Abstract
Background & Aims
The prevalence and significance of digestive manifestations in coronavirus disease 2019 (COVID-19) remain uncertain. We aimed to assess the prevalence, spectrum, severity, and significance of digestive manifestations in patients hospitalized with COVID-19.
Methods
Consecutive patients hospitalized with COVID-19 were identified across a geographically diverse alliance of medical centers in North America. Data pertaining to baseline characteristics, symptomatology, laboratory assessment, imaging, and endoscopic findings from the time of symptom onset until discharge or death were abstracted manually from electronic health records to characterize the prevalence, spectrum, and severity of digestive manifestations. Regression analyses were performed to evaluate the association between digestive manifestations and severe outcomes related to COVID-19.
Results
A total of 1992 patients across 36 centers met eligibility criteria and were included. Overall, 53% of patients experienced at least 1 gastrointestinal symptom at any time during their illness, most commonly diarrhea (34%), nausea (27%), vomiting (16%), and abdominal pain (11%). In 74% of cases, gastrointestinal symptoms were judged to be mild. In total, 35% of patients developed an abnormal alanine aminotransferase or total bilirubin level; these were increased to less than 5 times the upper limit of normal in 77% of cases. After adjusting for potential confounders, the presence of gastrointestinal symptoms at any time (odds ratio, 0.93; 95% CI, 0.76–1.15) or liver test abnormalities on admission (odds ratio, 1.31; 95% CI, 0.80–2.12) were not associated independently with mechanical ventilation or death.
Conclusions
Among patients hospitalized with COVID-19, gastrointestinal symptoms and liver test abnormalities were common, but the majority were mild and their presence was not associated with a more severe clinical course.
Keywords: COVID-19, SARS-CoV-2, Digestive Manifestations, Gastrointestinal Symptoms, Hepatic Manifestations
Abbreviations used in this paper: ALT, alanine aminotransferase; COVID-19, coronavirus disease 2019; IQR, interquartile range; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TB, total bilirubin
What You Need to Know.
Background
Emerging evidence has indicated that gastrointestinal and hepatic manifestations may play an important role in coronavirus disease 2019 (COVID-19), but their prevalence and significance remain uncertain. Two recent meta-analyses showed that gastrointestinal symptoms occur in less than 10% of affected patients, whereas other studies have reported rates in the range of 30% to 60%. Similarly, the prevalence of liver test abnormalities varies from 15% to 50%.
Findings
Using methodology aimed at limiting bias to the greatest extent possible, we found that gastrointestinal symptoms and liver test abnormalities occurred in approximately 50% of 1992 patients hospitalized with COVID-19 across 36 medical centers. These manifestations, however, were mild in the majority of cases and their presence was not associated independently with the need for mechanical ventilation or death.
Implications for patient care
Our findings affirm that digestive manifestations are common in COVID-19. However, gastrointestinal symptoms and liver test abnormalities do not appear to represent a principal aspect of this disease in terms of human suffering or resource utilization. Furthermore, our findings do not support a strong association between intestinal severe acute respiratory syndrome coronavirus 2 infection and severe pulmonary or systemic illness through gut–lung cross-talk or other mechanisms.
Even though coronavirus disease 2019 (COVID-19) is primarily a respiratory illness, the digestive system has been implicated in disease expression, transmission, and possible pathogenesis. The responsible virus—severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—gains cellular entry through the angiotensin-converting enzyme 2 receptor, which is present in the gastrointestinal tract at higher levels than in the respiratory system.1, 2, 3 Viral RNA has been detected in the stool of approximately 50% of affected patients,1 , 4 , 5 and it has been hypothesized that enteric infection might modulate the severity of pulmonary and systemic illness through alterations in the microbiome, dysregulated intestinal immunity, and/or increased gut permeability.6, 7, 8
Digestive manifestations may be common in patients with COVID-19, although reports have been conflicting, and the true prevalence remains uncertain. Early series from China and 2 recent meta-analyses suggested that gastrointestinal symptoms occur in less than 10% of patients,9, 10, 11, 12, 13 whereas other studies have suggested proportions in the range of 30% to 60%.14, 15, 16, 17 The prevalence of abnormal liver test results vary similarly from 15% to 50%.9 , 12 , 13 , 15 More importantly, the significance of digestive manifestations in COVID-19, in terms of impact on the alimentary tract and liver, and on overall outcomes, is unknown. The presence and magnitude of gastrointestinal symptoms and hepatic abnormalities have mirrored disease severity in some studies, but this observation has been inconsistent.18, 19, 20, 21
Reports on the digestive manifestations of COVID-19 have been limited in scope, reflecting the experience of a single hospital or isolated geographic region, and have used varying and potentially biased sampling strategies. We aimed to systematically and rigorously assess the prevalence, spectrum, and severity of digestive manifestations in consecutive patients hospitalized with COVID-19 across geographically diverse medical centers in North America. We also explored the association between the presence of digestive manifestations and overall outcomes.
Methods
Study Design
This was an observational cohort study conducted through an alliance of 36 medical centers in the United States and Canada. Any site in North America was eligible for participation by open invitation. Certain sites were specifically invited to maximize geographic, ethnic, and socioeconomic diversity, and to ensure representation of regions that were affected disproportionately by the early phase of the pandemic. Institutional review board approval was obtained at each center before patient identification and data collection.
Patients
Adult patients who were hospitalized with a confirmed diagnosis of COVID-19 according to local real-time polymerase chain reaction testing were considered eligible. To ensure an unbiased sample, we aimed to enroll the first 50 to 100 consecutive patients meeting eligibility criteria at each participating institution. Potentially eligible patients were identified by site investigators using multiple methods, including but not limited to data warehouse queries, electronic research subject identification tools, and lists provided by the infectious diseases service or other relevant hospital entities.
Data Collection and Quality Assurance
Demographic, clinical, laboratory, radiographic, and endoscopic data from symptom onset until discharge or death were abstracted manually through review of electronic health records by study personnel under the oversight of a designated clinician investigator. Deidentified data were entered directly into an electronic data collection form (Supplementary material; Data Collection Form). When patients had not been dispositioned by the end of the study period, data were collected within 3 days of study closure.
