Abstract
There is a strong association between obesity and colorectal cancer (CRC), especially in men, whereas estrogen protects against both the metabolic syndrome and CRC. Colon is the first organ to respond to high-fat diet (HFD), and estrogen receptor beta (ERβ) can attenuate CRC development. How estrogen impacts the colon under HFD and related sex differences has, however, not been investigated. To dissect this, mice were fed control diet or HFD for 13 weeks and administered receptor-selective estrogenic ligands for the last three weeks. We recorded impact on metabolism, colon crypt proliferation, macrophage infiltration, and the colon transcriptome. We found clear sex differences in the colon transcriptome and in the impact by HFD and estrogens, including on clock genes. ERα-selective activation reduced body weight and generated systemic effects, whereas ERβ-selective activation had local effects in the colon, attenuating HFD-induced macrophage infiltration and epithelial cell proliferation. We here demonstrate how HFD and estrogens modulate the colon microenvironment in a sex- and ER-specific manner.
Subject terms: Cell biology, Risk factors, Gastrointestinal diseases, Metabolism, Nutrition, Chronic inflammation
Introduction
Obesity represents a worldwide public-health issue and epidemiological studies highlight a strong association between body mass index (BMI) and risk of colorectal cancer (CRC)1–4. A high-fat diet (HFD) increases this risk, and the incidence of CRC is increasing among young adults due to altered life-style factors5, but exactly how is not clear. It is known that inflammatory bowel disease (IBD) increases the risk of CRC, and that both obesity and HFD induce a general low-grade inflammatory state6,7. The colon is the first organ to respond to HFD8, with reported increase of inflammation, PPARδ signaling, stem cell activity, and altered gut microbiota9–16, which may all contribute to initiation of CRC. Interestingly, while obesity is a risk factor for both sexes, this association appears stronger among men17. Men also have a higher incidence of CRC18,19. A role for sex hormones is supported by epidemiological studies (reviewed in20), and especially estrogen appears protective against CRC21,22. Numerous clinical studies have also indicated that estrogen protects against several aspects of obesity and the metabolic syndrome23,24, as well as impacts the immune system (reviewed in25).
The biological action of estrogen is mediated by three receptors, the nuclear receptors ERα (ESR1) and ERβ (ESR2), and the transmembrane G protein-coupled estrogen receptor 1 (GPER1). These receptors are expressed in both sexes, with a tissue-specific expression pattern. Both ERα and ERβ have been implicated to elicit anti-obesogenic effects26–29. Whereas ERα clearly improves the metabolic profile, the role of ERβ in this respect is controversial30. Most of the CRC protective effects, however, have been linked to ERβ. Although its mRNA expression in colon is low, treatment with ERβ-selective agonist, for example, resulted in anti-inflammatory and anti-tumorigenic effects in CRC mouse models31–34. Furthermore, loss of ERβ results in pro-inflammatory environment and increased tumor development in both APC Min/+ and colitis-associated tumor mouse model35,36. Notably, intestinal-specific deletion of ERβ increased tumor development and strongly enhanced inflammatory signaling in the colon of both male and female mice37. In fact, inflammatory signaling itself was shown to enhance transactivation of ERβ37. It is therefore plausible that this ERβ mechanism could protect against HFD-induced colonic inflammation. We hypothesize that estrogenic signaling, through ERα (systemic effects by improving overall metabolism), ERβ, or both; can attenuate the effect of HFD in colon, with possible sex differences.
In order to test this, and to characterize the impact of estrogen signaling on the colon microenvironment under HFD, we performed a detailed analysis of mice of both sexes. In order to compare sex differences and to obtain data comparable to premenopausal women, where obesity is thought to increase the incidence of CRC, none of the female mice in this study were ovariectomized. Mice were fed either a control diet (CD) or a HFD for 13 weeks, and administered different estrogenic ligands for the last three weeks. Importantly, by analyzing corresponding effects on the full colon transcriptome, we generated an unbiased overview of alterations mediated by HFD and estrogens. We observed sex differences in the colon transcriptome and in its response to HFD. In particular, ERβ opposed the HFD impact on colonic inflammation, epithelial cell proliferation and regulation of circadian rhythmicity. Altogether, we present the novel finding that estrogen signaling is indeed partially protective against HFD-induced alterations of the colon microenvironment, in part directly through intestinal ERβ. These findings open up new insights into prevention of obesity-associated CRC.
Results
Sex impacts obesity, fat distribution and fasting glucose levels
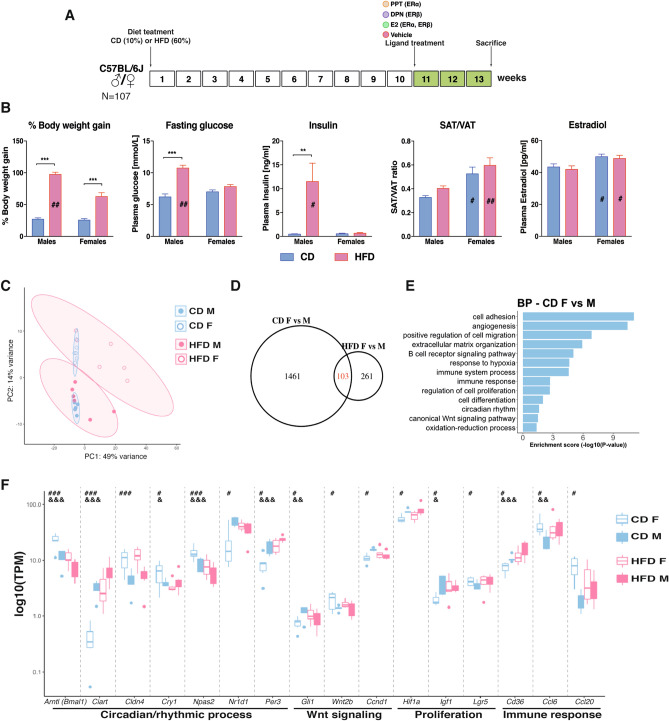
The impact of sex and 13-week HFD were characterized according to standard metabolic and endocrinologic measurements, including weight gain, fat distribution, fasting glucose, insulin, and circulating estrogen levels. HFD-induced weight gain was significantly higher in males compared to females (Fig. 1B). Obese males further presented significantly increased blood glucose level (after 6-h fasting) and insulin level (2-h fasting), which was not observed in obese females (Fig. 1B). These results support previous observations that obese males are more susceptible to glucose intolerance and insulin resistance compared to females38,39. As also noted previously39, the fat distribution was sex-dependent. Males had more visceral white adipose tissue (VAT) and less subcutaneous white adipose tissue (SAT) in relation to total fat during both dietary conditions, compared to females (Supplementary Fig. 1A). As a consequence, males had a substantially lower SAT/VAT ratio (Fig. 1B). A low SAT/VAT ratio has been correlated to poor metabolic outcomes. As expected, female mice had significantly higher circulating estradiol levels compared to males during both CD and HFD (Fig. 1B). Circulating estradiol levels were not significantly impacted by obesity (Fig. 1B). This contradicts one previous study which found increasing circulating estradiol levels upon HFD30. However, obesity is primarily known to increase local levels of estrogen in adipose tissue, which express aromatase40. We did not measure local adipose tissue estradiol levels. Overall, these results and sex differences replicate previous findings.
Figure 1.
