Abstract
Background & Aims
Colonoscopy is required following a positive fecal screening test for colorectal cancer (CRC). It remains unclear to what extent time to colonoscopy is associated with CRC-related outcomes. We performed a systematic review to elucidate this relationship.
Methods
An electronic search was performed through April 2020 for studies reporting associations between time from positive fecal testing to colonoscopy and outcomes including CRC incidence (primary outcome), CRC stage at diagnosis, and/or CRC-specific mortality. Our primary objective was to quantify these relationships following positive fecal immunochemical testing (FIT). Two authors independently performed screening, abstraction, and risk of bias assessments.
Results
From 1,612 initial studies, 8 were included in the systematic review, with 5 reporting outcomes for FIT. Although meta-analysis was not possible, consistent trends between longer time delays and worse outcomes were apparent in all studies. Colonoscopy performed beyond 9 months from positive FIT compared to within 1 month was significantly associated with a higher incidence of CRC, with adjusted odds ratios (AORs) of 1.75 and 1.48 in the two largest studies. These studies also reported significant associations between colonoscopy performed beyond 9 months and higher incidence of advanced stage CRC (stage III or IV) at diagnosis, with AORs of 2.79 and 1.55, respectively.
Conclusions
Colonoscopy for positive FIT should not be delayed beyond 9 months. Given the additional time required for urgent referrals and surgical planning for CRC, colonoscopy should ideally be performed well in advance of 9 months following a positive FIT.
Keywords: Colorectal Neoplasms, Mass Screening, Colonoscopy
Abbreviations used in this paper: AOR, adjusted odds ratio; COVID-19, coronavirus disease 2019; CRC, colorectal cancer; FIT, fecal immunochemical test; FOBT, fecal occult blood test; NOS, Newcastle-Ottawa Scale
Colorectal cancer (CRC) is a leading global cause of cancer-related mortality. In 2017, there were approximately 1.7 million incident cases and nearly 900,000 deaths attributable to CRC worldwide.1 Screening for CRC reduces the incidence of and mortality from CRC2 and is widely recommended in high-resource countries.3 Tests designed to detect occult blood in stool shed by cancers and precursor polyps are widely used for primary screening, including fecal immunochemical tests (FITs) and guaiac-based fecal occult blood tests (FOBTs).4 Five-year survival from CRC is largely determined by stage at diagnosis, ranging from approximately 90% for localized disease to <15% once metastatic.5 Therefore, in cases in which these initial screening tests are positive, timely colonoscopy is recommended to rule out the presence of cancer, detect it at an earlier stage, and remove polyps in an effort to ultimately lower the risk of subsequent colorectal neoplasia and death from CRC.2
Adherence to best practice timelines in healthcare can be impacted by a number of patient-, physician-, and system-related factors. Limited health care budgets create persistent challenges for all jurisdictions and in particular, single-payer health care systems. On the other end of the spectrum, unique events such as the coronavirus disease 2019 (COVID-19) pandemic can cause major time delays for all nonurgent care due to interruptions in health service supply or demand from suitable patients. Health care policymakers, physicians, and patients must understand the implications of delays in care. Furthermore, when health jurisdictions are required to cease or resume usual clinical practice following a shutdown, it is imperative that access be prioritized based on evidence-based health outcomes.
Studies have previously examined the relationship between time to colonoscopy following positive FIT or FOBT and CRC-related outcomes. However, a synthesis of the published literature on this important and timely topic has yet to be performed. As such, we performed a systematic review to assess the association between time interval from positive fecal testing to completion of colonoscopy and CRC outcomes.
Materials and Methods
Overview and Objectives
A systematic review adhering to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Supplementary Table 1) was conducted.6 Our primary objective was to determine whether longer time intervals from a positive FIT to colonoscopy were associated with a higher incidence of CRC, more advanced stage of CRC at diagnosis, or overall or CRC-specific mortality. In addition, the effect of time from FOBT to colonoscopy and these outcomes was assessed as a secondary study objective.
Search Strategy and Study Selection
An electronic search strategy was devised by a health research librarian (R.S.) with input from clinicians to guide relevant terminology. A full literature search of the databases EMBASE, Google Scholar, MEDLINE, and CENTRAL (Cochrane Central Registry of Controlled Trials) was performed from inception of the databases to April 23, 2020. Inclusion of conference abstracts was restricted from January 1, 2017, onward. The detailed search strategy is provided in the Supplementary Materials. The reference sections of any relevant articles were also reviewed to identify potential additional citations. Two reviewers (N.F., S.J.H.) independently screened all titles and abstracts in parallel to identify citations to be included in the full-text review stage. All included citations then underwent full-text review by the same 2 reviewers in parallel (N.F., S.J.H.). Discrepancies from either stage were resolved by consensus by including a third reviewer (R.J.H.).
Eligibility Criteria
We included a study if it met all of the following criteria: (1) it was an observational study (prospective or retrospective) or clinical trial, (2) it included data from patients having received a positive result from either FIT or FOBT, (3) it reported on 1 or more of the following outcomes of interest (CRC diagnosis at colonoscopy or beyond, stage of CRC at diagnosis, overall or CRC-specific mortality), and (4) outcomes data were separated by time from initial fecal test to colonoscopy. A study was excluded if it was a modeling study or a systematic or narrative review. However, the reference sections of such publications were also reviewed to identify potential citations.
Data Extraction and Study Quality
A data abstraction form was created to capture data from each included study, Following the final full-text review stage, 2 reviewers (N.F., S.J.H.) abstracted study data in parallel. Study-specific risk-of-bias assessments according to the Newcastle-Ottawa Scale (NOS)7 were also scored in parallel by 2 reviewers (N.F., M.M.), with discrepancies resolved by a third reviewer (S.J.H.).
