Abstract
The Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that originated in Chinese city of Wuhan has caused around 906,092 deaths and 28,040,853 confirmed cases worldwide (https://covid19.who.int/, 11 September 2020). In a life-threatening situation, where there is no specific and licensed anti-COVID-19 vaccine or medicine available; the repurposed drug might act as a silver bullet. Currently, more than 211 vaccines, 80 antibodies, 31 antiviral drugs, 35 cell-based, 6 RNA-based and 131 other drugs are in clinical trials. It is therefore utter need of the hour to develop an effective drug that can be used for the treatment of COVID-19 before a vaccine can be developed. One of the best-characterized and attractive drug targets among coronaviruses is the main protease (3CLpro). Therefore, the current study focuses on the molecular docking analysis of TAT-peptide47–57 (GRKKRRQRRRP)-conjugated repurposed drugs (i.e., lopinavir, ritonavir, favipiravir, and hydroxychloroquine) with SARS-CoV-2 main protease (3CLpro) to discover potential efficacy of TAT-peptide (TP) - conjugated repurposing drugs against SARS-CoV-2. The molecular docking results validated that TP-conjugated ritonavir, lopinavir, favipiravir, and hydroxychloroquine have superior and significantly enhanced interactions with the target SARS-CoV-2 main protease. In-silico approach employed in this study suggests that the combination of the drug with TP is an excelling alternative to develop a novel drug for the treatment of SARS-CoV-2 infected patients. The development of TP based delivery of repurposing drugs might be an excellent approach to enhance the efficacy of the existing drugs for the treatment of COVID-19. The predictions from the results obtained provide invaluable information that can be utilized for the choice of candidate drugs for in vitro, in vivo and clinical trials. The outcome from this work prove crucial for exploring and developing novel cost-effective and biocompatible TP conjugated anti-SARS-CoV-2 therapeutic agents in immediate future.
Keywords: SARS-CoV-2, TAT-peptide, 3CLpro main protease, COVID-19, In silico, Molecular docking, Repurposing drug
1. Introduction
The Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that originated in Chinese city of Wuhan (Chakraborty et al., 2020a) caused deadly human respiratory infection termed coronavirus disease 2019 (COVID-19) (Huang et al., 2020). World Health Organization (WHO) had declared SARS-CoV-2 a global health emergency on 30th January 2020 (Bhattacharya et al., 2020a) and on 11 March 2020, WHO declared it a pandemic (Rehman et al., 2020). Since the outbreak of COVID-19, as of 11 September 2020, the disease has caused around 906,092 deaths and 28,040,853 confirmed cases of COVID-19 infections worldwide (https://covid19.who.int/, Updated, n.d., 11 September 2020). SARS-CoV-2 belongs to the family Coronaviruses and subgenus beta-CoV (Chakraborty et al., 2020b, Saha et al., 2020a). Other previous known coronaviruses that cause severe respiratory diseases in human are MERS-CoV that caused MERS outbreak in the Middle East in 2012 (Chakraborty et al., 2020c) and SARS-CoV caused SARS outbreaks in Guangdong Province, China, in 2006 (Yin and Wunderink, 2018). SARS-CoV-2 is single-stranded positive-sense RNA (+ssRNA) virus and the genome size is ~30 kb which is the largest among all RNA viruses (Chen et al., 2020, Gralinski et al., 2020). SARS-CoV-2 maintains ~80% nucleotide identity with the original SARS-CoV epidemic viruses and 96% with bat coronavirus (Bhattacharya et al., 2020b, Gralinski et al., 2020). The genomic sequences of two bat SARS-related CoVs i.e., bat-SL-CoVZC45 and bat-SL-CoVZXC21 showed ~89% sequence similarity with novel SARS-CoV-2. Phylogenetic analysis has indicated that the SARS-CoV-2 is a viral recombinant of previously identified bat coronaviruses (Ansari et al., 2020, Chan et al., 2020).
The repurposing antiretroviral protease inhibitor drugs such as lopinavir/ritonavir, indinavir, saquinavir and antiviral RNA polymerase inhibitors drug such as remdesivir are currently being tested for the treatment of SARS-CoV-2 (Paules et al., 2020, Li and De Clercq, 2020, Liu et al., 2020). Recently, antiviral efficacy of remdesivir and chloroquine against SARS-CoV-2 clinical isolate has been investigated and was found that they can potentially inhibit SARS-CoV-2 at the low-micromolar concentration in vitro (Holshue et al., 2020, Wang et al., 2020a, Wang et al., 2020b). Nafamostat, Nitazoxanide, Ribavirin, Penciclovir and Favipiravir are other drugs that have been tested against SARS-CoV-2 also show potential inhibitory effects in vitro (Wang et al., 2020a, Wang et al., 2020b).
Currently, no vaccine or medicine has been developed that can be used for the treatment of SARS-CoV-2 infections, therefore, the repurposed drug could act as a brilliant alternative with potential to combat the disease effectively (Saha et al., 2020b). Though, preclinical and clinical trials are required to ensure their effectiveness, such treatment might be better and promising than a placebo. A number of clinical trials are being done to test the efficacy of protease inhibitors drugs lopinavir and ritonavir against SARS-CoV-2. These drugs are commonly used in the treatment of HIV infections. The combination of lopinavir and ritonavir with Chinese herbal medicines was used in preliminary clinical studies for the treatment of SARS-CoV-2 (Wang et al., 2020a, Wang et al., 2020b). In vitro studies show that hydroxychloroquine and chloroquine have potential anti-COVID-19 activity (Gautret et al., 2020, Gao et al., 2020). According to Milken Institute, 211 vaccines, 31 antiviral drugs, 35 cell-based, 80 antibodies, 6 RNA-based and 131 others drugs are at different phases of development and trials (https://milken-institute-covid-19-tracker.webflow.io/). It is therefore dire need to develop an effective drug that can be used for the treatment of COVID-19 before a vaccine can be developed.
