Abstract
PURPOSE
Risk-stratified therapy, which modifies treatment on the basis of clinical and biologic features, has improved 5-year overall survival of childhood acute lymphoblastic leukemia (ALL) to 90%, but its impact on long-term toxicity remains unknown.
METHODS
We assessed all-cause and health-related late mortality (including late effects of cancer therapy), subsequent malignant neoplasms (SMNs), chronic health conditions, and neurocognitive outcomes among 6,148 survivors of childhood ALL (median age, 27.9 years; range, 5.9-61.9 years) diagnosed between 1970 and 1999. Therapy combinations and treatment intensity defined 6 groups: 1970s-like (70s), standard- or high-risk 1980s-like (80sSR, 80sHR) and 1990s-like (90sSR, 90sHR), and relapse/transplantation (R/BMT). Cumulative incidence, standardized mortality ratios, and standardized incidence ratios were compared between treatment groups and with the US population.
RESULTS
Overall, 20-year all-cause late mortality was 6.6% (95% CI, 6.0 to 7.1). Compared with 70s, 90sSR and 90sHR experienced lower health-related late mortality (rate ratio [95% CI]: 90sSR, 0.2 [0.1 to 0.4]; 90sHR, 0.3 [0.1 to 0.7]), comparable to the US population (standardized mortality ratio [95% CI]: 90sSR, 1.3 [0.8 to 2.0]; 90sHR, 1.7 [0.7 to 3.5]). Compared with 70s, 90sSR had a lower rate of SMN (rate ratio [95% CI], 0.3 [0.1 to 0.6]) that was not different from that of the US population (standardized incidence ratio [95% CI], 1.0 [0.6 to 1.6]). The 90sSR group had fewer severe chronic health conditions than the 70s (20-year cumulative incidence [95% CI], 11.0% [9.7% to 12.3%] v 22.5% [19.4% to 25.5%]) and a lower prevalence of impaired memory (prevalence ratio [95% CI], 0.7 [0.6 to 0.9]) and task efficiency (0.5 [0.4 to 0.7]).
CONCLUSION
Risk-stratified therapy has reduced late morbidity and mortality among contemporary survivors of standard-risk ALL, represented by 90sSR. Health-related late mortality and SMN risks among 5-year survivors of contemporary, standard-risk childhood ALL are comparable to the general population.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy, accounting for 20% of all cancers before 20 years of age.1 Risk-stratified treatment, based on clinical and biologic risk factors as well as treatment response, has improved 5-year overall survival from only 10% in the 1960s to 90% in the 2000s.2-9 With the steady improvement in outcome, risk-stratified treatment has been designed not only to improve cure rates but also to minimize long-term sequelae, especially for standard-risk patients who constitute > 50% of patients with childhood ALL.3-8,10,11 To achieve this aim, there has been a reduction in anthracycline chemotherapy dose and the use of prophylactic cranial radiation therapy (CRT) over time, with a concurrent increase in the use of asparaginase, dexamethasone, and high-dose methotrexate.5,8,12-15
CONTEXT
Key Objective
We aimed to determine if risk stratification of therapy has modified patterns in late-health outcomes among acute lymphoblastic leukemia (ALL) survivors.
Knowledge Generated
Compared with survivors treated with therapy representative of the 1970s, those treated with therapy similar to contemporary standard-risk ALL regimens had decreased rates of health-related late mortality (includes death due to late effects of cancer therapy), subsequent malignancy, and many chronic health conditions. These rates of subsequent malignancy and health-related late mortality were similar to the US population. Survivors treated with therapy similar to high-risk regimens of the 1990s had an increase in specific chronic health conditions (diabetes, joint replacement).
Relevance
These findings can inform counseling of survivors and their families and demonstrate that although risk-stratified therapy has improved outcomes for standard-risk patients, there is still a need to decrease late complications of therapy, particularly among high-risk patients.
Long-term survivors of childhood ALL are at increased risk for late (> 5 years since diagnosis) mortality and excess morbidity as a result of their cancer treatment.16-21 However, there is limited information about late outcomes of survivors who received contemporary risk-stratified therapy. Recognition of late health outcomes for risk-stratified groups would inform counseling of newly diagnosed patients and survivors regarding expected long-term effects of therapy. Using the largest cohort of long-term survivors of childhood ALL in North America, we aimed to provide a comprehensive description of long-term outcomes for survivors diagnosed across 3 decades and determine whether risk-stratified therapy has improved late outcomes among survivors of standard-risk ALL treated on contemporary protocols. Furthermore, we aimed to identify changes in late morbidity and mortality for survivors treated with therapy for high-risk ALL, relapsed ALL, or hematopoietic cell transplantation.
METHODS
Study Population
The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort with longitudinal follow-up of survivors of childhood cancer treated at 31 institutions in North America.22 Eligibility included diagnosis of cancer, including ALL, before age 21 years, between January 1, 1970 and December 31, 1999, and alive at 5 years after diagnosis of a childhood cancer. The closest-age siblings of a random sample of survivors were recruited to serve as a comparison population. The CCSS was approved by institutional review boards at participating centers. Participants or their proxies provided informed consent. A detailed description of the cohort methodology and study design have been published previously.23
Cancer Treatment Information and Risk-Stratified Treatment Groups
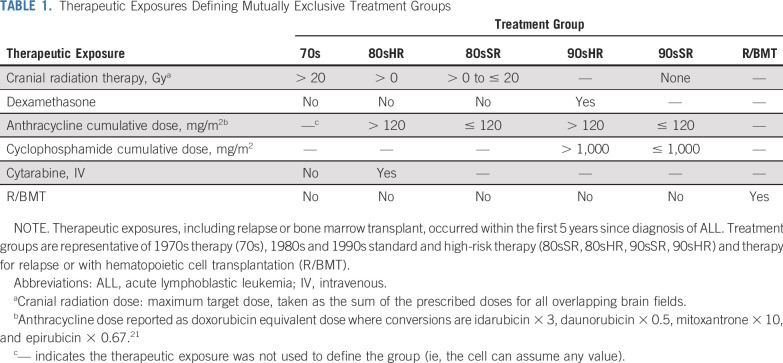
For survivors of childhood ALL who provided consent, cancer diagnosis and treatment data, including chemotherapy cumulative doses and body region–specific radiation dosimetry, were abstracted from medical records by treating institutions.24,25 CRT maximum target dose was determined by summing the prescribed dose to all overlapping treatment fields in the brain.26 Anthracycline drug doses were standardized as doxorubicin equivalents.27 We generated clinically meaningful and mutually exclusive treatment groups representative of risk-stratified therapy across different eras using multimodal therapy exposures and dose cut-points (Table 1) representative of 1970s therapy (70s), 1980s and 1990s standard- and high-risk therapy (80sSR, 80sHR, 90sSR, 90sHR), and therapy for relapse or with hematopoietic cell transplantation, regardless of era (R/BMT; Data Supplement). Survivors in the 90sSR group received therapy similar to contemporary (2005-2018) standard-risk ALL protocols of the Children’s Oncology Group (COG),10 Dana-Farber Cancer Institute Consortium,3,28,29 or St Jude Children’s Research Hospital,30 including no CRT, 0-1,000 mg/m2 cyclophosphamide, and 0-120 mg/m2 anthracycline.27,31 Nearly all patients with standard-risk ALL in North America are treated with one of these regimens.
