Abstract
Wastewater treatment plants (WWTPs) could emit a large amount of bioaerosols containing pathogenic bacteria. Assessing the health risks of exposure to these bioaerosols by using quantitative microbial risk assessment (QMRA) is important to protect workers in WWTPs. However, the relative impacts of the stochastic input variables on the health risks determined in QMRA remain vague. Hence, this study performed a Monte Carlo simulation-based QMRA case study for workers exposing to S. aureus or E. coli bioaerosols and a sensitivity analysis in two WWTPs with various aeration modes. Results showed that when workers equipped without personal protective equipment (PPE) were exposed to S. aureus or E. coli bioaerosol in the two WWTPs, the annual probability of infection considerably exceeded the U.S. EPA benchmark (≤10E-4 pppy), and the disease burden did not satisfy the WHO benchmark (≤10E-6 DALYs pppy) (except exposure to E. coli bioaerosol for disease health risk burden). Nevertheless, the use of PPE effectively reduced the annual infection health risk to an acceptable level and converted the disease health risk burden to a highly acceptable level. Referring to the sensitivity analysis, the contribution of mechanical aeration modes to the variability of the health risks was absolutely dominated in the WWTPs. On the aeration mode that showed high exposure concentration, the three input exposure parameters (exposure time, aerosol ingestion rate, and breathing rate) had a great impact on health risks. The health risks were also prone to being highly influenced by the various choices of the dose–response model and related parameters. Current research systematically delivered new data and a novel perspective on the sensitivity analysis of QMRA. Then, management decisions could be executed by authorities on the basis of the results of this sensitivity analysis to reduce related occupational health risks of workers in WWTPs.
Keywords: Quantitative microbial risk assessment, Sensitivity analysis, Staphylococcus aureus, Escherichia coli, Monte Carlo simulation, Personal protective equipment
Graphical abstract
Highlights
-
•
Even without PPE, DB of workers exposed to E. coli bioaerosol was still acceptable
-
•
The use of PPE effectively reduced the health risks to an acceptable level
-
•
With high exposure concentration, input exposure parameters highly impact health risk
-
•
Mechanical aeration modes’ contribution to health risks’ variability was dominated
-
•
Health risks were highly influenced by the various choices of the dose–response model
1. Introduction
Wastewater treatment plants (WWTPs) could emit a large amount of bioaerosols containing pathogenic bacteria (Szyłak-Szydłowski et al., 2016). Compared with other workers, workers in WWTPs have a particularly higher prevalence of the so-called “sewage worker's syndrome,” characterized by fatigue, headache, dizziness, gastrointestinal symptoms, and respiratory symptoms (Hung et al., 2010). These symptoms could be caused by work-related exposure to various bacterial bioaerosols that were liberated in wastewater treatment processes (Kowalski et al., 2017). Staphylococcus aureus and Escherichia coli bioaerosols, which had been frequently found in domestic wastewaters, are widely used as target indicator pathogens (Ikehata, 2013; Szyłak-Szydłowski et al., 2016; Shi et al., 2018; Kozajda et al., 2019). These bacteria from wastewater or sludge can infect people through inhalation (Szyłak-Szydłowski et al., 2016). Direct exposure to these bioaerosols causes gastrointestinal infection through bioaerosol capture in the upper respiratory tract by inhalation, where pathogenic bacterial bioaerosols move by ciliary action and pass into the digestive tract through the pharynx (Peccia et al., 2008). In general, the exposure of humans to WWTPs with pathogenic bacterial bioaerosols has significant health risks (Kozajda et al., 2019). Therefore, assessing the health risks of exposure to pathogenic bacterial bioaerosols is important to protect workers in WWTPs. In addition, the aeration mode in WWTPs and using personal protective equipment (PPE) could considerably influence the health risks of workers (Brandi et al., 2000; Teixeira et al., 2013). Pasalari et al. (2019) measured high health risks for workers exposed to Rotavirus and Norovirus bioaerosols in a WWTP equipped with various aeration tanks. Jones (2020) reported an increased contribution to health risks for patient care exposed to COVID-19 of the inhalation scenario equipped without PPE.
Health risk is usually quantified by the annual probability of infection (P(a)inf) and disease burden (DB) (Haas et al., 2014). The P(a)inf and DB of bioaerosol exposure could be estimated by quantitative microbial risk assessment (QMRA) (Haas et al., 2014; Shi et al., 2018; Esfahanian et al., 2019). QMRA commonly follows four classical working steps: (a) hazard identification, (b) exposure assessment, (c) dose–response assessment, and (d) risk characterization (Haas et al., 2014). Moreover, QMRA is often estimated from Monte Carlo simulations to assess the range and likelihood of the health risk quantitatively (Lim and Jiang, 2013; Shi et al., 2018; Liu et al., 2019). Furthermore, for risk characterization, the two most widely used health risk benchmarks are the acceptable annual infection risk level proposed by the U.S. EPA (2005) (≤10E−4 pppy) and the acceptable disability-adjusted life years (DALYs) by the WHO (2008) (≤10E−6 DALYs pppy). They are widely used in interpreting the magnitude of risk assessment outcomes (Lim et al., 2015; Fuhrimann et al., 2016; Shi et al., 2018). These two benchmarks were built around the concept of health-based targets that were grounded on well-defined health metrics (e.g., DALYs) and a level of tolerable health burdens (Fuhrimann et al., 2016).
In the QMRA, the importance of all input variables could be identified through a sensitivity analysis, which tests the relative impacts of stochastic input variables on health risks (Abhishek and Ashok, 2008; Federigi et al., 2019). Sensitivity analysis is usually performed in QMRA to: (a) identify the most influential input variables on the output so as to propose feasible management recommendations to the authorities; (b) improve the understanding and interpretation of the QMRA framework in order to extent of its analysis methodology; and (c) recognize input variable gaps and then prioritize future research priorities (Tesson et al., 2020). Haas et al. (2017) demonstrated health risk of Ebolavirus for sewer worker with or without PPE from inhalation exposure and sensitivity analysis from Monte Carlo simulation. Kowalski et al. (2017) analyzed the emission characterization of the bacteria and fungi bioaerosols collected in different aeration modes of WWTPs in Poland. Carducci et al. (2018) reported that the health risk for workers in WWTPs exposed to the human adenovirus (HAdV) was estimated by QMRA and the sensitivity analysis was employed to examine the impact of input parameters (breathing rate and concentration) on health risk.
However, given the ranking, significance, and contribution of these relative impacts remain vague, a series of open questions have been raised about the QMRA and its sensitivity analysis for stochastic input variables associated with workers using PPE and exposing under various aeration modes in WWTPs. Therefore, this research systematically investigates a Monte Carlo simulation-based QMRA case study for workers exposing to S. aureus and E. coli bioaerosols in two WWTPs. After that, the health risks (P(a)inf and DB) of the workers without or with PPE exposed to bioaerosols under various aeration modes in two WWTPs were discussed. Then, it focuses on the rank correlation coefficient values and contribution to variance of each input variable in QMRA which were assessed by sensitivity analysis. The current research can enrich the knowledge bases of the sensitivity analysis of QMRA for workers with PPE exposed to bioaerosols under various aeration modes in WWTPs and then provide an advanced understanding of the rank correlation coefficient values and contributions to variance of each input variable in QMRA framework. These results can inform efforts to establish rational management recommendations for reducing occupational health risks of workers in WWTPs.