Moreover, the efficiency of these potential anti-COVID drugs can be further enhanced by combining them with cell-penetrating peptides (CPPs), which can possibly help to enhance the cellular uptake of these drugs (Nori et al., 2003). CPPs are short peptides (less than 30 residues) consisting of excellent capability to cross cellular membranes with very limited toxicity, via energy-dependent and/or independent mechanisms, without the necessity of a chiral recognition by specific receptors (Bechara and Sagan, 2013, Ansari et al., 2020). The main antiviral approach of CPPs has been the conjugation of CPPs with potential drug molecules; however, some CPPs have even demonstrated antiviral properties by themselves (Pärn et al., 2015).
The cell-penetrating ability of TAT-peptide (TP) commenced a new era in intracellular drug delivery. TAT-peptide47-57 (GRKKRRQRRRP), a short cation richer with basic amino acid peptide is commonly used as research tool to enhance the delivery and transport of drugs, DNA, RNA, proteins, viruses and nanoparticles inside the cytoplasm (Quan et al., 2019, Skwarczynski and Toth, 2019, Ansari et al., 2020). We have suggested that the efficacy of antiviral activity of these repurposing drugs against COVID-19 can be improved and enhanced by conjugating it to the TP. Therefore, the current study focuses on the molecular docking analysis of TP (GRKKRRQRRRP)-conjugated repurposed drugs (lopinavir, ritonavir, favipiravir, and hydroxychloroquine) with SARS-CoV-2 main protease (3CL hydrolase, PDB: 6LU7) to discover potential efficacy of TP-conjugated repurposed drugs against SARS-CoV-2.
2. Methodology
2.1. Receptor molecule preparation
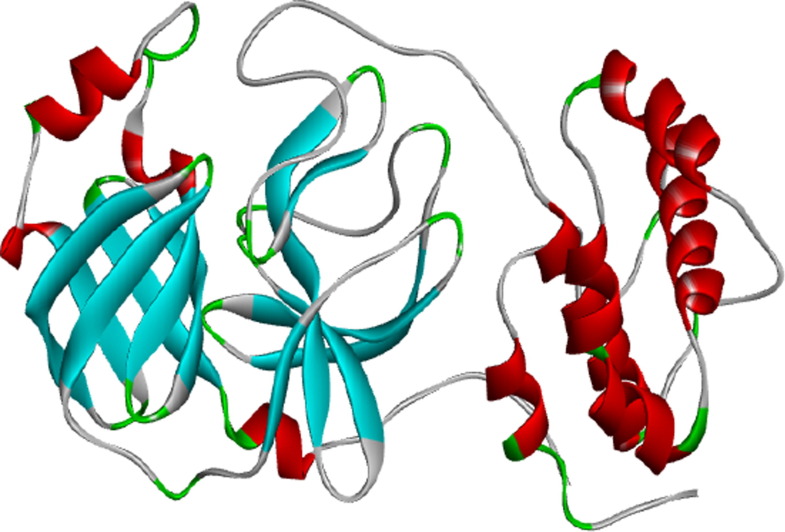
The crystal structure of COVID-19 main protease is complex with an inhibitor N3 (PDB ID: 6LU7) downloaded from Protein Data Bank (PDB), a well-known 3 Dimensional bio-macromolecular repository (Fig. 1 ). The 3D structure was developed by X-Ray diffraction method with the observed resolution of 2.16 Å, R-Value Free 0.235, R-Value Work 0.202, and R-Value observed 0.204 (PDB ID: 6LU7). N3 inhibitor, HETATOM, and water molecules were removed from the 3D structure PDB file (PDB ID: 6LU7). Active site amino acid residues (Asn142, Cys145, Gln189, Glu166, Gly143, His163, His164, Met165, Phe140, Thr190, Thr26) information of N3 inhibitor interaction with 6LU7 has been obtained for the docking analysis of selected drug molecules on same active site. After that CHARMM force field was applied on PDB ID: 6LU7 3D structure for the energy minimization process. Discovery Studio visualizer 2019 was used for afore mentioned editing of 3D structure.
Fig. 1.
3D structure of COVID-19 Protease (PDB ID: 6LU7).
2.2. 3D structure modeling of TAT-Peptide
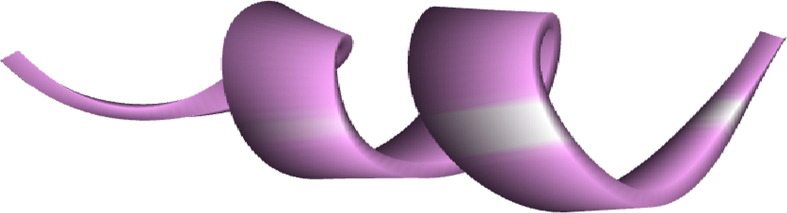
TAT-peptide (GRKKRRQRRRP) an 11 amino acid residue, rich in basic amino acid derived from nuclear transcription activator tat protein of human immunodeficiency virus-1 which penetrates various cell types (Fang et al., 2013) was submitted to PEP-FOLD3.5 webserver to generate the 3D structure of TP (Lamiable et al., 2016). PEP-FOLD3 server used the Hidden Markov Model sub-optimal conformation sampling approach for the prediction of the 3D structure of small peptide.
2.3. TAT-Peptide 3D structure validation
After successful generation of TP modeled, 3D structure was further assessed by MolProbity tool (Chenn et al., 2010) integrated in structure assessment module of SwissModel server (Waterhouse et al 2018).
2.4. Preparation of drug molecules
The performance of repurposed drugs Lopinavir, Ritonavir, Favipiravir, and Hydroxychloroquine with and without TP was explored to perform in silico interaction analysis with COVID-19 Protease (PDB ID: 6LU7). The chemical canonical SMILES IDs of selected drugs were extracted from PubChem Database (https://pubchem.ncbi.nlm.nih.gov/). Furthermore, we have generated a 3D structure of drug molecules using CORINA classic 3D structure generator server (https://www.mn-am.com/online_demos/corina_demo) (Table 1 ). Also discovery studio 2019 was used to implement the CHARMM force field in order to complete the energy minimization process for the generated 3D structures of drug molecules (Vanommeslaeghe et al. 2010). Apart from these, it has been suggested that nanotechnology could be an alternative therapeutic approach that can be used to counter COVID-19 and similar pandemics (Weiss et al., 2020, Gaurav et al., 2020).
