Abstract
Hepatorenal syndrome (HRS) occurs in patients with cirrhosis or fulminant hepatic failure and is a kind of pre-renal failure due to intense reduction of kidney perfusion induced by severe hepatic injury. While other causes of pre-renal acute kidney injury (AKI) respond to fluid infusion, HRS does not. HRS incidence is 5% in children with chronic liver conditions before liver transplantation. Type 1 HRS is an acute and rapidly progressive form that often develops after a precipitating factor, including gastrointestinal bleeding or spontaneous bacterial peritonitis, while type 2 is considered a slowly progressive form of kidney failure that often occurs spontaneously in chronic ascites settings. HRS pathogenesis is multifactorial. Cirrhosis causes portal hypertension; therefore, stasis and release of vasodilator substances occur in the hepatic vascular bed, leading to vasodilatation of splanchnic arteries and systemic hypotension. Many mechanisms seem to work together to cause this imbalance: splanchnic vasodilatation; vasoactive mediators; hyperdynamic circulation states and subsequent cardiac dysfunction; neuro-hormonal mechanisms; changes in sympathetic nervous system, renin-angiotensin system, and vasopressin. In patients with AKI and cirrhosis, fluid expansion therapy needs to be initiated as soon as possible and nephrotoxic drugs discontinued. Once HRS is diagnosed, pharmacological treatment with vasoconstrictors, mainly terlipressin plus albumin, should be initiated. If there is no response, other options can include surgical venous shunts and kidney replacement therapy. In this regard, extracorporeal liver support can be a bridge for liver transplantation, which remains as the ideal treatment. Further studies are necessary to investigate early biomarkers and alternative treatments for HRS.
Keywords: Hepatorenal syndrome, Physiopathology, Type 1 hepatorenal syndrome, Type 2 hepatorenal syndrome, Treatment, Hepatic failure, Liver transplant
Introduction
Treatment of children and adolescents with chronic hepatic conditions is a real challenge. Many situations can make this challenge even bigger, and the hepatorenal syndrome (HRS) is one of them. Hepatorenal syndrome occurs in patients with cirrhosis or fulminant hepatic failure and is a kind of pre-renal failure due to intense reduction of kidney perfusion induced by increasingly severe hepatic injury. HRS is considered a potentially reversible condition, but generally associated with very poor prognosis [1].
Reported HRS incidence is 5% for children with chronic liver conditions before liver transplantation. However, this percentage may be underestimated because specific pediatric diagnostic criteria are lacking [2]. It is also important to mention that there is a special need to differentiate HRS from other causes of acute kidney injury (AKI) [2]. Other causes of AKI in cirrhotic patients include the following: (a) pre-renal AKI, as the most common in children (i.e., hypovolemia due to gastrointestinal bleeding, aggressive diuretic treatment or infections), (b) intrinsic causes such as acute tubular necrosis, and, although very rare, (c) post-renal causes. While the pre-renal AKI responds to fluid infusion, the HRS does not improve following fluid therapy, and the other causes of AKI are associated with manifestations that allow identification.
The physiopathology is not entirely known; however, portal hypertension plays an important role. It is believed that the production of vasodilators and inflammatory cytokines in the splanchnic circulation results in a progressive rise in cardiac output and fall in systemic vascular resistance [1, 2]. Despite local increases of renal and femoral vascular resistance, the decline in kidney perfusion is persistent. This mechanism is associated with reduction in glomerular filtration rate (GFR) and sodium and water retention. Sodium excretion is often less than 10 mEq/day in advanced cirrhosis. A significant fall in mean arterial pressure can also be observed, despite the intense renal vasoconstriction. The renin angiotensin system (RAS) also plays an important role in all these pathways [1, 3].
HRS has two types. Type 1 is an acute and rapidly progressive form that often develops after a precipitating factor such as gastrointestinal bleeding or spontaneous bacterial peritonitis. Type 2 is a slowly progressive form of kidney failure that often occurs spontaneously in chronic ascites settings [4].
Some studies have shown that vasoconstrictor therapy with vasopressin analogues, mainly terlipressin, can improve kidney function and survival in adults [4]. On the other hand, few studies have evaluated the use of this medication in children. Liver transplantation is, to date, the only treatment that ensures long-term survival for children [4].
Therefore, this paper aims to review the literature and to address the latest findings on the pathophysiology, diagnosis, and treatment of HRS in children and adolescents.
Physiopathology
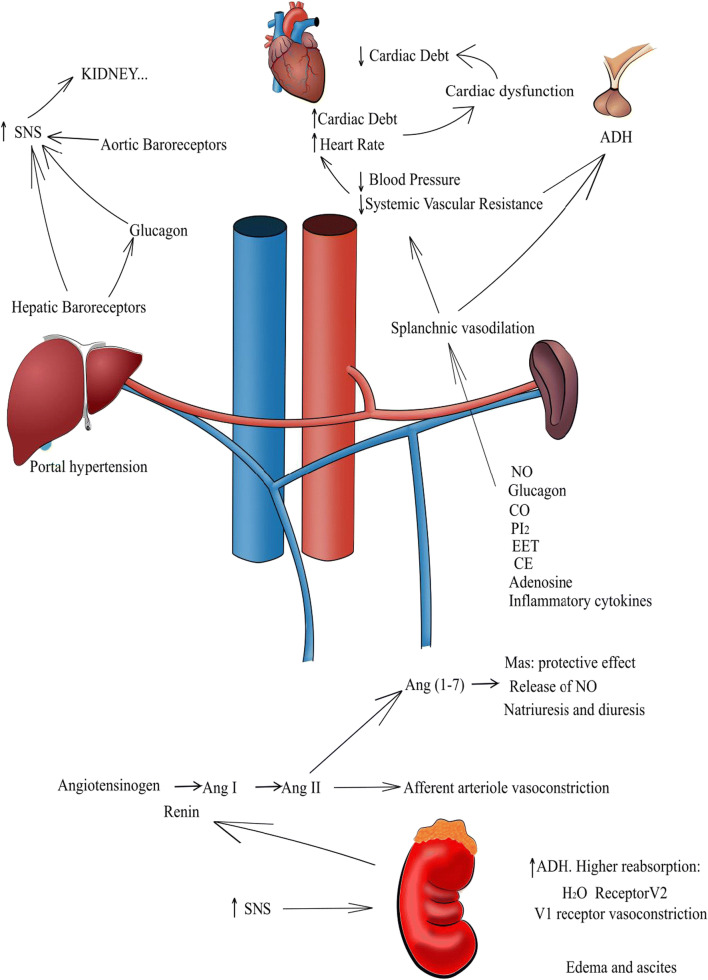
The pathogenesis of HRS is multifactorial. Many mechanisms are related to the deterioration of hepatic disease, leading to HRS (Fig. 1). It is important to point out that few studies have been conducted in children and, therefore, the physiopathological mechanisms are based on findings provided by adult patients [4–7].
Fig. 1.
Representative schema of the physiopathology of hepatorenal syndrome (HRS). HRS begins with portal hypertension, leading to permanent splanchnic vasodilation. As a compensatory mechanism for low vascular resistance, regulatory systems are activated, including renin angiotensin aldosterone system (RAAS) and sympathetic nervous system (SNS). Consequently, cardiac debt increases and renal vasoconstriction occurs. The persistence of these conditions may cause cardiac dysfunction and decreased kidney function, Legend: SNS, sympathetic nervous system; ADH, antidiuretic hormone; NO, nitric oxide; CO, carbon monoxide; PI2, prostaglandin I2; EET, epoxyeicosatrienoic acid; CE, endogenous cannabinoids; Ang I, angiotensin I; Ang II, angiotensin II; Ang-(1–7), angiotensin (1–7)
HRS physiopathology starts with cirrhosis, which has many etiologies in pediatric patients, the most common being biliary atresia, choledochal cysts, hepatitis by virus B (HBV) and C (HCV), and autoimmune hepatitis, among others [5]. Cirrhosis causes portal hypertension, i.e., higher resistance in the intrahepatic flow [6]. Therefore, stasis and release of vasodilator mediators occur in the hepatic vascular bed, leading to vasodilatation of splanchnic arteries and systemic hypotension, the latter because of the lower peripheral vascular resistance [7].