2. Materials and methods
2.1. Description of the WWTPs
This study was performed in two WWTPs (plants A and B), which were located in the central part of P.R. China. Their drainage pipe systems were similar. The collected domestic wastewater (occasionally mixed with a little industrial wastewater) was distributed into the WWTP by a series of variable-frequency pump stations. Plant A had a parallel wastewater treatment system equipped with a rotating disc aeration tank (RD) and a microporous aeration tank (M), treating 50,000 tons of wastewater per day, respectively. Similarly, plant B was also a parallel system. It had an inverted umbrella aeration tank (IU) and a microporous aeration tank (M), treating 100,000 tons of wastewater per day, respectively. Thus, there were three modes for aeration tanks (RD, IU, and M) in this research.
2.2. Sampling and analysis
2.2.1. Sampling procedure
Six bioaerosol sampling campaigns were conducted on 21th November 2019, 5th December 2019, 16th December 2019, 23rd December 2019, 7th January 2020, and 8th January 2020 in plants A and B by using an Andersen six-stage cascade impactor (FA-1, Hongchangxin Inc., Beijing, China) (Hung et al., 2010). Sterile agar media Egg-Yolk Mannitol Salt Agar Base and MacConkey-Agar-Medium were used as the collection media for culturing and colony enumeration of S. aureus and E. coli, respectively (Oppliger et al., 2005; Szyłak-Szydłowski et al., 2016; Nasir et al., 2018; Wang et al., 2019). A 27 mL aliquot of this sterile agar media (autoclaved at 121 °C for 15 min) was pipetted into sterile glass Petri dishes equipped with the cascade impactor (Jahne et al., 2015; Jahne et al., 2016).
The sampling point was set at 1.5 m above each aeration tank's ground (Szyłak-Szydłowski et al., 2016). The cascade impactor was operated for 10 min at a flow rate of 28.3 L/min (Hung et al., 2010; Kowalski et al., 2017). Each stage of the Andersen six-stage cascade impactor was decontaminated with 75% alcohol before and after use for air sampling on site (Hung et al., 2010). All samples were in triplicate and transported to the laboratory in a cold box before being cultivated in incubators for 24–48 h at 37 °C.
2.2.2. Colony enumeration
After cultivation, the samples were enumerated as colony-forming unit (CFU) by using an automatic colony enumeration instrument (HICC-B, Wanshen Inc., Hangzhou, China). The positive hole method was used to correct and then obtain the actual number of colonies measured at the each Petri dish stage on the basis of the enumeration results (Hung et al., 2010; Delort and Amato, 2017). Bioaerosol concentrations of S. aureus and E. coli in CFU/m3 were estimated by dividing the number of colonies in CFU by the sampled air volume in m3 (Hung et al., 2010). Then, the bioaerosol concentration was the sum of the concentrations of the six Petri dish stages of the Andersen six-stage cascade impactor (Katsivela et al., 2017).
2.3. Quantitative microbial risk assessment framework
2.3.1. Hazard identification
The indicator pathogens of concern in this research were S. aureus and E. coli bioaerosols in the two WWTPs. So, the workers in the WWTP aeration tanks were exposed to serious S. aureus and E. coli bioaerosols-related health risks.
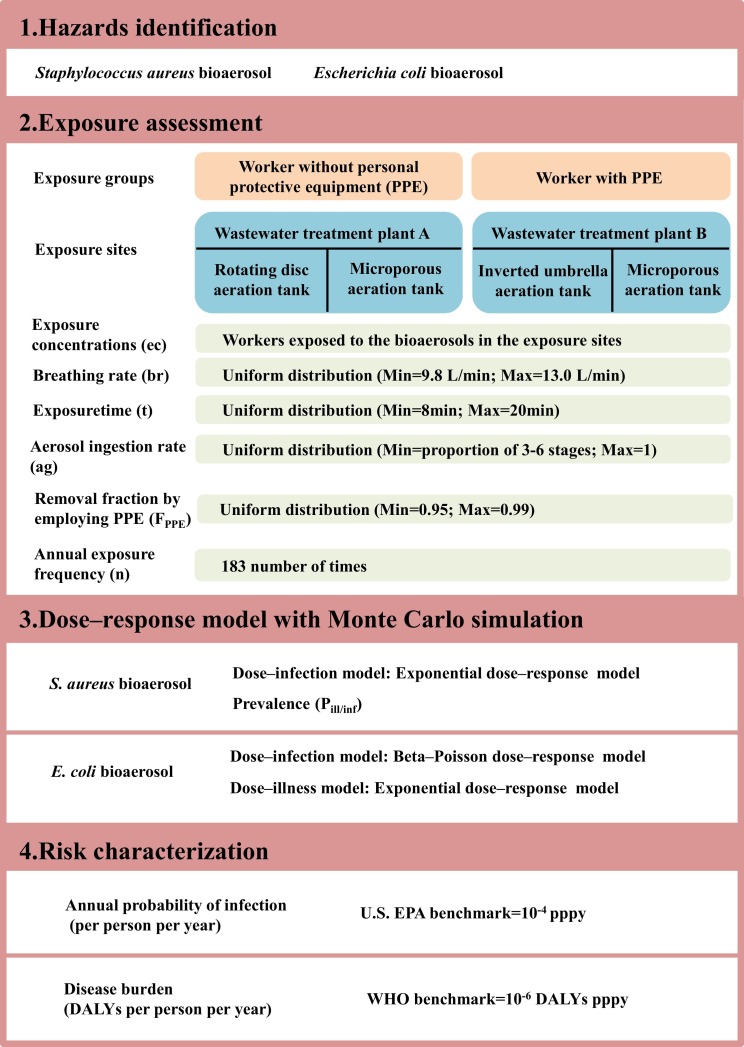
2.3.2. Exposure assessment
The parameters and flow chart for the exposure assessment referring to the QMRA calculation framework are presented in Table 1 and Fig. 1 , respectively. This research had eight exposure scenarios (Fig. 1): (a) workers without PPE exposed to S. aureus bioaerosol in plant A, (b) workers without PPE exposed to S. aureus bioaerosol in plant B, (c) workers with PPE exposed to S. aureus bioaerosol in plant A, (d) workers with PPE exposed to S. aureus bioaerosol in plant B, (e) workers without PPE exposed to E. coli bioaerosol in plant A, (f) workers without PPE exposed to E. coli bioaerosol in plant B, (g) workers with PPE exposed to E. coli bioaerosol in plant A, and (h) workers with PPE exposed to E. coli bioaerosol in plant B. The exposure concentrations (ec) of S. aureus and E. coli bioaerosols are calculated and shown in Supplementary Materials Table 1. The aerosol ingestion rate (ag) is shown in Supplementary Materials Table 2.
Table 1.
