ABSTRACT
Candida albicans
is abundant in the human gut mycobiota but this species does not colonize the mouse gastrointestinal tract. C. albicans administration in dextran-sulfate solution (DSS) induced-colitis mouse model (DSS+Candida) might resemble more to human condition, therefore, a DSS colitis model with Candida administration was studied; first, to test the influence of fungi in DSS model and second, to test the efficacy of Lactobacillus rhamnosus L34. We demonstrated serum (1→3)-β-D-glucan (BG) elevation in patients with IBD and endoscopic moderate colitis in clinical remission, supporting the possible influence of gut fungi toward IBD in human. Then, in mouse model, Candida gavage was found to worsen the DSS model indicated by higher mortality rate, more severe colon histology and enhanced gut-leakage (FITC-dextran assay, endotoxemia, serum BG and blood bacterial burdens) but did not affect weight loss and diarrhea. DSS+Candida induced higher pro-inflammatory cytokines both in blood and in intestinal tissue. Worsened systemic pro-inflammatory cytokine responses in DSS+Candida compared with DSS alone was possibly due to the more severe translocation of LPS, BG and bacteria (not fungemia) from gut into systemic circulation. Interestingly, bacteremia from Pseudomonas aeruginosa was more frequently isolated from DSS+Candida than DSS alone. In parallel, P. aeruginosa was also isolated from fecal culture in most of the mice in DSS+Candida group supported by prominent Gammaproteobacteria in fecal microbioata analysis. However, L. rhamnosus L34 attenuated both DSS+Candida and DSS model through the attenuation of gut local inflammation (cytokines and histology), gut-leakage severity, fecal dysbiosis (culture method and microbiome analysis) and systemic inflammation (serum cytokines). In conclusion, gut Candida in DSS model induced fecal bacterial dysbiosis and enhanced leaky-gut induced bacteremia. Probiotic treatment strategy aiming to reduce gut-fungi and fecal dysbiosis could attenuate disease severity. Investigation on gut fungi in patients with IBD is highly interesting.
KEYWORDS: Intestinal Candida, dextran sulfate solution induced colitis, dysbiosis, bacteremia, gut leakage
Introduction
Immune dysregulation toward gut microbiota1 is, at least in part, responsible for the pathogenesis of inflammatory bowel disease (IBD); Crohn’s disease (CD) and ulcerative colitis (UC).2 The maladaptation of gut microbiota (gut bacterial dysbiosis)3 in IBD is frequently demonstrated both in patients and in a representative mouse model (dextran sulfate sodium induced colitis; DSS-colitis).4 Besides increased bacterial dysbiosis, enhanced gut fungi in patients with IBD are also well-known4-6 which could be beneficial or detrimental to the host.7,8 In addition, intestinal barrier disruption (leaky-gut) in IBD as demonstrated by the spontaneous elevation of Gram-negative bacteria, endotoxin (LPS; a bacterial cell-wall component) and (1→3)-β-D-glucan (BG; a fungal cell-wall component), without systemic infection, in patients and in animal models are mentioned.9-12 The presentation of BG in serum of patients with active IBD12 implies the impact of gut Candida and leaky-gut upon IBD. As such, enhanced systemic inflammation by BG from gut translocation is demonstrated.13 Because i) the studies of gut fungi in IBD are still less in number,4 despite thorough studies in the impact of gut bacteria,14 ii) Candida albicans administration alters model characteristics in several mouse models7,15 and iii) C. albicans is not prominent in mouse gut and is undetectable by culture methods8 which differs from human16, therefore, DSS-colitis with C. albicans administration might be a more adequate representative model to resemble the human condition. But a single oral-gavage of C. albicans after DSS administration in wild-type mice demonstrated little impact on DSS mouse model, possibly due to a non-sustained or a subtle presentation of gut fungi.17 Hence, DSS model with several C. albicans administrations might be necessary for a model with sustained fecal Candida presentation.
On the other hand, probiotics as a therapeutic strategy against gut-dysbiosis, through the reduction of pathobionts both bacteria18 and fungi,19 has also been studied in IBD.20 Lactobacillus is one of the most common probiotics with several antagonistic mechanisms against Candida.21,22 Lactobacillus rhamnosus strain L34 attenuates interleukin-8 (IL-8) production in intestinal epithelial cells23 and in several mouse models13,24 which might also be beneficial in IBD. Here, 3% DSS induced colitis mouse model with or without orally administered C. albicans were explored and the effectiveness of L. rhamnosus L34 against these IBD models was tested.
Materials and methods
Patient samples
Serum samples of patients with IBD (18–60 y old) from the King Chulalongkorn Memorial Hospital, Bangkok, Thailand were collected following the approved protocol by the Ethical Institutional Review Board, Faculty of Medicine, Chulalongkorn University, according to the Declaration of Helsinki, with written informed consent from each individual patient. All patients had documented endoscopic-proven diagnosis and recorded severity of Crohn’s disease (CD) and ulcerative colitis (UC) with clinical disease remission by the clinical colitis activity index (for UC) and Harvey-Bradshaw index (for CD).25 Serum was taken within 1 month after the previous endoscopic examination. Patients with other conditions of possible gut leakage or elevated serum BG including (1) serum creatinine >1.5 mg/dL, (2) current infections or a history of infections within 2 weeks, (3) pregnancy and (4) liver injury were excluded. Serum endotoxin and serum BG were measured by HEK-Blue LPS Detection Kit 2 (InvivoGen, San Diego, CA, USA) and Fungitell assay (Associates of Cape Cod, Inc., East Falmouth, MA, USA), respectively. The lower limit of detection of the endotoxin and Fungitell assays was 0.01 EU/mL and 7.8 pg/mL, respectively, and values <0.01 EU/mL and <7.8 pg/mL were recorded as 0. Erythrocyte sedimentation rate (ESR) were determined by routine hospital standard protocol. Epidemiologic data is demonstrated in Table 1.
Table 1.
Epidemiologic data.
| Healthy |
Inflammatory bowel disease |
|||
|---|---|---|---|---|
| Volunteer | Remission | Mild colitis | Moderate colitis | |
| Number of patients | 8 | 11 | 13 | 6 |
| Age (years) | 31 ± 8 | 52 ± 15 | 50 ± 18 | 47 ± 23 |
| Female Gender (%) | 4 (50) | 7 (63) | 8 (62) | 3 (50) |
| Duration of disease (months) | 0 | 100 ± 55 | 77 ± 37 | 62 ± 58 |
| Ex-smoker (%) | 0 | 2 (18) | 0 | 0 |
| Crohn’s disease (%) | 0 | 5 (45) | 8 (62) | 5 (83) |
| History of colectomy (%) | 0 | 4 (36) | 3 (23) | 0 |
| Current Immunosuppression (%) | ||||
| Prednisolone | 0 | 3 (27) | 5 (38) | 4 (67) |
| Azathioprine or 6-MP | 0 | 6 (55) | 9 (69) | 5 (83) |
| 5-ASA | 0 | 6 (55) | 9 (69) | 4 (67) |
| Infliximab | 0 | 2 (18) | 0 | 0 |
Data in mean ± standard deviation (SD); 6-MP, 6-mercaptopurine; 5-ASA, 5-aminosalicylic acid
Animals and animal models
Animal care and animal use protocol based upon the National Institutes of Health (NIH) protocol, USA was approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. Mice were rested in the facility for 1 week before further use. Then, 8-week-old male C57BL/6 mice from the National Laboratory Animal Center, Nakhorn Pathom, Thailand were used and 3% (wt/vol) dextran sulfate sodium (Sigma-Aldrich, St. Louis, MO, USA, Cat. No. 42867-100G, Lot# BCBV6277) was diluted into drinking water for dextran sulfate solution (DSS) induced colitis mouse model as previously published.26 And C. albicans (American Type Culture Collection, ATCC90028, Fisher Scientific, Waltham, MA, USA) at 1 × 106 colonies forming unit (CFU; determined by hemocytometer) diluted in 0.3 ml of phosphate buffer solution (PBS) or PBS alone, were orally administered every 3 d to induce Candida presentation in gut (Figure 1a). The repeated Candida gavage was necessary because the lower doses and less frequent gavage did not consistently induce positive fecal Candida from culture (data not shown). Mice were sacrificed through cardiac puncture under isoflurane anesthesia when moribund or at 14 day – post DSS for survival analysis.