Ascites and edema develop due to a couple of mechanisms: “overflow,” which represents a primary event in the proximal kidney tubule, leads to water and salt retention, causing increased hydrostatic pressure and fluid extravasation. On the other hand, “underfilling,” proposed by Schrier et al. in 1988, considers that portal hypertension results in hypovolemia and in compensatory kidney responses that promote water and salt retention [8]. Hydrosaline retention produces edema by extravascular bound flow of intravascular fluids, leading to a positive feedback loop that aggravates both edema and ascites [8].
Splanchnic vasodilatation
Splanchnic vasodilatation is one of the most important features of HRS. The primary mechanism is still unknown, although it is directly related to the decrease in intrahepatic vascular resistance and to the opening of portosystemic shunts and minor arteriovenous fistulae [9]. Various vasoactive mediators were proposed to exert vasodilator effect. The main ones are nitric oxide, glucagon, carbon monoxide, prostacyclin (I2 prostaglandin), epoxyeicosatrienoic acids, endogenous cannabinoids, and adrenomedullin.
Nitric oxide has a short half-life and its increased production in mesenteric circulation is responsible for the reduction of splanchnic vascular resistance and, thus, for vasodilation [10]. The hyperactive production of NO and endothelial NO synthase (eNOS) in the endothelium of superior mesenteric arteries before the splanchnic circulation becomes hyperdynamic corroborates this hypothesis [11].
Glucagon is a peptide hormone released by pancreatic alpha cells, which has a known effect of dwindling vascular resistance by desensitizing smooth vascular muscle to the effects of vasoconstrictors such as catecholamine and angiotensin II. Thus, the mesenteric circulation undergoes peripheral vasodilatation under the effect of supraphysiological levels. The proposed mechanism for hyperglucagonism is functional hepatic failure and the numerous portosystemic collateral vessels that allow glucagon to escape hepatic degradation. In addition, there is hypersecretion of alpha pancreatic cells in portal hypertension [9, 12].
Carbon monoxide (CO) is a gaseous molecule resulting from endogenous metabolism. Its principal source is the heme oxidase enzyme (HO), which catalyzes the degradation of the heme group releasing CO, iron, and biliverdin. The main mechanism of vasodilation is the stimulation of soluble guanylyl cyclase, causing hyperpolarization of vascular myocytes leading to peripheral vasodilatation, mostly in the splanchnic circulation [13, 14]. Di Pascoli et al. [13] also emphasized that the vasodilation is related to CO capacity of inhibiting 20-hydroxyeicosatetraenoic acid, a precursor of vasoconstrictive leukotrienes. In cirrhotic rats with pre-hepatic portal hypertension, studies have shown an increase of HO-1 expression in mesenteric vasculature. Shear stress, glucagon, NO, angiotensin II, inflammatory agents, endotoxins, and cytokines might induce HO-1 [14, 15]. Overexpression of subunits of large-conductance Ca2+-activated K+ channels (BKCa α subunit) in cirrhotic rats as a response to CO stimulation was also reported [16].
Prostacyclin (prostaglandin I2) is a systemic vasodilator derived from the endothelium that contributes to vascular musculature relaxation by activating adenylyl cyclase and, thus, inducing cAMP production. In patients with cirrhosis, PGI2 increases in the gastric mucosa contributing to vasodilation in HRS [17]. On the other hand, a study by Moore et al. [17] detected that the amount of this vasodilator excreted is too little to produce systemic vasodilation. However, recent studies have shown that PGI2 inhibition by indomethacin administration significantly increases the peripheral vascular resistance and decreases the hepatic blood flow [18].
Epoxyeicosatrienoic acid (EET) is a product of arachidonic acid metabolism. Its behavior during the progression of portal hypertension is paradoxical: in the peripheral vascular bed, this molecule has a vasodilator effect, but, in the portal circulation, it exerts vasoconstrictive action [19]. The mechanism of action involves the interaction between EET and HO-1 isoenzyme [20]. Furthermore, EET also acts via calcium-activated potassium channels.
Endogenous cannabinoids (EC) are neurotransmitters originating from arachidonic acid metabolism. High levels of anandamide and CD1 receptors were observed in cirrhotic rats, indicating that anandamide is the main EC responsible for splanchnic vasodilatation in severe hepatic disease [21]. Other EC receptors related to splanchnic vasodilation can be found in cirrhosis, such as TRPV1 [22]. Some studies have shown that CB1 receptors are located mostly in the adventitia and the endothelial monolayer [22]. The same study did not find CB1 mRNA or protein in femoral arteries, indicating the selectivity of the substance.
Cytokines have a role in the inflammatory response triggered by intraluminal bacterial translocation, especially in HRS-2, that results in increased circulating levels of TNF and IL-6 [9].
Adenosine contributes to the development of HRS by causing vasodilation of splanchnic vessels and renal vasoconstriction. In animal models, adenosine analogues selective to A1 receptor, perfused at constant flux, cause vasoconstriction in the kidneys of rats. The probable mechanism is that adenosine increases the Ca+2 concentrations in the smooth muscle cells of kidney vessels, leading to vasoconstriction [23].
Hyperdynamic circulation states and subsequent cardiac dysfunction
The decreased peripheral vascular resistance and low blood pressure result in hyperdynamic circulation as a mechanism to restore homeostasis. Therefore, cardiac debt and cardiac rate are intensified. Many studies have shown the existence of hyperdynamic circulation in humans and rats with portal hypertension, as long as there would be a portosystemic shunt [8, 24, 25]. Benoit et al. [24] confirmed this hypothesis by verifying that collateral portal veins develop gradually as a portosystemic shunt after portal vein stenosis in rats.
If hepatic disease progresses and splanchnic vasodilatation increases, the initial increase in cardiac debt is not enough to maintain homeostasis. Even though neuro-hormonal activity enhances due to the release of chronotropic and inotropic positive mediators, the heart becomes less responsive, not providing the expected compensatory mechanism [26].
A condition known as cirrhotic cardiomyopathy has been described and is characterized by an enfeebled systolic and diastolic contraction in response to ventricular hypertrophy and chamber dilatation [6]. Loss of adrenergic signal transduction, changes in plasma membrane of the myocardium, and higher levels of cardiac depressant substances, including biliary acids, endotoxins, cytokines, NO, and carbon monoxide, are the proposed mechanisms [27]. This is a risk factor for the development of HRS [28].
On the other hand, it is as yet unconfirmed whether cardiac dysfunction in HRS is caused by cardiomyopathy. Cardiac dysfunction in HRS can be primarily functional, probably related to a decrease in venous return. The reduced cardiac debt may occur due to loss of cardiopulmonary pressure, which is compatible with downward tendency of cardiac preload. In addition, circulatory dysfunction in cirrhotic patients can be reversed by intravenous albumin in association with vasoconstrictors. The combination of these treatments boosts venous return and cardiac debt [29].
Studies verified that in type 1 HRS there was significant increase in cardiac debt and in cardiopulmonary pressure. However, these changes were not observed in type 2 HRS. In this latter condition, there was an increase in cardiac debt. The reduced chronotropic function was comparable in patients with both types of HRS [30]. One hypothesis that explains why cardiac function in type 1 is more affected than in type 2 is the occurrence of left ventricle failure caused by sepsis after spontaneous bacterial peritonitis (SBP) [27].