Outcomes and Analyses
Our primary outcome was CRC incidence. Secondary outcomes included CRC stage at diagnosis and overall or CRC-specific mortality. Outcomes from different studies were divided into respective groups and presented in tables where data could be considered according to time elapsed cutoffs from positive fecal screening test to colonoscopy. Given an inability to pool results via a formal meta-analysis, nonweighted curves of adjusted odds ratios (AORs) of outcomes over time were created for each study reporting on specific outcomes. For these plots, median times to colonoscopy were set as midpoints between time cutoffs, or 3 months after any open-ended final time cutoffs, if not explicitly provided in study data. Sensitivity analyses were also performed, in which data were considered separately according to the following methodologic or clinically relevant distinctions: (1) studies in which fecal testing was used along with formalized patient navigation to colonoscopy (deemed “programmatic” screening) vs with no patient navigation process (deemed “opportunistic” screening) and (2) studies in which ≥20% of eligible patients were not followed up with colonoscopy after their initial positive fecal test vs <20%.
Results
Study Selection
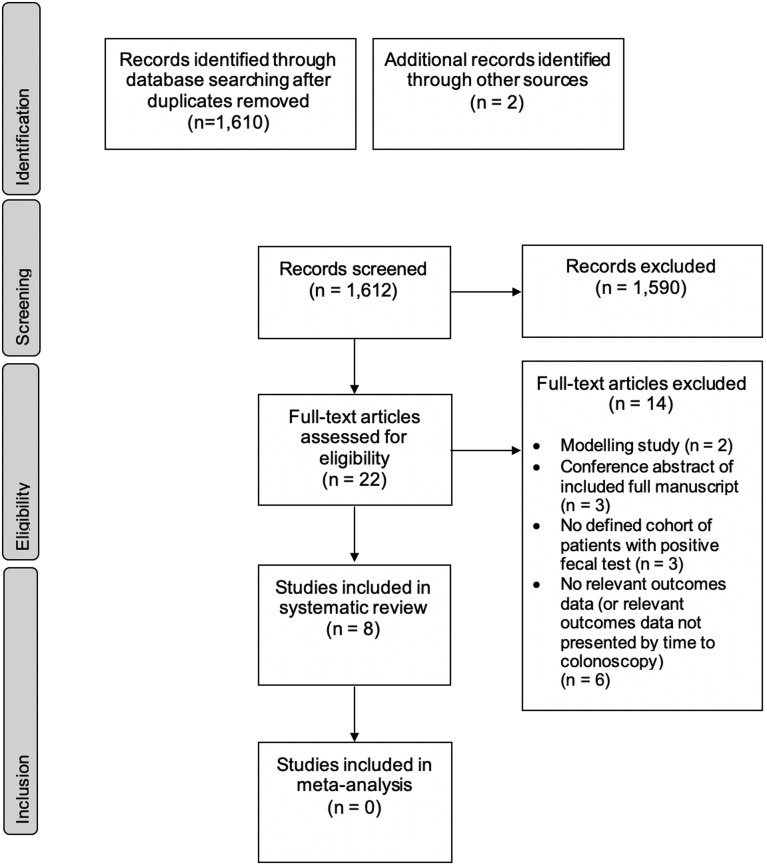
A PRISMA flowchart of the search results and study selection process is provided in Figure 1 .6 A total of 1610 citations were identified by the initial electronic database search after removing duplicates, with 2 additional citations identified via manual review of bibliographies from selected studies and informal searches. Of these, 22 papers were selected for full-text review following the initial title and abstract screen. Two modeling studies were reviewed but excluded.8 , 9 Eight studies were ultimately included in the final systematic review of primary or secondary outcomes, with 5 of these reporting outcomes based on time from positive FIT to colonoscopy. The remaining 3 studies reported on time from positive FOBT to colonoscopy.10, 11, 12 Detailed results relating to FOBT are presented in the Supplementary Table 3, while the results reported in the following subsections pertain to FIT.
Figure 1.
PRISMA flow diagram outlining study selection process.6
Study Characteristics and Quality
Baseline characteristics of studies included in the systematic review for FIT are presented in Table 1 . All included studies were in the form of fully published manuscripts. All 5 studies were observational. Included studies were published between 2017 and 2020. The baseline time comparators for patients to receive colonoscopy following FIT ranged from within 1 month to within 3 months, with 1 study excluding colonoscopies performed within 7 days13 and another excluding colonoscopies performed within 30 days,14 both in an attempt to eliminate procedures performed in an expedited fashion for heightened suspicion of CRC. Meta-analysis was not performed given the limited numbers of studies that would be comparable within each time cutoff. Summaries of study quality using the NOS are provided in Table 1, with full assessments provided in the Supplementary Table 4.7 Study quality was moderate to high overall, as per the NOS. The most common sources of potential bias were (1) calculation of CRC incidence during the index colonoscopy only, as opposed to an incidence calculation using a period of months or longer (to account for incomplete or poorly tolerated colonoscopies, or those with poor cleansing), and (2) relatively high rates of patients who did not undergo colonoscopy after positive fecal testing (or with incomplete or absent colonoscopy information), ranging from 8% to 38%. These aspects contributed to assessments concluding suboptimal length and/or adequacy of follow-up.
Table 1.
