Abstract
An increased prevalence of various filamentous fungi in sputum samples of patients with cystic fibrosis (CF) has been reported. The clinical significance, however, is mostly unclear. The aim of this study was to investigate the clinical relevance of Scedosporium spp. and Exophiala dermatitidis from sputum samples of patients with CF in the Netherlands. In this cross-sectional study, all CF patients of the Dutch national CF registry who were treated at five of the seven recognized CF centers during a 3-year period were included. We linked clinical data of the national CF registry with the national Dutch filamentous fungal database. We investigated the association between clinical characteristics and a positive sputum sample for Scedosporium spp. and E. dermatitidis, using logistic regression. Positive cultures for fungi were obtained from 3787 sputum samples from 699 of the 1312 patients with CF. Scedosporium spp. was associated with severe genotype, CF-related diabetes, several microorganisms, and inhaled antibiotics. E. dermatitidis was associated with older age, female sex, and Aspergillus spp. CF patients with and without Scedosporium spp. or E. dermatitidis seemed comparable in body mass index and lung function. This study suggests that Scedosporium spp. and E. dermatitidis are probably no major pathogens in CF patients in the Netherlands. Greater understanding of epidemiologic trends, risk factors, and pathogenicity of filamentous fungi in the respiratory tracts of patients with CF is needed.
Keywords: cystic fibrosis, filamentous fungi, Scedosporium, Exophiala, epidemiology
Introduction
Cystic fibrosis (CF) is the most common lethal autosomal recessive disease among populations with northern European ancestry.1 It is a multiorgan disorder and mostly affects the lungs. Progressive chronic lung inflammation and infection is responsible for at least 80% of CF-related deaths.1,2 The main cause of chronic lung inflammation and infection are bacteria, such as Staphylococcus aureus, Burkholderia cepacia, Stenotrophomonas maltophilia, and Pseudomonas aeruginosa.2 In addition to bacteria, filamentous fungi are increasingly recovered in respiratory cultures from patients with CF.3–5Aspergillus fumigatus is the fungus most frequently cultured in patients with CF. Reported prevalence rates based on sputum cultures vary from 16% to 57% in CF patients.3–8 It has been associated with allergic bronchopulmonary aspergillosis (ABPA), hospitalization, and lung function decline.7Scedosporium species have been reported as the second most prevalent filamentous fungus isolated in sputa from 2 to 9% of the patients with CF.3,5,9,10 Its isolation has been associated with older age, white race, and inhaled antibiotic use.10,11 The genus Scedosporium is an ubiquitous filamentous fungus present mainly in soil, sewage, and polluted waters.12 Several species of Scedosporium have been reported in CF: Scedosporium apiospermum, Scedosporium boydii, Lomentospora prolificans and Scedosporium aurantiacum, Scedosporium ellipsoideum, Scedosporium minutisporum.13 The black yeast Exophiala dermatitidis is able to grow as a filamentous fungus in humid environments such as dishwashers and steam baths14 and has been isolated from a significant percentage (1–16%) of CF patients.6,13,15
The clinical relevance of these two filamentous fungi is unclear, leading to a lack of guidelines for clinical management.16 Besides A. fumigatus, other filamentous fungi are increasingly reported as well, although mostly in small numbers and in single center studies.13,15,17 Whether presence of Scedosporium spp. and E. dermatitidis in the lungs only causes harmless colonization of the airways, or also constitutes a clinically relevant risk for patients with CF remains a matter of debate.10,16,18,19
The aim of this study was to investigate the clinical relevance of Scedosporium spp. and E. dermatitidis from sputum samples of patients with CF seen in Dutch CF centres during a 3-year period.
Methods
Design and procedures
In this cross-sectional study, all CF patients of the Dutch national CF registry who were treated at five of the seven recognized CF centers between March 22, 2010, and March 27, 2013 were included. Patients with a history of lung transplantation were excluded. We linked the national CF registry with the Dutch CF Fungal Collection Consortium (DCFFCC). Data were pseudonymized prior to analysis. Formal approval from the institutional review board was waived.
National CF registry
The national CF registry was established to support scientific research and improve CF care and treatment.20 It covers 98% of all patients with CF in the Netherlands. The five included centers represent 82% of the patients. The diagnosis of CF disease was based on typical clinical features associated with a positive sweat test (chloride >60 mmol/l) and/or the presence of two known pathogenic cystic fibrosis transmembrane conductance Rregulator (CFTR) mutations.
