Abstract
Objective
Encephalopathy is a major neurological complication of severe Coronavirus Disease 2019 (COVID-19), but has not been fully defined yet. Further, it remains unclear whether neurological manifestations are primarily due to neurotropism of the virus, or indirect effects, like cerebral hypoxia.
Methods
We analysed the electroencephalograms (EEGs) of 19 consecutive patients with laboratory-confirmed COVID-19, performed at peak disease severity as part of their clinical management. Disease severity, respiratory failure, immune and metabolic dysfunction, sedation status, and neurological examination on the day of the EEG were noted.
Results
Severe encephalopathy was confirmed in 13 patients, all with severe COVID-19; 10 remained comatose off sedation, and five of them had alpha coma (AC). Disease severity, sedation, immune and metabolic dysfunction were not different between those with AC and those without.
Conclusions
Severe COVID-19 encephalopathy is a principal cause of persisting coma after sedation withdrawal. The relatively high incidence of the rare AC pattern may reflect direct SARS-CoV-2 neurotropism with a predilection for the brainstem ascending reticular system.
Significance
Systematic early EEG detection of encephalopathy related to severe COVID-19 is important for the acute care and the management of long-term neurological and cognitive sequelae, and may help our better understanding of its pathophysiology.
Keywords: SARS-CoV-2, Neurotropism, Neurological manifestations, Brainstem, Ascending reticular formation, Encephalitis, Non-convulsive status, Seizures
1. Introduction
Encephalopathy is one of the main central nervous system (CNS) manifestations reported in patients with severe Coronavirus Disease 2019 (COVID-19) who require critical care (Helms et al., 2020 Apr 15, Mao et al., 2020). In these patients, the diagnosis of COVID-19 related encephalopathy has so far been based on clinical factors, such as a history of prodromal symptoms (Helms et al., 2020 Apr 15, Mao et al., 2020) and/or on abnormal neurological signs after sedation withdrawal, which may persist in the recovery phase (Helms et al., 2020). Potential causes may include neurotropic effects of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Li et al., 2020), or hypoxia due to severe pneumonitis and other possible confounders, such as superimposed bacterial sepsis or metabolic derangements.
The electroencephalogram (EEG) may objectively confirm suspected CNS dysfunction during the acute phase of COVID-19 (Gelisse et al., 2020), including patients on the intensive care unit (ICU) who remain comatose after sedation withdrawal because of possible non-convulsive status epilepticus (NCSE) or severe encephalopathy. Identification of the cause of persistent low conscious state is not only important for their acute management but also carries implications for their subsequent neurological recovery. EEG during the acute phase of COVID-19 might also contribute to our understanding of the pathophysiology of this novel disease; for example, demonstration of localised cerebral dysfunction might potentially be suggestive of SARS-CoV-2 neurotropism.
EEG data in COVID-19 has so far been limited. None was included in the large series of Mao and colleagues (Mao et al., 2020), the Strasbourg series contained only a brief description in one of their eight patients who had EEG amongst 58 with neurological manifestations (Helms et al., 2020), and the remainder are case reports concerning epileptic seizures and NCSE (Balloy et al., 2020, Flamand et al., 2020, Somani et al., 2020). We analysed the EEG findings in a cohort of consecutive patients who had EEG in the acute phase of COVID-19 as part of their clinical management.
2. Patients and methods
2.1. Setting of the study
This retrospective observational study was conducted at St. Thomas’ Hospital, an 1100 bed university-affiliated hospital in London, UK, which is a designated High Consequence Infectious Diseases referral centre, with a large ICU and extra-corporeal membrane oxygenation (ECMO) capability. Diagnosis of COVID-19 infection was confirmed by positive results on a reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay performed on nasopharyngeal and throat swab, or on lower respiratory tract specimens.
Standard care was provided at the discretion of the treating medical team. Typically, patients were admitted to ICU for hypoxaemic respiratory failure and underwent early invasive mechanical ventilation. Sedation was provided according to local protocols, based on infusions of propofol and fentanyl, but with modifications to include a greater use of intravenous or enteral benzodiazepines or longer acting enteral opioid substitutes due to drug shortages during the COVID-19 pandemic. The ICU had a protocol for daily sedation interruption although compliance was potentially impacted by the patient’s severe clinical condition as well as operational pressures during the pandemic. EEG was requested at physician discretion for indications including failure to wake from sedation or suspected epileptic seizures. Cross-sectional brain imaging was not protocolised.