Summary of Baseline Characteristics of FIT Studies Included in the Systematic Review
| Author, Year | Country/Countries | Study Design | Number and Type of Patients (Screening Model) | Patient Exclusions or Model Employed | FIT or FOBT Parameters | Proportion of Patients Not Receiving Colonoscopy (%) | Study Quality7 |
|---|---|---|---|---|---|---|---|
| Corley, 201713 | USA | Observational | 50–75 y 1,258,039 screened, 70,124 included (opportunistic) |
Those with: prior history of CRC; no record of colonoscopy during <1 y of membership after FIT screening; >3-mo gap in membership after screening; <1 y of membership prior to screening; colonoscopy within 10 y or sigmoidoscopy within 5 y before screening; colonoscopy or CRC diagnosis 1–7 d after positive FIT. | OC FIT-CHEK/ OC-Sensor Diana (Polymedco, Cortlandt, NY) Cutoff 20 ug/g (100 ng/mL) |
14.0 | NOS 9 |
| Kaalby, 201916 | Denmark | Observational | 50–74 y 899,411 screened, 53,171 included (programmatic) |
Those with: lack of colonoscopy findings reported; incomplete colonoscopy and lack of follow-up. | OC Sensor (Eiken Chemical, Tokyo, Japan) Cutoff 20 ug/g (100 ng/mL) |
8.3 | NOS 7 |
| Kim, 201917 | South Korea | Observational | 50 y and over 52,376 screened, 2362 included (programmatic) |
Those with: history of CRC or colorectal surgery; history of inflammatory bowel disease; poor bowel preparation. | OC Sensor Diana (Eiken Chemical) Cutoff 20 ug/g (100 ng/mL) |
26.9 | NOS 6 |
| Lee, 201914 | Taiwan | Observational | 50–69 y 2,914,855 screened, 39,346 included (programmatic) |
Those with: no or suboptimal diagnostic examination performed (including sigmoidoscopy and double-contrast barium enema; colonoscopy within 2 y before FIT; colonoscopy within 1 mo after positive FIT results. | OC Sensor (Eiken Chemical or Kyowa Medex [Tokyo, Japan]) Cutoff 20 ug/g (100 ng/mL) |
40.9 | NOS 6 |
| Zorzi, 202015 | Italy | Observational | 50–69 y 3,427,934 screened, 123,138 included (programmatic) |
N/R | OC Sensor (Eiken Chemical) Cutoff 20 ug/g (100 ng/mL) |
20.2 | NOS 6 |
CRC, colorectal cancer; FIT, fecal immunochemical test; FOBT, fecal occult blood test; N/R, not reported; NOS, Newcastle-Ottawa Scale.
CRC Incidence
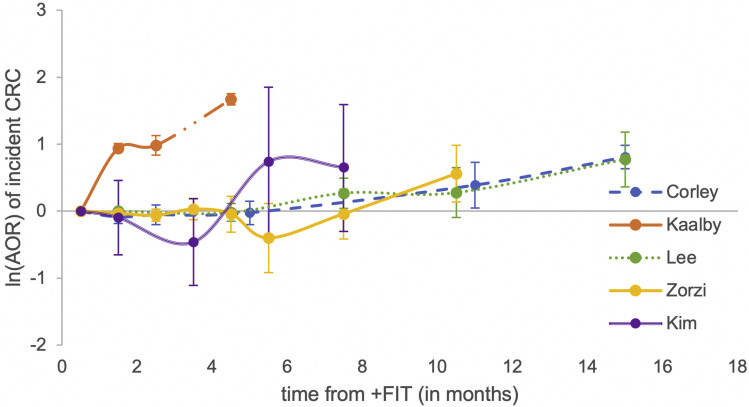
Most studies reported CRC detection at the time of colonoscopy, although 1 study reported cumulative incidence within the 6-month period following colonoscopy.13 Baseline CRC incidence (calculated from the earliest possible reference time from positive fecal test to colonoscopy) ranged from 30 to 50 per 1000 persons. There were significant increases in CRC incidence in patients undergoing colonoscopy at 12 months or later following the initial positive FIT compared with the baseline time cutoff, with incidences ranging from 76 to 98 per 1000 persons. AORs of CRC incidence associated with comparisons of colonoscopy at ≥12 months compared with the baseline time period ranged from 2.17 to 2.25. The 2 largest observational studies including 123,138 and 70,124 patients also found significant associations with colonoscopy at over 9 months compared with within 1 month, with AORs of and 1.75 and 1.48.13 , 15 Though 1 study reported significantly higher CRC incidence when colonoscopy was performed within 3 months compared with within 1 month (AOR, 2.68; 95% confidence interval, 2.31–3.10),16 2 other studies assessing this comparison observed no significant difference.13 , 17 Detailed associations between time to colonoscopy and CRC incidence are presented in Table 2 . An unweighted graphical representation of these associations by study is provided in Figure 2 .
Table 2.
Comparisons and Outcomes From FIT Studies Included in the Systematic Review
| Author, Year | Comparator Time From FIT | Alternate Time Cutoffs | Outcomes | Detailed Results | Summary |
|---|---|---|---|---|---|
| Corley, 201713 | Within 1 mo (excluding within 1–7 d) | Within 2, 3, 4–6, 7–9, 10–12, >12 mo | CRC incidence (within 6 mo of colonoscopy) CRC stage Advanced adenoma(s) |
|
Delays to colonoscopy of over 9 mo after positive FIT was significantly associated with higher CRC incidence and more advanced stage at diagnosis (compared with performing colonoscopy within 1 mo). |
| Kaalby, 201916 | Within 1 mo | Within 2, 3, >3 mo | CRC incidence (at colonoscopy) CRC stage Advanced adenoma(s) |
|
Delays to colonoscopy of 2 mo or more after positive FIT was significantly associated with higher incidence of CRC, more advanced stage at CRC diagnosis, and more advanced adenomas (compared with performing colonoscopy within 1 mo). |
| Kim, 201917 | Within 1 mo | Within 2, 3–5, 6, >6 mo | CRC incidence (at colonoscopy) CRC stage Advanced adenoma(s) |
|
Delays to colonoscopy of 6 mo or more after positive FIT was associated with a nonsignificant trend towards higher incidence of CRC and advanced adenomas combined (compared with performing colonoscopy within 1 mo). This study was limited by sample size. |
| Lee, 201914 | Within 3 mo (excluding within 30 d) | 4–6, 7–9, 10–12, >12 mo | CRC incidence (at colonoscopy) CRC stage Advanced adenoma(s) |
|
Delays to colonoscopy of 12 mo or more after positive FIT was significantly associated with higher CRC incidence (compared with performing colonoscopy within 3 mo). However, more advanced CRC stage at diagnosis was observed after 6 mo. |
| Zorzi, 202015 | Within 1 mo | Within 2, 3, 4, 5, 6, 7–9, >9 mo | CRC incidence (at colonoscopy) CRC stage Advanced adenoma(s) |
|
Delays to colonoscopy of over 9 mo after positive FIT were significantly associated with higher incidence of CRC and advanced stage of CRC at diagnosis (compared with performing colonoscopy within 1 mo). |
AOR, adjusted odds ratio; CI, confidence interval; CRC, colorectal cancer; FIT, fecal immunochemical test.
