Abstract
PURPOSE
Diffuse gliomas are malignant brain tumors that include lower-grade gliomas (LGGs) and glioblastomas. Transformation of low-grade glioma into a higher tumor grade is typically associated with contrast enhancement on magnetic resonance imaging. Mutations in the isocitrate dehydrogenase 1 (IDH1) gene occur in most LGGs (> 70%). Ivosidenib is an inhibitor of mutant IDH1 (mIDH1) under evaluation in patients with solid tumors.
METHODS
We conducted a multicenter, open-label, phase I, dose escalation and expansion study of ivosidenib in patients with mIDH1 solid tumors. Ivosidenib was administered orally daily in 28-day cycles.
RESULTS
In 66 patients with advanced gliomas, ivosidenib was well tolerated, with no dose-limiting toxicities reported. The maximum tolerated dose was not reached; 500 mg once per day was selected for the expansion cohort. The grade ≥ 3 adverse event rate was 19.7%; 3% (n = 2) were considered treatment related. In patients with nonenhancing glioma (n = 35), the objective response rate was 2.9%, with 1 partial response. Thirty of 35 patients (85.7%) with nonenhancing glioma achieved stable disease compared with 14 of 31 (45.2%) with enhancing glioma. Median progression-free survival was 13.6 months (95% CI, 9.2 to 33.2 months) and 1.4 months (95% CI, 1.0 to 1.9 months) for the nonenhancing and enhancing glioma cohorts, respectively. In an exploratory analysis, ivosidenib reduced the volume and growth rates of nonenhancing tumors.
CONCLUSION
In patients with mIDH1 advanced glioma, ivosidenib 500 mg once per day was associated with a favorable safety profile, prolonged disease control, and reduced growth of nonenhancing tumors.
INTRODUCTION
Diffuse gliomas represent the most common malignant primary brain tumor in adults and include glioblastoma (GBM) and WHO grade 2 and WHO grade 3 tumors. The latter are referred to as lower-grade gliomas (LGGs). LGGs grow at a slower rate, but eventually “transform” into a higher tumor grade.1 Patients with LGGs with long-term disease control suffer from treatment-related symptoms, including radiation-induced cognitive changes.2-5 Brain magnetic resonance imaging (MRI) plays a central role in disease monitoring.6,7 Malignant transformation of LGGs is often associated with the appearance of contrast enhancement.
CONTEXT
Key Objectives
To determine safety and tolerability of oral ivosidenib as a single agent in patients with glioma and to determine the recommended phase II dose.
Knowledge Generated
Ivosidenib was well tolerated, with no dose-limiting toxicities. 500 mg once per day was selected for the expansion cohort. In exploratory analyses, ivosidenib reduced the growth of nonenhancing tumors.
Relevance
Our findings point toward an important contribution of the mutant IDH1 enzyme to the growth of mIDH1 LGGs. Further evaluation of mIDH inhibitors for the treatment of mIDH LGGs appears warranted.
Mutations in the isocitrate dehydrogenase 1 (IDH1) gene, and less commonly in the IDH2 gene, are found in more than 70% of LGGs.8 IDH mutant (mIDH) gliomas have emerged as a separate glioma entity with a distinct molecular pathogenesis. IDH mutations in glioma occur early during tumor development, cluster in key arginine residues within the enzyme’s active site, are associated with a distinctive pattern of DNA hypermethylation, persist throughout the disease, and are associated with a better prognosis compared with IDH wildtype gliomas of the same tumor grade.8-15 Cancer-associated IDH1/2 mutations lead to the abnormal production of the oncometabolite D(-)-2-hydroxyglutarate (2-HG),16,17 which inhibits α-ketoglutarate–dependent enzymes, resulting in tumorigenesis.18-20
The contribution of mIDH enzymes to the growth of established cancers remains incompletely understood. Inhibition of the mIDH enzyme reduced tumor cell proliferation in experimental models of mIDH leukemia and mIDH glioma.21,22 In clinical trials for patients with advanced acute myeloid leukemia, another human cancer harboring IDH mutations,23,24 the first-in-class, Food and Drug Administration–approved inhibitors of mIDH2 (enasidenib) and mIDH1 (ivosidenib) induced clinical and molecular remissions.25,26
We designed a multicenter, open-label, phase I dose escalation and expansion study of ivosidenib in patients with mIDH1 advanced solid tumors. Data from cholangiocarcinoma and chondrosarcoma cohorts have been reported.27,28 Here we report results for the advanced glioma cohort in the phase I study, including LGG and GBM.
METHODS
Study Design
This phase I, multicenter, open-label study comprised a dose escalation and a dose expansion phase (Data Supplement, online only). The primary objectives were to assess the safety and tolerability of oral ivosidenib as a single agent and to determine the maximum tolerated dose or recommended phase II dose of ivosidenib in patients with solid tumors. Secondary objectives included evaluation of dose-limiting toxicities (DLTs) during cycle 1 of dose escalation, pharmacokinetic and pharmacodynamic findings (reported elsewhere29), and characterization of preliminary clinical response. DLTs were defined as any grade ≥ 3 event reported to be at least possibly related to ivosidenib. The data reported here are from patients with glioma who were enrolled in both phases.
Patients underwent baseline screening evaluations within 28 days before study day 1. Dose escalation was performed using a 3+3 design, with patients enrolled into sequential 3-patient cohorts of increasing doses from 100 mg twice per day (200 mg/d) to 1,200 mg once per day. Treatment with ivosidenib was continuous; 1 cycle was defined as 28 days.