Table 1.
Physiochemical description of 2D structure of repurposing drug molecules and 3D structure of TAT-peptide conjugated drugs used for molecular docking analysis with SARS-CoV-2 main protease (3CLpro).
| S.No | Drugs | Molecular formula | Molecular weight | Canonical SMILES IDs | 2D structure of drugs | 3D structure of TAT-peptide conjugated drugs |
|---|---|---|---|---|---|---|
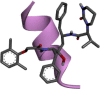
| 1. | Lopinavir | C37H48N4O5 | 628.8 g/mol | CC1 C(C( CC C1)C)OCC( O)NC(CC2 CC CC C2)C(CC(CC3 CC CC C3)NC( O)C(C(C)C)N4CCCNC4 O)O |  |
 |
| 2. | Ritonavir | C37H48N6O5S2 | 720.9 g/mol | CC(C)C1 NC( CS1)CN(C)C( O)NC(C(C)C)C( O)NC(CC2 CC CC C2)CC(C(CC3 CC CC C3)NC( O)OCC4 CN CS4)O | 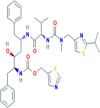 |
 |
| 3. | Favipiravir | C5H4FN3O2 | 157.1 g/mol | C1 C(N C(C( O)N1)C( O)N)F | 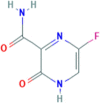 |
 |
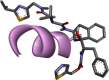
| 4. | Hydroxychloroquine | C18H26ClN3O | 335.8 g/mol | CCN(CCCC(C)NC1 C2C CC( CC2 NC C1)Cl)CCO | 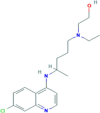 |
 |
2.5. In silico molecular interaction analysis
The in silico interaction analysis was executed into two parts:
2.5.1. Molecular docking of repurposed drugs without TAT-peptide with COVID-19 main protease
The docking experimentation between free drug (without TAT-peptide) and COVID-19 protease (PDB ID: 6LU7) was executed with the help of AutoDock 4.2 MGL tools version 1.5.6. AutoDock uses a Lamarckian Genetic Algorithm and empirical binding free energy function as a scoring function for the ligand-receptor interaction (Morris et al., 1998). The docking was performed on the active site after implementing the default AutoDock parameters. However, to cover the maximum area within the grid box 60x60x60 Å which can accommodate the selected active site residues in the grid box, the grid center point coordinates X, Y and Z were set as −15.217, 14.435 and 60.367, respectively with the default value of grid points spacing 0.375 Å.
2.5.2. Molecular docking of TAT-Peptide-conjugated repurposed drug with COVID-19 main protease
AutoDock 4.2 tool was used to prepare TAT-Peptide-conjugated drug complexes. After that TAT-Peptide-conjugated drug complexes were docked with COVID-19 protease (PDB ID: 6LU7) using the PatchDock online docking server (https://bioinfo3d.cs.tau.ac.il/PatchDock/). PatchDock uses a geometry-based molecular docking algorithm as a scoring function (Schneidman-Duhovny et al., 2005). After performing docking analysis, results were analyzed and 3D graphics was generated using discovery Studio Visualizer, 2019.
2.6. Results and discussion
Currently, 548 unique therapeutic compounds are in development and 176 of those are in the clinical stage i.e., 26 in phase I, 86 in phase II, 41 in phase III and 23 are in phase IV (https://www.bio.org/policy/human-health/vaccines-biodefense/coronavirus/pipeline-tracker). Among them, repurposed drugs such as remdesivir, lopinavir, ritonavir, favipiravir, and hydroxychloroquine show great therapeutic potential in the prevention and treatment of COVID-19 infections. However, the discovery of the cell-penetrating function of HIV1-TAT protein in 1988 quickly became a popular research tool used to enhance the intracellular delivery and transport of drugs and a large number of biomolecules (Skwarczynski and Toth, 2019). Thus, to improve the efficacy of repurposed drugs, CPPs can be conjugated to drug or formulation. CPPs can deliver drugs directly to the cytoplasm either by endocytic or nonendocytic pathways. At present, a large number of naturally derived as well as synthetic or artificial CPPs have been characterized and used for the intracellular delivery of a variety of cargos such as small molecules, drugs, DNA/RNA, peptide, proteins, liposomes and nanoparticles into cells (Skwarczynski and Toth, 2019). CPPs are also easy to prepare, cost-effective, and most importantly, they are usually non-toxic (Skwarczynski and Toth, 2019). It has been investigated that HIV1 TP directly penetrate the membranes by generating nanoscale pores (Ciobanasu et al., 2010). The preclinical and clinical trials on cancer diagnosis and treatment show that CPP-based drug delivery systems enhance the efficiency of the delivery of anti-cancer drugs and imaging reagents (Tripathi et al., 2018). Thus, the development of CPP-based delivery of repurposing drugs might be an excellent approach to enhance the efficacy of the existing drugs for the treatment of COVID-19.
One of the best-characterized and attractive drug targets among coronaviruses is the main protease (3CLpro) (Anand et al., 2003, Hang et al., 2020). Along with the papain-like protease(s), this enzyme is essential for processing the polyproteins that are translated from the viral RNA (Hilgenfeld, 2014). The 3CL protease of coronaviruses facilitates viral assembly by cleaving almost 11 sites on the large polyproteins. Inhibiting the activity of the protease enzyme would block viral replication and also prevent the progression of the disease (Hilgenfeld, 2014). The protein sequences of COVID-19 Main protease (2019-nCoV Mpro) and SARS-CoV Mpro are 96% identical (Bhattacharya et al., 2020b). In several studies, the similarities in the sequence of a potential target for COVID-19 to that of the SARS Mpro were utilized to build a model for the structure of SARS-CoV-2 Mpro. Homology based models were utilized to screen a library of compounds to predict potential drugs for COVID-19 (Nguyen et al., 2020; Xu et al., 2020; Liu and Wang, 2020).
