Abstract
Vitamin D deficiency, common in the population with irritable bowel syndrome (IBS), can induce the main factors that lead to IBS clinical symptoms, such as depression, anxiety, and inflammation. Serotonin (5-HT) plays an important role in the pathophysiology of IBS, and its production and secretion are increased from the lumen due to stress and inflammation. The aim of this study was to evaluate the effect of vitamin D3 supplementation on the pathogenesis of diarrhea-predominant IBS (IBS-D). Seventy-four IBS-D patients (age: 18-65 y) participated in a randomized, double-blind, placebo-controlled trial study from February 2017 to May 2018, at Rasoul-e-Akram Hospital, Tehran, Iran. Subjects were allocated into two groups receiving 50,000 IU/week of vitamin D3 or placebo for 9 weeks. IBS severity score system (IBS-SSS), IBS-quality of life questionnaire (QoL), hospital anxiety and depression Scale (HADs), visceral sensitivity index (VSI) and serum 25(OH) vitamin D3, serotonin, 5-hydroxy-indole acetic acid and ratio of 5-HIAA/5-HT were evaluated before and after the interventions. Symptoms severity, QoL, HADs-depression, and VSI score improved significantly in the vitamin D group as compared to the placebo group (P-values: <0.001, 0.049, 0.023, and 0.008; respectively). There were no significant differences in abdominal bloating, HADs-anxiety, serum 5-HT, 5-HIAA, and 5-HIAA/5-HT between the two groups at the end of the study. Based on our results, we recommend serum vitamin D be evaluated in the process of treatment of these patients to ameliorate symptoms and quality life of IBS-D patients with vitamin D deficiency and/or insufficiency.
Keywords: irritable bowel syndrome, vitamin D3, clinical symptoms, serotonin, randomized controlled trial
Abbreviations
IBS: Irritable Bowel Syndrome
IBS-D: diarrhea-predominant IBS
IBS-C: constipation-predominant IBS
IBS-SSS: IBS severity score system
IBS-QoL: IBS-quality of life questionnaire
HADs: Hospital Anxiety and Depression Scale
VSI: Visceral sensitivity index
5-HT: 5-hydroxytryptamine
5-HIAA: 5-hydroxy-indole acetic acid
TPH 1,2: tryptophan hydroxylase 1,2
SERT: serotonin reuptake transporter
Introduction
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder characterized by abdominal pain associated with altered bowel habits (Mearin et al., 2016[44]). Diagnosis of IBS is recognized by ROME IV criteria that is based on clinical symptoms (Drossman, 2016[11]; Ford et al., 2014[18]). Epidemiological studies have shown that IBS is more common in women and young people aged (25-54 years old) (Lacy and Moreau, 2016[35]), and its prevalence is 5 %-20 % (Ford and Talley 2012[19]). According to the bowel habits, IBS is classified to diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), and mixed-type IBS (IBS-M) which has alternating periods of diarrhea and constipation (Ford et al., 2014[18]).
IBS is a multifactorial disorder with the causes which are not still well understood. Genetic, gastrointestinal disorders, anxiety, disturbances in brain-gut axis, infection and inflammation in bowel, and changes in intestinal microbiota can be associated with IBS symptoms (Lacy and Moreau, 2016[35]; Nanayakkara et al., 2016[46]; Saha, 2014[58]). According to the psychological studies and American Association of Gastroenterology (AGA), depression and anxiety, which have been observed in 60 % of IBS patients, increase gastrointestinal symptoms including the severity of diarrhea and abdominal discomfort (Drossman et al., 2002[12]; Levy et al., 2006[38]). Anxiety is often the main cause of visceral pain hypersensitivity in patients with IBS (Labus et al., 2004[33]). IBS has a strong impact on quality of life such as diet, physical appearance, work, education, and interpersonal relationships (Phillips et al., 2013[54]).
Serotonin or 5-hydroxy tryptamine (5-HT) is a key neurotransmitter of the enteric nervous system (ENS) playing a significant role in the control of gastrointestinal motility, sensation, and secretion (Sen et al., 2011[60]). It seems that serotonin has a significant role in the pathophysiology of the IBS (Garvin and Wiley, 2008[22]). Approximately 95 % of total body 5-HT is found in the GI tract, 90 % of which is produced by enterochromaffin cells and 10 % by serotonergic neurons of the myenteric plexus (Sen et al., 2011[60]). Blood serotonin levels are often increased in IBS-D and decreased in IBS-C (Spiller et al., 2007[62]). The abnormal blood serotonin levels in patients with IBS is because all the 5-HT, found in the blood, is derived from the GI tract (Atkinson et al., 2006[4]). On the other hand, the number of enterochromaffin cells in the colon of patients with IBS-D is higher than that of healthy people, and even in patients with ulcerative colitis (Gershon et al., 1965[23]; Sen et al., 2011[60]). Upon the uptake of 5-HT into enterocyte and pre-synaptic neurons by the Serotonin Reuptake Transporter (SERT in the lumen), it is broken down to 5-hydroxy indole acetic acid (5-HIAA) by intracellular monoamine oxidase (MAO). Blood 5-HT is converted to 5-HIAA by MAO and aldehyde dehydrogenase in the liver and kidney and is then excreted in the urine (Thijssen et al., 2016[65]; Yazar et al., 2005[68]). The number of SERTs, their activities and the production of 5-HIAA are reported to be decreased in patients with IBS-D (Thijssen et al., 2016[65]). In addition, animal studies have shown that nonspecific inflammation can cause EC cells hyperplasia and decrease expression of SERT (Linden et al., 2003[41]), leading to an increase in 5-HT at the mucosal level and followed by the incremental gut motility and secretion, thereby causing IBS symptoms (Dunlop et al., 2005[13]).
1,25-dihydroxy vitamin D3 [1,25(OH)2D3] is a physiologically active form of vitamin D3 the main role of which is modulation of cell growth, immune function and reduction of inflammation (National Institutes of Health, Office of Dietary Supplements - Vitamin D, 2020[47]). It has been estimated that 1 billion people worldwide have vitamin D deficiency or insufficiency (Holick 2007[24]). Studies have shown that in patients with IBS, vitamin D deficiency is more common than in healthy population and severity of symptoms is inversely correlated with serum vitamin D concentration (Williams et al., 2018[67]). However, there are a few studies about the effect of vitamin D supplementation on IBS symptoms (Abbasnezhad et al., 2016[1]; El Amrousy et al., 2018[15]; Jalili et al., 2016[27]; Sprake et al., 2012[63]; Tazzyman et al., 2015[64]). Three recent randomized clinical trial studies have reported that treatment with vitamin D in all classification of IBS patients who had vitamin D deficiency could relief symptoms and quality of life compared to the placebo group (Abbasnezhad et al., 2016[1]; El Amrousy et al., 2018[15]; Jalili et al., 2016[27]). However, the mechanism of this effect is unclear. There are some hypotheses that vitamin D may improve clinical symptoms in patients with IBS by ameliorating anxiety and depression (Parker et al., 2017[49]), anti-inflammatory effects (Li et al., 2015[40]; Mangin et al., 2014[43]), and regulating the composition of the gut-microbiota and gut barrier function (Cantorna et al., 2014[8]; Luthold et al., 2017[42]).