The male metabolic profile is more sensitive to HFD and the colon trancriptome exhibits sex differences independent of diet. (A) Experimental study design. Mice of both sexes were given CD or HFD for 13 weeks and injected with different estrogenic ligands or vehicle for the last 3 weeks prior to sacrifice. (B) Percentage of BW gain, fasting glucose level, insulin level, SAT/VAT ratio, and plasma estradiol levels. (C) Principal component analysis of RNA-seq samples for male (unfilled) and female (filled) mice fed a CD (blue) or HFD (red) (n = 5–6). (D) Venn diagram comparing differentially expressed genes in colon between male and female mice fed a CD or a HFD. Genes were considered as differentially expressed when p value < 0.05 and log2FC > |0.4|. (E) Biological process enrichment analysis based on genes differentially expressed between the sexes during CD. (F) Boxplot of TPM values of genes differentially expressed between sexes and/or dietary conditions. The results are presented as mean ± SEM. Two-way ANOVA followed by fisher's LSD test, *p < 0.05; **p < 0.01; ***p < 0.001. # indicate significant sex differences during CD, ## during HFD and ### during both dietary conditions; & indicate significantly difference between CD and HFD in females, && in males, and &&& in both sexes. Blue color indicates CD and pink HFD.
The colon transcriptome exhibits clear sex differences
To specifically study sex differences in the colon, we performed RNA-seq of colon tissue from males and females. As evident from the principal component analysis (PCA) plot, the colon transcriptomes exhibited clear sex differences, especially during CD (Fig. 1C). Gene expression analysis also identified 1564 significantly differentially expressed genes (DEG) between male and female colon under CD (Fig. 1D). While the CD colon transcriptome clustered tightly together, the HFD was more spread out (Fig. 1C). As a result, the sex difference under HFD was less distinct, but 364 DEGs were still revealed (Fig. 1D). For animals fed a CD, enrichment analysis for biological processes (BP) disclosed that the genes responsible for the sex differences were related to immune response (e.g. Cd36, Ccl6 and Ccl20), cell proliferation (e.g. Hif1a, Igf1 and Lgr5) and canonical Wnt signaling (e.g. Ccnd1, Gli1 and Wnt2b, Fig. 1E,F). Although the mice had been sacrificed at the same time of the day, we found sex differences in expression of circadian genes, including the clock genes Arntl (Bmal1), Npas2, Cry1, Per3, and Nr1d1/Rev-ErbA (Fig. 1E,F). Over one fourth (28%) of the sex differences after HFD were also present under CD (Fig. 1D). The most overrepresented genes among these stable DEGs (103) belonged to the circadian/rhythmic pathway (e.g. Bmal1, Ciart, Cldn4 and Npas2, Fig. 1F). Thus, we report for the first time that mice fed CD, which lacks phytoestrogens, display clear sex differences in the colon transcriptome.
HFD modulates the colon transcriptome differently in males and females
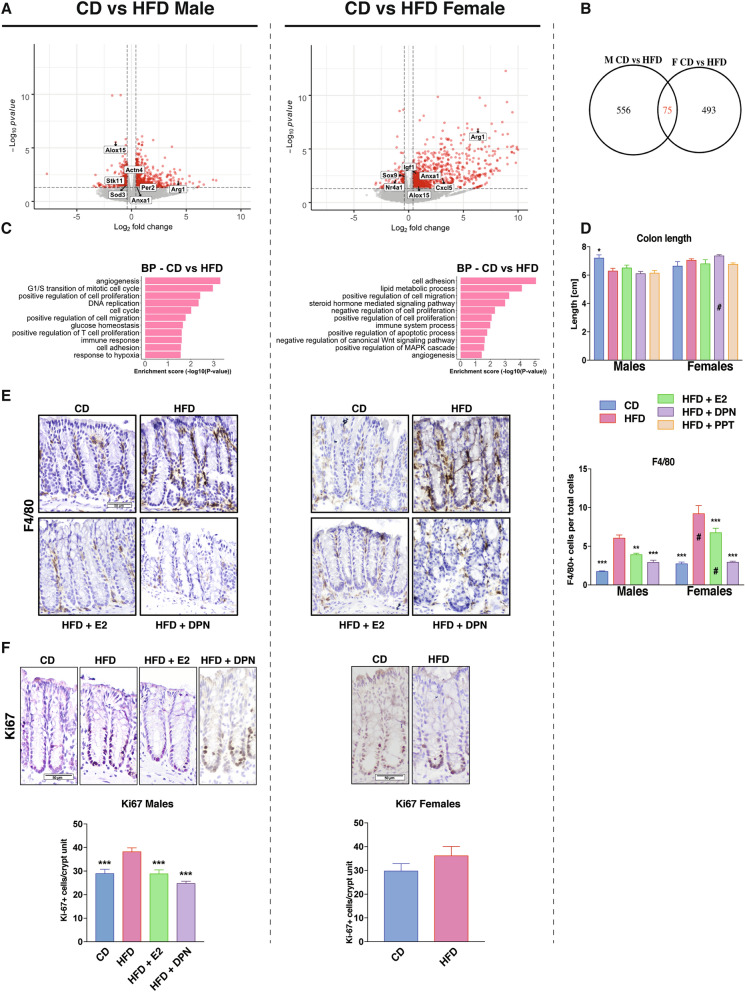
In order to explore how HFD impacted male and female colon, we compared the transcriptomes under HFD with corresponding expression under CD, for males and females separately. We identified 568 HFD-modulated DEGs in females and 631 in males (Fig. 2A). We noted that a larger proportion of genes was upregulated overall, but especially in females (85%, versus 51% in males), and that females exhibited stronger upregulation. Most of the modified genes differed between the sexes, only 75 genes responded to HFD in both (Fig. 2B). Among these common genes were, again, those related to the circadian rhythm (Bmal1, Ciart, Npas2 and Per3, Fig. 1F), oxidation–reduction process (Alox15), and inflammatory response (e.g. Arg1 and Anxa1, Fig. 2A). Both sexes altered expression of genes with functions in cell adhesion, cell proliferation, angiogenesis, migration, and immune system (Fig. 2C and Supplementary Table 2), but different genes were altered in males and females. Regulated genes involved in cell cycle (e.g. Stk11 and Wee1), hypoxia (e.g. Actn4 and Sod3) and glucose homeostasis (e.g. Stk11) were particularly enriched in males, whereas regulation of apoptosis (e.g. Nr4a1), inflammatory response (e.g. Cxcl5), MAPK cascade (e.g. Igf1) and Wnt signaling (e.g. Sox9) were enriched in females (Fig. 2A,C and Supplementary Table 2). Thus, while both sexes presented alterations within similar biological functions, including the immune system and cell adhesion, other changes appeared tied to sex, such as more pronounced changes of cell cycle and hypoxia in males, and of the lipid metabolism, steroid hormone and Wnt signaling in females. All together our data demonstrate, for the first time, that sex impacts the gene expression response to HFD in colon.
Figure 2.
The colon transcriptome responds to HFD in a sex-dependent manner. (A) Volcano plots show HFD-regulated genes in males (left) and females (right). Genes were considered differentially expressed when p < 0.05 and log2FC > |0.4|, indicated in red. (B) Venn diagram comparing differentially expressed genes in colon between CD and HFD in male and female mice. (C) Biological process enrichment analysis of HFD-regulated genes, separated by sex. (D) Colon length measured in cm. (E) Macrophage infiltration measured by F4/80+ IHC and quantified by stained cells per total cells ratio. (F) Intestinal epithelial cell proliferation measured with Ki67 IHC and quantified by number of positive cells per crypt unit. n = 5–15 per group. Results are presented as mean ± SEM. One-way and two-way ANOVA with uncorrected Fisher’s LSD test. *Indicate significant differences compared to HFD + vehicle, *p < 0.05; **p < 0.01; ***p < 0.001. #Indicate sex differences.