Data quality were ensured to the greatest extent possible using a 3-tiered system. First, formal instructions and consistent communications between the data coordinating center and co-investigators emphasized the importance of ensuring response accuracy at the site level and of seeking clinician investigator input for responses that required clinical interpretation. In addition, a manual of procedures for data collection and for the handling of special scenarios (eg, re-admissions or nosocomial infections) was circulated frequently to the sites throughout the study period. Second, all incoming data were reviewed manually by a data manager to identify missing or duplicate data, to verify that responses were within accepted boundaries, and to assess for discrepant or conflicting responses. Third, data were reviewed in aggregate by the study team primarily to assess for inconsistencies and outliers by center. Data concerns prompted direct queries to the sites, and were resolved before the final database freeze.
Definitions
Digestive manifestations were divided into gastrointestinal symptoms and liver test abnormalities. Because anorexia is a common and nonspecific symptom of viral illness, it was not considered a digestive manifestation in this study. Similarly, we did not include constipation because it has not been implicated previously as a symptom of acute or subacute viral infection. Therefore, the symptoms of interest in this study were diarrhea, nausea, vomiting, abdominal pain, gastrointestinal bleeding, dysphagia, and odynophagia. Patients were judged to have moderate–severe gastrointestinal symptoms when 1 or more of the following criteria were satisfied: (1) diarrhea with more than 4 bowel movements in any 24-hour period; (2) bloody diarrhea; (3) hematemesis, melena, or hematochezia; (4) abdominal computed tomography scan or endoscopic evaluation was performed; or (5) the gastroenterology consult service evaluated the patient. All other patients were considered to have mild symptoms.
Liver test abnormalities were defined as mild when the alanine aminotransferase level (ALT) or total bilirubin (TB) level was increased between 1.5 and 3 times the upper limit of normal, moderate when there was elevation between 3 and 5 times the upper limit of normal, and severe at more than 5 times the upper limit of normal. Liver tests less than 1.5 times the upper limit were considered normal in this study. The upper limits of normal for ALT and TB were considered to be 45 U/L and 1.2 mg/dL, respectively. Severe acute liver injury was defined as an ALT level higher than 1000 U/L, with an international normalized ratio greater than 2 or a factor 5 level less than 25%. For descriptive purposes, the proportion of patients with any liver test abnormality is reported, but only ALT and TB increases were used in the analyses.
Statistical Analysis
Digestive manifestations were reported using descriptive statistics. Categorical variables were expressed as counts or percentages with 95% CIs; continuous variables were expressed as means with SD or medians with interquartile range (IQR), depending on distribution. Liver test abnormality proportions were calculated using the full cohort as the denominator.
The association between digestive manifestations and the severity of COVID-19 was assessed using a multivariable logistic regression model that included the presence of gastrointestinal symptoms and liver test abnormalities as the independent variables of interest and that adjusted for prespecified baseline covariates. The primary outcome was the composite end point of mechanical ventilation and/or death. Potential covariates that were considered for the model are listed in the Supplementary material: Supplementary Table. If a univariable association with the outcome was observed (P < .10), the covariate was considered for inclusion in the final regression model. We also explored potential interactions between included covariates and the primary independent variables. Only liver tests at admission were assessed because hepatic injury during critical illness is associated consistently with multi-organ system failure and death regardless of etiology.22
In exploratory analyses, the associations between digestive manifestations and intensive care unit admission, the need for vasopressor support, and hospital length of stay (modeled as a continuous variable) were assessed using a similar approach.