To the best of our knowledge the present study is the first one assessing the effects of the increase in serum vitamin D on different dimensions of irritable bowel syndrome. The aim of this study was to evaluate the effect of vitamin D3 supplementation on improving clinical outcomes, quality of life, and anxiety, and decreasing serum serotonin (5-hydroxytryptamine), increasing 5-hydroxy-indole acetic acid and also 5-HIAA/5-HT ratio in patients with diarrhea-predominant irritable bowel syndrome.
Methods
Study design and participants
This was a randomized, double-blind, placebo-controlled trial study with parallel design performed in 9 weeks. The participants were informed about the study via printed posters on the notice boards of Rasoul-e-Akram Hospital, Tehran, Iran. We also explained the study objectives verbally to the patients with IBS-D, who were referred to Gastrointestinal Clinic of the hospital. Eventually, we recruited 88 adult male and female volunteers, with IBS-D, between the ages of 18- 65 years old. ROME IV criteria (Drossman, 2016[11]) and World Gastroenterology Organization (WGO) questionnaire, for healthcare professional (HCP) of IBS patients (Quigley et al., 2016[55]), were used as diagnostic criteria. The study was carried out from February 2017 to May 2018. The inclusion criteria for including the patients were: having irritable bowel syndrome with diarrhea-predominant, IBS-SSS score between 175 to 300, not being pregnant or lactating, having no GI disorders such as inflammatory bowel disease, celiac, GI infection or history of colon cancer, intestinal surgery or radiotherapy, and cholecystectomy, not taking vitamin D supplement in the last 6 months, no use of other supplements, NSIADs, Glucocorticoid and antidepressants drug containing serotonin resorptive antagonists, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants, no alcohol and caffeine intake and also no smoking 12 hours before the test. Exclusion criteria were: serum vitamin D higher than 30 ng/ml, any abnormal response or side effect to supplementation, blood in the stool, fast weight lost, using lower than 80 % of supplements, reluctance to continue cooperation with the researchers. Demographic data, medical history, measuring anthropometric indicators, physical activity [measured by International Physical Activity Questionnaire (IPAQ)], and 3-day food diary records (2 working day and 1 holiday) [their nutrients were calculated by dietary calculator software Nutritionist IV] were obtained from participants at baseline and the end of the study.
Ethics statements
The study was approved by ethic committee of Iran University of Medical Sciences (IR.IUMS.REC 1395.9413323001) and the study protocol was registered in the Iranian clinical trials Web site at: (http://www.irct.ir: IRCT201701162709N42). The objective of the study was explicitly stated to the participants and written informed consent form for acceptance of study details was signed by all participants.
Intervention
Following the randomization, participants received weekly Vitamin D3 (50,000 IU) or placebos (filled by edible paraffin) pearls (Zahravi Company, Tabriz, Iran) for 9 weeks. Placebos were similar to vitamin D in shape, color and package. Patients received 8 pearls weekly for 8 weeks and the last pearl used in 10th week of intervention. According to protocol treatment of vitamin D deficiency, the serum level of vitamin D can be improved by intake of 50,000 IU vitamin D3 in 8 weeks and after that using only 1 pearl monthly for maintaining serum vitamin D level in a normal range will suffice (Roth et al., 2012[56]) as 10-14 days after using the pearls, vitamin D reaches to peak rate in serum (Jones 2008[29]). For this reason, the blood sampling from participants were performed in week 12. In addition, all patients received Mebeverine 135 mg twice a day beside the supplementation.
Outcomes
The primary outcome was assessment of IBS severity score system. Secondary outcomes were assessment of quality of life, stress and depression, visceral sensitivity, serum serotonin and 5-hydroxyindole acetic acid.
Sample size calculation
According to our primary outcome and based on the result of similar previous study by Abbasnezhad et al. (2016[1]) the sample size was calculated to be 44 subjects in each group by considering 22 unit change in the average of IBS severity score system with a type I error of 5 % (α = 0.05), a type II error of 20 % (β = 0.2; power = 80 %), and considering 20 % of loss to follow up. We used G-Power software (http://www.gpower.hhu.de) for calculation of the sample size.
Randomization and allocation
The quadruple block randomization was used for allocation of the participants. According to the sample size of 88, 22 blocks were produced by using the online site (http://www.sealedenvelope.com). For making the concealment in the randomization process, dedicated codes were produced by the software and were used on the pharmaceutical sheets.
Information gathering tools
Gastrointestinal symptoms and severity scale
IBS symptoms severity score system (IBS-SSS) questionnaire (Francis et al., 1997[20]) was used for monitoring the IBS symptoms and severity at the baseline and end of the study. Data were collected by interview using visual analog scale (VAS). This questionnaire contains 5 items describing the severity of symptoms within the previous 10 days, such as: abdominal pain severity, abdominal pain duration, abdominal distention severity, bowel habit satisfaction, and life disruption. Each item was scored from 0 to 100 (total score from 0 to 500). The obtained scores were assigned “mild”, “moderate” and “severe” scores by scoring from 75 to 175, 175 to 300, and >300. Patients scoring below 75 were considered to be in remission.
Quality of life
IBS-QoL questionnaire (Patrick et al., 1998[50]) consists of 34 items and 8 subscales (dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual desire, and relationships) were filled for the participants. Each item has a 5-point response scale (1= not at all to 5= extremely). Similar to a previous study (Mokhtare et al., 2018[45]), we used the raw total score of the questionnaire with the range of 34 to 170, indicating that higher scores demonstrated lower quality of life in individuals.
Hospital Anxiety and Depression scale (HADs)
We used HADs (Larsen et al., 2014[36]; Zigmond and Snaith 1983[69]) questionnaire, which is a 14-item self-rating scale, assessing anxiety and depression symptoms in different diseases associated with psychological disorders. The questionnaire has two parts consisting of seven questions related to anxiety (HADs-ANX), and seven to depression (HADs-DEP). Each item has a rating of 0 to 3; the higher rates indicate greater anxiety or depression symptoms. The total score was calculated by the sum of the concession items, and ranged in each part from 0 to 21. The scores from 0 to 7 indicated a normal scale, 8 to 10 borderline, and the scores between 11 to 21 illustrated clinical problems.