2.2. EEG methodology
All video EEGs were performed during the increment of the pandemic in London, using XLTEK/Natus recording equipment. To reduce the time of technologists’ exposure and in view of the initial uncertainties surrounding personal protective equipment, we followed the American Clinical Neurophysiology Society recommendations at the start of the pandemic (Haines et al., 2020) and used a limited number of AMBU silver/silver chloride disposable scalp electrodes, placed according to the 10–20 international system. An 11-electrode montage, using Fp1, Fz, Fp2, T3, C3, Cz, C4, T4, O1 and O2 standard positions was deemed satisfactory for the assessment of diffuse encephalopathy, infectious encephalitides (with their typical predilection for the temporal and frontal areas in terms of both lateralized periodic discharges (Gupta and Seth, 1973) and epileptic seizures), and NCSE. Electrode impedance was kept below 5kOhms during recordings, which lasted 20–30 min. All patients had EEG while lying supine. Respiratory and other artefacts were annotated during the recording and clinical and EEG responsiveness was assessed in all patients. According to our protocol, stimulation was applied during epochs that probably represented lower level of vigilance, when spontaneous cycling was noted in the first half or two-thirds of the recording. It included calling out the patient’s name and clapping, passive eye-opening and closure, noxious stimuli in all limbs (at least twice per limb, each stimulus for ≥ 3 s to ensure temporal summation), trapezoid pinch or supraorbital pressure, and endotracheal suctioning. Analysis of the concurrent video recording helped with the identification of respiratory artefacts and of additional reactivity to sounds from the near-by ICU equipment. Pertinent clinical information relevant to acute EEG interpretation was collected at the time of the recording, such as clinical neurological status (including brainstem reflexes), sedation status, renal function and other laboratory measurements of metabolic and immune function, information regarding any concurrent complications (such as intercurrent bacterial sepsis) and results of recent brain imaging when available.
EEGs were independently analysed by four clinical neurophysiologists (in two groups of two), who were blind to patients’ clinical details, with any differences resolved by consensus. We noted the dominant and other activities, their distribution, and possible spontaneous fluctuations and reactivity. Voltage was measured from the Cz-C3 and Cz-C4 derivations. For the AC pattern in particular, we also compared its amplitude between anterior and posterior areas (on the common average derivation), noted possible voltage modulations (spindling) and reactivity, measured its frequency, and quantified its appearance as a percentage of the total EEG recording time.
2.3. Clinical, demographic and laboratory data
All pertinent data was collected from patients’ individual electronic medical records, including age, gender, ethnic background, the presence or absence of cardiopulmonary arrest (CPA), the severity of respiratory failure (using the Berlin acute respiratory distress syndrome (ARDS) classification) (ARDS Definition Task Force, 2012), and measures of other non-respiratory organ system dysfunction (utilising clinical parameters and/or worst laboratory values on the day of the EEG). Overall total organ dysfunction/sickness severity was measured using the sequential organ failure assessment (SOFA) score (Vincent et al., 1996) on the day of ICU admission and on the day the first EEG was performed. A state of “hyper-inflammation” associated with COVID-19 was ascribed pragmatically if the patient had been prescribed any form of immunomodulatory therapy (corticosteroids and/or the recombinant human interleukin-1 antagonist anakinra) for that indication by the treating physician. Sedation practices and use of anti-epileptic drugs was captured. Neurological status and brainstem reflexes were recorded on the day of the EEG. Because the clinical onset of encephalopathy was impossible to retrospectively ascertain due to the urgent need for mechanical ventilation and the lack of data on patients transferred from ICUs of other hospitals, the onset of “severe disease” and encephalopathy was pragmatically assigned to the day of intubation / sedation. Summary data is presented as median [interquartile range] (IQR) or n (%) as appropriate.
2.4. Ethical approval
This study was approved by the Ethics Committee of Guys and St Thomas’ NHS Trust (REC Number: 20/HRA/1871) and written informed consent was waived due to the rapid emergence of the disease and the urgent need to collect data.
3. Results
3.1. Clinical findings
At the time of writing 1574 patients have been admitted to our hospital with laboratory confirmed COVID-19 during the pandemic, of whom 317 were admitted to the ICU. Of these, 19 patients were referred for EEG between 3rd April and 10th May 2020, and are included in this study. Their demographic and clinical characteristics are presented in Table 1 . The median age was 52 [IQR 48.0 – 65.5] years, seven (37%) were female, and 11 (57.9%) belonged to Black, Asian and Minority Ethnic groups. Eleven patients had chronic co-morbidities, and many were obese, with median body mass index being 28.1 [IQR 26 – 34.4; data presented in the Supplementary Table 1].
Table 1.
Clinical, laboratory characteristics and EEG findings in the 19 patients with COVID-19.