All analyses were conducted using SAS 9.4 (SAS Institute, Inc, Cary, NC). The programming code for the final primary regression model is included in the Supplementary material.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
Patients
From April 15 to June 5, 2020, data were collected from 1992 subjects across 36 centers. The median number of patients enrolled per participating institution was 51 (IQR, 41–68). Characteristics of the study cohort are shown in Table 1 . The average age was 60 years (SD, 16.3 y); 57% were men; and 42% were black/African American. Eighty-nine percent of patients had at least 1 nondigestive comorbidity, and 9% had a pre-existing digestive disorder. Thirty-two percent of patients required mechanical ventilation and 19% died. The median hospital length of stay was 9 days (IQR, 4–17 d). Thirty patients (1.5%) still were hospitalized at the end of the study period.
Table 1.
Characteristics of the Study Cohort
| All (N = 1992) | 18–39 (n = 245) | 40–49(n = 270) | 50–59 (n = 384) | 60–69 (n = 499) | 70–79 (n = 358) | 80–89 (n = 178) | >89 (n = 58) | ||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Age, mean (SD) | 60.1 (16.3) | 31.2 (6) | 45.2 (2.9) | 54.9 (2.7) | 64.4 (2.9) | 74.2 (2.9) | 83.8 (2.9) | >89a (NA) | |
| Sex, n (%) | Male | 1128 (56.6) | 130 (53.1) | 175 (64.8) | 223 (58.1) | 294 (58.9) | 195 (54.5) | 86 (48.3) | 25 (43.1) |
| Female | 864 (43.4) | 115 (46.9) | 95 (35.2) | 161 (41.9) | 205 (41.1) | 163 (45.5) | 92 (51.7) | 33 (56.9) | |
| Race,b n (%) | American Indian/Alaska Native | 5 (0.3) | 1 (0.4) | 0 (0) | 2 (0.5) | 1 (0.2) | 0 (0) | 1 (0.6) | 0 (0) |
| Asian | 70 (3.5) | 6 (2.4) | 11 (4.1) | 20 (5.2) | 18 (3.6) | 10 (2.8) | 3 (1.7) | 2 (3.4) | |
| Black/African American | 842 (42.3) | 88 (35.9) | 119 (44.1) | 167 (43.5) | 223 (44.7) | 161 (45.1) | 68 (38.2) | 16 (27.6) | |
| White | 732 (36.8) | 76 (31) | 72 (26.7) | 125 (32.6) | 186 (37.3) | 148 (41.5) | 89 (50) | 36 (62.1) | |
| Multiple | 10 (0.5) | 1 (0.4) | 2 (0.7) | 3 (0.8) | 2 (0.4) | 1 (0.3) | 1 (0.6) | 0 (0) | |
| Unknown | 332 (16.7) | 73 (29.8) | 66 (24.4) | 67 (17.4) | 69 (13.8) | 37 (10.4) | 16 (9) | 4 (6.9) | |
| Ethnicity, n (%) | Hispanic or Latino | 290 (14.6) | 71 (29) | 67 (24.8) | 52 (13.5) | 51 (10.2) | 28 (7.8) | 16 (9) | 5 (8.6) |
| Not Hispanic or Latino | 1529 (76.8) | 146 (59.6) | 176 (65.2) | 300 (78.1) | 407 (81.6) | 301 (84.1) | 149 (83.7) | 50 (86.2) | |
| Unknown | 173 (8.7) | 28 (11.4) | 27 (10) | 32 (8.3) | 41 (8.2) | 29 (8.1) | 13 (7.3) | 3 (5.2) | |
| Health care worker, n (%) | Yes | 127 (6.4) | 26 (10.6) | 23 (8.5) | 41 (10.7) | 30 (6) | 3 (0.8) | 4 (2.2) | 0 (0) |
| No | 1653 (83) | 187 (76.3) | 212 (78.5) | 287 (74.7) | 410 (82.2) | 328 (91.6) | 172 (96.6) | 57 (98.3) | |
| Unknown | 212 (10.6) | 32 (13.1) | 35 (13) | 56 (14.6) | 59 (11.8) | 27 (7.5) | 2 (1.1) | 1 (1.7) | |
| Prior history | |||||||||
| Cigarette smoking, n (%) | Current smoker | 126 (6.3) | 21 (8.6) | 15 (5.6) | 23 (6) | 40 (8) | 20 (5.6) | 7 (3.9) | 0 (0) |
| Ex-smoker | 569 (28.6) | 20 (8.2) | 37 (13.7) | 88 (22.9) | 165 (33.1) | 151 (42.2) | 85 (47.8) | 23 (39.7) | |
| Nonsmoker | 1175 (59) | 195 (79.6) | 201 (74.4) | 253 (65.9) | 269 (53.9) | 161 (45) | 71 (39.9) | 25 (43.1) | |
| Unknown | 122 (6.1) | 9 (3.7) | 17 (6.3) | 20 (5.2) | 25 (5) | 26 (7.3) | 15 (8.4) | 10 (17.2) | |
| Alcoholism, n (%) | Current | 169 (8.5) | 26 (10.6) | 29 (10.7) | 37 (9.6) | 41 (8.2) | 26 (7.3) | 6 (3.4) | 4 (6.9) |
| Prior | 102 (5.1) | 7 (2.9) | 10 (3.7) | 23 (6) | 24 (4.8) | 24 (6.7) | 11 (6.2) | 3 (5.2) | |
| No | 1500 (75.3) | 194 (79.2) | 204 (75.6) | 283 (73.7) | 387 (77.6) | 260 (72.6) | 131 (73.6) | 41 (70.7) | |
| Unknown | 221 (11.1) | 18 (7.3) | 27 (10) | 41 (10.7) | 47 (9.4) | 48 (13.4) | 30 (16.9) | 10 (17.2) | |
| Obesity, n (%) | 968 (48.6) | 151 (61.6) | 151 (55.9) | 205 (53.4) | 264 (52.9) | 147 (41.1) | 46 (25.8) | 4 (6.9) | |
| Body mass index,c mean (SD) | 31.5 (8.5) | 35.2 (10.3) | 33.6 (8.9) | 32.5 (9.3) | 31.8 (7.5) | 29.1 (6.2) | 27.1 (6.4) | 23.9 (4.1) | |
| Charlson Comorbidity Index, median (IQR) | 1 (0–2) | 0 (0–1) | 0 (0–1) | 1 (0–2) | 1 (0–3) | 2 (1–3) | 2 (1–3) | 2 (1–3) | |
| Diabetes, n (%) | 722 (36.2) | 50 (20.4) | 75 (27.8) | 133 (34.6) | 221 (44.3) | 152 (42.5) | 79 (44.4) | 12 (20.7) | |