Visceral sensitivity index (VSI)
We also used VSI questionnaire (Labus et al., 2004[33]) to evaluate GI symptom-specific anxiety (GSA) in IBS patients. It has a 6-point Likert scale for each item (0= strongly disagree to 5= strongly agree) and total score ranges from 0 to 75, which a higher score indicates greater GSA. The validity and reliability of the VSI have been confirmed in several studies (Labus et al., 2007[34], 2004[33]; Saigo et al., 2014[59]); however, this questionnaire has not been evaluated in Iran. Thus, we designed the test-retest experiment with 2 weeks interval between two tests in 20 IBS-D patients. The original VSI questionnaire was translated into Persian by a fluent translator, and then a native English speaker back-translated it into English. Afterwards, four gastroenterologist experts confirmed the content of the questionnaire. At the end, Cronbach’s α was estimated 0.914 for reliability of VSI questionnaire in Persian.
Laboratory test
Following an overnight fasting, 5 ml blood samples were taken from the participants 90 minutes after ingestion of a carbohydrate-rich breakfast with 450 Kcal at the baseline and end of the study. Based on previous studies that evaluated 5-HT and 5-HIAA in IBS patients (Bearcroft et al., 1998[6]; Gershon et al., 1965[23]; Houghton et al., 2003[26]; Park et al., 2009[48]), subjects were given a breakfast containing 60 gr white bread, 35 gr cheese, 5 gr butter, 3 tablespoons of carrot jam and 150 ml water within 10 minutes.
Blood samples were collected in the clot activator tubes and serum samples were left at room temperature and then centrifuged twice at 4,000 rpm for 10 minutes for each centrifuge in order to, ensure no platelet contamination of supernatant in the samples because 5-HT is rapidly taken up by platelets which contain the largest 5-HT in the peripheral blood (Da Prada et al., 1972[10]). Serum samples were stored at -80 °C for later lab analysis.
Vitamin D
We used the LIAISON® 25 (OH) Vitamin D3 assay (DiaSorin, USA) which is a direct competitive chemiluminescence immunoassay (CLIA) for quantitative determination of total 25 (OH) vitamin D3 in serum. Functional sensitivity from the regression equation of dose concentration of 25 (OH) Vitamin D3 is ≤4.0 ng/mL. The classification of 25 (OH) Vitamin D3 status is <10 ng/mL deficiency, 10-30 ng/mL insufficiency, 30-100 ng/mL sufficiency, and >100 ng/mL toxicity (National Institutes of Health, Office of Dietary Supplements - Vitamin D, 2020[47]).
Serotonin (5-Hydroxy Tryptamine)
We used the ELISA method by IBL kit package (Hamburg, Germany) for the quantitative determination of Serotonin in human serum. Due to the dilution of samples, the values acquired from tests had to be multiplied by 107 to obtain the serotonin concentrations in ng/ml. To convert the serotonin unit from ng/ml to nmol/l, we multiplied the obtained value by 5.7. The intra-assay coefficient of variability (CV) was 3.8-6.6, and the inter-assay CV was 6.7-17.3 %.
5-HydroxyIndole Acetic Acid
5-HIAA was assessed by Bioassay ELISA kit (Shanghai Crystal Day Biotech CO., Ltd, China) based on the Biotin double antibody sandwich technology. The 5-HIAA unit was reported in mmol/l. The intra-assay CV was <10 % and, the inter-assay CV was <12 %.
5-HIAA to 5-HT ratio
Because of the difference in 5-HIAA and 5-HT units, both variables were converted to nmol/l by multiplying them into 10-6 and 5.7, respectively, and then the ratio was calculated.
Statistical analysis
The statistical analysis was performed by SPSS 25 for Windows. For comparison of the baseline characteristics and demographic data between the groups, we used chi-squared test (for qualitative variables) or independent t-test (for quantitative data). Data from the outcomes of the questionnaires (as IBS-SSS, IBS-QoL, HADs and VSI) and blood tests (as 5-HT, 5-HIAA, and 5-HIAA/5-HT) were analyzed by independent t-test or Mann-Whitney U-test for comparison between groups. Moreover, we used paired t-test or Wilcoxon paired rank test for within group comparisons. Normality distribution of data was assessed by Graphical methods, the degree of skewness and normality tests were determined by Kolmogorov–Smirnov and Shapiro-Wilk test. ANCOVA model was used for adjusting variables with significant differences at the baseline. The data are reported as numbers and percentages for qualitative variables, mean ± SD for parametric data and median (25th, 75th percentile) for non-parametric data in this article. Statistical significance was considered as P-values <0.05.
Results
Enrollment and study completion
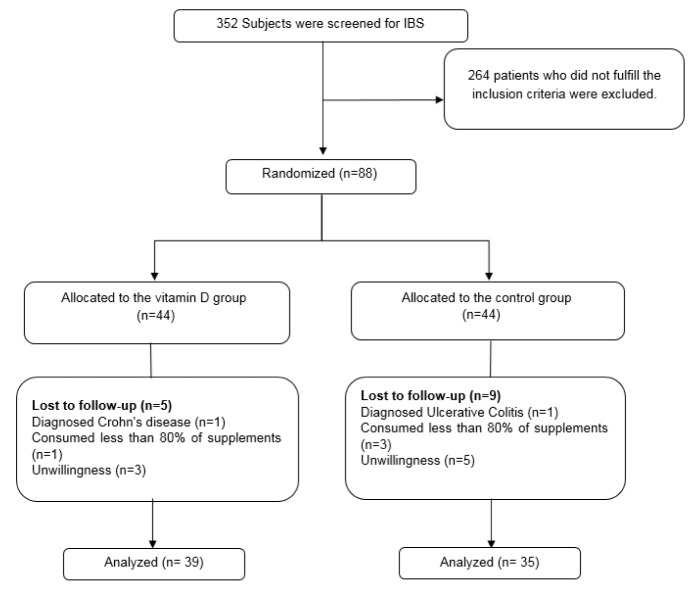
A total of the 88 IBS-D patients were included in the study, and 74 of them completed the study. Due to consuming less than 80 % of supplements, diagnosis of inflammatory bowel disease, and unwillingness to cooperate, 14 participants were excluded from the study. The flowchart of the patients enrolled in the study is presented in Figure 1(Fig. 1).
Figure 1. Flowchart of patients from enrollment to the end of the study.
