Abstract
MAS-related G protein coupled receptor-X2 (MRGPRX2), expressed in human mast cells, is associated with drug-induced pseudo-allergic reactions. Dogs are highly susceptible to drug-induced anaphylactoid reactions caused by various drugs; however, the distribution and physiological function of canine MRGPR family genes, including MRGPRX2, remain largely unknown. In the present study, we clarified the distribution of dog MRGPR family genes by real-time quantitative PCR and in situ hybridisation. We also investigated the stimulatory effects of various histamine-releasing agents, including fluoroquinolones, on HEK293 cells transiently transfected with dog MRGPR family genes to identify their physiological function. Dog MRGPRX2 and MRGPRG were distributed in a limited number of tissues, including the skin (from the eyelid, abdomen, and cheek), whereas MRGPRD and MRGPRF were extensively expressed in almost all tissues examined. Histochemical and in situ hybridisation analyses revealed that MRGPRX2 was expressed in dog connective tissue-type mast cells in the skin. Intracellular Ca2+ mobilisation assay revealed that HEK293 cells, expressing dog MRGPRX2 or human MRGPRX2, but not dog MRGPRD, MRGPRF, and MRGPRG, responded to histamine-releasing agents. Our results suggest that dog MRGPRX2 is the functional orthologue of human MRGPRX2 and plays an essential role in drug-induced anaphylactoid reactions in dogs.
Subject terms: Acute inflammation, Drug development, G protein-coupled receptors
Introduction
Pseudo-allergic drug reactions, including injection-site erythema and swelling, are one of the most commonly observed adverse events associated with intravenous administration of drugs, such as fluoroquinolones, antibacterial agents, and peptidergic drugs1,2. In certain cases, these drugs have been reported to induce more serious outcomes such as hypotension and shock-like syndrome3. Recently, pseudo-allergic adverse reactions have been demonstrated to be induced through the activation of MAS-related G protein coupled receptor-X2 (MRGPRX2), which is a Gi- or Gq-coupled receptor expressed in human mast cells4. The MRGPR family in rodents and humans comprises ~ 40 members and can be divided into several subfamilies (MRGPRA to -H and -X) because of sequence similarities5–9. Among rodents and primates, subfamilies A, B, C, and H exist only in rodents, whereas subfamily X is detected in primates, including humans, macaques, and rhesus monkeys10. Mrgprb2 and Mrgprb3 are the mouse and rat orthologues of human MRGPRX2, respectively11,12. Moreover, functional heterogeneity exists between human MRGPRX2 and mouse Mrgprb24,11. Sabramanian et al. have suggested that the Mrgprb2 mutant mouse may not be a suitable model to screen drugs with pseudo-allergic potential for human use4. This is because the half-maximum effective concentration (EC50) value of Ca2+ mobilisation, induced by substance P and fluoroquinolones, in cells transfected with human MRGPRX2 is markedly lower than that of cells transfected with mouse Mrgprb24,11.
Dogs are one of the most commonly used non-rodent species for the evaluation of preclinical toxicity during drug development13. In addition, dogs are highly susceptible to drug-induced anaphylactoid reactions including severe hypotension and shock-like syndrome caused by various drugs14–17. In fact, several fluoroquinolones, opioids, and neuromuscular blocking agents have been shown to produce severe hypotension in parallel to elevation of blood histamine when administered intravenously in bolus doses to dogs18–21. Furthermore, the dose levels of these drugs to induce histamine release and cardiovascular adverse effects were 30- to 100-fold lower in the dog than the rat16, suggesting that the dog may be a suitable model for detecting the pseudo-allergic potential including cardiovascular adverse reactions of candidate drugs in the preclinical phase. In dogs, MRGPRA, C, D, E, F, G, and H, in addition to X2, have been identified so far22,23, and among these genes, a total of four genes encoding MRGPR proteins (D, F, G, and X2) have been listed in the National Center for Biotechnology Information (NCBI). More recently, Grimes et al. have shown that U2OS cells expressing beagle dog MRGPRX2 responded to compound 48/80 and various peptidergic drugs24. However, the localisation and physiological function of dog MRGPR family genes, including MRGPRX2, remain largely unknown.
The present study was designed to identify the functional orthologue of human MRGPRX2 in canine mast cells. We evaluated the distribution of dog MRGPR family genes (D, F, G, and X2) in 21 tissues or organs obtained from male beagle dogs by quantitative reverse transcriptional PCR (RT-qPCR). We also investigated the expression of MRGPRX2 in dog mast cells from several tissues by histochemical and in situ hybridisation (ISH) analyses. Furthermore, we confirmed the stimulatory effects of compound 48/80 and several fluoroquinolones (ciprofloxacin [CPFX], gatifloxacin [GFLX], levofloxacin [LVFX], and pazufloxacin [PZFX]) on HEK293 cells transiently transfected with dog MRGPR family genes or human MRGPRX2 to identify the physiological function of dog MRGPR family genes by intracellular Ca2+ mobilisation assay.