HFD increases colon epithelial inflammation and proliferation in males
The above colon transcriptome analysis indicated that the immune system was impacted by HFD and that, especially in males, the cell cycle and hypoxia were modulated. A shortened colon is indicative of colonic inflammation, and we found that males but not females fed an HFD presented a significantly shorter colon (Fig. 2D). Next, we explored markers for macrophages in histological sections using immunohistochemistry (IHC). We found that mice of both sexes exhibited a significantly increased F4/80+ macrophage infiltration when fed HFD, but females had a stronger infiltration than males (Fig. 2E). Males on the other hand, presented significantly increased proliferation of crypt cells after HFD, which females did not (Fig. 2F). This reveals that HFD results in clear sex-dependent functional defects in the colon, in accordance with the above noted transcriptional differences.
Estrogen improves the metabolic profile of obese males via ERα
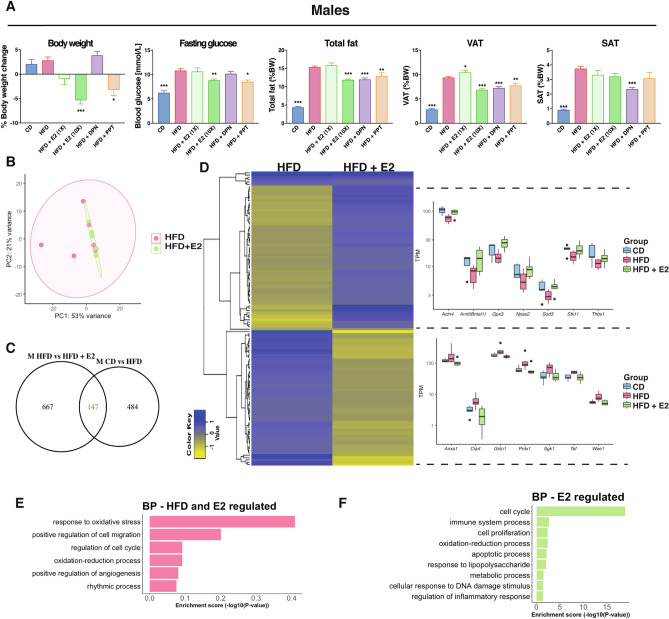
Several studies have shown that estradiol (E2) improves the metabolic profile of HFD-fed rodents of both sexes41,42. Further, knockout of ERα results in obese mice26. In our study, neither E2 nor ERα-selective ligand PPT treatment for the last 3 weeks (Fig. 1A) affected the body weight (BW)-gain in females compared to vehicle treatment (Supplementary Fig. 1C,D). However, male mice showed a dose-dependent BW loss during E2 treatment, with up to 5% weight reduction (0.5 mg/kg BW, Fig. 3A and Supplementary Fig. 1C). This was accompanied by a significant reduction of total fat and decreased blood glucose level upon fasting, not seen in females (Fig. 3A and Supplementary Fig. 1D). These effects were also evident with the selective ERα ligand PPT (Fig. 3A). Thus, our experimental set-up confirms that exogenous E2 via ERα impacts obesity, fasting glucose, total fat and VAT ratios in males.
Figure 3.
Estrogen treatment impacts the metabolic profile and colon gene expression in males. (A) BW change during treatment, fasting glucose level, total fat mass (reported to BW), VAT, and SAT (reported to BW) for males on a CD, HFD, and HFD treated with E2, DPN and PPT. (B) PCA of RNA-seq data for males fed a HFD with and without E2 treatment. (C) Venn diagram comparing HFD-regulated genes in males with E2-regulated genes in males under a HFD. (D) Heatmap of the homogenously regulated common genes (regulated by both HFD and E2 under HFD) and boxplots of TPM values of some genes. Biological process enrichment analysis of the (E) HFD-induced genes opposed by estrogen signaling and (F) estrogen-regulated genes under HFD feeding (n = 5–15 per group). Results are presented as mean ± SEM. One-way ANOVA with uncorrected Fisher’s LSD test. * Indicate significant differences compared to HFD + vehicle, *p < 0.05; **p < 0.01; ***p < 0.001.
Estrogen impacts the obese colon transcriptome
Next, we wanted to determine if E2 treatment impacted the colon, and specifically if it could modulate the HFD-induced colon inflammation signature. We focused on males, who had exhibited a shortening of the colon upon HFD and had shown a significant E2-mediated impact on their metabolic profile. The RNA-seq data, visualized in a PCA plot, showed that estrogen-treated HFD-fed male colon clustered tighter together compared to vehicle treated (Fig. 3B), just as CD samples did (Fig. 1C). We found that E2 treatment modified the expression of 814 genes in distal colon. Among these E2-regulated genes, we noted enrichment of cell cycle and cell proliferation (e.g. Cdk1, Melk, Mki67), and immune system process (e.g. Alcam, Irf7 and Bcl6) pathways (Fig. 3F). Of these, 147 genes were also impacted by HFD (compared to CD), nearly all (97%) in the opposite direction (Fig. 3C,D). BP enrichment analysis revealed that genes regulated by both HFD and E2 were involved in cell cycle and rhythmic processes (Fig. 3E). Two homogenous (regulated similarly in at least three animals from each group) clusters of DEG appeared, illustrated by a heatmap in Fig. 3D. One cluster (61 genes) was downregulated by HFD and then upregulated by E2, such as Actn4, Bmal1, Gpx3, Npas2, Sod3, Stk11 and Thbs1 (Fig. 3D, upper right). The second cluster included 58 genes that were upregulated by HFD and downregulated by E2, and comprised genes such as Anxa1, Ciart, Gsto1, Prdx1, Sgk1, Tef and Wee1 (Fig. 3D, lower right). qPCR supported these regulations. This demonstrates that both sex and estrogen have a substantial impact on the colon transcriptome, that estrogen may have a strong effect on proliferation and clearly opposes a fraction of the HFD-induced expression.
Estrogen reduces immune cell recruitment and proliferation of the HFD colon
Since we noted that E2 treatment could attenuate signs of inflammation and proliferation at the gene expression level, we investigated whether E2 impacted the length of colon, infiltration of macrophages, or proliferation. E2 did not significantly impact the colon length but did reduce F4/80+ macrophage infiltration in both sexes (Fig. 2D,E). We also noted a significant reduction of positive Ki67-stained cells upon E2 treatment of HFD males (Fig. 2F). These data clearly demonstrate that E2 impacts the colon, both in terms of immune cell recruitment and colonic epithelial cell proliferation.