Vasoconstriction mechanisms
Neuro-hormonal mechanisms are activated in order to diminish hypovolemia caused by peripheral vasodilation, with the goal of reestablishing circulatory homeostasis. These mechanisms act on cutaneous, muscular, and cerebral circulation. Femoral and brachial artery flows are also diminished in HRS, as detected by echo-Doppler ultrasound. The consequences of the decline in blood flow to these regions in HRS have not been explored in detail, but some studies suggest that patients with HRS type 2 and refractory ascites experience more episodes of muscle cramps. These episodes become less frequent through venous albumin infusion [29].
Vasoconstriction of cerebral arteries can also be observed, which is associated with renal vasoconstriction and high plasma renin activity [29]. A study analyzed the resistivity index (RI) of the middle cerebral artery of 37 patients with cirrhosis and compared it with the RI of healthy patients. The results showed that the cerebral arterial RI is higher in patients with cirrhosis and ascites, and that there is a correlation between renal arterial RI and cerebral arterial RI, suggesting a common mechanism of vasoconstriction for both organs [31]. This reduction of cerebral blood flow might be a risk factor for hepatic encephalopathy in HRS-1 patients [29].
The intrahepatic circulation is also influenced by the sympathetic nervous system (SNS), angiotensin II, and vasopressin. These mediators can cause vasoconstriction, thus elevating vascular resistance within the liver and worsening portal hypertension. The effects are even higher in the hepatic circulation, since the synthesis of NO is reduced in the liver of patients with cirrhosis.
The main mechanisms related to this process are as follows: (1) activation of the SNS; (2) activation of the renin-angiotensin-aldosterone system (RAAS); (3) non-osmotic hypersecretion of vasopressin; (4) higher production of vasodilator prostanoids in the kidney.
Activation of the SNS might be triggered by three mechanisms: (1) stimulus to pressure receptors in the carotid arch, which respond to hypotension, and to volume receptors in the atrium that are stimulated by hypovolemia; (2) stimulus of hepatic baroreceptors independently of volume; and (3) response to metabolic changes. All these mechanisms contribute to HRS [9]. The levels of plasma norepinephrine are increased in patients with HRS, especially in those with sodium retention and ascites [32]. The enhanced activity of the SNS is also associated with reductions of kidney blood flow and glomerular filtration rate (GFR), leading to low sodium urinary excretion. Following lumbar sympathetic block, sodium urinary excretion improves and kidney blood flow increases [6, 32].
Lang et al. [9] reported that the infusion of glutamine in the internal jugular vein did not alter kidney function when the amino acid was administered through the portal vein; however, it significantly reduced GFR and kidney blood flow. This effect is possibly mediated by the renal SNS [9]. The mechanism by which the SNS acts in the kidney is related to α-adrenergic mediated vasoconstriction of afferent arterioles, precipitating GFR decrease and sodium retention. Additionally, the SNS stimulates renin secretion via β-adrenergic receptors, further worsening sodium retention [9].
Activation of renin-angiotensin-aldosterone system (RAAS) is stimulated in 80% of patients with decompensated cirrhosis, and even more in patients with HRS [9]. The classical RAAS axis includes angiotensin-converting enzyme (ACE), the peptide angiotensin II (Ang II), and the angiotensin II type 1 receptor (AT1R). The ACE-Ang II-AT1R axis protects, initially, kidney function by promoting selective vasoconstriction of efferent glomerular arterioles, and thus increasing glomerular filtration pressure. Nonetheless, the exacerbated and imbalanced action of Ang II increases intra-glomerular hypertension, resulting in functional loss. Ang II also stimulates the release of aldosterone from the adrenocortical zona glomerulosa, which acts in the distal tubules and collecting ducts of the kidney, bolstering sodium retention. The last observed effect is higher sodium and water retention, as well as lower GFR [9]. Recent studies by Herath et al. [33], Tipnis et al. [34], and Donoghue et al. [35] have changed our understanding of RAAS function, by demonstrating that there is an alternative pathway including the enzyme homolog to ACE, named ACE2 that converts Ang II into Ang-(1–7), which, in turn, binds to the G-coupled receptor Mas [34–37].
In experimental biliary fibrosis with hepatic injury, Mas receptor expression was significantly increased. Paizis et al. [38] also detected an upregulation of ACE2 in the hepatic tissue of patients with cirrhosis and rats submitted to bile duct ligation. Simões e Silva et al. [39] described many actions of the alternative RAAS axis in the kidney, including vasodilation, anti-proliferative, anti-inflammatory, and anti-fibrotic effects. Regarding kidney function, Ang-(1–7) can enhance water reabsorption by acting in the distal nephron and by interacting with the vasopressin V2 receptor [40–42]. In contrast, there are studies showing that Ang-(1–7) may exhibit natriuretic and diuretic effects by inhibiting sodium reabsorption in the proximal tubules [43–45]. The vasodilator effect of Ang (1–7), acting on the Mas receptor, was described in vitro in pre constricted afferent arterioles of rabbits [46]. Ang-(1–7) also promotes the production of NO, which counteracts the response of Ang II in the renal vasculature [47–49]. Through these mechanisms, Ang-(1–7) has a protective effect on the kidneys, by raising blood flow and promoting vasodilation of renal arterioles.
Non-osmotic hypersecretion of vasopressin occurs at the initial stages of severe hepatic disease. Renal vasoconstrictors become active and sodium and water reabsorption are elevated, leading to ascites. At later stages, however, vasopressin is activated, which leads to hyponatremia [50]. Through V2 receptors, vasopressin causes water retention at the distal tubules and collecting ducts of the renal medulla. When blood pressure dysfunction is intense, however, vasopressin acts through V1 receptors exacerbating renal vasoconstriction. Nevertheless, V1 receptor agonists have been considered a viable treatment option for HRS, since some studies have shown that vasopressin causes preferably splanchnic vasoconstriction [51].
Endothelins are a group of three peptides that act through ETA and ETB receptors. The main mechanism of endothelins is the contraction of mesangial cells, decreasing filtration area. An increase in plasma concentrations of ET-1 and ET-3 has been observed, and the increase in ET-1 is higher in severe hepatic disease [52]. Furthermore, other studies show that there is an expressive increase of ET-1 following hepatic transplantation, confirming the importance of ET-1 in HRS physiopathology [53].
Cysteinyl leukotrienes (LTE) are lipids of the eicosanoid family that have a powerful effect upon smooth muscle contraction. A notably higher level of LTE4 and N-acetil-LTE4, its smaller metabolite, was detected in the urine of patients with HRS. These metabolites are potent vasoconstrictors and can lead in vitro to mesangial constriction [54]. Not only does a reduction in liver capture of LTE4 occur, but also an increase in its production through bacterial endotoxins, viral infections, and multiple tissue trauma [55]. In addition, kidney synthesis of cysteinyl leukotrienes is generally high. On the other hand, plasma levels are very low, and, because of that, unable to cause kidney effects [9].
Thromboxane A2 release is secondary to vasoconstriction and ischemia that increase its kidney excretion. However, studies suggested that thromboxane A2 is not a determinant factor in HRS pathogenesis [17].
Physiopathological differences between type 1 and type 2 HRS
HRS type 1 is characterized by a rapidly progressive impairment of kidney function and is associated with a precipitating factor, such as severe bacterial infection, gastrointestinal bleeding, surgical procedures, or acute hepatitis superimposed on cirrhosis [29]. Spontaneous bacterial peritonitis (SBP) is the main bacterial infection associated with HRS-1. Studies have shown that 25% of patients with SBP develop HRS-1, regardless of the infection resolution with non-nephrotoxic antibiotics [56]. The physiopathological mechanism relates to severe circulatory dysfunction caused by an acute inflammatory response. The higher levels of cytokines and polymorphonuclear leukocytes in plasma and ascites fluid cause an inflammatory response [57]. HRS-1 presents a poor prognosis, with patients having a survival rate of approximately 2 weeks [58].