Figure 2.
Nonweighted graphical representation of associations between time to colonoscopy and incidence of colorectal cancer between FIT studies. AORs are not directly comparable between studies given differences in reference populations.
CRC Stage and CRC-Specific Mortality
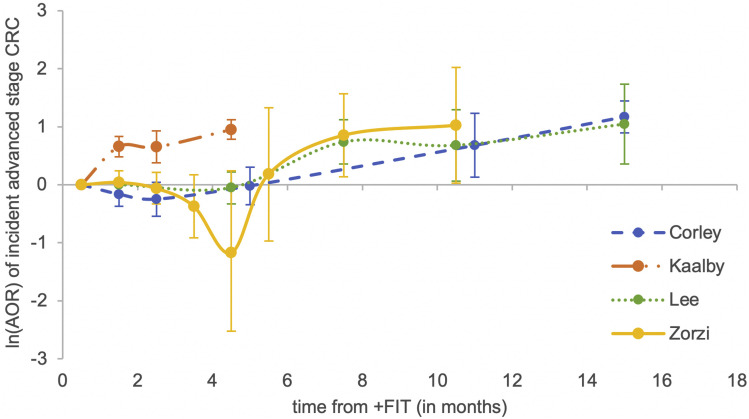
Advanced stage CRC was defined as stage III or stage IV carcinoma as per the American Joint Committee on Cancer Staging Manual.18 The incidence of advanced stage CRC at index colonoscopy was 4–15 per 1000 patients when performed within 1 month of a positive fecal test. Though 1 study found no significant increase in advanced stage CRC when colonoscopy was performed at >6 months compared with within 1 month,17 all others found significant associations between time to colonoscopy of 12 or more months and higher incidence of advanced stage CRC, with AORs ranging from 2.11 to 3.22. The 2 largest included studies also found significant associations between colonoscopy performed at >9 months vs within 1 month, with AORs of 2.79 and 1.55.13 , 15 Another study found significant associations between colonoscopy within or after 3 months and higher incidence of advanced stage CRC, compared with within 1 month (AORs, 1.92 and 2.59).16 Detailed associations between time to colonoscopy and advanced CRC stage are found in Table 2. An unweighted graphical representation of these associations by study is provided in Figure 3 . Only 1 study assessed CRC-specific mortality, reporting a significantly worse value with colonoscopy after 12 months (hazard ratio, 1.53; 95% confidence interval, 1.13–2.10).11
Figure 3.
Nonweighted graphical representation of associations between time to colonoscopy and incidence of advanced stage (stage III or IV) colorectal cancer between FIT studies. AORs are not directly comparable between studies given differences in reference populations.
Sensitivity Analyses
When considering programmatic vs opportunistic screening approaches between studies, it became clear that no study included patients screened from a purely opportunistic approach. The closest, the cohort in the study by Corley et al,13 did not undergo a formalized patient navigation process, and the results were comparable to those from the other studies in terms of all outcomes. Finally, noncompliance with follow-up colonoscopy ranged from 8.3% to 37.6% in included studies. Compared with studies reporting ≥20% noncompliance rates, there was no observable difference in outcomes or trends in studies reporting <20% noncompliance with colonoscopy.
Discussion
In this systematic review, we observed clear associations between longer time delay to colonoscopy after positive fecal-based CRC screening testing and increased incidence of CRC, more advanced cancer stage at diagnosis, and higher CRC-specific mortality. An understanding of these findings is crucial for primary care physicians, endoscopists, administrators of endoscopy units, and CRC program planners. While we have qualitatively summarized these important temporal trends, the more challenging task is to translate them into firm recommendations regarding an acceptable delay to colonoscopy following positive fecal testing.
A number of factors can result in potential delays to colonoscopy following positive fecal testing. These can be broadly divided into patient-, physician-, and system-related factors. Collectively, these contribute to wide variations in time intervals between positive FIT (or FOBT) and colonic evaluation. On the one hand, patient-related factors include issues with compliance,19 and socioeconomic influences,20 among other considerations. Physician-related factors can also result in delays to referral and workup, including inappropriate usage of stool-based CRC screening tests21 and premature endoscopic surveillance recommendations,22 both of which can lead to reduced resources for higher-risk patients in need. Many of these contributors to unnecessary delays are potentially modifiable, such as through educating patients on the importance of screening adherence and follow-up,23 or by informing primary care physicians on appropriate FIT and FOBT use.21 , 24 Patient-centered pathways can also support the timely performance of procedures.25
System-related factors, on the other hand, are most often beyond the control of patients, referring physicians, or endoscopists. These frequently involve limitations in the capacity of endoscopic resources, which vary between single-payer and multipayer systems. However, other extrinsic system-based factors can also create unexpected delays to colonoscopy, as is evident by the current COVID-19 pandemic having halted nonurgent endoscopy in an unprecedented and widespread manner across the world.26 In situations such as these, in which endoscopy resources are temporarily or permanently strained, it is crucial for referring physicians, endoscopists, and policymakers to have a clear plan on how to manage inevitable backlogs of FIT- or FOBT-positive patients waiting for colonoscopy. Although yet to be demonstrated, it is possible that systems employing patient-centered pathways25 may navigate the backlog more efficiently. Multiple societies and experts have issued guidance on the triaging or resumption of nonurgent endoscopic services,27 , 28 including expediting FIT-positive patients,29 an important endeavor to which we now provide evidence-based context. The burden of the COVID-19 pandemic has been overwhelming around the globe; hence, the measures taken by many countries to protect their health care systems are understandable. At the same time, we must not lose sight of the “bigger picture,” recognizing that many other important diseases continue to affect patients irrespective of the pandemic. Our findings underscoring the importance of time to colonoscopy are especially relevant today but will remain significant long after the pandemic is over.
