Abstract
Cell cycle progression is negatively regulated by the retinoblastoma family of pocket proteins and CDK inhibitors (CKIs). In contrast, CDKs promote progression through multiple phases of the cell cycle. One prominent way by which CDKs promote cell cycle progression is by inactivation of pocket proteins via hyperphosphorylation. Reactivation of pocket proteins to halt cell cycle progression requires dephosphorylation of multiple CDK-phosphorylated sites and is accomplished by PP2A and PP1 serine/threonine protein phosphatases. The same phosphatases are also implicated in dephosphorylation of multiple CDK substrates as cells exit mitosis and reenter the G1 phase of the cell cycle. This review is primarily focused on the role of PP2A and PP1 in the activation of pocket proteins during the cell cycle and in response to signaling cues that trigger cell cycle exit. Other functions of PP2A during the cell cycle will be discussed in brief, as comprehensive reviews on this topic have been published recently (De Wulf et al., 2009; Wurzenberger and Gerlich, 2011).
Keywords: p107, pRB, retinoblastoma, p130, B55alpha, E2F
1. Role of pocket proteins in negatively regulating passage through the restriction point and their inactivation by mitogenically activated CDKs
The cell cycle in metazoans is negatively regulated by members of the pocket protein family (reviewed in Sotillo and Graña, 2010; Wirt and Sage, 2010). In mammalian cells, pocket proteins consist of the tumor suppressor pRB and two closely related paralogs, p107 and p130. Pocket proteins are active in their hypophosphorylated state, which allows binding to a variety of proteins modulating their function. The primary target of pocket proteins to restrain cell cycle progression is the E2F family of transcription factors. Association of hypophosphorylated pocket proteins to heterodimers of E2F and DP (E2F dimerization partner) proteins forms repressor complexes that bind E2F-elements on the promoters of E2F-dependent genes required for cell cycle progression causing their silencing. Upon mitogenic stimulation, pocket proteins are phosphorylated by G1 and G1/S Cyclin/CDK complexes. p107 is primarily phosphorylated by D-type Cyclin/CDK complexes, while pRB and p130 are phosphorylated by the coordinated action of D-type Cyclin/CDK and Cyclin E/CDK2 complexes (reviewed in Sotillo and Graña, 2010; Wirt and Sage, 2010). Pocket protein hyperphosphorylation disrupts their interactions with E2F/DP complexes, eliminating repressor complexes coinciding with the expression of E2F dependent genes. Among these genes are those encoding activator E2Fs (E2F1–3), which are recruited to E2F-dependent promoters in the absence of pocket proteins and are associated with their transactivation. Activation of the E2F transcription program in late G1 corresponds with transition through the Restriction Point (Sotillo and Graña, 2010). Pocket protein inactivation is reversed by Protein Phosphatase 2A (PP2A), a serine/threonine phosphatase that opposes CDK-dependent phosphorylation of pocket proteins throughout the cell cycle (Garriga et al., 2004; reviewed in Graña, 2008)(Figure 1).
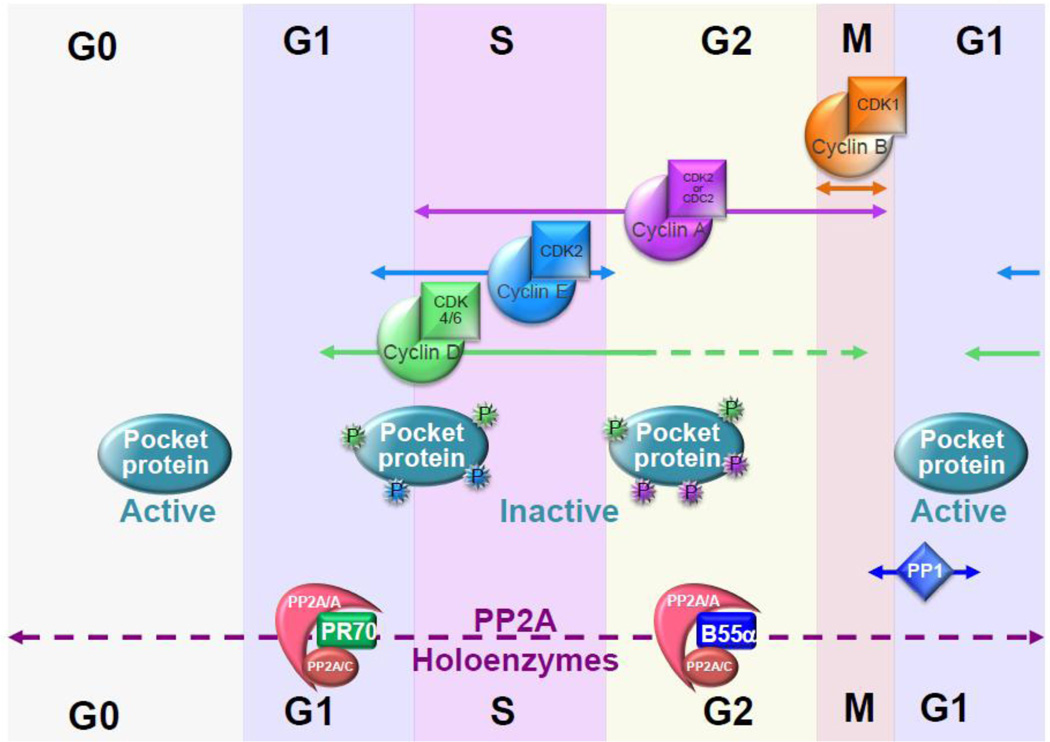
Figure 1. PP2A heterotrimeric holoenzymes modulate the phosphorylation status of the three pocket proteins throughout the cell cycle, whereas PP1 resets pRB phosphorylation in mitosis (based on Garriga et al., 2004 and Jayadeva et al., 2010).
An equilibrium between inducibly Cyclin/CDK complexes that are activated as cells progress through the cell cycle and PP2A determines the precise phosphorylation state of each protein during the cell cycle and in quiescent cells. In G0 or early G1, CDK activity is low and pocket proteins are hypophosphorylated (active) as consequence of PP2A activity. As CDKs become activated (indicated by arrows) pocket proteins become hyperphosphorylated (color coded P(s) according to responsible CDK). In late mitosis, PP1 becomes activated towards pRB leading to its hypophosphorylation (see text). B55α and PR70 trimeric PP2A holoenzymes are known to be involved in the dephosphorylation of pocket proteins and at least B55α PP2A holoenzymes appear to function throughout the cell cycle. Some of these holoenzymes appear to be also induced by specific signals. For instance, oxidative stress induces PR70 association with pRB and its dephosphorylation (see text for details). Recent work has also shown that B55/PP2A activity is low in mitosis to allow for phosphorylation of CDK1 substrates and becomes higher as cells exit mitosis ensuing dephosphorylation of CDK1 substrates and possibly p130/p107.
