Abstract
Background: New treatments combating bone and extraskeletal metastases are needed for patients with metastatic castration-resistant prostate cancer. The majority of metastases overexpress prostate-specific membrane antigen (PSMA), making it an ideal candidate for targeted radionuclide therapy.
Objective: The aim of this study was to test a novel liquid 224Ra/212Pb-generator for the rapid preparation of a dual-alpha targeting solution. Here, PSMA-targeting ligands are labelled with 212Pb in the 224Ra-solution in transient equilibrium with daughter nuclides. Thus, natural bone-seeking 224Ra targeting sclerotic bone metastases and 212Pb-chelated PSMA ligands targeting PSMA-expressing tumour cells are obtained.
Methods: Two PSMA-targeting ligands, the p-SCN-Bn-TCMC-PSMA ligand (NG001), specifically developed for chelating 212Pb, and the most clinically used DOTA-based PSMA-617 were labelled with 212Pb. Radiolabelling and targeting potential were investigated in situ, in vitro (PSMA-positive C4-2 human prostate cancer cells) and in vivo (athymic mice bearing C4-2 xenografts).
Results: NG001 was rapidly labelled with 212Pb (radiochemical purity >94% at concentrations of ≥15 µg/ml) using the liquid 224Ra/212Pb-generator. The high radiochemical purity and stability of [212Pb]Pb-NG001 were demonstrated over 48 hours in the presence of ascorbic acid and albumin. Similar binding abilities of the 212Pb-labelled ligands were observed in C4-2 cells. The PSMA ligands displayed comparable tumour uptake after 2 hours, but NG001 showed a 3.5-fold lower kidney uptake than PSMA-617. Radium-224 was not chelated and, hence, showed high uptake in bones.
Conclusion: A fast method for the labelling of PSMA ligands with 212Pb in the 224Ra/212Pb-solution was developed. Thus, further in vivo studies with dual tumour targeting by alpha-particles are warranted.
Keywords: 224Ra/212Pb-liquid generator, 212Pb, metastatic castration-resistant prostate cancer, NG001, PSMA-617, TCMC, targeted alpha therapy
1. INTRODUCTION
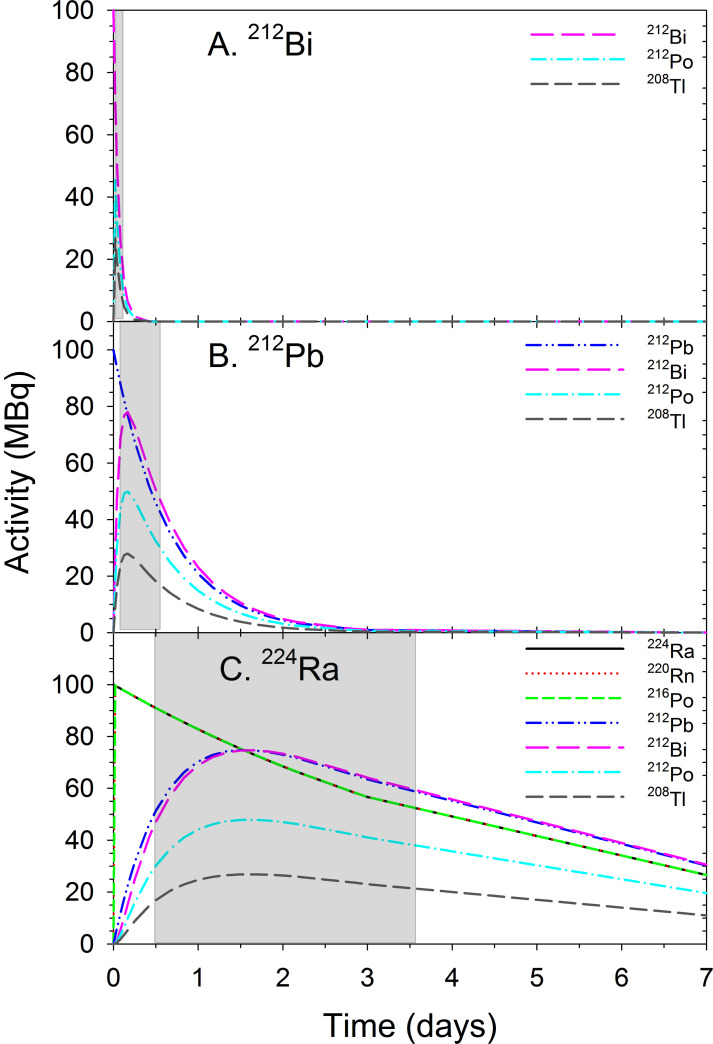
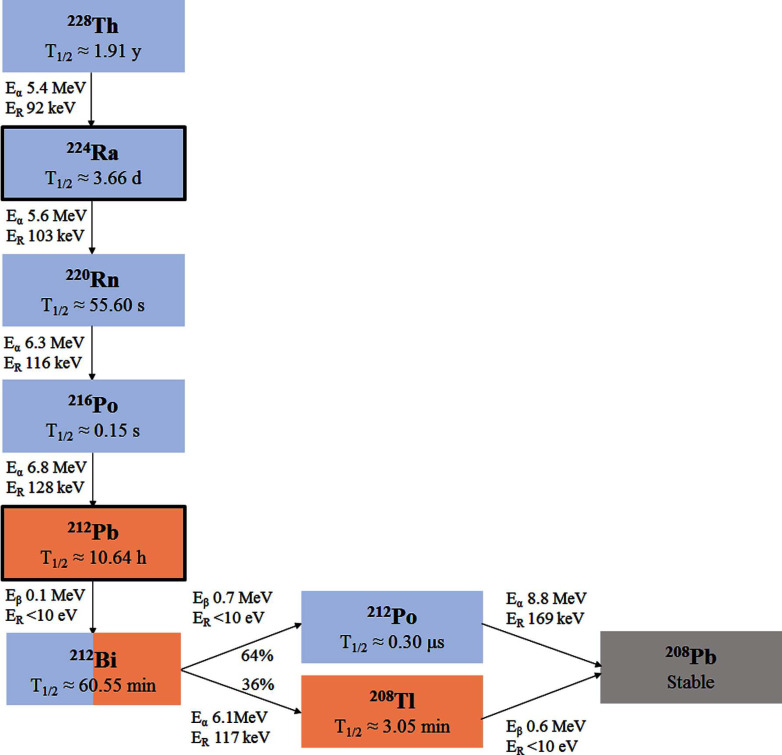
Radiopharmaceuticals have recently gained significant attention in the treatment of metastatic cancer [1]. One alpha-emitting and several beta-emitting radiopharmaceuticals have been approved for cancer therapy in the last decade [1]. Alpha-emitting radionuclides are particularly attractive as effector arms due to their short range (< 0.1 mm) in tissue and high-linear energy transfer (LET) properties, allowing the generation of strong and irreparable cell damage [2, 3]. However, there are only a few alpha emitters suitable for routine clinical use [3-6]. Two of the most promising nuclides in terms of half-life, decay properties and large-scale manufacturing potential, 223Ra (T1/2≈11.4 days) and 224Ra (T1/2≈3.7 days), are not suitable for conjugation to targeting molecules [7, 8]. Their use as natural bone-seekers has been limited to the targeting of osteosclerotic disease [9-11]. Bismuth-212 (t1/2≈60.6 min) can be produced in large quantities [4]. Unfortunately for clinical applications, the short half-life of 212Bi and the presence of high-energy gamma emission from 208Tl in the decay chain (Figs. 1 and 2A, Supplementary Table S1 (175.2KB, pdf) ) make this nuclide impractical to use. Therefore, several research groups have been looking into the use of its longer-lived mother nuclide, 212Pb (t1/2≈10.6 h, Figs. 1 and 2B) as a targetable in vivo generator of alpha particles [12-16]. Lead-212 is a beta emitter that produces alpha particle radiation via its short-lived progenies 212Bi and 212Po (t1/2≈0.3 µs). It should be noted that the needed activity levels of 212Pb would be much less than for a 212Bi-based product [17]. This is because 1 Bq of 212Pb would produce about 10 times the amount of alpha rays if integrated over its lifetime as compared to 1 Bq of 212Bi. Lead-212 has suitable properties in terms of chelation chemistry, and a highly effective bifunctional chelator, S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraaza-1,4,7,10-tetra(2-carbamoylmethyl)cyclododecane (TCMC), was developed specifically for this radionuclide [13, 18, 19]. Lead-212 can also be chelated by the versatile S-2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA) bifunctional chelator [13, 18, 19]. Furthermore, 212Pb can be obtained at an industrial scale from the 224Ra generator using 228Th (t1/2≈1.9 years) as a long-term generator [5-6, 17]. It is isolated in high yield and with high purity using, for example, actinide selective resin for immobilising or to catch 228Th while 224Ra is mobilised by HCl or HNO3 solutions [17]. Disadvantages include a non-optimal half-life of 212Pb, making centralised production and long-distance shipment difficult and impractical, and the considerable gamma rays associated with progenies. Another potential problem with 212Pb is the retention factor of daughter nuclides in the chelator after decay. The majority of 212Pb atoms are bound to the TCMC or DOTA chelator, but about one-third of 212Bi atoms could dissociate from the chelator after 212Pb decay [17, 20]. It is indicated that the use of 212Pb for developing radiotherapeutics would require a reliable method for supplying the radionuclide from a centralised production facility to the end user and a relatively rapid and simple procedure for preparing the radiopharmaceutical injectate for administration to patients.