Figure 1.
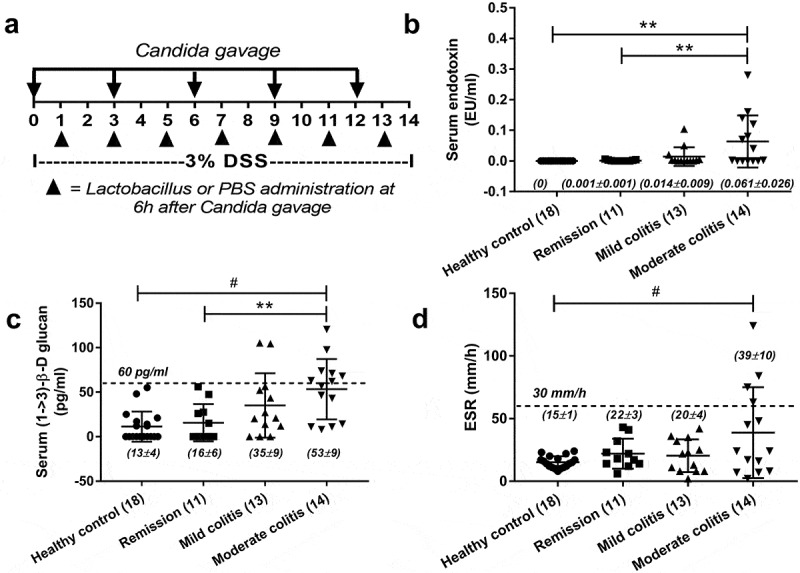
Schema of the experimental design (a) including Candida administration and Lactobacilli treatment is demonstrated. In parallel, a cross-sectional analysis demonstrated serum endotoxin (a), serum (1 → 3)-β-D-glucan (BG) (b), serum erythrocyte sedimentation rate (ESR) (c) in healthy volunteers and in patients with inflammatory bowel disease (IBD) of several endoscopic disease activities (number of cases indicated in figure) are demonstrated. The difference among groups was determined by ANOVA with Tukey’s analysis. **, p < .01; #, p < .001.
In addition, Lactobacillus rhamnosus L34 at 1 × 107 CFU/dose in PBS were administered every 3 d as in Figure 1a. L. rhamnosus L34 from stock previously isolated from Thai infant feces23,24 was cultured on deMan-Rogosa-Sharpe (MRS) agar (Oxoid, Hampshire, UK) under anaerobic conditions by gas generation sachets (AnaeroPack-Anaero; Mitsubishi Gas Chemical Co., INC, Japan) at 37°C for 24 h to 48 h before use. Blood was collected through tail vein nicking (25 µl) or cardiac puncture under isoflurane anesthesia (at sacrifice). This dose of Lactobacilli was selected because the dosage and qualities of probiotics are important, and a dose of a beneficial probiotic that is too high could be harmful,27,28 as previously demonstrated.24
Mouse blood sample analysis and gut leakage measurement
Blood (25 μl) was spread directly onto blood agar plates (Oxoid, Hampshire, UK), incubated at 37°C for 24 h before bacterial colony enumeration and colony identification was performed by mass-spectrometry analysis by ViTek MS (BioMerieux SA, Marcy-l’Etoile, France) following routine hospital protocol. Serum cytokines was performed with an enzyme-linked immunosorbent assay (ELISA) (Biolegend, San Diego, CA, USA). Gut leakage was analyzed by i) measurement of serum fluorescein isothiocyanate-dextran (FITC)-dextran, a non-absorbable high molecular weight molecule, after an oral administration and ii) spontaneously elevated endotoxemia and serum BG as previously reported.15,29
For FITC-dextran gut-leakage measurement, FITC-dextran (molecular weight 4.4 kDa) (Sigma-Aldrich, Cat. No. 46944-500MG-F, Lot#BCBT7927) at 50 mg/ml (0.25 ml) was orally administered. Then, 50 μl of blood was collected through tail vein at 3 h later, centrifuged and FITC-dextran in serum was detected by fluoro-spectrometry (Thermo Scientific Varioskan® Flash, Thermo Scientific, Ratastie 2, 01620 Vantaa Finland) with excitation and emission wavelength at 485 nm and 528 nm, respectively, using a standard serially diluted FITC-dextran (0–4,000 ng/ml). Serum from mice without FITC-dextran administration was used to determine the background. Serum endotoxin and serum BG were measured as the human samples.
Intestinal cytokines and histology
Several parts of mouse intestine including duodenum (2 cm from gastroduodenal junction), ilium (proximal 2 cm of cecum) and colon (distal 2 cm from cecum) were collected and separated in half longitudinally for cytokine measurement and for histology. For tissue cytokine detection, samples were weighed, sonicated thoroughly and the supernatant from homogenous tissue preparation was collected for cytokine measurement by ELISA assay (Biolegend) as serum samples. For histology, samples were put in 10% formalin-fixed, paraffin-embedded sections and stained with periodic acid-Schiff (PAS) reagent (Sigma-Aldrich). The modified semi-quantitative score at 200x magnification was used30 to determine histopathology based on mononuclear cell infiltration (in mucosa and sub-mucosa), epithelial hyperplasia (epithelial cell in longitudinal crypts), reduction of goblet cell and epithelial cell vacuolization in comparison with control as the following scores; 0; leukocyte < 5% and no epithelial hyperplasia (<10% of control), 1; leukocyte infiltration 5–10% or hyperplasia 10–25%, 2; leukocyte infiltration 10–25% or hyperplasia 25–50% or reduced globlet cells (>25% of control), 3; leukocyte infiltration 25–50% or hyperplasia >50% or intestinal vacuolization, 4; leukocyte infiltration >50% or ulceration.
Mouse fecal sample
An individual mouse was placed for 2 h in a metabolic cage (Hatteras Instrument, Cary, NC, USA) or a sterile empty cage for the collection of well-formed feces or diarrhea feces, respectively. Mouse feces were mixed with PBS at a ratio of 100 μg per 1 μl before plating onto 0.1% Chloramphenicol in Sabouraud Dextrose Agar (SDA) (Thermo Scientific, Waltham, MA, USA) in serial dilution and aerobically incubated at 35°C, for 72 h before colony enumeration to explore fecal fungi. In parallel, to detect only the most abundant fecal bacteria, feces were enormously diluted by mixing feces with PBS in different ratios at the maximum dilution of 1 μg per 100 μl before plating different volumes of the preparation directly onto blood agar (Oxoid) and 0.1% Chloramphenicol SDA (Thermo Scientific) for the identification of bacteria and Chloramphenicol-resistant bacteria, respectively. Due to the low abundance of fecal fungi and Chloramphenicol resistant bacteria in mouse feces, large amounts of feces (up to 1 gm) was necessary for culture. The plates were incubated at 35°C, for 72 h, before colony enumeration and 0 was recorded in case of no colony detectable. The identification of bacterial colonies was performed by mass-spectrometry analysis (BioMerieux SA) following routine hospital protocol as in blood sample mentioned previously. Fungal colony identification was performed by a standard method of Lactophenol Cotton Blue (LPBC) stain (the larger size of fungi in comparison with bacteria in the staining).
Fecal microbiome analysis
For fecal microbiome analysis, feces from nine mice (0.25 g per mouse) from different cages in each experimental group were divided into three samples per group (three mice per sample) before performing microbiota analysis as previously reported.7,8 In brief, fecal samples were processed for metagenomic DNA extractions by power DNA Isolation Kit (MoBio, Carlsbad, CA, USA). Universal prokaryotic 515F (forward; 5’-GTGCCAGCMGCCGCGGTAA-3’) and 806R (reverse; 5’-GGACTACHVGGGTWTCTAAT-3’) were used for 16S rRNA gene V4 library construction. Independently triplicate polymerase chain reactions (PCRs) were performed. The 16S rDNA amplicons of 381 basepairs (bp) were purified by GenepHlow™ Gel Extraction Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan), and quantified by Picogreen (Invitrogen, Eugene, Oregon, USA). Miseq300 platform sequencing (Illumina, San Diego, CA, USA) were used. Raw sequences were processed according to Mothur’s standard operating procedures for MiSeq.31 Quality screening included removal of sequences that have (i) <100 bases in length, (ii) >6 ambiguous bases, (iii) >6 homopolymers and (iv) chimera sequences.32,33 Sequences were aligned against Greengenes34 to remove contaminated sequences, including mitochondria and chloroplast sequences that also contain 16S rRNA gene. Operational taxonomic units (OTUs) were assigned based on phylotype and default parameters. Samples were normalized to an equal sequencing depth corresponding to the sample size of the fewest number of sequences (33,000). Good’s coverage to estimate a coverage index, bacterial richness, alpha diversity index (by Chao and Shannon),35 and beta diversity including J class similarity index and non-metric multidimensional scaling (NMDS) were conducted using Mothur tool.31 Of note, the nucleic acid sequences in this study were deposited in an NCBI open access of Sequence Read Archive database with accession number SRP189287.