2. Reactivation of pocket proteins by PP1 and PP2A in mitosis
The three pocket proteins remain hyperphosphorylated and thus inactive, through S, G2 and most of M phase and are concomitantly dephosphorylated as cells exit mitosis and reenter the next G1 phase (Calbó et al., 2002). Using a nocodazole block and release method to synchronize CV-1P cells, it was found that pRb dephosphorylation begins in anaphase and continues until completion in the next G1 phase of the cell cycle (Ludlow et al., 1993). Treatment of late-mitotic phase cell extracts with okadaic acid or protein phosphatase inhibitors 1 and 2 suggested that the phosphatase responsible for pRb dephosphorylation was PP1, as the concentrations needed to inhibit dephosphorylation were much higher than the sub-nanomolar concentrations needed to inhibit PP2A in vitro (Ludlow et al., 1993). Independently, a yeast two-hybrid screen identified an isoform of PP1α, designated PP1α2, as a pRB binding protein. This study also showed that PP1α2 associated with pRB from mitosis to early G1 coinciding with the period in which pRB is hypophosphorylated (Durfee et al., 1993). However, it has been proposed that PP1 is not a major regulator of the Rbf1/E2F1 pathway in Drosophila, based on PP1 dispensability for E2f1 inhibition by Rbf1 and G1 arrest in the embryonic epidermis and for the expression of E2f1 target genes in S phase in the embryo midgut and larval salivary gland; processes that require Rbf1 (Swanhart et al., 2007). This dispensability might be due to the presence of a different phosphatase or that PP1 cooperates with a redundant phosphatase to activate pRB. An obvious possibility given our results (Jayadeva et al., 2010) and those of the Martelli lab (Magenta et al., 2008), is PP2A. In addition, despite the apparent coordinated dephosphorylation of pocket proteins from mitosis to G1 (Calbó et al., 2002), others have reported that pRB, but not p130 and p107, binds strongly to PP1 (Dunaief et al., 2002). Of note, a peptide present in the C-terminus of pRB has been co-crystalyzed with PP1, identifying a docking site for PP1 that overlaps with a RxL Cyclin/CDK docking site also at the C-terminus of pRB (Hirschi et al., 2010). It has been proposed that PP1 competition with Cyclin/CDK complexes is sufficient to activate pRB independently of its phosphatase activity. While RxL sites that mediate binding to Cyclin/CDKs are present in the spacer domain of p130 and p107, these binding motifs failed to bind PP1 (Hirschi et al., 2010), further reinforcing the hypothesis that dephosphorylation of p130/p107 in mitosis is independent of PP1 (see also below). Thus, further experimentation is needed to test this hypothesis.
3. The phosphorylation steady state of pocket proteins is modulated by equilibrium between CDKs and PP2A through the cell cycle and in quiescent cells
It was originally believed that the dephosphorylation of pocket proteins phosphorylated from mid-G1 to M phase by phase specific Cyclin/CDK complexes occurred in mitosis. Cyclin/CDK complexes were “switched on” in mid-G1 as PP1 was “switched off”. This process was reversed in mitosis; PP1 was “switched on” while Cyclin/CDK complexes were “switched off” (Durfee et al., 1993; Nelson et al., 1997; Nelson and Ludlow, 1997). However, our lab noticed that pocket proteins were abruptly dephosphorylated upon treatment of asynchronous growing cells with the protein synthesis inhibitor cycloheximide or the CDK inhibitor flavopiridol (Garriga et al., 2004). This suggested that either a phosphatase was recruited to pocket proteins following CDK inhibition, or a phosphatase that is constitutively active throughout the cell cycle is responsible for the dephosphorylation of pocket proteins upon inhibition of CDK activity. Treatment of U2OS and T98G cells with PP2A-selective concentrations of okadaic acid and calyculin A strongly suggested that PP2A or a PP2A-like phosphatase is responsible for the dephosphorylation of pocket proteins. Additionally, this phosphatase was sensitive to the SV-40 small t antigen, suggesting that the pocket protein phosphatase was an heterotrimeric PP2A holoenzyme containing a B regulatory subunit (Garriga et al., 2004). Finally, the PP2A catalytic subunit was found to coimmunoprecipitate with p107 and/or p130 in quiescent cells and cells progressing thought the cell cycle. As depicted in Figure 1, our lab proposed a novel mechanism in which a dynamic equilibrium exists between Cyclin/CDK complexes and PP2A and is responsible for modulating the phosphorylation status of all three pocket proteins throughout the cell cycle (Garriga et al., 2004). However, while the implication of PP2A on the modulation of pocket protein phosphorylation state was critical, until more recently it remained unknown the identity of the B regulatory subunits targeting PP2A to pocket proteins (see section 5).
4. Subunit composition of PP2A holoenzymes and determinants of substrate specificity
Trimeric PP2A holoenzymes consist of an “A” scaffolding subunit, a “C” catalytic subunit, and a “B” regulatory subunit. The scaffolding and catalytic subunits, known as the core dimer, have two known isoforms each: α and β. The B subunit is the major determinant in substrate specificity and subcellular localization (Shi, 2009). To date, 15 separate B subunits in four major families (B, B’, B”, and B”’) have been identified (reviewed in Eichhorn et al., 2008; Virshup and Shenolikar, 2009). The structures of the B and B’ families are well characterized and have both similarities and differences in how they interact with the core dimer. The combination of these and other subunits result in the assembly of more than 200 distinct complexes in cells (reviewed in Virshup and Shenolikar, 2009).