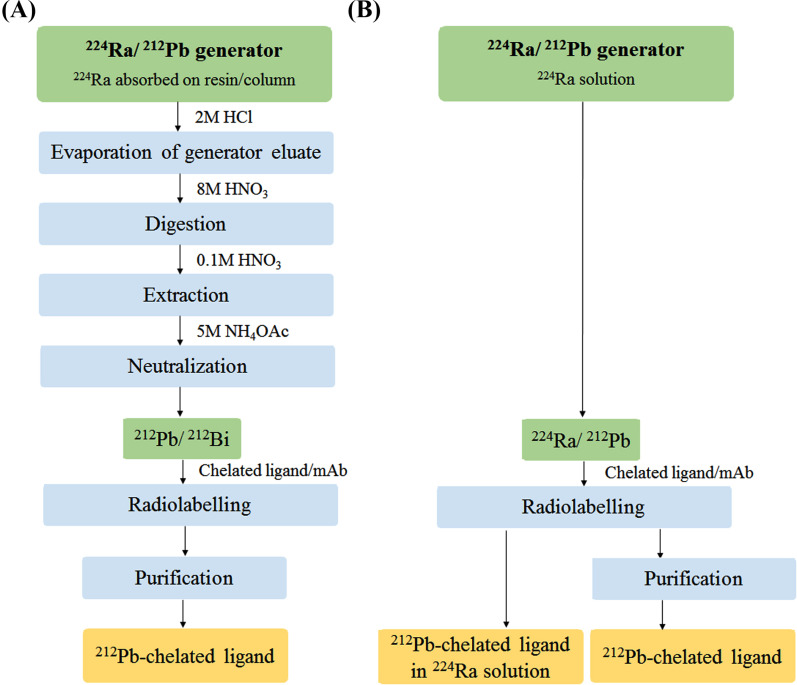
In recent preclinical and clinical studies, 212Pb was eluted with concentrated HCl from a 224Ra/212Pb-generator (Fig. 3A), as previously described by Baidoo et al. [19], where 224Ra is absorbed on a resin obtained from Orano Med (formerly Areva Med, Bessines-sur-Gartempe, France) or Oak Ridge National Laboratory (Oak Ridge, Tennessee, USA) [12, 15, 23, 24]. After the evaporation of the generator eluate, the residue is digested with concentrated HNO3 followed by the extraction of 212Pb with dilute HNO3 (Fig. 3A). The 224Ra/212Pb-generator facilitates the on-site production of 212Bi and 212Pb suitable for the radiolabelling of targeting ligands or monoclonal antibodies (mAb). This is in contrast to a 212Pb-chelated ligand or mAb prepared outside the hospital that must be delivered to the hospital for final use within a few hours after its preparation due to the short half-life [23].
Fig. (3).
A simplified scheme comparing the 212Pb production and labelling of chelated ligands or monoclonal antibodies (mAb) for the end user using (A) a standard 224Ra/212Pb-generator [19] and (B) a 224Ra/212Pb-liquid generator solution [17]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A simple and time-efficient method of producing 212Pb-labelled radioimmunoconjugates for the end user was suggested by Westrøm et al. [17]. The novel method presented here involves the in situ labelling of a tumour-targeted ligand using a 224Ra/212Pb-liquid generator, in which 224Ra is in transient equilibrium with daughter nuclides (Fig. 2C). The proposed procedure (Fig. 3B) may be advantageous compared to the 224Ra-based generator described by Baidoo et al. [19] (Fig. 3A) since the steps involving the handling and evaporation of concentrated acid solutions with high radioactivity levels can be avoided in the hospital, thereby reducing the on-site preparation time that only involves radiolabelling (∼30 min). After 212Pb-labelling, the removal of 224Ra may be performed by simple desalting gel exclusion separation (∼15 min) [17].
Fig. (2).
The decay of pure 212Bi (A) and 212Pb (B) and ingrowth/decay of their progenies. (C) Ingrowths and decays of 212Pb and other progenies from 224Ra in a liquid generator system starting with a pure 224Ra source [22]. Grey areas present time frames for the suitable clinical use of the radiopharmaceuticals. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Prostate cancer is the second most common cancer diagnosed in men worldwide [25]. The prostate-specific membrane antigen (PSMA) is highly expressed in androgen-independent prostate cancer and exhibits only limited expression in normal tissues [26]. Thus, PSMA presents both a therapeutic and diagnostic target in patients with metastasised castration-resistant prostate cancer (mCRPC) [27-29]. A series of small molecular PSMA ligands have been synthesised and radiolabelled with 177Lu, 225Ac and 213Bi [27, 28]. Radioligand therapy (RLT) with [177Lu]Lu-PSMA-617 or [177Lu]Lu-PSMA-I&T has shown high response rates, pain reduction and a favourable safety profile in patients with mCRPC [29-32]. However, about 30% of patients do not respond to beta-emitting [177Lu]Lu-PSMA therapy [33-35]. Recent clinical studies have reported that targeted alpha therapy (TAT) using 225Ac- and 213Bi-labelled PSMA ligands has a strong anticancer activity in mCRPC patients and, importantly, also in some cases in patients failing to respond to 177Lu-based PSMA RLT [29, 33, 34, 36, 37]. The high cytotoxicity including a high degree of non-re-joining DNA double-strand breaks and low oxygen enhancement ratio of high-LET radiation from alpha particles, may account for the increased anti-tumour response observed [2, 3, 38]. Unfortunately, the current supply situation for 225Ac and 213Bi may limit the use of these radionuclides for TAT [39]. Hence, there is a need for alternative clinical grade alpha emitters that can be produced in large scale.
Currently, bone metastases occur in up to 90% and extraskeletal metastases (regional lymph node and visceral metastases) in up to 50% of patients with mCRPC [40-42]. Hence, an ideal targeted RLT for mCRPC must combat the entire spectrum of metastases. The use of a liquid 224Ra/212Pb-generator would allow the preparation of a dual-alpha targeting solution, where a PSMA-targeting ligand is labelled with 212Pb in the 224Ra-solution without the subsequent removal of 224Ra (Fig. 3B). This solution will contain radionuclides with dual targeting properties, whereby the natural bone-seeking radionuclide 224Ra will target stromal elements in osteoblastic metastatic lesions [11, 43], and the tumour cell surface-seeking 212Pb-labelled PSMA ligand will target the extraskeletal metastases by selective binding to the PSMA-expressing cells.
The clinically most studied therapeutic PSMA-seeking carrier PSMA-617 gives relevant tumour to normal tissue ratios for longer-lived radionuclides, such as 177Lu (t1/2≈6.7 days) and 225Ac (t1/2≈9.9 days) but at early time points (typically a few hours after injection) shows relatively high uptake in kidneys [44, 45]. With shorter-lived radionuclides such as 212Pb and 211At (t1/2≈7.2 hours), the initial kidney uptake could represent a potential toxicity problem [46]. It therefore seems advantageous to have a PSMA ligand with more rapid pharmacokinetics, resulting in moderate kidney uptake and retention and high tumour-targeting capability.
The goal of this study was to label the p-SCN-Bn-TCMC PSMA-targeting ligand NG001 [47] and PSMA-617 with 212Pb by use of the 224Ra/212Pb-liquid generator to produce dual-targeting radiopharmaceutical solutions. We have characterised labelling efficiency with 212Pb in the 224Ra-solution in situ and investigated the targeting properties of the two radioligands in vitro and in vivo. Cell binding assays were performed to evaluate the radiolytic stability of the radioligands in dual targeting solutions. The biodistribution of the radioligands and 224Ra were studied in C4-2 tumour-bearing mice.
2. MATERIALS AND METHOD
2.1. Preparation of Radionuclides and Radioactivity Measurements
Radium-224 was extracted from a generator column containing actinide resin (Eichrom Technologies, Lisle, Illinois, USA) with immobilised 228Th (Eckert & Ziegler, Braunschweig, Germany) by eluting with 1 M HCl [17, 48]. Eichrom’s actinide resin is based on the DIPEX® extractant. A column-based generator was prepared by removing 80% of the actidine resin from a column and mixing this as a slurry with 228Th in 1 M HCl for approximately two hours and then the slurry was loaded onto the same column with the 20% acting as a catcher layer. Thereafter the column was washed thoroughly with 1 M HCl to ensure that only immobilised 228Th was present. After one or two weeks of ingrowth, the 224Ra generated was mobilised by eluting with 1 M HCl. The eluate was purified on a second actinide resin column, and the second eluate was evaporated to dryness using an evaporation vial with a cap with a gas inlet and outlet placed in a heater block at approximately 110 ◦C and a gentle stream of nitrogen gas. When the evaporation vial was empty of solvent, around 200-400 µl of 0.1 M HCl was added to dissolve the residue. The 224Ra-solution was left for 2-3 days to reach equilibrium with daughter radionuclides. The solution was then used for producing 212Pb-labelled ligands by labelling in situ, that is, 212Pb was complexed by the ligands in the presence of 224Ra.