Characteristics of the study participants
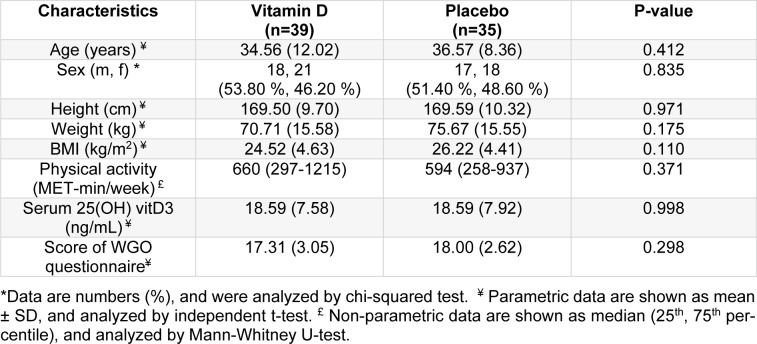
The data of the participants who completed the study (39 women and 35 men, age: 35.51±10.43 years, BMI: 25.32±4.58 kg/m2) were included for the analysis. All patients had diarrhea-predominant IBS, based on ROME IV criteria, and moderate disease severity (238.24±50.81) evaluated by the IBS-SSS questionnaire. There was no significant difference between the two groups regarding the baseline characteristics, score of WGO questionnaire, physical activity, and IBS-SSS scores between (Table 1(Tab. 1)). There was no significant difference between two groups in serum 25(OH) vitamin D3 at the beginning of the study. We found no differences in the intake of macronutrient and micronutrient between groups on the basis of the 3-day food records analysis, except for beta-carotene (P=0.04) which we adjusted for possible effect of antioxidant on the main study outcomes (data are not shown).
Table 1. Baseline characteristic of study participants.
Severity of IBS symptoms
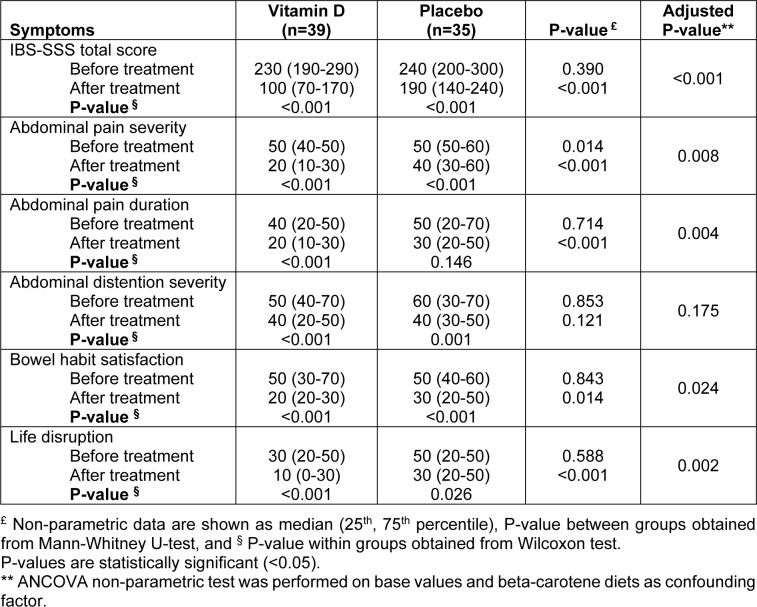
At the end of the study, the total score of IBS-severity score system was significantly improved in both groups compared to the beginning of the study (P<0.001 and P<0.001, respectively). This improvement was higher in the group receiving vitamin D and the difference between the groups was also significant (P<0.001). In subscale analysis, all symptoms ameliorated in both groups except for abdominal pain duration in the control group. Comparing this effect between the two groups, abdominal pain severity, abdominal pain duration, bowel habit satisfaction and life disruption significantly improved in vitamin D group compared to the control group. There was no significant difference in abdominal distention severity between the groups at the end of the study (Table 2(Tab. 2)).
Table 2. Comparisons of severity of symptoms before and after intervention in vitamin D and placebo group in patients with irritable bowel syndrome.
Quality of life
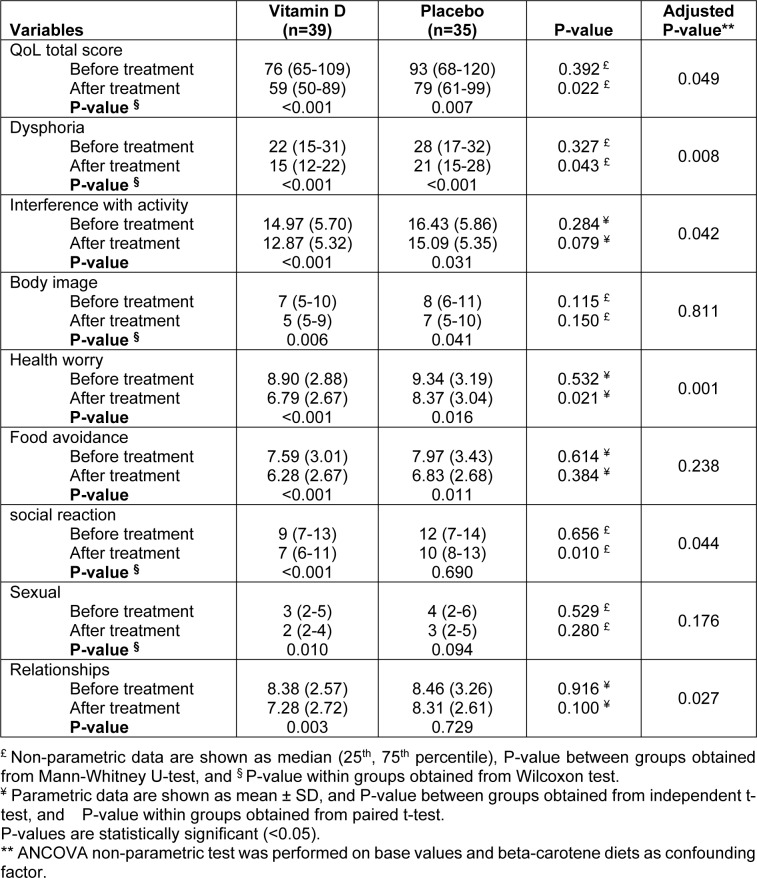
Overall score of IBS-QoL significantly improved in both groups of vitamin D and placebo at the end of the study (P<0.001 and P=0.007, respectively); however, the vitamin D group showed more improvement than the control group (P=0.049). Moreover, we observed improvement in quality of life subscales in both groups, except for social reaction and relationships in the control group. Dysphoria, interference with activity, health worry, social reaction, and relationships showed more favorable change in the group receiving vitamin D as compared to participants receiving placebo (Table 3(Tab. 3)).