TABLE 1.
Therapeutic Exposures Defining Mutually Exclusive Treatment Groups
Outcome Measures
Date and cause of death through December 2017 were obtained through linkage with the National Death Index and classified using the International Classification of Disease into 3 mutually exclusive categories: (1) recurrence or progression of ALL, (2) other health-related late mortality (subsequent neoplasms, cardiac, pulmonary, and other deaths due to a medical condition including late effects of cancer therapy), and (3) external causes (including accidents, suicides, and other external causes).
Subsequent neoplasms (SNs) were identified by self or proxy report or death certificate and classified as: (1) malignant neoplasms (SMNs), (2) nonmelanoma skin cancers (NMSC), and (3) benign meningiomas.18 Diagnoses were confirmed by pathology report or, when unavailable, death certificate, medical records, or both.
Ages at first occurrence of chronic health conditions were self-reported using a multi-item, organ system–based questionnaire. Conditions were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (v4.03) as mild (grade 1), moderate (grade 2), severe or disabling (grade 3), life-threatening (grade 4), or fatal (grade 5).32
Neurocognitive outcomes were measured using the CCSS Neurocognitive Questionnaire, previously validated to assess attention and processing speed (task efficiency), emotional regulation, organization, and memory.33 Impairment was defined using previously described methods.34-36
Sociodemographic outcomes including marital/relationship status, health insurance, employment, household income, and educational attainment were considered as indicators of psychosocial functioning among survivors ≥ 25 years of age.21
Statistical Analysis
Cumulative incidence of cause-specific death, SNs, and chronic health conditions were estimated, stratified by treatment group. Health conditions, including SNs, occurring before study entry at 5 years since cancer diagnosis were included as prevalent cases at cohort entry. Standardized mortality ratios (SMRs) were estimated for all-cause and cause-specific mortality compared with the age-, sex-, race-, and calendar year–matched US population.37 Standardized incidence ratios (SIRs) and absolute excess risk per 1,000 person-years were calculated for SMNs compared with those of the age-, sex-, race-, and year-matched US population using incidence rates from the SEER program. Follow-up time ended at the earlier of the date of death or December 31, 2017 for mortality; the earliest of an SN after 5 years since diagnosis, death, or last questionnaire for SNs; and the earliest of a condition meeting the grade of interest, death, or last questionnaire for chronic health conditions. The prevalence of neurocognitive impairment and sociodemographic outcomes were estimated overall and by treatment group. SMRs and SIRs were estimated overall and stratifying on 10-year intervals since diagnosis.
