Abstract
Custom polydimethylsiloxane (PDMS) microfluidic devices allow for small-volume human blood research under hemodynamic conditions of bleeding and clotting. However, issues of PDMS molding/assembly, bio-coating, and sample preparation often limit their point-of-care use. We aim to develop a microfluidic device that has the same utility as previously established PDMS devices but which is more usable in point-of-care operation. We designed an injection-molded 1 × 3 in.2 device with eight flow paths crossing a bio-printed surface of a collagen/tissue factor. The device is rapidly primed and compatible with multi-channel pipetting (<0.5 ml blood) and operates under venous or arterial shear rates using constant flow rate or constant pressure modes. Platelet and fibrin deposition were monitored dynamically by the imaging of immunofluorescence. For whole blood clotting at a wall shear rate of 200 s−1, the intrachip CV at 400 s for platelet and fibrin deposition was 10% and the interdonor CV at 400 s was 30% for platelet and 22% for fibrin deposition (across 10 healthy donors). No significant difference was detected for samples tested on a new chip vs a chip stored for 6 months at 4 °C. Using the fibrin signal, dose–response testing of whole blood revealed IC50's of 120 nM for rivaroxaban and apixaban, and 60 nM for dabigatran. A complete reversal of apixaban inhibition was observed for an equimolar addition of Xa DOAC reversal agent Andexanet Alfa. We demonstrate the ability to manufacture single-use, storage-stable eight-channel chips. In clinical settings, such chips may help evaluate patient bleeding risk, therapy choice, drug activity, or reversal.
BACKGROUND
One of the key functions of blood in the human body is to maintain hemostasis—that is, to keep blood in circulation and arrest leakage at the site of an injury when it occurs. The injury process exposes tissue factor and collagen from the vessel that stimulate clot formation. This is achieved by the activation and accumulation of platelets into a plug, and the formation of a mesh of fibrin generated from the polymerization of the circulating plasma protein fibrinogen, which is carried out by the coagulation cascade. In order to monitor and assess this function of blood, various assays exist that detect either the presence or action of either platelets or fibrin or both.
Over the last decade, microfluidic assays have proven to be an effective diagnostic tool for assessing platelet function and coagulation, creating relevant flow hemodynamics, while utilizing a minimal volume of blood.1–12 Polydimethylsiloxane (PDMS) is a popular choice for devices in these assays due to the speed and ease of fabrication, reusability, and fidelity to the design geometry.13,14 PDMS enables the replication of flow channel geometries on the scale of tens of micrometers, allowing physiological shear rates while utilizing only microliters of blood. Flow parallelization, as demonstrated in the previously developed eight-channel microfluidic device, allows for testing varied experimental conditions simultaneously, such as dose–response testing of platelet or coagulation inhibitors in a single donor blood sample.1,2,15,16 More specialized single-thrombus devices have also been demonstrated, which incorporate trans-thrombus transport into the assay.4,6 The versality of the microfluidic platform allows for variations in the particular thrombogenic surfaces and flow delivery methods used in each assay, providing more complete recreation of thrombotic and hemostatic processes.5,7,8,12,17,18
Although PDMS devices are reliable and easy to construct, they are typically only transiently sealed, often with vacuum or clamping, and need to be assembled prior to each use. Several studies have been performed with blood samples from clinical cohorts, but these experiments require a highly trained individual to perform them. Challenges associated with preparing devices ahead of time complicates experimental timing and typically necessitates the use of strong anti-coagulants.2,9,10,19 Furthermore, traditional soft lithography methods for producing PDMS devices from silicon masters do not have an obvious method of production scale-up. Although PDMS microfluidic devices offer significant design freedom and enable novel observations in thrombosis and hemostasis research, their application to the clinical environment remains an existing challenge.
Several point-of-care (POC) diagnostic techniques exist for assessing patient anti-coagulation status and platelet function, but many have very specific readouts, and none have demonstrated themselves as the clinical standard for hemostasis diagnostics. Thromboelastography and rotational thromboelastometry (TEG/ROTEM) are both POC viscoelastic tests that rapidly produce data from whole blood samples over a 30–60 min test.20 Viscoelastic methods emerged over 50 years ago and are beginning to find use in the field of trauma surgery. Viscoelastic methods monitor a whole blood clot but do not capture the impact of flow in a physiological way, due to their closed-system nature. Furthermore, viscoelastic measurements do not detect specific molecular entities (platelet receptors, fibrin) and only recently entered the stage of POC use.20,21 The Platelet Function Analyzer 100 (PFA-100) is a microfluidic assay that uses pressure sensors to detect the occlusion of an aperture coated with collagen and adenosine diphosphate (ADP), with the time to reach occlusion as the primary metric. This test is limited to the platelet function, however, and operates at very high shear rates (>5000 s−1), limiting its ability to capture physiological flow conditions and making the assay quite sensitive to variations in the von Willebrand factor (VWF).22 A second microfluidic technique that has been under recent investigation is the total thrombus formation analysis system (T-TAS). This assay has the advantage of operating at physiological shear rates and in an open system, but generates only a single clot per chip, has shown a poor correlation with direct oral anti-coagulant (DOAC) doses, and has poor agreement with the more established thromboelastographic methods.23,24
This study used validated flow experiments and reagents from PDMS microfluidic techniques and deployed an injection molded chip to create a versatile technology for medical professionals at the bedside.1–3,7,9,19 A POC microfluidic device needs to (1) utilize small volumes (<1 ml) of whole blood, (2) have a scalable production method, (3) be simple to prime and operate by non-expert users, (4) retain uniform flow behavior across all test conditions, (5) be storage stable in a prepared state, and (6) have a total experimental footprint suitable for benchtop use. Thus, by retaining the critical geometric parameters of prior PDMS devices, but manufacturing the parts via injection molding, and streamlining the operational procedures, a new microfluidic device serves clinical studies in thrombosis and hemostasis testing.1 Stable sealing and stable reagents are also required.25,26 We demonstrate a storage stable device that can be rapidly used with a fresh whole blood sample in a multiplexed eight-channel readout of platelet and fibrin function.