Results
Characteristic of MRGPR family in dogs
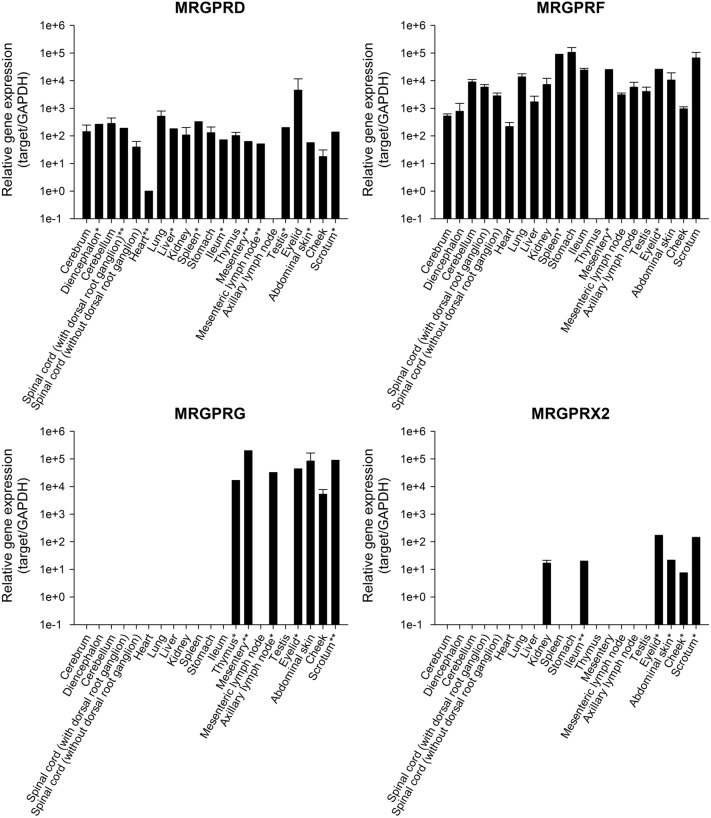
Homology analysis using BLASTP revealed that dog MRGPRX2 had the highest amino acid sequence homology to human MRGPRX2 (62% sequence homology), whereas dog MRGPRD, F, and G only shared 30–40% sequence identity with human MRGPRX2 (Table 1). Among four dog MRGPR family genes, MRGPRD and MRGPRF were widely expressed in almost all the tissues (Fig. 1). On the contrary, MRGPRG and MRGPRX2 were not found systemically and were mainly localised to the cutaneous tissues including the eyelid, abdominal skin, cheek, and scrotum (Fig. 1). MRGPRX2 was also found to be expressed in the kidney and ileum, and MRGPRG was found in the thymus, mesentery, and axillary lymph nodes (Fig. 1).
Table 1.
Amino acid sequence homology between members of dog MRGPR family and human MRGPRX2.
| % Identity | Size (amino acid) | Accession no. | |
|---|---|---|---|
| Dog MRGPRD | 41 | 349 | XP_540806 |
| Dog MRGPRF | 38 | 343 | NP_001300758 |
| Dog MRGPRG | 32 | 286 | NP_001300759 |
| Dog MRGPRX2 | 62 | 437 | XP_005633869 |
| Mouse Mrgprb2 | 53 | 338 | NP_780740 |
| Rat Mrgprb3 | 56 | 247 | AAQ08313 |
| Human MRGPRX2 | – | 330 | NP_001290544 |
Homology analysis (Protein BLAST, BLASTP) was performed using the Basic Local Alignment Search Tool (BLAST, https://blast.ncbi.nlm.nih.gov/Blast.cgi) of the National Center for Biotechnology Information.
Figure 1.
Localisation of dog MRGPR family genes. RT-qPCR analysis was performed to determine MRGPR family gene expression in various canine tissues. Relative gene expression level was calculated by 2−ΔΔCt method, using GAPDH as internal control. The ΔΔCt was calculated by subtracting the ΔCt of MRGPRD in the heart from the ΔCt of each sample. Data are expressed as the mean ± SD of three animals, except for the mean of two animals (*) or individual data of one animal (**) because of Ct > 40 or non-specific amplification.
Expression of MRGPRX2 in mast cells of dogs
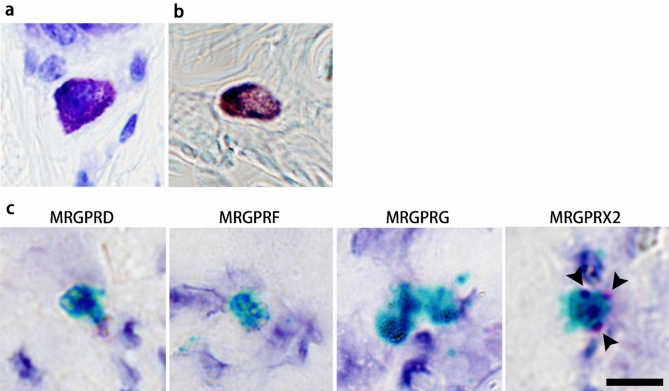
Histochemical analysis revealed that dog skin mast cells, which showed metachromatic staining with toluidine blue, were positive for both alcian blue and safranin O staining (Fig. 2a,b). Furthermore, ISH showed that dog skin mast cells co-expressed c-kit and MRGPRX2, but not MRGPRD, MRGPRF, and MRGPRG (Fig. 2c).
