not independently anchored to cell membranes. J Virol 69:4564–4568. doi: 10.1128/JVI.69.7.4564-4568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atanasiu D, Cairns TM, Whitbeck JC, Saw WT, Rao S, Eisenberg RJ, Cohen GH. 2013. Regulation of herpes simplex virus gB-induced cell-cell fusion by mutant forms of gH/gL in the absence of gD and cellular receptors. mBio 4:e00046-13. doi: 10.1128/mBio.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen GH, Isola VJ, Kuhns J, Berman PW, Eisenberg RJ. 1986. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol 60:157–166. doi: 10.1128/JVI.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gompels UA, Carss AL, Saxby C, Hancock DC, Forrester A, Minson AC. 1991. Characterization and sequence analyses of antibody-selected antigenic variants of herpes simplex virus show a conformationally complex epitope on glycoprotein H. J Virol 65:2393–2401. doi: 10.1128/JVI.65.5.2393-2401.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cairns TM, Friedman LS, Lou H, Whitbeck JC, Shaner MS, Cohen GH, Eisenberg RJ. 2007. N-terminal mutants of herpes simplex virus type 2 gH are transported without gL but require gL for function. J Virol 81:5102–5111. doi: 10.1128/JVI.00097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Showalter SD, Zweig M, Hampar B. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun 34:684–692. doi: 10.1128/IAI.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuller AO, Santos RE, Spear PG. 1989. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol 63:3435–3443. doi: 10.1128/JVI.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cairns TM, Milne RS, Ponce-de-Leon M, Tobin DK, Cohen GH, Eisenberg RJ. 2003. Structure-function analysis of herpes simplex virus type 1 gD and gH-gL: clues from gDgH chimeras. J Virol 77:6731–6742. doi: 10.1128/jvi.77.12.6731-6742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klyachkin YM, Stoops KD, Geraghty RJ. 2006. Herpes simplex virus type 1 glycoprotein L mutants that fail to promote trafficking of glycoprotein H and fail to function in fusion can induce binding of glycoprotein L-dependent anti-glycoprotein H antibodies. J Gen Virol 87:759–767. doi: 10.1099/vir.0.81563-0. [DOI] [PubMed] [Google Scholar]
- 39.Fan Q, Lin E, Spear PG. 2009. Insertional mutations in herpes simplex virus type 1 gL identify functional domains for association with gH and for membrane fusion. J Virol 83:11607–11615. doi: 10.1128/JVI.01369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saw WT, Matsuda Z, Eisenberg RJ, Cohen GH, Atanasiu D. 2015. Using a split luciferase assay (SLA) to measure the kinetics of cell-cell fusion mediated by herpes simplex virus glycoproteins. Methods 90:68–75. doi: 10.1016/j.ymeth.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa H, Meng F, Kondo N, Iwamoto A, Matsuda Z. 2012. Generation of a dual-functional split-reporter protein for monitoring membrane fusion using self-associating split GFP. Protein Eng Des Sel 25:813–820. doi: 10.1093/protein/gzs051. [DOI] [PubMed] [Google Scholar]
- 42.Atanasiu D, Saw WT, Lazear E, Whitbeck JC, Cairns TM, Lou H, Eisenberg RJ, Cohen GH. 2018. Using antibodies and mutants to localize the presumptive gH/gL binding site on HSV gD. J Virol 92:e01694-18. doi: 10.1128/JVI.01694-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bender FC, Samanta M, Heldwein EE, de Leon MP, Bilman E, Lou H, Whitbeck JC, Eisenberg RJ, Cohen GH. 2007. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J Virol 81:3827–3841. doi: 10.1128/JVI.02710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Q, Longnecker R, Connolly SA. 2014. Substitution of herpes simplex virus 1 entry glycoproteins with those of saimiriine herpesvirus 1 reveals a gD-gH/gL functional interaction and a region within the gD profusion domain that is critical for fusion. J Virol 88:6470–6482. doi: 10.1128/JVI.00465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atanasiu D, Whitbeck JC, Cairns TM, Reilly B, Cohen GH, Eisenberg RJ. 2007. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc Natl Acad Sci U S A 104:18718–18723. doi: 10.1073/pnas.0707452104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gianni T, Amasio M, Campadelli-Fiume G. 2009. Herpes simplex virus gD forms distinct complexes with fusion executors gB and gH/gL through the C-terminal profusion. J Biol Chem 284:17370–17382. doi: 10.1074/jbc.M109.005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez-Romero P, Perez A, Capul A, Montgomery R, Fuller AO. 2005. Herpes simplex virus entry mediator associates in infected cells in a complex with viral proteins gD and at least gH. J Virol 79:4540–4544. doi: 10.1128/JVI.79.7.4540-4544.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gompels UA, Minson AC. 1989. Antigenic properties and cellular localization of herpes simplex virus glycoprotein H synthesized in a mammalian cell expression system. J Virol 63:4744–4755. doi: 10.1128/JVI.63.11.4744-4755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 50.Backovic M, Jardetzky TS. 2009. Class III viral membrane fusion proteins. Curr Opin Struct Biol 19:189–196. doi: 10.1016/j.sbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, Cohen GH. 2011. Capturing the herpes simplex virus core fusion complex (gB-gH/gL) in an acidic environment. J Virol 85:6175–6184. doi: 10.1128/JVI.