Anti-inflammatory effects of Lactobacillus-conditioned medium on human colonic epithelial cells
Enterocyte was continuously activated by LPS (a major component of gut-bacterial cell wall of Gram-negative bacteria), Candida presentation in gut might introduce several fungal molecules, including BG (a major fungal cell wall component) upon enterocyte. Hence, the impact of Candida toward intestinal epithelial cell was tested in vitro. Human colorectal adenocarcinoma cells (HT-29) (ATCC HTB-38; Manassas) at 5 × 104 cells/well were plated in a 96-well plate for the co-culture assay. Heat-killed C. albicans and heat-killed Escherichia coli (E. coli ATCC 25922) were prepared at 65°C for 30 min and sonicated with an ultrasonic processor. HT-29 cells and C. albicans at ratios of 1:5 and 1:10),8 with and without heat-killed E. coli (1 × 109 cells/ml), were used. In parallel, LPS (Escherichia coli 026:B6; Sigma-Aldrich) at 100 ng/ml and/or BG (Pachyman) at 1 µg/ml were also used. The total volume was adjusted into 200 μl/well by PBS.
Moreover, the anti-inflammatory effect of L. rhamnosus L34 toward activated enterocytes was also tested. For preparation of the Lactobacillus-conditioned medium (LCM), L. rhamnosus L34 cells at an OD600 (optical density measured at a wavelength of 600 nm) of 0.1 were incubated anaerobically for 48 h before supernatant collection by centrifugation and filtered by 0.22 µm membrane-filter (Minisart; Sartorius Stedim Biotech GmbH, Göttingen, Germany). After that, 500 µL of the preparation was dried by speed vacuum at 40°C for 3 h (Savant Instruments, Farmingdale, NY, USA) then resuspended in an equal volume of McCoy’s 5a modified medium and stored at −20°C until use. HT-29 cells (5.0 × 104 cells/well) were then treated with LCM (5%, vol/vol) or McCoy’s 5a modified medium and co-incubated with heat-killed C. albicans (ratio of HT-29: C. albicans at 1:10) and/or heat-killed E. coli (1 × 109 cells/ml) under 5% CO2 at 37°C for 24 h. Supernatant was collected at 24 h of the incubation and IL-8 was measured by ELISA (Quantikine Immunoassay, R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Mean ± standard error (SE) was used for data presentation and the differences between groups were examined for statistical significance by one-way analysis of variance (ANOVA) followed by Tukey’s analysis or Student’s t-test for comparisons of multiple or two groups, respectively. Survival analysis was performed by Log-rank test. All statistical analyzes. were performed with SPSS 11.5 software (SPSS, IL, USA). A P-value of < .05 was considered statistically significant.
Results
A possible role of gut fungi in inflammatory bowel disease, patients and mice
The presence of BG (a major fungal component) in serum, without fungal infection, indirectly supports the influence of gut fungi and leaky-gut in patients with active IBD.12 And serum BG, at least in part, correlates with fungal burdens in gut.7 However, the data of serum BG in patients with IBD in remission have never been explored. Here, we demonstrated increased endotoxin (an important molecule of gram-negative bacteria in gut) and BG in serum, but not erythrocyte sedimentation rate (ESR), in patients with endoscopic moderate colitis even in clinical remission (Figure 1b–d). These data implied the importance of gut fungi and gut leakage in IBD pathophysiology. Thus, we further explored in mouse model. Because C. albicans is not a mouse commensal36 (different from human), DSS colitis mouse model with fungal administration might resemble more to patient condition. Indeed, C. albicans administration in DSS (DSS+Candida) increased serum endotoxin, serum BG and enhanced gut perm-selectivity injury as determined by FITC-dextran assay compared with DSS alone (Figure 2a–c) implying the influence of gut fungi in IBD pathophysiology.12 Thus, we further explored the importance of gut fungi in DSS mouse model.
Figure 2.
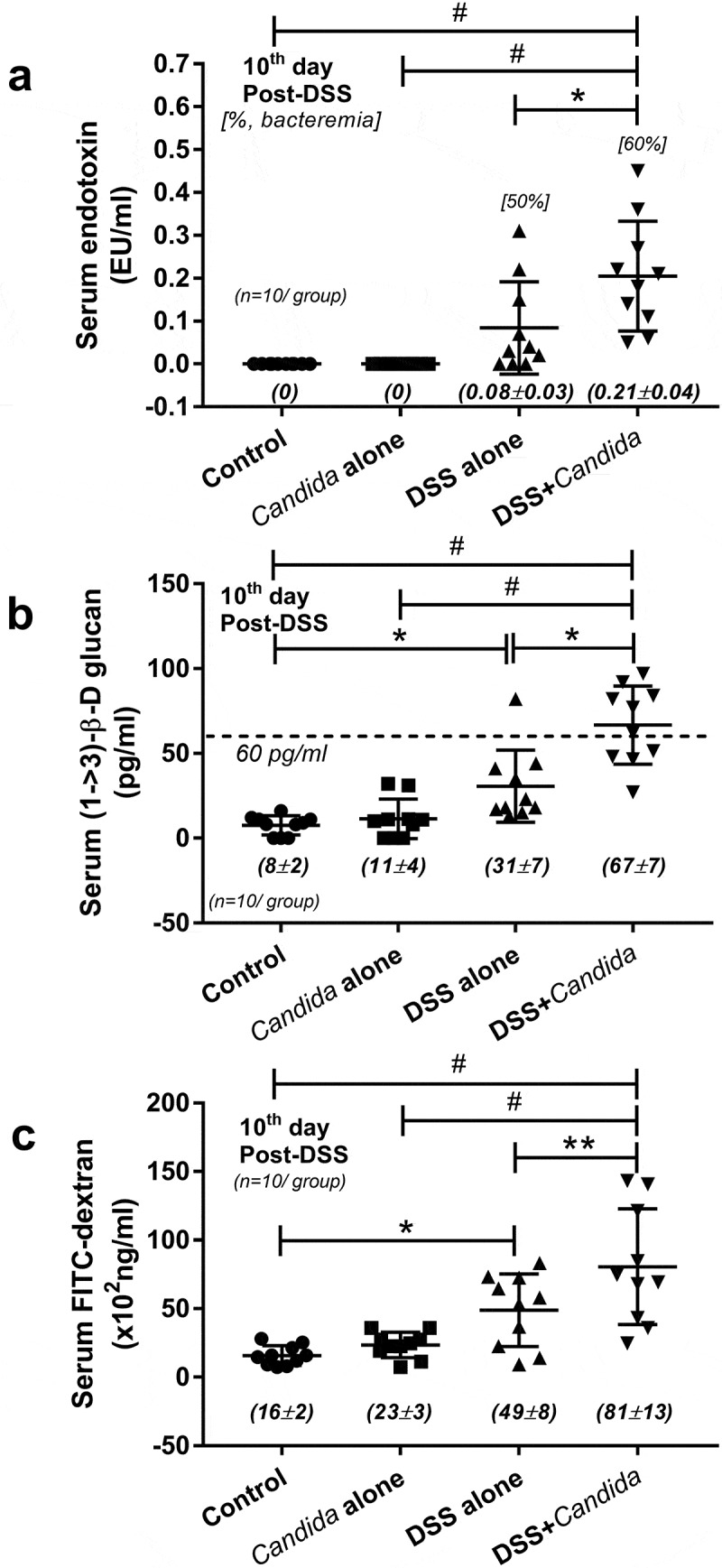
Serum endotoxin (b), serum (1 → 3)-β-D-glucan (BG) (c) and gut leakage (FITC-dextran assay) (d) in dextran sulfate solution (DSS) induced colitis mouse model, a representative model of IBD, is shown. Of note, the difference among groups was determined by ANOVA with Tukey’s analysis with n = 10/group for b–d. The [] in B demonstrated percentage of mice in each group that bacteria were detectable in blood (bacteremia). *, p < .05; **, p < .01; #, p < .001.