The B/PR55/PPP2R2 family contains at least six members transcribed from four different genes (reviewed in Eichhorn et al., 2008). These family members are highly conserved and share a β-propeller structure containing seven blades each formed by WD40 repeats. The crystal structure of the B55α subunit revealed a highly acidic groove at the top face of the β-propeller, which is believed to serve as a substrate binding site (Xu et al., 2008). The B55α subunit makes extensive contacts with the A scaffolding subunit but little contact with the C catalytic subunit. Mutations of residues on the acidic groove of the B subunit prevent in vitro dephosphorylation of Tau, a major substrate of B55α PP2A holoenzymes, in brain. One of these mutations, D197K, prevents binding of B55α to a second substrate, p107, as it will be described below. In addition to Tau and p107, several other substrates have been identified for B55α PP2A holoenzymes. β-catenin was found to directly interact with B55α, and this interaction mediates dephosphorylation and stabilization of β-catenin (Zhang et al., 2009). Ectopic expression of the B55α and B55δ subunits of PP2A increases EGF-dependent activation of the Raf1-MEK1/2-ERK1/2 signaling cascade, of which Raf1 was found to directly associate with B55α and B55δ PP2A holoenzymes triggering dephosphorylation of an inhibitory site (Adams et al., 2005). The type I TGF-β receptor (TGFβR1) was shown to associate with B55α, and this association is dependent on the kinase activity of the receptor. The type I receptor also phosphorylates B55α, and it is suggested that the B55α subunit itself regulates the TGF-β inhibitory response (Griswold-Prenner et al., 1998). Finally, B55γ was shown to target c-Src by dephosphorylating S12, which is needed for JNK activation, and this interaction is sensitive to UV irradiation (Eichhorn et al., 2007). For a more extensive review of B55-PP2A holoenzyme substrates see (Eichhorn et al., 2008).
The B’/PR61/PPP2R5 family has at least eight members transcribed from six different genes (Eichhorn et al., 2008). B56 family members are composed of a series of alpha helices known as HEAT (Huntington, Elongation Factor 3, PR65/A, TOR) repeats, which give these proteins a horseshoe-like structure. The scaffolding subunit A isoforms also share this structure. Like the B55α subunit, the B56γ subunit interacts with the N-terminus of the scaffolding subunit, however, B56γ makes also substantial contacts with the catalytic subunit (Xu et al., 2006; Cho and Xu, 2007). Many substrates are targeted by B56/PP2A holoenzymes; for an extensive list, refer to (Eichhorn et al., 2008).
B”/PR72/PPP2R3 family of subunits have at least 3 known members found in humans to date. PR72 and PR130 are transcribed from different promoters within the same gene (Hendrix et al., 1993). PR70 is the full-length form of a previously identified truncated variant designated PR48. PR48 was first identified in a yeast two-hybrid screen using the licensing factor CDC6 as bait (Yan et al., 2000). Each of these family members possesses Ca++-binding EF-hand motifs, which are needed for Ca++-dependent binding to the core enzyme (Janssens et al., 2003). PR59, another member of this family, was identified in murine cells (Voorhoeve et al., 1999). Another possible member of this family, G5PR, was identified in B cells and is believed to modulate the phosphorylation of the GANP/MCM3 complex throughout the cell cycle. It shares structural homology with the other members of the B” family and also contains EF hand motifs (Kono et al., 2002). PP2A/PR72 holoenzymes have been shown to dephosphorylate DARPP-32 at T75 in a Ca++-dependent manner (Ahn et al., 2007). In the absence of Wnt, PR72 negatively regulates the Wnt Signaling cascade by binding Naked, which binds and inhibits Dishevelled leading to β-catenin degradation (Creyghton et al., 2005). The longer isoform, PR130, appears to counteract PR72 activity and acts as a positive regulator of this pathway by preventing the Naked/Dishevelled interaction upon Wnt stimulation (Creyghton et al., 2006). Finally, PR70 is known to target pRB and CDC6, which will be discussed in the next section.
The B”’ family has two known members: striatin and SG2NA. These members also contain WD40 repeats and bind calmodulin in a calcium-dependent manner. Interestingly, striatin and SG2NA also share an epitope with the B’ family members, which may be important in core dimer binding (Moreno et al., 2000).
5. Identification of PP2A holoenzymes that modulate the phosphorylation state of pocket proteins
A great deal is known about cell cycle regulation by CDKs and many of their cell cycle phase specific substrates have been identified (Ubersax et al., 2003; reviewed in Sullivan and Morgan, 2007; Sotillo and Graña, 2010; Wurzenberger and Gerlich, 2011). Although recent work has identified B55α PP2A holoenzymes as key factors in reversing CDK mediated phosphorylation of pocket proteins (Jayadeva et al., 2010) and mitotic CDK substrates (Schmitz et al., 2009; Manchado et al., 2010) in mammalian cells, the role of PP2A in the cell cycle is comparatively much less understood. Therefore, identification and characterization of the specific holoenzymes and their cell cycle specific substrates is important for this field to progress further.
Martelli and collaborators showed that oxidative stress causes rapid and coordinated dephosphosphorylation of the three pocket proteins, which required PP2A (Cicchillitti et al., 2003) and subsequently identified a B” regulatory subunit designated PR70, as necessary and sufficient for dephosphorylation of pRB PP2A holoenzyme (Magenta et al., 2008)(Table I and Figure 2). PR48, a truncated form of PR70, was shown to interact preferentially with pRB, as compared to multiple B subunits of the B, B’ and B” families. PR70 was also found to bind pRB in cells and to be necessary for H2O2 induced pRB dephosphorylation and cell cycle arrest using loss of function studies in HUVEC cells. Of note, Ca++ mobilization increases with oxidative stress and is required for pRB dephosphorylation, which is consistent with the presence of EF-hand domains in PR70. Interestingly, the C-terminal end of pRB mediates its interaction with PR70 (Magenta et al., 2008). It is important, to point out that independent studies have also confirmed CDC6 as a bonafide substrate of PR70 trimeric holoenzymes, but no known common conserved domains have been found in these two substrates that could mediate binding to PR70. Interestingly, while Ca++ mobilization does not affect the PR70/CDC6 interaction, it increases the association of CDC6 with the catalytic and scaffold subunits (Davis et al., 2008). Our lab has identified B55α PP2A holoenzymes as major modulators of the phosphorylation state of p107 in human cells, to a lesser extent, p130 and with little if any effect on pRB (Figure 1 and Table I). These effects correlate with the binding preferences of the three pocket proteins in vitro, as we found p107 to interact strongly with B55α, and to a lesser extent with PR70, while the opposite is true for pRB. Interestingly, p130 appears to be able to similarly bind both B55α and PR70. Limited ectopic expression of B55α, but not other B subunits induces p107 dephosphorylation, and B55α knockdown results in p107 hyperphosphorylation (Jayadeva et al., 2010). Of note, we found that p107 binds B55α even if the PP2A A–C dimer is displaced in cells expressing SV40 st antigen. Our data suggests that members of the B and B” family, but not the B’ family cooperate to regulate the phosphorylation state of pocket proteins.
Table I.