Clinical characteristics
The following parameters were retrieved from the national CF registry: age, sex, body mass index (BMI), CFTR mutation, CF-related diabetes (CFRD), allergic bronchopulmonary aspergillosis (ABPA), lung function (forced expiratory volume in 1 second (FEV1) % predicted), presence of positive sputum sample(s) for Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Staphylococcus aureus, Burkholderia cepacia, nontuberculous mycobacteria, and Aspergillus spp., use of inhaled or oral corticosteroids, azithromycin or inhalation antibiotics, and history of lung transplantation. FEV1 was measured at least 4 times a year during routine visits, and the highest value per year was recorded. A patient receiving daily insulin during at least 1 year of the research period was classified as having CFRD. Continuous inhaled antibiotics or oral azithromycin use was defined as use for more than 3 months during at least 1 year. Sputum cultures of the year 2010 and 2011 were linked to clinical data from the national CF registry of the year 2011, and sputum cultures of the year 2012 and 2013 were linked to clinical data from the national CF registry of the year 2012.
Dutch CF fungal collection consortium
The DCFFCC was established to study the epidemiology, microbiological characteristics, and clinical relevance of fungal infection and colonization among patients with CF in the Netherlands. For this aim, we collected all sputum cultures positive for filamentous fungi from patients with CF who were treated at the five participating CF centers in the Netherlands between March 22, 2010, and March 27, 2013. At the local hospital, sputum samples were collected and examined for fungi 4 times a year during routine visits and during hospitalization. Therefore, we considered patients of whom we did not receive a sputum culture as negative. Measurement bias was avoided by excluding the two centers who participated only partially and did not send all positive sputum samples and by using one reference lab for molecular identification.
Fungal culture media and methods
Details on the fungal culture media and methods were previously published.13 Briefly, agar plates were provided to the participating laboratories from a central location. Every week, batches of filamentous fungi, recovered at the participating laboratories, were transported to a central microbiology laboratory of Canisius Wilhelmina Hospital Nijmegen on the original plates. Samples were inoculated onto Sabouraud dextrose agar (SDA, Oxoid, Basingstoke, UK) and medium B+ agar plates.21 All inoculated plates were incubated aerobically at 30°C for 3 weeks and checked for growth daily in the first week and twice per week in the second and third week. The reference laboratory identified the isolates with internal transcribed spacer sequencing and registered the fungus in the DCFFCC database.22
Statistical analyses
We compared patients with a positive sputum sample with patients without a positive sputum sample for Scedosporium spp. and Exophiala dermatitidis, using χ2, Fisher exact, and t tests. We investigated the association between clinical characteristics and a positive sputum sample for Scedosporium spp. and Exophiala dermatitidis using logistic regression analysis with adjustment for sex and age. We did a sensitivity analysis comparing patients with more than one positive sputum sample with patients without a positive sputum sample. We used STATA software (version 14; College Station, TX, USA) for all analysis.
Results
During the study period, 699 (53%) of the 1312 patients with CF had at least one positive sputum sample. In total, 3787 samples containing fungal isolates were obtained. A large variety of 107 species of filamentous fungi was encountered in CF patients. The median age was 21 years (range 0–72). The median number of positive sputum samples per patient in this study was three (range 1–35).
Scedosporium spp. were recovered from 94 (7%) of the 1312 patients with CF and from 225 sputum samples (6%) during the research period (Table 1). The mean age of these 94 patients was 22 years (range 0–56), and the majority was female and had two severe CFTR mutations (class I–III) (Table 2). The median number of sputum samples per patient with Scedosporium spp. was 11 (range 1–35).
Table 1.
Isolation rate of Scedosporium spp. and Exophiala dermatitides.
| Patients with CFaN = 1312 | Positive sputum samples N = 3787 | |
|---|---|---|
| n (%) | n (%) | |
| Total Scedosporium spp. | 94 (7) | 225 (6) |
| Scedosporium apiospermum | 23 (2) | 66 (2) |
| Scedosporium boydii | 16 (1) | 55 (2) |
| Scedosporium ellipsoideum | 11 (1) | 27 (1) |
| Scedosporium aurantiacum | 11 (1) | 19 (1) |
| Lomentospora prolificans | 4 (<1) | 8 (<1) |
| Scedosporium minutisporum | 1 (<1) | 1 (<1) |
| Scedosporium spp. – no species identification | 45 (3) | 49 (1) |
| Exophiala dermatitidis | 31 (2) | 57 (2) |
Cystic fibrosis.
Table 2.