| Hypertension, n (%) | 1245 (62.5) | 55 (22.4) | 121 (44.8) | 223 (58.1) | 362 (72.5) | 280 (78.2) | 156 (87.6) | 48 (82.8) | |
| Cardiac disease, n (%) | 435 (21.8) | 12 (4.9) | 14 (5.2) | 55 (14.3) | 135 (27.1) | 117 (32.7) | 74 (41.6) | 28 (48.3) | |
| Pulmonary disease, n (%) | 414 (20.8) | 46 (18.8) | 52 (19.3) | 74 (19.3) | 116 (23.2) | 75 (20.9) | 40 (22.5) | 11 (19) | |
| Active/current malignancy, excluding nonmelanoma skin cancer, n (%) | 123 (6.2) | 6 (2.4) | 7 (2.6) | 24 (6.3) | 42 (8.4) | 34 (9.5) | 7 (3.9) | 3 (5.2) | |
| Immunocompromised, n (%) | 265 (13.3) | 39 (15.9) | 30 (11.1) | 59 (15.4) | 76 (15.2) | 51 (14.2) | 7 (3.9) | 3 (5.2) | |
| Luminal gastrointestinal disease (nonmalignant), n (%) | 84 (4.2) | 5 (2) | 7 (2.6) | 16 (4.2) | 25 (5) | 22 (6.1) | 8 (4.5) | 1 (1.7) | |
| Pancreaticobiliary disease, n (%) | 56 (2.8) | 2 (0.8) | 8 (3) | 11 (2.9) | 15 (3) | 17 (4.7) | 3 (1.7) | 0 (0) | |
| Chronic liver disease, n (%) | 55 (2.8) | 2 (0.8) | 8 (3) | 14 (3.6) | 17 (3.4) | 10 (2.8) | 4 (2.2) | 0 (0) | |
| COVID-19 symptoms | |||||||||
| Fever (subjective or objective), n (%) | 1537 (77.2) | 209 (85.3) | 222 (82.2) | 303 (78.9) | 390 (78.2) | 254 (70.9) | 122 (68.5) | 37 (63.8) | |
| Cough, n (%) | 1476 (74.1) | 197 (80.4) | 220 (81.5) | 301 (78.4) | 362 (72.5) | 248 (69.3) | 112 (62.9) | 36 (62.1) | |
| Shortness of breath, n (%) | 1403 (70.4) | 186 (75.9) | 204 (75.6) | 279 (72.7) | 359 (71.9) | 232 (64.8) | 113 (63.5) | 30 (51.7) | |
| Fatigue or subjective weakness, n (%) | 851 (42.7) | 86 (35.1) | 105 (38.9) | 173 (45.1) | 216 (43.3) | 167 (46.6) | 77 (43.3) | 27 (46.6) | |
| Myalgia, n (%) | 580 (29.1) | 97 (39.6) | 111 (41.1) | 127 (33.1) | 141 (28.3) | 73 (20.4) | 24 (13.5) | 7 (12.1) | |
| COVID-19 treatments | |||||||||
| Hydroxychloroquine/chloroquine, n (%) | 1036 (52) | 115 (46.9) | 137 (50.7) | 207 (53.9) | 280 (56.1) | 185 (51.7) | 95 (53.4) | 17 (29.3) | |
| Remdesivir, n (%) | 109 (5.5) | 13 (5.3) | 20 (7.4) | 21 (5.5) | 37 (7.4) | 9 (2.5) | 7 (3.9) | 2 (3.4) | |
| Convalescent plasma, n (%) | 37 (1.9) | 4 (1.6) | 4 (1.5) | 9 (2.3) | 11 (2.2) | 7 (2) | 2 (1.1) | 0 (0) | |
| Glucocorticoids, n (%) | 240 (12) | 23 (9.4) | 31 (11.5) | 39 (10.2) | 69 (13.8) | 54 (15.1) | 22 (12.4) | 2 (3.4) | |
| Tocilizumab, n (%) | 109 (5.5) | 19 (7.8) | 16 (5.9) | 25 (6.5) | 32 (6.4) | 9 (2.5) | 7 (3.9) | 1 (1.7) | |
| COVID-19 outcomes | |||||||||
| Hospital length of stay, d, median (IQR) | 9 (4.17) | 6 (3.11) | 7 (4.15) | 8 (4.17) | 11 (5.23) | 10 (6.19) | 10.5 (6.18) | 8 (5.14) | |
| Intensive care unit admission, n (%) | 878 (44.1) | 80 (32.7) | 101 (37.4) | 157 (40.9) | 269 (53.9) | 178 (49.7) | 74 (41.6) | 19 (32.8) | |
| Mechanical ventilation, n (%) | 646 (32.4) | 53 (21.6) | 77 (28.5) | 113 (29.4) | 211 (42.3) | 134 (37.4) | 52 (29.2) | 6 (10.3) | |
| Vasopressor support, n (%) | 546 (27.4) | 40 (16.3) | 58 (21.5) | 94 (24.5) | 179 (35.9) | 125 (34.9) | 44 (24.7) | 6 (10.3) | |
| Death, n (%) | 375 (18.8) | 16 (6.5) | 20 (7.4 | 44 (11.5) | 93 (18.6) | 102 (28.5) | 66 (37.1) | 34 (58.6) | |
COVID-19, coronavirus disease 2019; IQR, interquartile range; NA, not available; SD, standard deviation.
Age was not collected for patients older than 89 years old.
There was 1 missing.
There were 172 missing.
Prevalence, Spectrum, and Severity of Gastrointestinal Symptoms
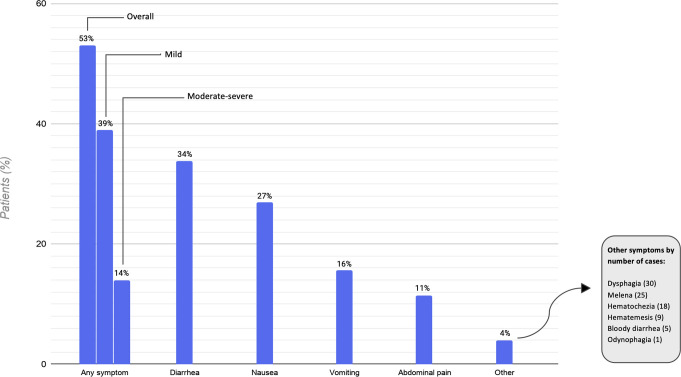
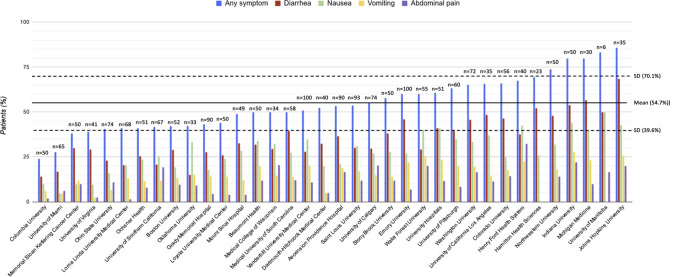
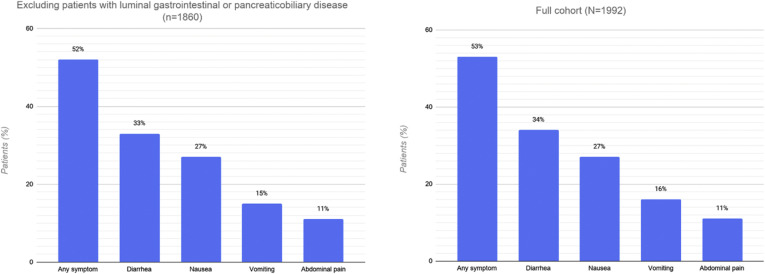
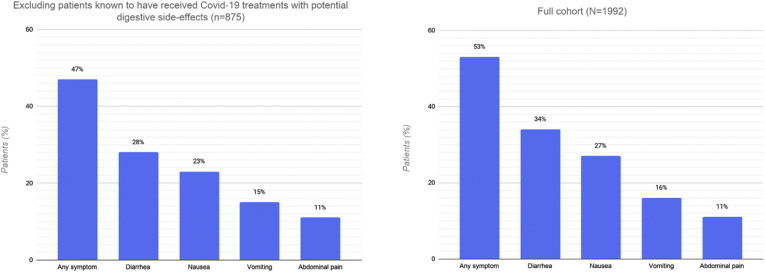
Overall, 1052 patients (53%; 95% CI, 51%–55%) experienced at least 1 gastrointestinal symptom at any time during their illness (Figure 1 ). Of these, 227 patients (11%; 95% CI, 10%–13%) experienced 3 or more gastrointestinal symptoms. The most common symptoms were diarrhea (34%; 95% CI, 32%–36%), nausea (27%; 95% CI, 25%–29%), vomiting (16%; 95% CI, 14%–17%), and abdominal pain (11%; 95% CI, 10%–13%). The prevalence of gastrointestinal symptoms and their distribution did not differ substantively after excluding patients with preexisting gastrointestinal luminal and pancreaticobiliary diseases. The overall proportion decreased to 47% (95% CI, 44%–50%) after excluding patients who were known to have received COVID-19 treatments that may be associated with gastrointestinal side effects, such as hydroxychloroquine or remdesivir (Supplementary material). The prevalence of gastrointestinal symptoms varied across sites (Figure 2 ).