Radioactive samples were measured on a Cobra II Autogamma Counter (Packard Instrument Company, Downer Grove, Illinois, USA) and a Hidex Automatic Gamma Counter (Hidex Oy, Turku, Finland) using the 50-120 keV and 60-110 keV counting windows, respectively. This setting mainly measures the 212Pb activity (34.9% relative to the mother nuclide 224Ra) with very little contribution from other radionuclides (1.2% relative to 224Ra) in the 224Ra series (Supplementary Table S1 (175.2KB, pdf) ) [17, 48]. Radium-224 activity was indirectly determined by measuring the 212Pb activity after 4-5 days when the initial 212Pb had decayed and the equilibrium between 224Ra and the newly formed 212Pb had been reached. A radioisotope dose calibrator Capintec CRC-25R (Capintec Inc., Ramsey, New Jersey, USA) was used during the radiolabelling procedure. To determine the distribution of 224Ra, 212Pb and 212Bi in samples, a liquid nitrogen cooled High-purity Germanium (HPGe) radiation detector (Canberra Industries Inc., Meriden, Connecticut, USA) combined with the Genie 2000 Spectroscopy Software (Canberra, Australia) was used.
2.2. Radiolabelling of NG001 and PSMA-617
The PSMA ligands NG001 and PSMA-617 were supplied by MedKoo Biosciences (Morrisville, North Carolina, USA) as a purchased synthesis service. The products were supplied as HPLC purified and dried trifluoroacetic acid salts with a purity of at least 98%. Before they were used in radiolabelling, the ligands were dissolved in 0.5 M ammonium acetate (NH4OAc) in 0.1 M HCl at a concentration of 1 mg/ml. For radiolabelling, NG001 and PSMA-617 were added to the 224Ra-solution (2-5 MBq/ml) with progenies in 0.5 M NH4OAc in 0.1 M HCl, adjusted to pH 5-6. Different concentrations of the ligands were radiolabelled, ranging from 2.5-500 µg/ml. The reaction mixture was incubated for 30 minutes on a thermomixer (Eppendorf, Hamburg, Germany) at 37 °C and 450 rpm. In an experiment, the effect of different incubation conditions (various temperatures and pH) on the radiolabelling of NG001 was tested. The labelling reactions were evaluated by thin layer chromatography (TLC).
2.3. Instant TLC Analysis
The radiochemical purity (RCP) of the radiolabelled ligands was measured by TLC using instant TLC (ITLC) strips (Tec-control, Biodex, New York, USA). A sample of the radiolabelled solution was diluted in a formulation buffer consisting of 7.5% human serum albumin and 5 mM EDTA in DPBS adjusted to pH 7. The mixture was left for 5-10 minutes to allow the chelation of unbound radioisotopes with EDTA. Around 1-5 µl of the reaction mixture was applied to the origin line of the ITLC strip and placed in a small beaker with 0.5 ml of 0.9% NaCl. When the solvent front had migrated to the front line, the strips were bisected and each half put in a counting tube for activity measurement using a gamma counter. In this system, unbound radioisotopes complex with EDTA and follow the solvent front (top half), while the radiolabelled ligand is retained at the baseline (bottom half). The RCP was calculated using the following equation:
RCP (%) = Activity in bottom half × 100 / (Activity in bottom half + activity in top half)
Bismuth-212 will also be present in the 224Ra-solution in equilibrium with progeny. Therefore, in a separate experiment, the chelation of 212Bi to NG001 was examined. The ITLC strips were measured in an HPGe detector to determine the radioactivity of 212Bi (727 keV, 4.3% relative abundance), and RCP was calculated as described above.
2.4. Cell Line
The PSMA-expressing prostate cancer cell line C4-2 [49], obtained from ATCC (ATCC® CRL3314™, Virginia, USA), was grown in RPMI 1640 medium (Sigma-Aldrich Norway AS, Oslo, Norway) supplemented with 10% heat-inactivated foetal bovine serum (FBS, GE Healthcare Life Sciences, Chicago, Illinois, USA) and 100 units/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich) at 37 °C in a humid atmosphere with 95% air and 5% CO2.
2.5. Radiolytic Stability of 212Pb-labelled NG001 and PSMA-617 in the Presence of 224Ra
The stability of 212Pb-labelled NG001 and PSMA-617 in the presence of 224Ra was tested by cell binding and TLC analysis after different time points up to 48 hours. Cell binding ability was measured by incubating 0.2 ml of C4-2 cells (5-6 × 107 cells/ml) with 0.5-2 ng of radioligand for 1 hour at 37 °C and 450 rpm. Non-specific binding was estimated by blocking cells with an excess of unlabelled ligand before the addition of radioligand. Added activities were measured in a gamma counter before the cells were washed 3 times with 0.5% BSA in PBS. Cell bound activities were then measured, and the cell-binding fraction (%) was estimated by subtracting the non-specific cell bound activity from the total cell bound activity.
The effect of the radioprotectants ascorbic acid and/or human serum albumin [16, 50, 51] on the cell binding ability of the radiolabelled NG001 was tested. Human serum albumin (HSA, Sigma-Aldrich) and/or L-ascorbic acid (Sigma-Aldrich) were added to the 224Ra-solution immediately after the radiolabelling of NG001. Stability and cell binding ability were tested at different time points up to 48 hours.
2.6. Separation of 212Pb-labelled NG001 from 224Ra
For one of the biodistribution studies, 212Pb-labelled NG001 and PSMA-617 were purified using PD Minitrap G-10 columns prepacked with Sephadex G-10 resin (exclusion limit, Mr cut-off of 0.7 kDa) (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) to remove 224Ra and other unconjugated daughter nuclides.
2.7. Animals
Twenty six Hsd: Athymic Nude-Foxn1nu mice bred at the Department of Comparative Medicine at the Norwegian Radium Hospital (Oslo University Hospital, Oslo, Norway) were used in this study and maintained under specific pathogen-free conditions with ad libitum access to food and water. Cages were housed in a scantainer, which was maintained at a constant temperature (24 °C) and humidity (60%). The mice were around 10-12 weeks in age, weighing between 25-35 g at the start of the study. The mice were inoculated subcutaneously in both flanks with 107 C4-2 cells in RPMI 1640 medium without supplements mixed 1:1 with Matrigel Matrix (Corning, New York, USA) in a total volume of 200 µl. Prior to cell inoculation, the absence of murine contaminations in the human cell line was verified by a PCR-based pathogen test (IMPACT I, IDEXX Bioanalytics, Ludwigsburg, Germany). The tumours were grown to reach a volume of 250-1000 mm3 before the animals were used in the studies. The mice were monitored two times per week for changes in weight and tumour size and for any sign of illness or discomfort. Humane end points were >15% weight loss from the initial weight, tumours that exceed 15 mm in any direction, ulcerate or interference with normal behaviour or any signs of severe sickness or discomfort. No mice became ill or died prior to the experimental end point.
2.8. Biodistribution of [212Pb]PbCl2, [212Pb]Pb-NG001, [212Pb]Pb-PSMA-617 and 224Ra in Mice with C4-2 Xenografts
The radiolabelled ligands were diluted in 0.9% NaCl and sterile-filtered immediately before injections. The mice bearing C4-2 tumours were injected intravenously via tail vein with 27 kBq (0.17 nmol) of [212Pb]Pb-NG001 in the presence of 224Ra (33 kBq), 18 kBq (0.20 nmol) of [212Pb]Pb-PSMA-617 in the presence of 224Ra (15 kBq), 44 kBq of [212Pb]PbCl2 in the presence of 224Ra (40 kBq), 29 kBq (0.24 nmol) of purified [212Pb]Pb-NG001 or 79 kBq (0.49 nmol) of purified [212Pb]Pb-PSMA-617 in a volume of 100 µl per mouse.
Two hours after injection, the mice were given gas anaesthesia (~3.5% Sevofluran [Baxter, Illinois, USA] in oxygen at a flow rate of 0.5 l/min) for blood collection by cardiac puncture. The mice were then euthanised by cervical dislocation and different organs/tissues were harvested. The weight and activity of each tissue sample were measured in a gamma counter. The obtained values were corrected for the decay of 212Pb and the ingrowth of 212Pb from 224Ra. Percentage injected dose per gram tissue (%ID/g) was calculated. Samples of the injectates were used as references in the measurement procedures.
2.9. Statistics
The datasets were analysed for significance using one-way ANOVA with Tukey’s multiple comparison post-test using SigmaPlot 14.0 software (Systat Software, Inc. San Jose, California, USA). A p-value of <0.05 was considered statistically significant.