Figure 2.
Characteristics of dog skin (cheek) mast cells. Skin mast cells were positive for alcian blue and safranin O and expressed MRGPRX2. (a) Toluidine blue staining. (b) Alcian blue (blue) and safranin O (red) staining. (c) In situ hybridisation for c-kit (green) and MRGPR family genes (red). No expression of MRGPRD, MRGPRF, or MRGPRG was found in canine skin mast cells. Scale bar: 10 μm.
Dog MRGPRX2 is the functional orthologue of human MRGPRX2
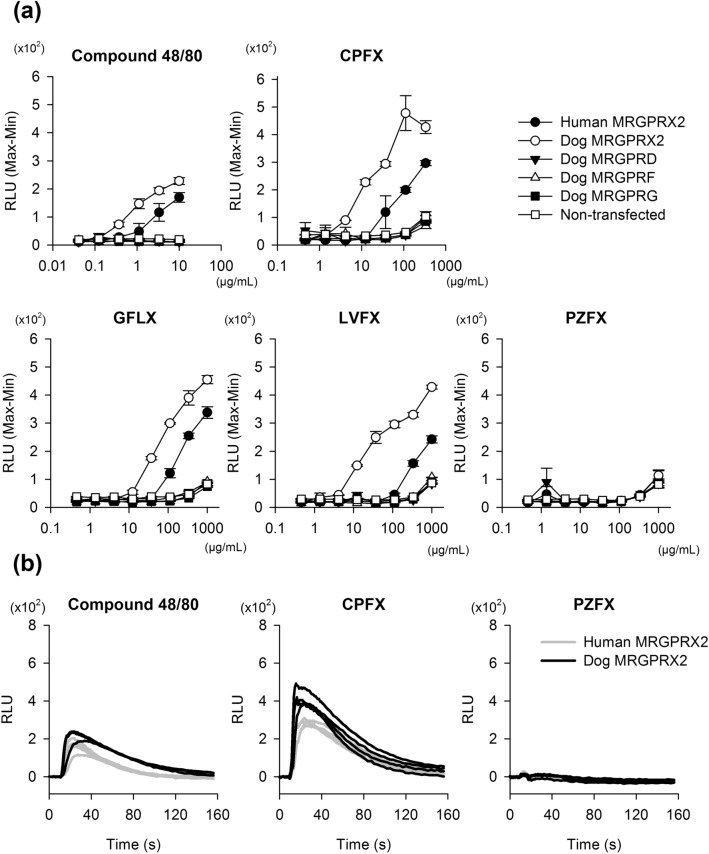
HEK293 cells transfected with dog MRGPRX2 responded to compound 48/80, CPFX, GFLX, and LVFX, whereas cells expressing dog MRGPRD, MRGPRF, and MRGPRG did not react to any of the test articles (Fig. 3a). On the contrary, PZFX, which does not induce histamine release in dogs25, did not activate dog or human MRGPRX2. The increase in intracellular Ca2+ levels, both in HEK293 cells transfected with dog MRGPRX2 or human MRGPRX2, caused by compound 48/80, CPFX, GFLX, and LVFX were concentration-dependent. Interestingly, the EC50 values of compound 48/80, CPFX, GFLX, and LVFX to activate MRGPRX2 were lower in dog MRGPRX2-expressing cells compared to those in human MRGPRX2-expressing cells (Table 2). Intracellular Ca2+ mobilisation in HEK293 cells expressing dog MRGPRX2 or human MRGPRX2 occurred immediately after treatment with the test articles (Fig. 3b).
Figure 3.
Various histamine-releasing agents activate dog MRGPRX2 and human MRGPRX2 expressed in HEK293 cells. HEK293 cells transiently transfected with dog MRGPR family genes (D, F, G, or X2) or human MRGPRX2 were exposed to compound 48/80 or fluoroquinolones (ciprofloxacin [CPFX], gatifloxacin [GFLX], levofloxacin [LVFX], and pazufloxacin [PZFX]). (a) Increase in intracellular Ca2+ levels in a concentration dependent manner. Data represent the mean of quadruplicate assays. Non-transfected HEK293 cells were used as a negative control. (b) Time-course changes of intracellular Ca2+ levels in dog or human MRGPRX2-expressing HEK293 cells. Traces show representative intracellular Ca2+ fluctuation following exposure to compound 48/80 (10 μg/mL), CPFX (333 μg/mL), and PZFX (333 μg/mL). Test articles were perfused from 10 s. RLU Relative light units, s second.
Table 2.
Half-maximum effective concentration (EC50) values of test articles on changes in intracellular Ca2+ levels in human or dog MRGPRX2-expressing cells.
| Test article | EC50 (μg/mL) ± SD | |
|---|---|---|
| Human MRGPRX2 | Dog MRGPRX2 | |
| Compound 48/80 | 3.0 ± 0.5 | 1.1 ± 0.4 |
| CPFX | 93.5 ± 10.3 | 14.5 ± 1.8 |
| GFLX | 198.5 ± 19.7 | 78.7 ± 10.9 |
| LVFX | 384.7 ± 101.3 | 58.9 ± 19.9 |
Data represent the mean ± SD of three independent experiments.