Calculation parameters of quantitative microbial risk assessment.
| Description | Unit | Value | Reference | ||
|---|---|---|---|---|---|
| Exposure concentrations (ec) | log10CFU/m3 | Supplementary materials Table 1 | – | ||
| Breathing rate (br) | L/min | Uniform distribution (Min = 9.8; Max = 13.0) | MEP-PRC, 2013 | ||
| Exposure time (t) | min | Uniform distribution (Min = 8; Max = 20) | According to the field survey in this research. | ||
| Aerosol ingestion rate (ag) | Unitless | Supplementary Materials Table 2 | – | ||
| Removal fraction by employing PPE (FPPE) | Unitless | Uniform distribution (Min = 0.95; Max = 0.99) | Haas et al., 2017 | ||
| Annual exposure frequency (n) | Number of times | 183 | According to the field survey in this research. | ||
| Staphylococcus aureus bioaerosol | Exponential dose–response model (dose–infection model) | k | Unitless | Uniform (Min = 6.46E−8; Max = 1.00E−7) | Esfahanian et al., 2019 |
| Prevalence | Pill/inf | Unitless | 1 | Busgang et al., 2018 | |
| Health burden (HB) | DALYs/case | 2.60E-3 | Havelaar et al., 2012 | ||
| Escherichia coli bioaerosol | Beta-Poisson dose–response model (dose–infection model) | α | Unitless | 1.55E-01 | Shi et al., 2018 |
| N50 | Unitless | 2.11E+06 | Shi et al., 2018 | ||
| Exponential dose–response model (dose–illness model) | k | Unitless | 1.22E-08 | Shi et al., 2018 | |
| Health burden (HB) | DALYs/case | 4.55E-2 | Shi et al., 2018 | ||
Fig. 1.
Flow chart of quantitative microbial risk assessment.
The removal fraction by employing PPE (FPPE) was conducted in two situations (the two exposure groups in Fig. 1): (a) workers in WWTPs used no face protection (i.e., workers without PPE) and (b) workers in WWTPs wore a properly fitted N-95 respirator at all times (i.e., workers with PPE) (Haas et al., 2017).
The dose of pathogens (Dose) per person per day was calculated in Eq. (1) (Dungan, 2014; Jahne et al., 2015; Haas et al., 2017):
| (1) |
where Dose represents the dose of bioaerosol inhaled per person per day (CFU/day), ec is the exposure concentration (Supplementary Materials Table 1), br is the breathing rate, ag is the aerosol ingestion rate (Supplementary Materials Table 2), and F PPE is the removal fraction by employing PPE (Table 1).
2.3.3. Dose–response models
For S. aureus bioaerosol, the exponential dose–response model as a dose–infection model was used to determine the relationship between the dose and the infection risks (Eq. (2)) (Esfahanian et al., 2019):
| (2) |
where P (d)inf is the estimated daily probability of infection, and k is the parameter of the model (Table 1).
For E. coli bioaerosol, the Beta–Poisson dose–response model as a dose–infection model was used to determine the relationship between the dose and the infection risks, which is shown in Eq. (3) (Shi et al., 2018):
| (3) |
where P (d)inf is the estimated daily probability of infection, and α and N 50 are the parameters of the model (Table 1).
2.3.4. Risk characterization
Risk characterization was carried out on the basis of the contaminant concentration to which individuals were exposed. Annual probability was estimated considering the number of exposure events per year with Eq. (4) (Haas et al., 2014; Sales-Ortells and Medema, 2014):
| (4) |
where P (a)inf is the annual probability of infection per person per year (pppy), and n is the annual exposure frequency (Table 1).
For S. aureus bioaerosol, the probability of infection was assumed equal to the probability of illness (Pill/inf = 1). The probability of illness, as a conditional of infection, was calculated in Eq. (5) (Busgang et al., 2018; Carducci et al., 2018):
| (5) |
where P (a)ill is the annual probability of illness, and P ill/inf is the specific conditional probability of illness given an infection (i.e., prevalence) (Table 1).
For E. coli bioaerosol, the exponential dose–response model was used as a dose–illness model to calculate the probability of illness, which was defined in Eq. (6) (Shi et al., 2018):
| (6) |
where P (a)ill is the annual probability of illness, and k is the parameter of the model, which are listed in Table 1.
The specific potential disease burden attributable to illness caused by exposure to S. aureus or E. coli bioaerosol was estimated in Eq. (7) (Havelaar et al., 2012; Shi et al., 2018):
| (7) |
where DB is the disease burden and expressed in DALYs pppy, and HB is the health burden and expressed in DALYs per illness case (DALYs/case) (Table1).
2.4. Monte Carlo simulation
Monte Carlo simulation was used to represent the propagation of variability in QMRA (Lim et al., 2015). It was run with 10,000 trials by using Oracle Crystal Ball and Microsoft Excel 2010 (Devleesschauwer et al., 2014; Haas et al., 2017; Liu et al., 2019). All inputted variables (exposure concentration, three input exposure parameters (exposure time, aerosol ingestion rate, and breathing rate), the removal fraction by employing PPE, and the model parameters of the dose–response model) were randomly selected from their probability distributions. Output health risks were computed over 10,000 iterations so that the distributions would reach a steady state (Lim and Jiang, 2013; Shi et al., 2018). The results of Monte Carlo simulation were shown by a box-and-whiskers chart. The lower whisker in the box chart represented optimistic estimate. The non-conservative estimate was originated from 25th percentile values to the lower whisker.
2.5. Sensitivity analysis
The rankings of each inputted variables were assessed using a sensitivity analysis with Oracle Crystal Ball. The significance of each parameter was characterized by its correlation coefficient values with the health risks, where a higher value (i.e. high ranking) indicated greater contribution (i.e., great impact) to the variability of the health risks and vice versa (Hamilton et al., 2006; Lim and Jiang, 2013; Vásquez et al., 2014; Pang et al., 2017; Pasalari et al., 2019).
Contribution to variance was calculated by squaring the rank correlation coefficient values and normalizing them to 100% (Zhou et al., 2014; Haas et al., 2017; Shi et al., 2018). Contribution to variance showed sensitivities as values that range from 0 to 100% and indicated relative importance by showing the percentage of the variance of the predicted variable contributed by each dose–response model input variable (Zhou et al., 2014; Haas et al., 2017; Shi et al., 2018).