Figure 1.
Gastrointestinal symptoms in patients hospitalized with coronavirus disease 2019 (COVID-19).
Figure 2.
Prevalence and distribution of gastrointestinal symptoms by study site.
Gastrointestinal symptoms preceded other COVID-19 symptoms in 13% of cases, started concurrently in 44%, and followed the other COVID-19 symptoms in 42%. In 7 patients (0.4%), gastrointestinal symptoms were the only manifestation of COVID-19.
In total, 74% of patients (781 of 1052) with gastrointestinal symptoms were judged to have mild symptoms according to our criteria. Among the 271 patients with moderate–severe symptoms, 21% had diarrhea with more than 4 bowel movements per 24 hours, 2% had bloody diarrhea, 18% had gastrointestinal hemorrhage, 63% underwent an abdominal computed tomography scan (159 patients) and/or endoscopic examination (19 patients), and 23% were evaluated by the gastroenterology consult service. Gastrointestinal symptoms were judged to be less prominent than other COVID-19 symptoms in 73% of patients, equally prominent in 20% of patients, and more prominent in 6% of patients.
Prevalence, Spectrum, and Severity of Liver Test Abnormalities
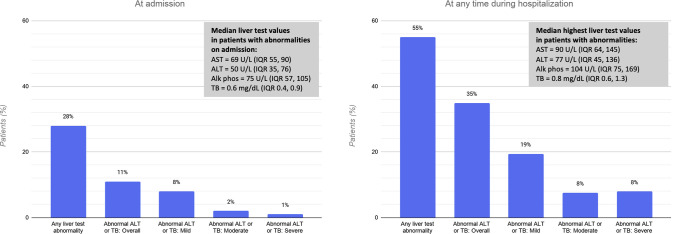
Liver tests were available in 1712 patients (86%) at presentation. At the time of admission, 554 patients (28% of the full cohort; 95% CI, 26%–30%) had at least 1 abnormal liver test (Figure 2). Of these, 215 (11%; 95% CI, 11%–14%) had an abnormal ALT or TB test result. Among patients with an abnormal ALT or TB test result, 77% (95% CI, 71%–82%) had a mild increase, 17% (95% CI, 12%–22%) had a moderate increase, and 6% (95% CI, 3%–9%) had a severe increase. The median abnormal liver test values are presented in Figure 3 .
Figure 3.
Liver test abnormalities in patients hospitalized with coronavirus disease 2019 (COVID-19), at admission and at any time during hospitalization. Proportions were calculated using the full cohort as the denominator (N = 1992). Alk phos, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IQR, interquartile range; TB, total bilirubin.
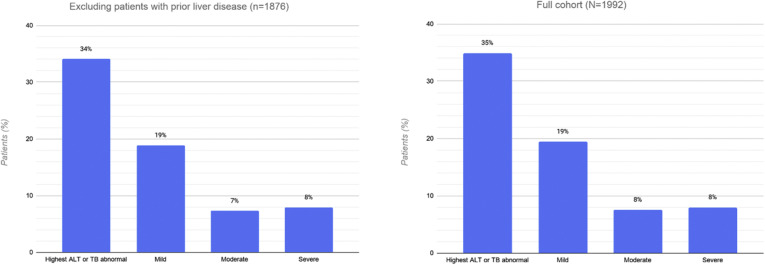
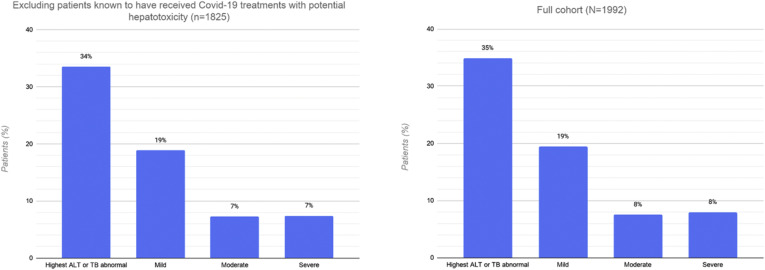
Liver tests were available in 1890 patients (95%) at any time during hospitalization. Among patients with normal liver tests at admission, 548 patients developed at least 1 abnormality during their hospitalization; of these, 480 (24%; 95% CI, 22%–26%) developed an abnormal ALT or TB test result. In total, 695 (35%, 95% CI, 33%–37%) had an increased ALT or TB test result; 56% (95% CI, 52%–59%) of these had a mild increase, 22% (95% CI, 18%–25%) had a moderate increase, and 23% (95% CI, 19%–26%) had a severe increase. Twenty-three patients (1%) developed an ALT level in excess of 1000 U/L during the hospitalization, and 5 of these (0.3%) met criteria for severe acute liver injury. No patients had an ALT level greater than 1000 U/L at admission. The overall proportion, pattern, and severity of abnormal ALT or TB levels did not differ after excluding patients with prior liver disease or those known to have received COVID-19 treatments that can cause hepatotoxicity (Supplementary material: Supplementary Figure).
Association Between Digestive Manifestations and Severe Coronavirus Disease 2019 Outcomes
After adjusting for potential confounders, the presence of gastrointestinal symptoms was not associated with the primary composite end point of mechanical ventilation and/or death (odds ratio [OR], 0.93; 95% CI, 0.76–1.15). Similarly, the presence of mild (OR, 1.05; 95% CI, 0.73–1.49), moderate (OR, 1.14; 95% CI, 0.56–2.32), or severe (OR, 1.89; 95% CI, 0.57–6.30) liver test abnormalities on admission were not associated with the primary end point. In the exploratory analyses, there was no association between digestive manifestations and intensive care unit admission, the need for vasopressor support, or hospital length of stay.