Clinical characteristics of patients with Scedosporium spp. positive and negative sputum samples.
| Scedosporium spp. positive N = 94 | Scedosporium spp. negative N = 1218 | ||
|---|---|---|---|
| n (%) | n (%) | P-valuea | |
| Demographic factors | |||
| Age, years (mean, SD) | 22 (12) | 21 (15) | 0.345 |
| Sex, female | 53 (56) | 575 (47) | 0.088 |
| BMI, (mean, SD) | 20 (3) | 20 (4) | 0.173 |
| Genotype, severe CFTR mutationsb | 83 (89) | 949 (78) | 0.010 |
| Disease characteristics | |||
| CF-related diabetes | 30 (33) | 232 (20) | 0.003 |
| ABPA | 14 (15) | 105 (9) | 0.049 |
| Lung function, FEV1 (mean, SD) | 71 (23) | 71 (25) | 0.950 |
| Microorganisms | |||
| Pseudomonas aeruginosa | 43 (47) | 387 (35) | 0.020 |
| Stenotrophomonas maltophilia | 16 (17) | 85 (8) | 0.001 |
| Staphylococcus aureus | 43 (47) | 369 (33) | 0.007 |
| Burkholderia cepacia | 2 (2) | 21 (2) | 0.692 |
| Nontuberculous mycobacteria | 3 (3) | 15 (1) | 0.150 |
| Aspergillus spp. | 47 (51) | 283 (26) | <0.001 |
| Medications | |||
| Inhalation corticosteroids | 32 (36) | 315 (31) | 0.370 |
| Azithromycin | 50 (56) | 485 (46) | 0.071 |
| Inhalation antibiotics | 48 (52) | 447 (39) | 0.012 |
T-test for continuous variables, Chi-2 for binary variables and Fisher's exact for binary variables with less than 10 cases.
Severe mutations was defined as 2 CFTR mutations from class I-III.
ABPA, allergic bronchopulmonary aspergillosis; BMI, body mass index; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; FEV1,. forced expiratory volume in 1 second.
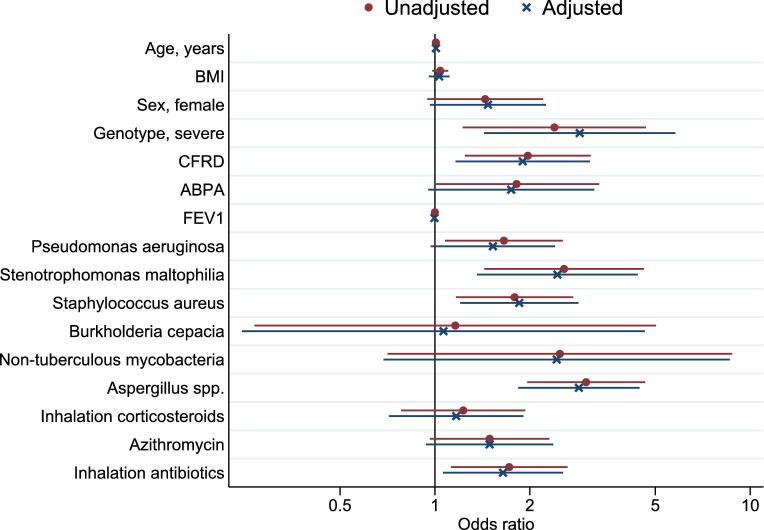
The clinical characteristics of patients with CF and at least one positive sputum sample of Scedosporium spp. compared to those without a positive sputum sample with Scedosporium spp. are shown in Figure 1. The two groups were comparable with respect to age and inhaled corticosteroid use. Severe genotype (odds ratio 2.86, 95% confidence interval [CI] 1.42–5.76), CF-related diabetes (1.80, 1.10–2.95), use of inhaled antibiotics (1.60, 1.03–2.48), and coinfection with Stenotrophomonas maltophilia (2.47, 1.37–4.45), Staphylococcus aureus (1.81, 1.17–2.79), and Aspergillus spp. (2.77, 1.77–4.32) were associated with Scedosporium spp. CF patients with or without Scedosporium spp. had almost a similar mean BMI (1.03, 0.96–1.11) and FEV1% predicted (1.00, 0.99–1.01), also when adjusted for age and sex.
Figure 1.
Clinical factors associated with Scedosporium species, odds ratio's unadjusted (red dot) and adjusted for age and sex (blue cross) (N = 1312). ABPA, allergic bronchopulmonary aspergillosi; BMI, body mass index; CFRD,cystic fibrosis related diabetes; FEV1, forced expiratory volume in 1 second. This Figure is reproduced in color in the online version of Medical Mycology.
Exophiala dermatitidis was cultured from 57 (2%) sputum samples of 31 (2%) of the 699 patients with CF (Table 1). The mean age of the 31 CF patients with Exophiala dermatitidis was 28 years (range 10–59), and the majority was female (Table 3). The median number of sputum samples with Exophiala dermatitidis per patient was five (range 1–29).