Table 3. Comparisons of quality of life before and after intervention in vitamin D and placebo group in patients with irritable bowel syndrome.
Anxiety and depression
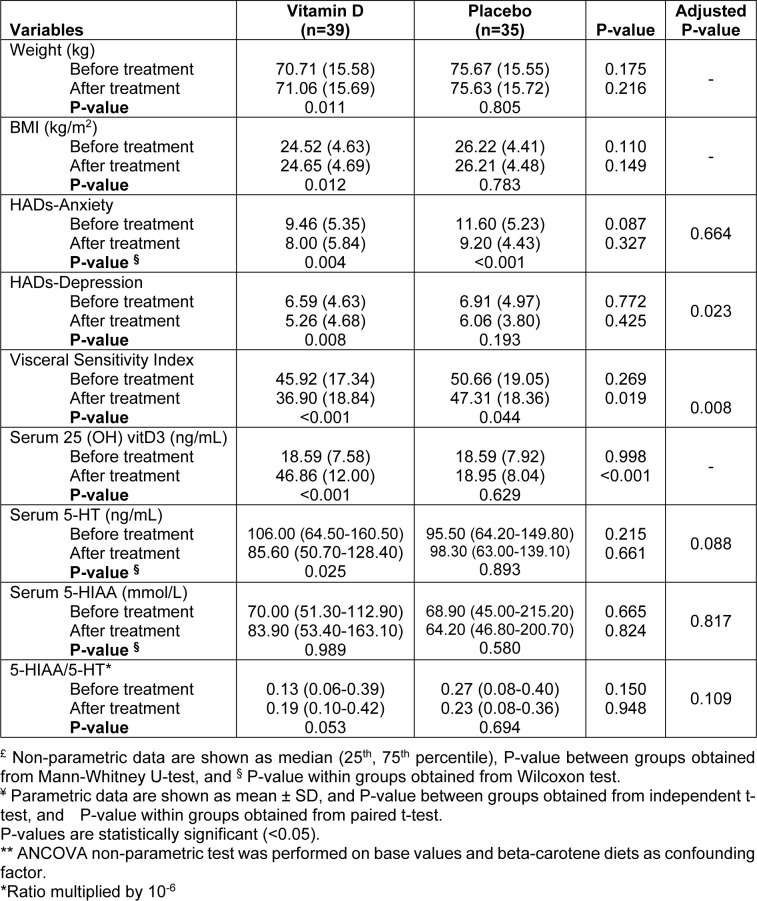
The results of HADs questionnaire showed that mean anxiety score and mean depression score of patients were 10.47±5.37 and 6.74±4.76, respectively at the baseline of the study. According to the results of the questionnaire, the total score of patients for depression scale was in normal range and for stress was in borderline range. At the end of the study, anxiety decreased significantly in both groups of vitamin D and control compared to the beginning of the study (P=0.004 and P<0.001, respectively); however, this improvement was not significant between the groups. The mean score of depression decreased significantly in vitamin D group (P=0.008), after modifying the effect of confounders; however, the reduction of depression symptoms was still significant between the groups (P=0.023) (Table 4(Tab. 4)).
Table 4. Comparisons of weight, BMI, HADs-Anxiety, HADs-depression, Visceral Sensitivity Index, serum 25 (OH) vitD3, 5-HT, 5-HIAA and 5-HIAA/5-HT ratio before and after intervention in vitamin D and placebo group in patients with irritable bowel syndrome.
Visceral Sensitivity Index
As Table 4(Tab. 4) displays, VSI has improved significantly in both groups of vitamin D and control (P<0.001, P=0.044, respectively). This improvement is significantly greater in the treatment group than in the control group (P=0.008).
Serum 25(OH) vitamin D3 levels
At the baseline, participants had an insufficient vitamin D level in both groups. After 9 weeks of supplementation with vitamin D, serum 25(OH) D3 significantly increased compared to the baseline values in the treatment group (P<0.001) and reached to normal levels. In addition, there was a significant increase in the vitamin D group compared to the control group at the end of the study (P<0.001) (Table 4(Tab. 4)).
Serum Serotonin (5-HT) and 5-Hydroxy-Indole Acetic Acid (5-HIAA)
5-HT significantly was reduced compared to the baseline values in patients receiving vitamin D (P=0.025). Despite this favorable outcome, the effect of vitamin D on serum serotonin, there was no significant statistical difference as compared to the control group at the end of the study. The result of serum samples assessment showed an increase in 5-HIAA in the vitamin D group; however, this rise was not statistically significant. Moreover, there was no significant difference between the two groups at the end of the study. For 5-HIAA/5-HT, same as 5-HIAA, we did not observe any significant change in serum samples of both groups after the intervention compared to the baseline (Table 4(Tab. 4)).
Discussion
The results of this randomized clinical trial showed that vitamin D3 supplementation improved IBS symptoms, quality of life, depression and visceral sensitivity index as compared to the control group. No significant differences in anxiety, serum concentration of serotonin, 5-HIAA, and 5-HIAA/5-HT were found between vitamin D and control groups.
The findings of this study showed that intake of one pearl of 50,000 IU vitamin D3 weekly for 9 weeks increased serum 25(OH) vitD3. In line with the findings of our study, it has been reported that nine weeks supplementation with 50,000 IU dose of vitamin D3, does not have any side effects for subjects with vitamin D deficiency (Holick et al., 2011[25]; Roth et al., 2012[56]). Similarly, assessing the effects of vitamin D on IBS, some researchers have shown the same results as our study (Abbasnezhad et al., 2016[1]; El Amrousy et al., 2018[15]; Jalili et al., 2016[27]).
The results of our study and some previous studies show, vitamin D can significantly improve symptoms and quality of life in IBS patients. El Amrousy et al. (2018[15]) evaluated the effect of 2,000 IU vitamin D3 daily in 112 IBS adolescents with vitamin D deficiency for 6 months. The authors reported improvement in serum 25(OH) vitD3, the severity of symptoms and quality of life of patients in the vitamin D group as compared to the control group. Abbasnezhad et al. (2016[1]) supplemented 50,000 IU vitamin D3 biweekly for 6 months in patients with IBS and noticed that taking vitamin D can reduce gastrointestinal symptoms and increase the quality of life of these patients as compared to the placebo-receiving group. In the study of Jalili et al. (2016[27]) on co-administration of soy isoflavones and vitamin D in the management of irritable bowel disease, it was observed that co-administration of soy isoflavones with vitamin D did not improve the IBS-SSS and IBS-QoL; however, prescription of vitamin D alone improved clinical symptoms and quality of life. The exact function of vitamin D in the improvement of clinical symptoms and quality of life of patients with IBS is unclear. It is assumed that vitamin D can control depression, anxiety, and mild inflammation which may be due to genetics, intestinal microbial, inflammation after infection, nutritional factors, etc., thereby improving the clinical symptoms and quality of life of IBS patients (Li et al., 2014[39]; Sinagra et al., 2016[61]). We observed patients receiving placebo significantly improved in symptoms from the baseline. This effect can be due to the psychological problems in IBS patients (Lee et al., 2015[37]). The systematic review of Flik et al. (2017[17]) remarks that the placebo could affect the psychiatric problems of IBS patients because their expectation from and willingness for treatment are more important than the drug.