ERβ-selective agonist impacts fat distribution and reduces colonic macrophages infiltration and cell proliferation
To investigate ERβ-mediated effects, we included treatment with a ligand selective for ERβ (DPN, Fig. 1A). DPN treatment did not result in weight reduction, in accordance with data assigning this phenotype to ERα. On the contrary, DPN-treated males and females exhibited a non-significant trend of increased weight gain, which was accompanied by a significant increase of fasting blood glucose levels in females but not males (Fig. 3A and Supplementary Fig. 1D). This also demonstrates that the ligands in the concentrations used in this set up retained receptor selectivity. Although the effects by DPN on weight were not significant in males, we noted clear reductions of total fat and VAT by DPN treatment, in a scale similar or higher than what was achieved with PPT treatment (Fig. 3A). Further, a reduction in SAT was specific for ERβ ligand activation (Fig. 3A). We did not see these effects in females where, on the contrary, VAT was increased by DPN (Supplementary Fig. 1D). This suggests that while obesity and HFD-induced BW can be decreased only through ERα, ERβ activation appears to modify the ratio of total fat, VAT, and SAT in males, and increase fasting glucose and VAT in females. Furthermore, estrogen through ERβ (E2 and DPN) opposed the HFD-induced Ki67 proliferation in males (Fig. 2F). The increased F4/80+ macrophage infiltration upon HFD was also significantly reduced by estrogen through ERβ (E2 and DPN) in both sexes (Fig. 2E). Our data thus establish that while an improved metabolic profile is mediated via ERα, ERβ impacts the colon by attenuating HFD-induced colonic proliferation in males and macrophage infiltration in both sexes.
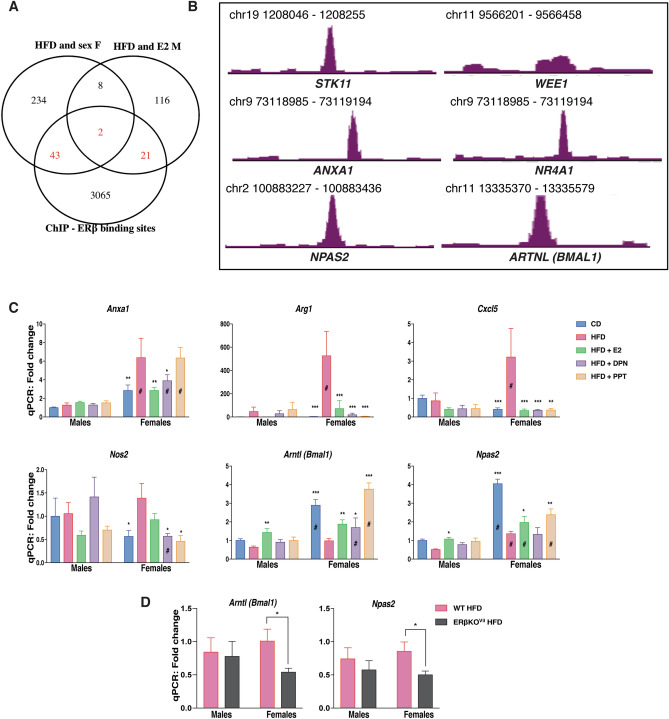
ERβ regulates gene expression in the colon
As shown above, E2 treatment opposed a set of HFD-induced colonic gene expression in males. This could be an indirect consequence of systemic effects (e.g. ERα improving the overall metabolism) or direct local effects through ERs expressed in the colon. We have previously shown that ERβ is expressed in intestinal epithelial at low levels, whereas there is not sufficient support that ERα is expressed in the colon43. While we did not sequence the colon transcriptome of DPN-treated mice, we evaluated the E2-mediated response of ERβ-target genes (as predicted from our ERβ chromatin immunoprecipitation (ChIP)-seq data from human CRC cell lines, with transduced ERβ, GSE149979) in the male colon transcriptome. This resulted in identification of 23 genes, which were regulated by both HFD and E2, and also have cis-chromatin ERβ-binding sites (Fig. 4A). These included the cell cycle genes Stk11 and Wee1 and the inflammatory related gene Anxa1 among others (Fig. 4B). Moreover, we compared the female-only HFD-regulated genes (potential female endogenous E2 regulation). This comprised of 287 genes of which 45 had ERβ-binding sites, including the orphan nuclear receptor Nr4a1 (Nur77, Fig. 4A,B). qPCR analysis of colon tissue corroborated the HFD-induced upregulation of Anxa1 gene expression and that this was indeed attenuated by both E2 and DPN (ERβ) but not by PPT in females, whereas it was not affected in males (Fig. 4C). Further, the HFD-induction of the M2 macrophage marker Arg1 was also stronger in females, and this was blocked by both ERα and ERβ activation (Fig. 4C). In addition, we have previously shown that ERβ can cross-talk with and regulate NFκB signaling in both human colon cell lines and mouse in vivo colon (ERβ-intestinal knockout mice)37. In line with this, we here observed that HFD-induced expression of several NFκB target genes (Cxcl5, Nos2) in females, which were blocked by both ERβ (DPN) and ERα (PPT). Our data suggest that systemic activation of ERα improves the overall metabolism, which helps oppose obesity-mediated colon dysfunction. ERβ, on the other hand, appears to locally downregulate proliferation and inflammatory genes (Cxcl5 and Nos2) in the colon. This data provides evidence that E2 through systemic and local effects via ERα and ERβ can partially attenuate inflammatory effects in the colon.
Figure 4.
Estrogen through both ERα and ERβ can modulate the expression of specific sets of genes under HFD in both sexes. (A) Venn diagram of the E2-regulated genes under HFD in males and the HFD and sex regulated genes in females compared to ERβ-binding chromatin sites in two different CRC cell lines (SW480 and HT29). (B) ERβ-binding sites revealed by ChIP-seq in CRC cell lines. (C) qPCR measurement of gene expression in the colon of female and male mice on a CD, HFD and HFD treated with E2, DPN and PPT (n = 5–15). (D) qPCR measurement of gene expression in colon of wild type and intestinal-specific ERβ KO mice fed a HFD. The results are presented as mean ± SEM. Two-way ANOVA with uncorrected Fisher’s LSD test. * Indicate significant differences compared to HFD + vehicle, *p < 0.05; **p < 0.01; ***p < 0.001. #Indicate sex differences.
Sex, hormones, and diet influence regulation of circadian clock genes in colon
The circadian rhythm is essential for intestinal homeostasis. Disruptions can increase intestinal permeability, proliferation, exacerbate colitis, modulate microbiota composition, and impact the immune system44–47. HFD impacts the circadian rhythmicity in both liver and adipose tissue, but very little is known about the impact of HFD on the circadian rhythm in the colon epithelium. Above, we identified sex differences in the expression of clock genes with higher expression of Bmal1, Npas2, and Cry1 in female colon and higher expression of Per3 and Nr1d1 (Rev-ErbA) in male colon (Fig. 1F). HFD downregulated Bmal1 and Npas2 and upregulated Per3 in both sexes, downregulated Cry1 specifically in females and upregulated Per2 specifically in males (Fig. 1F, 2A). In addition to exhibiting sex differences in their expression levels, ER ligands could oppose the HFD-induced downregulation of both Bmal1 and Npas2 in both sexes (Fig. 3D, 4C). ChIP-seq indicated that ERβ is a regulator of the clock genes Bmal1 and Npas2 (Fig. 4B). In males, both Bmal1 and Npas2 were upregulated in the colon by estrogen, but significant regulation by ERβ (DPN) were not observed (Fig. 4D). However, we could confirm that the circadian clock gene Bmal1 indeed was upregulated by DPN (ERβ) and, also, by E2 and PPT (ERα) in females (Fig. 4B). This evidence points towards local regulation of clock genes by ERβ in the colon. The circadian rhythm of the colon has essential impacts on the body metabolism. If this is impacted by ERβ, as our study shows, this has substantial implications for our understanding of how hormones affect the metabolism. To provide conclusive evidence of direct ERβ-mediated regulation of Bmal1 and Npas2 in the in vivo female colon, we fed intestinal-specific ERβ knockout mice HFD. We observed a significant decrease in the expression of both Bmal1 and Npas2 in female colon lacking intestinal ERβ (Fig. 4D). This was not observed in males. Our results demonstrate, that the expression of genes in colon, including clock genes, has clear sex differences, is impacted by HFD in both sexes, and is directly regulated by intestinal epithelial ERβ in the colon of females.