Hepatorenal syndrome type 2 is characterized by moderate and slowly progressive functional kidney impairment. The most important clinical finding is severe ascites, with little or no response to diuretics, a condition known as refractory ascites. Patient survival is, in general, about 4 to 6 months [58].
Recent studies have considered a third type of HRS to be included in the syndrome classification. This new type refers to cases of patients who already have a previous kidney disease and other patients who do not fulfill the HRS type 1 or 2 criteria [59]. Based on the studies conducted by McGuire et al. [60], which included adult patients with cirrhosis, it was shown that glomerulopathies are a common occurrence in patients with hepatitis C virus (HCV)–induced cirrhosis, who underwent liver transplantation [60]. Such patients could not have been classified in other HRS categories, because they presented previous kidney injuries. Nevertheless, they may still develop HRS due to circulatory disturbances secondary to the hepatic insufficiency. Hence, a new category was created for this specific subtype of HRS, named type 3. Considering that, all studies, until now, included only adult patients. It is still necessary to conduct studies with the pediatric population to understand HRS type 3 in this age group. Additionally, chronic kidney disease might also coexist with hepatic diseases in children and adolescents.
Diagnosis
The diagnosis of HRS is based on the International Club of Ascites (ICA) criteria. It is important to consider that the clinical setting and history of HRS in the pediatric population is often different from adult patients. Cirrhosis in children occurs mostly due to biliary atresia, a condition that, even with proper surgical treatment, increases the chance of needing a liver transplantation [58]. As such, these children should be closely monitored and should be included on the list for liver transplantation before the occurrence of HRS.
Accordingly, the percentage of occurrence of HRS in children is low and there is a need to differentiate it from other causes of AKI [61]. Other causes for AKI in cirrhotic patients comprise the following: (a) pre-renal AKI, as the most common etiology in children (i.e., hypovolemia due to gastrointestinal bleeding, aggressive diuretic treatment or infections); (b) intrinsic causes such as acute tubular necrosis; and, although very rare, (c) post-renal causes. While the pre-renal AKI responds to treatment with fluid infusion, the HRS is irresponsive to fluid therapy. The other causes of AKI may exhibit typical clinical findings that may help the diagnosis [61, 62].
The ICA criteria have changed and evolved over time, but it has the same principles, which are to establish the presence of advanced liver disease in conjunction with kidney function deterioration. The diagnosis is performed with the aid of serum creatinine (sCr) as a marker of kidney function. This approach poses some problems, such as an overestimation of kidney function in cirrhotic patients related to multiple factors, including the following: reduction in creatinine production, high levels of bilirubin interfering with the serum creatinine (sCr) dosage assay, and increased secretion of creatinine, among others [63].
Within these criteria, patients were classified as either type 1 HRS or type 2 HRS, the former being characterized as a rapid worsening of kidney function often secondary to a precipitating event, such as SBP. The latter is characterized by a slower worsening of kidney function, accompanied by refractory ascites [57]. Of note, most HRS in children will be secondary to a precipitating event [64].
Previously, the ICA criteria required a level of sCr > 2.5 mg/dL for therapy to be initiated [65]. This was problematic because it can increase the risk of making a late diagnosis, and consequently a late intervention of HRS [66]. The ICA criteria, however, have recently been revised. It adopted the concept of AKI to replace the old concept of acute renal failure. According to this new paradigm, clinicians are now encouraged to consider sCr change over baseline instead of an absolute cut-off value [65].
AKI is defined by the ICA as follows [65]:
Increase in sCr ≥ 0.3 mg/dL (≥ 26.5 μmol/L) within 48 h; or,
Increase over sCr ≥ 50% from baseline, which is known, or presumed, to have occurred within the prior 7 days.
There is still the question of how to measure the baseline creatinine level. The ICA recommends that, in the event that the patient has a previously recorded level of sCr measured at most 3 months prior to admission, this level should be used. If there is more than one value, the latest recorded level should be adopted as baseline sCr. If it is the case that the patient does not have a recent measurement of sCr, the value of sCr at admission can be set as baseline [65].
Subsequently, the revised AKI concept was incorporated for the diagnosis of HRS and it was proposed that the old HRS-1/HRS-2 criteria might be replaced by the new categories. HRS-acute kidney injury (HRS-AKI) and HRS-non acute kidney injury (HRS-NAKI), which encompasses HRS-CKD (HRS-chronic kidney disease) and HRS-AKD (HRS-acute kidney disease) [65, 67]. The detailed criteria, both old and new versions, will be further discussed.
Previously, type 1 HRS was classified based on a rapidly progressive kidney dysfunction and also on an elevation of the initial sCr to a level higher than 2.5 mg/dl. In addition, half reduction of the initial 24-h creatinine clearance to a level that was lower than 20 ml/min in a period of less than 2 weeks is considered sufficient to diagnose this syndrome [67]. Type 2 HRS, however, was determined by refractory ascites and slower, but still progressive, kidney dysfunction [67]. It is important to note that this specific cut-off level for serum creatinine is difficult to apply for the pediatric age group, particularly in young children [64].
The differences between type 1 and type 2 to AKI/NAKI classification were based on the cut-off value of sCr. However, it might result in late and inaccurate diagnosis. The new criteria should allow clinicians to intervene early in the progression of HRS and thus lead to a better prognosis [67] (Table 1).
Table 1.
Time changes in the classification of hepatorenal syndrome (HRS) subtypes
| Old definition | Criteria | New definition | Criteria | |
|---|---|---|---|---|
| HRS-1 | “Rapidly progressive reduction of renal function as defined by a doubling of the initial sCr to a level greater than 2.5 mg/dl or a 50% reduction of the initial 24-hour creatinine clearance to a level lower than 20 ml/min in less than 2 weeks.”1 | HRS-AKI |
“Defined by absolute increase in sCr 0.3 mg/dl within 48 hours and/or; Urinary output 0.5 mg/kg B.W. 6 h and/or; Percent increase in sCr 50% using the last available value of outpatient sCr within 3 months as the baseline value.”2 |
|
| HRS-NAKI | HRS-AKD | “Renal dysfunction that does not meet criteria for AKI and lasts for less than 90 days.”2 | ||
| HRS-2 | “Moderate renal failure (serum creatinine greater than 1.5 mg/dl or 133 μmol/l) which follows a steady or slowly progressive course. Type-2 HRS is frequently associated with refractory ascites.”1 | HRS-CKD | “A patient with cirrhosis and a GFR < 60 ml/min per 1.73 m2 for > 3 months (HRS-CKD) in whom other causes have been excluded.”2 |
HRS hepatorenal syndrome, AKI acute kidney injury, AKD acute kidney disease, CKD chronic kidney disease, HRS-AKI hepatorenal syndrome-acute kidney injury, HRS-NAKI hepatorenal syndrome non-AKI
1Criteria for the diagnosis of Hepatorenal Syndrome. Guideline from International Club of Ascites
2Angeli, Paolo; Garcia-Tsao, Guadalupe; Nadim, Mitra; Parikh, Chirag. News in pathophysiology, definition and classification of hepatorenal syndrome: A step beyond the International Club of Ascites (ICA) consensus document. Journal of Hepatology. (2019)
Recently, it was shown that the inclusion of urine output (UO) in the criteria for diagnosis of AKI improved its identification in patients with chronic liver diseases. In addition, patients identified based on UO criteria, without sCr elevation, had higher mortality rates, leading to the belief that this criteria might be of importance to determine prognosis [67].
Treatment
Since the underlying cause of HRS is severe liver impairment, liver transplantation (LTx) is often the best therapeutic option for HRS [68]. Nevertheless, the mortality rate is very high among HRS patients and many patients may not survive until transplantation. Additionally, pre-transplant kidney function is a predictor of postoperative survival in patients undergoing LTx [69]. Therefore, clinical therapies should be employed as a bridge until LTx is feasible, with the goal of increasing kidney function [70, 71]. With proper treatment and early diagnosis, the outcome of LTx in HRS patients can be similar to that of LTx in non-HRS patients [72].