Table 3.
Clinical characteristics of patients with Exophiala dermatitidis positive and negative sputum samples.
| Exophiala dermatitidis positive N = 31 | Exophiala dermatitidis negative N = 1281 | ||
|---|---|---|---|
| n (%) | n (%) | P-valueb | |
| Demographic factors | |||
| Age, years (mean, SD) | 28 (13) | 21 (14) | 0.005 |
| Sex, female | 21 (68) | 607 (47) | 0.025 |
| BMI, (mean, SD) | 22 (3) | 20 (4) | 0.003 |
| Genotype, severe CFTR mutationsa | 27 (87) | 1005 (78) | 0.246 |
| Disease characteristics | |||
| CF-related diabetes | 6 (19) | 256 (21) | 1.000 |
| ABPA | 4 (13) | 116 (9) | 0.529 |
| Lung function, FEV1 (mean, SD) | 65 (19) | 71 (25) | 0.167 |
| Microorganisms | |||
| Pseudomonas aeruginosa | 11 (37) | 420 (36) | 0.950 |
| Stenotrophomonas maltophilia | 3 (10) | 98 (8) | 0.733 |
| Staphylococcus aureus | 10 (33) | 404 (34) | 0.926 |
| Burkholderia cepacia | 1 (3) | 22 (2) | 0.442 |
| Non-tuberculous mycobacteria | 1 (3) | 17 (1) | 0.364 |
| Aspergillus spp. | 15 (50) | 314 (27) | 0.005 |
| Medications | |||
| Inhalation corticosteroids | 15 (48) | 330 (31) | 0.044 |
| Azithromycin | 18 (58) | 512 (47) | 0.208 |
| Inhalation antibiotics | 14 (45) | 483 (40) | 0.551 |
Severe mutations was defined as 2 CFTR mutations from class I-III.
T-test for continuous variables, Chi-2 for binary variables and Fisher's exact for binary variables with less than 10 cases.
BMI: body mass index, CFTR: Cystic fibrosis transmembrane conductance regulator, CF: Cystic fibrosis, ABPA: allergic bronchopulmonary aspergillosis, FEV1: forced expiratory volume in 1 second.
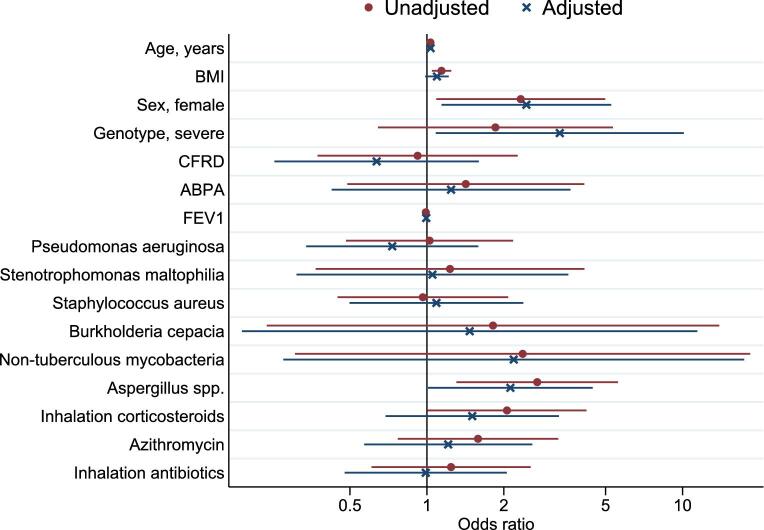
CF patients with Exophiala dermatitidis had more severe genotypes after adjusting for age and sex (3.31, 1.08–10.12), were slightly older (1.03, 1.01–1.06) and were mostly female (2.45, 1.14–5.26) (Fig. 2). Aspergillus spp. coinfection (2.12, 1.01–4.45) was more frequently observed in CF patients with Exophiala dermatitidis than in those without (Fig. 2). The frequency of co-infections with bacteria, use of medication did not differ between the two groups. CF patients with Exophiala dermatitidis had a weak association with the BMI compared to those without Exophiala dermatitidis, however, after adjustment for age and sex, BMI and FEV1% predicted were similar in both groups (1.09, 0.98–1.22 and 0.99, 0.98–1.01, respectively).
Figure 2.
Clinical factors associated with Exophiala dermatitidis, odds ratio's unadjusted (red dot) and adjusted for age and sex (blue cross) (N = 1312). ABPA, allergic bronchopulmonary aspergillosis; BMI, body mass index; CFRD, cystic fibrosis related diabetes; FEV1, forced expiratory volume in 1 second. This Figure is reproduced in color in the online version of Medical Mycology.