| Pt | Gender / age (years) | Primary and secondary diagnoses | Comorbidities | SOFA at EEG (0–24) | Peak serum urea (mmol/L) | Peak D-dimer (mg/L) | Sedation at EEG† | EEG | |
|---|---|---|---|---|---|---|---|---|---|
| Main rhythms / max voltage | Cycling / Reactivity** | ||||||||
| 1 | M / 37 | COVID-19 pneumonitis + sepsis | None reported | 10 | 37.6 | 29.4 | - | AC pattern | See table 2 |
| 2 | M / 47 | COVID-19 pneumonitis | End stage renal failure (on dialysis), hypertension, DM | 10 | 38.3 | 21.9 | - | AC pattern | See table 2 |
| 3 | F / 69* | COVID-19 pneumonitis | None reported | 11 | 39.8 | 80.0 | - | AC pattern | See table 2 |
| 4 | F / 67* | COVID-19 pneumonitis | None reported | 10 | 42.5 | 46.3 | - | AC pattern | See table 2 |
| 5 | F / 53* | COVID-19 pneumonitis | Hypertension, DM | 10 | 38.6 | 63.7 | - | δ, slow θ / 40–50 µV; also AC pattern | Yes / Yes See table 2 |
| 6 | M / 38* | COVID-19 pneumonitis + seizures*** + sepsis | None reported | 19 | 36.2 | 56.5 | Propofol Fentanyl | δ, θ / 20–25 µV | No / No |
| 7 | M / 67 | COVID-19 pneumonitis + seizures + SAH | Hypertension, chronic kidney disease, DM, transient ischaemic attack | 15 | 58.1 | 80.0 | Propofol Fentanyl | δ, θ / 30 µV | Yes / Yes |
| 8 | F / 67* | COVID-19 pneumonitis + multiple cerebral infarcts | End stage renal failure (on dialysis) | 14 | 40.1 | 80.0 | - | θ, δ, a / 25 µV | Yes / Yes |
| 9 | M / 51* | COVID-19 pneumonitis + sepsis + multiple cerebral infarcts | None reported | 10 | 51.6 | 80.0 | - | δ, some θ / 60 µV | Yes / Yes |
| 10 | M / 52 | COVID-19 pneumonitis + multiple cerebral infarcts + in-hospital cardiac arrest | None reported | 12 | 16.6 | 67.0 | - | δ alternating with periods of θ and a / 20–30 µV | Yes / paradoxical to diffuse δ) |
| 11 | F / 43* | COVID-19 pneumonitis | Asthma, DM | 4 | 35.2 | 4.2 | - | θ, a and δ / 60 µV | Yes / Yes |
| 12 | M / 51 | COVID-19 pneumonitis + left MCA territory infarct | Hypertension | 14 | 44.7 | 24.0 | - | δ, θ / 60 µV | No / No |
| 13 | M / 64 | COVID-19 pneumonitis + sepsis | None reported | 17 | 43.9 | 36.2 | - | δ, θ/ 10–15 µV | No / No |
| 14 | M / 63 | COVID-19 pneumonitis + in-hospital cardiac arrest | Asthma | 11 | 28.4 | 80.0 | - | Electrocerebral silence | NA |
| 15 | M / 43* | COVID-19 pneumonitis | None reported | 3 | 18.0 | 2.6 | - | Normal | |
| 16 | F / 90 | Delirium + COVID-19 infection | Vascular dementia, DM, atrial fibrillation, depression | 1 | 4.5 | Not done | Never on sedation | θ, some δ / 30 µV | Yes / Arousal to faster |
| 17 | F / 55* | Seizures*** + COVID-19 infection | HIV encephalopathy | 6 | 8.4 | 3.7 | Propofol Fentanyl | θ, a, some δ / 45–50 µV | Yes / Yes |
| 18 | M / 52* | COVID-19 pneumonitis + seizures | HIV encephalopathy | 2 | 10.6 | 12.0 | - | θ, a, some frontal δ / 20–30 µV | Yes / Arousal to faster |
| 19 | M / 49* | COVID-19 pneumonitis | Autism, DM | 5 | 53.3 | Not done | - | θ, a some δ / 30–40 µV | Yes / Arousal to faster |
| Median | 10 | 38.3 | 46.3 | ||||||
| IQR | 5.5–13 | 23.2–43.2 | 21.9–80 | ||||||
EEG: electroencephalography; COVID-19: Coronavirus Disease 2019; SOFA: Sepsis–related Organ Failure Assessment score; M: males; F: females; †: for time off sedation in the individual patients, see Supplementary Table 1; AC: alpha coma; DM: diabetes mellitus; SAH: sub-arachnoid haemorrhage; MCA: middle cerebral artery; HIV: human immunodeficiency virus; IQR = interquartile range; *: these eleven patients were from Black, Asian and other ethnic minority (BAME) backgrounds. All other patients were Caucasian; **: to lower voltage faster activities with the exception of patient 10; ***: Patients 6 and 17 had serial seizures on admission. All four patients with seizures were on anti-seizure treatment (Levetiracetam). Seizures were also suspected in patients 15, 16 and 19 (see text); a, θ and δ refer to the alpha, theta and delta EEG rhythms respectively.
Overall sickness severity was high: the median SOFA score on admission was 8 [IQR 6 – 12.5] and worsened to 10 [IQR 5.5 – 13] by the time of the EEG (see Supplementary Table 1 and Table 1 respectively for individual scores). Four patients had seizures (6, 7, 17 and 18) and two (10 and 14) suffered CRA secondary to hypoxaemia prior to the EEG. By the time of the EEG the severity of respiratory failure was classified as mild to moderate in 15 patients, with the remainder no longer fulfilling the criteria for ARDS by oxygenation (Supplementary Table 1). There was evidence of systemic hyperinflammation in many of the patients, with group median peak values for C-reactive protein (CRP) and Ferritin being 337 mg/L [IQR 301.5 – 432.5] and 2496 mg/L [IQR 1434.5 – 4172] respectively (Supplementary Table 1). In reflection of this, 11 patients (1–6, 9, 11, 12, 14 and 15, 57.9%) received immunomodulatory therapy for hyper-inflammation during their acute illness. There was concurrent evidence of significant hypercoagulability, with median peak D-dimer values being 46.3 mg/L [IQR 21.9 – 80], predisposing to vasculopathy. In addition, most patients also suffered significant metabolic disturbance, in particular uraemia from acute kidney injury: the group median peak value for serum urea was 38.3 mmol/L [IQR 23.2 – 43.2]. In reflection of this, 13 patients (68%, all except patients 11 and 14–18) were receiving renal replacement therapy at the time of EEG. Four patients (1, 6, 9 and 13) suffered superimposed bacterial sepsis from other pathogens. Cerebrospinal fluid (CSF) examination was performed only in patient 1, and was negative for SARS-CoV-2 ribonucleic acid (RNA), Herpes Simplex Virus (HSV) de-oxyribonucleic acid (DNA), varicella zoster DNA, enterovirus RNA and parechovirus RNA. Detailed laboratory and radiological results are presented in the Supplementary Table 1.