Candida albicans enhanced inflammatory process in DSS model, systemically and locally, but attenuated by Lactobacilli administration
The increased severity of DSS with Candida (DSS+Candida) mice over DSS alone was demonstrated by mortality rate and colon histopathology despite the non-difference in weight loss (Figures 3a–c and Figure S1) and bloody diarrhea (data not shown). In addition, increased serum IL-6 and TNF-α (pro-inflammation) and decreased serum IL-10 (anti-inflammation) was more prominent in DSS+Candida in comparison with DSS alone group (Figure 3d–f). According to previous reports on probiotic treatment in IBD,18,20 we tested a probiotic in Candida administered DSS model. Indeed, Lactobacilli attenuated DSS+Candida model as indicated by reduced mortality rate, less severe colon histology, lower serum pro-inflammatory cytokines and enhanced serum anti-inflammatory cytokine (Figure 3a–f). In addition, severity of local inflammation as determined by intestinal cytokines was also performed. DSS and DSS+Candida induced the elevation of IL-6 (cecum and colon) and TNF-α (ilium, cecum and colon) and reduced IL-10 in all examined intestinal parts (Figure 3g–i). Candida gavage alone in control mice (non DSS, intact gut perm-selectivity) did not induce clinical injury (no weight loss and normal serum cytokine) but activated intestinal cytokines locally (Figure 3g–i) and possibly increased the susceptibility toward other injury factors. With DSS induced gut perm-selectivity defect (DSS+Candida), Candida increased cytokines in both intestine and serum which were attenuated by Lactobacilli administration (Figure 3d–i). Hence, Candida enhanced the severity of DSS model as demonstrated by mortality rate (50% in DSS+Candida versus 0% in DSS alone) (Figure 3a), local inflammation (colon injury score and intestinal cytokines) (Figure 3c,g,h) and systemic inflammation (serum cytokines) (Figure 3d–f).
Figure 3.
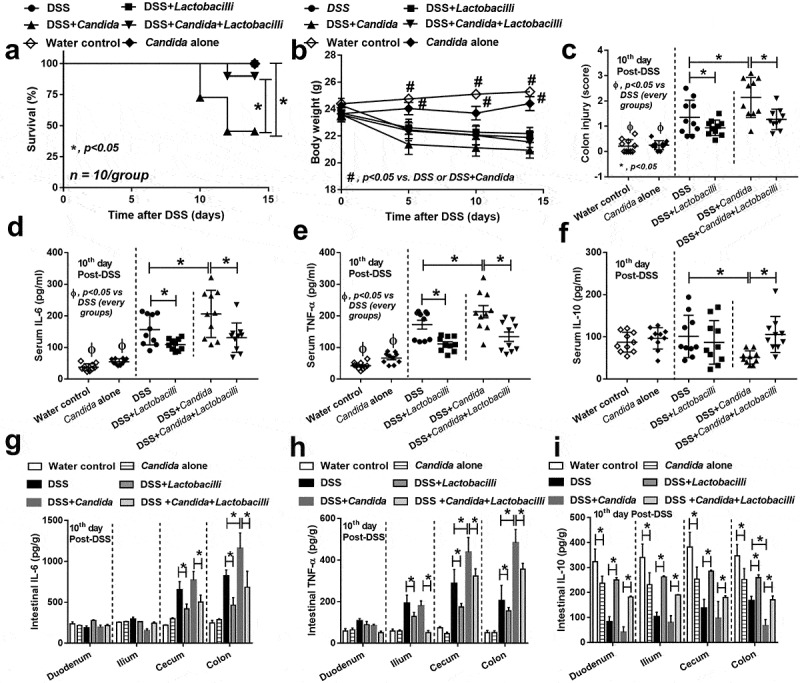
Characteristics of mice in group of drinking water with phosphate buffer solution (PBS) gavage (Control), Candida albicans (drinking water with Candida gavage; Candida alone), 3% dextran sulfate solution as drinking water with PBS gavage (DSS) or DSS plus C. albicans (DSS as drinking water with Candida gavage; DSS+Candida) with and without Lactobacillus rhamnosus L34 administration as determined by survival study (a) (n = 10/group), body weight (b) (n = 10/group initially, decreasing along with the survival study), colon injury score (c), systemic inflammation by serum cytokines (IL-6, TNF-α and IL-10) (d–f) and local inflammation by cytokines in the intestinal samples (g–i) are demonstrated (n = 10/group for c–i). Survival study and multiple group comparisons were determined by Log rank test and ANOVA with Tukey’s analysis, respectively. *, p < .05; #, p < .05 vs. DSS or DSS+Candida; Ф, p < .05 between non-DSS vs. each group with DSS.
Since the administration of Candida in DSS mouse model enhanced inflammation locally (in gut) and systemically (Figure 3), the molecular components of Candida, with and without gut bacteria (mostly Gram negative), might be associated with increased severity of the model. Heat-killed Candida (or BG) together with E. coli (or LPS) did enhance the production of IL-8, an important inflammatory enterocyte cytokine,37 from HT-29 intestinal cell line, in comparison with the individual activation (Figure 4a,b), supporting the additive effect of fungi and bacteria in gut against intestinal cell. However, the additive effect between E. coli and Candida was demonstrated only with a high dose of Candida (1:10) (Figure 4a) suggesting a possible dose-dependent characteristic. Because we previously demonstrated the in vitro anti-inflammation of Lactobacillus culture media (LCM),38 we tested LCM in the current model. While LCM showed only a tendency of IL-8 reduction as stimulated by E. coli with or without Candida (Figure 4c), LCM attenuated IL-8 in HT-29 cell incubated with LPS with or without BG (Figure 4d). Of note, the additive effect of LPS with BG was lost with the addition of culture media (both form control and from Lactobacilli) (Figure 4d) possibly due to the dilution interference against a sensitive dose-dependent characteristic of the additive effect.
Figure 4.
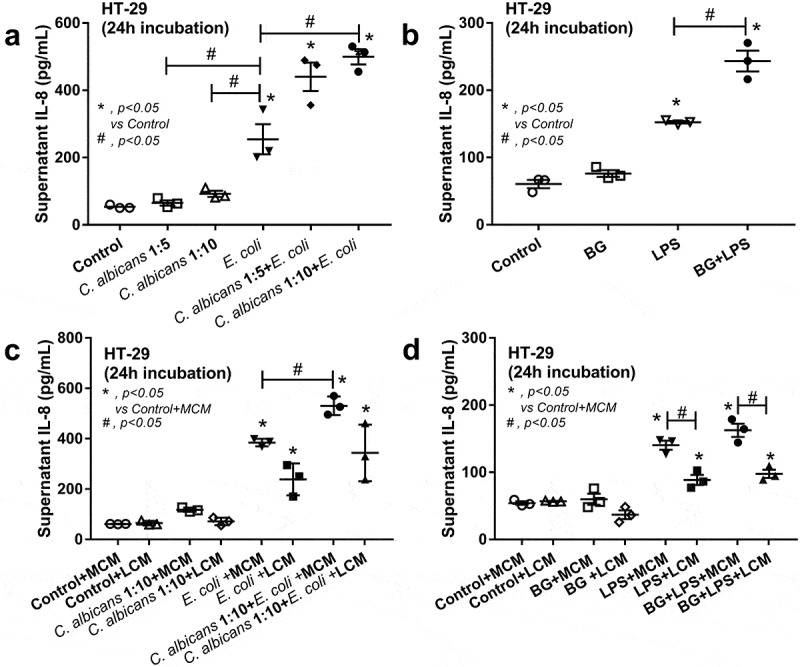
IL-8 cytokine in the supernatant of a human intestinal epithelial cell line (HT-29) after activated by Candida cell lysate at the dose of 1:5 and 1:10 (HT-29 cell: Candida) with and without cell lysate of heat-killed E. coli or E. coli alone (a) and after activated by (1→3)-β-D-glucan (BG) with or without endotoxin (LPS) (b) are demonstrated. Additionally, anti-inflammatory effect of Lactobacillus condition media (LCM) in HT-29 was explored by the co-incubation of LCM, or control McCoy’s 5a modified medium (MCM), into Candida cell lysate at 1:10 (as previously mentioned) with and without cell lysate of heat-killed E. coli (c) or BG with or without LPS (d) (see method). Independent experiments were performed in triplicate. The difference among groups was determined by ANOVA with Tukey’s analysis. *, p < .05 vs. control; #, p < .05.