MATERIALS AND METHODS
Device design and flow simulation
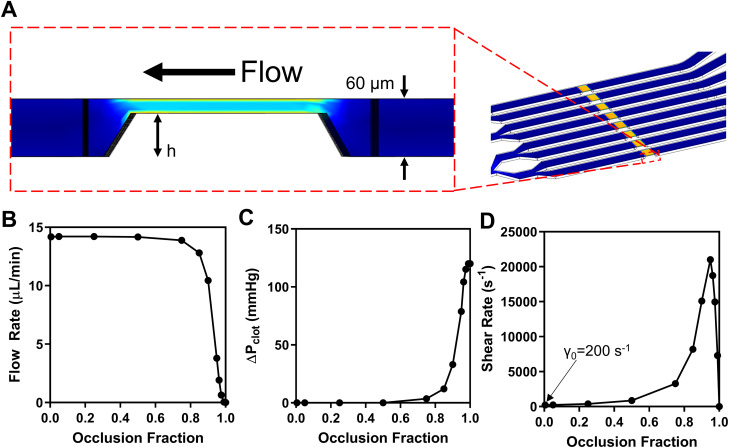
A previously published eight-channel microfluidic design deployed in PDMS devices was translated to fit on a 75.5 mm by 25.5 mm standard microscope slide footprint, to facilitate translation to an injection molding platform [Fig. 1(a)]. SolidWorks 2018 (Dassault Systèmes) was used to generate a full 3D model of the device flow paths. Steady state flow simulations (COMSOL 5.3a) were performed to verify uniformity of flow rates in each of the channels and to verify wall shear rates (Newtonian viscosity = 4 cP). To experimentally verify flow rate uniformity, solutions of FITC-conjugated flow cytometry beads (Spherotech) with four distinct peaks were added symmetrically to each of the four wells on both sides of the chip. The bead solutions were perfused through the device for 10 min and the effluent was collected and analyzed on an Accuri C6 flow cytometer (BD Biosciences). These results were compared with a uniformly pre-mixed control sample of all bead populations. Additional simulations were performed to monitor flow rate, pressure drop, and wall shear rate as a simulated clot formed and reduced the channel cross section.
FIG. 1.
(a) Schematic of injection molded chip with eight channels designed to have equal flow when applying a negative pressure at outlet. (b) Visual demonstration of priming, where priming fluid (green) injected in the dedicated priming inlet arrives at each inlet port and the outlet port simultaneously. (c) Blood added to inlet wells displaces priming fluid when a negative pressure applied at the outlet.
Device preparation and flow control
Experimental chips were assembled from pre-made, injection-molded cyclic-olefin-copolymer plastic components (ChipShop) with flow-paths molded into them as rectangular troughs. Type I equine fibrillar collagen (Chrono-Log) and lipidated tissue factor (TF) (Siemens) were coated as a 250 μm wide strip to a sheet of an optically clear substrate with an adhesive (ThorLabs), using a PDMS patterning device as previously described.1 The adhesive sheet was then used to form the fourth wall of the rectangular cross section (supplementary material, Fig. 1). Blood was perfused through the completed chips by applying a negative pressure at the outlet using a KPV14A miniature diaphragm vacuum pump (Koge Electronics) with a USB-controlled pressure controller (Fluigent). The pressure was calibrated to yield a centerline velocity of 0.004 m/s and an initial flow rate of 14 μl/min, corresponding to an initial wall shear rate of 200 s−1. The effluent blood from the device was captured in a liquid trap with a custom tubing adapter (Fluigent).
Blood collection
Blood was collected from consenting healthy donors self-reporting as free of any medications for at least 10 days. Blood was drawn via venipuncture into the corn trypsin inhibitor (CTI, Haematologic Technologies) to a final concentration of 4 mg/dl (40 μg/ml), in order to prevent Factor XIIa activation prior to the experiment, while allowing tissue factor-initiated thrombin generation in the surface test region of coated collagen/TF. All procedures were conducted in accordance with protocols approved by the University of Pennsylvania's Institutional Review Board and the Declaration of Helsinki.