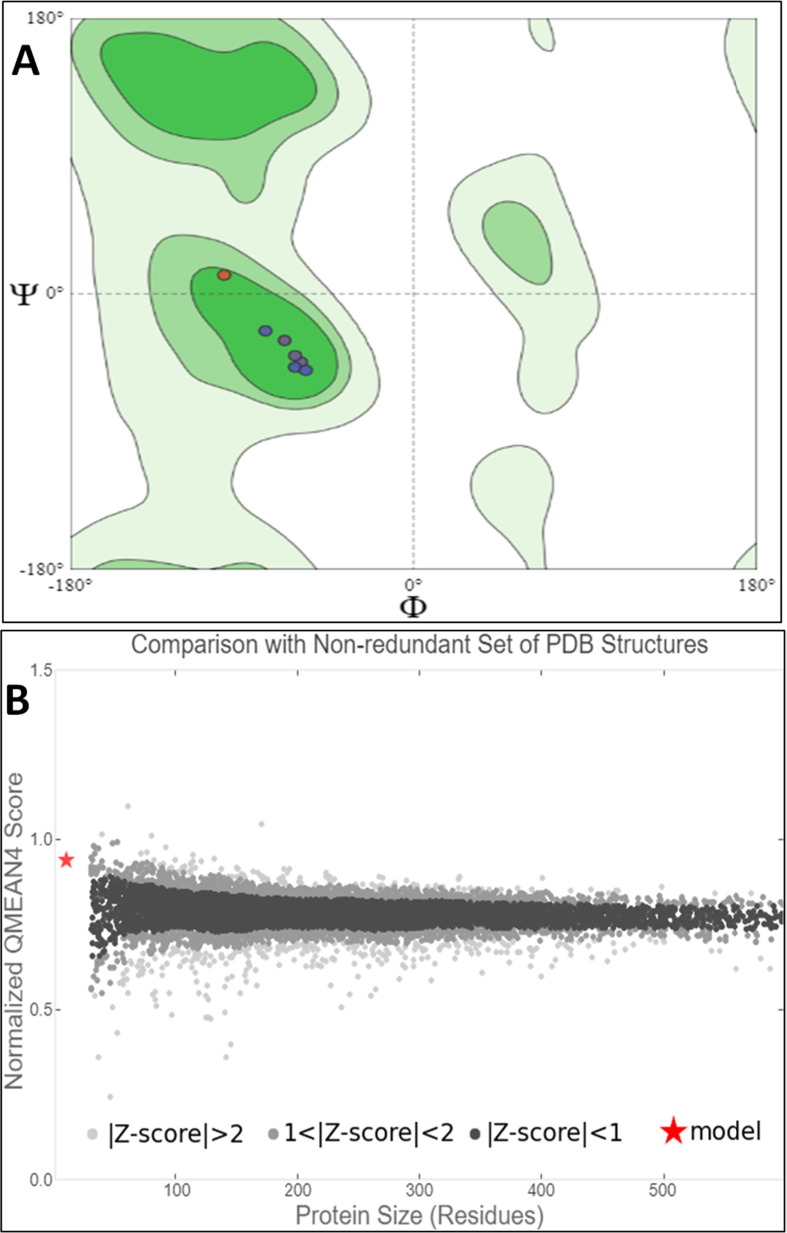
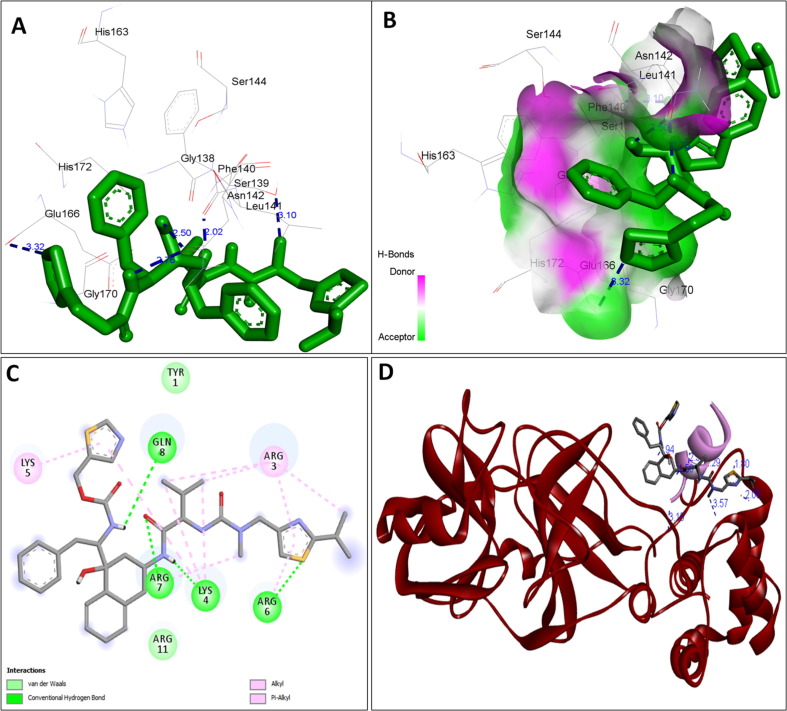
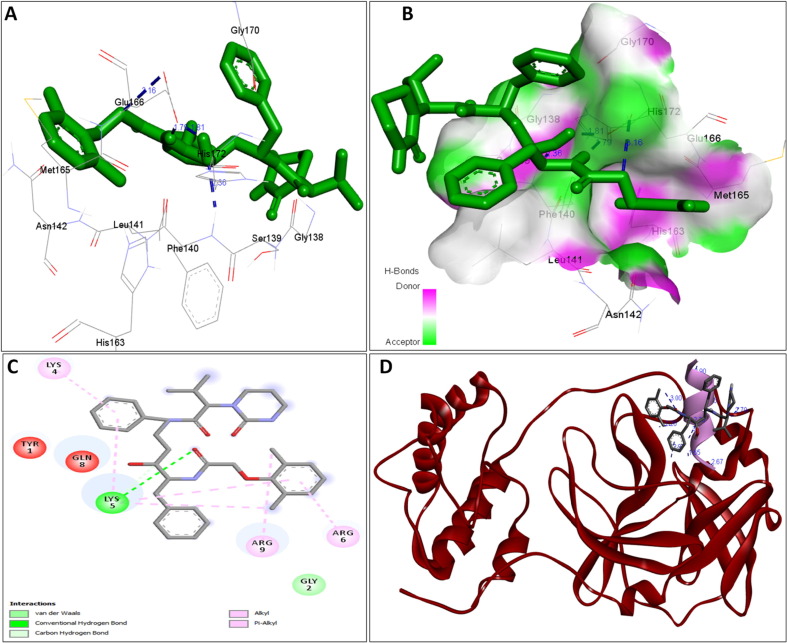
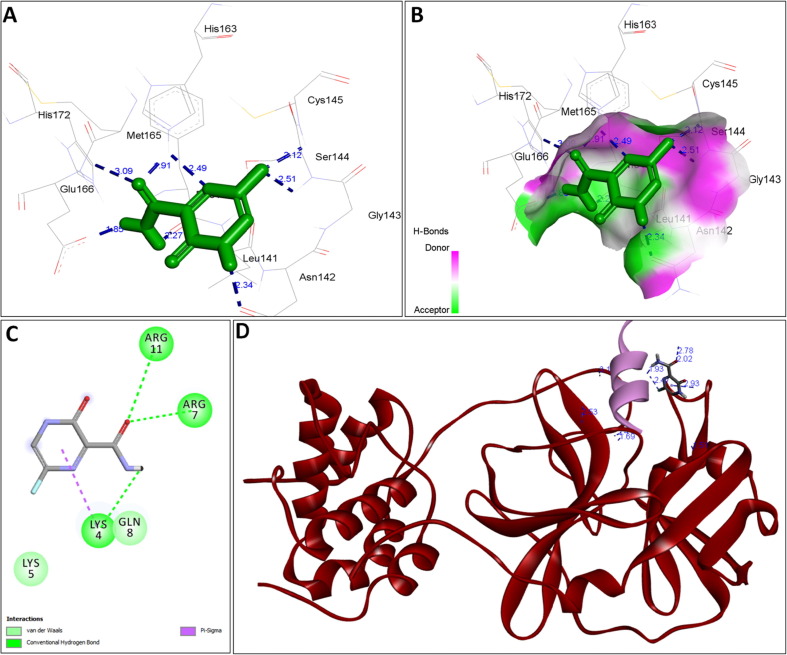
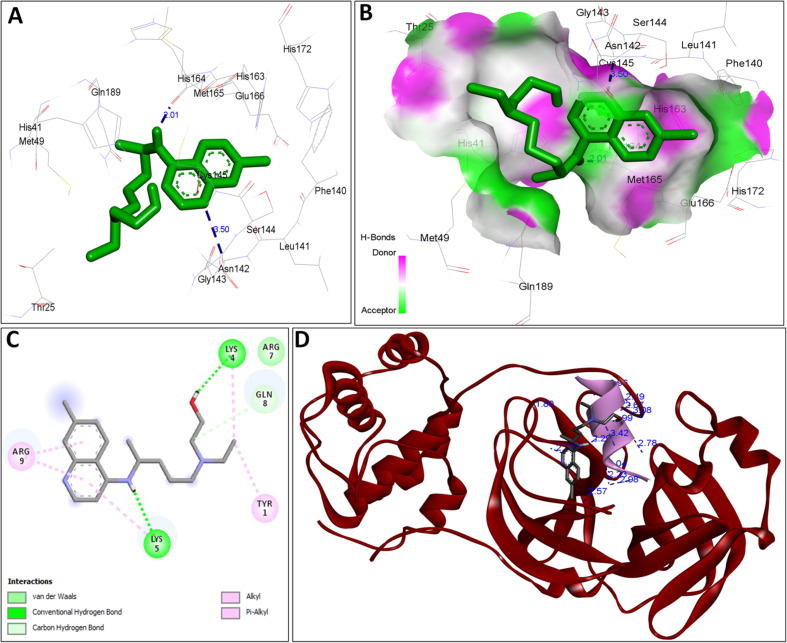
The availability of the high-resolution X-ray crystal structures of the target i.e., the main protease of SARS-CoV-2 Mpro (PDB ID: 6LU7), has been utilized in the current study as the target for molecular docking based virtual screening of TP-conjugated repurposing antiretroviral, antiviral and antimalarial drugs. The 3D modeled structure of TP used in this study was generated by PEP-FOLD3.5 (Fig. 2 ). Further, the 3D modeled structure of TP was validated by MolProbity tool integrated in structure assessment module of SwissModel server. It was observed that number of residues found in the favored region was ~100.0% and no residues were found in Ramachandran Outliers and Rotamer Outliers regions (Fig. 3 A). The MolProbity Score was 0.50 and QMEAN4 score was less than <1 as compared with standard ideal value that should be between 0 and 1 for good quality predicted structure (Benkert et al., 2011) (Fig. 3B). So, 3D structure of TP was found to be acceptable in order to perform further in silico interaction analysis. For the first time, TP was conjugated with