Our sensitivity analysis showed similar results in patients with more than one positive sputum sample for Scedosporium spp. (Table S1, and Fig. S1). CF patients with and without Scedosporium spp. had comparable BMI (1.06, 0.96–1.18) and FEV1% predicted (1.00–0.98–1.01). The number of patients with more than one positive sputum sample for Exophiala dermatitidis were too small to do logistic regression, but the comparison of proportions showed similar results as the main analysis (Table S2).
Discussion
We describe the clinical relevance of two species of non-Aspergillus filamentous fungi in a national cohort of Dutch CF patients. Our results showed that CF patients with and without Scedosporium spp. or Exophiala dermatitidis were comparable with regard to BMI and lung function. Scedosporium spp. was associated with severe genotype, CF-related diabetes, several microorganisms, and inhaled antibiotics. Exophiala dermatitidis was associated with older age, female sex, severe genotype, and Aspergillus spp.
Only one other multicenter study determined clinical factors associated with Scedosporium spp. isolation in patients with CF.10 This American retrospective cohort study found that FEV1% predicted was not associated with Scedosporium spp. isolation (odds ratio 0.99, 95% CI 0.96–1.04), which is in line with our findings (1.00, 0.99–1.01).10 An Australian single centre study did not find a difference in FEV1 between patients with and without Scedosporium spp. either.17 In the study by Hong et al., higher BMI was associated with lower probability of Scedosporium spp. in adults (0.90, 0.87–0.91) but not in children (0.96, 0.91–1.01). In comparison, we did not find a difference in BMI in our age-adjusted model (1.03, 0.96–1.11).
Our finding that inhaled antibiotic treatment was associated with positive sputum cultures for Scedosporium spp. (1.60, 1.03–2.48) is in line with findings by Hong et al. (2.01, 1.61–2.52) and Blyth et al.10,17 Both previous studies also found this association with other fungi such as Aspergillus, suggesting that the widespread use of broad-spectrum antibiotics has favored the selection and opportunities of growth and colonization of fungi in the respiratory tract.5
Blyth et al. and Kaur et al. both suggested that Scedosporium colonization/infection occurs less frequently in patients colonized by mucoid Pseudomonas aeruginosa.17,23 In contrast, we found an indication that Pseudomonas aeruginosa occurred more frequently in patients with a positive sputum culture for Scedosporium spp. which is in line with Hong et al.10 Synergistic or antagonistic interactions within the polymicrobial flora colonizing the airways in CF patients have been described, especially for Aspergillus fumigatus.7 An association between fungi, colonizing bacteria, and antimicrobial use has been observed for Aspergillus fumigatus, with increased risk for colonization of Aspergillus in combination with Pseudomonas aeruginosa and Burkholderia cepacia.7 The association of Burkholderia cepacia co-colonization was not found in our study for Scedosporium spp. due to very few Burkholderia cepacia co-colonizations, which is in line with Hong et al.10 We found an association between the presence of Stenotrophomonas maltophilia and a positive sputum sample for Scedosporium spp., which has also been found with Aspergillus fumigatus in a French single center study.3
Exophiala dermatitidis has been studied less, which could be due to the fact that routine isolation techniques are insufficient to detect this slow growing fungus in specimens from CF patients and that it needs a prolonged incubation time (of up to 4 weeks).6 Lebecque et al. did not find any associations between treatment with continuous antibiotic treatment and colonization with Exophiala dermatitidis and neither did we.15
It was also suggested that A. fumigatus coinfection is associated with an increased risk of Exophiala dermatitidis and that Pseudomonas aeruginosa is less frequently found in patients with Exophiala dermatitidis.15,24 In our study, a slight indication was found for the latter. A possible explanation could be that Pseudomonas aeruginosa has been reported to inhibit fungal growth by certain extracts from the extracellular mucus.6
A strength of this study is that it utilizes data of a large CF cohort followed for 3 years. Many other studies of fungal infection in CF patients have been limited by small sample size, short study time, or lack of correlating clinical details. Using data from a multicenter study improves the external validity of the study. Due to the reference laboratory, uniformity in the culturing, molecular typing and detection of filamentous fungi was assured.