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontana J, Atanasiu D, Saw WT, Gallagher JR, Cox RG, Whitbeck JC, Brown LM, Eisenberg RJ, Cohen GH. 2017. The fusion loops of the initial prefusion conformation of herpes simplex virus 1 fusion protein point toward the membrane. mBio 8:e01268-17. doi: 10.1128/mBio.01268-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeev-Ben-Mordehai T, Vasishtan D, Hernández Durán A, Vollmer B, White P, Prasad Pandurangan A, Siebert CA, Topf M, Grünewald K. 2016. Two distinct trimeric conformations of natively membrane-anchored full-length herpes simplex virus 1 glycoprotein B. Proc Natl Acad Sci U S A 113:4176–4181. doi: 10.1073/pnas.1523234113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrei SA, Sijbesma E, Hann M, Davis J, O'Mahony G, Perry MWD, Karawajczyk A, Eickhoff J, Brunsveld L, Doveston RG, Milroy LG, Ottmann C. 2017. Stabilization of protein-protein interactions in drug discovery. Expert Opin Drug Discov 12:925–940. doi: 10.1080/17460441.2017.1346608. [DOI] [PubMed] [Google Scholar]
- 55.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol 86:1563–1576. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicola AV, Willis SH, Naidoo NN, Eisenberg RJ, Cohen GH. 1996. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol 70:3815–3822. doi: 10.1128/JVI.70.6.3815-3822.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milne RS, Connolly SA, Krummenacher C, Eisenberg RJ, Cohen GH. 2001. Porcine HveC, a member of the highly conserved HveC/nectin 1 family, is a functional alphaherpesvirus receptor. Virology 281:315–328. doi: 10.1006/viro.2000.0798. [DOI] [PubMed] [Google Scholar]
- 58.Buckmaster EA, Gompels U, Minson A. 1984. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology 139:408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- 59.Parry C, Bell S, Minson T, Browne H. 2005. Herpes simplex virus type 1 glycoprotein H binds to alphavbeta3 integrins. J Gen Virol 86:7–10. doi: 10.1099/vir.0.80567-0. [DOI] [PubMed] [Google Scholar]
- 60.Minson AC, Hodgman TC, Digard P, Hancock DC, Bell SE, Buckmaster EA. 1986. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol 67:1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- 61.Eisenberg RJ, Long D, Pereira L, Hampar B, Zweig M, Cohen GH. 1982. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol 41:478–488. doi: 10.1128/JVI.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedman HM, Cohen GH, Eisenberg RJ, Seidel CA, Cines DB. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 63.Peng T, Ponce-de-Leon M, Jiang H, Dubin G, Lubinski JM, Eisenberg RJ, Cohen GH. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J Virol 72:65–72. doi: 10.1128/JVI.72.1.65-72.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cairns TM, Landsburg DJ, Whitbeck JC, Eisenberg RJ, Cohen GH. 2005. Contribution of cysteine residues to the structure and function of herpes simplex virus gH/gL. Virology 332:550–562. doi: 10.1016/j.virol.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Okuma K, Nakamura M, Nakano S, Niho Y, Matsuura Y. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235–244. doi: 10.1006/viro.1998.9530. [DOI] [PubMed] [Google Scholar]
- 66.Pertel PE, Fridberg A, Parish ML, Spear PG. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313–324. doi: 10.1006/viro.2000.0713. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Yang J, Jang R, Zhang Y. 2015. GPCR-I-TASSER: a hybrid approach to G protein-coupled receptor structure modeling and the application to the human genome. Structure 23:1538–1549. doi: 10.1016/j.str.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magrane M, UniProt Consortium. 2011. UniProt Knowledgebase: a hub of integrated protein data. Database (Oxford) 2011:bar009. doi: 10.1093/database/bar009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kondo N, Miyauchi K, Matsuda Z. 2011. Monitoring viral-mediated membrane fusion using fluorescent reporter methods. Curr Protoc Cell Biol Chapter 26:Unit 26.9. doi: 10.1002/0471143030.cb2609s50. [DOI] [PubMed] [Google Scholar]
- 70.Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Hirao L, Isaacs SN, Moss B, Eisenberg RJ, Cohen GH. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J Virol 79:6260–6271. doi: 10.1128/JVI.79.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Connolly SA, Whitbeck JJ, Rux AH, Krummenacher C, van Drunen Littel-van den Hurk S, Cohen GH, Eisenberg RJ. 2001. Glycoprotein D homologs in herpes simplex virus type 1, pseudorabies virus, and bovine herpes virus type 1 bind directly to human HveC(nectin-1) with different affinities. Virology 280:7–18. doi: 10.1006/viro.2000.0747. [DOI] [PubMed] [Google Scholar]