lopinavir, ritonavir, favipiravir and hydroxychloroquine using AutoDock tool to investigate their binding affinity and interaction with COVID-19 main protease. The 3D structure of individual drug with TP to perform in silico analysis with COVID-19 Protease was shown in Table 1. AutoDock analysis was also performed to illustrate the possible interaction between TP and drugs. The interaction between TP and drugs shows that a number of several others types of molecular contacts were also involved apart from hydrogen bonds that provide more stability to the complex. During Ritonavir and TP interaction Arg,3 Lys4, Lys5, Arg6, Arg7 and Gln8 were also involved in Alkyl and Pi-Alkyl bonding apart from conventional hydrogen bond and van der walls interactions (Fig. 4 C). During Lopinavir and TP interaction Lys4, Arg6 and Arg9 were involved in Alkyl and Pi-Alkyl interaction apart from hydrogen bond, carbon-hydrogen bond and van der walls interactions (Fig. 5 C). Favipiravir and TP interaction shows Pi-Sigma contact, hydrogen bond and van der Walls interaction (Fig. 6 C). In case of hydroxychloroquine and TP interaction Tyr1, Lys4, Lys5 and Arg9 were involved in Alkyl and Pi-Alkyl bonding apart from hydrogen bond, carbon-hydrogen bond and van der walls interactions (Fig. 7 C).