CPFX ciprofloxacin, GFLX gatifloxacin, LVFX levofloxacin.
Discussion
MRGPR family genes are expressed predominantly in the sensory neurons of the dorsal root ganglia (DRG) and trigeminal ganglia, and mast cells of mammals including rodents and primates10. Among MRGPR subfamilies (MRGPRA to H, and X), subfamily A to C and H are present in rodents10. MRGPRD to G subfamilies have been reported to be conserved between primates and rodents, and encode one protein per species5–7. MRGPRX subfamily exists in primates, and four proteins (MRGPRX1 to X4) are listed in NC-IUPHAR10. With regard to dog, which is highly susceptible to drug-induced anaphylactoid reactions, MRGPRA, C, D, E, F, G, and H, in addition to X2, have been identified so far22,23. In the present study, we characterised four dog MRGPR subfamilies (MRGPRD, MRGPRF, MRGPRG, and MRGPRX2), which were listed in the NCBI database. MRGPRD and MRGPRF were expressed in a variety of tissues and organs in dogs. In rodents, Mrgprd and Mrgprf have been reported to be expressed in a relatively limited number of tissues; Mrgprd was localised to the DRG, urinary bladder, testis, uterus, and arteries26, and Mrgprf was mainly distributed in the vas deferens, uterus, intestine, stomach, and aorta27, suggesting that dog MRGPRD and MRGPRF have distinct distribution characteristics from rodents. However, Mrgprd was reported to be expressed in aortic endothelial cells and leukocytes, including neutrophils, macrophages, and lymphocytes, in rodents28–30. Furthermore, human MRGPRF was identified in enteroendocrine cells31, in addition to enteric neurons32. We did not evaluate the localisation of dog MRGPRD and MRGPRF at the cellular level in this study, and hypothesise that they might be expressed in various cells or tissues, including arteries, blood cells, or enteroendocrine cells, on the basis of the information gathered. In this study, MRGPRG and MRGPRX2 were confirmed to be expressed in a limited number of tissues, including the skin (eyelid, abdomen, and cheek) of dogs. In the Genotype-Tissue Expression (GTEx) project, human MRGPRG has been reported to show high expression in the testis (GENE Code ID. ENSG00000182170.3, GTEx Analysis Release V8), where expression of dog MRGPRG was not detected in our study. Human MRGPRX2 has been shown to be highly expressed in the skin, adipose tissue, bladder, and colon12, and mast cells, sensory neurons, and keratinocytes5,33,34. The distribution profile of dog MRGPRX2 appeared to be consistent with that of human MRGPRX2, at least partially. In our study, quantitative PCR analysis showed that the expression levels of dog MRGPRX2 appeared to be lower than those of MRGPRD, MRGPRF, and MRGPRG; therefore, the expression profile of dog MRGPRX2 should be further investigated.
Dog MRGPRX2 has relatively higher sequence homology (62%) to human MRGPRX2 than that of rodents; homologies of Mrgprb2 and Mrgprb3, the human MRGPRX2 orthologues of mice and rats, are 53% and 56%, respectively. One can hypothesise that lower homologies between rodent and human MRGPR proteins result in different sensitives to drugs. In fact, McNeil et al. demonstrated that the EC50 of substance P in mouse Mrgprb2-expressing cells is 360-fold higher compared to that in human MRGPRX2-expressing cells (mouse: 54 μM, human: 152 nM)11. Therefore, it is crucial to select appropriate animal species to predict drug-induced pseudo-allergic reactions in humans.
As is the case with rodents35, dog mast cells are classified into two types; the mucosal-type mast cell (MMC) or connective tissue-type mast cell (CTMC)16,17. Histochemical analysis revealed that mast cells present in the skin from the cheek were positive for both alcian blue and safranin O, indicative of CTMC, in line with our previous work, which responded to basic secretagogues16,17. Furthermore, CTMC in the skin expressed c-kit and MRGPRX2, indicating that canine CTMC in the skin expressed the human MRGPRX2 orthologue.
More recently, Grimes et al. reported that compound 48/80 and several types of peptides activated dog MRGPRX224. Consistent with their research24, we also confirmed that compound 48/80 activated MRGPRX2, but not MRGPRD, F, and G, suggesting that dog MRGPRX2 expressed in canine CTMC is the functional orthologue of human MRGPRX2. In human MRGPRX2-expressing cells, the EC50 of certain drugs, such as compound 48/80, somewhat varied among the facilities11,12,24. This might have resulted from the difference in the host cells used, transfection methods, and co-expressed Gα proteins. For example, EC50 of compound 48/80 to activate human MRGPRX2 ranges from 0.47–3.75 μg/mL11,12,24. Grimes et al. reported that the EC50 of compound 48/80 for activating human MRGPRX2 with Gα16 and dog MRGPRX2 without Gα16 in their assay platform, was comparable24. However, the EC50 value of compound 48/80 in dog MRGPRX2-expressing cells was ca. three times lower than that in human MRGPRX2-expressing cells in this study. Therefore, further investigations are necessary to clarify the factors affecting distinct reactivity among assay platforms.