Discussion
In this large and geographically diverse cohort of patients hospitalized with COVID-19 in North America, 53% of patients experienced at least 1 gastrointestinal symptom and 35% developed an abnormal ALT or TB level at some point during their illness. The majority of gastrointestinal symptoms and hepatic abnormalities were mild in nature. The presence of gastrointestinal symptoms at any time or liver test abnormalities on admission were not associated with mechanical ventilation or death.
Although liver test abnormalities are objective, the assessment of gastrointestinal manifestations in COVID-19 is limited by uncertainties in symptom attribution and ascertainment. In this study, we considered any symptom without a clear alternative explanation (eg, abdominal pain resulting from a known postoperative complication) as potentially attributable to COVID-19. This approach likely overestimated prevalence because some symptoms may have been related to other factors such as medication effect (eg, diarrhea or nausea) or critical illness (eg, gastrointestinal hemorrhage or dysphagia). Indeed, a subgroup analysis that excluded patients who were documented to have received COVID-19 treatments that could cause digestive side effects showed a small decrease in the prevalence of gastrointestinal symptoms (47%; 95% CI, 44%–50% vs 53%; 95% CI, 51%–55% overall). Similarly, a manual review of endoscopy cases showed that approximately half were performed to address events related to critical illness (eg, feeding tube placement) rather than direct viral injury (data not shown). Conversely, because the focus of care in hospitalized patients with COVID-19 is typically the pulmonary process, it is possible that gastrointestinal symptoms were under-reported and/or underdocumented. Along these lines, abdominal imaging and endoscopy—criteria we used to determine the severity of gastrointestinal symptoms in this study—have been used judiciously during the pandemic to minimize in-hospital exposure, perhaps underestimating the significance of symptoms. Nevertheless, our findings provide valuable information on the overall burden of digestive manifestations and associated resource utilization in patients hospitalized with COVID-19, whether owing to direct viral effect, treatment of the infection, or a consequence of related systemic illness.
The prevalence of digestive manifestations in this study was reasonably consistent across the majority of participating institutions and in line with that observed in other Western studies.16 , 17 This is in contrast to much lower proportions of gastrointestinal symptoms reported in studies from China.9 , 10 This difference may be because the Chinese experience largely reflects the early phase of the pandemic, before widespread recognition of digestive symptoms as a frequent consequence of COVID-19. These early studies aimed to better understand the overall illness, whereas more recent studies from the West have focused specifically on identifying gastrointestinal symptoms. Alternatively, variable disease expression between patient populations as a result of genetic or epigenetic factors or prevalent virus mutations23 may explain the difference in proportions and deserves more attention.
The effect of digestive involvement on pulmonary and systemic illness through gut–lung cross-talk or other unknown mechanisms is of major potential importance. For example, microbiome-driven interferon signatures have been shown to suppress viral replication in the lung and can be disrupted by gut dysbiosis in experimental models of influenza infection.7 Moreover, the small intestine comprises a rich immune apparatus, the dysregulation of which by SARS-CoV-2 could potentiate or even drive systemic inflammatory response.8 Our findings, however, do not support such a hypothesis given the lack of association between gastrointestinal symptoms and overall severity of illness. Additional research is necessary to elucidate whether the gut–lung axis is influential in this disease independent of gastrointestinal symptoms.
Our findings are consistent with prior reports showing that liver test abnormalities are common in COVID-19. Fifty-five percent of patients in this cohort had an increased liver test at some point during their illness, including many with abnormal aminotransferase levels, raising the possibility of hepatocyte injury resulting from SARS-CoV-2. However, the large majority of patients had ALT or TB levels less than 5 times the upper limit of normal and only 23 patients had an ALT level greater than 1000 U/L, which was not present at the time of presentation, suggesting that clinically important liver injury in COVID-19 is uncommon. Future mechanistic investigations will be necessary to better understand whether infection leads to direct hepatic injury.
The findings of this study should be interpreted in the context of several limitations, some of which are inherent to observational research on COVID-19. As highlighted earlier, symptom attribution and ascertainment were influenced by several factors related to conducting research during a pandemic, such as retrospective data collection and reliance on medical records review rather than direct patient interviews. Furthermore, validated definitions for gastrointestinal symptom severity in COVID-19 are not available and thus we devised criteria that we believe reasonably reflects disease severity in terms of patient suffering and resource utilization. Alternative definitions of severity may have led to varying interpretations of the findings. Some of these limitations are mitigated by the large and geographically diverse sample, highly systematic approach to patient selection, and multilayered and rigorous strategy to ensure the veracity of collected data. It is also important to consider that this study was restricted to hospitalized patients and thus does not reflect the prevalence and significance of digestive manifestations in outpatients with COVID-19.
In summary, among patients hospitalized with COVID-19, gastrointestinal symptoms and liver test abnormalities were common, but the majority were mild in nature and their presence was not associated with worse clinical outcomes.