Patients
Eligible patients included men and women ≥ 18 years of age with an Eastern Cooperative Oncology Group performance status of 0 to 1 and an expected survival of ≥ 3 months. All patients had an established diagnosis of mIDH1 glioma that had recurred after, or not responded to, initial surgery, radiation, or chemotherapy. IDH1 mutation status was based on local laboratory testing with retrospective central confirmation. Because this study was initiated before the most recent revision of the WHO Classification of Tumors of the Central Nervous System,30 we used the 2007 classification.31
Transformation of LGGs to a higher tumor grade is frequently associated with the appearance of tumor contrast enhancement on T1-weighted brain MRI. For the dose expansion phase, patients were therefore separated into 2 cohorts on the basis of the presence or absence of tumor contrast enhancement at the time of enrollment according to the investigator. The “nonenhancing” glioma cohort comprised patients with mIDH1 glioma that had progressed within 12 months before enrollment and did not enhance on T1-weighted postgadolinium MRI. Patients in this cohort required at least 3 full sets of “historical” MRI examinations (not including screening), each separated by at least 2 months, and were ineligible if they had had surgery or radiation therapy within 6 months of enrollment. The second cohort comprised patients with progressive mIDH1 gliomas who did not meet these criteria.
Study Oversight
The study was designed by the sponsor in collaboration with the lead investigators. Clinical data were generated by investigators and research staff at each participating site. Safety data were reviewed at regular intervals by study investigators and the sponsor. All authors vouch for the accuracy and completeness of the data and analyses and for the adherence of the study to the protocol. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by relevant institutional review boards or ethics committees at each site. Written informed consent was provided by all the patients before screening and enrollment.
Study Assessments
Toxicity was evaluated by the collection of adverse events (AEs), serious AEs, and AEs leading to discontinuation, graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03. Treatment efficacy was assessed by investigators using MRI every 2 cycles (56 ± 3 days) according to Response Assessment in Neuro-Oncology (RANO) criteria for high-grade gliomas32 for all patients in the dose escalation phase and for those with enhancing glioma in the expansion phase. For patients with nonenhancing glioma in the expansion cohort, response was assessed using the RANO criteria for LGG (RANO LGG).33 End points included best overall response and objective response rate (defined as complete response plus partial response plus minor response). Progression-free survival (PFS) was defined as the interval from first dose to disease progression or death.
Exploratory Assessments
Tumor growth rate was assessed by volume in the nonenhancing glioma expansion cohort. Tumor volume measurements were performed at the same visits as the RANO assessments using either 2-dimensional T2-weighted images, 3-dimensional T2-weighted images, or fluid-attenuated inversion recovery (FLAIR) images in compliance with the international standardized brain tumor imaging protocol.34 All patients needed at least 3 “historical” pretreatment MRIs, each separated by ≥ 2 months, acquired with ≤ 5-mm slice thickness and up to 1-mm interslice gap. Tumor volumes were segmented using a semiautomated approach by an imaging contract research organization (MedQIA, Los Angeles, CA). A centralized review of coregistered MRIs was also performed. In a post hoc exploratory analysis, the tumor growth rate after treatment versus before treatment was determined using a linear mixed-effects model.35 Using this model, the percentage change in tumor volume per 6 months was derived from the slope estimates from the mixed-effects model, adjusted for 6 months.
Exploratory assessments also included confirmation of baseline mIDH1 status and identification of co-occurring mutations. Archival formalin-fixed paraffin-embedded samples were collected for analysis by next-generation sequencing using the FoundationOne panel (Foundation Medicine, Cambridge, MA),36 which includes 361 genes. Foundation Medicine provides a “known/likely oncogenic” call to identify known or likely oncogenic variants on the basis of current literature and likely somatic status of the variant.
Statistical Analysis
The safety analysis set comprised all patients with glioma who received at least 1 dose of study treatment. Patients who had received at least 1 dose of ivosidenib were included in the efficacy analysis. Efficacy results are reported separately for contrast-enhancing and nonenhancing tumors, and they combine the dose escalation and dose expansion cohorts. Descriptive statistics are reported for safety outcomes and other clinical parameters. PFS was estimated using Kaplan-Meier methods, and medians with associated 95% CIs were calculated. Statistical analyses were carried out (by L.J.) using SAS software version 9.3 or higher. Association of baseline gene or pathway mutation status and PFS was assessed using the log-rank test.
RESULTS
Patients
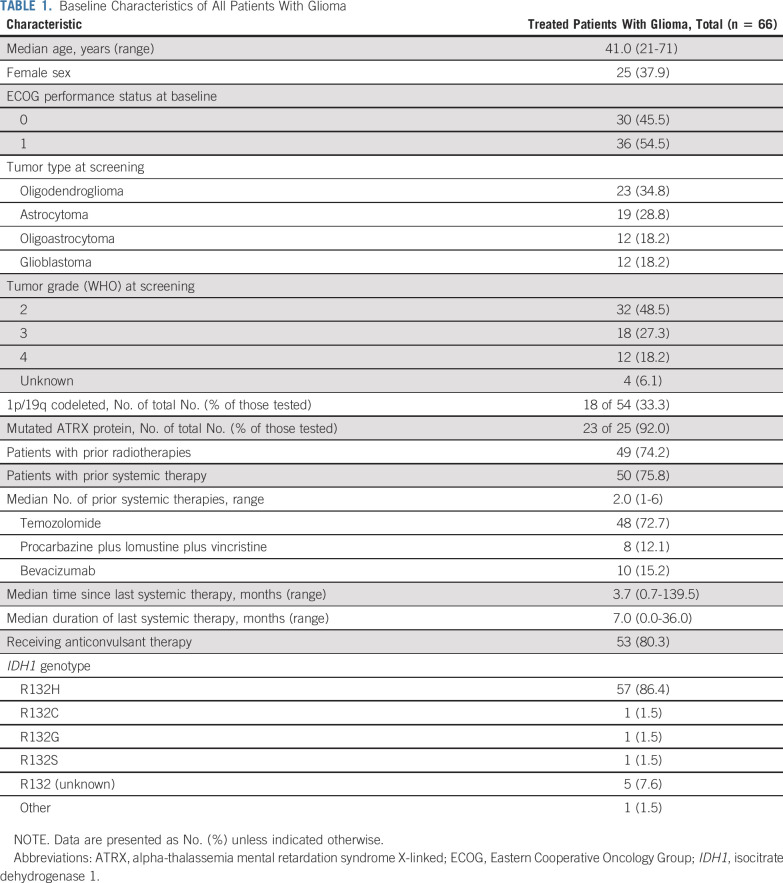
This study was initiated in March 2014 across 12 study sites in the United States and one in France, and 168 patients with mIDH1 solid tumors were enrolled, including 66 with glioma. At the data cutoff date (January 16, 2019), enrollment was complete, and the study was ongoing. Twelve of 66 patients (18.2%) had GBM; the remainder had LGGs. The median number of prior systemic therapies was 2 (range, 1 to 6) and included temozolomide (48 of 66 patients); combination procarbazine, lomustine, and vincristine (eight of 66 patients); and bevacizumab (10 of 66 patients). Forty-nine of 66 patients had received prior radiotherapy (Table 1).