Candida albicans enhanced gut perm-selectivity defect, bacteremia and gut-dysbiosis, but attenuated by Lactobacilli administration
Leaky-gut as determined by spontaneous elevation in serum of endotoxin and BG, which supported by FITC-dextran assay, was more severe in DSS+Candida mice over DSS alone (Figure 5a–c). In addition, bacterial burdens in blood (bacteremia) of DSS+Candida were also higher than DSS alone supporting the more severe gut-leakage (Figure 5d). The percentage of mice with bacteremia in DSS+Candida was 6 from 10 mice with predominantly Pseudomonas spp. while only 4 from 10 mice in DSS alone showed bacteremia, predominantly from E. coli and Staphyloccocus coagulase negative (Figure 5d,e). Surprisingly, some bacterial colonies were detectable in culture media for fungi (SDA with Chloramphenicol intended for fungal-detection) from blood samples of DSS+Candida (6 in 10 mice) and DSS alone (1 in 10 mice) (Figure 5f) possibly indicating the antibiotic resistant-property of these bacteria. These colonies from blood samples were identified as P. aeruginosa and Enterobacter cloacae by mass-spectrometry analysis in 4 and 2 mice, respectively (Figure 5f).
Figure 5.
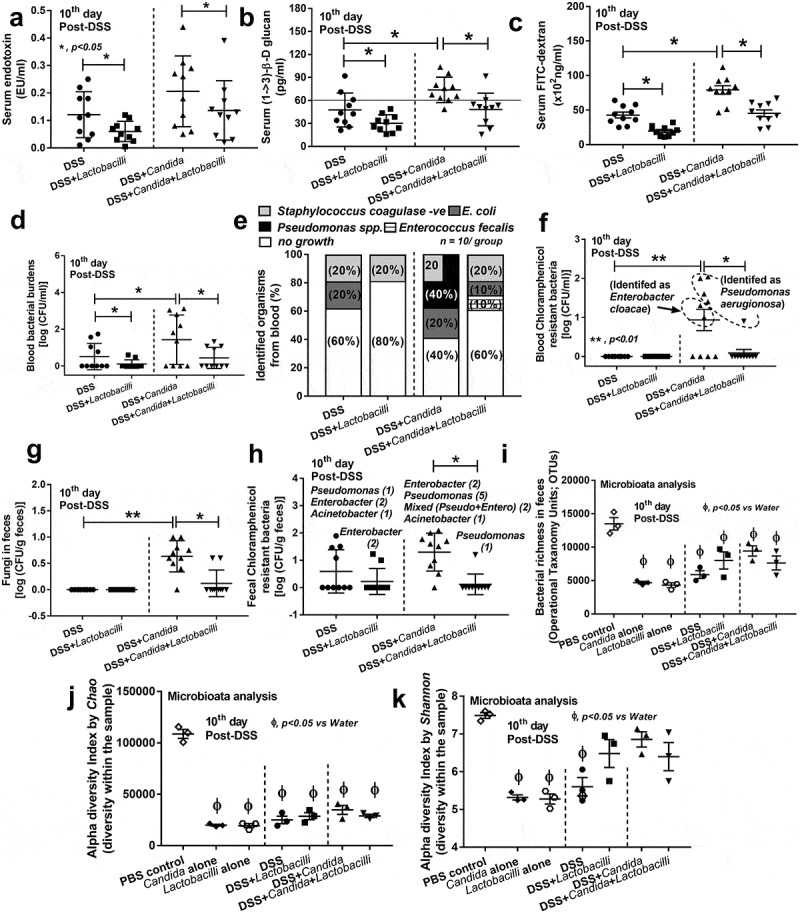
Characteristics of mice in group of drinking water with phosphate buffer solution (PBS) gavage (Control), Candida albicans (drinking water with Candida gavage; Candida alone), 3% dextran sulfate solution as drinking water with PBS gavage (DSS) or DSS plus C. albicans (DSS as drinking water with Candida gavage; DSS+Candida) with and without Lactobacillus rhamnosus L34 administration as determined by gut leakage [serum endotoxin, serum (1→3)-β-D-glucan (BG), Fluorescein isothiocyanate-dextran (FITC-dextran), and spontaneous bacteremia] (a–d), identified organisms from blood samples (e) and blood bacterial colonies isolated from Chloramphenicol in Sabouraud Dextrose Agar (Chloramphenicol resistant bacteria) (f) (circle with dot-line demonstrating the identified bacteria in each group) were demonstrated (n = 10/group for a–f). In (e), percentage (%) of each isolated bacteria was indicated in the stack bar and some stacked bar charts added up to more than 100% due to mixed organisms isolated from the same sample. In addition, fecal analysis as determined by fecal fungi (g) and fecal bacterial colonies isolated from Chloramphenicol in Sabouraud Dextrose Agar (Chloramphenicol resistant bacteria) (h) were demonstrated (n = 10/group for g–h). In (h), the list of identified bacteria are shown above the graph and [] refer to the number of samples that bacteria were isolated. In parallel, fecal bacterial richness (total read of the sequence that classified by Operation Taxanomy Units; OTUs) (i) and alpha diversity index by Chao and Shannon as calculated by Mothur tool (j and k) are shown (n = 3/group that retrieved from 9 mice; see method). Vertical dot-line in a–i separates experiment groups, with and without Candida gavage. The difference among groups was determined by ANOVA with Tukey’s analysis. *, p < .05; **, p < .01.
In parallel, fecal Candida was determined by culture and fecal fungi could be found only in mice with Candida administration (Figure 5g), supporting the relatively low fecal fungal burdens in mice. Then again, some bacterial colonies were demonstrated in fungal culture media (SDA with Chloramphenicol) from fecal samples in 4 mice (in 10) and 9 mice (in 10) of DSS alone and DSS+Candida mice, respectively (Figures 5h and Figure S2). These colonies from fecal samples were identified as Pseudomonas aeruginosa, Pseudomonas putida, Enterobacter cloacae and Acinetobacter baumanii by mass-spectrometry analysis (Figure 5h). It is possible that Candida presentation in mouse feces might enhance the fermentation of some specific fecal bacteria.39 More studies in this topic are worth exploring.
Interestingly, Lactobacilli administration attenuated disease severity in both DSS and DSS+Candida as determined by endotoxemia, glucanemia, leaky-gut, bacteremia, incidence of bacteremia, fecal fungal burdens and possible anti-biotic resistant bacteria in feces (Figure 5a–h). Only 1 in 10 mice of DSS+Candida with Lactobacilli administration showed detectable Candida in feces (Figure 5g,h) with Pseudomonas bacteremia (Figure 5e,f).
Further, fecal microbiota analysis was performed for additional exploration in fecal-dysbiosis. Although limited numbers of samples were used in the microbiome analysis due to a pilot characteristic of the experiment, some differences from this small-scale experiment were demonstrated. For example, bacterial richness at Operational Taxanomy Units (OTUs), which classified groups of closely related individuals sequences, with Mothur method was decreased by all interventions (bacterial or Candida gavage with and without DSS) compared with PBS control (Figure 5i). Also, alpha diversity index (the diversity of bacterial 16s rRNA within the sample) by Chao was lower than control group with all interventions (Figure 5j). Further, alpha diversity index by Shannon (the more strict method for diversity determination than Chao) was also lower than control in Candida alone, Lactobacilli alone and DSS (Figure 5k). These data support the impact of ingested microorganism in the alteration of gut microbiota.40 Additionally, dominant Proteobacteria (examples of bacteria in this group; E. coli, Enterobacter spp. and Pseudomonas spp.) was demonstrated in 2 out of 3 samples in comparison with control feces (PBS) (Figure 6a). Venn diagram at the genus level demonstrated the possible unique bacterial strains in feces of different experimental groups (Figure 6b). To emphasize the impact of Candida in DSS model, the list of bacterial groups and the percent relative abundance of unique OTUs in DSS (49 bacterial species) and DSS+Candida (140 bacterial species) were demonstrated in Supplemental Table 1A and 1B, respectively.