3. Results and discussion
3.1. Dose–response assessment and risk characterization
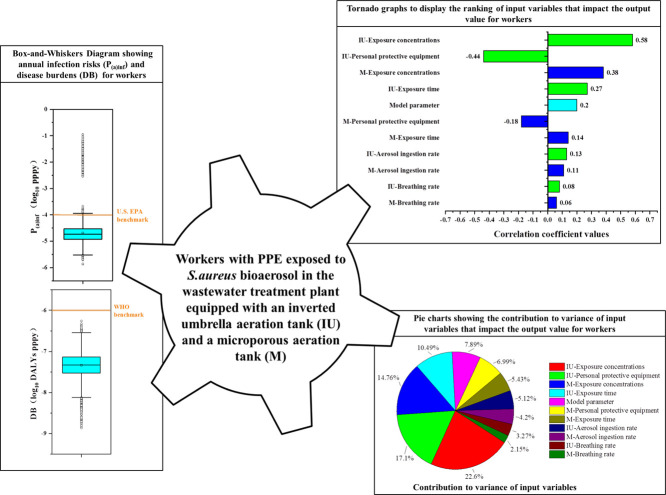
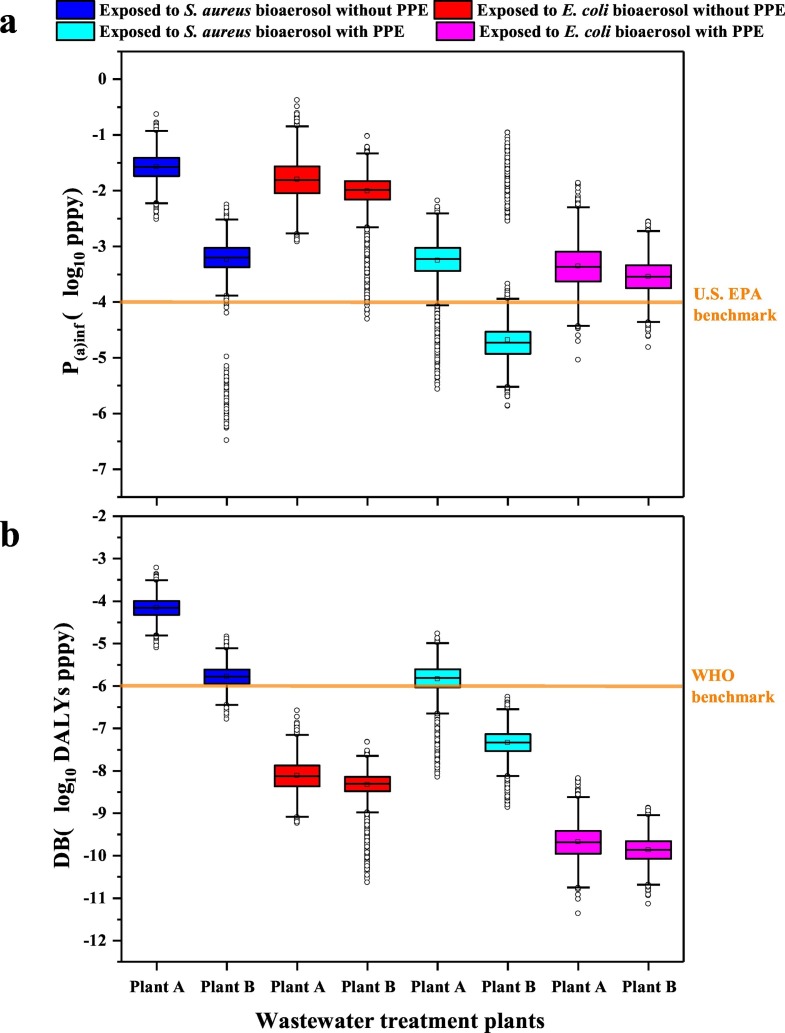
Fig. 2 demonstrates the annual infection risks (P(a)inf) and the disease burdens (DB) that were estimated from the Monte Carlo simulations with 10,000 iterations under the eight exposure scenarios where workers (without or with PPE) were exposed to S. aureus or E. coli bioaerosols in the two WWTPs.
Fig. 2.
Box-and-Whiskers Diagram showing (a) annual infection risks (P(a)inf) and (b) disease burdens (DBs) under eight exposure scenarios of workers (without or with PPE) exposed to S. aureus or E. coli bioaerosols in the two wastewater treatment plants.
The bottom and top of the box represent the first and third quartiles (25th and 75th percentile values), the band inside the box represents the second quartile (median), and the tetragon inside the box represents the average value. The whiskers extend 1.5 interquartile ranges (75th percentile value–25th percentile value) from each end of the box, and markers plotted outside each whisker are considered as outliers.
S. aureus = Staphylococcus aureus,
E. coli = Escherichia coli,
PPE = Personal Protective Equipment,
U.S. EPA = United States Environmental Protection Agency,
WHO = World Health Organization.
For exposing to S. aureus bioaerosol, the P(a)inf of the workers in plant A were always much higher than that of the workers in plant B (Fig. 2a). This finding could be explained by the theory that the different aeration modes between the two WWTPs lead to huge differences in the concentration of S. aureus bioaerosol emissions, which would largely affect the annual infection health risks for workers (Haas et al., 2014; Dungan, 2014; Jahne et al., 2015). Nevertheless, the P(a)inf of the workers without PPE in plants A and B both considerably exceeded the U.S. EPA benchmark (≤10E-4 pppy) (Fig. 2a). However, the P(a)inf of the workers with PPE in plant A (median = 6.04E-04) was on the same order of magnitude as the benchmark, and the P(a)inf of the workers with PPE in plant B clearly satisfied the benchmark. These results indicated that using PPE can effectively reduce the annual infection health risks of S. aureus bioaerosol to an acceptable level (Ikehata, 2013; Haas et al., 2017; Carducci et al., 2018).
For E. coli bioaerosol, the P(a)inf of the workers without or with PPE in plant A slightly differed from that of the workers in plant B (Fig. 2a). Furthermore, the P(a)inf of the workers without PPE in the two plants both considerably exceeded the U.S. EPA benchmark (≤10E-4 pppy). However, the P(a)inf values of the workers with PPE in plant A (median = 4.36E-04) and plant B (median = 2.88E-04) were just on the same order of magnitude as the benchmark. The annual infection health risk of the workers was even acceptable under the optimistic estimate because the lower whisker of the P(a)inf of the workers with PPE satisfied the benchmark. This result disclosed that the use of PPE reduced the annual infection health risks of E. coli bioaerosol in the two plants to an acceptable level, but the risks were still far from negligible.
For S. aureus bioaerosol, the DB of the workers in plant A was much higher than that of the workers in plant B (Fig. 2b). The DB of the workers without PPE in plant A significantly exceeded the WHO benchmark (≤10E-6 DALYs pppy). However, the DB values of the workers with PPE in plant A (median = 1.57E-06) and that of the workers without PPE in plant B (median = 1.67E-06) were roughly on the same order of magnitude as the benchmark. Under non-conservative estimate, the disease health risk burden of those workers was even acceptable since the benchmark was satisfied by the DB from the 25th percentile values to the lower whisker. Furthermore, the DB of the workers with PPE in plant B completely satisfied the WHO benchmark. Therefore, wearing of PPE improved the disease health risk burden of workers exposed to S. aureus bioaerosol from low acceptable level to high acceptable level.
For E. coli bioaerosol, the DB of the workers in plant A was similar to that of the workers in plant B. However, all DBs of the workers without or with PPE in the two plants satisfied the WHO benchmark. This result can be ascribed to the fact that the dose–illness model used in E. coli bioaerosol QMRA made the calculated DB demonstrate a non-conservative health risk estimate and therefore fulfilled the WHO benchmark (Shi et al., 2018). Thus, even without PPE, the disease health risk burden of the workers exposed to E. coli bioaerosol was still acceptable. Similar result had been reported. Shi et al. (2018) found that even in the worst-case scenario, where all E. coli bioaerosols were assumed to be pathogenic, the health risks were still far below the benchmark.
What was noteworthy was that, as expected, the health risks (P(a)inf and DB) of the workers exposed to S. aureus bioaerosol with PPE reduced by approximately two orders of magnitude compared with those of the workers without PPE in plants A and B. This result was because the N-95 respirators utilized in this research were engineered to filter at least 95% of the particles that would be inhaled (Haas et al., 2017). The results of the reduction of health risk of workers with PPE exposed to E. coli bioaerosol were similar.