TABLE 1.
Baseline Characteristics of All Patients With Glioma
Twenty patients were treated in the dose escalation phase, and 46 were treated in the dose expansion phase (24 with nonenhancing disease). In the dose escalation phase, patients received ivosidenib doses of 100 mg twice per day (n = 1), 300 mg once per day (n = 6), 500 mg once per day (n = 4), 600 mg once per day (n = 5), and 900 mg once per day (n = 4). Fifty patients received 500 mg once per day (4 in dose escalation and all 46 patients in dose expansion). At the data cutoff date, 15 patients (22.7%) were still receiving treatment and 51 (77.3%) had discontinued; all but one discontinued for disease progression (Data Supplement).
Safety
No DLTs were reported, and the maximum tolerated dose was not reached. A dose of 500 mg once per day was selected for expansion on the basis of the pharmacokinetic/pharmacodynamic data from all solid tumor cohorts, including less-than-dose-proportional increases in exposure and maximum suppression of plasma 2-HG at 500 mg in patients with nonglioma solid tumors, as well as the safety profile and preliminary clinical activity observed in the dose escalation phase. Plasma 2-HG was not elevated above normal levels in patients with glioma.29
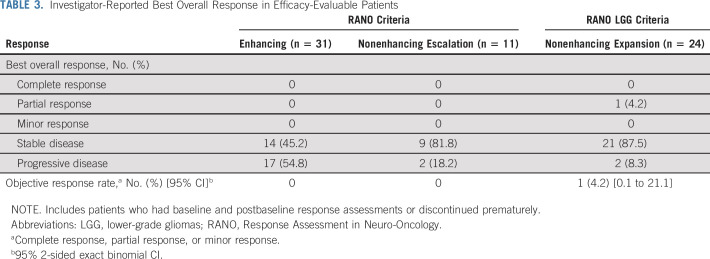
Most patients (63 of 66 [95.5%]) experienced at least 1 AE of any grade or causality. The most common AEs (≥ 10%) were headache (39.4%), nausea (22.7%), fatigue (22.7%), vomiting (19.7%), seizure (18.2%), diarrhea (16.7%), hyperglycemia (15.2%), aphasia (15.2%), neutrophil count decreased (12.1%), depression (10.6%), hypophosphatemia (10.6%), and paresthesia (10.6%; Table 2; Data Supplement). Grade ≥ 3 AEs were observed in 13 of 66 patients (19.7%). These included headache (4.5%), hypophosphatemia (3.0%), and seizure (3.0%; Table 2; Data Supplement). Treatment-related AEs were observed in 39 of 66 patients (59.1%); most were grade 1 or grade 2. The most common treatment-related AEs of any grade were fatigue (13.6%), decreased neutrophil count (12.1%), and diarrhea (10.6%; Data Supplement). Grade ≥ 3 treatment-related AEs were reported in 2 patients (neutropenia, decreased weight, hyponatremia, and arthralgia). Serious AEs were reported for 11 patients (16.7%), but none were considered related to treatment. No patients discontinued study treatment owing to an AE. Eight patients (12.1%) had a dose interruption because of an AE; no patients required dose reduction for AEs. Two patients (3.0%) died within 30 days of the last dose (unrelated to AEs; both had enhancing glioma and both had received ivosidenib 500 mg once per day). There were no clinically meaningful changes in hematology parameters, coagulation parameters, vital signs, physical examination assessments, left ventricular ejection fraction, or Eastern Cooperative Oncology Group performance status.
TABLE 2.
Adverse Events Occurring in ≥ 10% of Patients With Glioma
Investigator-Reported Response
All 66 patients in the dose escalation and dose expansion phases were evaluable for efficacy. According to the investigator’s assessment of response, 1 patient had a partial response, 44 patients (66.7%) had a best response of stable disease, and 21 patients (31.8%) had a best response of progressive disease.
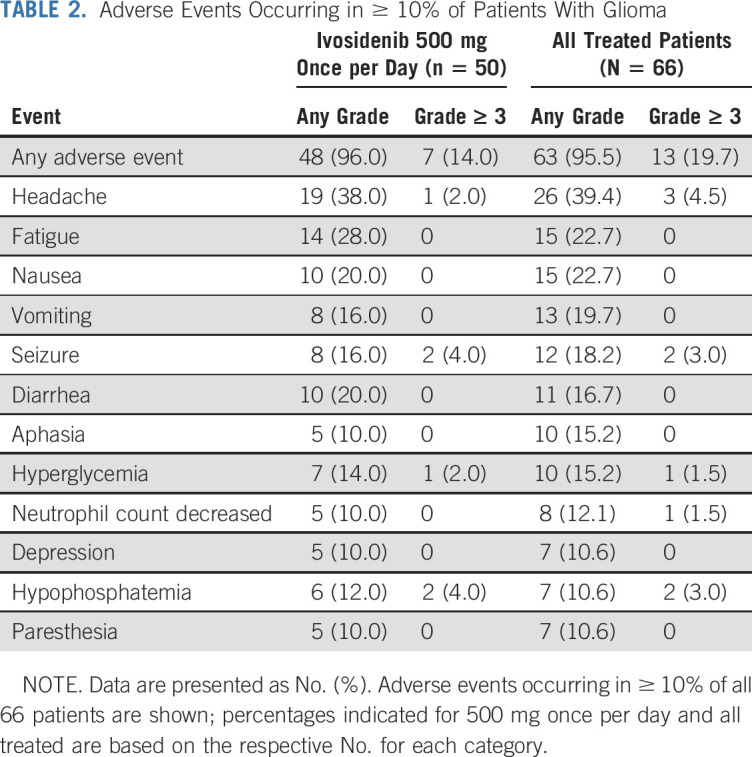
As of the data cutoff, patients with nonenhancing tumors had a median treatment duration of 18.4 months (range, 1.4-47.2 months) compared with a treatment duration of 1.9 months (range, 0.4-39.9 months) for patients with enhancing tumors. Fifteen (22.7%) remained on treatment (Figs 1A and 1B). In patients with measurable disease at baseline, tumor measurements decreased from baseline in 22 of 33 nonenhancing tumors (66.7%) and in 9 of 27 enhancing tumors (33.3%; Fig 1C). The patient with a partial response had a nonenhancing tumor and received ivosidenib 500 mg once per day. The majority of patients had disease control, with a best response of stable disease observed in 30 of 35 patients with nonenhancing tumors (85.7%) and 14 of 31 patients with enhancing tumors (45.2%; Table 3). The median PFS times were 13.6 months (95% CI, 9.2 to 33.2 months) and 1.4 months (95% CI, 1.0 to 1.9 months) for the nonenhancing and enhancing glioma cohorts, respectively, across all doses (Fig 1D). PFS curves for patients receiving 500 mg were similar (Data Supplement).