Multivariable piecewise-exponential models for rates of time-to-event outcomes and multivariable log-binomial models for prevalence of cross-sectional outcomes were used to estimate associations of treatment group, as well as specific therapeutic exposures, adjusted for age at diagnosis, attained age, sex, and race, and are reported as rate ratios (RRs) and prevalence ratios (PRs), respectively, with 95% CIs. To account for the undersampling of survivors of ALL diagnosed in 1987-1999, all CCSS analyses use sampling weights through inverse probability weighting. Additional description of outcome measures and statistical methods are available in the Data Supplement.
RESULTS
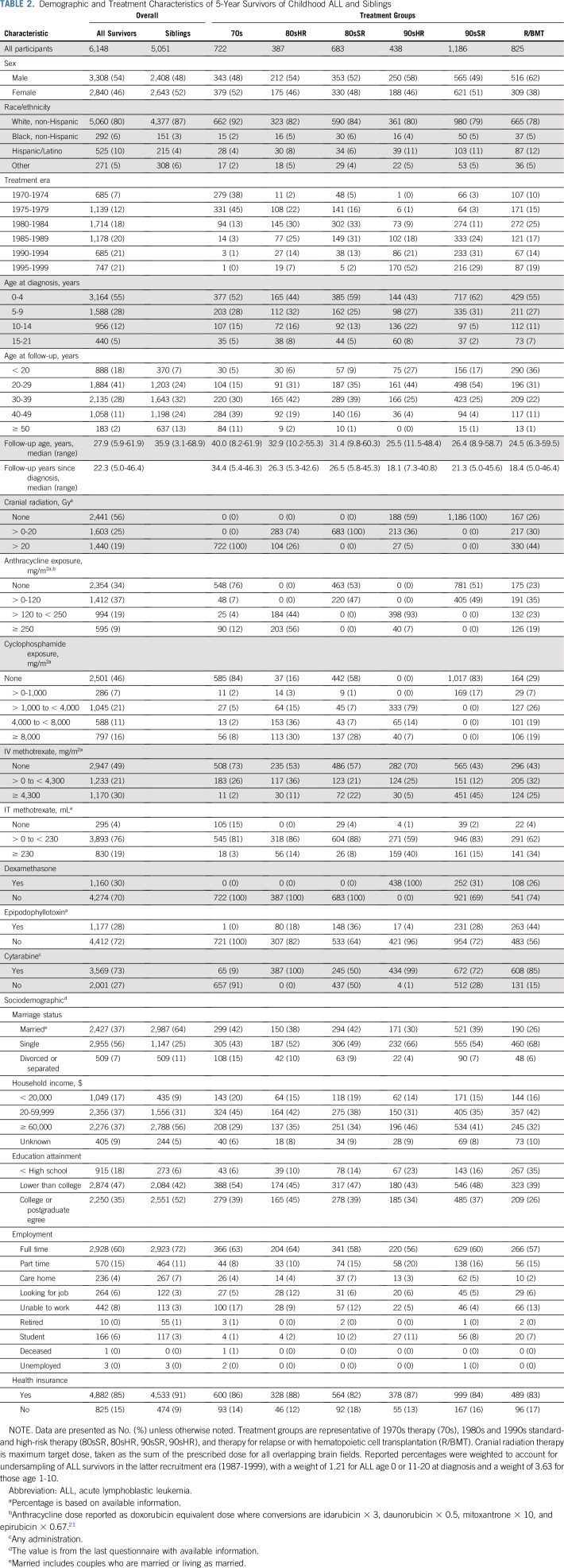
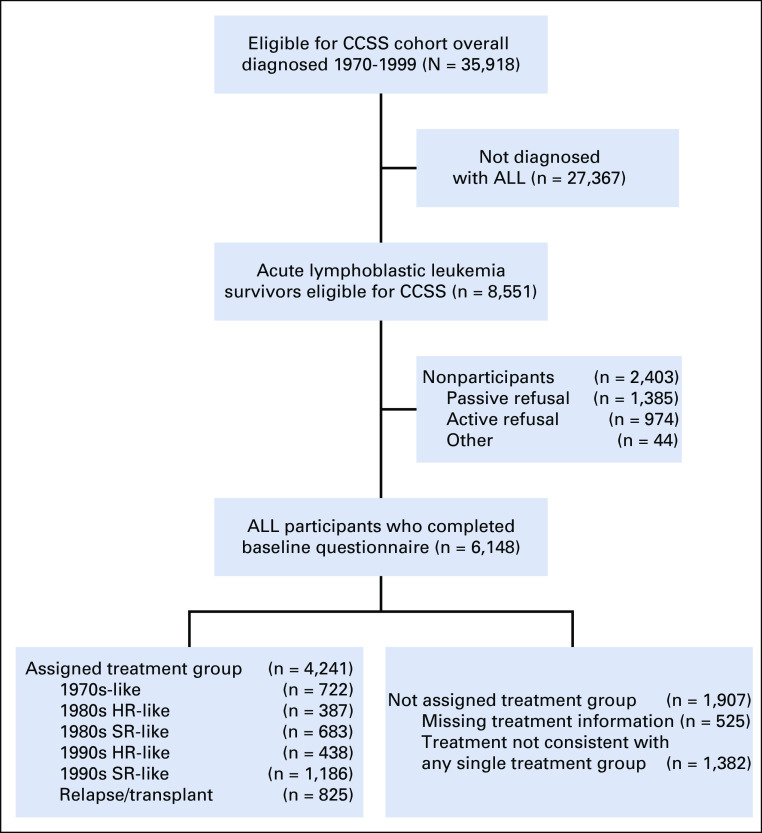
Among 6,148 survivors and 5,051 siblings, the median age at last follow-up or death was 27.9 years (range, 5.9-61.9 years) and 35.9 (3.1-68.9 years), respectively (Table 2). Of the 5,623 survivors with complete treatment information, 4,241 (75.4%) could be assigned to one of the predefined treatment groups (Appendix Figure A1, online only). Treatment group assignment was concordant with year of diagnosis (Table 2; Data Supplement). Most 90sHR received no CRT, whereas all 80sHR and 70s received CRT. Although all high-risk survivors received > 120 mg/m2 anthracycline, 90sHR received a lower cumulative dose (7% received ≥ 250 mg/m2) compared with 80sHR (56% received ≥ 250 mg/m2). A larger proportion of 90sSR received high-dose methotrexate (45%) compared with all other treatment groups (< 25%).
TABLE 2.
Demographic and Treatment Characteristics of 5-Year Survivors of Childhood ALL and Siblings
Late Mortality
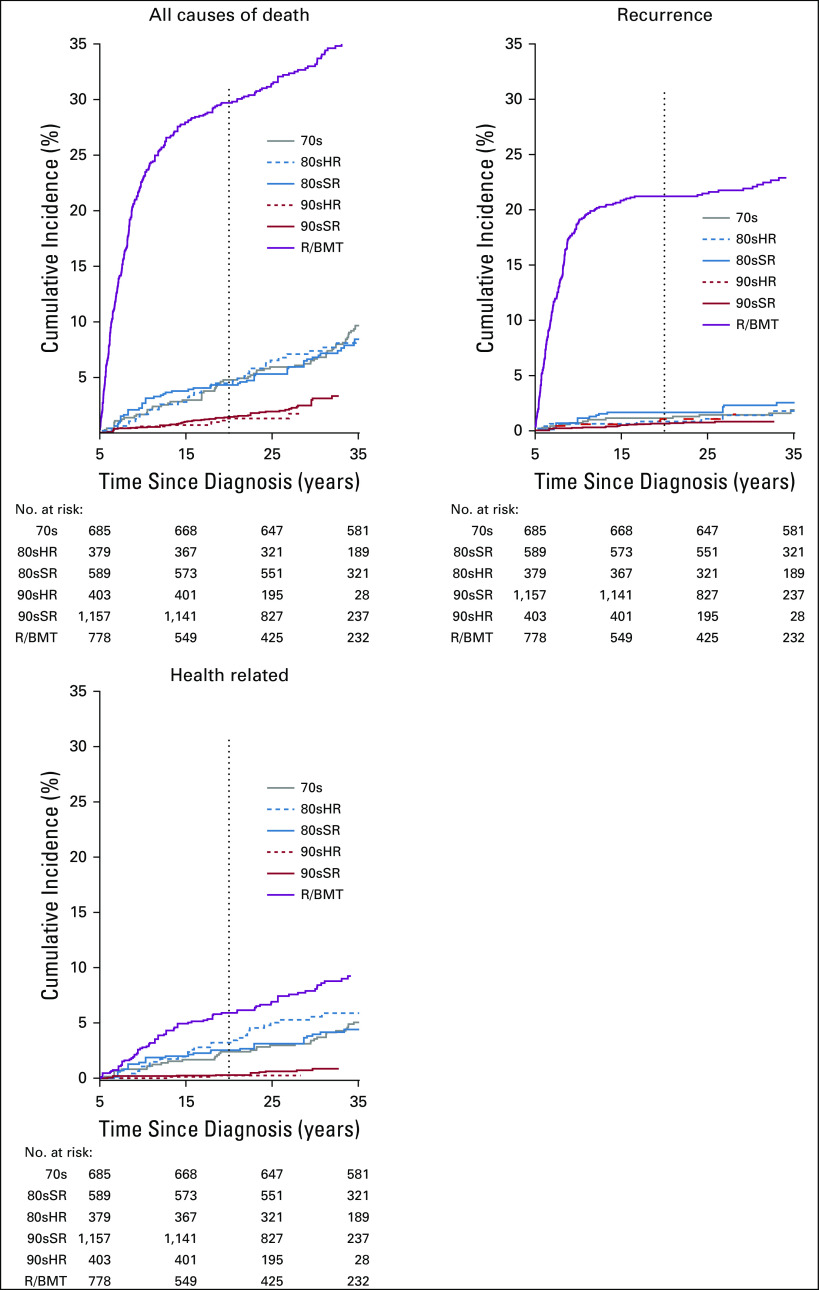
At 20 years since diagnosis, the cumulative incidence of all-cause late mortality was 6.6% overall (95% CI, 6.0 to 7.1; Fig 1; Data Supplement). Health-related late mortality was 2.0% (1.7% to 2.3%) overall and was similar for 70s (2.4% [1.3% to 3.5%]), 80sSR (2.5% [1.5% to 3.6%]), and 80sHR (3.2% [1.6% to 4.8%]) groups. Compared with 70s, the cumulative incidence of health-related late mortality was lower for 90sSR (0.3% [0.1% to 0.6%]) and 90sHR (0.6% [0.0% to 1.1%]) and higher for R/BMT (5.6% [4.2% to 6.9%]). Notably, with the exception of R/BMT, the 20-year cumulative mortality due to disease recurrence/progression was < 2% among all treatment groups and lowest among 90sSR (0.6% [0.3% to 0.9%]). External causes of death included 10 suicides among 70s (n = 3), 90sSR (n = 1), and participants not in a treatment group (n = 6).
FIG 1.