3.2. EEG findings
We recorded 22 EEGs on 19 patients. In 13 patients (patients 1–13) the EEG was requested for suspected encephalopathy because they remained comatose after sedation had been stopped, or to exclude NCSE (Table 1). The question of NCSE arose particularly for patients 6 and 7, who had seizures, and patient 12, who had left middle cerebral artery territory infarction during the acute phase of the disease. Patient 14 had an in-hospital CPA, and the EEG was requested for neurological prognostication. In the remaining five patients (patients 15–19) the EEG was requested for suspected focal seizures: Patient 15 with reported vacant spells had no clinical evidence of encephalopathy. Patient 16 with advanced neurodegenerative dementia had the EEG on the first day of her admission for delirium and possible seizures; she never needed respiratory support or admission to ICU. Patients 17 and 18 had pre-existing established diagnoses of human immunodeficiency virus (HIV)-related encephalopathy. Patient 19 had severe cognitive impairment from birth; he had an EEG for possible focal seizures after he had already been discharged from ICU to a standard medical ward, and 10 days after a repeat COVID-19 swab test was negative. Aside from patients 16 and 19, the remaining 17 patients had their EEGs recorded during their ICU admissions.
The median time from ICU admission to the EEG was 17 days [IQR 9–21]. At the time of the EEG, sedation had been stopped for median duration of 2 days [IQR 1 – 4.75], and none of the patients were receiving medications to induce neuromuscular blockade. None of the patients received barbiturates at any stage. At the time of EEG recording, 12 patients were comatose (median Glasgow Coma Scale score was 5 [IQR 3.5 – 9]); of those, 10 were off sedation, and none were in a clinically locked-in state (Supplementary Table 1).
The EEG showed features of encephalopathy in 17 patients (patients 1–13 and 16–19), complete electro-cerebral silence in patient 14, and was normal in patient 15 (Table 1). In patients 16–19, EEG abnormalities were mild to moderately severe, and in keeping with their pre-existing pathologies. Therefore, notwithstanding potential confounding factors, the EEG supported a clinical diagnosis of COVID-19 related encephalopathy in 13 patients (patients 1–13).
Five of these 13 patients (patients 1–5) had alpha coma (AC) pattern, defined as a predominant, generalized (recorded over all areas of the cerebrum), and symmetrical between homologous areas rhythm within the alpha frequency band (Kaplan et al., 1999). The AC pattern was recorded between day 14 and day 27 of their ICU stay (median 19 days [IQR 19–21]) (Supplementary Table 1). Its distribution was generalised with anterior predominance in four patients (Table 2 ). In patients 1 and 2 the AC pattern was the sole EEG rhythm and showed no reactivity or amplitude modulations (Fig. 1 ). In patients 3 and 4 it occurred for most of the recording time and showed minimal reactivity to stimulation only in patient 4, changing from a clearly spindling pattern at rest to a less modulated pattern of similar frequency but slightly higher voltage upon stimulation (Fig. 2 ). In patient 5 it occurred intermittently in brief epochs of up to 10–15 seconds for about 20% of the total recording time. Due to the brevity of the AC epochs its reactivity was impossible to ascertain, but the rest of the EEG rhythms were clearly reactive and showed spontaneous cycling (Table 1). Patient 1 had a second EEG 6 days after the first. Clinically he was slightly more responsive (all brainstem reflexes were normal, he opened and closed his eyes spontaneously and in response to auditory stimulation, but did not follow commands), and the EEG showed delta and theta rhythms of generally low voltage, with reactivity. Representative EEG traces of AC pattern in patients 2, 3 and 5 can be found in the relevant EEG figures in the Supplementary Material.
Table 2.
Characteristics of the alpha coma pattern in patients 1–5.