Platelet and fibrin detection during clotting
Platelets were labeled in whole blood by the addition of a non-function blocking, AlexaFluor 488-conjugated anti-CD61 molecule (Bio-Rad Laboratories), while fibrin was quantified by the addition of AlexaFluor 594-conjugated human fibrinogen (Life Technologies). Chips were mounted on a three-color, LED-based Lumascope 620 (Etaluma) benchtop epifluorescence microscope with a 2.5× objective and primed with 150 μl of a solution 0.5% bovine serum albumin (BSA) in HEPES-buffered saline (HBS). A volume of 35 μl of blood was added to each of the eight inlet wells, with a negative pressure applied to the single outlet to initiate flow at t = 0. Fluorescence images were captured at 10 s intervals for green and red LED channels to obtain kinetic data for platelet and fibrin accumulation. Image sequences were analyzed with ImageJ to calculate the average pixel brightness of a 200 μm by 200 μm region center within each clot zone, in order to exclude sidewall effects of the rectangular channels.
Stability testing
In order to assess the ability of collagen and tissue factor reagents to retain activity over time, chips were prepared as described and then stored in a 4 °C refrigerator in an air-tight chamber with a desiccant. After 6 months, chips were removed from the chamber and tested in the microfluidic assay. For comparison, a second device prepared immediately before use was also tested with the same blood sample.
DOAC testing
To test the effects of DOACs added ex vivo, dry rivaroxaban or apixaban (SelleckChem) were dissolved in DMSO (20 mM stock solution) and diluted to 100 μM in HBS, then further diluted for final concentrations of 0.05, 0.2, 0.5, and 5 μM after the addition of 90 μl of whole blood in separate microcentrifuge tubes. HBS was added to each tube such that the total volume of drug solution and additional buffer was 10 μl in each, to ensure the hematocrit remained consistent across all conditions and whole blood composed 90% of the final volume.
To compare the Xa inhibitors with a direct thrombin inhibitor, Dabigatran (active form) (SelleckChem), dry powder was dissolved in a solution of 10% trifluoroacetic acid in de-ionized water, to an initial concentration of 40 mM. This stock was then diluted to 100 μM in HBS and again then further diluted for final concentrations of 0.05, 0.2, 0.5, and 5 μM after the addition of 90 μl of whole blood in separate microcentrifuge tubes.
The platelet and fibrin accumulation assay described above was then performed for each DOAC, with one well of the control condition (no drug), one well of 5 μM drug, and two wells each for the remaining concentrations. Chips were run immediately after DOAC dosing of blood.
To evaluate the extent that DOAC reversal can be observed using this assay, Andexanet Alfa (Portola Pharmaceuticals) was added to whole blood containing Apixaban. Samples were prepared for 0.02 μM Apixaban, 0.02 μM Apixaban with a 1:1 molar ratio of Andexanet Alfa, and 0.02 μM Apixaban with a 1:5 molar ratio. For each chip, two wells each were allocated for the control condition (no drug, no reversal agent), and two each of the aforementioned drug combinations. Chips were run immediately after dosing of blood.
Statistical analysis
Statistical significance was calculated for an unpaired t-test, using GraphPad Prism (GraphPad Software). Shaded error regions on plots represent the standard deviation from the mean. Coefficients of variation (CV) are reported for the 400 s time points in each experiment. Concentrations for 50% inhibition (IC50) were calculated using the t = 400 s time point for each concentration, with the “log(inhibitor) vs response” 3-parameter routine in Prism, with a fixed baseline at the fluorescence intensity noise level of the microscope (FI = 2.5 A.U.).
RESULTS
Injection-molded, disposable chip
A previously described eight-channel PDMS device was successfully converted to an injection molded 1 × 3 in.2 microscope slide footprint.1 The 60 μm height × 250 μm width of each channel was retained from the original device. The inlet well locations were altered to be linearly oriented, with 96-well spacing for convenient pipetting. Serpentine elements were added to the flow-paths such that the fluidic resistance of each channel was identical, ensuring that the flow rate through each channel was identical [Fig. 1(a)]. The final chip (up to 5 chips at a time) was bonded with manual pressure (single pass with moderate pressure) applied by “ink” roller, a processing step highly amenable to roller automation. Failure of bonding indicated by fluid leakage was never observed in manufacturing runs up to 100 chips. An additional port was added to the design for back-priming of the device with 0.5% BSA for the passivation of the wetted surfaces to minimize FXIIa generation and platelet adhesion [Fig. 1(b)]. Extra flow resistance was added to the converged outlet channel such that priming fluid was evenly split toward the inlet channels and the single outlet port. Priming was achieved by injecting 150 μl priming fluid with a 1 ml plastic syringe through a luer check valve (supplementary material, Fig. 1).