Cumulative incidence of late mortality as (A) all-cause, (B) recurrence, and (C) health-related late mortality among 5-year survivors of childhood acute lymphoblastic leukemia by treatment group. 70s, 1970s therapy; 80sHR, 1980s high-risk therapy; 80sSR, 1980s standard-risk therapy; 90sHR, 1990s high-risk therapy; 90sSR, 1990s standard-risk therapy; R/BMT, relapse or with hematopoietic cell transplantation.
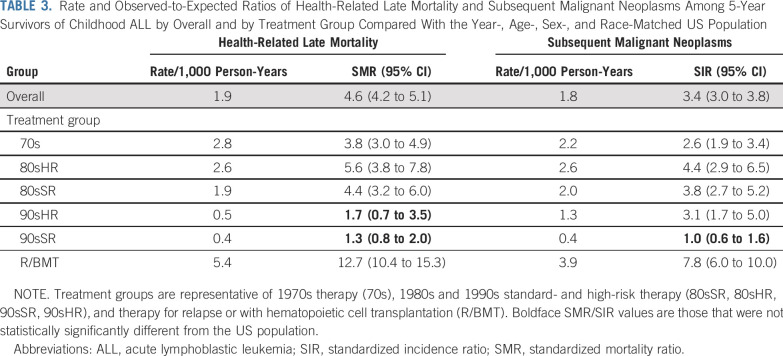
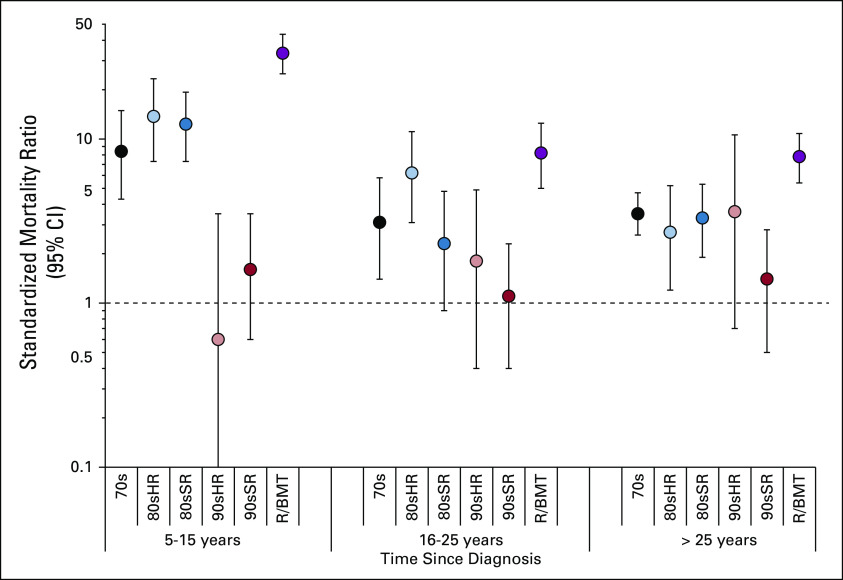
In multivariable analysis, 90sSR had a 5-fold reduction in risk for health-related late mortality compared with 70s (RR [95% CI], 90sSR: 0.2 [0.1 to 0.4]), and risk among 90sHR was reduced 3-fold (90sHR: 0.3 [0.1 to 0.7]; Data Supplement). This was attributable largely to reductions in SMN and cardiac death. Compared with 70s, no significant change in the rate of health-related late mortality was identified for 80sSR (RR, 0.9 [0.5 to 1.5]) or 80sHR (1.2 [0.8 to 1.9]), whereas R/BMT had nearly 3 times the rate (2.8 [1.9 to 4.1]). Overall, health-related late mortality among survivors in 90sSR and 90sHR did not differ from that observed in the general US population (SMR [95% CI], 90sSR: 1.3 [0.8 to 2.0], 90sHR: 1.7 [0.7 to 3.5]; Table 3; Data Supplement). When stratified by years since diagnosis, similar findings were observed within each stratum, with no significant difference in health-related late mortality for survivors in 90sSR or 90sHR compared with the general population (Data Supplement; Fig 2).
TABLE 3.
Rate and Observed-to-Expected Ratios of Health-Related Late Mortality and Subsequent Malignant Neoplasms Among 5-Year Survivors of Childhood ALL by Overall and by Treatment Group Compared With the Year-, Age-, Sex-, and Race-Matched US Population
FIG 2.
Standardized mortality ratios (SMRs) for health-related late mortality stratified by time since diagnosis and treatment group compared with the age-, sex-, race-, and year-matched US population. Treatment groups are representative of 1970s therapy (70s), 1980s and 1990s standard- and high-risk therapy (80sSR, 80sHR, 90sSR, 90sHR), and therapy for relapse or with hematopoietic cell transplantation (R/BMT). The dashed line at an SMR of 1.0 represents an observed rate of death that is no different from the expected rate in the general US population. Vertical bars represent 95% CIs.
Subsequent Neoplasms
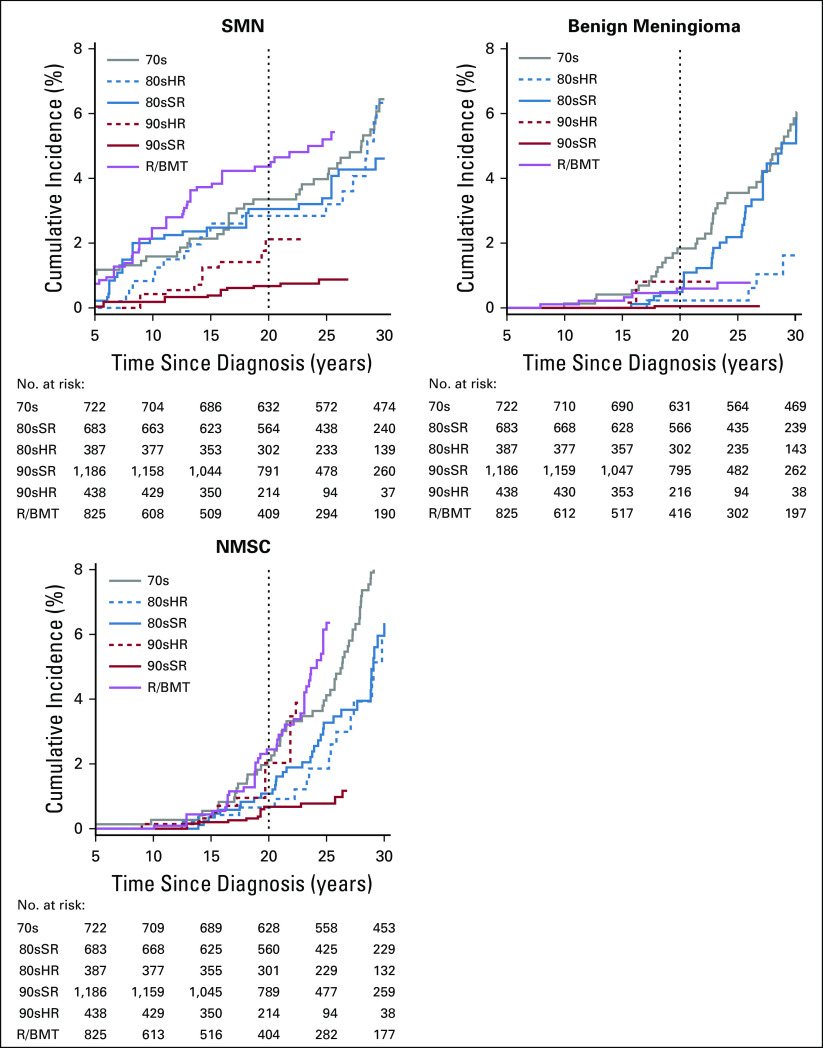
At 20 years since diagnosis, the cumulative incidence of an SN was 3.8% overall (95% CI, 3.3% to 4.2%), including SMN (2.3% [2.0% to 2.6%]), benign meningioma (0.5% [0.3% to 0.6%]), and NMSC (1.1% [0.9% to 1.4%]; Data Supplement; Appendix Fig A2, online only). Compared with 70s, only 90sSR had a reduced cumulative incidence of SMN (70s: 3.4% [2.0% to 4.7%], 90sSR: 0.7% [0.3% to 1.0%]), whereas the difference from 90sHR was not statistically significant (2.1% [0.9% to 3.3%]). In multivariable analysis, 90sSR experienced a significant reduction in rate of SMN (RR [95% CI], 0.3 [0.1 to 0.6]), benign meningioma (0.0 [0.0 to 0.1]), and NMSC (0.2 [0.1 to 0.5]) compared with 70s (Data Supplement). In a separate analysis by treatment exposure, no single exposure accounted for normalization of this risk (Data Supplement).