Although we were unable to pool data from the studies in this systematic review, several important conclusions can be derived from our work. First, time matters; this has been consistently shown across the evidence base that we have summarized. Based on the data from the 2 largest studies,13 , 15 patients with positive FIT have a higher risk of both incident CRC and advanced CRC when colonoscopy is delayed beyond 9 months from their initial screening test. Though it is intuitive that longer delays to colonoscopy should be associated with worse outcomes, this relatively short time frame may be surprising from a purely biologic perspective, given the established and typically lengthy adenoma to carcinoma sequence.30 However, considering that FIT positivity predicts the presence of advanced adenoma(s) or CRC in up to 54% and 8% of patients, respectively,4 it is understandable that the impact of delays is more pronounced in these higher-risk patients.31 Therefore, in the absence of additional data to guide the field otherwise, time from positive fecal test to colonoscopy should not exceed 9 months. When faced with extenuating circumstances such as a hospital admission for comorbidities, patients can be considered for other forms of full colonic examination such as computed tomography colonography on an individualized basis.
While a 9-month time frame is supported by evidence, patients diagnosed with CRC at colonoscopy who are suitable for potential curative treatment must also complete additional investigations, be referred for surgery, and undergo resection when appropriate, all of which take additional time.32 In addition, a primary aim of CRC screening is to identify earlier stage cancers and to intervene before the disease advances in stage. Furthermore, associations between time to colonoscopy and worse CRC outcomes were observed far earlier than 9 months in 1 included study.16 It is not entirely clear why this more pronounced effect of time was observed in this study, but possibilities could include the influence of a more comorbid or screening-naïve population.16 Given these considerations, we propose that wherever possible, colonoscopy should not be delayed beyond 6 months of positive fecal testing as an aspirational target (with 9 months as an upper limit). However, in situations in which resources remain pressured, future risk prediction models may offer the potential to select those most in need of urgent colonoscopy. Alternatively, adjustments in FIT thresholds could represent another mechanism to ensure timely access to colonoscopy by matching supply and demand.17
Our review has several limitations, primarily the result of the study designs and available data presented in the included studies. All of the input studies were observational, and consequently could not account for unmeasured confounders. For instance, 1 study reported an AOR of CRC of 2.7 when colonoscopy was performed at 3 months compared with within 1 month.16 Given the dubious biological relationship implicit in this association, this is instead likely representative of important methodological limitations with this input study. Furthermore, only 1 study adjusted for or excluded patients with signs or symptoms suggestive of CRC.13 Thus, future studies should aim to adjust for these factors. As an added example, patient-related factors associated with time delays including higher comorbidity20 could also have been associated with the outcomes of interest. In addition, attrition rates—in this case, those never completing colonoscopy despite a positive fecal test—were relatively high. These patients were excluded from all analyses, and therefore, the CRC rates in these patients are unknown. If patients undergoing colonoscopy in the open-ended upper time categories were more likely to have CRC (eg, due to the presence of symptoms) than those who never underwent a colonoscopy (possibly due to a lack of symptoms), the CRC outcomes would be an overestimate, and therefore biased. As mentioned, most studies reported an open-ended upper range of time to colonoscopy. For instance, if time to colonoscopy was >12 months, a patient having waited 13 months would be included alongside a patient having waited 3 years. Accordingly, we had to assume a median time to colonoscopy in such situations if not provided with a value in the study’s results. Therefore, interpretation of data from these open-ended time cutoffs should be performed with caution. Additionally, we performed a sensitivity analysis comparing the results from studies with less than or greater than 20% noncompliance to follow-up. While this did not yield any additional findings, it should be noted that given the low positive CRC rate at colonoscopy, even a much lower nonadherence rate could result in significant biases in results. Finally, we were ultimately unable to perform a meta-analysis of pooled data as a result of the limited number of studies included in our review and owing to differences in time cutoffs between the studies. Therefore, future research is still needed in this area, and researchers performing this important work should strive to adhere to similar time cutoffs.