P(a)inf and DB were adopted as health risk indicators throughout the analysis in this research, considering that the DALYs approach can add values to health risk management (Haas et al., 2014; Lim et al., 2015; Shi et al., 2018). However, DALYs might be blighted by the lack of data to support its development in China because of its rare local practical application. Therefore, DALYs data specific to China were thought to be less readily available. Lim et al. (2015) put forth that the U.S. EPA P(a)inf benchmark is regionally bounded because it was proposed according to the disease surveillance data only in the U.S. Therefore, this benchmark might not be representative of the whole world. Moreover, the WHO DB benchmark should be treated cautiously in a similar manner to the U.S. EPA P(a)inf benchmark, and these two indicators ought to be used as complements rather than opposites in health risk assessment (Lim et al., 2015). In addition, the U.S. EPA P(a)inf benchmark and the WHO DB benchmark are considered to be overly conservative (Lim et al., 2015).
In this research, the P(a)inf and DB were calculated by using different dose–response models for the QMRA of S. aureus and E. coli bioaerosols (Shi et al., 2018). For S. aureus bioaerosol QMRA, the metrics used for P(a)inf and DB were directly related to each other, and the DB was calculated via P(a)inf and DALYs (Havelaar et al., 2012; Busgang et al., 2018). By contrast, uncorrelation of the dose–response models for E. coli bioaerosol QMRA led to the variability of the health risk calculations. P(a)inf was calculated using the Beta–Poisson dose–response model (Eqs. (3), (4)), and the DB was calculated using the exponential dose–response model (Eqs. (6), (7)). Thus, this research implied that the health risks (P(a)inf and DB) were prone to being highly influenced by the various dose–response models of choice. In general, accurate health risk estimation called for additional field studies and clinical infection data (Shi et al., 2018). But there remain also need to understand that an efficient and rigorous validation of the dose–response model and its relevant parameters for QMRA is warranted (Haas, 2015).
3.2. Sensitivity analysis for quantitative microbial risk assessment results
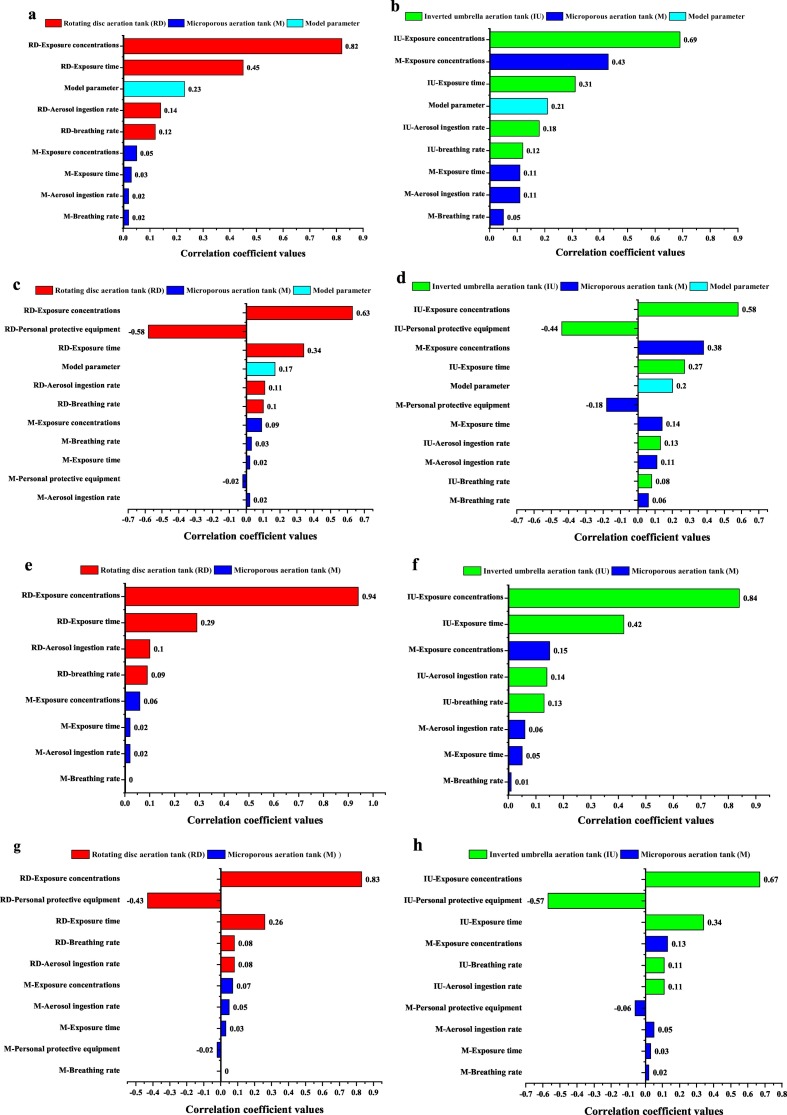
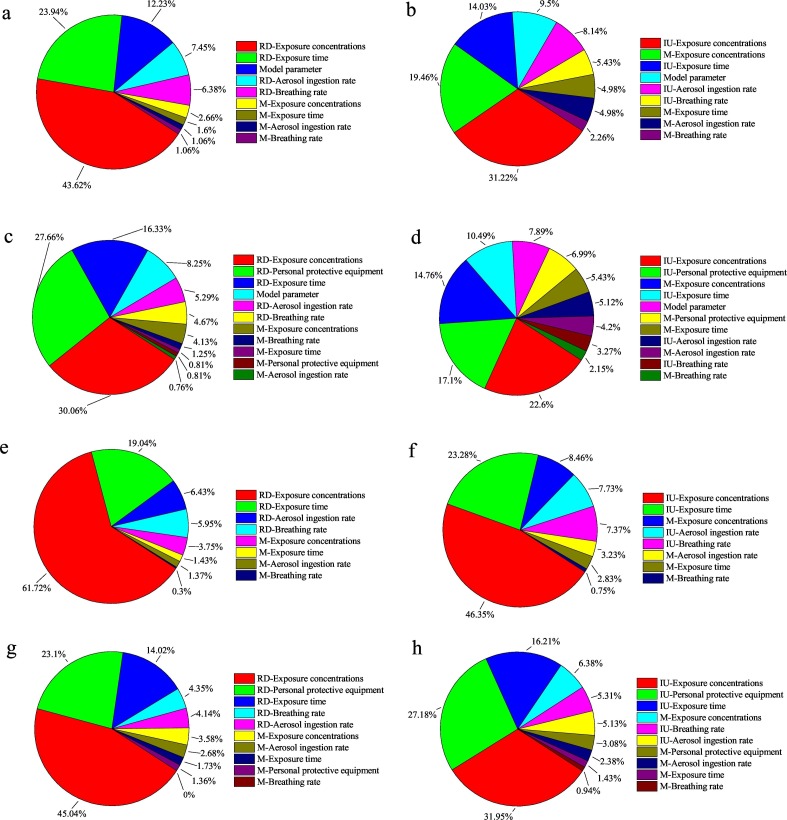
Fig. 3 shows the input variable ranking of the sensitivity of the exposure concentration, the three input exposure parameters (exposure time, aerosol ingestion rate, and breathing rate), the removal fraction by employing PPE, and the dose-response model parameters to the health risks (P(a)inf and DB). Each aeration mode was individually analyzed in the Fig. 3. Fig. 4 demonstrates the contribution to variance of the input variables that impact the output value of the health risks.
Fig. 3.