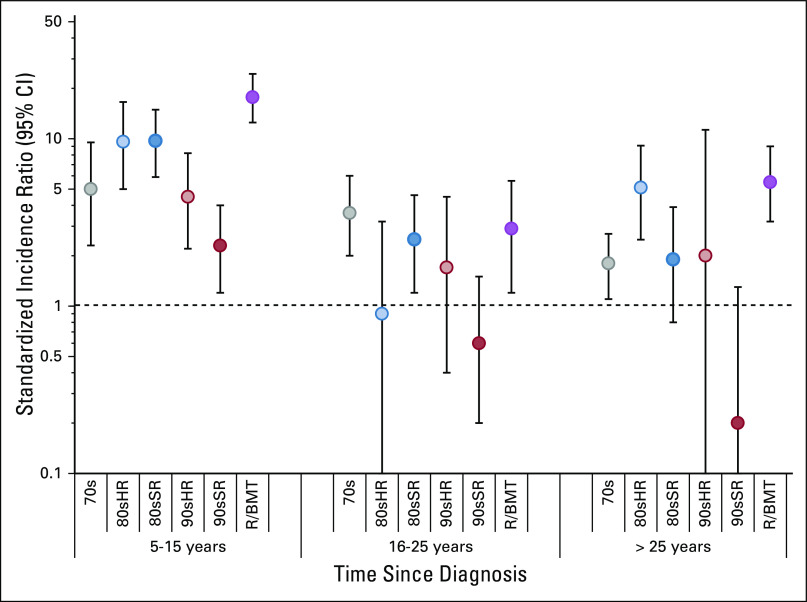
We did not identify an increase in risk for a malignancy after primary ALL among 90sSR compared with the general population (SIR [95% CI], 1.0 [0.6 to 1.6]; Table 3; Data Supplement). However, when stratified by years since diagnosis, 90sSR had an increase in risk for SMN earlier in survivorship, from 5-15 years (SIR [95% CI], 2.3 [1.2 to 4.0]), but not in later strata (SIR [95% CI] 16-25 years: 0.6 [0.2 to 1.5]; > 25 years: 0.2 [0.0 to 1.3]; Data Supplement; Appendix Fig A3, online only).
Chronic Health Conditions
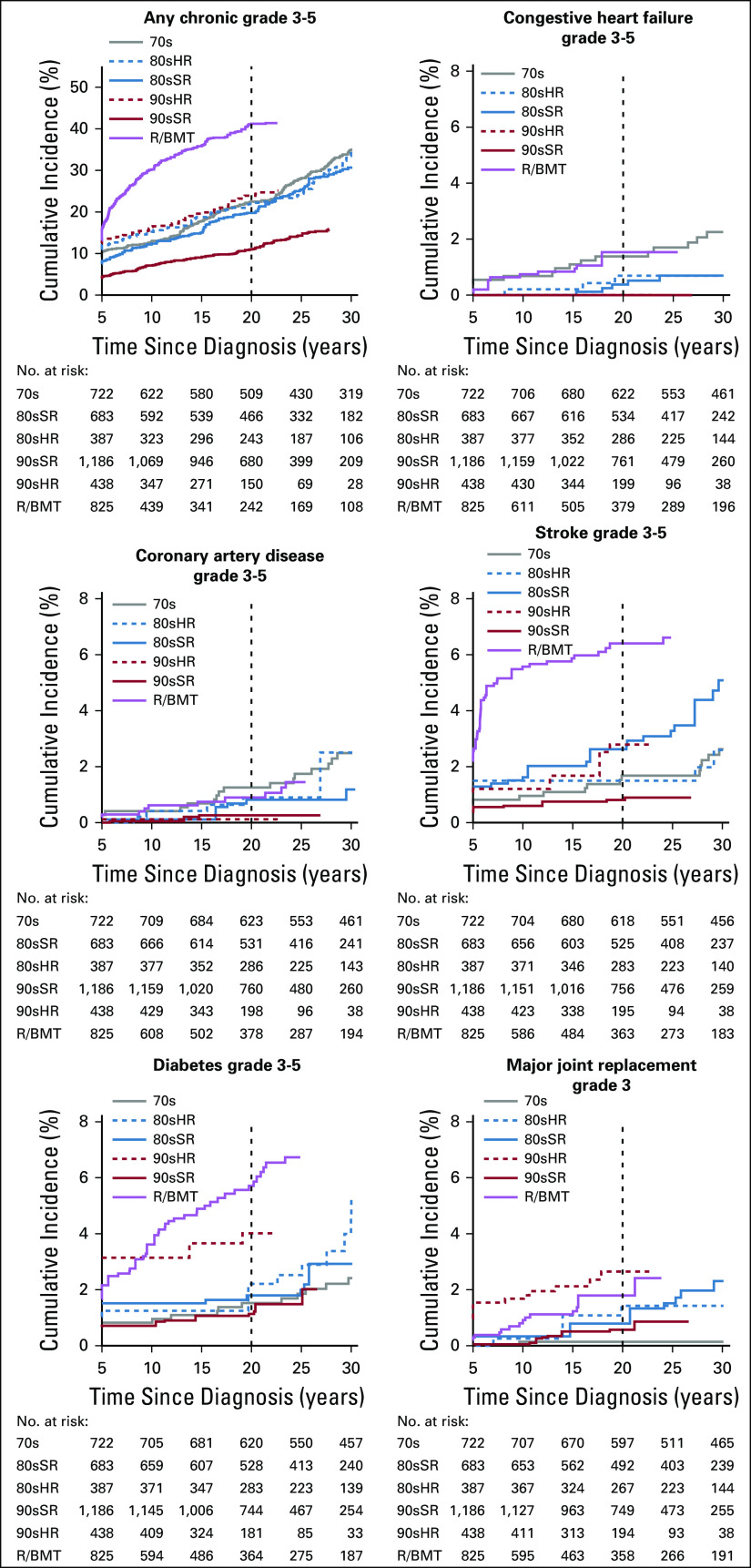
At 20 years since diagnosis, the cumulative incidence of any severe, disabling, life-threatening, or fatal (grade 3-5) chronic health condition was 21.1% (95% CI, 20.3% to 22.0%) overall (Data Supplement; Appendix Fig A4, online only). Compared with 70s, only 90sSR had a reduced cumulative incidence of any grade 3-5 condition (70s: 22.5% [19.4% to 25.5%]; 90sSR: 11.0% [9.7% to 12.3%]), whereas that of R/BMT was increased (41.2% [38.2% to 44.2%]). Compared with 70s, both 90sHR and R/BMT had an increase in major joint replacement (70s: 0.1% [0.0% to 0.4%]; 90sHR: 2.7% [1.5% to 3.8%]; R/BMT: 1.8% [1.0% to 2.6%]) and diabetes (70s: 1.5% [0.6% to 2.4%]; 90sHR: 4.0% [2.6% to 5.4%]; R/BMT: 5.7% [4.3% to 7.1%]).