Vitamin D and its metabolites have the ability to cross the blood-brain barriers and their receptors are present in different regions of the brain which can contribute to the pathophysiology of depression (Eyles et al., 2005[16]). A systematic review and meta-analysis conducted by Anglin et al. (2013[2]) showed that there was a direct correlation between vitamin D deficiency and adult depression. However, some trial studies have indicated that supplementation with vitamin D cannot affect depression symptoms. It has been reported that the effect is associated with severity of clinical symptoms of depression due to the finding that vitamin D is more effective in people with more severe symptoms (Li et al., 2014[39]). In our study, patients in vitamin D group had a significant reduction in depression symptoms, and the difference between the groups was significant. Some recent studies have shown that there is a relationship between vitamin D levels and anxiety. The animal study of Kalueff et al. (2004[31]) on mice lacking vitamin D receptors in the brain has shown increased stress in these mice compared with the control group. A direct correlation between the severity of anxiety symptoms and low levels of serum vitamin D has also been observed in some human studies (Armstrong et al., 2007[3]; Bičíková et al., 2015[7]). A high prevalence of anxiety and depression in people with IBS has been reported as one of the main causes of this disease. For this reason, the treatment of anxiety and depression in these patients can help to reduce the clinical symptoms (Banerjee et al., 2017[5]). Our results showed that stress score of HADs questionnaire reduced in both groups but the difference was not significant between the groups. The similar borderline range score of anxiety at the baseline led to the absence of a meaningful difference between the two groups at the end of the study; patients did not show severe symptoms of depression and anxiety. It is likely that we might have observed a significant difference between the groups if the duration of the study was longer. Jorde et al. (2008[30]) found that overweight and obese individuals had a significant improvement in symptoms of depression by vitamin D intake of 20,000 and 40,000 IU weekly over a period of one year.
About half of people with IBS report increasing visceral sensitivity (Kanazawa et al., 2011[32]). Psychological disorders such as stress and depression are reported to be the main causes of this sensitivity (Garland et al., 2012[21]). The mechanism of the effect is not clear, but as discussed in some studies on visceral sensitivity in IBS patients, changes in neuromuscular immunity and disorders of intestinal microbial regulation can be the causes of this sensitivity (Cong et al., 2018[9]). There is no specific treatment for this disturbance and the treatment can be in the improvement of clinical symptoms, anxiety, depression, dietary regulation and physical activity of patients (Trinkley and Nahata 2014[66]). Improvement in visceral sensitivity seems reasonable, as our participants had an improvement in clinical symptoms in both groups receiving vitamin D and placebo. On the other hand, we found a better status in the vitamin D group than in the control group, which can be due to the effects of the vitamin on inflammatory and intestinal microbial (Li et al., 2015[40]).
Serotonin is one of the key factors in gastrointestinal movements and secretions the production of which will be increased in people with IBS-D and cause diarrhea and spasms in these patients (Saha 2014[58]; Sen et al., 2011[60]). Any mild inflammation and immune response can lead to hyperplasia of enterochromaffin cells, increased serotonin production and secretion and reduced SERT expression, as a result of which IBS clinical symptoms appear (Thijssen et al., 2016[65]). In addition to increased production, some studies have shown that impaired serotonin metabolism occurs in IBS, thereby decreasing the production of 5-HIAA and its level in the blood and urine levels (Houghton et al., 2003[26]; Jin et al., 2016[28]). A high incidence of vitamin D deficiency in these individuals can worsen inflammatory status (Li et al., 2015[40]; Williams et al., 2018[67]). The study of Dussik et al. (2018[14]) on gene expression profiling and assessment of vitamin D and serotonin pathway variations in patients with irritable bowel syndrome showed that IBS patients’ derived RNA exhibited lower levels of tryptophan hydroxylase-1 (TPH1) expression, the enzyme that catalyzes the rate-limiting step in serotonin biosynthesis in enterochromaffin cells. Also, the level of 25 (OH) vitD3 in patients with IBS (particularly in the IBS-D subtype) was lower than in the non-IBS control. As a result, the expression of selected IBS genetic biomarkers (TPH1) was shown to be modulated by vitamin D. The results of that study suggest that IBS pathogenesis and pathophysiology may be involved in dysregulation of serotonin production and vitamin D insufficiency (Dussik et al., 2018[14]). Moreover, studies on brain monoamine oxidase (Serotonin degrading enzyme to 5-hydroxyindol acetic acid) have shown that treatment with vitamin D can increase the level of expression of this enzyme (Pertile et al., 2016[53]; Sabir et al., 2018[57]). In addition, vitamin D increases the expression of SERT in brain neurons (Sabir et al., 2018[57]). Patrick and Ames's studies on patients with mental disorders have illustrated that vitamin D has a regulatory effect on tryptophan hydrolysis enzymes, indicating that more tryptophan is converted to serotonin in the brain by increasing the activity of the TPH2 enzyme. In non-brain tissues the production of serotonin is reduced and regulated by decreasing the activity of TPH1 (Patrick and Ames, 2015[51], 2014[52]). According to those research findings, and despite the decrease and increase in serum serotonin and 5-HIAA, there was no significant difference between the two groups in our study. Considering a significant improvement in clinical symptoms of patients compared to the control group, there is a strong likelihood that if the duration of the intervention had been longer or biochemical evaluations had been assessed more accurately, we might have seen a significant difference with the control group.
The strengths of the present study are a first study which has evaluated the effects of vitamin D on anxiety, depression and the biochemical parameters of serotonin and 5-HIAA in patients with IBS. A VSI questionnaire was used to show the status of the patients, and to assess the effect of stress on IBS patients more precisely. This study was performed only in the group with diarrhea-predominant IBS. However, the study has some limitations. Due to time constraints, the duration of our study was 3 months. Lack of evaluation of 5-HT and 5-HIAA in platelet poor plasma, which according to other studies is the best method to measure these factors, is another limitation of our study (Bearcroft et al., 1998[6]). This method requires the employment of a higher number of kits; however, due to budget limitation, we could not carry out this method.