In conclusion, our study demonstrates clear associations between time from positive fecal screening test to colonoscopy and worse CRC outcomes. Further research is urgently needed to elucidate the optimal time frame within which those with positive fecal testing should undergo colonoscopy. However, it is clear even now that incident CRC and advanced stage CRC are both higher beyond 9 months. Thus, it is incumbent on practitioners and the health system to support completion of colonoscopy well in advance of this time point.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.09.048.
Supplementary File 1. Detailed Search Strategy
Database: Embase Classic+Embase <1947 to 2020 April 23>, Ovid MEDLINE(R) ALL <1946 to April 23, 2020> Search Strategy:------------------
-
1
exp Colorectal Neoplasms/bl, di (32474)
-
2
((colorectal∗ or CRC or colon∗ or bowel∗ or rectal or rectum or sigmoid or anal or anus) adj2 (cancer or neoplasm∗ or tumor∗ or tumour or carcinom∗ or sarcom∗ or adenocarcinom∗ or adeno?carcinom∗ or adenom∗ or lesion∗) adj3 (screen∗ or diagnosis)).tw. (33413)
-
3
((colorectal∗ or CRC or colon∗ or bowel∗ or rectal or rectum or sigmoid or anal or anus) and (cancer or neoplasm∗ or tumor∗ or tumour or carcinom∗ or sarcom∗ or adenocarcinom∗ or adeno?carcinom∗ or adenom∗ or lesion∗) and (screen∗ or diagnosis)).kf. (2719)
-
4
Occult Blood/ and feces/ (1423)
-
5
((f?ece∗ or f?ecal) adj3 (immunochemic∗ or blood)).tw. (19215)
-
6
((f?ece∗ or f?ecal) and (immunochemic∗ or blood)).kf. (788)
-
7
(gFOBT or FOBT or FOB or haemoccult or hemoccult or sensa or heamoccultsensa or hemocare or hema screen or hemascreen or hemacheck or hema check or hemawipe or hema wipe or hemofec or hemofecia or fecatest or fecatwin or coloscreen or seracult or ez?detect or colocare or flexsure or hemmoquant or immocare or hemochaser or bayer detect or hemeselect or immudia or monohaem or insure or hemodia or instant?view or immocare or magstream or guaiac).tw,kw. (17627)
-
8
or/1–7 (84049)
-
9
Colonoscopy/ or Colonoscop∗.tw,kw. (127896)
-
10
8 and 9 (23267)
-
11
Time Factors/ (1198728)
-
12
(time adj3 (diagnosis or factor∗ or wait or delay∗)).tw. (205915)
-
13
time.ti,kf. (559679)
-
14
Delayed Diagnosis/ (18587)
-
15
or/11-14 (1888109)
-
16
10 and 15 (1322)
-
17
Occult Blood/ and feces/ (1423)
-
18
((f?ece∗ or f?ecal) adj3 (immunochemic∗ or blood)).tw. (19215)
-
19
((f?ece∗ or f?ecal) and (immunochemic∗ or blood)).kf. (788)
-
20
(gFOBT or FOBT or FOB or haemoccult or hemoccult or sensa or heamoccultsensa or hemocare or hema screen or hemascreen or hemacheck or hema check or hemawipe or hema wipe or hemofec or hemofecia or fecatest or fecatwin or coloscreen or seracult or ez?detect or colocare or flexsure or hemmoquant or immocare or hemochaser or bayer detect or hemeselect or immudia or monohaem or insure or hemodia or instant?view or immocare or magstream or guaiac).tw,kw. (17627)
-
21
17 or 18 or 19 or 20 (32796)
-
22
Colonoscopy/ or Colonoscop∗.tw,kw. (127896)
-
23
21 and 22 (8372)
-
24
positiv∗.tw,kw. (4209560)
-
25
23 and 24 (4411)
-
26
follow up.mp. (3296612)
-
27
25 and 26 (912)
-
28
16 or 27 (2165)
-
29
28 use medall (1177) Medline
-
30
exp colon cancer/di [Diagnosis] (37277)
-
31
rectum cancer/di [Diagnosis] (7824)
-
32
((colorectal∗ or CRC or colon∗ or bowel∗ or rectal or rectum or sigmoid or anal or anus) adj2 (cancer or neoplasm∗ or tumor∗ or tumour or carcinom∗ or sarcom∗ or adenocarcinom∗ or adeno?carcinom∗ or adenom∗ or lesion∗) adj3 (screen∗ or diagnosis)).tw. (33413)
-
33
colorectal cancer/ and cancer screening/ (18656)
-
34
occult blood test/ or occult blood/ (19075)
-
35
((f?ece∗ or f?ecal) adj3 (immunochemic∗ or blood)).tw. (19215)
-
36
(gFOBT or FOBT or FOB or haemoccult or hemoccult or sensa or heamoccultsensa or hemocare or hema screen or hemascreen or hemacheck or hema check or hemawipe or hema wipe or hemofec or hemofecia or fecatest or fecatwin or coloscreen or seracult or ez?detect or colocare or flexsure or hemmoquant or immocare or hemochaser or bayer detect or hemeselect or immudia or monohaem or insure or hemodia or instant?view or immocare or magstream or guaiac).tw. (17499)
-
37
30 or 31 or 32 or 33 or 34 or 35 or 36 (102515)
-
38
colonoscopy/ (104116)
-
39
colonoscop∗.tw. (87108)
-
40
38 or 39 (127032)
-
41
37 and 40 (28803)
-
42
time factor/ (1212516)
-
43
(time adj3 (diagnosis or factor∗ or wait or delay∗)).tw. (205915)
-
44
time.ti. (531099)
-
45
∗follow up/ or follow up.ti. (241112)
-
46
delayed diagnosis/ (18587)
-
47
or/43-46 (968755)
-
48
41 and 47 (1190)
-
49
conference abstract.pt. (3752439)
-
50
48 and 49 (260)
-
51
limit 50 to yr="2017 -Current" (71)
-
52
48 not 49 (930)
-
53
51 or 52 (1001)
-
54
53 use emczd (666) Embase
-
55
29 or 54 (1843)
-
56
remove duplicates from 55 (1610)
-
57
56 use medall (1174)
-
58
56 use emczd (436)
Supplementary Material
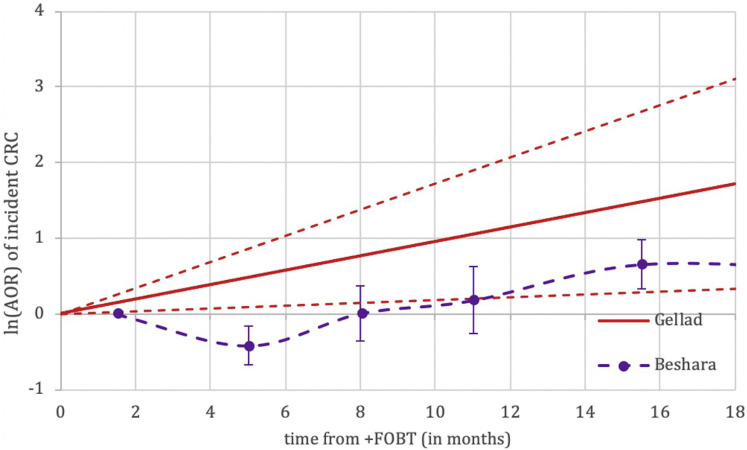
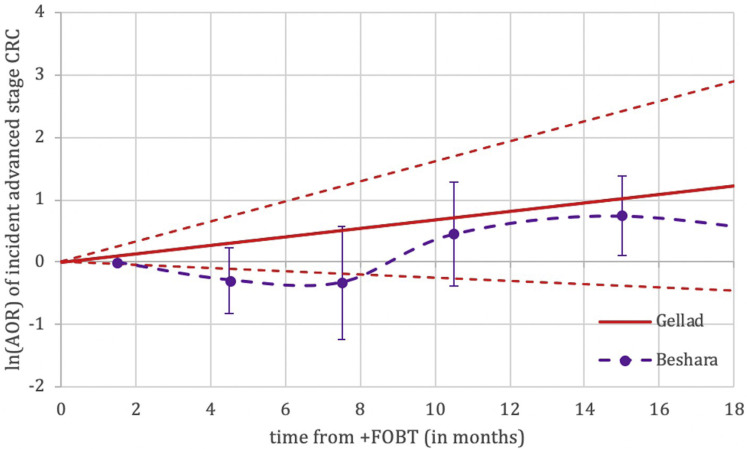
Supplementary Figure 1.