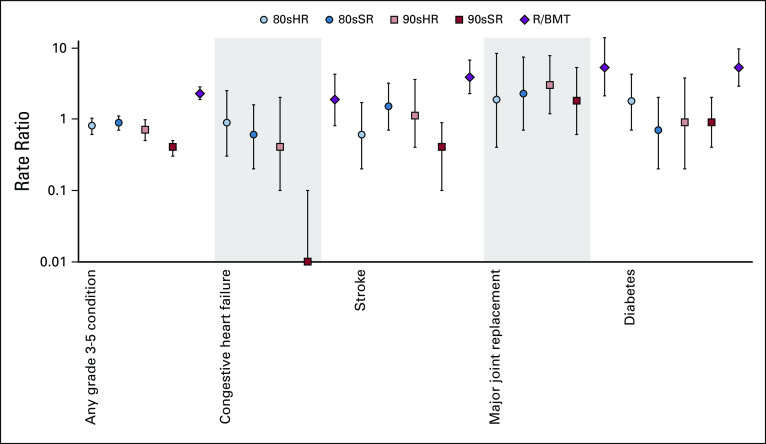
In multivariable analysis compared with 70s, 90sSR experienced a reduced rate of any grade 3-5 condition (RR [95% CI], 0.4 [0.3 to 0.5]), stroke (0.4 [0.1 to 0.9]), and congestive heart failure (0.0 [0.0 to 0.1]), with no grade 3-5 heart failure events reported in 90sSR survivors (Fig 3; Data Supplement). Compared with 70s, both 90sHR and R/BMT had at least 3 times the rate of major joint replacement (RR, 90sHR: 3.0 [1.2 to 7.8]; R/BMT: 5.3 [2.1 to 13.8]). No significant difference in self-reported prevalence of infertility was identified between treatment groups overall (Data Supplement). In an analysis assessing risk for specific therapeutic exposures, only exposure to CRT or total body irradiation were significant for risk of any grade 3-5 chronic health condition (Data Supplement).
FIG 3.
Rate ratios of any and specific grade 3-5 chronic health conditions by treatment group compared with 70s, adjusted for sex, race, age at diagnosis, and attained age. No grade 3-5 heart failure events were observed in the 90sSR group; RR = 0.0. 70s, 1970s therapy; 80sHR, 1980s high-risk therapy; 80sSR, 1980s standard-risk therapy; 90sHR, 1990s high-risk therapy; 90sSR, 1990s standard-risk therapy; R/BMT, relapse or with hematopoietic cell transplantation. The dashed line at a RR of 1.0 represents no difference from 70s. Vertical bars represent 95% CIs.
Compared with siblings, all treatment groups experienced an increased rate of any grade 3-5 chronic health condition (RR, 90sSR: 1.9 [1.5 to 2.3]; Data Supplement). However, although 90sSR had no increase in rate of heart failure compared with siblings (RR, 0.0 [0.0 to 0.6]), 90sHR had 3 times the rate (3.1 [0.9 to 11.2]) and R/BMT had 13 times the rate (13.0 [5.9 to 28.5]).
Neurocognitive Impairment
In multivariable analysis compared with 70s, 90sSR had reduced prevalence of impaired memory (PR [95% CI], 0.7 [0.6 to 0.9]), emotional regulation (0.5 [0.4 to 0.7]), and task efficiency (0.5 [0.4 to 0.7]; Data Supplement). In an analysis assessing risk for specific therapeutic exposures, associations between neurocognitive impairment with female sex and CRT were confirmed (Data Supplement).
Sociodemographic Outcomes
The 90sSR group was no different from siblings on the basis of income, uninsured status, education, or employment. However, a higher proportion had never married or lived as married (PR, 1.4 [95% CI, 1.2 to 1.6]; Data Supplement).
DISCUSSION
Risk-stratified therapy for childhood ALL has focused on improving survival by increasing the intensity of therapy in patients with high-risk features while reducing therapy in standard-risk patients to minimize their long-term toxicities. The success of this approach in improving disease-free survival has been well established,3,5,8,9,11 but our analysis directly demonstrates that contemporary risk-stratified therapy has succeeded in reducing late morbidity and mortality for standard-risk patients. Specifically, to our knowledge, our analysis is the first to show that survivors treated similarly to contemporary standard-risk ALL therapies (represented by > 1,100 survivors in the 90sSR group, with a median follow-up of > 25 years for mortality and > 21 years for all other outcomes) have no statistically significant increase in risk for health-related late mortality or SMN compared with the general population. Furthermore, reductions in treatment intensity have not led to an increase in late mortality due to relapse. Although Essig et al31 previously presented results of 556 survivors treated in the 1970s and early 1980s with therapy similar to standard-risk therapy of the 2000s, they did not include any survivors treated after 1986 or comparisons with survivors treated with high-risk therapy or therapy reflective of an earlier era. Furthermore, although prior studies found no decrease in risk for chronic health conditions among ALL survivors treated in more recent decades,16,17 we have now shown that, compared with the 70s cohort, standard-risk patients treated with contemporary risk-stratified protocols had a significant reduction in risk for developing a severe chronic health condition, including congestive heart failure, stroke, and neurocognitive impairment. However, a larger proportion developed a chronic condition than similar-age siblings.