Discussion
Our objective with this study was to determine whether estrogen could improve the colon microenvironment and thereby attenuate the negative effects of HFD and, if so, to dissect this mechanism and identify sex differences. Several studies have suggested that both ERα and ERβ can improve the metabolic syndrome, a risk factor for CRC26–28. Other studies support a protective role of ERβ against CRC (reviewed in19). The role of ERβ, or ERα, in the colon during HFD-induced obesity has, however, not been investigated. We here demonstrate that estrogen indeed modulates the colon microenvironment in mice fed HFD, and that ERα- and ERβ-selective ligands can in part counteract different facets of HFD-induced phenotypes, including at the level of colonic gene expression.
As the metabolic data show, males appeared more sensitive to HFD, and the higher-dose E2 and ERα-selective activation improved their glucose metabolism, in line with previous studies38,41. Although both doses of E2 injections resulted in similar plasma concentrations, the low-dose E2 did not significantly impact glucose metabolism. It is possible that the higher dose resulted in increased local concentrations in certain tissues, especially as lipophilic steroid hormone can be sequestered in the visceral adipose tissue, or that it was higher at certain time points, not reflected in the plasma concentrations at the point of sacrifice. Some studies have also reported a protective effect through ERβ against HFD-induced obesity, but the data are conflicting. Foryst-Ludwig et al. found that ERβ protected against HFD-induced weight gain but still elicited pro-diabetogenic effects30. We could not confirm that ERβ protected against HFD-induced weight gain, but we did find that its activation (E2 and DPN, not PPT) impaired glucose levels in females (Supplementary Fig. 1D).
Our study detail several other significant sex differences. In particular, a notable sex difference in the colon transcriptome of mice (CD), which was not expected. As this diet did not contain phytoestrogens, which standardized soy-based chow diets are rich in, this enabled us to observe the impact of endogenous estrogen signaling without external interference. The sex differences that were observed in both dietary conditions (CD and HFD) were related to hypoxia, immune response, cell proliferation, circadian rhythm and Wnt signaling. Thus, our data bring in a role of sex hormones in the basal regulation of these pathways in colon. Intriguingly, we found that estrogen treatment in males indeed regulated the immune response, cell proliferation and rhythmic processes in colonic tissue.
Interestingly, HFD-induced pro-inflammatory modifications are known to occur earlier in the colon than in the adipose tissue. These changes are characterized by increased infiltration of pro-inflammatory macrophages and by epithelia permeability in the colon8. Estrogen treatment has been shown to elicit an anti-inflammatory response in the colon of mouse models for colitis32,48. Our transcriptomic data reveal that both sexes' immune response were altered upon HFD feeding, but with clear sex differences. This was supported by a significant increase of F4/80+ macrophage infiltration in the colon upon HFD in both sexes, but with a significantly higher overall macrophage count in females. The F4/80+ macrophage infiltration was significantly reduced by ERβ activation (E2 and DPN) in both males and females. In addition, our mechanistic data from ChIP-seq in CRC cell lines demonstrated that ERβ could bind to cis-regulatory chromatin regions of both Anxa1 and the orphan nuclear receptor Nr4a1 (Nur77). Anxa1 is known to recruit monocytes49 and Nur77 can regulate the immune system and suppress DSS-induced colitis50. In females, we found that ERβ activation could reduce the expression of Anxa1 and markers for anti-inflammatory M2 (Arg1) and pro-inflammatory M1 (Nos2) macrophages. Thus, our study delineates how ERβ contributes to anti-inflammatory effects mediated by estrogen in the in vivo colon. ERβ is not detected in macrophages, but is expressed in intestinal epithelial cells51,52. Cross-talk between the colonic epithelial cells and macrophages has been demonstrated in IBD53 and with HFD in an obesity context8. Although the etiology of obesity and IBD is different, both are characterized by gut inflammation with common inflammatory pathways including TNFα, IL6, and IL1b54–57. We have recently shown in a colitis-induced CRC mouse model that ERβ in intestinal epithelial cells can downregulate the TNFα/NFkB signaling37. Together, our data suggest that specific activation of intestinal ERβ may have a beneficial impact to modulate gut inflammation in both pathologies.
Obesity is a well-known risk factor for CRC, and HFD-induced obesity exacerbates colon tumor development in mice by enhancing colonic cell proliferation58. We reveal that the colon transcriptome, which responded to HFD-feeding in a sex-dependent manner, were particularly enriched for pathways involved in cell proliferation. HFD clearly increased and E2 treatment opposed the HFD-induced proliferation, both at the transcriptome and functional (Ki67) level, in males. While we saw a trend of HFD-increased proliferation in females, this was not significant and we assume that they may be protected through their already higher levels of endogenous estrogen. Previous in vitro results have demonstrated anti-proliferative effects by exogenously expressed ERβ in CRC cell lines59–61. In line with these data, we found a significant decrease in HFD-induced cell proliferation upon ERβ activation (DPN). These results demonstrate that ERβ can protect against the HFD-induced cell proliferation in males, and potentially undermine the promotion of colon tumor development.
The most unexpected finding was that key circadian clock genes were differentially expressed between the sexes in the colon, during both dietary conditions. The circadian clock regulates critical processes involved in inflammation and proliferation. The circadian rhythm has been shown to be dysregulated in both obesity and IBD62,63. HFD feeding is known to impact the circadian rhythm in the hypothalamus, liver and fat tissue, and to disrupt the eating behavior. However, timed HFD-feeding can reset the circadian metabolism and help prevent obesity and metabolic disorders64,65, indicating a major contribution of the circadian rhythm in the pathogenesis of the disease. The core circadian clock is regulated by different factors including sex hormones such as estrogen and there are evidences of sex-dependent regulation66. In breast cancer cell lines, ERα was shown to increase the protein and mRNA expression of Clock67, indicating the potential of estrogen receptors to regulate clock genes at the tissue level. However, the impact of HFD and estrogen on the circadian rhythm in the colon epithelium has not been investigated previously. Here we show that mice fed HFD, of both sexes, alter expression of key clock genes Npas2 and Bmal1, and that estrogen can modulate this expression. Estrogen is also known to impact the feeding behavior68. The food intake was not successfully monitored in this study, due to problems with the HFD breaking into powder, and we cannot exclude the possibility that some of the effects mediated by estrogen could be through decreased diet consumption. However, our mechanistic data demonstrated that ERβ could bind to cis-regulatory chromatin regions of both Npas2 and Bmal1. This suggests that ERβ acts locally in the colon and directly regulates these genes, and we could confirm this using our intestinal-specific ERβ knockout mice. The expression of colonic Bmal1, which was enhanced by selective-ERβ activation in females, was indeed significantly decreased upon colon epithelia ERβ knockout in female mice on a HFD. It is worth noting that ERα activation by PPT also regulated the expression of Npas2 and Bmal1 in the colon. Available data do not support significant expression of ERα in the colon51, and we therefore assume that these effects are systemic through its effect on the overall metabolism. However, we did notice a two-fold increase of colonic ERα mRNA levels in female colon upon HFD-feeding (data not shown), and we cannot completely exclude local effects of ERα in colon, possibly via immune cells. The ERβ-binding chromatin site by Bmal1 included an estrogen response element (ERE), which ERα should also be able to bind, if expressed. However, the Npas2 ERβ-binding site was indicated to be through tethering with JUN/AP-1. Since the two ERs differ in their protein interactions, because of less conserved AF1 and AF2 domains, this site is more likely to be specific for ERβ. It should be emphasized that although all mice were sacrificed at the same time of the day, this was not a circadian experiment, and we also did not monitor the estrous cycle of the females. Hence, while our results highlight the implication of ERβ in the regulation of core clock genes in the colon, further experiments investigating different zeitgeber times are needed to conclude its impact on their rhythmicity. Additionally, further studies are needed to investigate the impact of different estrous phases on the colon transcriptome, since our results in females presents the average response of diet, independent of the estrous cycle.