Flow simulations
Uniformity of flow was verified by both simulation (supplementary material, Fig. 2) and experiment (supplementary material, Fig. 3), ensuring identical hemodynamics in each lane. In order to characterize the changes to the flow as experiments progress for a new fluid delivery method, COMSOL simulations were performed for an inlet pressure of 16 000 Pa (120 mmHg) over each inlet and an outlet pressure of 0 Pa (atmosphere) in order to match the total ΔP to the negative pressure typically applied to the outlet in experiments (160 mbar vacuum). Pressure was maintained as constant, as the hydrostatic pressure is a negligible contribution (∼20 Pa) and, therefore, changes in inlet well height do not alter the value meaningfully. Furthermore, the reservoir hydrostatic head never drove blood flow spontaneously in the primed chip prior to operation. Blood was modeled as a Newtonian fluid with a density of 1060 kg/m3 and a viscosity of 4 cP, as blood's non-Newtonian properties are considered unimportant for shear rates in excess of 100 s−1.27 The impact of thrombus growth on the overall flow was modeled by enforcing a reduction in the lumen area of the channel over the 250 μM × 250 μM collagen-intersection region in discrete steps and running a separate flow simulation for each thrombus height [Fig. 2(a)]. This height restriction was represented as an occlusion fraction between 0 and 1. The flow rate, pressure drop across the clot region, and wall shear rate at the top surface of the thrombus were calculated for occlusion fraction [Figs. 2(b) and 2(c)]. In all experiments, clot height changes during the experiment if platelets are functional, causing the fluidics to evolve. When full occlusion is reached, this corresponds with platelet signal reaching its maximal value and, in this way, the instantaneous clot height relative to the channel height can be interpreted by the fluorescence intensity in proportion to the maximal value.5 Although experiments for this study were not run to full occlusion, this value has been observed as ∼60 A.U. for the lighting conditions and label concentrations used in this work.
FIG. 2.
(a) 2D side view of the focal region of a single channel from a 3D model of chip. (b) Volumetric flow rate as a function of occlusion fraction (h/60), for a constant outlet vacuum pressure. (c) Pressure drop across the clot as a function of occlusion fraction. (D) Shear rate at the top surface of the clot as a function of clot height.
For experiments with <70% occlusion (∼70% maximal platelet FI), the pressure drop is essentially constant, as is the flow rate, however, the wall shear rate increases as shown in Fig. 2. As the clotting event approaches full occlusion, the wall shear rate on the exposed surface of the clot becomes pathological reaching 20 000 s−1, a shear rate sufficient to extend and deposit von Willebrand factor (VWF) and mediate shear induced platelet activation [Fig. 2(d)].5,28
Healthy whole blood clotting on collagen and tissue factor
In order to establish the baseline performance of the newly designed device, whole blood from N = 10 healthy donors was perfused through devices prepared as described at 200 s−1, with all n = 8 channels running simultaneously [Figs. 3(a) and 3(b)]. For the 10 healthy donors (8 male), the platelet signal measured by image analysis of immunofluorescence showed an average interdonor CV of 30%, with the fibrin signal displaying a CV of 22%. No differences were observed between the platelet or fibrin signals and the specific inlet well from which the blood originated, verifying that the flow in each channel was identical.
FIG. 3.
Average platelet intensity (a) and fibrin intensity (b) vs time for N = 10 donors, and n = 8 clots per donor for a constant outlet pressure yielding an initial wall shear rate of 200 s−1. (c) Fluorescence microscopy images for platelets (AF488 CD-61) and fibrin (AF594-conjugated human fibrinogen) at t = 400 s.
Chip stability
Prefabricated microfluidic chips retained activity when stored at 4 °C for 6 months in dry conditions when tested at the same 200 s−1 venous condition as previously described. Both platelet and fibrin signal showed similar response when compared to a chip prepared immediately prior to use (NS, p > 0.05), indicating minimal degradation of the collagen or tissue factor reagents during the storage period (Fig. 4). Interestingly, chips stored at room temperature showed significant reduction in the platelet signal after less than one week, likely indicating loss activity in the GPVI binding domains of the collagen.
FIG. 4.
Comparison of a chip used immediately after preparation (day 1) and a chip used 6 months after preparation (stored at 4 degrees Celsius in a desiccated chamber) for platelet (a) and fibrin (b) intensity. Data for N = 1 donor, and n = 8 clots per chip, where blood is perfused with constant outlet pressure yielding an initial wall shear rate of 200 s−1. No significance was detected in the fresh vs stored chips for either platelets or fibrin (p > 0.05).