Fig. 2.
3D structure of Modeled TAT-peptide.
Fig. 3.
(A) Ramachandran Plot generated by MolProbity tool for the Modeled 3D TP validation. (B) Showing the modeled TP QMean4 Score and comparison with non-redundant set of PDB structures.
Fig. 4.
A: showing Ritonavir (green color stick pattern) interaction with COVID-19 Main protease (PDB ID: 6LU7) amino acid residues (grey color stick pattern) involved in hydrophobic interaction. Blue dotted lines represents hydrogen bonds; B: showing COVID-19 protease (PDB ID: 6LU7) pocket that accommodated the Ritonavir (green color stick pattern); C: 2D visualization of TP interaction with Ritonavir; D- showing TP (pink color ribbon pattern) conjugated Ritonavir complex (grey color stick pattern) interaction with COVID-19 protease (PDB ID: 6LU7) (maroon color ribbon pattern). Blue dotted lines are showing hydrogen bonds formation.
Fig. 5.
A: showing Lopinavir (green color stick pattern) interaction with COVID-19 Main protease (PDB ID: 6LU7) amino acid residues (grey color stick pattern) involved in hydrophobic interaction. Blue dotted lines represents hydrogen bonds; B: showing COVID-19 protease (PDB ID: 6LU7) pocket that accommodated the Lopinavir (green color stick pattern); C: 2D visualization of TP interaction with Lopinavir; D: showing TP (pink color ribbon pattern) conjugated Lopinavir complex (grey color stick pattern) interaction with COVID-19 protease (PDB ID: 6LU7) (maroon color ribbon pattern). Blue dotted lines are showing hydrogen bonds formation.
Fig. 6.
A: showing Favipiravir (green color stick pattern) interaction with COVID-19 Main protease (PDB ID: 6LU7) amino acid residues (grey color stick pattern) involved in hydrophobic interaction. Blue dotted lines represents hydrogen bonds; B: showing COVID-19 protease (PDB ID: 6LU7) pocket that accommodated the Favipiravir (green color stick pattern); C: 2D visualization of TP interaction with Favipiravir; D: showing TP (pink color ribbon pattern) conjugated Favipiravir complex (grey color stick pattern) interaction with COVID-19 protease (PDB ID: 6LU7) (maroon color ribbon pattern). Blue dotted lines are showing hydrogen bonds formation.
Fig. 7.
A: showing hydroxychloroquine (green color stick pattern) interaction with COVID-19 Main protease (PDB ID: 6LU7) amino acid residues (grey color stick pattern) involved in hydrophobic interaction. Blue dotted lines represents hydrogen bonds; B: showing COVID-19 protease (PDB ID: 6LU7) pocket that accommodated the hydroxychloroquine (green color stick pattern); D: 2D visualization of TP interaction with hydroxychloroquine; D: showing TP (pink color ribbon pattern) conjugated hydroxychloroquine complex (grey color stick pattern) interaction with COVID-19 protease (PDB ID: 6LU7) (maroon color ribbon pattern). Blue dotted lines are showing hydrogen bonds formation.
We postulated that after conjugating repurposed drug to TAT-peptide, the binding affinity was enhanced to counter the COVID-19 protease. Further, it was also hypothesized that the TP conjugated repurposing drugs interact more strongly and efficiently than drugs without TAT conjugate. In the present study, the obtained results support our experimental hypothesis. The molecular docking study showed that the interaction of repurposed drugs ritonavir, lopinavir, favipiravir and hydroxychloroquine (without TP conjugation) formed 5, 4, 8 and 2H-bonds, respectively, when docked with COVID-19 main protease (Tables S1 and S3; Figs. 4ab, 5ab, 6ab, 7ab). The visualization of full 3D structures of COVID-19 main protease docked with the individual drugs without TP was shown in supplementary figure (S1-S5). The observed binding energy score was −9.16 kcal/mol for ritonavir, −7.57 kcal/mol for lopinavir, −4.23 kcal/mol for favipiravir and −6.61 kcal/mol for hydroxychloroquine (Table S1). The results of molecular docking for compounds currently in clinical trials predict that ritonavir and lopinavir are in Phase IV of clinical trials, has the best binding energy for inhibition of Mpro of SARS-CoV-2 (-9.16 and −7.57 kcal/mol, Table S1). The favipiravir, an antiviral drug that inhibits viral RNA-dependent RNA-polymerase is currently in Phase 2, 3 and 4 for treatment of COVID-19, has the binding energy for inhibition of Mpro of SARS-CoV-2 (i.e., −4.23 kcal/mol, Table S1) are weaker binders than ritonavir & lopinavir (-9.16 & −7.57 kcal/mol). Lopinavir and ritonavir have been reported in earlier studies as potential drug candidates that target Mpro of SARS-CoV-2. In this study, the binding energies for ritonavir and lopinavir (−9.16 & −7.57 kcal/mol) are in good agreement with previous molecular docking study (Sekhar 2020) and are consistent with preliminary clinical data indicating effectiveness for these drugs (Wang et al., 2020a, Wang et al., 2020b). Hydroxychloroquine, an antimalarial drug has been found to be efficient on SARS-CoV-2 and reported to be beneficial in Chinese COV-19 patients (Gautret et al., 2020). Hydroxychloroquine has been approved for human clinical trials and currently in Phase I, II, III and IV for treatment of COVID-19, has the binding energy of −6.61 kcal/mol (Table S1), indicating a potential for increased efficacy. Based on the modeled structure of SARS-CoV-2 (Mpro), molecular docking and free energy, it was predicted that ritonavir, lopinavir, and hydroxychloroquine are the most potent drug candidates for COVID-19. In contrary to the drug without TP-conjugation, the molecular docking study of TP-conjugated ritonavir, lopinavir, favipiravir and hydroxychloroquine complex showed enhanced binding affinity with COVID-19 Main protease (Figs. 4D, 5D, 6D, 7D; Table S2). It has been observed that the efficacy of repurposed drugs have been enhanced after conjugating with TP when compared to drug without TP. When TP was interacted with ritonavir, lopinavir, favipiravir, and hydroxychloroquine only 4, 1, 3, and 2H-bond were formed (Table S3). However, when TP-conjugated ritonavir, lopinavir, favipiravir, and hydroxychloroquine drug complex were docked with COVID-19 main protease 8, 10, 10, and 15H-bonds were formed which are comparatively much higher than that of the drug without the TP (Figs. 4D, 5D, 6D, 7D; Table S2, S3). After comparing the molecular docking data of drugs without TP with COVID-19 protease (Figs. 4ab, 5ab, 6ab, 7ab; Table S1) and TP- conjugated drug complex (Figs. 4D, 5D, 6D, 7D; Table S2), it has been found that TP- conjugated drugs interacted most efficiently with COVID-19 main protease (Table S3). The molecular docking results revealed and validated that TP-conjugated drugs have superior and significantly enhanced interaction with the target SARS-CoV-2 