| Distribution / Predominance / Max voltage | Appearance (other rhythms) | Frequency | Reactivity | spindling | % of total EEG time | |
|---|---|---|---|---|---|---|
| Pt. 1 | Diffuse bilateral / even over all areas / 20 µV | Unremitting (random anterior delta 1–1.5 Hz, <20 µV) | 11–13 Hz | No | No | 100 |
| Pt. 2 | Diffuse bilateral / anterior / 30 µV | Unremitting (scattered anterior theta < 20 µV) | 12–14 Hz | No | No | 100 |
| Pt. 3 | Diffuse bilateral / anterior / 25 µV | Almost continuous (some anterior 1.5–2 Hz delta that became hyper-synchronous delta in resting state) | 11–13 Hz | No | No | 80–85 |
| Pt. 4 | Diffuse bilateral / anterior / 25 µV | In long epochs (anterior theta at 20 µV) | 12–13 Hz | Yes (from clear spindling to slightly higher voltage less modulated) | Yes | 75 |
| Pt. 5 | Diffuse bilateral / anterior / 20 µV | In brief epochs (long periods of delta, slow theta / 40–50 µV) | 12 (11–13)Hz | Not clear because of the brevity of the alpha epochs | Yes | 25 |
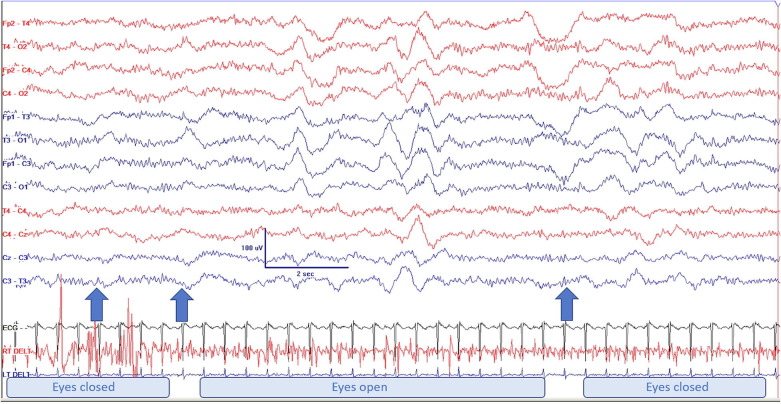
Fig. 1.
Continuous unresponsive AC pattern in patient 1. Left arrow: protracted painful stimulation (right trapezius pinch); middle arrow: eyes slowly open and remain open until passive closure, as shown by the artefact marked by the arrow on the right. A respiration belt was not applied, but the bilateral bursts of delta appear on the video to reflect respiratory movements rather than biological activity. Montage is limited due to safety considerations at the start of the pandemic (see methods). AC: alpha coma.
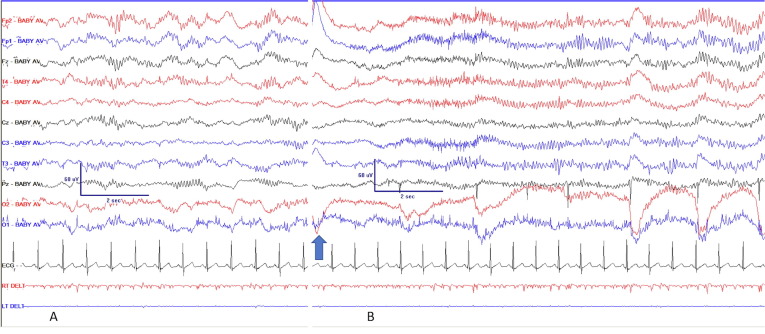
Fig. 2.
AC pattern in patient 4. A: the rhythm shows clear spindling while she is resting; B: spontaneous biting on tube after an increased respiratory effort (arrow), following which the AC pattern appears less modulated and with slightly increased voltage. Noxious stimulation had identical effect. The movement artefacts in the O2 and O1 leads reflect increased respiratory efforts. Common average derivation, using limited montage as in Fig. 1. AC: alpha coma.
The EEG features in the remaining patients with COVID-19 related encephalopathy (6–13) showed generalised slowing of generally low voltage without periodic suppression. Reactivity to lower voltage faster rhythms was noted in seven patients, and paradoxical reactivity to higher voltage delta in one. Spontaneous fluctuations were noted in six patients (Table 1). No generalised or lateralised periodic discharges, subclinical seizures or interictal epileptic activity were noted in any of the 22 EEGs.
3.3. Clinical EEG correlations
The 13 patients with EEG features of COVID-19 related encephalopathy had more severe disease than those without encephalopathy (median SOFA scores at time of EEG were 11 [IQR 10–14] for patients 1–13 and 3 [IQR 2–5] for patients 15–19 respectively). Amongst those with COVID-19 related encephalopathy there was no difference in disease severity, sedation type or time off sedation prior to EEG between those with AC pattern (patients 1–5) and those without (patients 6–13).
3.4. Brain imaging findings
Concurrent to EEG, computed tomography (CT) brain imaging was performed in 12 of these 13 patients. Three patients (1, 8 and 9) had also magnetic resonance imaging (MRI) of the brain (Supplementary Table 1). CT findings included acute sub-arachnoid haemorrhage in patient 7, multifocal embolic infarcts in patients 9 and 10 (confirmed on subsequent MRI in patient 9), large infarction of the left middle cerebral artery territory with haemorrhagic transformation in patient 12, and appeared normal in the others. Initial CT in Patient 8 appeared normal, but subsequent MRI showed bilateral cerebral and cerebellar infarcts. The brain MRI in patient 1 (performed five days after his first EEG which demonstrated AC pattern) showed bilateral symmetrical signal abnormalities descending from the pallidi through the corticospinal tracts to the cerebral peduncles, as well as diffuse signal abnormalities in the bilateral insular, cingulate and hippocampal cortices (brain MRI figure in the Supplementary Material).
4. Discussion
We found severe encephalopathic EEG abnormalities at/around the time of peak disease severity in 13 patients with laboratory-confirmed severe COVID-19. At the time of the EEG, 10 of those remained comatose following cessation of sedation. No periodic discharges or subclinical seizures occurred in any of these patients. These findings would suggest that, in most patients with severe COVID-19 who remain comatose on the ICU after sedation withdrawal, the principal causative factor is COVID-19 related encephalopathy, rather than encephalitis or NCSE.