DOAC detection
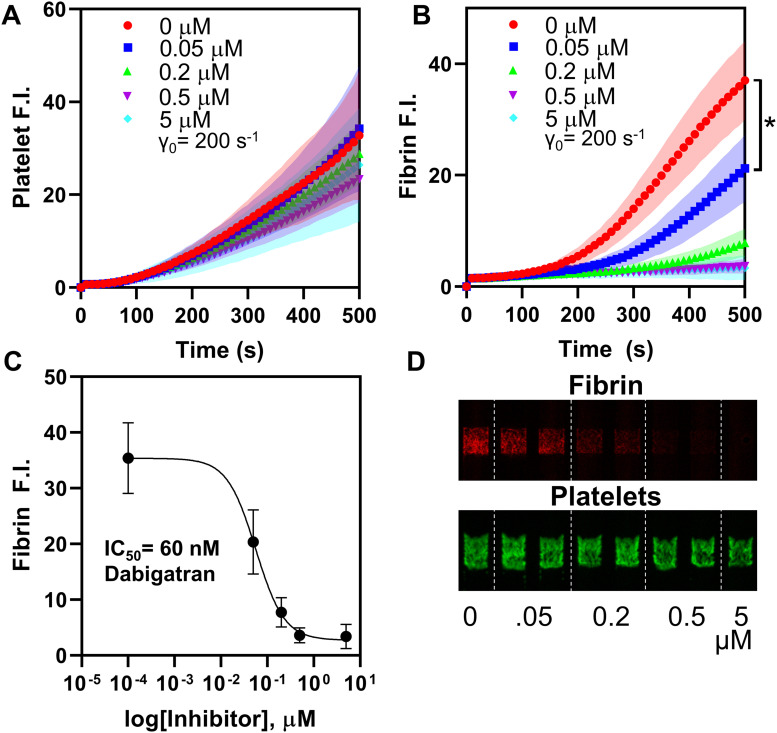
To assess the ability of our assay to determine the anti-coagulation status of blood sample, the platelet and fibrin accumulation assay was run at four concentrations of rivaroxaban. For N = 3 healthy donors, a strong dose dependent response in fibrin signal was observed for each DOAC added to blood ex vivo, yielding an IC50 of 120 nM for rivaroxaban and apixaban, and 60 nM for dabigatran [Figs. 5(c), 5(f), and 6(c)]. The lowest concentrations of rivaroxaban, apixaban, and dabigatran (50 nM) all showed a statistically significant difference in fibrin signal at 500 s (p < 0.01). The platelet deposition signal had minimal correlation with drug concentration, indicating that in this assay, collagen activation of platelet GPVI dominated the platelet deposition.
FIG. 5.
Fluorescence data for whole blood treated with Factor Xa inhibitors rivaroxaban (a)–(c) and apixaban (d)–(f), showing limited platelet response (a) but a strong dose-dependent response for fibrin signal (b). (c) and (f) Log[inhibitor] vs fibrin intensity indicating an IC50 of 120 nM for both inhibitors in this assay. A statistically significant difference in fibrin signal was observed at 500 s for both drugs (*p < 0.01).
FIG. 6.
Fluorescence data for whole blood treated with Factor IIa inhibitor Dabigatran, showing limited platelet response (a) but a strong dose-dependent response for fibrin signal (b), and micrographs of the same at t = 400 s (d). (c) Log[inhibitor] vs fibrin intensity indicating an IC50 of 60 nM in this assay. A statistically significant difference in fibrin signal was observed at 500 s (*p < 0.01).
Dabigatran was significantly more potent than either of the factor Xa inhibitors in these assays for fibrin signal (Fig. 6). Controls run with TFA alone confirmed that this difference was not due to the action of the vehicle solvent but rather the drug itself. Platelet signal was similarly limited in response to drug concentration for each DOAC.
DOAC reversal
To evaluate the ability of the assay to detect reversal of DOAC drug action in whole blood, platelet and fibrin accumulation assays were run for three combinations of apixaban and the mutant inactive factor Xa molecule Andexanet Alfa. For N = 3 healthy donors, the anticipated ∼50% inhibition of fibrin signal was observed for 0.2 μM apixaban alone, which was completely reversed to control behavior by an equimolar ratio of Andexanet Alfa [Fig. 7(b)]. A 1:5 ratio of Andexanet Alfa to apixaban yielded partial restoration of fibrin signal. For all combinations of drugs, no clear trend was observed in the platelet signal [Fig 7(a)].
FIG. 7.
Fluorescence data for whole blood treated with 200 nM Factor Xa inhibitor apixaban with and without mutant inactive factor Xa molecule Andexanet Alfa (Andexxa) as a reversal agent. Platelets (a) show no dependence on either drug or reversal agent, whereas fibrin (b) is partially inhibited by apixaban, but is recovered completely by an equimolar ratio of Andexanet Alfa, and partially recovered by 1:5 deficit of Andexanet Alfa.
DISCUSSION
Custom microfluidic devices are widely used in thrombosis/hemostasis research laboratories for the interrogation of small blood samples under diverse hemodynamic flows and real time multi-color imaging. PDMS devices have allowed the study of hemophiliac blood, neonatal blood, and trauma patient blood.9,19,29 Additionally drugs such as COX inhibitors, P2Y12 inhibitors, reFVIIa, and polyphosphate inhibitors have been studied using microfluidics.1–3,9,15,16 However, such PDMS devices are difficult to use in the emergency room (ER) or bedside point-of-care (POC) diagnostics due to obstacles in (a) manufacturing scale-up and (b) ease of operation.