One limitation of the study is that we did not use a Scedosporium-selective medium, such as the Scesel + medium, which might have led to an underestimation of the Scedosporium spp. positive sputum samples.25,26 In our study, we used medium B+, which has better bacterial inhibition than the routinely used Sabouraud followed by polymerase chain reaction based identification. We did not have clinical data from the national CF registry for the whole study period. Therefore, we used clinical data from 2011 and 2012 as a proxy. This could have let to misclassification bias. In patients with CF, the lung function and BMI usually decrease over time, because disease progresses. Therefore, the clinical parameters (such as lung function) of 2011 are likely to be worse for the cases with Scedosporium spp. and Exophiala dermatitidis identified in 2010, than they actually were in 2010, so the impact of a positive sputum culture could be overestimated in these patients. Only three new cases with Scedosporium spp. were identified in 2013, so this will have had little impact. Detection bias may have occurred in sicker patients who had more respiratory tract cultures per quarter compared to other patients. Increased respiratory sampling in this group may have led to a higher fungal yield, providing an overestimation of the association between decreased lung function and filamentous fungi. We aimed to correct for differences in the number of (outpatient) clinic visits by analysing unique patients instead of analysing the number of sputum samples. Finally, it could be that the number of patients with Scedosporium spp. or Exophiala dermatitidis was not big enough to show slight differences.
In clinical practice, although there was no association with FEV1 and BMI, Scedosporium spp. or Exophiala dermatitidis may still be important at an individual level. For example, Scedosporium apiospermum, Scedosporium prolificans, and Scedosporium auranticum were suggested as emerging pathogens among immuno-compromised patients and in immuno-competent patients with a chronic lung disease.11,27,28 It has also been suggested that the presence of Scedosporium apiospermum in the respiratory tract is clinically relevant because it may trigger an inflammatory response that can manifest as an allergic bronchopulmonary disease.9 Furthermore, Scedosporium species are reportedly difficult to treat because of their resistance to antifungal agents, with Scedosporium prolificans being the most resistant.22,29 However, since the prevalence of filamentous fungi is very high among patients with CF and resistance is a serious problem, it becomes increasingly important to identify the individual patient who requires antifungal treatment.13 A careful selection of patients is necessary to avoid unintentional selection of more viable but resistant fungi in the respiratory system.30 We believe that watchful waiting in the case of positive cultures with Scedosporium spp. and Exophiala dermatitidis in CF is warranted because starting, switching, and stopping azole therapy has the potential risk of selecting for resistant fungal species with wild-type fitness.30
Our study showed that CF patients with and without Scedosporium spp. or Exophiala dermatitidis were comparable in terms of clinical parameters, that is, BMI and lung function, suggesting that Scedosporium spp. and Exophiala dermatitidis are probably no major pathogens for CF patients on a population level. These findings should be confirmed in a larger study.
As the prevalence of filamentous fungi in the sputum of patients with CF is very high, greater understanding of epidemiologic trends, risk factors, and pathogenicity of filamentous fungi in the respiratory tracts of patients with CF is needed to develop evidence based management guidelines. This study suggests that in general, it seems wise to withhold antifungal treatment when encountering Scedosporium spp. or Exophiala dermatitidis in patients with CF.
Supplementary Material
Acknowledgments
We thank our collaborators of the Dutch Cystic Fibrosis Fungal Collection Consortium*. This work was supported by the Dutch CF Foundation. The funding source had no involvement in study design and collection, analysis, and interpretation of data. They did not influence the writing of the report or the decision to submit the article for publication.
Notes
The Dutch Cystic Fibrosis Fungal Collection Consortium is a collaboration among seven Dutch CF Centres, the Canisius Wilhelmina Hospital Nijmegen and the Dutch Cystic Fibrosis Foundation. Academic Medical Centre Amsterdam: NW Rutjes, department of Pediatrics; EJM Weersink, department of Pulmonology; L Spanjaard, department of Medical Microbiology. Canisius Wilhelmina Hospital Nijmegen: JF Meis, department of Medical Microbiology and Infectious Diseases; P Gerrits, department of Pediatrics. Dutch CF Foundation: VAM Gulmans. Erasmus Medical Centre Rotterdam: HM Janssens, department of Pediatrics and Department of Pediatric Pulmonology, Sophia Children's Hospital; M Bakker, department of Pulmonology; M van Westreenen, department of Medical Microbiology. Haga Hospital the Hague: M Nuijsink, department of Pediatrics; R van der Meer, department of Pulmonology; RW Brimicombe, department of Medical Microbiology. Maastricht University Medical Centre Maastricht: HJ Hendriks, department of Pediatrics; E Dompeling, department of Pediatrics; GJ Wesseling, department of Pulmonology; HA van Dessel, department of Medical Microbiology. Radboud university medical centre Nijmegen: P Merkus, department of Pediatrics; JB Yntema, department of Pediatrics; M Reijers, department of Pulmonology. University Medical Centre Utrecht: K van der Ent, department of Pediatrics; E van de Graaf, department of Pulmonology; HGM Heijerman, department of Pulmonology; PJ Haas, department of Medical Microbiology. University Medical Centre Groningen: BL Rottier, department of Pediatrics; H van der Vaart, department of Pulmonology; HLJ Winter, department of Medical Microbiology.