Nonweighted graphical representation of associations between time to colonoscopy and incidence of colorectal cancer between fecal occult blood test (FOBT) studies. Adjusted odds ratios (AORs) not directly comparable between studies given differences in reference populations. CRC, colorectal cancer.
Supplementary Figure 2.
Nonweighted graphical representation of associations between time to colonoscopy and incidence of advanced stage (Stage III or IV) colorectal cancer between FOBT studies. AORs not directly comparable between studies given differences in reference populations.
Supplementary Table 1.
PRISMA Checklist12
| Section/Topic | # | Checklist item | Page Reported on |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | Title page |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 3–4 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 5–6 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 6–7 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | N/A |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 7–8 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 7 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | Supp Mat |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 7 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 8 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | Tables |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 8 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 8–9 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 8–9 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 10, Table 1, Supp Mat |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 8–9 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 9, Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 9–10, Tables 1 and 2 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 10, Table 1, Supp Mat |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 10–12, Tables 1 and 2 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | Figures 2 and 3 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 10, Table 1, Supp Mat |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 12–13 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 13–16 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 16–17 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 17 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | Title Page |
N/A, ▪▪▪.
Supplementary Table 2.
Summary of Baseline Characteristics of FOBT Studies Included in the Systematic Review
| Author, Year | Country/Countries | Study Design | Number and Type of Patients (Screening Model) | Patient Exclusions or Model Employed | FIT or FOBT Parameters | Proportion of Patients Not Receiving Colonoscopy (%) | Study Quality7 |
|---|---|---|---|---|---|---|---|
| Gellad, 200942 | USA | Observational | 45 y and over 231 included (opportunistic) |
Those with: FOBT sent for indications other than CRC screening; no colonoscopy within 18 mo of FOBT; unavailable colonoscopy pathology results. | Hemoccult SENSA (Beckman Coulter, Fullerton, CA) 2 tests each from 3 stool samples |
50.024 | NOS 6 |
| Flugelman, 201918 | Israel | Observational (CRC cases only) | 50–74 y 740,259 screened, 1749 included (all CRC cases) (opportunistic) |
Those with known anemia prior to FOBT. | Hemoccult SENSA (Beckman Coulter) 2 tests each from 3 stool samples |
N/R | NOS 8 |
| Beshara, 202017 | Israel | Observational | 50–74 y 17,958 included (opportunistic) |
Those with: no colonoscopy after positive FOBT within 24 mo; not belonging to the health service continuously from 5 y before FOBT to 24 mo after FOBT; prior CRC. | Hemoccult SENSA (Beckman Coulter) 2 tests each from 3 stool samples |
30.7 | NOS 8 |
CRC, colorectal cancer; FIT, fecal immunochemical test; FOBT, fecal occult blood test; N/R, not reported; NOS, Newcastle-Ottawa Scale.
Supplementary Table 3.
Comparisons and Outcomes From FOBT Studies Included in the Systematic Review
| Author, Year | Comparator Time From FIT | Alternate Time Cutoffs | Outcomes | Detailed Results | Summary |
|---|---|---|---|---|---|
| Gellad, 200942 | Continuous | Continuous | Incidence of CRC or advanced adenoma(s) (at colonoscopy) |
|
Incremental delays to colonoscopy of 1 mo after positive FOBT were associated with a nonsignificant trend toward higher incidence of CRC and advanced adenomas combined. This study was limited by sample size. |
| Flugelman, 201918 | Within 3 mo | Within 4–6, 7–12, >12 mo | CRC-specific mortality |
|
Delays to colonoscopy of 12 mo or more after positive FOBT were significantly associated with higher CRC-specific mortality (compared with performing colonoscopy within 3 mo). |
| Beshara, 202017 | Within 3 mo | 4–6, 7–9, 10–12, 13–18, 19–24 mo |
CRC incidence (at colonoscopy) CRC stage |
|
Delays to colonoscopy of 12 mo or more after positive FOBT were significantly associated with higher CRC incidence and more advanced stage of diagnosis (compared with performing colonoscopy within 3 mo). |
AOR, adjusted odds ratio; CI, confidence interval; CRC, colorectal cancer; FIT, fecal immunochemical test; FOBT, fecal occult blood test; HR, hazard ratio; OR, odds ratio.
Supplementary Table 4.
Risk of Bias Assessments Using the Newcastle-Ottawa Scale
| Author, Year | Selection (Max 4) | Comparability (Max 2) | Outcome Assessment (Max 3) | Overall Assessment |
|---|---|---|---|---|
| Positive FIT | ||||
| Meester 2016 |
N/A | N/A | N/A | N/A (modeling study) |
| Corley 2017 |
4 | 2 | 3 | High quality |
| Rutter 2018 |
N/A | N/A | N/A | N/A (modeling study) |
| Zorzi 2020 |
3 | 1 | 2 | Moderate quality |
| Kaalby 2019 |
4 | 1 | 2 | Moderate quality |
| Kim 2019 |
3 | 1 | 2 | Moderate quality |
| Lee 2019 |
3 | 1 | 2 | Moderate quality |
| Positive FOBT | ||||
| Gellad 2009 |
3 | 1 | 2 | Moderate quality |
| Flugelman 2019 |
4 | 2 | 2 | High quality |
| Beshara 2020 |
4 | 2 | 2 | High quality |
FIT, fecal immunochemical test; FOBT, fecal occult blood test; N/A, ▪▪▪.
References
- 1.GBD 2017 Colorectal Cancer Collaborators The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913–933. doi: 10.1016/S2468-1253(19)30345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zauber A.G., Winawer S.J., O'Brien M.J. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bénard F., Barkun A.N., Martel M. Systematic review of colorectal cancer screening guidelines for average-risk adults: summarizing the current global recommendations. World J Gastroenterol. 2018;24:124–138. doi: 10.3748/wjg.v24.i1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson D.J., Lee J.K., Boland C.R. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: a Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152:1217–1237.e3. doi: 10.1053/j.gastro.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 6.Moher D., Shamseer L., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells G., Shea B., O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2018. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at: Accessed •••.