Using only 250 μl of whole blood, this single use and disposable chip allowed eight independent clotting events to proceed simultaneously under venous shear rates, while monitoring platelet deposition and fibrin production over time. A chip design was developed to demonstrate (1) the ability to injection mold small features into plastic; (2) successful collagen/TF microprinting; (3) successful mechanical bonding of the chip layers; (4) successful priming fluidics; (5) collagen/TF integrity during bonding; (6) retention of collagen and tissue factor activity in a dry state at 4 °C; and (7) blood compatibility. Operation of the chip required eight-channel pipetting of blood into reagent wells (detectors, inhibitors, etc.) and then onto the chip, suitable for manual or automated pipetting.
Injection molding enables this chip design to be manufactured at scales of thousands per run, rather than the tens per day possible by PDMS replica molding, while maintaining a low cost per chip. By altering the flow delivery to a constant negative pressure applied through a dry tubing line, the assay preparation process has been simplified by removing the need for a prefilled syringe to mount in a syringe pump. Furthermore, clots formed under a constant outlet pressure show reduced ballistic embolization of near-occlusive clots, compared to clots formed under constant flow rate perfusion.
Several alternative microfluidic sealing methods exist to create multilayer devices, such as ultrasonic welding, solvent bonding, and thermal bonding.30–32 The need to retain the activity of biological reagents within the chip through sealing limits options, however, and was verified in this study. The use of collagen pre-patterned in a localized, ready-to-use dried state in this format offers an unique advantage to this new device. In other assays, alternative agonists such as adenosine diphosphate are used (TEG-6S) or collagen is typically added from a solution either stored refrigerated or frozen (aggregometry).33 The pressure sensitive adhesive used in this study provided a robust seal that held for >6 months and showed no observable off-target platelet adhesion or fibrin formation, when used with a passivating BSA priming solution. The new device retained uniform behavior channel to channel, as indicated by the 10% intrachip CV. In addition, manufacturing is scalable to mass manufacturing levels of 102–103 chips per run.
More than 4 × 106 U.S. patients are currently prescribed DOACs for diseases such as atrial fibrillation (A-fib) and deep vein thrombosis (DVT).34 With increasing use of DOACs comes a corresponding increase in patients at risk of bleeding, making monitoring patients for bleeding risk an ever more critical task. There does not exist, however, a reliable, standardize laboratory method of monitoring the anticoagulation status for DOAC patients in clinical settings, a need that the assay described in this work may help address. Expected values for drug concentration (Cmax and Ctrough) levels for patients on dabigatran (150 mg twice daily), rivaroxaban (20 mg once daily), and apixaban (5 mg twice daily) are known from plasma measurements, providing a range of interest for detection.35 Typical rivaroxaban plasma concentration ranges between approximately 115 and 320 nM, depending on the dosage, a range that is within the detectable range of the described assay, based on the measured 120 nM IC50.36 The IC50 for rivaroxaban has previously been reported at lower values (21 nM) in other assays, by directly quantifying Factor Xa activity rather than correlating with fibrin signal, in assays carried out in plasma under static conditions.37 The ISTH recommended maximum rivaroxaban concentration prior to urgent intervention is 30 ng/ml (∼70 nM) for patients at risk of bleeding, where Factor Xa inhibitor reversal agents are recommended at all higher concentrations.38 With 50 nM rivaroxaban detectable in the assay reported (corresponding to ∼22 ng/ml), the device presented may be useful in quantifying DOACs to help assess patient risk relative to ISTH guidelines. Comparable detection ranges have been reported for the TEG-6s (30 ng/ml), but without the ability to run multiplexed assays to the same extent, the ability to independently monitor platelets and fibrin, and without physiological flow conditions.39 With the high cost of Factor Xa reversal agents (>$50 000 per full dose), the ability to provide fast, point-of-care measurements of a patient's current inhibition levels could potentially improve decision making for management of bleeding patients.40 In addition, since the assay is quantifying fibrin rather than Xa activity, IIa inhibitors can be detected without modifying the assay, as shown in Fig. 6, unlike in TEG assays.
In addition to patients on DOACs, traumatic injury patients present another case where monitoring current anticoagulation status is critical. Trauma is the leading cause of death in the U.S. and accounted for approximately 1.7 × 106 hospital visits a year for the period of 2000–2011 and can lead to platelet dysfunction and bleeding risk from trauma induced coagulopathy (TIC).41 The ability to independently monitor platelet and fibrin signal may also help differentiate DOAC anticoagulation and trauma induced coagulopathy (TIC), as TIC is typically associated with platelet hypofunction as well as coagulation dysfunction, which has been previously observed in previous microfluidic assays.19,42 Direct inhibition of Factor Xa, contrastingly, does not show a significant impact on platelet signal in this assay, except the highest concentrations—far in excess of therapeutic doses [Figs. 5(a) and 5(d)]. Other currently utilized methods such as prothrombin time (PT) and activated partial-thromboplastin time (aPTT) are not able to differentiate DOAC anticoagulation and dysfunction due to TIC.40 Although sensitivity to DOACs has been demonstrated in TEG, the single “amplitude” output signal similarly makes distinguishing between platelet and coagulation dysfunction more ambiguous without performing additional comparative experiments in the presence of platelet α2bβ3 inhibitors.43
Detection of the action of a reversal agent in a DOAC measurement assay is critically important to distinguish drug-related anticoagulant behavior from disease or trauma associated coagulopathy. The ability of the microfluidic assay described in this work to detect the action of reversal agent on therapeutic dosages of a DOAC using less than 0.5 ml of blood, and ∼1 μg of reversal agent could provide utility in bedside applications for bleeding risk assessment.