3. RESULTS
3.1. Radiolabelling of Ligands
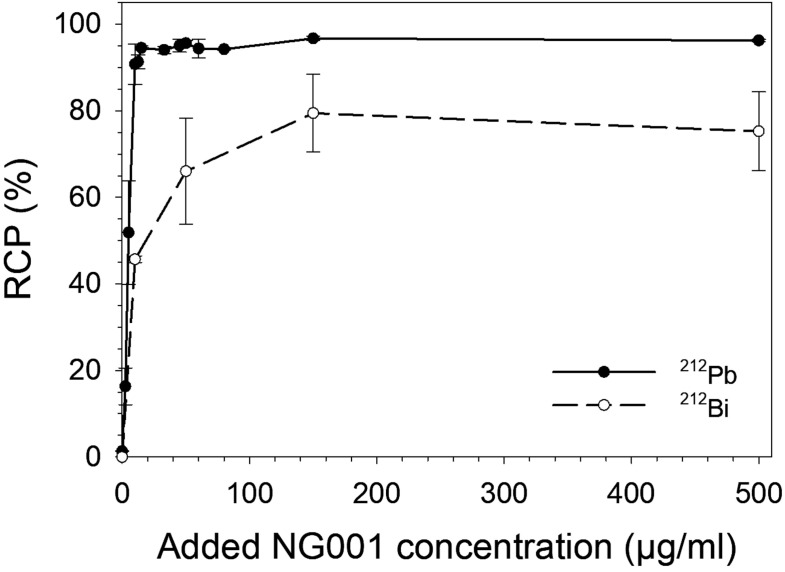
The 212Pb-labelling of NG001 and PSMA-617 in the 224Ra-solution in transient equilibrium with progeny was tested at different ligand concentrations (2.5-500 µg/ml), temperatures and pH values. Radiolabelling was efficient with RCP above 90% at a ligand concentration of 10 µg/ml and above 94% at concentrations of 15 µg/ml and higher (Fig. 4, Supplementary Table S2 (175.2KB, pdf) ), indicating that a variety of specific activities can be achieved. For NG001, the RCP of 212Bi was around 45% at a ligand concentration of 10 µg/ml and around 65-75% at concentrations above 50 µg/ml, demonstrating that 212Bi is also conjugated by the TCMC chelator though not to the same extent as 212Pb (Fig. 4, Supplementary Table S2 (175.2KB, pdf) ). The radiolabelling of NG001 was successful with RCP >93% at a range of various temperatures and pH values (Table 1). There was a slight decline in RCP at pH 8, but RCP was above 88%.
Fig. (4).
Radiochemical purity (RCP) values as mean ± SD of 212Pb and 212Bi after the radiolabelling of different concentrations of NG001 in the 224Ra-solution in transient equilibrium with progeny. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Radiochemical purity (RCP) values as mean ± SD of 212Pb-labelled NG001 (33 µg/ml) after radiolabelling in the 224Ra-solutions of different pH values at 37 ºC or at different incubation temperatures (pH=5-6). N, the number of independent experiments.
| Incubation Conditions | RCP (%) | N |
|---|---|---|
| pH | ||
| 4 | 94.59±0.89 | 2 |
| 5-6 | 94.04±0.87 | 7 |
| 8 | 88.25±0.82 | 2 |
| Temperature (°C) | ||
| 4 | 93.81±0.40 | 2 |
| 20 | 94.86±0.43 | 2 |
| 37 | 94.04±0.87 | 7 |
3.2. Radiolytic Stability
The radiolytic stability of 212Pb-labelled NG001 and PSMA-617 in the 224Ra-solution was tested by performing cell binding and TLC analysis at different time points up to 48 hours. The initial activity of 224Ra in the solutions was between 2.8-5 MBq/ml, causing absorbed radiation doses to the solutions of 1-1.8 kGy after 24 hours and 1.8-3.2 kGy after 48 hours. The radioligands maintained a high RCP even after 48 hours (Table 2), indicating that the PSMA ligands are capable of complexing 212Pb continuously as it is generated from 224Ra during storage. The chelation of 212Bi to NG001 was also stable up to 48 hours (Supplementary Table S3 (175.2KB, pdf) ).
Table 2.
Radiochemical purity (RCP) values and cell binding fraction expressed as mean ± SD of 212Pb-labelled NG001 and PSMA-617 in the 224Ra-solution when incubated at activity concentrations of 2.8-5 MBq/ml up to 48 h (at room temperature, pH 5-6, n=2-6).
| Incubation Time | RCP (%) | P-value | Cell Binding Fraction (%) | P-Value | ||
|---|---|---|---|---|---|---|
| [212Pb]Pb-NG001 | [212Pb]Pb-PSMA-617 | [212Pb]Pb-NG001 | [212Pb]Pb-PSMA-617 | |||
| 1 h | 94.04±1.23 | 95.49±1.65 | 0.140 | 57.62±4.59 | 51.62±4.84 | 0.122 |
| 4 h | 94.79±0.59 | 94.91±1.31 | 0.893 | 52.27±0.05 | 48.35±5.45 | 0.495 |
| 24 h | 93.46±0.67 | 94.67±1.75 | 0.269 | 45.92±5.46 | 46.07±5.30 | 0.969 |
| 48 h | 92.52±1.39 | 94.55±1.32 | 0.095 | 40.12±5.31 | 44.86±3.76 | 0.194 |
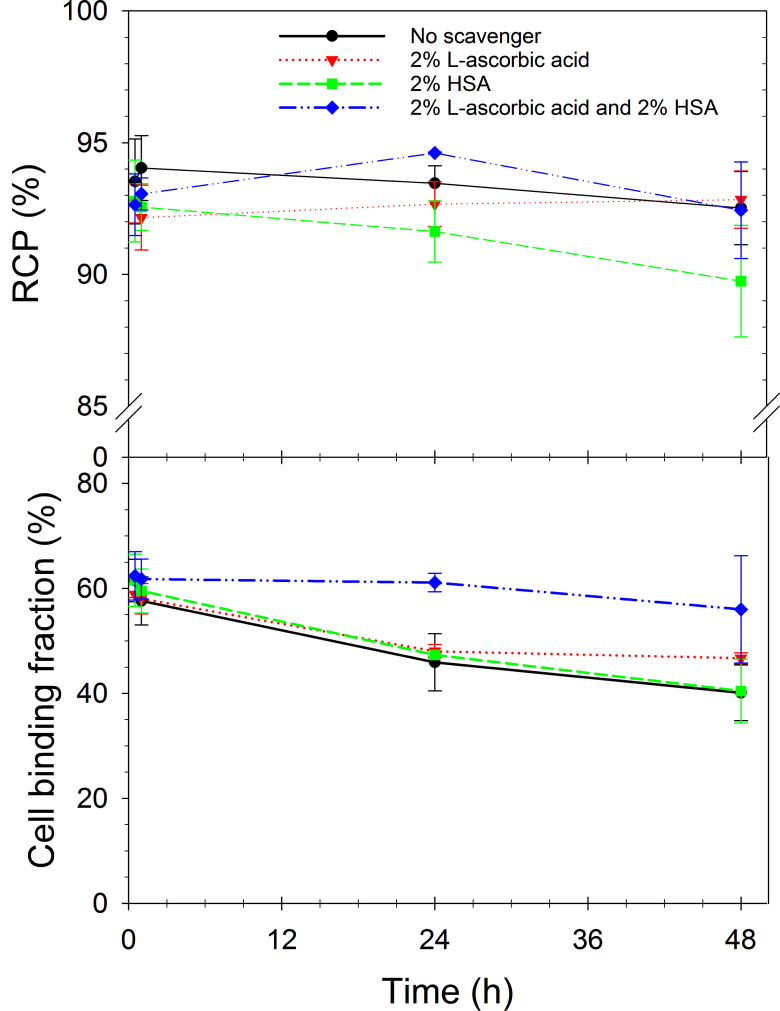
Both radioligands were stable over 4 hours at room temperature but showed decreased cell binding ability when incubated for 24 and 48 hours (Table 2). Therefore, the effect of the radioprotectants L-ascorbic acid and HSA, alone or in combination, on the stability of 212Pb-labelled NG001 was evaluated.
The addition of scavengers did not interfere with the continuous complexing of 212Pb by the ligand, indicated by the high RCP values at all time points (Fig. 5, Supplementary Table S4 (175.2KB, pdf) ). However, only the combination of L-ascorbic acid and HSA prevented radiolysis and resulted in the radioligand maintaining a higher cell binding ability after 24 and 48 hours (cell binding fraction of 61.14±1.77% and 56.00±10.24, respectively).
Fig. (5).
Radiochemical purity (RCP) values (top) and cell binding fraction (bottom) expressed as mean ± SD of 212Pb-labelled NG001 in 224Ra-solution containing no scavengers, 2% L-ascorbic acid, 2% HSA or both scavengers when incubated at activity concentrations of 2.8-5 MBq/ml up to 48 hours (at room temperature, n=2-3). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.3. Biodistributions of 212Pb and 224Ra in Mice Bearing Human Prostate C4-2 Xenografts
Before injection into the mice, the binding of the radiolabelled ligands was verified by measuring cell binding ability in C4-2 cells. The cell binding fraction was between 45-65% for both radioligands. The mice were euthanised 2 hours after the injection of radioligands.