Figure 6.
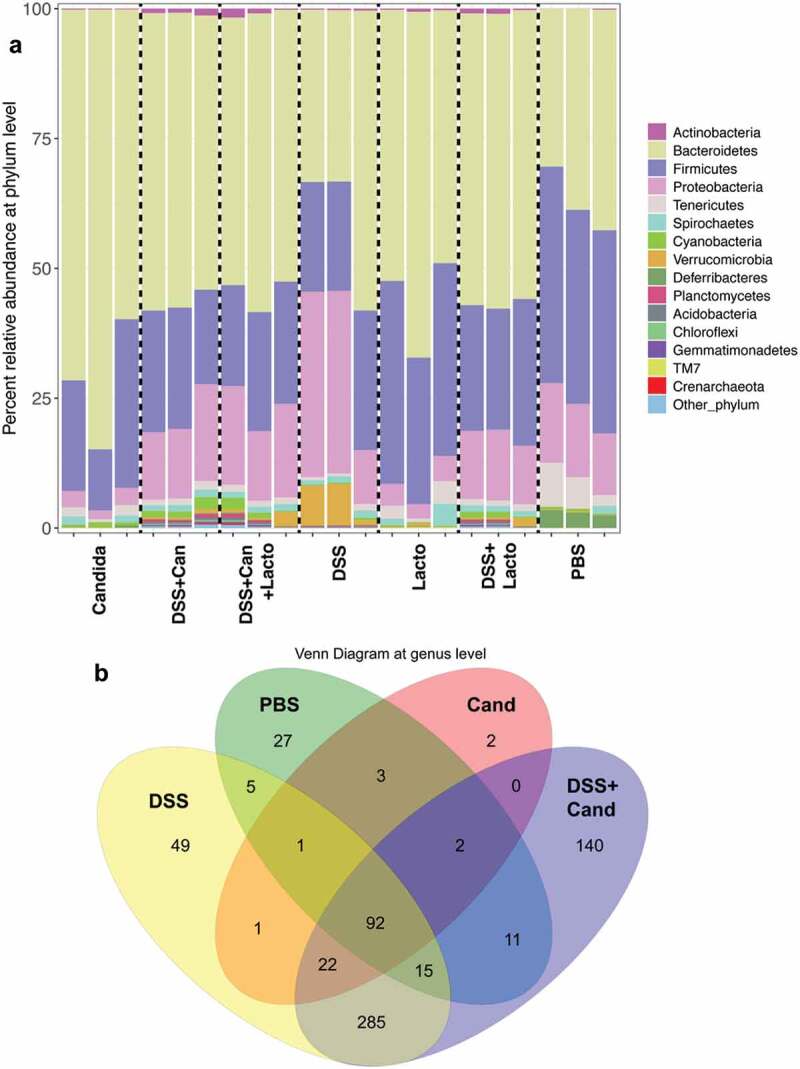
Gut microbiota analysis from feces of mouse administered with Candida albicans alone (Candida), dextran sulfate solution with C. albicans (DSS+Can), DSS+Can with Lactobacilli rhamnosus L34 administration (DSS+Can+Lacto), dextran sulfate solution alone (DSS), DSS with Lactobacilli (DSS+Lacto) and control gavage with phosphate buffer solution (PBS) (n = 3/group that retrieved from 9 mice; see methods) by relative abundance of bacterial diversity at phylum (a) and Venn diagram at genus level (b) and relative abundance at genus (c) are shown (unclassified-phyla were removed). Abbreviation “p”, “c” and “o” means unclassified family sequences in phylum, class and order, respectively, and genera that contain <0.01% relative abundance were removed. Downward and upward arrows demonstrate prominent Gammaproteobacteria in DSS+Candida and Enterobacteriaceae in DSS alone, respectively.
In addition, relative abundances of the bacterial genera demonstrated increased Enterobacteriaceae (Enterobacter spp.) and Gammaproteobacteria (Pseudomonas spp.) in DSS alone model (2 in 3 samples) and in DSS+Candida, respectively (Figure 6c). Lactobacilli administration reduced Enterobacteriaceae and Gammaproteobacteria in DSS and DSS+Candida model (2 in 3 samples), respectively (Figure 6c). Moreover, the non-metric multidimensional scaling function based on bacterial community membership (J class index) showed the difference between non colitis group (control, Candida alone and Lactobacilli alone) (Figure 7 right side) and colitis group (DSS and DSS+Candida with and without Lactobacilli) (Figure 7 left side).
Figure 6.
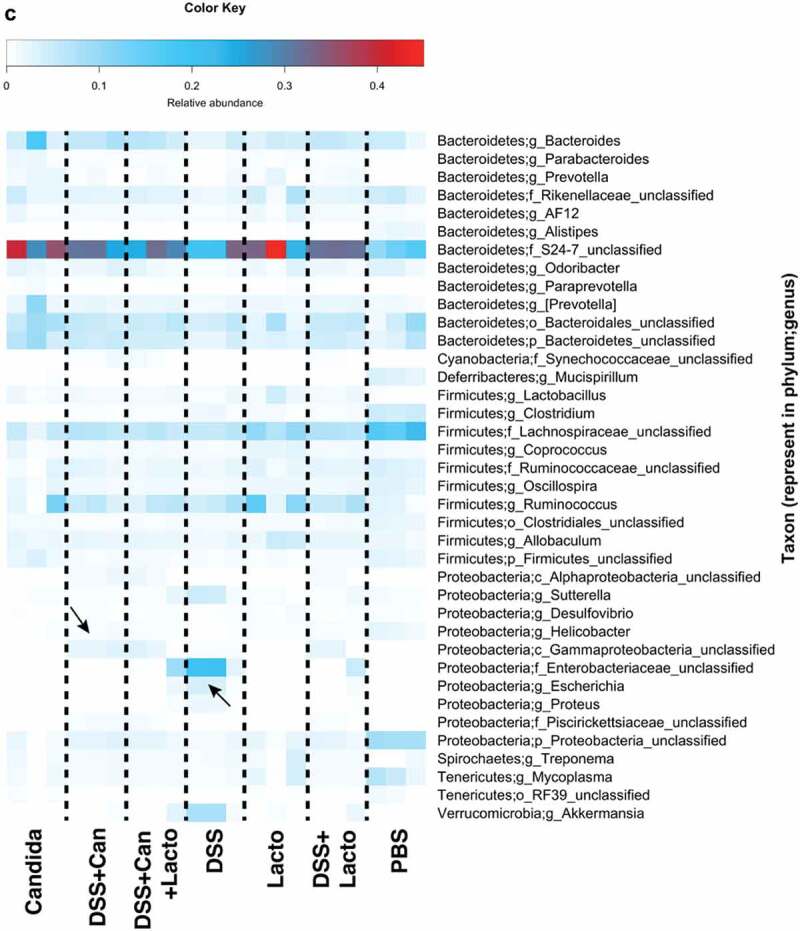
(Continue.)
Figure 7.