Dog MRGPRX2 was also activated with several fluoroquinolones, including CPFX, in the present study. The EC50 values of CPFX and LVFX required to activate dog MRGPRX2 were approximately 15 μg/mL and 60 μg/mL, respectively. These values were comparable to results in our previous report, where CPFX and LVFX induced histamine release from dispersed canine skin mast cells at 10 μg/mL or more and 30 μg/mL or more, respectively16. In addition, the EC50 value of CPFX required to activate human MRGPRX2 was approximately 100 μg/mL, which was comparable to the concentration (200 μg/mL) required to induce histamine release from dispersed mast cells of human skin36. Further, PZFX does not cause histamine release even in the dog, the most susceptible species to fluoroquinolones25. In the present study, PZFX did not activate dog MRGPRX2, indicating our assay platform using HEK293 cells expressing dog MRGPRX2 could mimic the histamine release assay using dispersed dog mast cells.
The difference in EC50 values of compound 48/80 between dog MRGPRX2 and human MRGPRX2 was smaller (ca. threefold) than the values of CPFX or LVFX (ca. sevenfold) in this study, suggesting that there might be differences in ligand selectivity between dog and human MRGPRX2. A basic substituent at position 7 of fluoroquinolones is suggested to associate with its histamine-releasing property37. Thus, it might be valuable to compare the interaction between the basic substituent at position 7 of each fluoroquinolone and each receptor by structure–activity relationships to elucidate the factors causing differences in EC50 values between human and dog proteins. Recently, it has been reported that the binding of compound 48/80 or substance P to MRGPR genes was remarkably lowered by replacing the amino acid at position 164 of human MRGPRX2 and the amino acid at position 171 of murine Mrgprb2, which are located in equivalent tertiary structural regions38. To clarify the factors contributing to species-based differences in sensitivity, further investigation on the structures of the receptors, including comparisons of amino acid sequences and tertiary structures, and the identification of binding sites using recombinant mutants is required.
It has been reported that human and dog mast cells showed high similarity; protease content and histamine releasing property including degranulation process by several drugs to date39. In addition, as we have shown in this study, dog MRGPRX2 has high similarities to human MRGPRX2 both in distributions and functions. Therefore, in vivo and in vitro systems using dogs have the potential to be the best models for elucidating and predicting the mechanisms of mast cell-related adverse effects in human clinical.
In summary, dog MRGPRX2 was distributed in a limited number of tissues, including the skin, similar to human MRGPRX2, and expressed in CTMC. Basic secretagogue compound 48/80 and fluoroquinolones activated dog MRGPRX2, indicating that dog MRGPRX2 is the functional orthologue of human MRGPRX2. As shown here, dog MRGPRX2 was found to be highly susceptible to certain drugs, including fluoroquinolones. Based on the similarity between human and dog mast cells including MRGPRX2, and on the susceptibility of dogs to anaphylactoid reactions, dog is a suitable model to predict the potential risk for human use and elucidate the mechanism of drug-induced pseudo-allergic reactions.
Materials and methods
Homology analysis of dog MRGPR family with human MRGPRX2
Homology analysis of the amino acid sequence (Protein BLAST, BLASTP) was carried out using Basic Local Alignment Search Tool (BLAST, https://blast.ncbi.nlm.nih.gov/Blast.cgi) of NCBI.
Reagents
Compound 48/80 was purchased from Sigma-Aldrich Co. LLC. (St. Louis, MO, USA). CPFX and LVFX were obtained from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan), and GFLX and PZFX were obtained from LKT Laboratories Inc. (St. Paul, MN, USA).
Animals
A total of three male beagle dogs were purchased from Marshall BioResources Japan (Tsukuba, Japan). The animals weighing 10–13 kg were 2–3 years old. The animals were housed individually in stainless steel cages with a controlled temperature of 18–28 °C and humidity of 30–70%, and a 12-h light (from 07:00 to 19:00, 300 luces or more) and 12-h dark cycle. Certified canine diet (CD-5M, Clea Japan, Inc., Tokyo, Japan) and chlorinated water were provided to each animal ad libitum. The experimental protocol was approved in advance by the Ethics Review Committee for Animal Experimentation of Daiichi Sankyo Co., Ltd. (Tokyo, Japan). All animal procedures were performed in accordance with the guidelines of the Animal Care and Use Committee of Daiichi Sankyo Co., Ltd.
Sampling of the tissues/organs
Animals were euthanised humanely under anaesthesia with an intravenous injection of sodium pentobarbital (25 mg/kg, Somnopentyl Injection, Kyoritsu Seiyaku Corporation, Tokyo, Japan). The brain (cerebrum, diencephalon, and cerebellum), spinal cord (lumbar, with or without dorsal root ganglion), heart (ventricular papillary muscle), lung (right lower lobe), liver (left lateral lobe), kidney, spleen, stomach, ileum, thymus, mesentery, mesenteric lymph node, axillary lymph node, testis, eyelid, abdominal skin, cheek, and scrotum were collected. A portion of each tissue (approximately 200 mg) was excised, snap-frozen in liquid nitrogen, and stored at − 80 °C until RNA extraction. The remaining tissue samples were fixed in 10 vol% neutral buffered formalin and embedded in paraffin for immunohistochemistry and in situ hybridisation.