- 8.Meester R.G., Zauber A.G., Doubeni C.A. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol. 2016;14:1445–1451.e8. doi: 10.1016/j.cgh.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutter C.M., Kim J.J., Meester R.G.S. Effect of time to diagnostic testing for breast, cervical, and colorectal cancer screening abnormalities on screening efficacy: a modeling study. Cancer Epidemiol Biomarkers Prev. 2018;27:158–164. doi: 10.1158/1055-9965.EPI-17-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gellad Z.F., Almirall D., Provenzale D. Time from positive screening fecal occult blood test to colonoscopy and risk of neoplasia. Dig Dis Sci. 2009;54:2497–2502. doi: 10.1007/s10620-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flugelman A.A., Stein N., Segol O. Delayed colonoscopy following a positive fecal test result and cancer mortality. JNCI Cancer Spectr. 2019;3:pkz024. doi: 10.1093/jncics/pkz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beshara A., Ahoroni M., Comanester D. Association between time to colonoscopy after a positive guaiac fecal test result and risk of colorectal cancer and advanced stage disease at diagnosis. Int J Cancer. 2020;146:1532–1540. doi: 10.1002/ijc.32497. [DOI] [PubMed] [Google Scholar]
- 13.Corley D.A., Jensen C.D., Quinn V.P. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317:1631–1641. doi: 10.1001/jama.2017.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y.C., Fann J.C., Chiang T.H. Time to colonoscopy and risk of colorectal cancer in patients with positive results from fecal immunochemical tests. Clin Gastroenterol Hepatol. 2019;17:1332–1340.e3. doi: 10.1016/j.cgh.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 15.Zorzi M., Hassan C., Capodaglio G. Colonoscopy later than 270 days in a fecal immunochemical test-based population screening program is associated with higher prevalence of colorectal cancer. Endoscopy. 2020;52:871–876. doi: 10.1055/a-1159-0644. [DOI] [PubMed] [Google Scholar]
- 16.Kaalby L., Rasmussen M., Zimmermann-Nielsen E. Time to colonoscopy, cancer probability, and precursor lesions in the Danish colorectal cancer screening program. Clin Epidemiol. 2019;11:659–667. doi: 10.2147/CLEP.S206873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim N.H., Lim J.W., Kim S. Association of time to colonoscopy after a positive fecal test result and fecal hemoglobin concentration with risk of advanced colorectal neoplasia. Dig Liver Dis. 2019;51:589–594. doi: 10.1016/j.dld.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Amin M, Edge S, Greene F, et al. AJCC Cancer Staging Manual, 8th ed. New York: Springer International Publishing.
- 19.Fisher D.A., Jeffreys A., Coffman C.J. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006;15:1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 20.Morris S., Baio G., Kendall E. Socioeconomic variation in uptake of colonoscopy following a positive faecal occult blood test result: a retrospective analysis of the NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107:765–771. doi: 10.1038/bjc.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rijn A.F., Stroobants A.K., Deutekom M. Inappropriate use of the faecal occult blood test in a university hospital in the Netherlands. Eur J Gastroenterol Hepatol. 2012;24:1266–1269. doi: 10.1097/MEG.0b013e328313bbd3. [DOI] [PubMed] [Google Scholar]
- 22.Zorzi M., Senore C., Turrin A. Appropriateness of endoscopic surveillance recommendations in organised colorectal cancer screening programmes based on the faecal immunochemical test. Gut. 2016;65:1822–1828. doi: 10.1136/gutjnl-2015-310139. [DOI] [PubMed] [Google Scholar]
- 23.Powell A.A., Nugent S., Ordin D.L. Evaluation of a VHA collaborative to improve follow-up after a positive colorectal cancer screening test. Med Care. 2011;49:897–903. doi: 10.1097/MLR.0b013e3182204944. [DOI] [PubMed] [Google Scholar]
- 24.Forbes N., Hilsden R.J., Heitman S.J. The appropriate use of fecal immunochemical testing. CMAJ. 2020;192:E68. doi: 10.1503/cmaj.190901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thamarasseril S., Bhuket T., Chan C. The need for an integrated patient navigation pathway to improve access to colonoscopy after positive fecal immunochemical testing: a safety-net hospital experience. J Community Health. 2017;42:551–557. doi: 10.1007/s10900-016-0287-2. [DOI] [PubMed] [Google Scholar]
- 26.Forbes N., Smith Z.L., Spitzer R.L. Changes in gastroenterology and endoscopy practices in response to the COVID-19 pandemic: results from a North American survey. Gastroenterology. 2020;159:772–774.e13. doi: 10.1053/j.gastro.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gralnek I.M., Hassan C., Beilenhoff U. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020;52:483–490. doi: 10.1055/a-1155-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ménard C., Waschke K., Tse F. COVID-19: Framework for the resumption of endoscopic activities from the Canadian Association of Gastroenterology. J Can Assoc Gastroenterol. 2020;3:243–245. doi: 10.1093/jcag/gwaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouillard S., Liu V.X., Corley D.A. COVID-19: Long-term planning for procedure-based specialties during extended mitigation and suppression strategies. Gastroenterology. 2020 May 18 doi: 10.1053/j.gastro.2020.05.047. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner H., Altenhofen L., Katalinic A. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German national screening colonoscopy database. Am J Epidemiol. 2011;174:1140–1146. doi: 10.1093/aje/kwr188. [DOI] [PubMed] [Google Scholar]
- 31.Wattacheril J., Kramer J.R., Richardson P. Lagtimes in diagnosis and treatment of colorectal cancer: determinants and association with cancer stage and survival. Aliment Pharmacol Ther. 2008;28:1166–1174. doi: 10.1111/j.1365-2036.2008.03826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilimoria K.Y., Ko C.Y., Tomlinson J.S. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253:779–785. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]