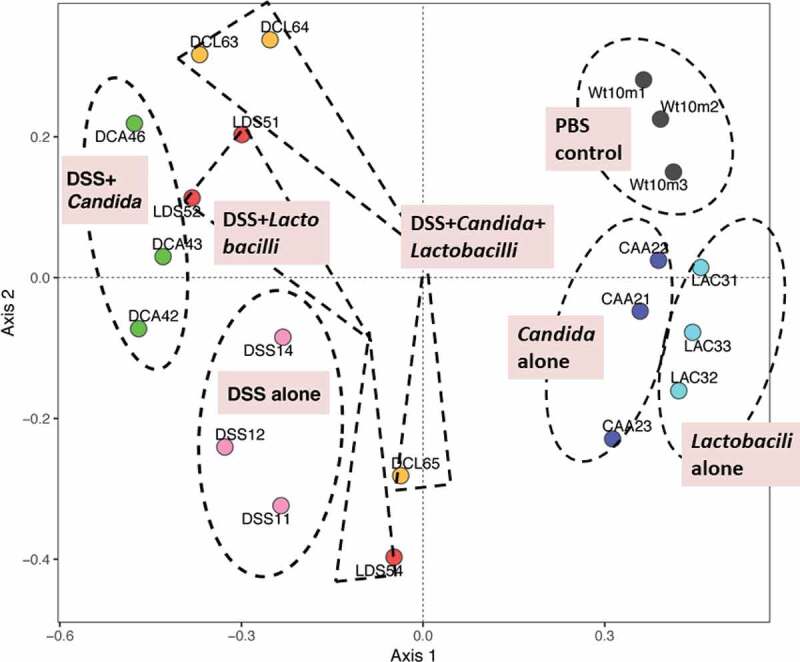
Gut microbiota analysis from feces of mouse administered with Candida albicans alone (Candida), dextran sulfate solution with C. albicans (DSS+Can), DSS+Can with Lactobacilli rhamnosus L34 administration (DSS+Can+Lacto), dextran sulfate solution alone (DSS), DSS with Lactobacilli (DSS+Lacto) and control gavage with phosphate buffer solution (PBS) (n = 3/group that retrieved from 9 mice; see method) by non-metric multidimensional scaling function (the difference among experimental groups) based on J class index (community membership) are demonstrated (the dotted line indicating a possible area of similarity within each group; these postulation lines were not derived from the calculation). CAA, Candida alone; CTL (Wt10m3), Control (PBS); DCA, DSS+Candida; DCL, DSS+Candida+Lactobacilli; DSS, DSS alone; LAC, Lactobacilli administration alone; LDS, DSS+Lactobacilli.
Discussion
Despite leaky-gut in patients with clinically active IBD as a result of spontaneous elevation of endotoxin and BG (a polyglucoside component of fungal cell wall)41 in serum,12,42 leaky-gut in clinically inactive IBD has never been reported. We demonstrated endotoxemia and glucanemia in patients with IBD and endoscopic active characteristics during clinical remission. Although Candida induced gut-dysbiosis in gastrointestinal tract inflammation is well-known,5,16,26,43 a representative mouse model of IBD (DSS-induced colitis) might not well mimic patients due to the less abundance of Candida in mouse gut than in humans.36
The influence of Candida in DSS mouse model
Indeed, DSS with Candida gavage (DSS+Candida) demonstrated more severe leaky-gut and higher serum level of LPS and BG with increased mortality in comparison with DSS alone. Candida in DSS model also locally worsened colon histopathology, enhanced inflammatory cytokines locally in intestinal tissue and systemically in serum. Interestingly, reduced intestinal IL-10 (an anti-inflammatory cytokine), without the elevation of pro-inflammatory cytokines, was demonstrated from duodenum to colon even with Candida administration alone. Also, there was non-diarrhea and no weight-loss in mice with Candida gavage without DSS, suggesting the effective immune responses against the administered Candida.
In addition, Candida gavage alone (without DSS) also increased gut bacterial-burdens (gut dysbiosis) (Figure 5h) and altered gut microbiota despite non-diarrhea in these mice. Moreover, fecal microbiota analysis demonstrated increased Bacteroides bacteria (Figure 6a) even with Candida gavage alone. Bacteroides administration induced colitis in susceptible mice has been reported.44 Hence, the presentation of gut Candida might increase susceptibility toward gut inflammation through the reduction of intestinal IL-10 together with increased pathobionts. Further, with an additional DSS in Candida gavage, Proteobacteria (pathogenic Gram-negative bacteria) increased in comparison with Candida alone in 2 out of 3 samples supporting a possibility that gut inflammation induced the more gut dysbiosis.45 Additionally, emergence of Gammaproteobacteria (Figure 6c) which were mostly Chloramphenicol-resistant Pseudomonas aeruginosa (detectable by Chloramphenicol-SDA media) (Figure 5h) in DSS+Candida implies the relationship between C. albicans and P. aeruginosa in this model. Likewise, the co-operation between C. albicans and P. aeruginosa induced mucosal injury is reported in a zebrafish model.46 To understand more about these relationships, further studies are warranted.
IBD activity associated with systemic inflammation (as determined by ESR) is well-known.47 Here, we demonstrated enhanced systemic inflammation (serum cytokines) in DSS+Candida over DSS alone supporting the influence of gut Candida in enhancing systemic inflammation. It is possible that the systemic inflammation in IBD is, at least in part, due to the systemic activation of BG and LPS from gut translocation as the synergistic effect of these molecules on macrophages are demonstrated.7,8,48,49 Hence, C. albicans administration in DSS model enhanced local gut inflammation, induced gut dysbiosis and possibly increased systemic inflammation.
Probiotic treatment in DSS model with Candida administration
Although DSS+Candida was more severe than DSS alone, Lactobacillus rhamnosus L34 could attenuate model severity as determined by survival study, local and systemic cytokines. In addition, Lactobacilli attenuated leaky-gut in both DSS and DSS+Candida model as evaluated by FITC-dextran, endotoxemia, serum BG and blood bacterial burdens. Interestingly, Lactobacilli decreased both gut fungi and pathogenic bacteria, reduced Pseudomonas bacteremia and attenuated antibiotic-resistant bacteria in DSS+Candida possibly through the attenuation of gut dysbiosis. Indeed, the attenuation on gut dysbiosis was also demonstrated with microbiota analysis as there was a decrease in Proteobacteria, especially Enterobactereiaceae, in DSS+Lactobacilli in comparison with DSS alone (Figure 6a,c). The similarity between non-colitis mice of control, Candida gavage and Lactobacilli administration were also observed.
Translation and future directions
The difference between DSS+Candida and DSS mouse model was demonstrated in several aspects, supporting the influence of gut Candida in IBD. Due to the association between fecal Candida and serum BG level,7,8 clinical utilization of serum BG in IBD is interesting. While ESR associates with systemic inflammation, serum BG might be more specific to gut lesions (leaky-gut) and gut dysbiosis (fungal burdens). Hence, the evaluation and reduction of fecal fungal burdens might be beneficial in patients with IBD. More studies are warranted to better understand the benefits of serum BG in IBD.
Conclusion
Candida enhances DSS colitis severity through the alteration of gut bacteria (dysbiosis) and additive inflammatory induction against intestinal cells which enhances gut translocation of pathogen molecules and systemic inflammatory responses. In translation, probiotic treatment and determination on gut fungal burdens and/or gut leakage as new biomarkers or novel treatment strategies against IBD should be studied further.
Supplementary Material
Acknowledgments
AL worked at the Translational Research in Inflammation and Immunology Research Unit (TRIRU), Department of Microbiology, Chulalongkorn University, Bangkok, Thailand.
Funding Statement
This study was supported by Thailand Government Fund (RSA-6080023), Thailand Research Fund (RES_61_202_30_022) and Ratchadaphiseksomphot Endowment Fund 2017 (76001-HR), Chulalongkorn University. WP was supported by Rachadapisek Sompote Fund for Postdoctoral Fellowship, Chulalongkorn University.
Supplementary Material
Supplementary data for this article can be accessed publisher’s website.