Five of these 13 patients showed AC pattern (Kaplan et al., 1999, Fernández-Torre et al., 2018). Although residual sedation due to concurrent kidney disease could, at least partly, account for the low voltage slow rhythms in the other eight patients, it cannot explain the occurrence of the AC pattern. Propofol (the main anaesthetic agent we used, often in combination with fentanyl) increases the alpha power frontally (alpha anteriorization), but simultaneously, it also increases the low-frequency EEG power, again anteriorly (Purdon et al., 2013, Numan et al., 2019). When consciousness is lost, these effects result in the familiar row EEG pattern of frontally predominant slowing with over-riding alpha and faster rhythms (Hagihira 2015), which is clearly dissimilar to the AC pattern. The EEG effects of fentanyl are consistent and characterized by high-voltage slow delta waves (Sebel et al., 1981), hence they would be expected to enhance the slow component of the EEG pattern in propofol-induced anaesthesia. Further, it would be difficult to understand why the same anaesthetic agents would produce AC pattern in some patients only.
The relatively frequent occurrence of the AC pattern amongst our patients merits particular attention. AC occurred in patients with severe encephalopathy, but it did not appear to be per se an indicator of its severity, or the severity of COVID-19 disease. AC is a rare EEG encephalopathic pattern, yet we saw it in five of our 13 patients with COVID-19 associated encephalopathy within just six weeks. Kaplan and colleagues (Kaplan et al., 1999) described 36 patients with AC from two big centres specialising in critical care, over a period of 10 years; in their meta-analysis, they found 335 cases with AC since its original description (Loeb and Poggio, 1953), equating to a reported global incidence of just over 7 cases per year. Given the unsystematic timing of our EEG recordings and our small sample size, it is uncertain whether the relatively high occurrence of AC pattern in our patients is representative of its true prevalence in COVID-19 related encephalopathy. However, AC is also a transient and rather short-lived EEG phenomenon. In our patient 1 it had completely disappeared in his second EEG, a week later. In their large series, Young and colleagues reported that the AC changed to an alternate pattern after 6 days on average (Young et al., 1994), and in another patient AC occurred 2 hours after the onset of post-myocardial infarction hypoxic encephalopathy and had disappeared 12 hours later (Iragui and McCutchen, 1983). It is therefore also possible that we might have seen more patients with AC pattern had we performed more frequent recordings at regular time intervals.
Another unusual finding was the apparently late occurrence of AC pattern in comparison with the evidence from the literature. We observed the AC pattern after 14 to 27 days from ICU admission, at or around the peak severity of COVID-19. In contrast, all 36 patients of Kaplan and colleagues had the AC pattern between days 1 and 5 post-insult (Kaplan et al., 1999), while in another series, only two of the 20 patients had AC rhythms after the 10th day post-insult, both on day 12 (Uldry et al., 1991). In another big series of 24 patients, the AC pattern was also noted within the first few days (in most of the patients on day 1) after insult (Grindal et al., 1977). Such late documentation in our study may reflect a truly late occurrence of the AC pattern in COVID-19, but – again – it may also suggest that more patients with COVID-19 related encephalopathy could have had AC pattern if their EEGs had been recorded earlier.
What is the significance of the relatively high occurrence of AC pattern in our patients, and could it shed more light on the nature of COVID-19 related encephalopathy? The 1999 meta-analysis showed that AC was associated with CRA in around 2/3 of the cases (Kaplan et al., 1999). The newest literature confirms this strong association (Fernández-Torre et al., 2018). The remainder are associated with stroke, hypoxia without cardiac arrest, infection and metabolic derangements, head injury, and sedatives overdose (barbiturates, benzodiazepines, amitriptyline, chlormethiazole, meprobamate) in several case reports (Kaplan et al., 1999). In the present context, potential causal factors from the list above include infection and confounding metabolic derangements, but the EEG changes in the latter typically consist of non-specific slowing.
The AC pattern has also been described in localised pathologies involving the pontine and caudal mesencephalic tegmentum (Loeb and Poggio, 1953, Chatrian et al., 1964, Otomo, 1966, Westmoreland et al., 1975), with neurophysiological evidence in keeping with damage to the brainstem reticular formation (Obeso et al., 1980). Lugaresi and colleagues described AC pattern in a patient with fatal familial insomnia and bilateral anterior and dorsolateral thalamic nuclei neuronal loss (Lugaresi et al., 1986). If SARS-CoV-2 has direct neurotropism, it may access the brainstem with possible impact on the respiratory drive (Li et al., 2020) directly via the olfactory system, as indicated by the well-documented initial symptom of anosmia (Giacomelli et al., 2020, Xydakis et al., 2020), or indirectly through increased blood–brain barrier permeability due to hyperinflammatory state. The recent detection of anti-SARS-CoV-2 antibodies in the CSF of two patients with COVID-19 related encephalopathy (Andriuta et al., 2020) attests to such neurotropism. Interestingly, the second patient in that report was very similar to our patient 1: he did not awaken after sedation withdrawal, had flaccid tetraparalysis with retained brainstem reflexes, and his brain MRI showed bilateral pallidal lesions. It seems therefore plausible that the occurrence of the AC pattern in our patients relates to a neurotropic effects of SARS-CoV-2 on the ascending reticular formation within the brainstem-thalamus axis, lending support to the hypothesis that the virus can directly affect the CNS.