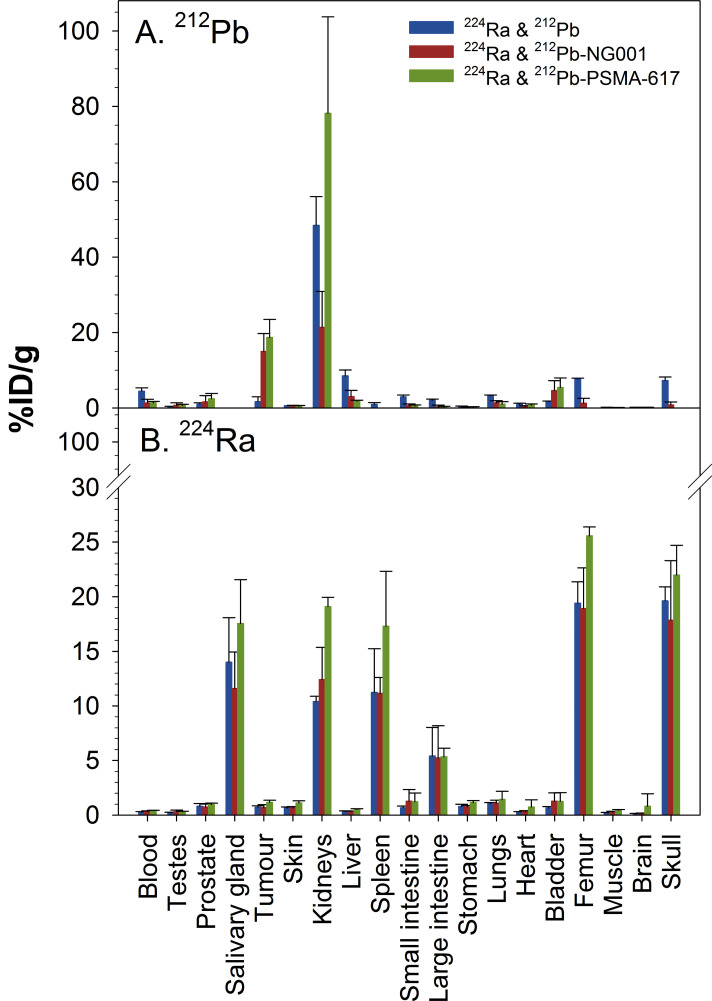
The measured activity values were corrected for the decay of 212Pb and ingrowth of 212Pb from 224Ra, and %ID/g values of 212Pb without 212Pb ingrowth from 224Ra (Fig. 6A, Supplementary Table S5 (175.2KB, pdf) ), 212Pb ingrowth from 224Ra (Supplementary Table S5 (175.2KB, pdf) ) and 224Ra (Fig. 6B, Supplementary Table S6 (175.2KB, pdf) ) were calculated. The highest activities of the carrier-free 212Pb (224Ra&212Pb group) were observed in the kidneys, urine, liver, bones and blood, while the activities of [212Pb]Pb-NG001 and [212Pb]Pb-PSMA-617 were highest in the urine, kidneys and tumour (Fig. 6A). The uptake values (%ID/g) for the tumour and kidneys at 2 hours post-injection were 1.71±1.29 and 48.47±7.57 for [212Pb]PbCl2, 15.01±4.73 and 21.39±9.55 for [212Pb]Pb-NG001 and 18.71±4.78 and 74.17±25.59 for [212Pb]Pb-PSMA-617 after intravenous injection of the 224Ra-solutions (Fig. 6A, Supplementary Table S5 (175.2KB, pdf) ). The tumour-to-kidney ratios for [212Pb]PbCl2, [212Pb]Pb-NG001 and [212Pb]Pb-PSMA-617 were 0.04, 0.70 and 0.25, respectively. Compared with [212Pb]Pb-PSMA-617, [212Pb]Pb-NG001 showed comparable tumour uptake (P>0.05) and 3.5-fold lower kidney uptake (P<0.01). Radium-224 had high skeletal uptake with similar uptake in the femur and skull in all groups (P>0.05): 19.40±1.97 and 19.62±1.28%ID/g for [212Pb]PbCl2, 18.90±3.74 and 17.85±5.45%ID/g for [212Pb]Pb-NG001, and 25.57±0.81 and 21.98±2.73%ID/g for [212Pb]Pb-PSMA-617 (Fig. 6B, Supplementary Table S6 (175.2KB, pdf) ).
Fig. (6).
Percentage of injected activity per gram of tissue (%ID/g) ± SD of [212Pb]PbCl2, [212Pb]Pb-NG001 and [212Pb]Pb-PSMA-617 (top) and radium-224 (bottom) at 2 hours after intravenous injection of 224Ra-solution containing [212Pb]PbCl2, [212Pb]Pb-NG001 or [212Pb]Pb-PSMA-617 in athymic mice bearing human prostate C4-2 cancer xenografts (n=3-5 mice per group). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. DISCUSSION
The current study has evaluated the feasibility of using 224Ra as a liquid generator solution for 212Pb in the preparation of 212Pb-labelled PSMA-binding ligands. It indicates that the 224Ra-based solution is suitable for such use. A relatively fast method (∼30 min) for the labelling of NG001 or PSMA-617 with 212Pb was developed. Here, 224Ra is in transient equilibrium with progeny at pH 4-6. It was shown that the labelling reaction would occur in situ when the reaction solution was stored up to at least 48 hours (Table 2) at room temperature. The use of an 224Ra-solution generator system provides an opportunity for centralised production and long-distance shipment within a time window of three to four days (Fig. 3B).
The use of 212Pb to label PSMA-targeting ligands may be a promising option for TAT. PSMA has an internalisation function, a feature which is very attractive for 212Pb-labelled products as this would minimise the translocalisation of 212Pb progenies due to the decay-induced release of 212Bi from the chelator [17, 20]. The decay-induced release of daughters from the chelator may be a problem for alpha-emitting radionuclides. In the case of 225Ac, the four consecutive decay daughters (221Fr, 217At, 212Bi and 209Pb) receive high recoil energies (typically about 100 keV) when the alpha particle is emitted [52, 53]. This energy is about 1000 times higher than the chemical bond energies of any chelating agent, which means that the recoiling daughter will always break free from chelating agents [52, 53]. Thus, free daughter radionuclides may accumulate in healthy organs and cause high toxicity [53, 54]. The recoil energies from beta emitters are low (< 10 eV) [55]. However, conversion electron cascades associated with the 212Pb decay are probably responsible for some release of 212Bi from the chelator [20].
The rapid elimination of unbound radioligands from normal tissues is mandatory when using shorter-lived radionuclides such as 212Pb. A potential problem is the high initial kidney uptake of these small radioligands, but it appears that the use of NG001 instead of PSMA-617 as a carrier for 212Pb significantly reduced the kidney exposure (Fig. 6A).
The 224Ra/212Pb-based generator used here was designed to overcome challenges with the original 228Th/212Pb-based generators, including the radiolytic damage of the parent nuclide materials that decreased the yield as well as serious radiation safety issues [17, 56, 57]. To avoid the potential radiolysis of the radioligand, the mixing of the ligand and the 224Ra/212Pb-liquid generator solution could take place just before injection (Fig. 3B). The 212Pb-labelled ligand could be used as a purified single-agent product and not only as a dual-targeting product in tandem with 224Ra.
An interesting feature with the 212Pb-labelling of the PSMA-targeting molecule is the great variation in labelling conditions that can be used for preparing the radioligand (Fig. 4 and Table 1). With the 177Lu-labelling of PSMA-617, the product has to be heated (80-98 °C) to obtain efficient radiolabelling of the ligand [58, 59]. Regarding 212Pb-labelling, the current data show that NG001 and PSMA-617 can be radiolabelled in an efficient way using a 224Ra-solution in transient equilibrium with daughters at pH 4-6 and without the need for heating the reaction solution (Fig. 4, Table 1, Supplementary Table S2 (175.2KB, pdf) ). It was found that the majority of 212Bi present in the labelling solutions was also complexed by the chelators (Fig. 4). This observation is compatible with that reported by Westrøm et al. for TCMC-conjugated mAb [17].
A noteworthy aspect with the 224Ra-based generator solution is that the activity level curve for 212Pb is quite flat from about 20 to about 96 hours when starting with a pure 224Ra source (Fig. 2C). For example, an 224Ra source of 100 MBq at day 0 can be mixed with the radioligand for in situ 212Pb-labelling, which would yield a product of 60 MBq of 212Pb-labelled radioligand three days later (Fig. 1C). Thereby, the liquid generator system significantly improves the suitability of the centralised production and shipment of the 212Pb product kit.
Fig. (1).
The decay chain of 228Th to stable 208Pb. Alpha-emission energies (Eα), beta-emission energies (Eβ), recoil energies (ER) and the half-lives for 228Th and daughter nuclides are specified [21]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
When using RLT, radiolysis could be a potential problem for product integrity. The oxidation of PSMA ligands has been shown to occur when the PSMA ligands are labelled with the 177Lu isotope and can be minimised by the addition of ascorbic acid [60]. The presence of the oxidised forms of NG001 and PSMA-617 was also observed in our studies after 24 and 48 hours. This oxidation somewhat influenced the binding of 212Pb-labelled NG001 and PSMA-617 to cells (Table 2). The addition of ascorbic acid alone did not improve cell binding at 24 and 48 hours (Fig. 5), nor did the addition of another potential radioprotectant, HSA [51], alone proved efficient to prevent the radiolysis of NG001 (Fig. 5). However, when both of them were added to the 224Ra-solution, they prevented the radiolysis of [212Pb]Pb-NG001 even at higher activity concentrations (2.8-5 MBq/ml) without interfering with the continuous complexing of 212Pb by the ligand (Fig. 5).