Few studies have been reported regarding the disease in the pediatric population and some existing treatment algorithms specific for children were based on extrapolations of the data about HRS in adults [62, 64].
In children, it is known that the use of agents that can induce nephrotoxicity should be discontinued, as well as non-steroidal anti-inflammatory drugs (NSAIDs) and diuretic treatments [64, 70]. If SBP is detected, early treatment with albumin infusion plus antibiotic therapy can preserve kidney function in HRS [70]. Ascites should be managed with paracentesis followed by albumin infusion. It is also important to ensure that the intravascular status of the patient is maintained, with aggressive treatment of hypovolemia with fluids/blood products and use of cardiac output monitoring (ultrasound or invasive) [61, 62].
In patients at high risk for HRS, such as cirrhotic patients with arterial hypotension, gastrointestinal bleeding, and severe hyponatremia, it is recommended they should be monitored closely for possible development of HRS [70]. Pharmacological options can act by decreasing splanchnic vasodilation and reverting the effects of systemic hypovolemia, increasing mean arterial pressure, and reversing the conditions that lead to renal vasoconstriction [73]. The most often used and studied medications are albumin in association with terlipressin (vasopressin analogue), norepinephrine, and a combination of midodrine and octreotide (somatostatin analogue). Albumin also can be used alone, as well as terlipressin [74]. A recent meta-analysis included 24 articles with 1429 participants and found that terlipressin plus albumin is the best treatment for HRS [74]. The reversal of HRS, defined as a decrease of 30% in serum creatinine levels, and in adults to values below 1.5 mg/dL, may be reached. Furthermore, the authors point out that the association of norepinephrine and albumin exhibited no significant difference from terlipressin plus albumin, making it a viable candidate for treatment when terlipressin may be not available or when the patient does not respond to it [74].
Nevertheless, a multivariate analysis of a controlled trial of terlipressin versus placebo revealed that the only variable that may predict HRS reversal was baseline serum creatinine. When serum creatinine was below 3 mg/dL in adults, the probability of HRS reversal when treated with terlipressin was optimal, but it worsens as baseline serum creatinine increases, becoming negligible at 7 mg/dL or higher [66]. The contribution of vasoactive drugs to patient survival is still a matter of debate in the context of HRS.
It has also been noted that the association of terlipressin plus albumin is best when used in HRS-AKI (previously type 1 HRS) patients due to the high rate of recurrence in patients with HRS-NAKI (previously type 2). Therefore, it might not be a recommended treatment for the latter, even in patients waiting for LTx [64].
The aquaretic agents Vaptans can promote water excretion and diuresis leading to urine dilution and the blockade of V2-mediated vasodilatation. Acting like this, these agents can improve hyponatremia and ascites, and, by so doing, increase plasma vasopressin concentrations. Recent studies show that Vaptans might also play a role in elevating serum sodium concentration in cirrhotic patients [75].
Extracorporeal kidney replacement therapies may also be useful for the control and treatment of HRS. Hemodialysis is the most recommended form for critically ill and pre-transplant patients, who have not responded to other therapies. It can be intermittent or continuous, depending on the severity of the patient’s condition. [61]
Liver assist devices are also available and can be useful in specific situations. These devices work by removing toxins from the circulation, and available procedures include the following: (a) plasmapheresis combined with HD; (b) molecular adsorbent recirculating system (MARS); and (c) Prometheus dialysis [76–78].
Tandem plasmapheresis and HD (TPH) is useful for critically ill pediatric patients who present with grade 3 hepatic encephalopathy and/or severe hepatic failure with important coagulation disturbance [76].
Molecular adsorbent recirculating system (MARS) is an extracorporeal system, which combines high-flux HD, filtration, and adsorption and uses an albumin-enriched dialysate to remove toxins. Studies with adult patients have shown benefits on kidney function and survival of patients with acute liver failure. Studies in children are still lacking [77].
Another innovating extracorporeal system is Prometheus® (Fresenius Medical Care, Bad Homburg, Germany), which is based on the principles of fractional plasma separation with high-flux HD. It promotes a higher reduction of toxins, including ammonia, urea, bilirubin, and cytokines. On the other hand, coagulation factors and platelets remain unaltered, differing from MARS. Its use in children is still incipient and studies are necessary to know the potential benefits for this age group. [78]
Other therapeutic options might be considered such as transjugular intrahepatic portosystemic shunt (TIPS). This procedure can increase preload, thus increasing cardiac output and lowering portal system blood pressure, one of the basic conditions of HRS [71]. Although the beneficial effects of TIPS in reducing the mortality rate of HRS type 1 and 2 patients have been shown [79], it is still the last option due to its higher number of contraindications and the higher risk of complications, such as hepatic encephalopathy [59, 79].
Despite this, LTx is still the ideal treatment for patients with HRS type 1, even children, irrespective of any previous pharmacological treatment, as it removes the fundamental cause of the disease. This was evident in a clinical trial that analyzed the 6-month survival rate of various groups of patients. The group that received terlipressin plus albumin, but not LTx, had a survival rate of 34%. However, the group that received only albumin but did undergo LTx had a survival rate of 94% [68]. This indicates that patients who do not receive a liver transplant have a worse prognosis, even when they are treated with the most effective drugs, whereas patients that receive the transplantation have a much better prognosis even if their pharmacological treatment is only albumin.
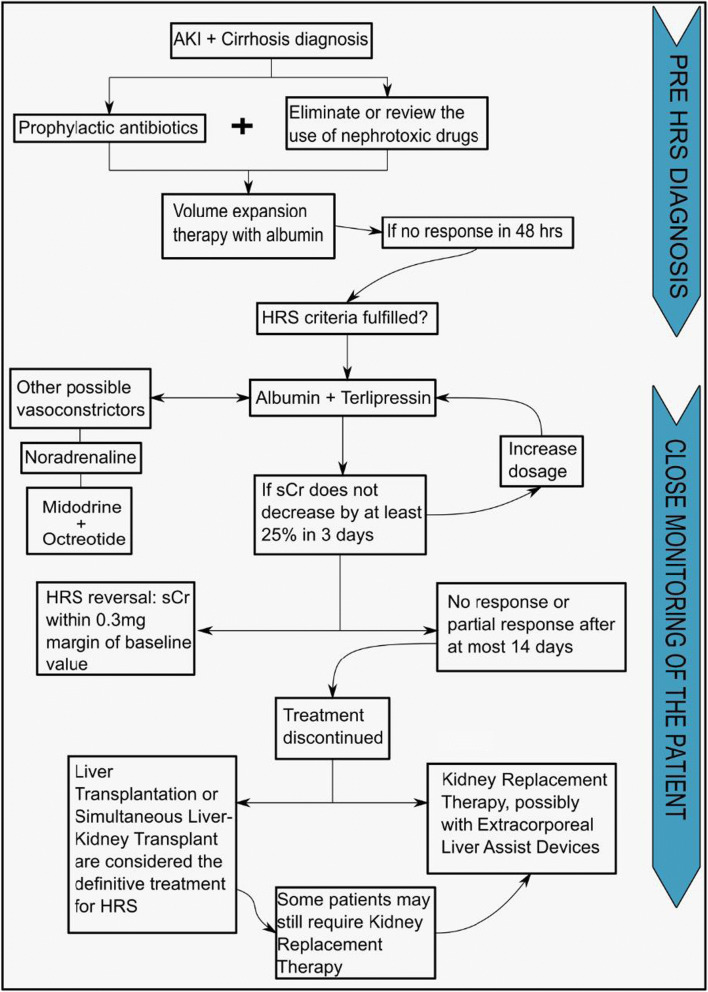
More recently, simultaneous liver-kidney transplant has become an option for patients with liver transplant indication who also have severe kidney conditions and who have no (or minimal) chance of recovering kidney function after liver transplantation. Many studies and also guidelines have tried to establish the indication criteria, but to date, the individual evaluation of each case scenario is the best recommendation [80]. Figure 2 shows the therapeutic steps for HRS. It is important to emphasize that these options are based on a low level of evidence, mainly observational data in children. Therefore, individualized approaches should be considered for specific patients.