One methodological limitation in our study is the paucity of data about the type and time of onset of encephalopathic symptoms. Difficulty in obtaining this information is largely due to the urgency of the ICU admission and is perhaps innate to retrospective studies on patients with severe COVID-19 who were admitted during the peak of the pandemic; in the series of Mao and colleagues, pre-treatment symptoms were provided by “conscious and cognitive and mentally normal patients” and included non-specific symptoms of dizziness and headache. Impaired consciousness was noted in only eight of 78 patients on admission (Mao et al., 2020). Similarly, neurological findings on admission were recorded in only eight of 58 patients of the Strasbourg series (Helms et al., 2020). Another important limitation is that our EEG recordings were not protocolised in respect of their timing and follow up, due to them being prompted by acute clinical needs. Both these restrains limit our understanding of the time course of the encephalopathic EEG findings, and in particular that of the AC pattern. The use of limited EEG montage for safety reasons has been acknowledged in the methods section.
5. Conclusions
COVID-19 associated CNS manifestations appear to be diverse, including encephalopathy (Helms et al., 2020 Apr 15, Mao et al., 2020), encephalitis (Pilotto et al., 2020) and related epileptic seizures or NCSE (Balloy et al., 2020, Somani et al., 2020), and cerebrovascular disorder (Helms et al., 2020 Apr 15, Mao et al., 2020). In this first cohort of patients with EEG studies at/around the peak of severe COVID-19 with neurological manifestations we have shown that encephalopathy is both common and a principal cause of persisting comatose state after sedation withdrawal. Furthermore, we observed a relatively high incidence of the rare AC pattern, which could reflect a specific predilection of SARS-CoV-2 for the ascending reticular formation in the brainstem, and thereby support the hypothesis that the virus can directly affect the CNS. Further protocolised (Gelisse et al., 2020), serial EEG recordings are needed to more accurately identify the prevalence of COVID-19 related encephalopathy in this pandemic, and the time course between the onset of neurological symptoms and occurrence of alpha coma pattern.
Contributors
MK, JG and GG wrote the manuscript, planned the study, and were responsible for data collection.
GG and JG were treating physicians.
SS and AW performed the EEGs on most patients.
MK, SS, AW and VT analysed the EEGs.
All authors approved the final version of the report.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgments
Acknowledgements
We thank all the medical and nursing staff of the Intensive Care Unit and the physiologists of the EEG department of St Thomas’ Hospital for the excellent care and exemplary dedication to their patients during the COVID-19 pandemic.
Conflict of Interest Statement
None of the authors have potential conflicts of interest to be disclosed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data sharing statement.
All data relevant to the study are included in the article or uploaded as supplementary information.
See Article, pages 202–203
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinph.2020.09.008.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Andriuta D, Roger PA, Thibault W, Toublanc B, Sauzay C, Castelain S et al. COVID-19 encephalopathy: detection of antibodies against SARS-CoV-2 in CSF [published online ahead of print, 2020 Jun 11]. J Neurol. 2020;10.1007/s00415-020-09975-1. doi:10.1007/s00415-020-09975-1. [DOI] [PMC free article] [PubMed]
- ARDS Definition Task Force, Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- Balloy G, Mahé PJ, Leclair-Visonneau L, Péréon Y, Derkinderen P, Magotet A et al. Non-lesional status epilepticus in a patient with coronavirus disease 2019 [published online ahead of print, 2020 May 13]. Clin Neurophysiol. 2020;131:2059–61. doi:10.1016/j.clinph.2020.05.005. [DOI] [PMC free article] [PubMed]
- Chatrian G.E., White L.E., Jr, Shaw C.M. EEG pattern resembling wakefulness in unresponsive decerebrate state following traumatic brain-stem infarct. Electroencephalogr Clin Neurophysiol. 1964;16:285–289. doi: 10.1016/0013-4694(64)90111-7. [DOI] [PubMed] [Google Scholar]
- Fernández-Torre J.L., López-Delgado A., Hernández-Hernández M.A., Paramio-Paz A., Pía-Martínez C., Orizaola P. Postanoxic alpha, theta or alpha-theta coma: Clinical setting and neurological outcome. Resuscitation. 2018;124:118–125. doi: 10.1016/j.resuscitation.2017.12.022. [DOI] [PubMed] [Google Scholar]
- Flamand M., Perron A., Buron Y., Szurhaj W. Pay more attention to EEG in COVID-19 pandemic [published online ahead of print, 2020 May 22] Clin Neurophysiol. 2020;S1388–2457(20):30326–30336. doi: 10.1016/j.clinph.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelisse P., Rossetti A.O., Genton P., Crespel A., Kaplan P.W. How to carry out and interpret EEG recordings in COVID-19 patients in ICU? [published online ahead of print, 2020 May 13] Clin Neurophysiol. 2020;131:2023–2031. doi: 10.1016/j.clinph.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomelli A., Pezzati L., Conti F. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa330. published online March 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindal A.B., Suter C., Martinez A.J. Alpha-pattern coma: 24 cases with 9 survivors. Ann Neurol. 1977;1(4):371–377. doi: 10.1002/ana.410010408. [DOI] [PubMed] [Google Scholar]