The current study is the first presentation in the scientific literature regarding the labelling of NG001 and PSMA-617 with 212Pb in the 224Ra-solution in transient equilibrium with daughter nuclides and where [212Pb]Pb-NG001 and [212Pb]Pb-PSMA-617 in the 224Ra-solution efficiently bind to PSMA-positive C4-2 cells in vitro and in vivo (Table 2, Figs. 5 and 6A). Recently, several novel PSMA-seeking ligands (CA008, CA009/NG001, CA011, CA012, L1, L2, L3, L4 and L5) were synthesised and labelled with 203Pb, a surrogate for 212Pb [14, 15]. Two PSMA ligands, 203Pb-labelled CA009 (identical to the structure of NG001, which is patented by Larsen [61]) and CA012, were presented by Dos Santos et al. [14]. In C4-2 tumour-bearing BALB/c nu/nu mice, [203Pb]Pb-CA012 showed a tumour uptake of 8.4%ID/g at 1 hour after injection [14], which was considerably lower than the tumour uptake of 22.5%ID/g reported for [203Pb]Pb-L2 in NOD-SCID mice bearing PC3-PIP tumours [15]. In the present work, [212Pb]Pb-NG001 and [212Pb]Pb-PSMA-617 showed similar tumour uptakes of 15.01%ID/g and 18.71%ID/g in C4-2 tumour-bearing Hsd: Athymic Nude-Foxn1nu mice after 2 hours, respectively (Fig. 6A). The obtained values are higher than those reported for 203Pb-CA012 [14] for the same tumours and lower than those for [203Pb]Pb-L2 where PC3-PIP tumours were studied [15]. PC3-PIP cells have higher PSMA expression than C4-2 cells [49, 62, 63]. The direct comparison of new PSMA ligands is not easy since different mouse strains and cell lines were used in the various studies. However, our results indicate that the efficient targeting of PSMA-tumours can be achieved with radioligands labelled in the 224Ra-solution.
Herein, 224Ra showed high uptake in the femur and skull in all groups (Fig. 6B), demonstrating that it is possible to effectively chelate 212Pb without reducing the bone-seeking properties of 224Ra in radiopharmaceutical solutions containing the two radionuclides. The novel 224Ra/212Pb liquid generator opens opportunities to use 212Pb-labelled NG001 either as a purified compound by itself for targeting prostate cancer cells or together with 224Ra in a dual bone and soft-tissue targeting solution. Such solutions could be used to extend TAT to prostate cancer patient groups which are not eligible for Xofigo ([223Ra]RaCl2) as bone metastases therapy since Xofigo is not indicated for patients with documented extraskeletal disease [9].
The main route of excretion of radiolabelled PSMA ligands is via renal excretion/elimination [64]. Kidneys receive the highest radiation doses, and they are considered the dose-limiting organ [14, 15, 65]. Importantly, kidney uptake was 3.5-fold lower for [212Pb]Pb-NG001 compared to [212Pb]Pb-PSMA-617 at the 2 hour time point (Fig. 6A) without compromising tumour-targeting, indicating that NG001 may be better suited for 212Pb-targeting than PSMA-617.
Salivary gland toxicity is a common side effect of [225Ac]Ac-PSMA-617 therapy [29, 35, 44]. Preventative approaches to mitigate this side effect have included intraglandular injection of botulinum toxin, performing sialendoscopy or deescalating the dose schedule of [225Ac]Ac-PSMA-617 [29, 37, 66, 67]. Only traces of [212Pb]Pb-NG001 and [212Pb]Pb-PSMA-617 were found in the salivary glands (Fig. 6A). It is unclear the extent to which mouse data can be indicative of salivary gland uptake of the PSMA-targeting ligands in humans. A significant uptake of 224Ra was observed in the kidneys and salivary glands (Fig. 6B). Radium-224 was used earlier for many years for the treatment of different non-cancerous diseases [43]. To our knowledge, salivary gland toxicities have not been linked to the use of 224Ra. Kidney uptake is not a problem in humans as more than 90% of radium is excreted by intestinal clearance in man compared with about 70% renal clearance of radium in mice [68, 69].
Radium-224 has a high affinity for sclerotic bone tumours while PSMA binding ligands have shown a great affinity for PSMA-positive tumour lesions. The current work demonstrates the possibility to combine these two modes of targeting in one radiopharmaceutical solution using the 224Ra and 212Pb radionuclide combination.
CONCLUSION
A fast and efficient method for the labelling of NG001 or PSMA-617 with 212Pb in the 224Ra-solution in transient equilibrium with progeny at pH 4-6 and without the need for heating the reaction solution was developed. High RCP and stability of [212Pb]Pb-NG001 were demonstrated over 48 hours in the presence of ascorbic acid and albumin. Thus, the use of shippable 224Ra sources for the preparation of 212Pb would allow shipment to the end user of a ready-to-use product or a simple kit that can be used to prepare the injectable radiopharmaceutical just prior to administration. Biodistribution data indicated similar tumour-targeting and less kidney uptake of radiolabelled NG001 compared with PSMA-617. [212Pb]Pb-NG001 could be prepared as a purified product by simple desalting gel exclusion separation. Alternatively, a dual-alpha solution of [212Pb]Pb-PSMA ligand for cell surface targeting and cationic 224Ra for bone-targeting could be prepared by using the reaction solution without purification. Thus, further in vivo studies with [212Pb]Pb-NG001 in the absence or presence of 224Ra are warranted.
Table 3.
Percentage of injected activity per gram of tissue (%ID/g) ± SD of purified [212Pb]Pb-NG001 and [212Pb]Pb-PSMA-617 at 2 hours after intravenous injection of [212Pb]Pb-NG001 and [212Pb]Pb-PSMA-617 in athymic mice bearing human prostate C4-2 cancer xenografts (n=3-11 mice).
| Organ | %ID/g | P-value | |
|---|---|---|---|
| [212Pb]Pb-NG001 | [212Pb]Pb-PSMA-617 | ||
| Tumour | 17.61±6.76 | 17.93±2.90 | 0.931 |
| Kidney | 21.07±10.33 | 52.82±26.62 | 0.013 |
Purified 212Pb-labelled ligands were prepared from the 224Ra/212Pb-solution using a standard gel filtration column. The removal of 224Ra and other unconjugated daughter nuclides from the solutions with NG001 or PSMA-617 did not influence their biodistribution (Table 3). The uptake values (%ID/g) of purified 212Pb-labelled ligands for tumour and kidneys at 2 hours post-injection were similar to the corresponding values of 212Pb-labelled ligands in 224Ra-solution (Table 3).
ACKNOWLEDGEMENTS
Declared none.
Supplementary Material
Supplementary material is available on the publisher’s website along with the published article.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Committee on Research Animal Care, Department of Comparative Medicine, Oslo University Hospital, Norway and the Norwegian Food Safety Authority, Brumunddal, Norway (Approval No: FOTS ID 8724, 2016/111707).
HUMAN AND ANIMAL RIGHTS
No humans were involved in this study. All procedures and experiments involving animals in this study were performed in accordance with the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Marketing, and Education (New York Academy of Sciences, New York, NY) and the EU Directive 2010/63/EU for animal experiments.
CONSENT FOR PUBLICATION
Not applicable.
Availability of Data and Materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
FUNDING
This study was funded by the Norwegian Research Council (Grant No: 290639), Oslo, Norway.