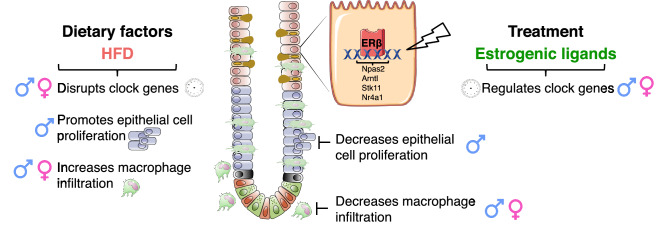
In conclusion, our data provide evidence that the colon microenvironment responds to HFD in both sexes, but with apparent sex differences, as illustrated in Fig. 5. Both sexes present an altered expression of core clock genes, although this was stronger in females, and increased macrophage infiltration. Males, on the other hand, showed significant effects on epithelial cell proliferation, increased by HFD and decreased through ERβ. All of the above functions play important roles in the pathogenesis of HFD-induced metabolic disorders and colitis. Entirely novel findings in our study demonstrate that estrogen signaling can partially modulate the colon microenvironment during HFD, and that core clock genes, colonic cell proliferation and macrophage infiltration can be modulated by estrogen via ERβ in this context, in a sex-dependent manner. Intestinal-specific ERβ knockout mice and ChIP-seq data demonstrate that ERβ in the colon is a regulator of both Bmal1 and Npas2. This study opens up new insights, where ERβ exhibits beneficial action against deleterious effects of diet-induced obesity on colon microenvironment.
Figure 5.
Schematic illustration; proposed model for estrogen regulation of the colon microenvironment during HFD-induced obesity. The HFD-induced impaired colonic circadian clock genes and increased cell proliferation was opposed by ERβ in males. The HFD-induced increase in macrophage infiltration was repressed by ERβ activation in both females and males.
Methods
Animal experiment and tissue collection
Five to six-week old male and non-ovariectomized female C57BL/6J mice were obtained from in-house breeding. Mice lacking ERβ specifically in the epithelial cells in the intestine were generated by crossing ERβflox/flox mice (B6.129X1- Esr2tm1Gust, Jan-Åke Gustafsson’s laboratory) with the transgenic mice bearing Cre recombinase expressed under the control of the enterocyte-specific Villin promoter (B6.SJL-Tg(Vil-cre)997 Gum/J; Jackson Laboratory, Bar Harbor, ME). Littermates (ERβflox/flox) lacking the Cre allele were used as controls (referred to as WT). The genotype was confirmed with standard PCR protocol. Animals were housed under controlled environment at 20 °C with a 12-h light–dark cycle. The animals were fed HFD (D12492, 60% kcal fat, Research Diet) or a control diet corresponding to a matched low-fat diet (D12450J, 10% kcal fat, Research Diet) and water was provided ad libitum. For the treatment with estrogenic ligands, at 10 weeks of diet the mice were injected i.p. every other day for a total of 9 injections with 0.0569 (N = 10 for males and 11 for females) or 0.5 mg/kg70 (N = 10 for males) BW for 17β-estradiol (E2, Sigma-Aldrich ), 2.5 mg/kg BW for 4,4′,4′'-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT, Tocris, N = 5 per group for both females and males)71, 5 mg/kg BW for 2,3-bis(4-Hydroxyphenyl)-propionitrile (DPN, Tocris, N = 10 for males and 9 for females)71, or vehicle. The ligands were prepared in a solution of 40% PEG400, 5% DMSO and 55% water. Mice were fasted for 6 h prior the measurement of the glucose level. Briefly, a small piece of the tail was cut with a scalpel, a drop of blood was removed, and glucose level immediately measured with a OneTouch Ultra glucometer (AccuChek Sensor, Roche Diagnostics). Mice were dissected and fat pads from the abdominal and posterior subcutaneous regions carefully removed and weighted. Total fat (TF) was calculated as the sum of all fat depots including gonadal fat, retroperitoneal fat, omental fat and inguinal/gluteal fat depots. Visceral adipose (VAT) comprised of the sum of gonadal fat, retroperitoneal fat and omental fat. The subcutaneous fat (SAT) comprised of the inguinal/gluteal fat depots. All experiments were performed in accordance with ARRIVAL guidelines and EU Directive 2010/63/EU, for the care and use of laboratory animals. The local ethical committee of the Swedish National Board of Animal Research (Stockholm ethical committee, N230/15) approved all experimental protocols. Colons were harvested, washed, measured and opened along the long axis. The colons were then either embedded in OCT (Tissue-tek, Sakura) and prepared for IHC analyses using the “swiss roll” method, or snap frozen in liquid nitrogen for further analysis.
ELISA
Blood was collected by cardiac puncture at sacrifice using EDTA-treated tubes and plasma was collected after centrifugation and stored at -80 for further analysis. Estradiol levels were measured in mouse plasma by mouse/rat estradiol kit (ES180S-100, Calbiotech) according to manufacturer’s instructions. Plasma samples were diluted 1:5 for vehicle treated CD and HFD and 1:12 for E2 treated mice with assay diluent. Insulin level was measured in plasma by mouse/rat Elisa kit (EZRMI-13K) according to manufacturer’s instructions with plasma samples dilution of 1:2. Calculations were performed using standard controls provided with the kit.
IHC
Colonic samples embedded in OCT were cryo-sectioned at 8 μm thickness. Sections were fixed in 4% formaldehyde for 10 min and endogenous peroxidase activity was blocked with 3% hydrogen peroxidase in 50% methanol and PBS for 30 min. Slides were blocked with 5% normal goat serum for 30 min at 4 °C and blocked for unspecific avidin/biotin binding (DAKO). The sections were incubated overnight at 4 °C with the primary antibodies, anti-F4/80+ (1:100, cat# MCA497R, lot# 1608, RRID: AB_323279, Bio-Rad), anti-Ki67 (1:100, SP6, cat# MA5-14520, lot# TC2542944A, RRID AB_10979488, Invitrogen) in 0.1% IGEPAL in PBS. Negative controls without primary antibodies were used for each slide. The sections were incubated with appropriate biotin conjugated anti IgG secondary antibodies (1:500) for 1 h at room temperature (RT), and then incubated with avidin–biotin complex (ABC, Thermofisher) for 1 h at RT. Sections were developed with Liquid DAB + (3,3-diaminobenzidine) Substrate Chromogen System (DAKO) and counterstained with Mayer’s Hematoxylin (Sigma-Aldrich). After dehydration in ethanol and xylene, the slides were cover-slipped using Pertex (Histolab). Ten to fifteen random images from three fully stained sections per animal were randomly taken using a BX53 light microscope and CAM-SC50 camera (Olympus). Positive stained cells per crypts were counted using QuPath and approximately 30 crypts per animal were analyzed.