Protein Phosphatases targeting pocket proteins
| Signal | Phosphatase | Pocket Protein (or smads) |
Reference |
|---|---|---|---|
| Inducible Signals | |||
| H2O2/ Oxidative Stress | PP2A/PR48/PR70 | pRb | Magenta et al., 2008 |
| PP2A/? | p107, p130 | Cicchillitti et al., 2003 | |
| UV Irradiation | PP2A/PR59 | p107 | Voorhoeve et al., 1999b |
| All-trans- Retinoic Acid | PP2A/? | p130 | Purev et al., 2006 |
| FGF | PP2A/B or B' | p107 | Dailey et al., 2003, Kolupaeva et al., 2008 |
| TGFβ | PP2A/? | p107 | Chen et al., 2002 |
| BMP | PP2A/B55β | Smads | Bengtsson et al., 2009 |
| Cell Cycle | |||
| Throughout | PP2A/B55α | p107, p130 | Jayadeva et al., 2010 |
| PP2A/PR70? | pRb | Jayadeva et al., 2010 | |
| Mitosis/Early G1 | PP1α2 | pRb | Ludlow et al., 1993, Durfee et al., 1993 |
| PP2A + CDK inhibition | pRb?, p107?, p130? | Calbó et al., 2002, Garriga et al., 2004 |
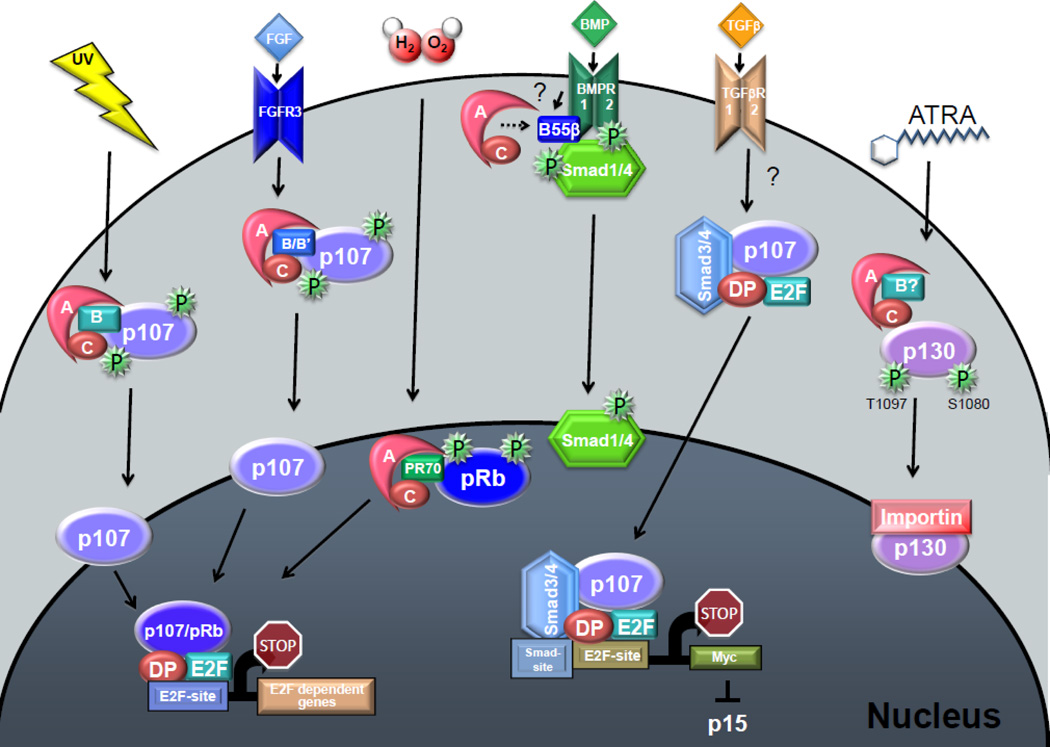
Figure 2. Upregulation of PP2A activity in response to various signals is associated with pocket protein dephosphorylation and cell cycle exit and maturation.
PP2A holoenzymes are activated in response to various signals including UV irradiation, FGF stimulation, H2O2 treatment, and BMP and all-trans-retinoic acid (ATRA) ligand/receptor binding. UV irradiation leads to specific dephosphorylation of p107, but the B subunit of the holoenzyme is unknown. FGF stimulation in chondrocytes leads to specific p107 dephosphorylation and the B subunit of the PP2A holoenzyme is likely a member of the B or B’ families. Oxidative stress results in pRB dephosphorylation by PR70 PP2A holoenzymes. While no specific phosphatase has been implicated in the formation of the p107/Smad/E2F complexes in response to TGFβ signaling (question mark), it was previously demonstrated that B55β interacts with the BMP receptor recruiting the PP2A/A–C dimer to ensure dephosphorylation of a linker site on SMADS that triggers nuclear translocation. Similar mechanisms could conceivably lead to TGFβ-mediated formation of the SMAD/p107/E2F/DP complex that translocates to the nucleus to repress Myc expression, in turn leading to derepression of p15 and growth arrest in epithelial cells. Finally, ATRA stimulates dephosphorylation of p130 and recruitment of p130 to de nucleus by importins. However, whether a B subunit is involved it is not known. Activation of pocket proteins via dephosphorylation results in complex formation with E2F/DP transcription factors leading to the repression of the E2F dependent gene program and eventual cell cycle exit. In these signaling pathways, association of pocket proteins with differentiation or maturation factors is also possible (see text for additional details).
Another B subunit capable of binding p107, PR59 (ppp2r3d in mouse), was identified through a yeast two-hybrid screen using the pocket domain of p107 as bait (Voorhoeve et al., 1999). PR59 associates with mammalian p107, but not pRB, and over-expression of PR59 in U2-OS cells results in the dephosphorylation of p107. PR59 and PR72 (ppp2r3a) mRNAs are differentially expressed in mouse tissues and both are located on murine chromosome 9, however, PR72 does not bind to any of the pocket proteins (Voorhoeve et al., 1999). Notably, PR59 is expressed in murine cells, but has not been identified in humans. Conversely, human PR70 (ppp2r3b) has not been identified in murine cells, and both PR59 and PR70 exhibit higher identity to each other than to PR72, their species paralog. However, human PR70 and mouse PR59 have not been classified as orthologs, perhaps because PR70 is located on the X chromosome in humans, which is not an equivalent chromosome location to where PR59 maps in the mouse. Nevertheless, it appears likely that they share functional roles, although they seem to display distinct specificities for pocket proteins (Voorhoeve et al., 1999; Magenta et al., 2008).