FIG 1.
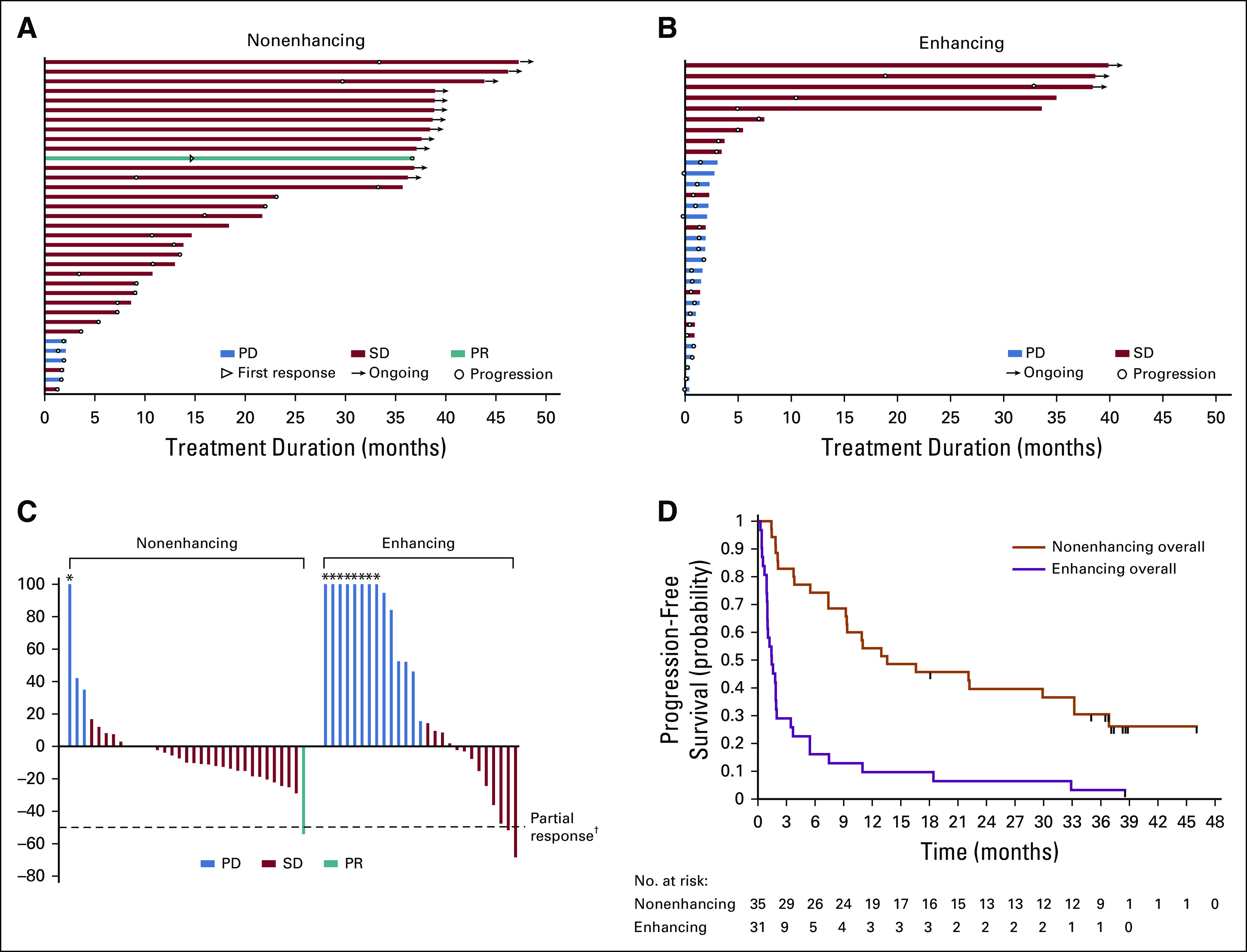
Clinical activity and efficacy of ivosidenib in patients with glioma. (A) Time receiving ivosidenib for the 35 patients with nonenhancing glioma. Twelve patients remain on treatment as of the data cutoff. (B) Time receiving ivosidenib for the 31 patients with enhancing glioma. Three patients remain on treatment as of the data cutoff. (C) Best response in evaluable patients with measurable disease (27 enhancing and 33 nonenhancing), expressed as the percent change in sum of products of the diameters from the target lesions at start of treatment. (D) Investigator-assessed progression-free survival according to glioma type for all evaluable patients with glioma (n = 66). Tick marks indicate censored data. PD, progressive disease; PR, partial response; SD, stable disease. (*) Lesion growth > 100%. (†) Two patients with enhancing disease had decreases of > 50% that were not confirmed and are indicated as SD.
TABLE 3.