Tornado graphs to display the ranking of input variables that impact the output value for workers (without or with PPE) exposed to S. aureus or E. coli bioaerosols in various aeration tanks of the two wastewater treatment plants referring to (a) workers without PPE exposed to S. aureus bioaerosol in wastewater treatment plant A, (b) workers without PPE exposed to S. aureus bioaerosol in wastewater treatment plant B, (c) workers with PPE exposed to S. aureus bioaerosol in wastewater treatment plant A, (d) workers with PPE exposed to S. aureus bioaerosol in wastewater treatment plant B, (e) workers without PPE exposed to E. coli bioaerosol in wastewater treatment plant A, (f) workers without PPE exposed to E. coli bioaerosol in wastewater treatment plant B, (g) workers with PPE exposed to E. coli bioaerosol in wastewater treatment plant A, and (h) workers with PPE exposed to E. coli bioaerosol in wastewater treatment plant B.
Correlation coefficient values were obtained from @ Oracle Crystal Ball sensitivity analyses and are shown next to each bar.
S. aureus = Staphylococcus aureus,
E. coli = Escherichia coli,
PPE = Personal Protective Equipment.
Fig. 4.
Pie charts showing the contribution to variance of input variables that impact the output value for workers (without or with PPE) exposed to S. aureus or E. coli bioaerosols in various aeration tanks of the two wastewater treatment plants referring to (a) workers without PPE exposed to S. aureus bioaerosol in wastewater treatment plant A, (b) workers without PPE exposed to S. aureus bioaerosol in wastewater treatment plant B, (c) workers with PPE exposed to S. aureus bioaerosol in wastewater treatment plant A, (d) workers with PPE exposed to S. aureus bioaerosol in wastewater treatment plant B, (e) workers without PPE exposed to E. coli bioaerosol in wastewater treatment plant A, (f) workers without PPE exposed to E. coli bioaerosol in wastewater treatment plant B, (g) workers with PPE exposed to E. coli bioaerosol in wastewater treatment plant A, and (h) workers with PPE exposed to E. coli bioaerosol in wastewater treatment plant B.
Contribution to variance values were obtained from @ Oracle Crystal Ball sensitivity analyses and are shown next to each pie.
S. aureus = Staphylococcus aureus,
E. coli = Escherichia coli,
PPE = Personal Protective Equipment,
RD = Rotating disc aeration tank,
IU=Inverted umbrella aeration tank,
M = Microporous aeration tank.
For S. aureus bioaerosol, the exposure concentration for workers (with or without PPE) on mechanical aeration modes (the rotating disc aeration tank (RD) in plant A or the inverted umbrella aeration tank (IU) in plant B) was the most sensitive to the health risks (Fig. 3a, b, c, and d). On the RD aeration tank in plant A, the exposure concentration for workers contributed the maximum variability of health risks associated with S. aureus bioaerosol. Among workers without or with PPE, the contribution to variance of the exposure concentration accounted for 43.62% or 30.06%, respectively (Fig. 4a and c). The exposure time, aerosol ingestion rate, and breathing rate on the RD aeration tank showed lower input variables ranking than the exposure concentration (Fig. 3a and c). On the RD aeration tank, the fraction of the contribution to variance of the exposure concentration was approximately 2, 6, and 7 times as large as the exposure time, the aerosol ingestion rate, and the breathing rate, respectively (Fig. 4a and c). The exposure concentration and the three input exposure parameters on the RD aeration tank were all more sensitive than those on the microporous aeration tank (M) in plant A (Fig. 3a and c). This result disclosed that on the aeration mode, which characterized high exposure concentration, the three input exposure parameters had a great impact on the workers' health risks. In plant B, the exposure concentration for the workers on the IU aeration tank showed a large contribution to the health risks, accounting for 31.22% (without PPE) or 22.6% (with PPE) (Fig. 4b and d). Moreover, the contribution to variance of the exposure concentration for the workers on the M aeration tank exerted minor effect on the health risk with fraction >10% (Fig. 4b and d). The three input exposure parameters (exposure time, aerosol ingestion rate, and breathing rate) for the workers on the IU aeration tank all showed a slightly higher ranking than those on the M aeration tank (Fig. 3b and d). These results reflected that the contribution of mechanical aeration modes to the variability of the health risks was absolutely dominated in the two WWTPs, especially for the contribution of the RD aeration tank in plant A. The ranking of the dose–response model parameters was just lower than those of the exposure concentration, the exposure time, and the removal fraction by employing PPE. The contribution of choice of the dose–response model parameters to health risks was far from negligible.
For E. coli bioaerosol, the rankings of the input variables were nearly the same as those for S. aureus bioaerosol. The exposure concentration on mechanical aeration modes, rather than mode of the microporous aeration tanks, accounted for most of the health risk's variability, with the fraction >40% (without PPE) or >30% (with PPE) (Fig. 4e, f, g, and h).
This result could be due to the fact that the microporous aeration mode completely differs from the mechanical aeration mode (Li et al., 2016). Several studies reported similar results. Stellacci et al. (2010) detected bioaerosols in the WWTP, which is often related to the surface mechanical aeration bioreactors. Another study in Poland obtained comparable results with this research, and they explained that the blast aeration technology with microporous aerators does not cause any large turbulence because it is situated at the bottom of the tank and accordingly does not generate large amounts of bioaerosols as the mechanical aeration mode did (Gotkowska-Płachta et al., 2013; Li et al., 2016).
When the PPE employed workers exposing to the mechanical aeration tanks in plants A and B, the input variable “removal fraction by employing with PPE” contributed the second ranking for health risks. This result illustrated that the PPE can largely affect health risks associated with bioaerosol (Haas et al., 2017; Carducci et al., 2018). Therefore, workers exposed to the mechanical aeration modes are strongly suggested to wear PPE. However, the effects of employing PPE on the M aeration tank in plants A and B showed weaker impact on the variability of the health risks. This result disclosed that the microporous aeration mode did not exert obvious effects on the health risks of the workers wearing PPE as large as that on the mechanical aeration modes. This finding is consistent with previous studies that QMRA could be used to indicate the most suitable scenario to employ PPE by considering its efficiency of protection (Carducci et al., 2018). In addition, the effective use of PPE can significantly decrease the worker's health risks (Ikehata, 2013; Haas et al., 2017).
4. Conclusion
The P(a)inf of the workers equipped without PPE exposed to S. aureus or E. coli bioaerosols in the two WWTPs considerably exceeded the U.S. EPA benchmark (≤10E-4 pppy), and the DB also did not satisfy the WHO benchmark (≤10E-6 DALYs pppy) except exposure to E. coli bioaerosol for disease health risk burden. However, the use of PPE can effectively reduce the annual infection health risk to an acceptable level and convert the disease health risk burden to a high acceptable level. In general, the health risks (P(a)inf and DB) of the workers with PPE were reduced approximately two orders of magnitude compared with those of the workers without PPE. The PPE could largely affect the health risk associated with bioaerosol, especially on the mechanical aeration modes. In addition, the different aeration modes between the two WWTPs led to the higher health risks of the workers in plant A than those of the workers in plant B. Under exposure to S. aureus bioaerosol, the contribution of mechanical aeration modes to the variability of the health risks was absolutely dominated in the WWTPs, especially the contribution of the RD aeration tank in plant A. The exposure concentration of the workers exposed to E. coli bioaerosol on the mechanical aeration modes, rather than the microporous aeration mode, accounted for most of the health risk variability. Therefore, the mechanical aeration should be managed as priority. On the aeration mode characterized with high exposure concentration, the three input exposure parameters (exposure time, aerosol ingestion rate, and breathing rate) had a great impact on health risks. Of note, the health risks were also prone to being highly influenced by the various choices of the dose–response model and related parameters. Therefore, accurate health risk estimation called for additional field studies and clinical infection data, and the dose–response model should be chosen discreetly.