References
- 1.Kaser A, Blumberg RS.. The road to Crohn’s disease. Science. 2017;357:976–977. doi: 10.1126/science.aao4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 3.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53:1–4. doi: 10.1136/gut.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoarau G, Mukherjee PK, Gower-Rousseau C, Hager C, Chandra J, Retuerto MA, Neut C, Vermeire S, Clemente J, Colombel JF, et al. Bacteriome and mycobiome interactions underscore microbial dysbiosis in familial Crohn’s disease. MBio. 2016;7:e01250-16. doi: 10.1128/mBio.01250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerard R, Sendid B, Colombel JF, Poulain D, Jouault T. An immunological link between Candida albicans colonization and Crohn’s disease. Crit Rev Microbiol. 2015;41:135–139. doi: 10.3109/1040841X.2013.810587. [DOI] [PubMed] [Google Scholar]
- 7.Panpetch W, Somboonna N, Bulan DE, Issara-Amphorn J, Finkelman M, Worasilchai N, Chindamporn A, Palaga T, Tumwasorn S, Leelahavanichkul A, et al. Oral administration of live- or heat-killed Candida albicans worsened cecal ligation and puncture sepsis in a murine model possibly due to an increased serum (1–>3)-beta-D-glucan. PLoS One. 2017;12:e0181439. doi: 10.1371/journal.pone.0181439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panpetch W, Somboonna N, Bulan DE, Issara-Amphorn J, Worasilchai N, Finkelman M, Chindamporn A, Palaga T, Tumwasorn S, Leelahavanichkul A. Gastrointestinal colonization of Candida albicans increases serum (1–>3)-beta-D-Glucan, without Candidemia, and worsens cecal ligation and puncture sepsis in murine model. Shock. 2017;49:62–70. doi: 10.1097/SHK.0000000000000896. [DOI] [PubMed] [Google Scholar]
- 9.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54 e42. quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Kreuzpaintner G, Horstkotte D, Heyll A, Losse B, Strohmeyer G. Increased risk of bacterial endocarditis in inflammatory bowel disease. Am J Med. 1992;92:391–395. doi: 10.1016/0002-9343(92)90269-h. [DOI] [PubMed] [Google Scholar]
- 11.Vaishnavi C. Translocation of gut flora and its role in sepsis. Indian J Med Microbiol. 2013;31:334–342. doi: 10.4103/0255-0857.118870. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Zhou G, He C, Yang W, He Z, Liu Z. Serum levels of lipopolysaccharide and 1,3-beta-D-Glucan refer to the severity in patients with Crohn’s disease. Mediators Inflamm. 2015;2015:843089. doi: 10.1155/2015/125380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Issara-Amphorn J, Surawut S, Worasilchai N, Thim-Uam A, Finkelman M, Chindamporn A, Palaga T, Hirankarn N, Pisitkun P, Leelahavanichkul A. The Synergy of Endotoxin and (1–>3)-beta-D-Glucan, from gut translocation, worsens sepsis severity in a lupus model of Fc gamma receptor IIb-deficient mice. J Innate Immun. 2018;10:189–201. doi: 10.1159/000486321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michielan A, D’Inca R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015;2015:628157. doi: 10.1155/2015/125380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leelahavanichkul A, Worasilchai N, Wannalerdsakun S, Jutivorakool K, Somparn P, Issara-Amphorn J, Tachaboon S, Srisawat N, Finkelman M, Chindamporn A. Gastrointestinal leakage detected by serum (1–>3)-beta-D-Glucan in mouse models and a pilot study in patients with sepsis. Shock. 2016;46:506–518. doi: 10.1097/SHK.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 16.Kumamoto CA. Inflammation and gastrointestinal Candida colonization. Curr Opin Microbiol. 2011;14:386–391. doi: 10.1016/j.mib.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strijbis K, Yilmaz OH, Dougan SK, Esteban A, Grone A, Kumamoto CA, Ploegh HL, Andes DR. Intestinal colonization by Candida albicans alters inflammatory responses in Bruton’s tyrosine kinase-deficient mice. PLoS One. 2014;9:e112472. doi: 10.1371/journal.pone.0112472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boskoski I, Bruno G, Petito V, Laterza L, Cammarota G, et al. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. Biomed Res Int. 2013;2013:435268. doi: 10.1155/2013/435268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hager CL, Ghannoum MA. The mycobiome: role in health and disease, and as a potential probiotic target in gastrointestinal disease. Dig Liver Dis. 2017;49:1171–1176. doi: 10.1016/j.dld.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol. 2018;9:2247. doi: 10.3389/fmicb.2018.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy MJ, Volz PA. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985;49:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21:334–341. doi: 10.1016/j.tim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boonma P, Spinler JK, Venable SF, Versalovic J, Tumwasorn S. Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells. BMC Microbiol. 2014;14:177. doi: 10.1186/1471-2180-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leelahavanichkul A, Panpetch W, Worasilchai N, Somparn P, Chancharoenthana W, Nilgate S, Finkelman M, Chindamporn A, Tumwasorn S, Flock J-I. Evaluation of gastrointestinal leakage using serum (1 -> 3)-beta-D-glucan in a Clostridium difficile murine model. FEMS Microbiol Lett. 2016;363. doi: 10.1093/femsle/fnw204. [DOI] [PubMed] [Google Scholar]
- 25.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Heinsbroek SE, Williams DL, Welting O, Meijer SL, Gordon S, de Jonge WJ. Orally delivered beta-glucans aggravate dextran sulfate sodium (DSS)-induced intestinal inflammation. Nutr Res. 2015;35:1106–1112. doi: 10.1016/j.nutres.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XQ, Zhu YH, Zhang HF, Yue Y, Cai ZX, Lu QP, Zhang L, Weng X-G, Zhang F-J, Zhou D, et al. Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhoea: intestinal microbiota and immune imbalances. PLoS One. 2012;7:e40666. doi: 10.1371/journal.pone.0040666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen K, Li G, Bui T, Liu F, Li Y, Kocher J, Lin L, Yang X, Yuan L. High dose and low dose Lactobacillus acidophilus exerted differential immune modulating effects on T cell immune responses induced by an oral human rotavirus vaccine in gnotobiotic pigs. Vaccine. 2012;30:1198–1207. doi: 10.1016/j.vaccine.2011.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erben U, Loddenkemper C, Doerfel K, Spieckermann S, Haller D, Heimesaat MM, Zeitz M, Siegmund B, Kühl AA. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol. 2014;7:4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 31.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huse SM, Welch DM, Morrison HG, Sogin ML. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol. 2010;12:1889–1898. doi: 10.1111/j.1462-2920.2010.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. Isme J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung SP, Kang H. Assessment of microbial diversity bias associated with soil heterogeneity and sequencing resolution in pyrosequencing analyses. J Microbiol. 2014;52:574–580. doi: 10.1007/s12275-014-3636-9. [DOI] [PubMed] [Google Scholar]
- 36.Koh AY. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryot Cell. 2013;12:1416–1422. doi: 10.1128/EC.00196-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. [DOI] [PubMed] [Google Scholar]
- 38.Panpetch W, Chancharoenthana W, Bootdee K, Nilgate S, Finkelman M, Tumwasorn S, Leelahavanichkul A. Lactobacillus rhamnosus L34 attenuates gut translocation-induced bacterial sepsis in murine models of leaky gut. Infect Immun. 2018;86:86: e00700-17. doi: 10.1128/IAI.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiengrach P, Panpetch W, Worasilchai N, Chindamporn A, Tumwasorn S, Jaroonwitchawan T, Somboonna N, Wilantho A, Chathanathon P, Leelahavanichkul A. Administration of Candida albicans to dextran sulfate solution treated mice causes intestinal dysbiosis, emergence and dissemination of intestinal Pseudomonas aeruginosa and lethal sepsis. Shock. 2019;1. doi: 10.1097/SHK.0000000000001339. [DOI] [PubMed] [Google Scholar]
- 40.Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7. doi: 10.3390/microorganisms7080214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zekovic DB, Kwiatkowski S, Vrvic MM, Jakovljevic D, Moran CA. Natural and modified (1–>3)-beta-D-glucans in health promotion and disease alleviation. Crit Rev Biotechnol. 2005;25:205–230. doi: 10.1080/07388550500376166. [DOI] [PubMed] [Google Scholar]
- 42.Gardiner KR, Halliday MI, Barclay GR, Milne L, Brown D, Stephens S, Maxwell RJ, Rowlands BJ. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jawhara S, Thuru X, Standaert-Vitse A, Jouault T, Mordon S, Sendid B, Desreumaux P, Poulain D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J Infect Dis. 2008;197:972–980. doi: 10.1086/528990. [DOI] [PubMed] [Google Scholar]
- 44.Bloom SM, Bijanki VN, Nava GM, Sun L, Malvin NP, Donermeyer DL, Dunne WM, Allen PM, Stappenbeck TS. Commensal bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res. 2016;14:127–138. doi: 10.5217/ir.2016.14.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergeron AC, Seman BG, Hammond JH, Archambault LS, Hogan DA, Wheeler RT. Candida albicans and Pseudomonas aeruginosa interact to enhance virulence of mucosal infection in transparent zebrafish. Infect Immun. 2017;85. doi: 10.1128/IAI.00475-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iskandar HN, Ciorba MA. Biomarkers in inflammatory bowel disease: current practices and recent advances. Transl Res. 2012;159:313–325. doi: 10.1016/j.trsl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 49.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