Conclusion
The findings of the present study revealed that supplementation with vitamin D3 in patients with diarrhea-predominant irritable bowel syndrome could improve severity of symptoms, quality of life, depression and visceral sensitivity. The findings also showed that intake of vitamin D reduced anxiety and serum serotonin; however, this decrease was not significant compared to the control group.
We recommend that serum 25(OH) vitamin D3 should be evaluated in the treatment process of these patients. Further studies are needed to confirm the effect of vitamin D on IBS patients and determine the exact mechanism.
Acknowledgements
We are owed to the IBS patients for participating in this study; and for their collaboration in the implementation and completion of this project. We are so grateful for the cooperation of Dr. Nasseri Moghadam, and Dr. Merat (Tehran University of Medical Sciences) to accomplish this project.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions
The authors' responsibilities were as follows: MKS and FS designed the research; MKS, MM, MM, AHFK, SA and NA contributed to sampling; MKS and LJ analyzed and interpreted the data; MKS and FS wrote the original draft; MKS, AD, FS and SS contributed to reviewing and revising the paper. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that there were no conflicts of interest.
References
- 1.Abbasnezhad A, Amani R, Hajiani E, Alavinejad P, Cheraghian B, Ghadiri A. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: a randomized double-blind clinical trial. Neurogastroenterol Motil. 2016;28:1533–44. doi: 10.1111/nmo.12851. [DOI] [PubMed] [Google Scholar]
- 2.Anglin RES, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–7. doi: 10.1192/bjp.bp.111.106666. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DJ, Meenagh GK, Bickle I, Lee ASH, Curran E-S, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26:551–4. doi: 10.1007/s10067-006-0348-5. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee A, Sarkhel S, Sarkar R, Dhali GK. Anxiety and depression in Irritable Bowel Syndrome. Indian J Psychol Med. 2017;39:741–5. doi: 10.4103/IJPSYM.IJPSYM_46_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bearcroft CP, Perrett D, Farthing MJ. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut. 1998;42:42–6. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bičíková M, Dušková M, Vítků J, Kalvachová B, Řípová D, Mohr P, et al. Vitamin D in anxiety and affective disorders. Physiol Res. 2015;64(Suppl 2):S101–S103. doi: 10.33549/physiolres.933082. [DOI] [PubMed] [Google Scholar]
- 8.Cantorna MT, McDaniel K, Bora S, Chen J, James J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp Biol Med (Maywood) 2014;239:1524–30. doi: 10.1177/1535370214523890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cong X, Perry M, Bernier KM, Young EE, Starkweather A. Effects of self-management interventions in patients with Irritable Bowel Syndrome: Systematic review. West J Nurs Res. 2018;40:1698–1720. doi: 10.1177/0193945917727705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Da Prada M, Tranzer JP, Pletscher A. Storage of 5-hydroxytryptamine in human blood platelets. Experientia. 1972;28:1328–9. doi: 10.1007/BF01965326. [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA. Functional gastrointestinal disorders: History, pathophysiology, clinical features, and Rome IV. Gastroenterology. 2016;150:1262–79.e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 12.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–31. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–57. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 14.Dussik CM, Hockley M, Grozić A, Kaneko I, Zhang L, Sabir MS, et al. Gene expression profiling and assessment of vitamin D and serotonin pathway variations in patients with irritable bowel syndrome. J Neurogastroenterol Motil. 2018;24:96–106. doi: 10.5056/jnm17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Amrousy D, Hassan S, El Ashry H, Yousef M, Hodeib H. Vitamin D supplementation in adolescents with irritable bowel syndrome: Is it useful? A randomized controlled trial. Saudi J Gastroenterol. 2018;24:109–14. doi: 10.4103/sjg.SJG_438_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Flik CE, Bakker L, Laan W, van Rood YR, Smout AJPM, de Wit NJ. Systematic review: The placebo effect of psychological interventions in the treatment of irritable bowel syndrome. World J Gastroenterol. 2017;23:2223–33. doi: 10.3748/wjg.v23.i12.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2–26. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 19.Ford AC, Talley NJ. Irritable bowel syndrome. BMJ. 2012;345:e5836. doi: 10.1136/bmj.e5836. [DOI] [PubMed] [Google Scholar]
- 20.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 21.Garland EL, Gaylord SA, Palsson O, Faurot K, Mann JD, Whitehead WE. Therapeutic mechanisms of a mindfulness-based treatment for IBS: Effects on visceral sensitivity, catastrophizing, and affective processing of pain sensations. J Behav Med. 2012;35:591–602. doi: 10.1007/s10865-011-9391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garvin B, Wiley JW. The role of serotonin in irritable bowel syndrome: implications for management. Curr Gastroenterol Rep. 2008;10:363–8. doi: 10.1007/s11894-008-0070-3. [DOI] [PubMed] [Google Scholar]
- 23.Gershon MD, Drakontides AB, Ross LL. Serotonin: synthesis and release from the myenteric plexus of the mouse intestine. Science. 1965;149(3680):197–9. doi: 10.1126/science.149.3680.197. [DOI] [PubMed] [Google Scholar]
- 24.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–70. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalili M, Hekmatdoost A, Vahedi H, Poustchi H, Khademi B, Saadi M, et al. Co-administration of soy isoflavones and vitamin D in management of irritable bowel disease. PLOS One. 2016;11(8):e0158545. doi: 10.1371/journal.pone.0158545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin D-C, Cao H-L, Xu M-Q, Wang S-N, Wang Y-M, Yan F, et al. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J Gastroenterol. 2016;22:8137–48. doi: 10.3748/wjg.v22.i36.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 30.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. J Intern Med. 2008;264:599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 31.Kalueff AV, Lou Y-R, Laaksi I, Tuohimaa P. Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport. 2004;15:1271–4. doi: 10.1097/01.wnr.0000129370.04248.92. [DOI] [PubMed] [Google Scholar]
- 32.Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26(Suppl 3):119–21. doi: 10.1111/j.1440-1746.2011.06640.x. [DOI] [PubMed] [Google Scholar]
- 33.Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, et al. The Visceral Sensitivity Index: Development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 34.Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: Further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- 35.Lacy BE, Moreau JC. Diarrhea-predominant irritable bowel syndrome: Diagnosis, etiology, and new treatment considerations. J Am Assoc Nurse Pract. 2016;28:393–404. doi: 10.1002/2327-6924.12387. [DOI] [PubMed] [Google Scholar]
- 36.Larsen AR, Engsbro AL, Bytzer P. Screening instruments for anxiety and depression in patients with irritable bowel syndrome are ambiguous. Dan Med J. 2014;61:A4785. [PubMed] [Google Scholar]
- 37.Lee Y-T, Hu L-Y, Shen C-C, Huang M-W, Tsai S-J, Yang AC, et al. Risk of psychiatric disorders following irritable bowel syndrome: A nationwide population-based cohort study. PLOS One. 2015;10(7):e0133283. doi: 10.1371/journal.pone.0133283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy RL, Olden KW, Naliboff BD, Bradley LA, Francisconi C, Drossman DA, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–58. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 39.Li G, Mbuagbaw L, Samaan Z, Falavigna M, Zhang S, Adachi JD, et al. Efficacy of vitamin D supplementation in depression in adults: A systematic review. J Clin Endocrinol Metab. 2014;99:757–67. doi: 10.1210/jc.2013-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YC, Chen Y, Du J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J Steroid Biochem Mol Biol. 2015;148:179–83. doi: 10.1016/j.jsbmb.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linden DR, Chen J-X, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G207–G216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- 42.Luthold RV, Fernandes GR, Franco-de-Moraes AC, Folchetti LGD, Ferreira SRG. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism. 2017;69:76–86. doi: 10.1016/j.metabol.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: The infection connection. Inflamm Res. 2014;63:803–19. doi: 10.1007/s00011-014-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150:1393–407.e5. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 45.Mokhtare M, Asadipanah M, Bahardoust M, Chaharmahali A, Sikaroudi MK, Khoshdelnezamiha M, et al. Efficacy of adding Luvos® Healing Earth supplementation to mebeverine in improving symptoms and quality of life of patients with diarrhea-predominant irritable bowel syndrome: A randomized clinical trial. Biomed Res Ther. 2018;5:2776–83. [Google Scholar]
- 46.Nanayakkara WS, Skidmore PM, O’Brien L, Wilkinson TJ, Gearry RB. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: the evidence to date. Clin Exp Gastroenterol. 2016;9:131–42. doi: 10.2147/CEG.S86798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institutes of Health, Office of Dietary Supplements. Vitamin D. 2020. Available from: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/.