Investigator-Reported Best Overall Response in Efficacy-Evaluable Patients
Exploratory Evaluation of Tumor Genetics
We examined tumor genetic profiles by targeted sequencing for 15 patients with enhancing glioma and for 16 with nonenhancing glioma. In the nonenhancing glioma group, the presence of genetic alterations in cell cycle pathway genes was associated with shorter PFS (P < .001; Data Supplement).
Exploratory Evaluation of Tumor Volume Growth Rates
We supplemented the investigator-based assessment of tumor response with a quantitative evaluation of tumor volumes before and during treatment with ivosidenib for all 24 patients in the nonenhancing expansion cohort. As defined by the study protocol, this analysis included at least 3 brain MRIs before enrollment, each separated by at least 2 months. No patient had received surgery or radiation within 6 months before enrollment. In total, this analysis included 239 MRI scans from 24 patients, including 63 historical MRIs. The estimated tumor growth rate per 6 months was 26% (95% CI, 9% to 46%) in the pretreatment period and 9% (95% CI, 1% to 20%) with ivosidenib (Data Supplement). The percentage change of tumor growth rate after treatment versus before treatment estimated from the model was –14% (95% CI, –25% to –0.4%).
We also performed a centralized review of MRIs after image coregistration to minimize scan-to-scan variability related to head tilt.37 Figure 2 and Data Supplement show brain MRIs and manually segmented tumor volume growth curves for selected patients with nonenhancing glioma. Patient 1 had an anaplastic oligodendroglioma that was initially treated with surgery, radiation, and temozolomide. Following this initial tumor therapy, the patient was off therapy for 3 years and developed a slowly progressive T2/FLAIR signal abnormality. Visual inspection of coregistered images and volume growth curves showed tumor shrinkage after the initiation of ivosidenib (Fig 2A). Despite a best response of stable disease according to the investigator, this patient subsequently achieved partial response by RANO LGG. Patient 2 had an astrocytoma and had undergone tumor resection 6 years before enrollment and had received no additional therapy in the interim. MRIs demonstrated an increase in tumor volume before enrollment. Visual inspection of coregistered images and volume growth curves showed tumor shrinkage after initiation of ivosidenib (Fig 2B). Best response by investigator for this patient was stable disease. Patient 3 had an oligodendroglioma diagnosed 4 years before enrollment and was observed without additional therapy since the initial surgery. Treatment with ivosidenib resulted in reduction of tumor volumes (Fig 2C). Best response by investigator for this patient was stable disease. Patient 4 had an oligodendroglioma diagnosed by biopsy 8 years before enrollment, was initially treated with surgery and 1 year of temozolomide, and then was observed for 7 years without additional therapy. The gradual increase in tumor volume before enrollment stabilized after initiation of ivosidenib (Fig 2D). Best response by investigator for this patient was stable disease. All of these patients were receiving ivosidenib at the time of analysis.
FIG 2.
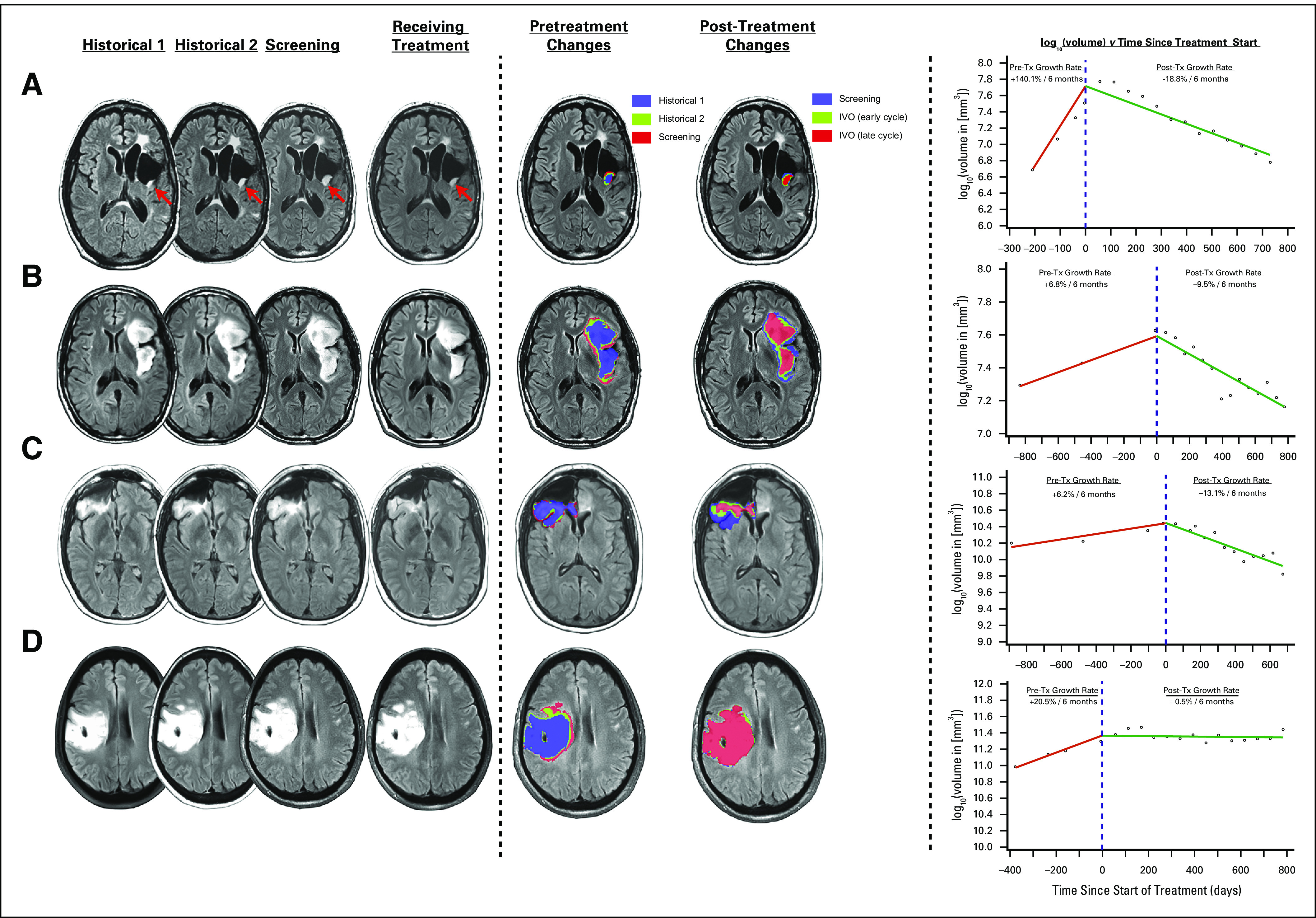
(A-D) Examples of brain magnetic resonance images and manually segmented tumor volume growth curves in 4 patients with nonenhancing glioma treated with ivosidenib. IVO, ivosidenib; Tx, treatment with ivosidenib.