Fig. 2.
Therapeutic flowchart for hepatorenal syndrome (HRS) in the pediatric population. In patients with AKI and cirrhosis, volume expansion therapy is initiated as soon as possible. To prevent bacterial translocation, prophylactic antibiotics are employed. Nephrotoxic drugs are discontinued. Once HRS is diagnosed, pharmacological treatment with vasoconstrictors, mainly terlipressin plus albumin, should be promptly initiated. The main goal of pharmacological therapy is to act as a bridge therapy until liver transplantation is possible. If there is no response to pharmacological treatment, other options can be considered such as kidney replacement therapy. These therapeutic options are based on studies with low level of evidence, mainly observational data in children. Individualized approaches should be considered for specific patients
Prognosis
Patients with chronic liver diseases suffer from the consequences of the hemodynamic changes of progressive cirrhosis that can result in ascites, hyponatremia, and AKI. The relationship between the hemodynamic changes caused by cirrhosis and kidney function is very important, as changes in kidney blood flow can disturb renal handling of sodium and free water excretion. Regardless of the cause of AKI, the prognosis of children who have impaired kidney function is worse than that of those who do not [81].
Lal et al. [82] studied 84 children with acute-on-chronic liver failure, and between them found in 31.6% of cases that HRS was the cause of AKI. Also, the authors found that 5 patients (26.3%) survived with native liver, 10 (52.6%) died, and 4 (21.1%) underwent liver transplantation. Although liver transplantation is the best choice for these patients, nearly 40% of pediatric liver transplant recipients develop chronic kidney disease post-transplant and approximately 25% develop clinical hypertension [83, 84]. This fact reinforces the importance of early identification of patients at risk for changes in kidney function and the adequacy of treatment for this condition.
Concluding remarks
Despite the importance of HRS for pediatric patients with liver diseases, there are very few studies with children. Therefore, most information was provided by data obtained in adult patients. The pathophysiology of this disease begins with portal hypertension, leading to permanent splanchnic vasodilation. As a compensatory mechanism for low vascular resistance, regulatory systems are activated, including RAAS and CNS. As a consequence, cardiac debt increases and renal vasoconstriction occurs. The persistence of these conditions may cause cardiac dysfunction and reduced kidney function. Several molecules take part in the physiopathology of HRS and this line of investigation may allow the discovery of novel biomarkers and therapeutic targets. The new diagnostic criteria are based on kidney function parameters and propose the terms HRS-AKI, HRS-AKD, and HRS-CKD. HRS still has a very poor prognosis and liver transplantation remains to be the ideal treatment. Further studies are necessary to investigate early biomarkers and alternative treatments for HRS.
Authors’ contributions
STC, PFF, MLBC, and RGBC equally contributed to this article. These authors made the literature revision and selection of main articles, defined the topics of this review, and wrote the first draft. PMFL and ACSS equally contributed to this article, both conceptualized the study, made general supervision, and revised the manuscript. ACSS submitted the final version of the manuscript, which is approved by all authors.
Funding
This study was partially supported by the Brazilian National Council of Research Development (CNPq-Grant # 302153/2019-5), the Coordination of High Education Level Personnel (CAPES), and the Foundation of Research of Minas Gerais (FAPEMIG).
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflicts of interest.
Data availability
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sarah Tayná de Carvalho, Pollyanna Faria Fradico, Maria Luiza Barreto Cazumbá and Ramon Gustavo Bernardino Campos contributed equally to this work.
References
- 1.Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819–1827. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 2.Debray D, Yousef N, Durand P. New management options for end-stage chronic liver disease and acute liver failure: potential for pediatric patients. Paediatr Drugs. 2006;8:1–13. doi: 10.2165/00148581-200608010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Vilas-Boas WW, Ribeiro-Oliveira A, Jr, Pereira RM, Ribeiro RC, Almeida J, Nadu AP, Simões e Silva AC, Santos RAS. Relationship between angiotensin-(1-7) and angiotensin II correlates with hemodynamic changes in human liver cirrhosis. World J Gastroenterol. 2009;15:2512–2519. doi: 10.3748/wjg.15.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousef N, Habes D, Ackermann O, Durand P, Bernard O, Jacquemin E. Hepatorenal syndrome: diagnosis and effect of terlipressin therapy in 4 pediatric patients. J Pediatr Gastroenterol Nutr. 2010;51:100–102. doi: 10.1097/MPG.0b013e3181d60e73. [DOI] [PubMed] [Google Scholar]
- 5.Cordova J, Jericho H, Azzam RK. An overview of cirrhosis in children. Pediatr Ann. 2016;45:e427–e432. doi: 10.3928/19382359-20161117-01. [DOI] [PubMed] [Google Scholar]
- 6.Shah N, Silva RG, Kowalski A, Desai C, Lerma E. Hepatorenal syndrome. Dis Mon. 2016;62:364–375. doi: 10.1016/j.disamonth.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Moore CM, Van Thiel DH. Cirrhotic ascites review: pathophysiology, diagnosis andmanagement. World J Hepatol. 2013;5:251. doi: 10.4254/wjh.v5.i5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 9.Van Roey G, Moore K. The hepatorenal syndrome. Pediatr Nephrol. 1996;10:100–107. doi: 10.1007/BF00863460. [DOI] [PubMed] [Google Scholar]
- 10.Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926–931. doi: 10.1002/hep.510280405. [DOI] [PubMed] [Google Scholar]
- 11.Wiest R, Shah V, Sessa WC, Groszmann RJ. NO overproduction by eNOS precedes hyperdynamic splanchnic circulation in portal hypertensive rats. Am J Phys. 1999;276:G1043–G1051. doi: 10.1152/ajpgi.1999.276.4.G1043. [DOI] [PubMed] [Google Scholar]
- 12.Pak JM, Lee SS. Glucagon in portal hypertension. J Hepatol. 1994;20:825–832. doi: 10.1016/S0168-8278(05)80156-4. [DOI] [PubMed] [Google Scholar]
- 13.Di Pascoli M, Zampieri F, Quarta S, Sacerdoti D, Merkel C, Gatta A, Bolognesi M. Heme oxygenase regulates renal arterial resistance and sodium excretion in cirrhotic rats. J Hepatol. 2011;54:258–264. doi: 10.1016/j.jhep.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Di Pascoli M, Sacerdoti D, Pontisso P, Angeli P, Bolognesi M. Molecular mechanism leading to splanchnic vasodilation in liver cirrhosis. J Vasc Res. 2017;54:92–99. doi: 10.1159/000462974. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez M, Bonkovsky HL. Increased heme oxygenase-1 gene expression in liver cells and splanchnic organs from portal hypertensive rats. Hepatology. 1999;29:1672–1679. doi: 10.1002/hep.510290621. [DOI] [PubMed] [Google Scholar]
- 16.Bolognesi M, Sacerdoti D, Piva A, Di Pascoli M, Zampieri F, Quarta S, Motterlini R, Angeli P, Merkel C, Gatta A. Carbon monoxide-mediated activation of large-conductance calcium-activated potassium channels contributes to mesenteric vasodilatation in cirrhotic rats. J Pharmacol Exp Ther. 2007;321:187–194. doi: 10.1124/jpet.106.116665. [DOI] [PubMed] [Google Scholar]