Contributor Information
C C M de Jong, Department of Pediatrics, Radboud University Medical Center, Nijmegen, The Netherlands.
L Slabbers, Department of Pediatrics, Radboud University Medical Center, Nijmegen, The Netherlands.
T G P Engel, Department of Medical Micriobiology, Radboud University Medical Center, Nijmegen, The Netherlands; Centre of Expertise in Mycology Radboudumc/CWZ, Nijmegen, The Netherlands.
J B Yntema, Department of Pediatrics, Radboud University Medical Center, Nijmegen, The Netherlands.
M van Westreenen, Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Centre, Rotterdam, The Netherlands.
P D Croughs, Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Centre, Rotterdam, The Netherlands.
N Roeleveld, Department for Health Evidence, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, The Netherlands.
R Brimicombe, Department of Medical Microbiology, HagaZiekenhuis, The Hague, The Netherlands.
P E Verweij, Department of Medical Micriobiology, Radboud University Medical Center, Nijmegen, The Netherlands; Department of Medical Microbiology and Infectious Diseases, Canisius Wilhelmina Hospital (CWZ), Nijmegen, The Netherlands.
J F Meis, Department of Medical Micriobiology, Radboud University Medical Center, Nijmegen, The Netherlands; Centre of Expertise in Mycology Radboudumc/CWZ, Nijmegen, The Netherlands; Department of Medical Microbiology and Infectious Diseases, Canisius Wilhelmina Hospital (CWZ), Nijmegen, The Netherlands.
P J Merkus, Department of Pediatrics, Radboud University Medical Center, Nijmegen, The Netherlands.
Declaration of interest
P.E.V. reports grants from Gilead Sciences, grants from MSD, grants from Pfizer, grants from F2G, nonfinancial support from OLM, nonfinancial support from IMMY, outside the submitted work. J.F.M. reports grants from Scynexis, Gilead Sciences, United Medical, and TEVA, outside the submitted work. The other authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
References
- 1. Elborn JS. Cystic fibrosis. Lancet. 2016; 388: 2519–2531. [DOI] [PubMed] [Google Scholar]
- 2. O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009; 373: 1891–1904. [DOI] [PubMed] [Google Scholar]
- 3. Paugam A, Baixench MT, Demazes-Dufeu N et al.. Characteristics and consequences of airway colonization by filamentous fungi in 201 adult patients with cystic fibrosis in France. Med Mycol. 2010; 48: 32–36. [DOI] [PubMed] [Google Scholar]
- 4. Saunders RV, Modha DE, Claydon A, Gaillard EA. Chronic Aspergillus fumigatus colonisation of the cystic fibrosis airway is common and may be associated with a more rapid decline in lung function. J Cyst Fibros. 2011; 10: 37. [DOI] [PubMed] [Google Scholar]
- 5. Sudfeld CR, Dasenbrook EC, Merz WG, Carroll KC, Boyle MP. Prevalence and risk factors for recovery of filamentous fungi in individuals with cystic fibrosis. J Cyst Fibros. 2010; 9: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bakare N, Rickerts V, Bargon J, Just-Nübling G. Prevalence of Aspergillus fumigatus and other fungal species in the sputum of adult patients with cystic fibrosis. Mycoses. 2003; 46: 19–23. [DOI] [PubMed] [Google Scholar]
- 7. Amin R, Depuis A, Aaron SD, Ratjen F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest. 2010; 137: 171–176. [DOI] [PubMed] [Google Scholar]
- 8. Seufert R, Sedlacek L, Kahl B et al.. Prevalence and characterization of azole-resistant Aspergillus fumigatus in patients with cystic fibrosis: a prospective multicentre study in Germany. J Antimicrob Chemother. 2018; 73: 2047–2053. [DOI] [PubMed] [Google Scholar]
- 9. Cimon B, Carrere J, Vinatier JF et al.. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2000; 19: 53–56. [DOI] [PubMed] [Google Scholar]
- 10. Hong G, Lechtzin N, Hadjiliadis D, Kawut SM. Inhaled antibiotic use is associated with Scedosporium/Lomentospora species isolation in cystic fibrosis. Pediatr Pulmonol. 2019; 54: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cortez KJ, Roilides E, Quiroz-Telles F et al.. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008; 21: 157–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rougeron A, Giraud S, Alastruey-Izquierdo A et al.. Ecology of Scedosporium species: present knowledge and future research. Mycopathologia. 2018; 183: 185–200. [DOI] [PubMed] [Google Scholar]