DISCUSSION
The majority of human LGGs harbor IDH mutations.30 Standard treatment of LGG consists of radiation and chemotherapy. There are no approved molecularly targeted therapies for LGG, and IDH mutations represent a novel opportunity for early therapeutic intervention. Our study shows that continuous daily oral therapy with ivosidenib was well tolerated and was not associated with DLTs in patients with advanced mIDH1 glioma. An ivosidenib dose of 500 mg once per day was selected for the expansion phase.
The median PFS for patients with nonenhancing gliomas in our study compares favorably to that reported for temozolomide in advanced mIDH1 LGG (approximately 7 months).38 However, comparisons with earlier LGG studies, and in particular retrospective single-center studies, should be made with caution because these studies often included patients with both IDH wildtype and mIDH LGGs and used variable definitions of disease progression (ie, treatment-naïve progressive disease v progression after standard therapy).39 More direct evidence for the antitumor activity of ivosidenib in mIDH LGG stems from our exploratory analysis of tumor volumes, which documented shrinkage in several patients. Compared with conventional 2-dimensional measurements, tumor volume measurements that incorporate changes in tumor growth rates may represent the diffuse intracranial growth of LGG with greater confidence and accuracy,7,40 but broader implementation of this approach for LGG will require harmonization of image acquisition and analysis,34,41 as well as regulatory guidance.
Despite the heterogeneous patient population in our trial, the nonrandomized design, and the lack of central pathology review, the data from our trial suggest that ivosidenib has greater activity against nonenhancing gliomas than against enhancing gliomas. This finding may seem surprising because the absence of contrast enhancement is typically associated with impaired drug delivery. In a perioperative clinical trial (ClinicalTrials.gov identifier: NCT03343197), we recently observed that ivosidenib (at 500 mg once per day orally) reduces intratumoral 2-HG levels in nonenhancing gliomas by > 90%42 and is associated with objective responses. We hypothesize that ivosidenib may be more effective in nonenhancing gliomas because these tumors represent an earlier disease stage with fewer genetic alterations, reminiscent of the greater antitumor activity of the BCR-ABL inhibitor imatinib in earlier stages of chronic myeloid leukemia.43,44 In support of this hypothesis, we found that the presence of genetic alterations in cell cycle genes (lesions that are associated with LGG progression)5,45 was associated with shorter PFS within the subgroup of nonenhancing gliomas. On the basis of these data, additional clinical development of mIDH inhibitors for mIDH low-grade gliomas is warranted.
ACKNOWLEDGMENT
We thank the participating patients and their families, and the nurses, research coordinators, and study management team. Tara Nimkar of Agios Pharmaceuticals provided operational support for this study. MedQIA (Los Angeles, CA) assisted with image analysis. Medical writing support was provided by Mark Poirier of Excel Scientific Solutions, Fairfield, CT.
SUPPORT
Supported by Agios Pharmaceuticals. Translational research studies were supported by National Institutes of Health Grant Nos. 1 R35 NS105109 01 and P30CA008748 (I.K.M.) and the National Brain Tumor Society Defeat GBM Initiative (I.K.M. and T.F.C.).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Ingo K. Mellinghoff, Benjamin M. Ellingson, Mehdi Touat, Gregory M. Cote, Howard Burris, Robert J. Young, Raymond Huang, Chris Bowden, Shuchi S. Pandya, Timothy F. Cloughesy, Patrick Y. Wen
Provision of study material or patients: Mehdi Touat, Howard Burris, Filip Janku, Timothy F. Cloughesy, Patrick Y. Wen
Collection and assembly of data: Ingo K. Mellinghoff, Benjamin M. Ellingson, Mehdi Touat, Elizabeth Maher, Macarena I. De La Fuente, Matthias Holdhoff, Howard Burris, Filip Janku, Robert J. Young, Raymond Huang, Lori Steelman, Shuchi S. Pandya, Timothy F. Cloughesy, Patrick Y. Wen
Data analysis and interpretation: Ingo K. Mellinghoff, Mehdi Touat, Gregory M. Cote, Howard Burris, Raymond Huang, Liewen Jiang, Sung Choe, Bin Fan, Katharine Yen, Min Lu, Chris Bowden, Lori Steelman, Shuchi S. Pandya, Timothy F. Cloughesy, Patrick Y. Wen
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ivosidenib in Isocitrate Dehydrogenase 1–Mutated Advanced Glioma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ingo K. Mellinghoff
Honoraria: Roche
Consulting or Advisory Role: Agios, Puma Biotechnology, Debiopharm Group, Black Diamond Therapeutics, Voyager Therapeutics
Research Funding: General Electric, Amgen, Lilly
Travel, Accommodations, Expenses: Voyager Therapeutics, AstraZeneca, Roche, Puma Biotechnology, Agios
Benjamin M. Ellingson
Consulting or Advisory Role: Siemens, Roche/Genentech, Bristol-Myers Squibb, Northwest Biotherapeutics, Nativis, Omniox, Agios, Medicenna, MedQIA, Novogen, Tocagen, Imaging Endpoints
Research Funding: Siemens, Roche/Genentech, Agios
Travel, Accommodations, Expenses: Siemens
Mehdi Touat
Consulting or Advisory Role: Agios, Taiho Pharmaceutical, Integragen
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Elizabeth Maher
Consulting or Advisory Role: Agios, Curadev, Curadev (I), FORMA Therapeutics
Research Funding: Curadev, Curadev (I)
Travel, Accommodations, Expenses: Agios, FORMA Therapeutics
Macarena I. De La Fuente