- 17.Moore K, Ward PS, Taylor GW, Williams R. Systemic and renal production of thromboxane A2 and prostacyclin in decompensated liver disease and hepatorenal syndrome. Gastroenterology. 1991;100:1069–1077. doi: 10.1016/0016-5085(91)90284-r. [DOI] [PubMed] [Google Scholar]
- 18.Ohta M, Kishihara F, Hashizume M, Kawanaka H, Tomikawa M, Higashi H, Tanoue K, Sugimachi K. Increased prostacyclin content in gastric mucosa of cirrhotic patients with portal hypertensive gastropathy. Prostaglandins Leukot Essent Fatty Acids. 1995;53:41–45. doi: 10.1016/0952-3278(95)90081-0. [DOI] [PubMed] [Google Scholar]
- 19.Sacerdoti D, Pesce P, Di Pascoli M, Brocco S, Cecchetto L, Bolognesi M. Arachidonic acid metabolites and endothelial dysfunction of portal hypertension. Prostaglandins Other Lipid Mediat. 2015;120:80–90. doi: 10.1016/j.prostaglandins.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Sacerdoti D, Pesce P, Di Pascoli M, Bolognesi M. EETs and HO-1 crosstalk. Prostaglandins Other Lipid Mediat. 2016;125:65–79. doi: 10.1016/j.prostaglandins.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, Urbaschek R, Garcia N, Jr, Sanyal AJ, Kunos G. Endocannabinoids acting at vascular CB 1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827–832. doi: 10.1038/89953. [DOI] [PubMed] [Google Scholar]
- 22.Domenicali M, Ros J, Fernández-Varo G, Cejudo-Martin P, Crespo M, Morales-Ruiz M, Briones AM, Campistol J-M, Arroyo V, Vila E, Rodés J, Jiménez W. Increased anandamide induced relaxation in mesenteric arteries of cirrhotic rats: role of cannabinoid and vanilloid receptors. Gut. 2005;54:522–527. doi: 10.1136/gut.2004.051599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi N, Churchill P, Ellis V, Amore B. Mechanism of adenosine receptor-induced renal vasoconstriction in rats. Am J Phys. 1988;255:H885–H890. doi: 10.1152/ajpheart.1988.255.4.H885. [DOI] [PubMed] [Google Scholar]
- 24.Benoit JN, Granger DN. Splanchnic hemodynamics in chronic portal hypertension. Semin Liver Dis. 1986;6:287–298. doi: 10.1055/s-2008-1040611. [DOI] [PubMed] [Google Scholar]
- 25.Vorobioff J, Bredfeldt JE, Groszmann RJ. Hyperdynamic circulation in portalhypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Phys. 1983;244:G52–G57. doi: 10.1152/ajpgi.1983.244.1.G52. [DOI] [PubMed] [Google Scholar]
- 26.Arroyo V, Fernandez J, Gines P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis. 2008;28:081–095. doi: 10.1055/s-2008-1040323. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-del-Arbol L, Urman J, Fernández J, González M, Navasa M, Monescillo A, Albillos A, Jiménez W, Arroyo V. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 28.Barbano B, Sardo L, Gigante A, Ludovica Gasperini M, Liberatori M, Di Lazzaro G, Lacanna A, Amoroso A, Cianci R. Pathophysiology, diagnosis and clinical management of hepatorenal syndrome: from classic to new drugs. Curr Vasc Pharmacol. 2014;12:125–135. doi: 10.2174/157016111201140327163930. [DOI] [PubMed] [Google Scholar]
- 29.Arroyo V, Terra C, Ginès P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935–946. doi: 10.1016/j.jhep.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Gines P, Moreira V, Milicua M, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 31.Guevara M, Bru C, Ginès P, Fernández-Esparrach G, Sort P, Bataller R, Jiménez W, Arroyo V, Rodés J. Increased cerebrovascular resistance in cirrhotic patients with ascites. Hepatology. 1998;28:39–44. doi: 10.1002/hep.510280107. [DOI] [PubMed] [Google Scholar]
- 32.Solis-Herruzo JA, Duran A, Favela V, Castellano G, Madrid JL, Muñoz-Yagüe MT, Morillas JD, Estenoz J. Effects of lumbar sympathetic block on kidney function in cirrhotic patients with hepatorenal syndrome. J Hepatol. 1987;5:167–173. doi: 10.1016/S0168-8278(87)80569-X. [DOI] [PubMed] [Google Scholar]
- 33.Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, Smith AI, Burrell LM, Angus PW. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin- (1–7) levels in experimental biliary fibrosis. J Hepatol. 2007;47:387–395. doi: 10.1016/j.jhep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 35.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Acton S, Breitbart RE. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 36.Simões e Silva AC, Teixeira MM. ACE inhibition, ACE2 and angiotensin-(1-7) axis in kidneyand cardiac inflammation and fibrosis. Pharmacol Res. 2016;107:154–162. doi: 10.1016/j.phrs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Santos RA, Simões e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SVB, Lopes MT, Bader M, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T, Mendes EP. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simões e Silva AC, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin- (1-7) and M as receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169:477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos RA, Simões e Silva AC, Magaldi AJ, Khosla MC, Cesar KR, Passaglio KT, Baracho NC. Evidence for a physiological role of angiotensin-(1-7) in the control of hydroelectrolyte balance. Hypertension. 1996;27:875–884. doi: 10.1161/01.HYP.27.4.875. [DOI] [PubMed] [Google Scholar]
- 41.Simões e Silva AC, Baracho NCV, Passaglio KT, Santos RAS. Renal actions of angiotensin-(1-7) Braz J Med Biol Res. 1997;30:503–513. doi: 10.1590/S0100-879X1997000400012. [DOI] [PubMed] [Google Scholar]
- 42.Magaldi AJ, Cesar KR, de Araújo M, Simões e Silva AC, Santos RA. Angiotensin-(1–7) stimulates water transport in rat inner medullary collecting duct: evidence for involvement of vasopressin V 2 receptors. Pflugers Arch. 2003;447:223–230. doi: 10.1007/s00424-003-1173-1. [DOI] [PubMed] [Google Scholar]
- 43.Andreatta-van Leyen S, Romero MF, Khosla MC, Douglas JG. Modulation of phospholipase A2 activity and sodium transport by angiotensin-(1-7) Kidney Int. 1993;44:932–936. doi: 10.1038/ki.1993.334. [DOI] [PubMed] [Google Scholar]
- 44.DelliPizzi A, Hilchey SD, Bell-Quilley CP. Natriuretic action of angiotensin (1– 7) Br J Pharmacol. 1994;111:1–3. doi: 10.1111/j.1476-5381.1994.tb14014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lara LS, Vives D, Correa JS, Cardozo FP, Marques-Fernades MF, Lopes AG, Caruso-Neves C. PKA-mediated effect of MAS receptor in counteracting angiotensin II-stimulated renal Na+-ATPase. Arch Biochem Biophys. 2010;496:117–122. doi: 10.1016/j.abb.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Simões e Silva ACS, Miranda AS, Rocha NP, Teixeira AL. Renin angiotensin system in liver diseases: friend or foe? World J Gastroenterol. 2017;23:3396–3406. doi: 10.3748/wjg.v23.i19.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–H1994. doi: 10.1152/ajpheart.01145.2002. [DOI] [PubMed] [Google Scholar]
- 48.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 49.Botelho-Santos GA, Sampaio WO, Reudelhuber TL, Bader M, Campagnole-Santos MJ, Souza dos Santos RA. Expression of an angiotensin-(1-7)-producing fusion protein in rats induced marked changes in regional vascular resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2485–H2490. doi: 10.1152/ajpheart.01245.2006. [DOI] [PubMed] [Google Scholar]
- 50.John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol. 2015;21:3197–3205. doi: 10.3748/wjg.v21.i11.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Claria J, Jiménez W, Arroyo V, La Villa G, López C, Asbert M, Castro A, Gaya J, Rivera F, Rodés J. Effect of V1-vasopressin receptor blockade on arterial pressure in conscious rats with cirrhosis and ascites. Gastroenterology. 1991;100:494–501. doi: 10.1016/0016-5085(91)90222-7. [DOI] [PubMed] [Google Scholar]