6. Selective pocket protein PP2A holoenzyme interactions may obey structural determinants
As mentioned previously, the B subunit of the PP2A holoenzyme is responsible for substrate specificity. There are four known major families of B substrates, and the members of each family are highly homologous. The four members of the B55 family have β-propeller structures containing WD40 repeats, while the B56 family members are comprised of α-helices similar in structure to the PR65 scaffolding subunit. The diversity of these structures alone most likely affect which substrates are able to bind specific holoenzymes.
The crystal structure of the PP2A/B55α holoenzyme was recently solved. From this structure, a potential substrate binding site on the acidic central groove of the top face of the B55α subunit was identified (Xu et al., 2008). Mutations of several residues in this site lead to a decreased ability of the PP2A/B55α holoenzyme to dephosphorylate the microtubule-associated protein Tau in vitro. The region in Tau that is responsible for PP2A/B55α binding was also identified- a basic region encompassing the tubulin-binding domains (Xu et al., 2008). B55α D197K, a point mutant that was shown to be deficient in Tau dephosphorylation, is also unable to bind p107 or the PP2A core enzyme (Jayadeva et al., 2010). However, other B55α mutants defective on Tau dephosphorylation in vitro, bind p107 similarly to wild type B55α, suggesting that substrate specific residues dictate the selectivity for different substrates. On the other hand, it was shown that the spacer region of p107 interacts with B55α, and this interaction is enhanced by the C-terminus of p107, which does not bind B55α on its own (Jayadeva et al., 2010). This is different from the interaction of PR70 with pRB that seems to contact both the pocket and the C-terminus of pRB independently (Magenta et al., 2008). Also, as p107 and p130 are more similar to each other than to pRb, specifically in the spacer region, this may explain why p107 and p130 are preferentially targeted by PP2A/B55α holoenzymes, while pRb is primarily targeted by PR70 (Magenta et al., 2008; Jayadeva et al., 2010)(Table I). In another study, the C-terminus but not the pocket of p130 was shown to associate with purified PP2A/C or PP2A/C form lysates of all-trans-retinoic acid treated ovarian cells in vitro (Purev et al., 2011), suggesting a mode of recruiting PP2A by p130 likely independent of B55α. Interestingly, it has been recently shown that the interaction between pRB and PP1 occurs through an overlapping kinase and phosphatase docking site located at residues 870–882 on pRb (Hirschi et al., 2010). In this same study, it was shown that CDKs and PP1 compete for access to this site, shedding light on a new mechanism on the control of pRb activity. Considering that these motifs are in the spacer region of p107 and p130, where B55α complexes bind (Zhu et al., 1995a; Zhu et al., 1995b; Lacy and Whyte, 1997; Woo et al., 1997), it is tempting to speculate that PP2A holoenzymes may interact with p107 and p130 in a fashion similar to PP1/pRB.
7. Upregulation of PP2A activity is associated with pocket protein dephosphorylation and cell cycle exit and maturation
PP2A holoenzymes maintain an equilibrium with CDKs targeting pocket proteins throughout the cell cycle and even in quiescent cells poised to restrict hyperphosphorylation of pocket proteins and prompting rapid pocket protein dephosphorylation following CDK inactivation (Figure 1)(Garriga et al., 2004). It is conceivable that various distinct holoenzymes act coordinately in this equilibrium, as B55α knockdown on its own does not prevent dephosphorylation induced by abrupt inhibition with FVP (Jayadeva and Graña, unpublished observations). As pointed above, however, progress has been made in identification of cellular instances where hypophosphorylation of pocket proteins precedes CDK inactivation, making a strong case for PP2A playing a regulatory rate-limiting role in these situations (Figure 2 and Table I).
As mentioned in a previous section, one example is oxidative stress induced by the addition of H2O2 in HUVEC cells where PP2A/PR70 holoenzymes become rapidly activated as result of Ca++ mobilization coinciding with Ca++-dependent dephosphorylation of pRB. Since ectopic expression of PR70 is sufficient to trigger pRB dephosphorylation, PR70 knockdown prevents H2O2-mediated dephosphorylation, and p130 and p107 are dephosphorylated with similar kinetics, it is conceivable that the same holoenzyme also targets p130/p107 under these conditions (Cicchillitti et al., 2003). However, this possibility is intriguing considering that p107 apparently shows a relative lower affinity for PR70 (Jayadeva et al., 2010).
In contrast to oxidative stress that leads to coordinated dephosphorylation of the three pocket proteins, other signals described so far modulate the phosphorylation status of just one pocket protein, suggesting that specific activation of a particular pocket protein is important in those situations (Figure 2 and Table I). UV irradiation results in the preferential dephosphorylation of p107 by PP2A holoenzymes (Voorhoeve et al., 1999b). NIH3T3 cells arrest transiently in G1 following exposure to UV irradiation. Extracts from these cells revealed the formation of an E2F/p107 complex, as well as an increase in the hypophosphorylated from of p107. Pre-treatment of these cells with okadaic acid and calyculin A, or overexpression of a B regulatory subunit prior to irradiation prevented dephosphorylation of p107 and/or the formation of E2F/p107 complexes, implicating a yet to be characterized unknown PP2A holoenzyme in this process (Voorhoeve et al., 1999b).
Treatment with all-trans-retinoic acid has been shown to stabilize p130 in CAOV cells, which are arrested in G1 after treatment. This stabilization correlates with a decrease in ubiquitination of p130, as well as an increase in PP2A activity and PP2A/C protein levels (Purev et al., 2006). Decreased ubiquitination likely results from dephosphorylation of CDK sites that are important for binding of the SKP2 ubiquitin ligase to p130 (Tedesco et al., 2002; Bhattacharya et al., 2003). Growth inhibition in ATRA treated CAOV cells was reduced with siRNAs against PP2A/C (Purev et al., 2006). A mechanism was later elucidated. PP2A dephosphorylates the S1080 and T1097 residues at the C-terminal end of p130. These residues lie adjacent to two nuclear localization signals, which then interact with importins after dephosphorylation, resulting in the translocation of p130 to the nucleus and cell cycle arrest (Purev et al., 2011). No B subunit has been implicated in this process (Figure 2).