Survival of childhood ALL has improved dramatically over the time frame presented in this study,3-6,8,18 which has led to increased numbers of survivors of high-risk leukemia who received more intensive therapy.3,5,8 Although survivors treated with more recent high-risk therapy, 90sHR, had reduced late mortality compared with survivors treated in earlier eras, we did not identify reductions in the rate of SMN or chronic health conditions. Furthermore, survivors treated for relapse or requiring transplantation (R/BMT) were the only group to experience worse overall outcomes than the 70s, with more than twice the rate of health-related late mortality, SMNs, and chronic health conditions. The absence of consistent improvements in late health outcomes for 90sHR and poor outcomes for R/BMT likely explain the findings of prior studies that did not stratify by risk and identified improved late mortality19 but no improvement in rates of SN and chronic health conditions among more contemporary survivors of ALL.17,18 This phenomenon, where intensively treated patients with high-risk disease who would not have survived in an earlier era alter the morbidity profile of the overall population, has recently been described among survivors of medulloblastoma. The introduction of adjuvant chemotherapy in the 1980s and risk stratification in the 1990s dramatically improved 5-year survival of patients with medulloblastoma to > 70% at a cost of increased risk for severe chronic health conditions and SNs among survivors.38 The high incidence of chronic conditions including major joint replacement and diabetes among 90sHR and R/BMT survivors identifies potential opportunities for continued improvement through therapy modification, use of novel agents, or earlier monitoring and intervention for late effects.
We determined that the spectrum of chronic health conditions experienced by the contemporary survivor differs from that of survivors treated in previous eras. In the 1990s, prophylactic CRT was largely replaced by intensified intrathecal and CNS-targeted chemotherapy regimens (dexamethasone, high-dose methotrexate), resulting in a significantly decreased risk for neurocognitive deficits, stroke, and SMN.16,36 Dose restriction of anthracycline chemotherapy has resulted in a significant reduction in risk of cardiomyopathy, even among high-risk survivors. By contrast, contemporary therapy for high-risk patients and those with relapse or requiring transplantation has increased treatment-related morbidity. The 90sHR and R/BMT groups had a higher incidence of major joint replacement and diabetes than 70s or 90sSR. Although no single therapy exposure was shown to increase risk for major joint replacement in our study, prior studies have established an association between increased corticosteroid exposure, as well as l-asparaginase, and osteonecrosis among patients with high-risk ALL.39,40 This may explain the increased incidence of joint replacement, a major functional morbidity, observed in 90sHR, highlighting the changing spectrum of chronic health conditions among survivors of high-risk disease.
Limitations to this study include that all outcomes, with the exception of mortality and SMN, were derived only from self-report. In addition, because survival has improved dramatically over the 30 years of the study, particularly for high-risk and relapse/transplantation groups, it may be difficult to differentiate the impact of improved survival on late health outcomes by treatment group. Finally, although median follow-up was at least 18 years since diagnosis for all treatment groups and 21 years for 90sSR, this is still relatively short follow-up for the 90s groups and R/BMT. As morbidity and mortality increase with age, comparisons presented between groups and to the general population may change over time.
In summary, we identified that the largest group of survivors (90sSR) treated with risk-adapted regimens similar to contemporary standard-risk ALL therapy have improved outcomes across all domains compared with those treated with therapy reflective of an earlier era or for high-risk disease. Furthermore, the risk for health-related late mortality and subsequent malignancy of the 90sSR group are no greater than the general US population, and their socioeconomic outcomes are similar to those of siblings. However, continued follow-up is needed to determine if these encouraging findings are maintained as these survivors age. Our findings also emphasize the need to encourage efforts to mitigate the known risk of chronic health conditions, particularly for survivors of high-risk ALL, where specific treatment-related morbidity has increased (joint replacement, diabetes). Continued improvements in risk-stratified treatment, addition of targeted agents and immunotherapy, and attention to modifiable risk factors are needed to optimize life span and health span. Our findings can inform both discussions regarding long-term expectations of newly diagnosed patients and risk-based follow-up care priorities of aging, long-term survivors of ALL, outlined in the COG Long-Term Follow up Guidelines.41 Taken together, these results demonstrate that the goal of risk-stratified therapy for children with standard-risk ALL, to reduce late morbidity and mortality while maintaining excellent outcomes, has been realized. However, there remains an urgent need to decrease the burden of chronic health conditions experienced in this aging population.
APPENDIX
FIG A1.
Diagram of study population. ALL, acute lymphoblastic leukemia; CCSS, Childhood Cancer Survivor Study; HR, high risk; SR, standard risk.
FIG A2.
Cumulative incidence of subsequent neoplasms as (A) subsequent malignant neoplasm (SMN), (B) benign meningioma, and (C) nonmelanoma skin cancer (NMSC) among 5-year survivors of childhood acute lymphoblastic leukemia by treatment group. 70s, 1970s therapy; 80sHR, 1980s high-risk therapy; 80sSR, 1980s standard-risk therapy; 90sHR, 1990s high-risk therapy; 90sSR, 1990s standard-risk therapy; R/BMT, relapse or with hematopoietic cell transplantation.
FIG A3.
Standardized incidence ratios comparing rate of subsequent malignant neoplasm by time since diagnosis and treatment group to the age-, sex-, race-, and year-matched US population. 70s, 1970s therapy; 80sHR, 1980s high-risk therapy; 80sSR, 1980s standard-risk therapy; 90sHR, 1990s high-risk therapy; 90sSR, 1990s standard-risk therapy; R/BMT, relapse or with hematopoietic cell transplantation.
FIG A4.
Cumulative incidence of severe or disabling, life-threatening, or fatal (Common Terminology Criteria for Adverse Events grade 3-5) chronic health conditions (A) overall, and of selected conditions including (B) congestive heart failure, (C) coronary artery disease, (D) stroke, (E) diabetes, and (F) major joint replacement among 5-year survivors of childhood acute lymphoblastic leukemia by treatment group. 70s, 1970s therapy; 80sHR, 1980s high-risk therapy; 80sSR, 1980s standard-risk therapy; 90sHR, 1990s high-risk therapy; 90sSR, 1990s standard-risk therapy; R/BMT, relapse or with hematopoietic cell transplantation.
PRIOR PRESENTATION
Presented at the ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019; the North American Symposium on Late Complications After Childhood Cancer, Atlanta, GA, June 20-22, 2019; and the International Society of Paediatric Oncology Annual Meeting, Lyon, France, October 23-26, 2019.