Consulting or Advisory Role: Agios, Puma Biotechnology, Foundation Medicine, FORMA Therapeutics
Other Relationship: Targeted Oncology (I), OncLIve (I), OncInfo (I)
Matthias Holdhoff
Consulting or Advisory Role: Celgene, AbbVie, BTG, Newlink Genetics, DPClinical
Travel, Accommodations, Expenses: Arbor Pharmaceuticals
Gregory M. Cote
Consulting or Advisory Role: Agios, PharmaMar, Epizyme
Research Funding: Macrogenics (Inst), Boston Biomedical (Inst), PharmaMar (Inst), Epizyme (Inst), Agios (Inst), Eisai (Inst), Merck (Inst), Plexxikon (Inst), CBA (Inst), Bavarian Nordic
Research Funding: Bayer (Inst), Springworks Therapeutics (Inst)
Travel, Accommodations, Expenses: PharmaMar
Howard Burris
Employment: HCA Healthcare/Sarah Cannon
Leadership: HCA Healthcare/Sarah Cannon
Stock and Other Ownership Interests: HCA Healthcare/Sarah Cannon
Consulting or Advisory Role: AstraZeneca (Inst), FORMA Therapeutics (Inst), Celgene (Inst), Incyte (Inst)
Research Funding: Roche/Genentech (Inst), Bristol-Myers Squibb (Inst), Incyte (Inst), AstraZeneca (Inst), MedImmune (Inst), Macrogenics (Inst), Novartis (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Seattle Genetics (Inst), Merck (Inst), Agios (Inst), Jounce Therapeutics (Inst), Moderna Therapeutics (Inst), CytomX Therapeutics (Inst), GlaxoSmithKline (Inst), Verastem (Inst), Tesaro (Inst), Millennium (Inst), BioMed Valley Discoveries (Inst), TG Therapeutics (Inst), Vertex (Inst), eFFECTOR Therapeutics (Inst), Janssen (Inst), Gilead Sciences (Inst), BioAtla (Inst), CicloMed (Inst), Harpoon therapeutics (Inst), Arch (Inst), Arvinas (Inst), Revolution Medicines (Inst), Array BioPharma (Inst), Bayer (Inst), BIND Therapeutics (Inst), Kymab (Inst), miRNA Therapeutics (Inst), Pfizer (Inst)
Expert Testimony: Novartis (Inst)
Uncompensated Relationships: Daiichi Sankyo (Inst), Pfizer (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/201030/summary
Filip Janku
Stock and Other Ownership Interests: Trovagene
Consulting or Advisory Role: Deciphera, Trovagene, Novartis, Sequenom, Foundation Medicine, Guardant Health, Synlogic, Valeant/Dendreon, IFM Therapeutics, Sotio, PureTech, Jazz Pharmaceuticals, Immunomet, IDEAYA Biosciences
Research Funding: Novartis (Inst), BioMed Valley Discoveries (Inst), Roche (Inst), Agios (Inst), Astellas Pharma (Inst), Deciphera (Inst), Plexxikon (Inst), Piqur (Inst), Fujifilm (Inst), Symphogen (Inst), Bristol-Myers Squibb (Inst), Asana Biosciences (Inst), Astex Pharmaceuticals (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), Proximagen (Inst)
Other Relationship: Bio-Rad
Robert J. Young
Stock and Other Ownership Interests: Alexion, Agios, Biogen, Celgene, Gilead Sciences, Karyopharm Therapeutics, Spark Therapeutics, Regeneron, Stemline Therapeutics, Vertex, Merck, Amgen
Consulting or Advisory Role: Agios, Puma Biotechnology, NordicNeuroLab, ICON Clinical Research
Research Funding: Agios (Ins)
Raymond Huang
Consulting or Advisory Role: Agios
Research Funding: Agios
Liewen Jiang
Employment: Agios
Stock and Other Ownership Interests: Agios
Sung Choe
Employment: Agios
Stock and Other Ownership Interests: Agios
Patents, Royalties, Other Intellectual Property: Patents derived from my work at Agios
Travel, Accommodations, Expenses: Agios
Bin Fan
Employment: Agios
Stock and Other Ownership Interests: Agios
Travel, Accommodations, Expenses: Agios
Katherine Yen
Employment: Agios Pharmaceuticals
Leadership: Auron Therapeutics
Stock and Other Ownership Interests: Agios Therapeutics, Auron Therapeutics
Consulting or Advisory Role: Agios Therapeutics
Research Funding: Auron Therapeutics, Auron Therapeutics
Patents, Royalties, Other Intellectual Property: Patents around IDH mutant inhibitors and methods of treatment
Travel, Accommodations, Expenses: Agios Therapeutics, Auron Therapeutics
Min Lu
Employment: Agios
Stock and Other Ownership Interests: Agios
Chris Bowden
Employment: Agios
Leadership: Agios, Miragen, Ziopharm
Stock and Other Ownership Interests: Agios
Lori Steelman
Employment: Agios
Stock and Other Ownership Interests: Infinity Pharmaceuticals
Shuchi S. Pandya
Employment: Agios Pharmaceuticals
Stock and Other Ownership Interests: Agios
Research Funding: Agios
Travel, Accommodations, Expenses: Agios
Timothy F. Cloughesy
Stock and Other Ownership Interests: Notable Labs, Katmai Pharmaceuticals
Consulting or Advisory Role: Roche/Genentech, Celgene, Tocagen, VBL Therapeutics, NewGen Therapeutics, Novartis, Agios, Cortice, Novocure, AbbVie, Oxigene, Wellcome Trust, Pfizer, Notable Labs, Bristol-Myers Squibb, Merck, Insys Therapeutics, Human Longevity, Sunovion, Boston Biomedical, Novogen, Alexion Pharmaceuticals, GW Pharmaceuticals, Lilly, Genocea Biosciences, Puma Biotechnology, Deciphera, Boehringer Ingelheim, KIYATEC, VBI Vaccines, Bayer, DelMar Pharmaceuticals, QED, Amgen, Pascal Bio, Karyopharm Therapeutics
Patents, Royalties, Other Intellectual Property: U.S. Provisional application No. 62/819,322: Compositions and methods for treating cancer; Filing date: March 15, 2019; Inventor(s): David A. Nathanson et al. FH Reference No. UCH-17760 (32246-17760)Your Reference No. [UCLA 2019-630-1] US
Other Relationship: Global Coalition for Adaptive Research 501(c)(3)
Patrick Y. Wen
Consulting or Advisory Role: Agios, AstraZeneca, Vascular Biogenics, Immunomic Therapeutics, Kayatec, Puma Biotechnology, Taiho Pharmaceutical, Deciphera, VBI Vaccines, Tocagen, Bayer, Blue Earth Diagnostics, Karyopharm, Deciphera, Voyager, Taiho Pharmaceutical, QED, Imvax, Elevate Bio, Integral Health
Speakers' Bureau: Merck, Prime Oncology