This research systematically delivered new data and a novel perspective on the sensitivity analysis of QMRA for workers with PPE exposed to pathogenic bacterial bioaerosols under various aeration modes. Furthermore, it significantly aided in advancing the understanding of the rank correlation coefficient values and contributions to variance of each input variable in QMRA. Then, management decisions can be implemented by authorities on the basis of the results of the sensitivity analysis for the workers to abate the related occupational health risks. Generally, this research could be an educational tool to fill the gap between the QMRA framework and feasible rational management recommendations and to offer proposals that could be executed by authorities to protect public health.
CRediT authorship contribution statement
Conceptualization, Y.C. and C.Y.; Sampling and data curation, Y.C., C.Y., Y.Y., and J.M.; Formal analysis, Y.C. Y.Y., and C.Y.; Investigation, Y.C. Y.Y., and C.Y.; Methodology, Y.C., Y.Y., C.Y., and J.M.; Resources, C.Y.; Supervision, C.Y.; Visualization, Y.C., C.Y., and J.M.; Writing—original draft, Y.C. and C.Y.; and Writing—review and editing, Y.C., C.Y., and J.M.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was sponsored by the National Natural Science Foundation of China (51608497), the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (CUGL170409, CUG170103, CUGGC07), the Special Fund from the State Key Joint Laboratory of Environment Simulation and Pollution Control (Research Center for Eco-environmental Sciences, Chinese Academy of Sciences) (18K03ESPCR), the Major Science and Technology Program for Water Pollution Control and Treatment (2018ZX07110004), as well as the Program of Geological Processes Resources and Environment in the Yangtze Basin (CUGCJ1702).
Editor: Frederic Coulon
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.142615.
Appendix A. Supplementary data
Supplementary material
References
- Abhishek B., Ashok K. 2008. Application of the Crystal Ball Software for Uncertainty and Sensitivity Analyses for Predicted Concentration and Risk Levels. (Wiley InterScience) [Google Scholar]
- Brandi G., Sisti M., Amagliani G. Evaluation of the environmental impact of microbial aerosols generated by wastewater treatment plants utilizing different aeration systems. J. Appl. Microbiol. 2000;88(5):845–852. doi: 10.1046/j.1365-2672.2000.01024.x. [DOI] [PubMed] [Google Scholar]
- Busgang A., Friedler E., Gilboa Y., Gross A. Quantitative microbial risk analysis for various bacterial exposure scenarios involving greywater reuse for irrigation. Water-Sui. 2018;10(4):413. [Google Scholar]
- Carducci A., Donzelli G., Cioni L., Federigi I., Lombardi R., Verani M. Quantitative microbial risk assessment for workers exposed to bioaerosol in wastewater treatment plants aimed at the choice and setup of safety measures. Int. J. Environ Res Public Health. 2018;15(7) doi: 10.3390/ijerph15071490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delort A., Amato P. John Wiley & Sons; Inc: 2017. Microbiology of Aerosols. [Google Scholar]
- Devleesschauwer B., Havelaar A.H., Maertens De Noordhout C., Haagsma J.A., Praet N., Dorny P., Duchateau L., Torgerson P.R., Van Oyen H., Speybroeck N. DALY calculation in practice: a stepwise approach. Int. J. Public Health. 2014;59(3):571–574. doi: 10.1007/s00038-014-0553-y. [DOI] [PubMed] [Google Scholar]
- Dungan R.S. Estimation of infectious risks in residential populations exposed to airborne pathogens during center pivot irrigation of dairy wastewaters. Environ. Sci. Technol. 2014;48(9):5033–5042. doi: 10.1021/es405693v. [DOI] [PubMed] [Google Scholar]
- Esfahanian E., Adhikari U., Dolan K., Mitchell J. Construction of a new dose-response model for Staphylococcus aureus considering growth and decay kinetics on skin. Pathogens. 2019;8(4) doi: 10.3390/pathogens8040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federigi I., Verani M., Donzelli G., Cioni L., Carducci A. The application of quantitative microbial risk assessment to natural recreational waters: a review. Mar. Pollut. Bull. 2019;144:334–350. doi: 10.1016/j.marpolbul.2019.04.073. [DOI] [PubMed] [Google Scholar]
- Fuhrimann S., Winkler M.S., Stalder M., Niwagaba C.B., Babu M., Kabatereine N.B., Halage A.A., Utzinger J., Cissé G., Nauta M. Disease burden due to gastrointestinal pathogens in a wastewater system in Kampala, Uganda. Microbial Risk Analysis. 2016;4:16–28. [Google Scholar]
- Gotkowska-Płachta A., Filipkowska Z., Korzeniewska E., Janczukowicz W., Dixon B., Gołas I., Szwalgin D. Airborne microorganisms emitted from wastewater treatment plant treating domestic wastewater and meat processing industry wastes. Clean-Soil Air Water. 2013;5(41):429–436. [Google Scholar]
- Haas C.N. Microbial dose response modeling: past, present, and future. Environ. Sci. Technol. 2015;49(3):1245–1259. doi: 10.1021/es504422q. [DOI] [PubMed] [Google Scholar]
- Haas C.N., Rose J.B., Gerba C.P. John Wiley & Sons; Inc. New Jersey: 2014. Quantitative Microbial Risk Assessment. [Google Scholar]
- Haas C.N., Rycroft T., Bibby K., Casson L. Risks from ebolavirus discharge from hospitals to sewer workers. Water Environ. Res. 2017;89(4):357–368. doi: 10.2175/106143017X14839994523181. [DOI] [PubMed] [Google Scholar]
- Hamilton A.J., Stagnitti F., Premier R., Boland A.M., Hale G. Quantitative microbial risk assessment models for consumption of raw vegetables irrigated with reclaimed water. Appl. Environ. Microbiol. 2006;5(72):3284–3290. doi: 10.1128/AEM.72.5.3284-3290.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar A.H., Haagsma J.A., Mangen M.J., Kemmeren J.M., Verhoef L.P.B., Vijgen S.M.C., Wilson M., Friesema I.H.M., Kortbeek L.M., van Duynhoven Y.T.H.P., van Pelt W. Disease burden of foodborne pathogens in the Netherlands, 2009. Int. J. Food Microbiol. 2012;156(3):231–238. doi: 10.1016/j.ijfoodmicro.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Hung H.F., Kuo Y.M., Chien C.C., Chen C.C. Use of floating balls for reducing bacterial aerosol emissions from aeration in wastewater treatment processes. J. Hazard. Mater. 2010;175(1–3):866–871. doi: 10.1016/j.jhazmat.2009.10.090. [DOI] [PubMed] [Google Scholar]
- Ikehata K. Business Media; Dordrecht: 2013. Wastewater Reuse and Management, Springer Science. [Google Scholar]
- Jahne M.A., Rogers S.W., Holsen T.M., Grimberg S.J. Quantitative microbial risk assessment of bioaerosols from a manure application site. Aerobiologia. 2015;31(1):73–87. [Google Scholar]