- 13. Engel TGP, Slabbers L, de Jong C et al.. Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients: a Dutch, multicentre study. J Cyst Fibros. 2019; 18: 221–226. [DOI] [PubMed] [Google Scholar]
- 14. Dögen A, Kaplan E, Oksüz Z et al.. Dishwashers are a major source of human opportunistic yeast-like fungi in indoor environments in Mersin, Turkey. Med Mycol. 2013; 51: 493–498. [DOI] [PubMed] [Google Scholar]
- 15. Lebecque P, Leonard A, Huang D et al.. Exophiala (Wangiella) dermatitidis and cystic fibrosis: prevalence and risk factors. Med Mycol. 2010; 48: 4–9. [DOI] [PubMed] [Google Scholar]
- 16. Boyle M, Moore JE, Whitehouse JL, Bilton D, Downey DG. The diagnosis and management of respiratory tract fungal infection in cystic fibrosis: a UK survey of current practice. Med Mycol. 2019; 57: 155–160. [DOI] [PubMed] [Google Scholar]
- 17. Blyth CC, Middleton PG, Harun A et al.. Clinical associations and prevalence of Scedosporium spp. in Australian cystic fibrosis patients: identification of novel risk factors? Med Mycol. 2010; 48: 37–44. [DOI] [PubMed] [Google Scholar]
- 18. Liu JC, Modha DE, Gaillard EA. What is the clinical significance of filamentous fungi positive sputum cultures in patients with cystic fibrosis. J Cyst Fibros. 2013; 12: 187–193. [DOI] [PubMed] [Google Scholar]
- 19. Chotirmall SH, McElvaney NG. Fungi in the cystic fibrosis lung: bystanders or pathogens? Int J Biochem Cell Biol. 2014; 52: 161–173. [DOI] [PubMed] [Google Scholar]
- 20. Dutch Cystic Fibrosis Registry 2017 https://www.ncfs.nl/bestanden/report-cf-registry-2017.pdf. [Date last updated: 2018] [Date last accessed 11 November 2019]. [Google Scholar]
- 21. Nagano Y, Millar BC, Goldsmith CE et al.. Development of selective media for the isolation of yeasts and filamentous fungi from the sputum of adult patients with cystic fibrosis. J Cyst Fibros. 2008; 7: 566–572. [DOI] [PubMed] [Google Scholar]
- 22. Lackner M, Klaassen CH, Meis JF, van den Ende AH, de Hoog GS. Molecular identification tools for sibling species of Scedosporium and Pseudallescheria. Med Mycol. 2012; 50: 497–508. [DOI] [PubMed] [Google Scholar]
- 23. Kaur J, Pethani BP, Kumar S et al.. Pseudomonas aeruginosa inhibits the growth of Scedosporium aurantiacum, an opportunistic fungal pathogen isolated from the lungs of cystic fibrosis patients. Front Microbiol. 2015; 6: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondori N, Gilljam M, Lindblad A et al.. High recovery rate of Exophiala dermatitidis in the airways of cystic fibrosis patients is associated with pancreatic insufficiency. J Clin Microbiol. 2011; 49: 1004–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sedlacek L, Graf B, Schwarz C et al.. Prevalence of Scedosporium species and Lomentospora prolificans in patients with cystic fibrosis in a multicenter trial by use of a selective medium. J Cyst Fibros. 2015; 14: 237–241. [DOI] [PubMed] [Google Scholar]
- 26. Blyth CC, Harun A, Middleton PG et al.. Detection of occult Scedosporium species in respiratory tract specimens from patients with cystic fibrosis by use of selective media. J Clin Microbiol. 2010; 48: 314–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heath CH, Slavin MA, Sorrell TC et al.. Population-based surveillance for scedosporiosis in Australia: epidemiology, disease manifestations and emergence of Scedosporium aurantiacum infection. Clin Microbiol Infect. 2009; 15: 689–693. [DOI] [PubMed] [Google Scholar]
- 28. Schwarz C, Brandt C, Whitaker P et al.. Invasive pulmonary fungal infections in cystic fibrosis. Mycopathologia. 2018; 183: 33–43. [DOI] [PubMed] [Google Scholar]
- 29. Schwarz C, Brandt C, Melichar V et al.. Combined antifungal therapy is superior to monotherapy in pulmonary scedosporiosis in cystic fibrosis. J Cyst Fibros. 2019; 18: 227–232. [DOI] [PubMed] [Google Scholar]
- 30. Verweij PE, Zhang J, Debets AJM et al.. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: a dilemma for clinical management. Lancet Infect Dis. 2016; 16: e251–e260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.