In response to FGF1 treatment in rat chondrosarcoma (RCS) cells, a PP2A holoenzyme complex rapidly dephosphorylates p107 within 30 minutes, which leads to a potent G1 cell cycle arrest by 24 hours. FGF1 signaling in chondrocytes is key to skeletal bone development (Dailey et al., 2003) and p107/p130 are required for endochondral bone formation in vivo (Cobrinik et al., 1996). Subsequently, p107 was shown to be essential for FGF-induced cell cycle exit in primary chondrocyte micromass cultures (Laplantine et al., 2002). Interestingly, p107, but not pRB or p130, is dephosphorylated by PP2A within minutes of FGF1 stimulation, and PP2A activity is required for both p107 dephosphorylation and subsequent pocket protein dependent cell cycle exit (Kolupaeva et al., 2008)(Figure 2 and Table I). p130 and pRb are dephosphorylated ~10–15 hours after treatment. The implication of PP2A holoenzymes is strongly suggested by the sensitivity of the PP2A/p107 interaction and PP2A dependent growth arrest to the expression of SV40 st antigen and adenovirus E4orf4, which are know to displace B and B’ subunits from the core PP2A A–C dimer (Kolupaeva et al., 2008).
Finally, in epithelial cells, hypophosphorylated p107 associates with SMADs (transcriptional regulators induced by TGF-β) in the cytoplasm forming complexes that modulate gene expression upon TGF-β -induced translocation to the nucleus, leading to cell cycle exit (Chen et al., 2002). SMADs themselves are targeted by PP2A upon TGF-β/BMP stimulation (Bengtsson et al., 2009), but the role of these phosphatases ensuring that p107 remains active has not been explored (Figure 2 and Table I).
8. PP2A holoenzymes implicated in the activation of pocket proteins and cell cycle exit are also involved in mitotic exit
The B55α PP2A holoenzymes implicated in the dephosphorylation and, thus activation of p107 and p130, would be expected to negatively regulate cell cycle progression. However, these complexes also play a critical role in promoting mitotic exit in higher eukaryotes from Drosophila to mice and humans. An RNAi screen using live-cell imaging implicated B55α and the nuclear transport factor importin-β as key regulators in post mitotic spindle disassembly, Golgi apparatus assembly and chromatin decondensation (Schmitz et al., 2009). Also in this study, it was found that B55α PP2A holoenzyme activity towards CDK1 substrates is higher in interphase than in mitotic cells extracts. In keeping with this observation, B55α PP2A holoenzyme subunits are more phosphorylated in mitosis than in interphase cells and phosphorylation of B55α on S167 reduces PP2A complex assembly in vitro (Schmitz et al., 2009), unveiling a possible mechanism for activation of B55α PP2A holoenzymes as cells exit mitosis. Biochemical studies in Xenopus extracts has also shown that PP2A activity towards CDK substrates is reduced in mitosis. B55δ PP2A holoenzymes appear to be responsible for dephosphorylation of many CDK substrates in interphase and the activity of B55δ holoenzymes is negatively regulated by the Greatwall (Gwl) kinase, which is activated in mitosis (Castilho et al., 2009; Mochida et al., 2009; Vigneron et al., 2009). Indeed, Gwl depletion, which inhibits mitotic entry, is rescued by depletion of B55δ. More recently two substrates of Gwl have been identified as novel inhibitors of the B55δ holoenzyme in mitosis (Gharbi-Ayachi et al., 2010; Mochida et al., 2010). Moreover, other B55 family members have been previously shown to exhibit anti-mitotic activity in Xenopus oocytes and extracts (Lee et al., 1994; Iwashita et al., 1997). Another study in mice showed that cells null for the activating subunit of the Anaphase Promoting Complex, Cdc20, which is essential for anaphase, exit mitosis upon forced inactivation of Cdk1 and the kinase Mastl/Gwl. In particular, treatment of Cdc20 null cells with siRNA against Mastl and B55α, B55β and β55δ revealed that the B55α and B55δ subunits, but not the B55β subunit, are needed for mitotic exit (Manchado et al., 2010).
Thus, in sum, B55 PP2A activity is inhibited as higher eukaryotic cells enter mitosis to allow for efficient phosphorylation of CDK1 substrates, and subsequently needs to be activated for cells to exit mitosis and enter the next G1 phase. Given our results showing the sensitivity of p107, and to a lesser extent p130, phosphorylation state to forced modulation of B55α expression in human cells (Jayadeva et al., 2010) and the absence of efficient docking sites for PP1 (Hirschi et al., 2010), it appears likely that dephosphorylation of p130 and p107 in mitosis is mediated by PP2A/B55 holoenzymes. There might be a different scenario for pRB, as it contains domains that interact with PP1 that are not present in p130/p107, as discussed above. Additional experimentation is required to definitively demonstrate which phosphatase targets p130 and p107 in mitosis.
A final though is that while activation of PP2A late in mitosis resets CDK1 substrates to their hypophosphorylated state to complete mitosis and in preparation for initiation of a new cell cycle, activation of PP2A activity towards pocket proteins via specific signals is associated with sustained pocket protein dephosphorylation and G1 arrest. It remains to be elucidated if similar trimeric holoenzymes can selectively induce distinct responses as result of unique upstream modulators responding to extracellular signals or environmental cues.
Highlights.
Cell cycle progression is negatively regulated by the pRB pocket protein family.
Pocket proteins also promote differentiation in a context dependent manner.
Reactivation of pocket proteins requires dephosphorylation of multiple CDK sites.
PP2A/PP1 phosphatases dephosphorylate pocket proteins and mitotic CDK substrates.