The device defined in this study allows rapid POC use for the multiplexed assay design (8 channels ×2 or 3 colors), ideal for blood phenotyping, drug dose–response, and risk assessment.
SUPPLEMENTARY MATERIAL
See the supplementary material for figures regarding construction of the chip and experiments confirming the uniformity of flow in each channel.
AUTHORS' CONTRIBUTIONS
All simulations and experiments were conducted by J.M.R. Both authors contributed to research design, data analysis, and manuscript preparation.
ACKNOWLEDGMENTS
This work was performed with support by the National Institutes of Health (NIH) Grants [Nos. R01-HL-103419, U01-HL131053 (S.L.D.)] and pre-doctoral training Grant [No. 5T32HL007439-38 (J.M.R)]. Andexanet Alfa was provided by Dr. Rodney Camire, CHOP. Scott Diamond and Jason Rossi are inventors on a patent licensed to FloBio LLC. Jason Rossi is a consultant for FloBio LLC.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Maloney S. F., Brass L. F., and Diamond S. L., Integr. Biol. 2, 183 (2010). 10.1039/b919728a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R., Fries S., Li X., Grosser T., and Diamond S. L., Clin. Chem. 59, 1195 (2013). 10.1373/clinchem.2012.198101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu S., Travers R. J., Morrissey J. H., and Diamond S. L., Blood 126, 1494 (2015). 10.1182/blood-2015-04-641472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muthard R. W. and Diamond S. L., Lab Chip 13, 1883 (2013). 10.1039/c3lc41332b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colace T. V., Muthard R. W., and Diamond S. L., Arterioscler. Thromb. Vasc. Biol. 32, 1466 (2012). 10.1161/ATVBAHA.112.249789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoeman R. M., Rana K., Danes N., Lehmann M., Di Paola J. A., Fogelson A. L., Leiderman K., and Neeves K. B., Cell. Mol. Bioeng. 10, 3 (2017). 10.1007/s12195-016-0469-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu S. and Diamond S. L., Thromb. Res. 134, 1335 (2014). 10.1016/j.thromres.2014.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen R. R., Tipnis A. A., White-Adams T. C., Di Paola J. A., and Neeves K. B., Langmuir 27, 13648 (2011). 10.1021/la2023727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R., Panckeri K. A., Fogarty P. F., Cuker A., and Diamond S. L., Haemophilia 23, 759 (2017). 10.1111/hae.13259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabowski E. F., Curran M. A., and Van Cott E. M., Thromb. Res. 129, e18 (2012). 10.1016/j.thromres.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 11.Branchford B. R., Ng C. J., Neeves K. B., and Di Paola J., Thromb. Res. 136, 13 (2015). 10.1016/j.thromres.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Z., Lu J., Zhang C., Hsia I., Yu X., Marecki L., Marecki E., Asmani M., Jain S., Neelamegham S., and Zhao R., Nat. Commun. 10, 2051 (2019). 10.1038/s41467-019-10067-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mcdonald J. C., Duffy D. C., Anderson J. R., and Chiu D. T., Electrophoresis 21, 27 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Zhang C. and Neelamegham S., Platelets 28, 434 (2017). 10.1080/09537104.2017.1319047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M., Hotaling N. A., Ku D. N., and Forest C. R., PLoS One 9, e82493 (2014). 10.1371/journal.pone.0082493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosokawa K., Ohnishi T., Sameshima H., Miura N., Ito T., Koide T., and Maruyama I., Thromb. Haemost. 109, 102 (2013). 10.1160/TH12-06-0441 [DOI] [PubMed] [Google Scholar]
- 17.De Witt S. M., Swieringa F., Cavill R., Lamers M. M. E., Van Kruchten R., Mastenbroek T., Baaten C., Coort S., Pugh N., Schulz A., Scharrer I., Jurk K., Zieger B., Clemetson K. J., Farndale R. W., Heemskerk J. W. M., and Cosemans J. M. E. M., Nat. Commun. 5, 4257 (2014). 10.1038/ncomms5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onasoga-Jarvis A. A., Puls T. J., O’Brien S. K., Kuang L., Liang H. J., and Neeves K. B., J. Thromb. Haemost. 12, 373 (2014). 10.1111/jth.12491 [DOI] [PubMed] [Google Scholar]