Acknowledgments
CRediT Authorship Contributions
Badih Joseph Elmunzer, MD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Supervision: Lead; Writing — original draft: Lead)
Rebecca L. Spitzer (Data curation: Supporting; Formal analysis: Supporting; Project administration: Lead; Writing — review & editing: Equal)
Lydia D. Foster (Formal analysis: Lead; Writing — review & editing: Equal)
Ambreen A. Merchant (Data curation: Equal; Writing — review & editing: Equal)
Eric F. Howard (Data curation: Equal; Writing — review & editing: Equal)
Vaishali A. Patel (Data curation: Supporting; Writing — review & editing: Equal)
Mary K. West (Data curation: Equal; Writing — review & editing: Equal)
Emad Qayad (Data curation: Equal; Investigation: Equal; Writing — review & editing: Equal)
Rosemary Nustas (Data curation: Supporting; Writing — review & editing: Equal)
Ali Zakaria (Data curation: Equal; Writing — review & editing: Equal)
Marc S. Piper (Data curation: Equal; Writing — review & editing: Equal)
Jason R. Taylor (Data curation: Equal; Investigation: Equal; Writing — review & editing: Equal)
Lujain Jaza (Data curation: Equal; Writing — review & editing: Equal)
Nauzer Forbes (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Millie Chau (Data curation: Equal; Writing — review & editing: Equal)
Luis F. Lara (Data curation: Equal; Investigation: Equal; Writing — review & editing: Equal)
Georgios I. Papachristou (Data curation: Equal; Investigation: Equal; Writing — review & editing: Equal)
Michael L. Volk (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Liam G. Hilson (Data curation: Equal; Writing — review & editing: Equal)
Selena Zhou (Data curation: Supporting; Writing — review & editing: Equal)
Vladimir M. Kushnir (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Alexandria M. Lenyo (Data curation: Equal; Writing — review & editing: Equal)
Caroline G. McLeod (Data curation: Equal; Writing — review & editing: Equal)
Sunil Amin (Data curation: Equal; Writing — review & editing: Equal)
Gabriela N. Kuftinec (Data curation: Equal; Writing — review & editing: Equal)
Dhiraj Yadav (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Charlie Fox (Data curation: Equal; Writing — review & editing: Equal)
Jennifer M. Kolb (Data curation: Equal; Writing — review & editing: Equal)
Swati Pawa (Data curation: Equal; Investigation: Equal; Writing — review & editing: Equal)
Rishi Pawa (Data curation: Supporting; Writing — review & editing: Equal)
Andrew Canakis (Data curation: Equal; Writing — review & editing: Equal)
Christopher Huang (Data curation: Supporting; Writing — review & editing: Equal)
Laith H. Jamil (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Andrew M. Aneese (Data curation: Equal; Writing — review & editing: Equal)
Benita K. Glamour (Data curation: Equal; Writing — review & editing: Equal)
Zachary L. Smith (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Katherine A. Hanley (Data curation: Equal; Writing — review & editing: Equal)
Jordan Wood (Data curation: Equal; Writing — review & editing: Equal)
Harsh K. Patel (Data curation: Equal; Writing — review & editing: Equal)
Janak N. Shah (Data curation: Supporting; Writing — review & editing: Equal)
Emil Agarunov (Data curation: Equal; Writing — review & editing: Equal)
Amrita Sethi (Data curation: Equal; Writing — review & editing: Equal)
Evan L. Fogel (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Gail McNulty (Data curation: Equal; Writing — review & editing: Equal)
Abdul Haseeb (Data curation: Equal; Writing — review & editing: Equal)
Judy A. Trieu (Data curation: Equal; Writing — review & editing: Equal)
Rebekah E. Dixon (Data curation: Equal; Writing — review & editing: Equal)
Jeong Yun Yang (Data curation: Equal; Writing — review & editing: Equal)
Robin B. Mendelsohn (Data curation: Equal; Investigation: Equal; Writing — review & editing: Equal)
Delia Calo (Data curation: Equal; Writing — review & editing: Equal)
Olga C. Aroniadis (Data curation: Equal; Writing — review & editing: Equal)
Joseph F. LaComb (Data curation: Equal; Writing — review & editing: Equal)
James M. Scheiman (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Bryan G. Sauer (Data curation: Supporting; Writing — review & editing: Equal)
Duyen T. Dang (Data curation: Equal; Writing — review & editing: Equal)
Cyrus R. Piraka (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Eric D. Shah (Data curation: Equal; Writing — review & editing: Equal)
Heiko Pohl (Data curation: Equal; Investigation: Equal; Writing — review & editing: Equal)
William M. Tierney (Data curation: Supporting; Investigation: Equal; Writing — review & editing: Equal)
Stephanie Mitchell (Data curation: Equal; Writing — review & editing: Equ
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.09.041.
Supplementary Material
Supplementary Figure 1.
Prevalence and distribution of gastrointestinal symptoms in 1860 patients without pre-existing luminal gastrointestinal or pancreaticobiliary disease, compared with the full cohort.
Supplementary Figure 2.
Prevalence and distribution of gastrointestinal symptoms in 875 patients who were not known to have received any of the following medications that are associated with potential gastrointestinal side effects: hydroxychloroquine, chloroquine, remdesivir, oseltamivir, lopinavir/ritonavir, and interferon alpha, compared with the full cohort. COVID-19, coronavirus disease 2019.
Supplementary Figure 3.
Prevalence and distribution of liver test abnormalities in 1876 patients without pre-existing liver disease, compared with the full cohort. ALT, alanine aminotransferase; TB, total bilirubin.
Supplementary Figure 4.
Prevalence and distribution of abnormal liver tests in 1825 patients who were not known to have received any of the following medications with potential hepatotoxicity: remdesivir, oseltamivir, lopinavir/ritonavir, and interferon alpha, compared with the full cohort. ALT, alanine aminotransferase; COVID-19, coronavirus disease 2019; TB, total bilirubin.
Supplementary Table 1.