Research Funding: Agios (Inst), AbbVie (Inst), AstraZeneca (Inst), Merck (Inst), Novartis (Inst), Oncoceutics (Inst), Lilly (Inst), AstraZeneca (Inst), Beigene (Inst), Kazia (Inst), MediciNova (Inst), Vacular Biogenics (Inst), VBI Vaccines (Inst), Puma (Inst), Celgene (Inst), Bayer (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53:524–528. doi: 10.1002/ana.10528. [DOI] [PubMed] [Google Scholar]
- 2.van den Bent MJ, Smits M, Kros JM, et al. Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol. 2017;35:2394–2401. doi: 10.1200/JCO.2017.72.6737. [DOI] [PubMed] [Google Scholar]
- 3.Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30194-8. [DOI] [PubMed] [Google Scholar]
- 4.Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374:1344–1355. doi: 10.1056/NEJMoa1500925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonsson P, Lin AL, Young RJ, et al. Genomic correlates of disease progression and treatment response in prospectively characterized gliomas. Clin Cancer Res. 2019;25:5537–5547. doi: 10.1158/1078-0432.CCR-19-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairncross JG, Pexman JH, Rathbone MP, et al. Postoperative contrast enhancement in patients with brain tumor. Ann Neurol. 1985;17:570–572. doi: 10.1002/ana.410170607. [DOI] [PubMed] [Google Scholar]
- 7.Pallud J, Taillandier L, Capelle L, et al. Quantitative morphological magnetic resonance imaging follow-up of low-grade glioma: A plea for systematic measurement of growth rates. Neurosurgery. 2012;71:729–739, discussion 739-740. doi: 10.1227/NEU.0b013e31826213de. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: A report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16:1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- 12.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 14.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower grade gliomas. N Engl J Med. 2015;372:2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward PS, Patel J, Wise DR, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury R, Yeoh KK, Tian YM, et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losman JA, Looper RE, Koivunen P, et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Travins J, DeLaBarre B, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 22.Rohle D, Popovici-Muller J, Palaskas N, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosmider O, Gelsi-Boyer V, Slama L, et al. Mutations of IDH1 and IDH2 genes in early and accelerated phases of myelodysplastic syndromes and MDS/myeloproliferative neoplasms. Leukemia. 2010;24:1094–1096. doi: 10.1038/leu.2010.52. [DOI] [PubMed] [Google Scholar]
- 24.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 27.Lowery MA, Burris HA, III, Janku F, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: A phase 1 study. Lancet Gastroenterol Hepatol. 2019;4:711–720. doi: 10.1016/S2468-1253(19)30189-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. doi: 10.1200/JCO.19.02492. Tap WD, Villalobos VM, Cote GM, et al: Phase 1 study of the mutant IDH1 inhibitor ivosidenib: Safety and clinical activity in patients with advanced chondrosarcoma. J Clin Oncol 38:1693-1701, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan B, Mellinghoff IK, Wen PY, et al. Clinical pharmacokinetics and pharmacodynamics of ivosidenib, an oral, targeted inhibitor of mutant IDH1, in patients with advanced solid tumors. Invest New Drugs. 2020;38:433–444. doi: 10.1007/s10637-019-00771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 31.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 33.van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12:583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 34.Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro-oncol. 2015;17:1188–1198. doi: 10.1093/neuonc/nov095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. ed 2. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 36.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuter M, Gerstner ER, Rapalino O, et al. Impact of MRI head placement on glioma response assessment. J Neurooncol. 2014;118:123–129. doi: 10.1007/s11060-014-1403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubbink HJ, Taal W, van Marion R, et al. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73:1792–1795. doi: 10.1212/WNL.0b013e3181c34ace. [DOI] [PubMed] [Google Scholar]
- 39.Nahed BV, Redjal N, Brat DJ, et al. Management of patients with recurrence of diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2015;125:609–630. doi: 10.1007/s11060-015-1910-2. [DOI] [PubMed] [Google Scholar]
- 40.Rees J, Watt H, Jäger HR, et al. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009;72:54–64. doi: 10.1016/j.ejrad.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Freyschlag CF, Krieg SM, Kerschbaumer J, et al. Imaging practice in low-grade gliomas among European specialized centers and proposal for a minimum core of imaging. J Neurooncol. 2018;139:699–711. doi: 10.1007/s11060-018-2916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mellinghoff IK, Cloughesy TF, Wen PY, et al: A phase 1, open-label, perioperative study of AG-120 and AG-881 in recurrent IDH1 mutant, low-grade glioma: Results from cohort 1. J Clin Oncol 37(15_suppl abstr):2003, 2019.
- 43.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 44.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 45.Shirahata M, Ono T, Stichel D, et al. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136:153–166. doi: 10.1007/s00401-018-1849-4. [DOI] [PubMed] [Google Scholar]