CONFLICT OF INTEREST
R. H. L., Ø. S. B. and V. Y. S. hold an ownership interest in Nucligen AS.
REFERENCES
- 1.Dolgin E. Radioactive drugs emerge from the shadows to storm the market. Nat. Biotechnol. 2018;36(12):1125–1127. doi: 10.1038/nbt1218-1125. [DOI] [PubMed] [Google Scholar]
- 2.Marcu L., Bezak E., Allen B.J. Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit. Rev. Oncol. Hematol. 2018;123:7–20. doi: 10.1016/j.critrevonc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y.S., Brechbiel M.W. An overview of targeted alpha therapy. Tumour Biol. 2012;33(3):573–590. doi: 10.1007/s13277-011-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garashchenko B.L., Korsakova V.A., Yakovlev R.Y. Radiopharmaceuticals based on alpha emitters: preparation, properties, and application. Phys. At. Nucl. 2018;81(10):1515–1525. doi: 10.1134/S1063778818100071. [DOI] [Google Scholar]
- 5.Kozempel J., Mokhodoeva O., Vlk M. Progress in targeted alpha-particle therapy. what we learned about recoils release from in vivo generators. Molecules. 2018;23(3):E581. doi: 10.3390/molecules23030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makvandi M., Dupis E., Engle J.W., Nortier F.M., Fassbender M.E., Simon S., Birnbaum E.R., Atcher R.W., John K.D., Rixe O., Norenberg J.P. Alpha-emitters and targeted alpha therapy in oncology: from basic science to clinical investigations. Target. Oncol. 2018;13(2):189–203. doi: 10.1007/s11523-018-0550-9. [DOI] [PubMed] [Google Scholar]
- 7.Henriksen G., Hoff P., Larsen R.H. Evaluation of potential chelating agents for radium. Appl. Radiat. Isot. 2002;56(5):667–671. doi: 10.1016/S0969-8043(01)00282-2. [DOI] [PubMed] [Google Scholar]
- 8.Gott M., Yang P., Kortz U., Stephan H., Pietzsch H.J., Mamat C.A. 224Ra-labeled polyoxopalladate as a putative radiopharmaceutical. Chem. Commun. (Camb.) 2019;55(53):7631–7634. doi: 10.1039/C9CC02587A. [DOI] [PubMed] [Google Scholar]
- 9.Deshayes E., Roumiguie M., Thibault C., Beuzeboc P., Cachin F., Hennequin C., Huglo D., Rozet F., Kassab-Chahmi D., Rebillard X., Houédé N. Radium 223 dichloride for prostate cancer treatment. Drug Des. Devel. Ther. 2017;11:2643–2651. doi: 10.2147/DDDT.S122417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruland O.S., Nilsson S., Fisher D.R., Larsen R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006;12(20 Pt 2):6250–6257. doi: 10.1158/1078-0432.CCR-06-0841. [DOI] [PubMed] [Google Scholar]
- 11.Juzeniene A., Bernoulli J., Suominen M., Halleen J., Larsen R.H. Antitumor Activity of Novel Bone-seeking, α-emitting 224Ra-solution in a breast cancer skeletal metastases model. Anticancer Res. 2018;38(4):1947–1955. doi: 10.21873/anticanres.12432. [DOI] [PubMed] [Google Scholar]
- 12.Meredith R.F., Torgue J.J., Rozgaja T.A., Banaga E.P., Bunch P.W., Alvarez R.D., Straughn J.M., Jr, Dobelbower M.C., Lowy A.M. Safety and Outcome Measures of First-in-human intraperitoneal α radioimmunotherapy with 212Pb-TCMC-trastuzumab. Am. J. Clin. Oncol. 2018;41(7):716–721. doi: 10.1097/COC.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong K., Brechbiel M. Application of 212Pb for Targeted α-particle Therapy (TAT): Pre-clinical and mechanistic understanding through to clinical translation. AIMS Med. Sci. 2015;2(3):228–245. doi: 10.3934/medsci.2015.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos J.C., Schäfer M., Bauder-Wüst U., Lehnert W., Leotta K., Morgenstern A., Kopka K., Haberkorn U., Mier W., Kratochwil C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging. 2019;46(5):1081–1091. doi: 10.1007/s00259-018-4220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee S.R., Minn I., Kumar V., Josefsson A., Lisok A., Brummet M., Chen J., Kiess A.P., Baidoo K., Brayton C., Mease R.C., Brechbiel M., Sgouros G., Hobbs R.F., Pomper M.G. Preclinical evaluation of 203/212Pb-labeled low-molecular-weight compounds for targeted radiopharmaceutical therapy of prostate cancer. J. Nucl. Med. 2020;61(1):80–88. doi: 10.2967/jnumed.119.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stallons T.A.R., Saidi A., Tworowska I., Delpassand E.S., Torgue J.J. Preclinical investigation of 212Pb-DOTAMTATE for peptide receptor radionuclide therapy in a neuroendocrine tumor model. Mol. Cancer Ther. 2019;18(5):1012–1021. doi: 10.1158/1535-7163.MCT-18-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westrøm S., Generalov R., Bønsdorff T.B., Larsen R.H. Preparation of 212Pb-labeled monoclonal antibody using a novel 224Ra-based generator solution. Nucl. Med. Biol. 2017;51:1–9. doi: 10.1016/j.nucmedbio.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Chappell L.L., Dadachova E., Milenic D.E., Garmestani K., Wu C., Brechbiel M.W. Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl. Med. Biol. 2000;27(1):93–100. doi: 10.1016/S0969-8051(99)00086-4. [DOI] [PubMed] [Google Scholar]
- 19.Baidoo K.E., Milenic D.E., Brechbiel M.W. Methodology for labeling proteins and peptides with lead-212 (212Pb). Nucl. Med. Biol. 2013;40(5):592–599. doi: 10.1016/j.nucmedbio.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirzadeh S., Kumar K., Gansow O.A. The chemical fate of 212Bi-DOTA formed by b-decay of 212Pb(DOTA)2-***. Radiochim. Acta. 1993;60:1–10. doi: 10.1524/ract.1993.60.1.1. [DOI] [Google Scholar]
- 21.Templeman T., Shandalov M., Schmidt M., Tal A., Sarusi G., Yahel E., Kelson I., Golan Y. A new solid solution approach for the study of self-irradiating damage in non-radioactive materials. Sci. Rep. 2017;7(1):2780. doi: 10.1038/s41598-017-03150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WISE Uranium Project Universal Decay Calculator. http://www.wise-uranium.org/
- 23.Meredith R.F., Torgue J., Azure M.T., Shen S., Saddekni S., Banaga E., Carlise R., Bunch P., Yoder D., Alvarez R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014;29(1):12–17. doi: 10.1089/cbr.2013.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasten B.B., Gangrade A., Kim H., Fan J., Ferrone S., Ferrone C.R., Zinn K.R., Buchsbaum D.J. 212Pb-labeled B7-H3-targeting antibody for pancreatic cancer therapy in mouse models. Nucl. Med. Biol. 2018;58:67–73. doi: 10.1016/j.nucmedbio.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. https://gco.iarc.fr/
- 26.Cimadamore A., Cheng M., Santoni M., Lopez-Beltran A., Battelli N., Massari F., Galosi A.B., Scarpelli M., Montironi R. New prostate cancer targets for diagnosis, imaging, and therapy: focus on prostate-specific membrane antigen. Front. Oncol. 2018;8:E653. doi: 10.3389/fonc.2018.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wüstemann T., Haberkorn U., Babich J., Mier W. Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Med. Res. Rev. 2019;39(1):40–69. doi: 10.1002/med.21508. [DOI] [PubMed] [Google Scholar]
- 28.Lütje S., Slavik R., Fendler W., Herrmann K., Eiber M. PSMA ligands in prostate cancer - Probe optimization and theranostic applications. Methods. 2017;130:42–50. doi: 10.1016/j.ymeth.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Sachpekidis C., Alberts I., Rominger A., Afshar-Oromieh A. PSMA radioligand therapy in prostate cancer: overview, latest advances and remaining challenges. Immunother. 2019;11(5):1267–1271. doi: 10.2217/imt-2019-0146. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y.J., Kim Y.I. Therapeutic responses and survival effects of 177lu-psma-617 radioligand therapy in metastatic castrate-resistant prostate cancer: a meta-analysis. Clin. Nucl. Med. 2018;43(10):728–734. doi: 10.1097/RLU.0000000000002210. [DOI] [PubMed] [Google Scholar]
- 31.von Eyben F.E., Roviello G., Kiljunen T., Uprimny C., Virgolini I., Kairemo K., Joensuu T. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur. J. Nucl. Med. Mol. Imaging. 2018;45(3):496–508. doi: 10.1007/s00259-017-3895-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fendler W.P., Reinhardt S., Ilhan H., Delker A., Böning G., Gildehaus F.J., Stief C., Bartenstein P., Gratzke C., Lehner S., Rominger A. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget. 2017;8(2):3581–3590. doi: 10.18632/oncotarget.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakravarty R., Siamof C.M., Dash A., Cai W. Targeted α-therapy of prostate cancer using radiolabeled PSMA inhibitors: a game changer in nuclear medicine. Am. J. Nucl. Med. Mol. Imaging. 2018;8(4):247–267. [PMC free article] [PubMed] [Google Scholar]