Quantitative reverse transcription PCR (RT-qPCR)
Total RNA was extracted from each frozen tissue sample using RNeasy Mini Kit (QIAGEN, Hilden, Germany) or TRIzol Reagent (Thermo Fisher Scientific Inc.) with RNase-Free DNase Set (Thermo Fisher Scientific Inc.), according to the manufacturer's protocol. The cDNA samples were synthesised using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific Inc.). Expression of dog MRGPRD, MRGPRF, MRGPRG, MRGPRX2, and GAPDH was analysed using the 7900HT Fast Real Time PCR System (Thermo Fisher Scientific Inc.) with the Fast SYBR Green Master Mix (Thermo Fisher Scientific Inc.) and primer pairs for each gene (see Table 3). qPCR amplifications were performed in duplicate as follows: initial denaturation at 95 °C for 20 s, followed by 40 cycles of amplification at 95 °C for 1 s and 60 °C for 20 s. The results of the target genes (MRGPRD, MRGPRF, MRGPRG, and MRGPRX2) were normalised against GAPDH. Gene expression levels were represented as relative gene expression using 2−ΔΔCt method. The ΔΔCt was calculated by subtracting the ΔCt of MRGPRD in the heart from the ΔCt of each sample.
Table 3.
Sequences of the primer pairs for dog MRGPR family genes for RT-qPCR.
| Target gene (Accession no.) | Forward primer 5′–3′ | Reverse primer 5′–3′ |
|---|---|---|
| Dog MRGPRD (XM_540806) | GGAAGTCCTACATGGCATTG | CCAGATCACCAGGCTGTTC |
| Dog MRGPRF (NM_001313829) | GAGATGGTGGGGAACTGTTC | GAGACAAAGGAGCAGGAAGATG |
| Dog MRGPRG (NM_001313830) | CCTTCACCAACGTGCTCTTC | GAAGCCGAGGAACAGGAAG |
| Dog MRGPRX2 (XM_005633812) | GACGCTGCAGTCACAGTC | CTGGTCACTTGCATTCTTTG |
| Dog GAPDH (NM_001003142) | GGTCGGAGTGAACGGATTTG | GGAACATGTACACCATGTAGTTGAG |
Histochemical analysis and in situ hybridisation
Histochemical analysis and in situ hybridisation were conducted using skin samples, based on the present distribution study of MRGPRX2 and our previous study using dispersed mast cells17. The tissue specimens were stained with 0.1% toluidine blue (pH 3) or 0.1% alcian blue (pH 0.3) and 0.1% safranin O (pH 1). ISH was performed using the RNAscope® 2.5 HD Duplex Reagent Kit and RNAscope probes obtained from Advanced Cell Diagnostics Inc. (Newark, CA, USA), according to the manufacturer’s instructions. Signals for c-kit were detected with horseradish peroxidase-based green chromogens, and signals for dog MRGPR family (D, F, G, and X2) were detected with alkaline phosphatase-based Fast Red chromogens.
Transfection of HEK293 cells with dog or human MRGPR genes
HEK293 cells obtained from the JCRB Cell Bank (Osaka, Japan) were transfected transiently with dog MRGPRD, MRGPRF, MRGPRG, or MRGPRX2 or human MRGPRX2. Lipofectamine 2000 Reagent (Thermo Fisher Scientific Inc.) and pcDNA3.1(+) vector containing each gene were diluted and mixed using Opti-MEM I Reduced Serum Medium (Thermo Fisher Scientific Inc.) to prepare lipid-DNA complexes (final concentrations: lipofectamine 2.5 μL/mL and DNA 2,500 ng/mL). HEK293 cells were detached using TrypLE™ Express (Thermo Fisher Scientific Inc.) and prepared to 7 × 105 cells/mL with the lipid-DNA complex. Thereafter, 25 μL cells (1.75 × 104 cells/well) were seeded per well in 384-well flat-bottomed plates (Corning Incorporated, Corning, NY, USA) and incubated overnight at 37 °C under 5% CO2 conditions. Cells treated with plasmid-free lipid solution were used as negative control (non-transfected cells).