Main protease (Tables S2 and S3). The results of the current study confirm the therapeutic potential of TP-conjugated drugs complex against SARS-CoV-2 Mpro; therefore, these TP-conjugated drug complexes are reassuring and more suitable for therapeutic application of COVID-19 treatment than the existing free drugs. Further, the therapeutic potential TP-conjugated repurposed drugs can be enhanced and improved by utilizing various nanomedicine-based drug delivery approaches e.g., lipid-based nanoparticles (solid lipid nanoparticle, nanoemulsion, liposome), polymeric nanoparticles (poly lactic-co-glycolic acid, poly ε-caprolactone), dendrimers, polymeric micelles, etc. (Ansari et al., 2020). Nanomedicine-based delivery techniques possibly augment the efficacy of the drugs by facilitating controlled-release and may also enhance the bioavailability and reduce side effects (Lembo et al., 2018). The manufactured nanocarriers may easily get to specific extracellular/intracellular targets site and thus can compete with virus for their attachment to the cell surface receptors (Lembo et al., 2018). As a result, nanomedicine based-drug delivery approach is promising alternative strategies that can be explored to develop the broad-spectrum antiviral drugs against current COVID-19 infection (Lembo et al., 2018). Moreover, organometallic complexes and nanocomposites can also be evaluated for anti-SARS-CoV-2 therapeutic agents. However, further extensive research is required before these nanomedicines based TP-conjugated drugs will be ready for advanced clinical trials.
3. Conclusion
CPPs are promising immune enhancers when incorporated into appropriate drug delivery systems. CPPs has a number of advantages over other translocation and delivery methods as it is easy to prepare, inexpensive and normally have low cell toxicity with no immunological response. Drug development against SARS-CoV-2 is considered urgent in order to fight COVID-19. The present study suggests that TP-conjugated drugs will be effective in treating COVID-19. The molecular docking results validated that TP-conjugated ritonavir, lopinavir, favipiravir, and hydroxychloroquine have superior and significantly enhanced interactions with the target SARS-CoV-2 Main protease (Mpro). TAT-peptide has a higher capability to translocate into a wide range of cell types, higher rate of cellular permeability and uptake, easier to pass through other biological barriers. In conclusion, in-silico approach employed in this study suggests that the combination of the drug with TP is an excelling alternative to develop a novel drug for the treatment of SARS-CoV-2 infected patients. The predictions from the results obtained provide invaluable information that can be utilized for the choice of candidate drugs for in vitro, in vivo and clinical trials. The outcomes from this work prove crucial for exploring and developing novel cost-effective and biocompatible TP-conjugated anti-SARS-CoV-2 therapeutic agents in immediate future.
Funding
Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia, Grant number-Covid19-2020-002-IRMC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.09.037.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- Ansari M.A., Almatroudi A., Alzohairy M.A., AlYahya S., Alomary M.N., Al-Dossary H.A., Alghamdi S. Lipid-based nano delivery of Tat-peptide conjugated drug or vaccine–promising therapeutic strategy for SARS-CoV-2 treatment. Exp. Opin. Drug Deliv. 2020;31:1–4. doi: 10.1080/17425247.2020.1813712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara C., Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013;587:1693–1702. doi: 10.1016/j.febslet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Benkert P., Biasini M., Schwede T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics. 2011;27:343–350. doi: 10.1093/bioinformatics/btq662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C., Saha R.P., Lee S.S., Chakraborty C. A SARS-CoV-2 vaccine candidate: In-silico cloning and validation. Inf. Med. Unlocked. 2020;20 doi: 10.1016/j.imu.2020.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Sharma A.R., Patra P., Ghosh P., Sharma G., Patra B.C., Lee S.S., Chakraborty C. Development of epitope-based peptide vaccine against novel coronavirus 2019 (SARS-COV-2): Immunoinformatics approach. J. Med. Virol. 2020;92:618–631. doi: 10.1002/jmv.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty C., Sharma A.R., Sharma G., Bhattacharya M., Lee S.S. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): diagnostic and proposed therapeutic options. Eur Rev Med Pharmacol Sci. 2020;24:4016–4026. doi: 10.26355/eurrev_202004_20871. [DOI] [PubMed] [Google Scholar]
- Chakraborty C., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S. The 2019 novel coronavirus disease (COVID-19) pandemic: A zoonotic prospective. Asian Pacif. J. Trop. Med. 2020;13:242–246. [Google Scholar]
- Chakraborty C., Sharma A.R., Sharma G., Bhattacharya M., Saha R.P., Lee S.S. Extensive Partnership, Collaboration, and Teamwork is Required to Stop the COVID-19 Outbreak. Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.W., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:18–23. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn V.B., Arendall W.B., Headd J.J., Keedy D.A., Immormino R.M., Kapral G.J., Murray L.W., Richardson J.S., Richardson D.C. All-atom structure validation for macromolecular crystallography. Acta Cryst. 2010;D66:16–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciobanasu C., Siebrasse J.P., Kubitscheck U. Cell-penetrating HIV1 TAT peptides can generate pores in model membranes. Biophys. J. 2010;7:153–162. doi: 10.1016/j.bpj.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S.L., Fan T.C., Fu H.W., Chen C.J., Hwang C.S., Hung T.J., Lin L.Y., Chang M.D.T. A novel cell-penetrating peptide derived from human eosinophil cationic protein. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0057318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gaurav C., Marc J.M., Sourav K., Vianni C., Deepa G., Sergio O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano. 2020 doi: 10.1021/acsnano.0c04006. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Age. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gralinski L.E., Menachery V.D. Return of the Coronavirus: 2019-nCoV. Viruses. 2020;12:135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgenfeld R. From SARS to MERS: Crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281:4085–4096. doi: 10.1111/febs.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://milken-institute-covid-19-tracker.webflow.io/ LAST UPDATED:SEPTEMBER 10, 2020 8:26 AMPACIFIC.