Potential Covariates That Were Considered for the Primary Regression Analysis
| Mechanical ventilation or death (n = 760) | No mechanical ventilation or death (n = 1232) | P value | ||
|---|---|---|---|---|
| Age, y, mean (SD) | 64.1 (15.1) | 57.6 (16.5) | <.0001 | |
| Sex, n (%) | Male | 464 (61.1) | 664 (53.9) | .0017 |
| Female | 296 (38.9) | 568 (46.1) | ||
| Race, n (%) | American Indian/Alaska Native | 2 (0.3) | 3 (0.2) | .1938 |
| Asian | 35 (4.6) | 35 (2.8) | ||
| Black/African American | 327 (43) | 515 (41.8) | ||
| White | 281 (37) | 451 (36.6) | ||
| Multiple | 3 (0.4) | 7 (0.6) | ||
| Unknown | 112 (14.7) | 220 (17.9) | ||
| Cigarette smoking status, n (%) | Current/ex-smoker | 306 (40.3) | 389 (31.6) | <.0001 |
| Nonsmoker | 383 (50.4) | 792 (64.3) | ||
| Unknown | 71 (9.3) | 51 (4.1) | ||
| Alcoholism, n (%) | Current/prior | 93 (12.2) | 178 (14.4) | <.0001 |
| No | 547 (72) | 953 (77.4) | ||
| Unknown | 120 (15.8) | 101 (8.2) | ||
| Obesity, n (%) | No | 381 (50.1) | 643 (52.2) | .3715 |
| Yes | 379 (49.9) | 589 (47.8) | ||
| Body mass index, mean (SD) | 31.8 (8.9) | 31.3 (8.2) | .2681 | |
| Hypertension, n (%) | No | 226 (29.7) | 521 (42.3) | <.0001 |
| Yes | 534 (70.3) | 711 (57.7) | ||
| Diabetes, n (%) | No | 448 (58.9) | 822 (66.7) | .0005 |
| Yes | 312 (41.1) | 410 (33.3) | ||
| Cardiac disease, n (%) | No | 557 (73.3) | 1000 (81.2) | <.0001 |
| Yes | 203 (26.7) | 232 (18.8) | ||
| Pulmonary disease, n (%) | No | 591 (77.8) | 987 (80.1) | .2091 |
| Yes | 169 (22.2) | 245 (19.9) | ||
| Immunocompromised, n (%) | No | 645 (84.9) | 1082 (87.8) | .0591 |
| Yes | 115 (15.1) | 150 (12.2) | ||
| Active/current malignancy, excluding nonmelanoma skin cancer, n (%) | No | 704 (92.6) | 1165 (94.6) | .0821 |
| Yes | 56 (7.4) | 67 (5.4) | ||
| Moderate to severe kidney disease (creatinine >3 mg/dL before admission, end-stage renal disease, or dialysis), n (%) | No | 663 (87.2) | 1141 (92.6) | <.0001 |
| Yes | 97 (12.8) | 91 (7.4) | ||
| Luminal gastrointestinal disease, n (%) | No | 740 (97.4) | 1168 (94.8) | .0057 |
| Yes | 20 (2.6) | 64 (5.2) | ||
| Chronic liver disease, n (%) | No | 737 (97) | 1200 (97.4) | .5704 |
| Yes | 23 (3) | 32 (2.6) | ||
| Recent (within 1 month of admission) or current (at admission) ACE or ARB use, n (%) | Yes | 232 (30.5) | 364 (29.5) | .0008 |
| No | 501 (65.9) | 854 (69.3) | ||
| Unknown | 27 (3.6) | 14 (1.1) | ||
| Recent (within 1 month of admission) or current (at admission) NSAIDs use, n (%) | Yes | 190 (25) | 337 (27.4) | .0193 |
| No | 425 (55.9) | 718 (58.3) | ||
| Unknown | 145 (19.1) | 177 (14.4) | ||
| Recent (within 1 month of admission) or current (at admission) PPI or H2 blocker use, n (%) | Yes | 235 (30.9) | 317 (25.7) | <.0001 |
| No | 451 (59.3) | 860 (69.8) | ||
| Unknown | 74 (9.7) | 55 (4.5) | ||
| Recent (within 1 month of admission) or current (at admission) antibiotic use, n (%) | Yes | 241 (31.7) | 346 (28.1) | <.0001 |
| No | 472 (62.1) | 852 (69.2) | ||
| Unknown | 47 (6.2) | 34 (2.8) | ||
ACE, Angiotensin converting enzyme; ARB, Angiotensin Receptor Blocker; H2, Histamine 2; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitor.
References
- 1.Xiao F., Tang M., Zheng X. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du M., Cai G., Chen F. Multi-omics evaluation of gastrointestinal and other clinical characteristics of SARS-CoV-2 and COVID-19. Gastroenterology. 2020;158 doi: 10.1053/j.gastro.2020.03.045. 2298–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACE2 angiotensin I converting enzyme 2 [Homo sapiens (human)]. Gene ID: 59272. https://www.ncbi.nlm.nih.gov/gene/59272 Available from: Accessed May 9, 2020.
- 4.Han C., Duan C., Zhang S. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115:916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Y., Guo C., Tang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley K.C., Finsterbusch K., Schnepf D. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28:245–256. doi: 10.1016/j.celrep.2019.05.105. [DOI] [PubMed] [Google Scholar]
- 7.Marsland B.J., Trompette A., Gollwitzer E.S. The gut-lung axis in respiratory disease. Ann Am Thorac Soc. 2015;12(Suppl 2):S150–S156. doi: 10.1513/AnnalsATS.201503-133AW. [DOI] [PubMed] [Google Scholar]
- 8.Mönkemüller K., Fry L., Rickes S. COVID-19, coronavirus, SARS-CoV-2 and the small bowel. Rev Esp Enferm Dig. 2020;112:383–388. doi: 10.17235/reed.2020.7137/2020. [DOI] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin X., Lian J.S., Hu J.H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sultan S., Altayar O., Siddique S. AGA Institute rapid review of the GI and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159:320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parasa S., Desai M., Thoguluva Chandrasekar V. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Amico F., Baumgart D.C., Danese S. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aroniadis O.C., DiMaio C.J., Dixon R.E. Current knowledge and research priorities in the digestive manifestations of COVID-19. Clin Gastroenterol Hepatol. 2020;18:1682–1684. doi: 10.1016/j.cgh.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redd W.D., Zhou J.C., Hathorn K.E. Prevalence and characteristics of gastrointestinal symptoms in patients with SARS-CoV-2 infection in the United States: a multicenter cohort study. Gastroenterology. 2020;159:765–767.e2. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajifathalian K., Krisko T., Mehta A. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020;159:1137–1140.e2. doi: 10.1053/j.gastro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan L., Yang P., Sun Y. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei X.-S., Wang X., Niu Y.-R. Diarrhea is associated with prolonged symptoms and viral carriage in COVID-19. Clin Gastroenterol Hepatol. 2020;18:1753–1759.e2. doi: 10.1016/j.cgh.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao R., Qiu Y., He J.S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lone N.I., Walsh T.S. Impact of intensive care unit organ failures on mortality during the five years after a critical illness. Am J Respir Crit Care Med. 2012;186:640–647. doi: 10.1164/rccm.201201-0059OC. [DOI] [PubMed] [Google Scholar]
- 23.Coppée F., Lechien J.R., Declèves A.E. Severe acute respiratory syndrome coronavirus 2: virus mutations in specific European populations. New Microbes New Infect. 2020;36:10069. doi: 10.1016/j.nmni.2020.100696. [DOI] [PMC free article] [PubMed] [Google Scholar]