This review focuses on PP2A’s role in pocket protein activation by cellular cues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DG, Coffee RL, Jr, Zhang H, Pelech S, Strack S, Wadzinski BE. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem. 2005;280:42644–42654. doi: 10.1074/jbc.M502464200. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Sung JY, McAvoy T, Nishi A, Janssens V, Goris J, Greengard P, Nairn AC. The B"/PR72 subunit mediates Ca2+dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc Natl Acad Sci U S A. 2007;104:9876–9881. doi: 10.1073/pnas.0703589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson L, Schwappacher R, Roth M, Boergermann JH, Hassel S, Knaus P. PP2A regulates BMP signalling by interacting with BMP receptor complexes and by dephosphorylating both the C-terminus and the linker region of Smad1. J Cell Sci. 2009;122:1248–1257. doi: 10.1242/jcs.039552. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Garriga J, Calbo J, Yong T, Haines DS, Graña X. SKP2 associates with p130 and accelerates p130 ubiquitylation and degradation in human cells. Oncogene. 2003;22:2443–2451. doi: 10.1038/sj.onc.1206339. [DOI] [PubMed] [Google Scholar]
- Calbó J, Parreño M, Sotillo E, Yong T, Mazo A, Garriga J, Graña X. G1 cyclin/CDK coordinated phosphorylation of endogenous pocket proteins differentially regulates their interactions with E2F4 and E2F1 and gene expression. J Biol Chem. 2002;277:50263–50274. doi: 10.1074/jbc.M209181200. [DOI] [PubMed] [Google Scholar]
- Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell. 2009;20:4777–4789. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CR, Kang Y, Siegel PM, Massague J. E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell. 2002;110:19–32. doi: 10.1016/s0092-8674(02)00801-2. [DOI] [PubMed] [Google Scholar]
- Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445:53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- Cicchillitti L, Fasanaro P, Biglioli P, Capogrossi MC, Martelli F. Oxidative stress induces PP2A-dependent de-phosphorylation of the pocket proteins pRb p107 and p130. J Biol Chem. 2003 doi: 10.1074/jbc.M300511200. [DOI] [PubMed] [Google Scholar]
- Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N, Harlow E, Beach D, Weinberg RA, Jacks T. Shared role of the pRb-related p130 and p107 proteins in limb development. Genes & Development. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- Creyghton MP, Roel G, Eichhorn PJ, Hijmans EM, Maurer I, Destree O, Bernards R. PR72, a novel regulator of Wnt signaling required for Naked cuticle function. Genes Dev. 2005;19:376–386. doi: 10.1101/gad.328905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton MP, Roel G, Eichhorn PJ, Vredeveld LC, Destree O, Bernards R. PR130 is a modulator of the Wnt-signaling cascade that counters repression of the antagonist Naked cuticle. Proc Natl Acad Sci U S A. 2006;103:5397–5402. doi: 10.1073/pnas.0507237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey L, Laplantine E, Priore R, Basilico C. A network of transcriptional and signaling events is activated by FGF to induce chondrocyte growth arrest and differentiation. J Cell Biol. 2003;161:1053–1066. doi: 10.1083/jcb.200302075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AJ, Yan Z, Martinez B, Mumby MC. Protein phosphatase 2A is targeted to cell division control protein 6 by a calcium-binding regulatory subunit. J Biol Chem. 2008;283:16104–16114. doi: 10.1074/jbc.M710313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wulf P, Montani F, Visintin R. Protein phosphatases take the mitotic stage. Curr Opin Cell Biol. 2009;21:806–815. doi: 10.1016/j.ceb.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Dunaief JL, King A, Esumi N, Eagen M, Dentchev T, Sung CH, Chen S, Zack DJ. Protein Phosphatase 1 binds strongly to the retinoblastoma protein but not to p107 or p130 in vitro and in vivo. Curr Eye Res. 2002;24:392–396. doi: 10.1076/ceyr.24.5.392.8524. [DOI] [PubMed] [Google Scholar]
- Durfee T, Becherer K, Chen PL, Yeh SH, Yang Y, Kilburn AE, Lee WH, Elledge SJ. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes & Development. 1993;7:555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Eichhorn PJ, Creyghton MP, Wilhelmsen K, van Dam H, Bernards R. A RNA interference screen identifies the protein phosphatase 2A subunit PR55gamma as a stress-sensitive inhibitor of c-SRC. PLoS Genet. 2007;3:e218. doi: 10.1371/journal.pgen.0030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga J, Jayaraman AL, Limon A, Jayadeva G, Sotillo E, Truongcao M, Patsialou A, Wadzinski BE, Grana X. A Dynamic Equilibrium Between CDKs and PP2A Modulates Phosphorylation of pRB p107 and p130. Cell Cycle. 2004;3 doi: 10.4161/cc.3.10.1183. [DOI] [PubMed] [Google Scholar]
- Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- Graña X. Downregulation of the phosphatase nuclear targeting subunit (PNUTS) triggers pRB dephosphorylation and apoptosis in pRB positive tumor cell lines. Cancer Biol Ther. 2008;7:842–844. doi: 10.4161/cbt.7.6.6298. [DOI] [PubMed] [Google Scholar]
- Griswold-Prenner I, Kamibayashi C, Maruoka EM, Mumby MC, Derynck R. Physical and functional interactions between type I transforming growth factor beta receptors and Balpha, a WD-40 repeat subunit of phosphatase 2A. Mol Cell Biol. 1998;18:6595–6604. doi: 10.1128/mcb.18.11.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix P, Mayer-Jackel RE, Cron P, Goris J, Hofsteenge J, Merlevede W, Hemmings BA. Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J Biol Chem. 1993;268:15267–15276. [PubMed] [Google Scholar]
- Hirschi A, Cecchini M, Steinhardt RC, Schamber MR, Dick FA, Rubin SM. An overlapping kinase and phosphatase docking site regulates activity of the retinoblastoma protein. Nat Struct Mol Biol. 2010;17:1051–1057. doi: 10.1038/nsmb.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita J, Shima H, Nagao M, Sagata N. cDNA cloning of a novel B subunit of Xenopus protein phosphatase 2A and its biological activity in oocytes. Biochem Biophys Res Commun. 1997;232:218–222. doi: 10.1006/bbrc.1997.6259. [DOI] [PubMed] [Google Scholar]
- Janssens V, Jordens J, Stevens I, Van Hoof C, Martens E, De Smedt H, Engelborghs Y, Waelkens E, Goris J. Identification and functional analysis of two Ca2+binding EF-hand motifs in the B"/PR72 subunit of protein phosphatase 2A. J Biol Chem. 2003;278:10697–10706. doi: 10.1074/jbc.M211717200. [DOI] [PubMed] [Google Scholar]
- Jayadeva G, Kurimchak A, Garriga J, Sotillo E, Davis AJ, Haines DS, Mumby M, Grana X. B55alpha PP2A holoenzymes modulate the phosphorylation status of the retinoblastoma-related protein p107 and its activation. J Biol Chem. 2010;285:29863–29873. doi: 10.1074/jbc.M110.162354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolupaeva V, Laplantine E, Basilico C. PP2A-mediated dephosphorylation of p107 plays a critical role in chondrocyte cell cycle arrest by FGF. PLoS One. 2008;3:e3447. doi: 10.1371/journal.pone.0003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y, Maeda K, Kuwahara K, Yamamoto H, Miyamoto E, Yonezawa K, Takagi K, Sakaguchi N. MCM3-binding GANP DNA-primase is associated with a novel phosphatase component G5PR. Genes Cells. 2002;7:821–834. doi: 10.1046/j.1365-2443.2002.00562.x. [DOI] [PubMed] [Google Scholar]