- 48.Park S-Y, Park M-H, Yoon K-W, Cho S-B, Lee W-S, Park C-H, et al. Plasma 5-hydroxytryptamine concentration and its correlation with psychopathology in patients with irritable bowel syndrome. Gut Liver. 2009;3(1):26–30. doi: 10.5009/gnl.2009.3.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208:56–61. doi: 10.1016/j.jad.2016.08.082. [DOI] [PubMed] [Google Scholar]
- 50.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 51.Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, Part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29:2207–22. doi: 10.1096/fj.14-268342. [DOI] [PubMed] [Google Scholar]
- 52.Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014;28:2398–413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- 53.Pertile RAN, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016;333:193–203. doi: 10.1016/j.neuroscience.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 54.Phillips K, Wright BJ, Kent S. Psychosocial predictors of irritable bowel syndrome diagnosis and symptom severity. J Psychosom Res. 2013;75:467–74. doi: 10.1016/j.jpsychores.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Quigley EMM, Fried M, Gwee K-A, Khalif I, Hungin APS, Lindberg G, et al. World Gastroenterology Organisation Global Guidelines Irritable Bowel Syndrome: A Global Perspective Update September 2015. J Clin Gastroenterol. 2016;50:704–13. doi: 10.1097/MCG.0000000000000653. [DOI] [PubMed] [Google Scholar]
- 56.Roth DE, Al Mahmud A, Raqib R, Black RE, Baqui AH. Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non-pregnant women. Nutr J. 2012;11(1):114. doi: 10.1186/1475-2891-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabir MS, Haussler MR, Mallick S, Kaneko I, Lucas DA, Haussler CA, et al. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018;13:19. doi: 10.1186/s12263-018-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saha L. Irritable bowel syndrome: Pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759–73. doi: 10.3748/wjg.v20.i22.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saigo T, Tayama J, Hamaguchi T, Nakaya N, Tomiie T, Bernick PJ, et al. Gastrointestinal specific anxiety in irritable bowel syndrome: validation of the Japanese version of the visceral sensitivity index for university students. Biopsychosoc Med. 2014;8(1):10. doi: 10.1186/1751-0759-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sen F, Pinarbasi B, Issever H, Akyuz F, Mungan Z, Kaymakoglu S. Postprandial platelet-poor plasma 5-hydroxytryptamine concentrations during diarrhea and constipation periods of alternatingtype irritable bowel syndrome patients. Turk J Gastroenterol. 2011;22:270–8. doi: 10.4318/tjg.2011.0212. [DOI] [PubMed] [Google Scholar]
- 61.Sinagra E, Pompei G, Tomasello G, Cappello F, Morreale GC, Amvrosiadis G, et al. Inflammation in irritable bowel syndrome: Myth or new treatment target? World J Gastroenterol. 2016;22:2242–55. doi: 10.3748/wjg.v22.i7.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–98. doi: 10.1136/gut.2007.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sprake EF, Grant VA, Corfe BM. Vitamin D3 as a novel treatment for irritable bowel syndrome: single case leads to critical analysis of patient-centred data. BMJ Case Rep. 2012;2012:bcr–2012. doi: 10.1136/bcr-2012-007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tazzyman S, Richards N, Trueman AR, Evans AL, Grant VA, Garaiova I, et al. Vitamin D associates with improved quality of life in participants with irritable bowel syndrome: outcomes from a pilot trial. BMJ Open Gastroenterol. 2015;2(1):e000052. doi: 10.1136/bmjgast-2015-000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thijssen AY, Mujagic Z, Jonkers DMAE, Ludidi S, Keszthelyi D, Hesselink MA, et al. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment Pharmacol Ther. 2016;43:272–82. doi: 10.1111/apt.13459. [DOI] [PubMed] [Google Scholar]
- 66.Trinkley KE, Nahata MC. Medication management of irritable bowel syndrome. Digestion. 2014;89:253–67. doi: 10.1159/000362405. [DOI] [PubMed] [Google Scholar]
- 67.Williams CE, Williams EA, Corfe BM. Vitamin D status in irritable bowel syndrome and the impact of supplementation on symptoms: what do we know and what do we need to know? Eur J Clin Nutr. 2018;72:1358–1363. doi: 10.1038/s41430-017-0064-z. [DOI] [PubMed] [Google Scholar]
- 68.Yazar A, Büyükafpar K, Polat G, Pata C, Kanýk A, Tiftik EN, et al. The urinary 5-hydroxyindole acetic acid and plasma nitric oxide levels in irritable bowel syndrome: a preliminary study. Scott Med J. 2005;50(1):27–9. doi: 10.1177/003693300505000111. [DOI] [PubMed] [Google Scholar]
- 69.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]