- 34.Kratochwil C., Bruchertseifer F., Giesel F.L., Weis M., Verburg F.A., Mottaghy F., Kopka K., Apostolidis C., Haberkorn U., Morgenstern A. 225Ac-PSMA-617 for PSMA-Targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016;57(12):1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 35.Kratochwil C., Bruchertseifer F., Rathke H., Bronzel M., Apostolidis C., Weichert W., Haberkorn U., Giesel F.L., Morgenstern A. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J. Nucl. Med. 2017;58(10):1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- 36.Sathekge M., Knoesen O., Meckel M., Modiselle M., Vorster M., Marx S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2017;44(6):1099–1100. doi: 10.1007/s00259-017-3657-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sathekge M., Bruchertseifer F., Knoesen O., Reyneke F., Lawal I., Lengana T., Davis C., Mahapane J., Corbett C., Vorster M., Morgenstern A. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur. J. Nucl. Med. Mol. Imaging. 2019;46(1):129–138. doi: 10.1007/s00259-018-4167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahle J., Abbas N., Bruland O.S., Larsen R.H. Toxicity and relative biological effectiveness of alpha emitting radioimmunoconjugates. Curr. Radiopharm. 2011;4(4):321–328. doi: 10.2174/1874471011104040321. [DOI] [PubMed] [Google Scholar]
- 39.Robertson A.K.H., Ramogida C.F., Schaffer P., Radchenko V. Development of (225). Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018;11(3):156–172. doi: 10.2174/1874471011666180416161908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N., Oudard S., Théodore C., James N.D., Turesson I., Rosenthal M.A., Eisenberger M.A. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 41.Rahbar K., Boegemann M., Yordanova A., Eveslage M., Schäfers M., Essler M., Ahmadzadehfar H. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur. J. Nucl. Med. Mol. Imaging. 2018;45(1):12–19. doi: 10.1007/s00259-017-3848-4. [DOI] [PubMed] [Google Scholar]
- 42.Pezaro C., Omlin A., Lorente D., Rodrigues D.N., Ferraldeschi R., Bianchini D., Mukherji D., Riisnaes R., Altavilla A., Crespo M., Tunariu N., de Bono J., Attard G. Visceral disease in castration-resistant prostate cancer. Eur. Urol. 2014;65(2):270–273. doi: 10.1016/j.eururo.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lassmann M., Nosske D., Reiners C. Therapy of ankylosing spondylitis with 224Ra-radium chloride: dosimetry and risk considerations. Radiat. Environ. Biophys. 2002;41(3):173–178. doi: 10.1007/s00411-002-0164-5. [DOI] [PubMed] [Google Scholar]
- 44.Kratochwil C., Giesel F.L., Stefanova M., Benešová M., Bronzel M., Afshar-Oromieh A., Mier W., Eder M., Kopka K., Haberkorn U. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016;57(8):1170–1176. doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 45.Baum R.P., Kulkarni H.R., Schuchardt C., Singh A., Wirtz M., Wiessalla S., Schottelius M., Mueller D., Klette I., Wester H.J. 177Lu-Labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J. Nucl. Med. 2016;57(7):1006–1013. doi: 10.2967/jnumed.115.168443. [DOI] [PubMed] [Google Scholar]
- 46.Kiess A.P., Minn I., Vaidyanathan G., Hobbs R.F., Josefsson A., Shen C., Brummet M., Chen Y., Choi J., Koumarianou E., Baidoo K., Brechbiel M.W., Mease R.C., Sgouros G., Zalutsky M.R., Pomper M.G. (2S)-2-(3-(1-Carboxy-5-(4-211At-Astatobenzamido) Pentyl)Ureido)-Pentanedioic Acid for PSMA-Targeted α-Particle Radiopharmaceutical Therapy. J. Nucl. Med. 2016;57(10):1569–1575. doi: 10.2967/jnumed.116.174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stenberg V.Y., Juzeniene A., Chen Q., Yang X., Bruland O.S., Larsen R.H. Preparation of the alpha-emitting PSMA targeted radioligand [212Pb]Pb-NG001 for prostate cancer. J. Labelled Comp. Radiopharm. 2020;63(3):129–143. doi: 10.1002/jlcr.3825. [DOI] [PubMed] [Google Scholar]
- 48.Larsen R.H. 2016.
- 49.Fan X., Wang L., Guo Y., Tu Z., Li L., Tong H., Xu Y., Li R., Fang K. Ultrasonic nanobubbles carrying anti-PSMA nanobody: construction and application in prostate cancer-targeted imaging. PLoS One. 2015;10(6):e0127419. doi: 10.1371/journal.pone.0127419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chakrabarti M.C., Le N., Paik C.H., De Graff W.G., Carrasquillo J.A. Prevention of radiolysis of monoclonal antibody during labeling. J. Nucl. Med. 1996;37(8):1384–1388. [PubMed] [Google Scholar]
- 51.Salako Q.A., O’Donnell R.T., DeNardo S.J. Effects of radiolysis on yttrium-90-labeled Lym-1 antibody preparations. J. Nucl. Med. 1998;39(4):667–670. [PubMed] [Google Scholar]
- 52.Schwartz J., Jaggi J.S., O’Donoghue J.A., Ruan S., McDevitt M., Larson S.M., Scheinberg D.A., Humm J.L. Renal uptake of bismuth-213 and its contribution to kidney radiation dose following administration of actinium-225-labeled antibody. Phys. Med. Biol. 2011;56(3):721–733. doi: 10.1088/0031-9155/56/3/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Kruijff R.M., Wolterbeek H.T., Denkova A.G. A Critical review of alpha radionuclide therapy-how to deal with recoiling daughters? Pharmaceuticals (Basel) 2015;8(2):321–336. doi: 10.3390/ph8020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poty S., Carter L.M., Mandleywala K., Membreno R., Abdel-Atti D., Ragupathi A., Scholz W.W., Zeglis B.M., Lewis J.S. Leveraging bioorthogonal click chemistry to improve 225ac-radioimmunotherapy of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2019;25(2):868–880. doi: 10.1158/1078-0432.CCR-18-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartoś B., Lyczko K., Kasperek A., Krajewski S., Bilewicz A. Search of ligands suitable for 212Pb/212Bi in vivo generators. J. Radioanal. Nucl. Chem. 2013;295(1):205–209. doi: 10.1007/s10967-012-2238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atcher R.W., Friedman A.M., Hines J.J. An improved generator for the production of 212Pb and 212Bi from 224Ra. Int. J. Rad. Appl. Instrum. [A] 1988;39(4):283–286. doi: 10.1016/0883-2889(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 57.Hassfjell S.A. 212Pb generator based on a 228Th source. Appl. Radiat. Isot. 2001;55(4):433–439. doi: 10.1016/S0969-8043(00)00372-9. [DOI] [PubMed] [Google Scholar]
- 58.Benešová M., Bauder-Wüst U., Schäfer M., Klika K.D., Mier W., Haberkorn U., Kopka K., Eder M. Linker modification strategies to control the prostate-specific membrane antigen (PSMA)-targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J. Med. Chem. 2016;59(5):1761–1775. doi: 10.1021/acs.jmedchem.5b01210. [DOI] [PubMed] [Google Scholar]
- 59.de Zanger R.M.S., Chan H.S., Breeman W.A.P., de Blois E. Maintaining radiochemical purity of [177Lu]Lu-DOTA-PSMA-617 for PRRT by reducing radiolysis. J. Radioanal. Nucl. Chem. 2019;321(1):285–291. doi: 10.1007/s10967-019-06573-y. [DOI] [Google Scholar]
- 60.Umbricht C.A., Benešová M., Schibli R., Müller C. Preclinical development of novel PSMA-targeting radioligands: modulation of albumin-binding properties to improve prostate cancer therapy. Mol. Pharm. 2018;15(6):2297–2306. doi: 10.1021/acs.molpharmaceut.8b00152. [DOI] [PubMed] [Google Scholar]
- 61.Larsen R.H. 2019.
- 62.Castanares M.A., Copeland B.T., Chowdhury W.H., Liu M.M., Rodriguez R., Pomper M.G., Lupold S.E., Foss C.A. Characterization of a novel metastatic prostate cancer cell line of LNCaP origin. Prostate. 2016;76(2):215–225. doi: 10.1002/pros.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michalska M., Schultze-Seemann S., Bogatyreva L., Hauschke D., Wetterauer U., Wolf P. In vitro and in vivo effects of a recombinant anti-PSMA immunotoxin in combination with docetaxel against prostate cancer. Oncotarget. 2016;7(16):22531–22542. doi: 10.18632/oncotarget.8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yordanova A., Becker A., Eppard E., Kürpig S., Fisang C., Feldmann G., Essler M., Ahmadzadehfar H. The impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2017;44(9):1473–1479. doi: 10.1007/s00259-017-3681-9. [DOI] [PubMed] [Google Scholar]
- 65.Sarnelli A., Belli M.L., Di Iorio V., Mezzenga E., Celli M., Severi S., Tardelli E., Nicolini S., Oboldi D., Uccelli L., Cittanti C., Monti M., Ferrari M., Paganelli G. Dosimetry of 177Lu-PSMA-617 after mannitol infusion and glutamate tablet administration: preliminary results of EUDRACT/RSO 2016-002732-32 IRST protocol. Molecules. 2019;24(3):E621. doi: 10.3390/molecules24030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baum R.P., Langbein T., Singh A., Shahinfar M., Schuchardt C., Volk G.F., Kulkarni H. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: an empirical proof of a promising concept. Nucl. Med. Mol. Imaging. 2018;52(1):80–81. doi: 10.1007/s13139-017-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rathke H., Kratochwil C., Hohenberger R., Giesel F.L., Bruchertseifer F., Flechsig P., Morgenstern A., Hein M., Plinkert P., Haberkorn U., Bulut O.C. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur. J. Nucl. Med. Mol. Imaging. 2019;46(1):139–147. doi: 10.1007/s00259-018-4135-8. [DOI] [PubMed] [Google Scholar]
- 68.Nilsson S., Larsen R.H., Fosså S.D., Balteskard L., Borch K.W., Westlin J.E., Salberg G., Bruland O.S. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin. Cancer Res. 2005;11(12):4451–4459. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 69.Henriksen G., Fisher D.R., Roeske J.C., Bruland O.S., Larsen R.H. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J. Nucl. Med. 2003;44(2):252–259. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.