- Lacy S, Whyte P. Identification of a p130 domain mediating interactions with cyclin A/cdk 2 and cyclin E/cdk 2 complexes. Oncogene. 1997;14:2395–2406. doi: 10.1038/sj.onc.1201085. [DOI] [PubMed] [Google Scholar]
- Laplantine E, Rossi F, Sahni M, Basilico C, Cobrinik D. FGF signaling targets the pRb-related p107 and p130 proteins to induce chondrocyte growth arrest. J Cell Biol. 2002;158:741–750. doi: 10.1083/jcb.200205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Turck C, Kirschner MW. Inhibition of cdc2 activation by INH/PP2A. Molecular Biology of the Cell<. 1994;5:323–338. doi: 10.1091/mbc.5.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow JW, Glendening CL, Livingston DM, DeCarprio JA. Specific enzymatic dephosphorylation of the retinoblastoma protein. Molecular & Cellular Biology. 1993;13:367–372. doi: 10.1128/mcb.13.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magenta A, Fasanaro P, Romani S, Di Stefano V, Capogrossi MC, Martelli F. Protein phosphatase 2A subunit PR70 interacts with pRb and mediates its dephosphorylation. Mol Cell Biol. 2008;28:873–882. doi: 10.1128/MCB.00480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchado E, Guillamot M, de Carcer G, Eguren M, Trickey M, Garcia-Higuera I, Moreno S, Yamano H, Canamero M, Malumbres M. Targeting mitotic exit leads to tumor regression in vivo: Modulation by Cdk1, Mastl, and the PP2A/B55alpha,delta phosphatase. Cancer Cell. 2010;18:641–654. doi: 10.1016/j.ccr.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. Embo J. 2009;28:2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- Moreno CS, Park S, Nelson K, Ashby D, Hubalek F, Lane WS, Pallas DC. WD40 repeat proteins striatin and S/G(2) nuclear autoantigen are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J Biol Chem. 2000;275:5257–5263. doi: 10.1074/jbc.275.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Krucher NA, Ludlow JW. High molecular weight protein phosphatase type 1 dephosphorylates the retinoblastoma protein. J Biol Chem. 1997;272:4528–4535. doi: 10.1074/jbc.272.7.4528. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Ludlow JW. Characterization of the mitotic phase pRb-directed protein phosphatase activity. Oncogene. 1997;14:2407–2415. doi: 10.1038/sj.onc.1201081. [DOI] [PubMed] [Google Scholar]
- Purev E, Giordano A, Soprano DR, Soprano KJ. Interaction of PP2A catalytic subunit with Rb2/p130 is required for all-trans retinoic acid suppression of ovarian carcinoma cell growth. J Cell Physiol. 2006;206:495–502. doi: 10.1002/jcp.20490. [DOI] [PubMed] [Google Scholar]
- Purev E, Soprano DR, Soprano KJ. PP2A interaction with Rb2/p130 mediates translocation of Rb2/p130 into the nucleus in all-trans retinoic acid-treated ovarian carcinoma cells. J Cell Physiol. 2011;226:1027–1034. doi: 10.1002/jcp.22418. [DOI] [PubMed] [Google Scholar]
- Schmitz MH, Held M, Janssens V, Hutchins JR, Hudecz O, Ivanova E, Goris J, Trinkle-Mulcahy L, Lamond AI, Poser I, Hyman AA, Mechtler K, Peters JM, Gerlich DW. Live-cell imaging RNAi screen identifies PP2A-B55alpha and importin-beta1 as key mitotic exit regulators in human cells. Nat Cell Biol. 2009;12:886–893. doi: 10.1038/ncb2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Sotillo E, Graña X. Cell Cycle Deregulation in Cancer, Contemporary Cancer Research. Springer Publishing; 2010. Escape from cellular quiescence; pp. 3–22. [Google Scholar]
- Sullivan M, Morgan DO. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol. 2007;8:894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]
- Swanhart LM, Sanders AN, Duronio RJ. Normal regulation of Rbf1/E2f1 target genes in Drosophila type 1 protein phosphatase mutants. Dev Dyn. 2007;236:2567–2577. doi: 10.1002/dvdy.21265. [DOI] [PubMed] [Google Scholar]
- Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, Morgan DO. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425:859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- Vigneron S, Brioudes E, Burgess A, Labbe JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. Embo J. 2009;28:2786–2793. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Voorhoeve PM, Hijmans EM, Bernards R. Functional interaction between a novel protein phosphatase 2A regulatory subunit, PR59, and the retinoblastoma-related p107 protein. Oncogene. 1999;18:515–524. doi: 10.1038/sj.onc.1202316. [DOI] [PubMed] [Google Scholar]
- Voorhoeve PM, Watson RJ, Farlie PG, Bernards R, Lam EW. Rapid dephosphorylation of p107 following UV irradiation. Oncogene. 1999b;18:679–688. doi: 10.1038/sj.onc.1202289. [DOI] [PubMed] [Google Scholar]
- Wirt SE, Sage J. p107 in the public eye: an Rb understudy and more. Cell Div. 2010;5:9. doi: 10.1186/1747-1028-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MS, Sanchez I, Dynlacht BD. p130 and p107 use a conserved domain to inhibit cellular cyclin- dependent kinase activity. Mol Cell Biol. 1997;17:3566–3579. doi: 10.1128/mcb.17.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzenberger C, Gerlich DW. Phosphatases: providing safe passage through mitotic exit. Nat Rev Mol Cell Biol. 2011;12:469–482. doi: 10.1038/nrm3149. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chen Y, Zhang P, Jeffrey PD, Shi Y. Structure of a protein phosphatase 2A holoenzyme: insights into B55-mediated Tau dephosphorylation. Mol Cell. 2008;31:873–885. doi: 10.1016/j.molcel.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y. Structure of the protein phosphatase 2A holoenzyme. Cell. 2006;127:1239–1251. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Yan Z, Fedorov SA, Mumby MC, Williams RS. PR48, a novel regulatory subunit of protein phosphatase 2A, interacts with Cdc6 and modulates DNA replication in human cells. Mol Cell Biol. 2000;20:1021–1029. doi: 10.1128/mcb.20.3.1021-1029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yang J, Liu Y, Chen X, Yu T, Jia J, Liu C. PR55 alpha, a regulatory subunit of PP2A, specifically regulates PP2A-mediated beta-catenin dephosphorylation. J Biol Chem. 2009;284:22649–22656. doi: 10.1074/jbc.M109.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Enders G, Lees JA, Beijersbergen RL, Bernards R, Harlow E. The pRB-related protein p107 contains two growth suppression domains: independent interactions with E2F and cyclin/cdk complexes. EMBO Journal. 1995a;14:1904–1913. doi: 10.1002/j.1460-2075.1995.tb07182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Harlow E, Dynlacht BD. p107 uses a p21CIP1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes & Development. 1995b;9:1740–1752. doi: 10.1101/gad.9.14.1740. [DOI] [PubMed] [Google Scholar]