- 19.Li R., Elmongy H., Sims C., and Diamond S. L., J. Trauma Acute Care Surg. 80, 440 (2016). 10.1097/TA.0000000000000915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen A. and Teruya J., Clin. Lab. Med. 29, 391 (2009). 10.1016/j.cll.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 21.Dunham C. M., Rabel C., Hileman B. M., Schiraldi J., Chance E. A., Shima M. T., Molinar A. A., and Hoffman D. A., Thromb. J. 12, 1 (2014). 10.1186/1477-9560-12-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward C. P. M., Harrison P., Cattaneo M., Ortel T. L., and Rao A. K., J. Thromb. Haemost. 4, 312 (2006). 10.1111/j.1538-7836.2006.01771.x [DOI] [PubMed] [Google Scholar]
- 23.Lawson P. J., Moore H. B., Moore E. E., Gerich M. E., Stettler G. R., Banerjee A., Schulick R. D., and Nydam T. L., J. Surg. Res. 231, 54 (2018). 10.1016/j.jss.2018.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishii M., Kaikita K., Ito M., Sueta D., Arima Y., Takashio S., Izumiya Y., Yamamoto E., Yamamuro M., Kojima S., Hokimoto S., Yamabe H., Ogawa H., and Tsujita K., Sci. Rep. 7, 1 (2017). 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serra M., Pereiro I., Yamada A., Viovy J. L., Descroix S., and Ferraro D., Lab Chip 17, 629 (2017). 10.1039/C6LC01319H [DOI] [PubMed] [Google Scholar]
- 26.Khashayar P., Amoabediny G., Larijani B., Hosseini M., Van Put S., Verplancke R., and Vanfleteren J., J. Brazilian Soc. Mech. Sci. Eng. 39, 1469 (2017). 10.1007/s40430-016-0684-6 [DOI] [Google Scholar]
- 27.Berger S. A. and Jou L., Annu. Rev. Fluid Mech. 32, 347 (2000). 10.1146/annurev.fluid.32.1.347 [DOI] [Google Scholar]
- 28.Brass L. F. and Diamond S. L., J. Thromb. Haemost. 14, 906 (2016). 10.1111/jth.13280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaza E. A., Egalka M. C., Zhou H., Chen J., Evans D., Prats J., Li R., Diamond S. L., Vincent J. A., Bacha E. A., and Diacovo T. G., JACC Basic Transl. Sci. 2, 465 (2017). 10.1016/j.jacbts.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Y., Zhang Z., Wang X., and Zheng Y., Microelectron. Eng. 87, 2429 (2010). 10.1016/j.mee.2010.04.020 [DOI] [Google Scholar]
- 31.Brown L., Koerner T., Horton J. H., and Oleschuk R. D., Lab Chip 6, 66 (2006). 10.1039/B512179E [DOI] [PubMed] [Google Scholar]
- 32.Sun Y., Kwok Y. C., and Nguyen N. T., J. Micromech. Microeng. 16, 1681 (2006). 10.1088/0960-1317/16/8/033 [DOI] [Google Scholar]
- 33.Ranucci M. and Baryshnikova E., J. Clin. Med. 9, 189 (2020). 10.3390/jcm9010189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rawal A., Ardeshna D., Minhas S., Cave B., Ibeguogu U., and Khouzam R., Ann. Transl. Med. 7, 411 (2019). 10.21037/atm.2019.07.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuker A., Burnett A., Triller D., Crowther M., Ansell J., Van Cott E. M., Wirth D., and Kaatz S., Am. J. Hematol. 94, 697 (2019). 10.1002/ajh.25475 [DOI] [PubMed] [Google Scholar]
- 36.Harenberg J., Krämer S., Du S., Zolfaghari S., Schulze A., Krämer R., Weiss C., Wehling M., and Lip G. Y. H., Eur. J. Clin. Invest. 44, 743 (2014). 10.1111/eci.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perzborn E., Strassburger J., Wilmen A., Pohlmann J., Roehrig S., Schlemmer K. H., and Straub A., J. Thromb. Haemost. 3, 514 (2005). 10.1111/j.1538-7836.2005.01166.x [DOI] [PubMed] [Google Scholar]
- 38.Levy J. H., Ageno W., Chan N. C., Crowther M., Verhamme P., and Weitz J. I., J. Thromb. Haemost. 14, 623 (2016). 10.1111/jth.13227 [DOI] [PubMed] [Google Scholar]
- 39.Artang R., Anderson M., and Nielsen J. D., Res. Pract. Thromb. Haemost. 3, 391 (2019). 10.1002/rth2.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunt B. J., Neal M. D., and Stensballe J., Blood 132, 2441 (2018). 10.1182/blood-2018-06-850396 [DOI] [PubMed] [Google Scholar]
- 41.Dimaggio C., Ayoung-Chee P., Shinseki M., Wilson C., Marshall G., Lee D. C., Wall S., Maulana S., Leon Pachter H., and Frangos S., Injury 47, 1393 (2016). 10.1016/j.injury.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee M. Y., Verni C. C., Herbig B. A., and Diamond S. L., J. Thromb. Haemost. 15, 2396 (2017). 10.1111/jth.13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornblith L. Z., Kutcher M. E., Redick B. J., Calfee C. S., Vilardi R. F., and Cohen M. J., J. Trauma Acute Care Surg. 76, 255 (2014). 10.1097/TA.0000000000000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See the supplementary material for figures regarding construction of the chip and experiments confirming the uniformity of flow in each channel.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.