RNA isolation and qPCR
Frozen colon distal tissue and colon epithelial layer were homogenized with a tissue lyser (Qiagen, Chatsworth, CA). Total RNA was isolated with QIAzol and purified using miRNeasy Mini Kit (Qiagen, Chatsworth, CA) according to the standard protocol and on-column DNAse treatment was applied. Quantitative and qualitative analyses of the RNA were performed with NanoDrop 1000 spectrophotometer and Agilent Tapestation 2200 (Agilent Technologies, Palo Alto, CA), respectively. All samples had RNA integrity > 6.5. One microgram RNA was reversed transcribed using iScript cDNA synthesis kit (Biorad) according to standard protocol. Ten nanogram of cDNA were used to perform qPCR in the CFX96 Touch System (Biorad), with iTaq universal SYBR Green supermix (Biorad) as recommended by the supplier. Samples were run in duplicates and the relative gene expression was calculated as the mean per group using the ΔΔCt method, normalized to the geometric mean of three reference genes (Actb, Eef2 and Tbp). Primer sequences are provided in supplementary table 1.
RNA-seq
RNA from colon tissue was prepared from 6 biological replicates, from male and female fed with CD and HFD, and males fed with HFD and treated with E2 (0.5 mg/kg BW). Library preparation (Illumina RiboZero) and sequencing (Illumina NovaSeq6000) was performed at Sweden’s National Genomics Infrastructure (NGI). At least 17 M paired-end reads (2 × 51 bp in length) were generated for each sample, and mapped against mouse genome (GRCm38) using STAR. FeatureCounts and StringTie were used to generate gene counts and TPM values. DESeq2 was used to calculate differentially expressed genes (DEG) with raw counts as input and the Benjamini–Hochberg procedure was used to estimate FDR. Genes were considered as significantly differentially expressed if p value < 0.05 and log2FC >|0.4|. Gene ontology/biological function was performed with DAVID bioinformatics website.
Statistical analysis
GraphPad Prism was used for statistical analysis (GraphPad Software Inc, La Jolla, CA). The results are presented as mean ± SEM. A (two-tailed) Welch's t-test was used for comparison between two groups. One-way analysis of variance (ANOVA) was used for comparison between multiple conditions followed by fisher's LSD test. Two-way ANOVA was used for comparison between multiple conditions in two different groups followed by fisher's LSD test. All conditions were compared to HFD vehicle and a p value < 0.05 was considered being statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001).
Supplementary information
Acknowledgements
We thank master student Srinidhi Rangarajan for assistance with qPCR, IHC, and quantification. The authors acknowledge support from the National Genomics Infrastructure (NGI) in Stockholm funded by Science for Life Laboratory, the Knut and Alice Wallenberg Foundation and the Swedish Research Council, and SNIC/Uppsala Multidisciplinary Center for Advanced Computational Science for assistance with massively parallel sequencing and access to the UPPMAX computational infrastructure. Additionally, Viktor Jonsson from NBIS/SciLifeLab advised on the RNA-seq analysis.
Author contributions
L.H. and A.A. designed the in vivo study; L.H., A.A., R.I., M.B., C.S. and M.K.-A. performed all the in vivo experiments; A.A. and M.B. performed the histology experiments; R.I. performed ChIP experiments; L.H., and A.A. performed the qPCR experiments; L.H. performed the RNA-seq analyses; L.H., A.A. and C.W. interpreted results; L.H., R.I., and A.A. prepared figures; L.H., A.A. and C.W wrote manuscript; M.K.-A. advised on the metabolic parameters; A.A. and C.W. initiated, coordinated the study and supervised. All authors discussed the results and approved final version of manuscript.
Funding
Open Access funding provided by Kungliga Tekniska Högskolan. This work was supported by the Swedish Cancer Society (CAN 2018/596), Swedish Research Council (2017-01658), Stockholm County Council (2017-0578), Karolinska Institutet PhD student support Grants (KID 2-3591/2014, 2-5586/2017) to CW and Novo Nordisk Foundation (NNF14OC0010705), Lisa and Johan Grönbergs Foundation (2019-00173) and AstraZeneca to MKA.
Data availability
Gene expression data are deposited in the NCBI Gene Expression Omnibus database [GSE149811].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: L. Hases and A. Archer.
Supplementary information
is available for this paper at 10.1038/s41598-020-73166-1.
References
- 1.Guh DP, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris KC, Kuramoto LK, Schulzer M, Retallack JE. Effect of school-based physical activity interventions on body mass index in children: a meta-analysis. CMAJ. 2009;180:719–726. doi: 10.1503/cmaj.080966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J. Gastroenterol. 2007;13:4199–4206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol. Biomarkers Prev. 2007;16:2533–2547. doi: 10.1158/1055-9965.Epi-07-0708. [DOI] [PubMed] [Google Scholar]
- 5.Loomans-Kropp HA, Umar A. Increasing incidence of colorectal cancer in young adults. J. Cancer Epidemiol. 2019;2019:9841295. doi: 10.1155/2019/9841295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietrzyk L, Torres A, Maciejewski R, Torres K. Obesity and obese-related chronic low-grade inflammation in promotion of colorectal cancer development. Asian Pac. J. Cancer Prev. 2015;16:4161–4168. doi: 10.7314/apjcp.2015.16.10.4161. [DOI] [PubMed] [Google Scholar]
- 7.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933–947. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- 8.Kawano Y, et al. Colonic pro-inflammatory macrophages cause insulin resistance in an intestinal Ccl2/Ccr2-dependent manner. Cell Metab. 2016;24:295–310. doi: 10.1016/j.cmet.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, et al. Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J. Nutr. Biochem. 2012;23:1207–1213. doi: 10.1016/j.jnutbio.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luck H, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–542. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Beyaz S, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53–58. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie Y, et al. Impact of a high-fat diet on intestinal stem cells and epithelial barrier function in middle-aged female mice. Mol. Med. Rep. 2020;21:1133–1144. doi: 10.3892/mmr.2020.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildebrandt MA, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serino M, et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut. 2012;61:543–553. doi: 10.1136/gutjnl-2011-301012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H, Giovannucci EL. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. 2017;28:1–4. doi: 10.1007/s10552-016-0831-5. [DOI] [PubMed] [Google Scholar]
- 18.Zheng D, et al. Sexual dimorphism in the incidence of human cancers. BMC Cancer. 2019;19:684. doi: 10.1186/s12885-019-5902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams C, DiLeo A, Niv Y, Gustafsson JA. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016;372:48–56. doi: 10.1016/j.canlet.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lobo RA. Hormone-replacement therapy: current thinking. Nat. Rev. Endocrinol. 2017;13:220–231. doi: 10.1038/nrendo.2016.164. [DOI] [PubMed] [Google Scholar]
- 21.Botteri E, et al. Menopausal hormone therapy and colorectal cancer: a linkage between nationwide registries in Norway. BMJ Open. 2017;7:e017639. doi: 10.1136/bmjopen-2017-017639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simin J, Tamimi R, Lagergren J, Adami HO, Brusselaers N. Menopausal hormone therapy and cancer risk: An overestimated risk? Eur. J. Cancer. 2017;84:60–68. doi: 10.1016/j.ejca.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Stachowiak G, Pertynski T, Pertynska-Marczewska M. Metabolic disorders in menopause. Prz Menopauzalny. 2015;14:59–64. doi: 10.5114/pm.2015.50000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salpeter SR, et al. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006;8:538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 25.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum. Reprod. Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 26.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yepuru M, et al. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J. Biol. Chem. 2010;285:31292–31303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Granillo M, et al. Selective estrogen receptor (ER)beta activation provokes a redistribution of fat mass and modifies hepatic triglyceride composition in obese male mice. Mol. Cell Endocrinol. 2019;502:110672. doi: 10.1016/j.mce.2019.110672. [DOI] [PubMed] [Google Scholar]