Ca2+ mobilisation assay
Test articles were dissolved in HBSS (pH 7.4, Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 20 mM HEPES (Sigma-Aldrich Co. LLC.) and 0.05 vol% bovine serum albumin (BSA, Sigma-Aldrich Co. LLC.). The highest concentration of the fluoroquinolones was set at 1000 μg/mL based on previous reports at which the test substances induced marked intracellular Ca2+ mobilisation in human MRGPRX2-expressing HEK293 cells11 or caused histamine release in rat or human mast cells16,25,36. Intracellular Ca2+ levels were analysed using Calcium Kit II-iCellux (Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according to the manufacturer's instructions. HEK293 cells (1.75 × 104 cells/well) were loaded with 1.25 mM probenecid and calcium probe for 45 min at 25 °C. Changes in fluorescence intensities before and after addition of the test articles were measured over time using FLIPR Tetra (Molecular Devices, LLC., Sunnyvale, CA, USA) with excitation at 470–495 nm and emission at 515–575 nm. The test articles were added at 10 s after beginning the measurements. Samples were measured in duplicate or quadruplicate. The data were analysed using ScreenWorks (Molecular Devices, LLC. Version 3.2.0.14) to determine the difference between maximal and minimal fluorescence intensity (max–min). As CPFX (1,000 μg/mL) induced nonspecific increase in intracellular Ca2+ levels in non-transfected cells, these data were excluded from the analysis.
Statistical analysis
Data represent the mean ± SD of three animals for distribution study or three independent assays for Ca2+ mobilisation. EC50 of each test article used in the Ca2+ mobilisation assay was calculated using the sigmoid Emax model. These analyses were performed by using the SAS System Release 8.2 software (SAS Institute Inc., Cary, NC, USA).
Acknowledgements
The authors would like to thank Mr. Junya Matsushita, Mr. Satoshi Tamai, Ms. Noriyo Niino, Ms. Shinobu Hakamata, and Mr. Toyo Sakurai for the excellent support in performing the experiments.
Author contributions
E.H.-Y. designed the research, performed the experiments, analysed the results, and wrote the article. T.I. and K.K. performed the experiments, analysed the results, and reviewed the article. T.I., K.K., Y.T. and K.M. contributed to planning and performing the study and writing and editing the article.
Data availability
All relevant data are present within the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kelesidis T, Fleisher J, Tsiodras S. Anaphylactoid reaction considered ciprofloxacin related: A case report and literature review. Clin. Ther. 2010;32:515–526. doi: 10.1016/j.clinthera.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumry WR, et al. Randomized placebo-controlled trial of the bradykinin B(2) receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: The FAST-3 trial. Ann. Allergy Asthma Immunol. 2011;107:529–537. doi: 10.1016/j.anai.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Sivagnanam S, Deleu D. Red man syndrome. Crit. Care. 2003;7:119–120. doi: 10.1186/cc1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016;138:700–710. doi: 10.1016/j.jaci.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 6.Lembo PM, et al. Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat. Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- 7.Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, et al. Cloning and expression of MRG receptors in macaque, mouse, and human. Brain Res. Mol. Brain Res. 2005;133:187–197. doi: 10.1016/j.molbrainres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Burstein ES, et al. Characterization of the Mas-related gene family: Structural and functional conservation of human and rhesus MrgX receptors. Br. J. Pharmacol. 2006;147:73–82. doi: 10.1038/sj.bjp.0706448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solinski HJ, Gudermann T, Breit A. Pharmacology and signaling of MAS-related G protein-coupled receptors. Pharmacol. Rev. 2014;66:570–597. doi: 10.1124/pr.113.008425. [DOI] [PubMed] [Google Scholar]
- 11.McNeil BD, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519:237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatemoto K, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 13.Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals M3(R2). International Conference on Harmonization (2009). [PubMed]
- 14.Ennis M, Lorenz W, Kapp B, Luben L, Schmal A. Comparison of the histamine-releasing activity of cremophor E1 and some of its derivatives in two experimental models: The in vivo anaesthetized dog and in vitro rat peritoneal mast cells. Agents Actions. 1985;16:265–268. doi: 10.1007/BF01983156. [DOI] [PubMed] [Google Scholar]
- 15.Masini E, Planchenault J, Pezziardi F, Gautier P, Gagnol JP. Histamine-releasing properties of Polysorbate 80 in vitro and in vivo: Correlation with its hypotensive action in the dog. Agents Actions. 1985;16:470–477. doi: 10.1007/BF01983649. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Maru C, Takasuna K. Characterization of histamine release induced by fluoroquinolone antibacterial agents in-vivo and in-vitro. J. Pharm. Pharmacol. 2000;52:577–584. doi: 10.1211/0022357001774228. [DOI] [PubMed] [Google Scholar]