- 52.Moore K, Wendon J, Frazer M, Karani J, Williams R, Badr K. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med. 1992;327:1774–1778. doi: 10.1056/NEJM199212173272502. [DOI] [PubMed] [Google Scholar]
- 53.Bachmann-Brandt S, Bittner I, Neuhaus P, Frei U, Schindler R. Plasma levels of endothelin- 1 in patients with the hepatorenal syndrome after successful liver transplantation. Transpl Int. 2000;13:357–362. doi: 10.1111/j.1432-2277.2000.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 54.Moore KP, Taylor GW, Maltby NH, Siegers D, Fuller RW, Dollery CT, Williams R. Increased production of cysteinyl leukotrienes in hepatorenal syndrome. J Hepatol. 1990;11:263–271. doi: 10.1016/0168-8278(90)90123-9. [DOI] [PubMed] [Google Scholar]
- 55.Huber M, Kästner S, Schölmerich J, Gerok W, Keppler D. Analysis of cysteinyl leukotrienes in human urine: enhanced excretion in patients with liver cirrhosis and hepatorenal syndrome. Eur J Clin Investig. 1989;19:53–60. doi: 10.1111/j.1365-2362.1989.tb00195.x. [DOI] [PubMed] [Google Scholar]
- 56.Follo A, Llovet JM, Navasa M, Planas R, Forns X, Francitorra A, Rimola A, Gassull MA, Arroyo V, Rodés J. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495–1501. doi: 10.1002/hep.1840200619. [DOI] [PubMed] [Google Scholar]
- 57.Toledo C, Salmerón JM, Rimola A, Navasa M, Arroyo V, Llach J, Ginés A, Ginès P, Rodés J. Spontaneous bacterial peritonitis in cirrhosis: predictive factors of infection resolution and survival in patients treated with cefotaxime. Hepatology. 1993;17:251–257. doi: 10.1002/hep.1840170215. [DOI] [PubMed] [Google Scholar]
- 58.Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V, Rodés J. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 59.Munoz SJ. The hepatorenal syndrome. Med Clin North Am. 2008;92:813–837. doi: 10.1016/j.mcna.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 60.McGuire BM, Julian BA, Bynon JJS, Cook WJ, King SJ, Curtis JJ, Accortt NA, Eckhoff DE. Brief communication: glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med. 2006;144:735–741. doi: 10.7326/0003-4819-144-10-200605160-00007. [DOI] [PubMed] [Google Scholar]
- 61.Deep A, Saxena R, Jose B. Acute kidney injury in children with chronic liver disease. Pediatr Nephrol. 2019;34:45–59. doi: 10.1007/s00467-018-3893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–2077. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 63.Acevedo JG, Cramp ME. Hepatorenal syndrome: update on diagnosis and therapy. World J Hepatol. 2017;9:293–299. doi: 10.4254/wjh.v9.i6.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Govindan S, Venkataraman C (2019) Hepatorenal syndrome in children: diagnosis and management. Pediatric Liver Intensive Care:49–52. 10.1007/978-981-13-1304-2_7
- 65.Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G, Moore K. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 66.Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, Teuber P, Terlipressin Study Group Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315–321. doi: 10.1016/j.jhep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–822. doi: 10.1016/j.jhep.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Boyer TD, Sanyal AJ, Garcia-Tsao G, Regenstein F, Rossaro L, Appenrodt B, Güilberg V, Sigal S, Bexon AS, Teuber P, Terlipressin Study Group Impact of liver transplantation on the survival of patients treated for hepatorenal syndrome type 1. Liver Transpl. 2011;17:1328–1332. doi: 10.1002/lt.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–1185. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 70.Dundar HZ, Yılmazlar T. Management of hepatorenal syndrome. World J Nephrol. 2015;4:277. doi: 10.5527/wjn.v4.i2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Angeli P, Merkel C. Pathogenesis and management of hepatorenal syndrome in patients with cirrhosis. J Hepatol. 2008;48:S93–S103. doi: 10.1016/j.jhep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 72.Restuccia T, Ortega R, Guevara M, Ginès P, Alessandria C, Ozdogan O, Navasa M, Rimola A, Garcia-Valdecasas JC, Arroyo V, Rodés J. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol. 2004;40:140–146. doi: 10.1016/j.jhep.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 73.Amin AA, Alabsawy EI, Jalan R, Davenport A. Epidemiology, pathophysiology, and management of hepatorenal syndrome. Semin Nephrol. 2019;39:17–30. doi: 10.1016/j.semnephrol.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Wang L, Long Y, Li KX, Xu GS. Pharmacological treatment of hepatorenal syndrome: a network meta-analysis. Gastroenterol Rep. 2020;8:111–118. doi: 10.1093/gastro/goz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cárdenas A, Ginès P, Marotta P, Czerwiec F, Oyuang J, Guevara M, Afdhal NH. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol. 2012;56:571–578. doi: 10.1016/j.jhep.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 76.Yuan J, Ye Q, Zhang H, Ming Y, Gui M, Ji Y, Sun J, Wang JW, Ren ZH, Cheng K, Zhao YJ, Sun PL, Wu K, Ji LZ. Evaluation of the renal replacement therapy on the liver transplant patients with acute renal failure. Zhonghua Gan Zang Bing Za Zhi. 2009;17:334–337. [PubMed] [Google Scholar]
- 77.Schaefer B, Schmitt CP. The role of molecular adsorbent recirculating system dialysis for extracorporeal liver support in children. Pediatr Nephrol. 2013;28:1763–1769. doi: 10.1007/s00467-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 78.Evenepoel P, Laleman W, Wilmer A, Claes K, Kuypers D, Bammens B, Nevens F, Vanrenterghem Y. Prometheus versus molecular adsorbents recirculating system: comparison of efficiency in two different liver detoxification devices. Artif Organs. 2006;30:276–284. doi: 10.1111/j.1525-1594.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 79.Charilaou P, Devani K, Petrosyan R, Reddy C, Pyrsopoulos N (2020) Inpatient mortality benefit with transjugular intrahepatic portosystemic shunt for hospitalized hepatorenal syndrome patients. Dig Dis Sci. 10.1007/s10620-020-06136-2 [DOI] [PubMed]
- 80.Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, Feng S, Friedewald JJ, Hong JC, Kellum JA, Kim WR, Lake JR, Melton LB, Pomfret EA, Saab S, Genyk YS. Simultaneous liver–kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12:2901–2908. doi: 10.1111/j.1600-6143.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- 81.Berg UB, Németh A. Well preserved renal function in children with untreated chronic liver disease. J Pediatr Gastroenterol Nutr. 2018;66:575–580. doi: 10.1097/MPG.0000000000001862. [DOI] [PubMed] [Google Scholar]
- 82.Lal BB, Alam S, Sood V, Rawat D, Khanna R. Profile, risk factors and outcome of acute kidney injury in paediatric acute-on-chronic liver failure. Liver Int. 2018;38:1777–1784. doi: 10.1111/liv.13693. [DOI] [PubMed] [Google Scholar]
- 83.Alam S, Lal BB, Sood V, Rawat D. Pediatric acute-on-chronic liver failure in a specialized liver unit: prevalence, profile, outcome, and predictive factors. J Pediatr Gastroenterol Nutr. 2016;63:400–405. doi: 10.1097/MPG.0000000000001179. [DOI] [PubMed] [Google Scholar]
- 84.Matloff RG, Arnon R. The kidney in pediatric liver disease. Curr Gastroenterol Rep. 2015;17:36. doi: 10.1007/s11894-015-0457-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.