- Gupta P.C., Seth P. Periodic complexes in herpes simplex encephalitis. A clinical and experimental study. Electroencephalogr Clin Neurophysiol. 1973;35(1):67–74. doi: 10.1016/0013-4694(73)90132-6. [DOI] [PubMed] [Google Scholar]
- Hagihira S. Changes in the electroencephalogram during anaesthesia and their physiological basis. Br J Anaesth. 2015;115(Suppl 1):i27–i31. doi: 10.1093/bja/aev212. [DOI] [PubMed] [Google Scholar]
- Haines S., Caccamo A., Chan F., Galaso G., Catinchi A., Gupta P.K. Practical considerations when performing neurodiagnostic studies on patients with COVID-19 and other highly virulent diseases [published online ahead of print, 2020 May 6] Neurodiagn J. 2020;1–18 doi: 10.1080/21646821.2020.1756132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iragui V.J., McCutchen C.B. Physiologic and prognostic significance of “alpha coma”. J Neurol Neurosurg Psychiatry. 1983;46(7):632–638. doi: 10.1136/jnnp.46.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P.W., Genoud D., Ho T.W., Jallon P. Etiology, neurologic correlations, and prognosis in alpha coma. Clin Neurophysiol. 1999;110(2):205–213. doi: 10.1016/s1388-2457(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online ahead of print, 2020 Feb 27]. J Med Virol. 2020;10.1002/jmv.25728. doi:10.1002/jmv.25728. [DOI] [PMC free article] [PubMed]
- Loeb C., Poggio G. Electroencephalograms in a case with ponto-mesencephalic haemorrhage. Electroencephalogr Clin Neurophysiol. 1953;5(2):295–296. doi: 10.1016/0013-4694(53)90017-0. [DOI] [PubMed] [Google Scholar]
- Lugaresi E., Medori R., Montagna P., Baruzzi A., Cortelli P., Lugaresi A. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N Engl J Med. 1986;315(16):997–1003. doi: 10.1056/NEJM198610163151605. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan. China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan T., van Dellen E., Vleggaar F.P., van Vlieberghe P., Stam C.J., Slooter A.J.C. Resting state EEG characteristics during sedation with midazolam or propofol in older subjects. Clin EEG Neurosci. 2019;50(6):436–443. doi: 10.1177/1550059419838938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J.A., Iragui M.I., Marti-Masso J.F., Maravi E., Teijeira J.M., Carrera N. Neurophysiological assessment of alpha pattern coma. J Neurol Neurosurg Psychiatry. 1980;43(1):63–67. doi: 10.1136/jnnp.43.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo E. Beta wave activity in the electroencephalogram in cases of coma due to acute brain-stem lesions. J Neurol Neurosurg Psychiatry. 1966;29(5):383–390. doi: 10.1136/jnnp.29.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilotto A, Odolini S, Stefano Masciocchi S, Comelli A, Volonghi I, Gazzina S et al. Steroid-responsive encephalitis in Covid-19 disease [published online ahead of print, 2020 May 17]. Ann Neurol. 2020;10.1002/ana.25783. doi:10.1002/ana.25783. [DOI] [PMC free article] [PubMed]
- Purdon P.L., Pierce E.T., Mukamel E.A., Prerau M.J., Walsh J.L., Wong K.F.K. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110(12):E1142–E1151. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebel P.S., Bovill J.G., Wauquier A., Rog P. Effects of high-dose fentanyl anesthesia on the electroencephalogram. Anesthesiology. 1981;55(3):203–211. doi: 10.1097/00000542-198109000-00004. [DOI] [PubMed] [Google Scholar]
- Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S. De Novo Status Epilepticus in patients with COVID-19 [published online ahead of print, 2020 May 14]. Ann Clin Transl Neurol. 2020;10.1002/acn3.51071. doi:10.1002/acn3.51071. [DOI] [PMC free article] [PubMed]
- Uldry P.A., Despland P.A., Regli F. Alpha-coma: présentation rétrospective de 20 cas [Alpha coma: rectrospective presentation of 20 cases] Neurophysiol Clin. 1991;21(2):85–94. doi: 10.1016/s0987-7053(05)80063-2. [DOI] [PubMed] [Google Scholar]
- Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonça A., Bruining H. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- Westmoreland B.F., Klass D.W., Sharbrough F.W., Alpha-coma Reagan TJ. Electroencephalographic, clinical, pathologic, and etiologic correlations. Arch Neurol. 1975;32(11):713–718. doi: 10.1001/archneur.1975.00490530035001. [DOI] [PubMed] [Google Scholar]
- Xydakis MS, Dehgani-Mobaraki P, Holbrook EH, Geisthoff UW, Bauer C, Hautefort C et al. Smell and taste dysfunction in patients with COVID-19 [published online ahead of print, 2020 Apr 15]. Lancet Infect Dis. 2020;S1473-3099(20)30293-0. doi:10.1016/S1473-3099(20)30293-0. [DOI] [PMC free article] [PubMed]
- Young G.B., Blume W.T., Campbell V.M., Demelo J.D., Leung L.S., McKeown M.J. Alpha, theta and alpha-theta coma: a clinical outcome study utilizing serial recordings. Electroencephalogr Clin Neurophysiol. 1994;91(2):93–99. doi: 10.1016/0013-4694(94)90030-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.