- 17.Mori K, Shibano M, Satoh H, Takasuna K, Furuhama K. Differential response of mast cells separated from various organs and basophils of dogs to the fluoroquinolone antimicrobial levofloxacin. Arch. Toxicol. 2001;75:227–233. doi: 10.1007/s002040100230. [DOI] [PubMed] [Google Scholar]
- 18.Takasuna K, et al. General pharmacology of the new quinolone antibacterial agent levofloxacin. Arzneimittelforschung. 1992;43:408–418. [PubMed] [Google Scholar]
- 19.Furuhata K, et al. Histamine-releasing properties of T-3762, a novel fluoroquinolone antimicrobial agent in intravenous use. I. Effects of doses and infusion rate on blood pressure, heart rate and plasma histamine concentration. Biol. Pharm. Bull. 1998;21:456–460. doi: 10.1248/bpb.21.456. [DOI] [PubMed] [Google Scholar]
- 20.Robinson EP, Faggella AM, Henry DP, Russell WL. Comparison of histamine release induced by morphine and oxymorphone administration in dogs. Am. J. Vet. Res. 1988;49:1699–1701. [PubMed] [Google Scholar]
- 21.Lee D, Johnson DL. Effect of D-tubocurarine and anaesthesia upon cardiac output in normal and histamine-depleted dogs. Can. Anaesth. Soc. J. 1971;18:157–165. doi: 10.1007/BF03025445. [DOI] [PubMed] [Google Scholar]
- 22.Premzl M. Comparative genomic analysis of eutherian Mas-related G protein-coupled receptor genes. Gene. 2014;540:16–19. doi: 10.1016/j.gene.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 23.Haitina T, Fredriksson R, Foord SM, Schioth HB, Gloriam DE. The G protein-coupled receptor subset of the dog genome is more similar to that in humans than rodents. BMC Genom. 2009;10:24. doi: 10.1186/1471-2164-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimes J, et al. MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions. Pharmacol. Res. Perspect. 2019;7:e00547. doi: 10.1002/prp2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuhata K, et al. Histamine-releasing properties of T-3762, a novel fluoroquinolone antimicrobial agent in intravenous use. II. Dermovascular permeability-increasing effect and action on peritoneal mast cells. Biol. Pharm. Bull. 1998;21:461–464. doi: 10.1248/bpb.21.461. [DOI] [PubMed] [Google Scholar]
- 26.Shinohara T, et al. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J. Biol. Chem. 2004;279:23559–23564. doi: 10.1074/jbc.M314240200. [DOI] [PubMed] [Google Scholar]
- 27.Ross PC, et al. RTA, a candidate G protein-coupled receptor: Cloning, sequencing, and tissue distribution. Proc. Natl. Acad. Sci. U. S. A. 1990;87:3052–3056. doi: 10.1073/pnas.87.8.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Etelvino GM, Peluso AA, Santos RA. New components of the renin-angiotensin system: Alamandine and the MAS-related G protein-coupled receptor D. Curr. Hypertens. Rep. 2014;16:433. doi: 10.1007/s11906-014-0433-0. [DOI] [PubMed] [Google Scholar]
- 29.Da Silva AR, et al. Alamandine abrogates neutrophil degranulation in atherosclerotic mice. Eur. J. Clin. Invest. 2017;47:117–128. doi: 10.1111/eci.12708. [DOI] [PubMed] [Google Scholar]
- 30.Zhou C, et al. Expression and localization of MrgprD in mouse intestinal tract. Cell Tissue Res. 2019;377:259–268. doi: 10.1007/s00441-019-03017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora R, et al. Activation of human Mas-related G protein-coupled receptor F (MRGPRF) by the cysteine protease Cathepsin S: Implication in neuro-immune communication within the gut. FASEB J. 2019;33:585.1. doi: 10.1096/fj.201800754RR. [DOI] [Google Scholar]
- 32.Avula LR, et al. The effect of inflammation on the expression and distribution of the MAS-related gene receptors MrgE and MrgF in the murine ileum. Histochem. Cell Biol. 2011;136:569–585. doi: 10.1007/s00418-011-0862-7. [DOI] [PubMed] [Google Scholar]
- 33.Azimi E, Reddy VB, Lerner EA. Brief communication: MRGPRX2, atopic dermatitis and red man syndrome. Itch (Phila.) 2017 doi: 10.1097/itx.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiatsurayanon C, et al. Angiogenic peptide (AG)-30/5C activates human keratinocytes to produce cytokines/chemokines and to migrate and proliferate via MrgX receptors. J. Dermatol. Sci. 2016;83:190–199. doi: 10.1016/j.jdermsci.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Stevens RL, Rothenberg ME, Levi-Schaffer F, Austen KF. Ontogeny of in vitro-differentiated mouse mast cells. Fed. Proc. 1987;46:1915–1919. [PubMed] [Google Scholar]
- 36.Nakagawa T, Shimada J, Mizushima Y, Takaishi T, Morita Y. Effect of ciprofloxacin on histamine release from human and rat mast cells. Jpn. J. Inflamm. 1995;15:337–338. [Google Scholar]
- 37.Mori K, Maru C, Takasuna K, Furuhama K. Mechanism of histamine release induced by levofloxacin, a fluoroquinolone antibacterial agent. Eur. J. Pharmacol. 2000;394:51–55. doi: 10.1016/S0014-2999(00)00147-3. [DOI] [PubMed] [Google Scholar]
- 38.Reddy VB, Graham TA, Azimi E, Lerner EA. A single amino acid in MRGPRX2 necessary for binding and activation by pruritogens. J. Allergy Clin. Immunol. 2017;140:1726–1728. doi: 10.1016/j.jaci.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Mora F, Puigdemont A, Torres R. The role of mast cells in atopy: What can we learn from canine models? A thorough review of the biology of mast cells in canine and human systems. Br. J. Dermatol. 2006;155:1109–1123. doi: 10.1111/j.1365-2133.2006.07494.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are present within the paper.