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamiable A., Thévenet P., Rey J., Vavrusa M., Derreumaux P., Tufféry P. PEP-FOLD3: faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016;44:W449–W454. doi: 10.1093/nar/gkw329. https://mobyle.rpbs.univ-paris-diderot.fr/cgi-bin/portal.py#forms::PEP-FOLD3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo D., Donalisio M., Civra A., Argenziano M., Cavalli R. Nanomedicine formulations for the delivery of antiviral drugs: a promising solution for the treatment of viral infections. Expert Opin. Drug Deliv. 2018;15:93–114. doi: 10.1080/17425247.2017.1360863. [DOI] [PubMed] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Liu W., Morse J.S., Lalonde T., Xu S. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020 doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang X.J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Gen. Genom. 2020;47:119. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.M., Goodsell D.S., Halliday R.S., Huey R., Hart W.E., Belew R.K., Olson A.J. Automate Docking Using a Lamarckian Genetic Algorithm and and Empirical Binding Free Energy Function. J. Comput. Chem. 1998;1998(19):1639–1662. [Google Scholar]
- https://covid19.who.int/ Updated: September 11, 2020.
- Nguyen, D., Gao, K., Chen, J., Wang, R., Wei, G., 2020. Potentially highly potent drugs for 2019-nCoV. bioRxiv. 10.1101/2020.02.05.936013. [DOI]
- Nori A., Jensen K.D., Tijerina M., Kopecková P., Kopeček J. Tat-conjugated synthetic macromolecules facilitate cytoplasmic drug delivery to human ovarian carcinoma cells. Bioconjug. Chem. 2003;14:44–50. doi: 10.1021/bc0255900. [DOI] [PubMed] [Google Scholar]
- Pärn K., Eriste E., Langel Ü. Cell-Penetrating Peptides. Humana Press; New York, NY: 2015. The antimicrobial and antiviral applications of cell-penetrating peptides; pp. 223–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Quan X., Sun D., Zhou J. Molecular mechanism of HIV-1 TAT peptide and its conjugated gold nanoparticles translocating across lipid membranes. PCCP. 2019;21:10300–10310. doi: 10.1039/c9cp01543d. [DOI] [PubMed] [Google Scholar]
- Rehman S., Majeed T., Ansari M.A., Ali U., Sabit H., Al-Suhaimi E.A. Current scenario of COVID-19 in pediatric age group and physiology of immune and thymus response. Saudi J. Biol. Sci. 2020 doi: 10.1016/2Fj.sjbs.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S., Chakraborty C. Tocilizumab: A therapeutic option for the treatment of cytokine storm syndrome in COVID-19. Arch. Med. Res. 2020;51:595–597. doi: 10.1016/j.arcmed.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S., Chakraborty C. Probable Molecular Mechanism of Remdesivir for the Treatment of COVID-19: Need to Know More. Arch. Med. Res. 2020;51:585–586. doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidman-Duhovny D., Inbar Y., Nussinov R., Wolfson H.J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucl. Acids. Res. 2005;33:W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar, T., 2020. Virtual Screening based prediction of potential drugs for COVID-19. 10.20944/preprints202002.0418.v2. Preprints (www.preprints.org). [DOI]
- Skwarczynski M., Toth I. Cell-penetrating peptides in vaccine delivery: facts, challenges and perspectives. Ther. Deliv. 2019;10:465–467. doi: 10.4155/tde-2019-0042. [DOI] [PubMed] [Google Scholar]
- Tripathi P.P., Arami H., Banga I., Gupta J., Gandhi S. Cell penetrating peptides in preclinical and clinical cancer diagnosis and therapy. Oncotarget. 2018;9:37252–37267. doi: 10.18632/oncotarget.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanommeslaeghe K., Hatcher E., Acharya C., Kundu S., Zhong S., Shim J., Darian E., Guvench O., Lopes P., Vorobyov I., Mackerell A.D., Jr CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Bioscience Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- Weiss C., Carriere M., Fusco L., Capua I., Regla-Nava J.A., Pasquali M., Scott J.A., Vitale F., Unal M.A., Mattevi C., Bedognetti D. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L., Lepore R., Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z., Peng, C., Shi, Y., Zhu, Z., Mu, K., Wang, X., Zhu, W., 2020. Nelfinavir was predicted to be a potential inhibitor of 2019-nCov main protease by an integrative approach combining homology modelling, molecular docking and binding free energy calculation. bioRxiv. 10.1101/2020.01.27.921627. [DOI]
- Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.