- 29.González-Granillo M, et al. ERβ activation in obesity improves whole body metabolism via adipose tissue function and enhanced mitochondria biogenesis. Mol. Cell Endocrinol. 2019;479:147–158. doi: 10.1016/j.mce.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Foryst-Ludwig A, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giroux V, Bernatchez G, Carrier JC. Chemopreventive effect of ERbeta-selective agonist on intestinal tumorigenesis in Apc(Min/+) mice. Mol. Carcinog. 2011;50:359–369. doi: 10.1002/mc.20719. [DOI] [PubMed] [Google Scholar]
- 32.Son HJ, et al. Effect of estradiol in an azoxymethane/dextran sulfate sodium-treated mouse model of colorectal cancer: implication for sex difference in colorectal cancer development. Cancer Res. Treat. 2019;51:632–648. doi: 10.4143/crt.2018.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song CH, et al. Effects of 17beta-estradiol on colonic permeability and inflammation in an azoxymethane/dextran sulfate sodium-induced colitis mouse model. Gut Liver. 2018;12:682–693. doi: 10.5009/gnl18221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song CH, et al. Effects of 17beta-estradiol on colorectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice. Biochem. Pharmacol. 2019;164:139–151. doi: 10.1016/j.bcp.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Saleiro D, et al. Estrogen receptor-beta protects against colitis-associated neoplasia in mice. Int. J. Cancer. 2012;131:2553–2561. doi: 10.1002/ijc.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giroux V, Lemay F, Bernatchez G, Robitaille Y, Carrier JC. Estrogen receptor beta deficiency enhances small intestinal tumorigenesis in ApcMin/+ mice. Int. J. Cancer. 2008;123:303–311. doi: 10.1002/ijc.23532. [DOI] [PubMed] [Google Scholar]
- 37.Hases L, et al. Intestinal estrogen receptor beta suppresses colon inflammation and tumorigenesis in both sexes. Cancer Lett. 2020;492:54–62. doi: 10.1016/j.canlet.2020.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE. 2012;7:e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Granillo M, et al. Sex-specific lipid molecular signatures in obesity-associated metabolic dysfunctions revealed by lipidomic characterization in ob/ob mouse. Biol. Sex Differ. 2019;10:11. doi: 10.1186/s13293-019-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hetemäki N, et al. Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J. Clin. Endocrinol. Metab. 2017;102:4588–4595. doi: 10.1210/jc.2017-01474. [DOI] [PubMed] [Google Scholar]
- 41.Dakin RS, Walker BR, Seckl JR, Hadoke PW, Drake AJ. Estrogens protect male mice from obesity complications and influence glucocorticoid metabolism. Int. J. Obes. (Lond.) 2015;39:1539–1547. doi: 10.1038/ijo.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riant E, et al. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 43.Andersson S, et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat. Commun. 2017;8:15840. doi: 10.1038/ncomms15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagel R, et al. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J. 2017;31:4707–4719. doi: 10.1096/fj.201700141RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyoko OO, et al. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS ONE. 2014;9:e98016. doi: 10.1371/journal.pone.0098016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deaver JA, Eum SY, Toborek M. Circadian disruption changes gut microbiome taxa and functional gene composition. Front. Microbiol. 2018;9:737. doi: 10.3389/fmicb.2018.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voigt RM, et al. Circadian disorganization alters intestinal microbiota. PLoS ONE. 2014;9:e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song CH, et al. Effects of 17β-estradiol on colorectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice. Biochem. Pharmacol. 2019;164:139–151. doi: 10.1016/j.bcp.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 49.McArthur S, et al. Definition of a novel pathway centered on lysophosphatidic acid to recruit monocytes during the resolution phase of tissue inflammation. J. Immunol. 2015;195:1139–1151. doi: 10.4049/jimmunol.1500733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamers AA, et al. Deficiency of nuclear receptor Nur77 aggravates mouse experimental colitis by increased NFkappaB activity in macrophages. PLoS ONE. 2015;10:e0133598. doi: 10.1371/journal.pone.0133598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uhlén M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 52.Uhlen M, et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science. 2019 doi: 10.1126/science.aax9198. [DOI] [PubMed] [Google Scholar]
- 53.Al-Ghadban S, Kaissi S, Homaidan FR, Naim HY, El-Sabban ME. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 2016;6:29783. doi: 10.1038/srep29783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gulhane M, et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016;6:28990. doi: 10.1038/srep28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 57.Reinecker HC, et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin. Exp. Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuominen I, et al. Diet-induced obesity promotes colon tumor development in azoxymethane-treated mice. PLoS ONE. 2013;8:e60939. doi: 10.1371/journal.pone.0060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edvardsson K, Strom A, Jonsson P, Gustafsson JA, Williams C. Estrogen receptor beta induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol. Endocrinol. 2011;25:969–979. doi: 10.1210/me.2010-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edvardsson K, et al. Estrogen receptor beta expression induces changes in the microRNA pool in human colon cancer cells. Carcinogenesis. 2013;34:1431–1441. doi: 10.1093/carcin/bgt067. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen-Vu T, et al. Estrogen receptor beta reduces colon cancer metastasis through a novel miR-205 - PROX1 mechanism. Oncotarget. 2016;7:42159–42171. doi: 10.18632/oncotarget.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sobolewska-Włodarczyk A, et al. Circadian rhythm abnormalities—association with the course of inflammatory bowel disease. Pharmacol. Rep. 2016;68:847–851. doi: 10.1016/j.pharep.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Froy O. Circadian rhythms and obesity in mammals. ISRN Obes. 2012;2012:437198. doi: 10.5402/2012/437198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohsaka A, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Sherman H, et al. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- 66.Yan L, Silver R. Neuroendocrine underpinnings of sex differences in circadian timing systems. J. Steroid Biochem. Mol. Biol. 2016;160:118–126. doi: 10.1016/j.jsbmb.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao L, et al. Induction of the CLOCK gene by E2-ERα signaling promotes the proliferation of breast cancer cells. PLoS ONE. 2014;9:e95878. doi: 10.1371/journal.pone.0095878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rivera HM, Stincic TL. Estradiol and the control of feeding behavior. Steroids. 2018;133:44–52. doi: 10.1016/j.steroids.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dragin N, et al. Balance between estrogens and proinflammatory cytokines regulates chemokine production involved in thymic germinal center formation. Sci. Rep. 2017;7:7970. doi: 10.1038/s41598-017-08631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim HJ, et al. Identification of estradiol/ERα-regulated genes in the mouse pituitary. J. Endocrinol. 2011;210:309–321. doi: 10.1530/joe-11-0098. [DOI] [PubMed] [Google Scholar]
- 71.Frasor J, et al. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) alpha activity by ERbeta in the uterus. Endocrinology. 2003;144:3159–3166. doi: 10.1210/en.2002-0143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gene expression data are deposited in the NCBI Gene Expression Omnibus database [GSE149811].