SUPPORT
Supported by the National Cancer Institute Grant No. CA55727 (G.T.A., Principal Investigator). Support to St Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) Grant No. CA21765 (C. Roberts, Principal Investigator) and the American Lebanese Syrian Associated Charities.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Leslie L. Robison, Gregory T. Armstrong
Administrative support: Gregory T. Armstrong
Provision of study material or patients: Ching-Hon Pui, Joseph P. Neglia, Melissa M. Hudson, Leslie L. Robison, Gregory T. Armstrong
Collection and assembly of data: Yutaka Yasui, Ching-Hon Pui, Rebecca M. Howell, Joseph P. Neglia, Melissa M. Hudson, Leslie L. Robison, Gregory T. Armstrong
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Reduced Morbidity and Mortality in Survivors of Childhood Acute Lymphoblastic Leukemia: A Report From the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stephanie B. Dixon
Employment: Medtronic (I)
Stock and Other Ownership Interests: Medtronic (I)
Travel, Accommodations, Expenses: Medtronic (I)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/2791772/summary
Ching-Hon Pui
Leadership: Adaptive Biotechnologies
Honoraria: Amgen, Servier
Consulting or Advisory Role: Adaptive Biotechnologies
Research Funding: National Cancer Institute
Stephen P. Hunger
Stock and Other Ownership Interests: Amgen, Merck (I)
Honoraria: Amgen
Consulting or Advisory Role: Novartis
Lewis B. Silverman
Consulting or Advisory Role: Takeda, Servier
Research Funding: Baxalta/Shire (Inst), Servier (Inst)
Nina S. Kadan-Lottick
Honoraria: Medtronic (I), Boston Scientific (I)
Consulting or Advisory Role: Medtronic (I), Boston Scientific (I)
Speakers' Bureau: Medtronic (I), Boston Scientific (I)
Kevin R. Krull
Patents, Royalties, Other Intellectual Property: Royalties from Wolters Kluwer
Melissa M. Hudson
Consulting or Advisory Role: Oncology Research Information Exchange Network, Princess Máxima Center
No other potential conflicts of interest were reported.
REFERENCES
- 1. Howlader N, Noone AM, Krapcho M, et al: SEER Cancer Statistics Review, 1975-2014, (ed April 2017). Bethesda, MD, National Cancer Institute, 2017. [Google Scholar]
- 2.Steinhorn SC, Myers MH. Progress in the treatment of childhood acute leukemia: A review. Med Pediatr Oncol. 1981;9:333–346. doi: 10.1002/mpo.2950090405. [DOI] [PubMed] [Google Scholar]
- 3.Silverman LB, Stevenson KE, O’Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000) Leukemia. 2010;24:320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzer WL, Devidas M, Carroll WL, et al. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: A report from the children’s oncology group. Leukemia. 2010;24:355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pui CH, Pei D, Sandlund JT, et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24:371–382. doi: 10.1038/leu.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Möricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. doi: 10.1038/leu.2009.257. [DOI] [PubMed] [Google Scholar]
- 7.Madanat-Harjuoja LM, Pokhrel A, Kivivuori SM, et al. Childhood cancer survival in Finland (1953-2010): A nation-wide population-based study. Int J Cancer. 2014;135:2129–2134. doi: 10.1002/ijc.28844. [DOI] [PubMed] [Google Scholar]
- 8.Gaynon PS, Angiolillo AL, Carroll WL, et al. Long-term results of the children’s cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: A Children’s Oncology Group Report. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: Acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957–963. doi: 10.1002/pbc.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 12.Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 13.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58:334–343. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung YT, Krull KR. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci Biobehav Rev. 2015;53:108–120. doi: 10.1016/j.neubiorev.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacola LM, Edelstein K, Liu W, et al. Cognitive, behaviour, and academic functioning in adolescent and young adult survivors of childhood acute lymphoblastic leukaemia: A report from the Childhood Cancer Survivor Study. Lancet Psychiatry. 2016;3:965–972. doi: 10.1016/S2215-0366(16)30283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulrooney DA, Hyun G, Ness KK, et al. The changing burden of long-term health outcomes in survivors of childhood acute lymphoblastic leukaemia: A retrospective analysis of the St Jude Lifetime Cohort Study. Lancet Haematol. 2019;6:e306–e316. doi: 10.1016/S2352-3026(19)30050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19:1590–1601. doi: 10.1016/S1470-2045(18)30537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970-2015. JAMA. 2017;317:814–824. doi: 10.1001/jama.2017.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374:833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Cheung YT, Conklin HM, et al. Evolution of neurocognitive function in long-term survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. J Cancer Surviv. 2018;12:398–406. doi: 10.1007/s11764-018-0679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood. 2008;111:5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Childhood Cancer Survivor Study, St Jude Children’s Research Hospital, 2020 https://ccss.stjude.org/
- 23.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 25.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell RM, Smith SA, Weathers RE, et al. Adaptations to a generalized radiation dose reconstruction methodology for use in epidemiologic studies: An update from the MD Anderson Late Effect Group. Radiat Res. 2019;192:169–188. doi: 10.1667/RR15201.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019;5:864–871. doi: 10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverman LB, Blonquist TM, Hunt SK, et al. Randomized study of pegasparagase (SS-PEG) and calaspargase pegol (SC-PEG) in pediatric patients with newly diagnosed acute lymphoblastic leukemia or lymphoblastic lymphoma: Results of DFCI ALL Consortium protocol 11-001. Blood. 2016;128:175. [Google Scholar]
- 29.Vrooman LM, Blonquist TM, Harris MH, et al. Refining risk classification in childhood B acute lymphoblastic leukemia: Results of DFCI ALL Consortium protocol 05-001. Blood Adv. 2018;2:1449–1458. doi: 10.1182/bloodadvances.2018016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pui CH, Relling MV, Sandlund JT, et al. Rationale and design of Total Therapy Study XV for newly diagnosed childhood acute lymphoblastic leukemia. Ann Hematol. 2004;83(suppl 1):S124–S126. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 31.Essig S, Li Q, Chen Y, et al. Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: A report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2014;15:841–851. doi: 10.1016/S1470-2045(14)70265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 33.Kenzik KM, Huang IC, Brinkman TM, et al. The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) revised: Item response analysis and concurrent validity. Neuropsychology. 2015;29:31–44. doi: 10.1037/neu0000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong GT, Jain N, Liu W, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro-oncol. 2010;12:1173–1186. doi: 10.1093/neuonc/noq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung YT, Brinkman TM, Li C, et al. Chronic health conditions and neurocognitive function in aging survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2018;110:411–419. doi: 10.1093/jnci/djx224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102:881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Compressed Mortality File (CMF) on CDC WONDER Online Database. https://wonder.cdc.gov/
- 38.Salloum R, Chen Y, Yasui Y, et al. Late morbidity and mortality among medulloblastoma survivors diagnosed across three decades: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37:731–740. doi: 10.1200/JCO.18.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albertsen BK, Grell K, Abrahamsson J, et al. Intermittent versus continuous PEG-asparaginase to reduce asparaginase-associated toxicities: A NOPHO ALL2008 randomized study. J Clin Oncol. 2019;37:1638–1646. doi: 10.1200/JCO.18.01877. [DOI] [PubMed] [Google Scholar]
- 40.Mattano LA, Jr, Devidas M, Nachman JB, et al. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: Results from the CCG-1961 randomised cohort trial. Lancet Oncol. 2012;13:906–915. doi: 10.1016/S1470-2045(12)70274-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Children’s Oncology Group: Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers, version 5.0. Monrovia, CA: Children’s Oncology Group; October 2018 http://www.survivorshipguidelines.org/