- Jahne M.A., Rogers S.W., Holsen T.M., Grimberg S.J., Ramler I.P., Kim S. Bioaerosol deposition to food crops near manure application: quantitative microbial risk assessment. J. Environ. Qual. 2016;45(2):666–674. doi: 10.2134/jeq2015.04.0187. [DOI] [PubMed] [Google Scholar]
- Jones R.M. Relative contributions of transmission routes for COVID-19 among healthcare personnel providing patient care. J. Occup. Environ. Hyg. 2020;17(9):408–415. doi: 10.1080/15459624.2020.1784427. [DOI] [PubMed] [Google Scholar]
- Katsivela E., Latos E., Raisi L., Aleksandropoulou V., Lazaridis M. Particle size distribution of cultivable airborne microbes and inhalable particulate matter in a wastewater treatment plant facility. Aerobiologia. 2017;33(3):297–314. [Google Scholar]
- Kowalski M., Wolany J., Pastuszka J.S., Płaza G., Wlazło A., Ulfig K., Malina A. Characteristics of airborne bacteria and fungi in some Polish wastewater treatment plants. Int. J. Environ. Sci. Te. 2017;14(10):2181–2192. [Google Scholar]
- Kozajda A., Jezak K., Kapsa A. Airborne Staphylococcus aureus in different environments-a review. Environ. Sci. Pollut. Res. Int. 2019;26(34):34741–34753. doi: 10.1007/s11356-019-06557-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhou L., Zhang X., Xu C., Dong L., Yao M. Bioaerosol emissions and detection of airborne antibiotic resistance genes from a wastewater treatment plant. Atmos. Environ. 2016;124:404–412. [Google Scholar]
- Lim K., Jiang S.C. Reevaluation of health risk benchmark for sustainable water practice through risk analysis of rooftop-harvested rainwater. Water Res. 2013;47(20):7273–7286. doi: 10.1016/j.watres.2013.09.059. [DOI] [PubMed] [Google Scholar]
- Lim K., Hamilton A.J., Jiang S.C. Assessment of public health risk associated with viral contamination in harvested urban stormwater for domestic applications. Sci. Total Environ. 2015;523:95–108. doi: 10.1016/j.scitotenv.2015.03.077. [DOI] [PubMed] [Google Scholar]
- Liu Y., Lu W., Wang H., Gao X., Huang Q. Improved impact assessment of odorous compounds from landfills using Monte Carlo simulation. Sci. Total Environ. 2019;648:805–810. doi: 10.1016/j.scitotenv.2018.08.213. [DOI] [PubMed] [Google Scholar]
- MEP-PRC . 2013. Exposure Factors Hankbook of Chinese Population.(Chinese) [Google Scholar]
- Nasir Z., Rolph C., Collins S., Stevenson D., Gladding T., Hayes E., Williams B., Khera S., Jackson S., Bennett A., Parks S., Kinnersley R., Walsh K., Pollard S., Drew G., Alcega S., Coulon F., Tyrrel S. A controlled study on the characterisation of bioaerosols emissions from compost. Atmosphere-Basel. 2018;9(10):379. [Google Scholar]
- Oppliger A., Hilfiker S., Vu D.T. Influence of seasons and sampling strategy on assessment of bioaerosols in sewage treatment plants in Switzerland. Ann. Occup. Hyg. 2005;49(5):393–400. doi: 10.1093/annhyg/meh108. [DOI] [PubMed] [Google Scholar]
- Pang H., Lambertini E., Buchanan R.L., Schaffner D.W., Pradhan A.K. Quantitative microbial risk assessment for Escherichia coli O157:H7 in fresh-cut lettuce. J. Food Protect. 2017;80(2):302–311. doi: 10.4315/0362-028X.JFP-16-246. [DOI] [PubMed] [Google Scholar]
- Pasalari H., Ataei-Pirkooh A., Aminikhah M., Jafari A.J., Farzadkia M. Assessment of airborne enteric viruses emitted from wastewater treatment plant: atmospheric dispersion model, quantitative microbial risk assessment, disease burden. Environ. Pollut. 2019;253:464–473. doi: 10.1016/j.envpol.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Peccia J., Milton D.K., Reponen T., Hill J. A role for environmental engineering and science in preventing bioaerosol-related disease. Environ. Sci. Technol. 2008;42(13):4631–4637. doi: 10.1021/es087179e. [DOI] [PubMed] [Google Scholar]
- Sales-Ortells H., Medema G. Screening-level microbial risk assessment of urban water locations: a tool for prioritization. Environ. Sci. Technol. 2014;48(16):9780–9789. doi: 10.1021/es5020407. [DOI] [PubMed] [Google Scholar]
- Shi K., Wang C., Jiang S.C. Quantitative microbial risk assessment of Greywater on-site reuse. Sci. Total Environ. 2018;635:1507–1519. doi: 10.1016/j.scitotenv.2018.04.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellacci P., Liberti L., Notarnicola M., Haas C.N. Hygienic sustainability of site location of wastewater treatment plants. Desalination. 2010;253(1–3):106–111. [Google Scholar]
- Szyłak-Szydłowski M., Kulig A., Miaśkiewicz-Pęska E. Seasonal changes in the concentrations of airborne bacteria emitted from a large wastewater treatment plant. Int. Biodeterior. Biodegradation. 2016;115:11–16. [Google Scholar]
- Teixeira J.V., Miranda S., Monteiro R.A.R., Lopes F.V.S., Madureira J., Silva G.V., Pestana N., Pinto E., Vilar V.J.P., Boaventura R.A.R. Assessment of indoor airborne contamination in a wastewater treatment plant. Environ. Monit. Assess. 2013;185(1):59–72. doi: 10.1007/s10661-012-2533-0. [DOI] [PubMed] [Google Scholar]
- Tesson V., Federighi M., Cummins E., de Oliveira Mota J., Guillou S., Boué G. A systematic review of beef meat quantitative microbial risk assessment models. Int. J. Env. Res. Pub. He. 2020;17(3):688. doi: 10.3390/ijerph17030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA . 2005. Occurrence and exposure assessment for the final Long Term 2 Enhanced Surface Water Treatment Rule. Office of Water, Washington. DC. United States Environmental Protection Agency. EPA 815-R-06-002. [Google Scholar]
- Vásquez G.A., Busschaert P., Haberbeck L.U., Uyttendaele M., Geeraerd A.H. An educationally inspired illustration of two-dimensional quantitative microbiological risk assessment (QMRA) and sensitivity analysis. Int. J. Food Microbiol. 2014;190:31–43. doi: 10.1016/j.ijfoodmicro.2014.07.034. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li L., Xiong R., Guo X., Liu J. Effects of aeration on microbes and intestinal bacteria in bioaerosols from the BRT of an indoor wastewater treatment facility. Sci. Total Environ. 2019;648:1453–1461. doi: 10.1016/j.scitotenv.2018.08.244. [DOI] [PubMed] [Google Scholar]
- WHO . vol. 1. World Health Organization; Switzerland: 2008. Guidelines for Drinking-Water Quality: Incorporating 1st and 2nd Addenda. [Google Scholar]
- Zhou L., Echigo S., Ohkouchi Y., Itoh S. Quantitative microbial risk assessment of drinking water treated with advanced water treatment process. J. Water Supply Res. Technol. AQUA. 2014;63(2):114–123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material