Abstract
Ashwagandha (W. somnifera Dunal. Linn.), known as Indian ginseng, contains three major bioactive compounds, withaferin-A (WA), 12-deoxywithastramonolide (WO) and withanoloide A (WD). In a field experiment, the impacts of foliar application of growth retardants/promoters was assessed with respect to biomass allocation pattern and major withanoloide content at different phenological stages in W. somnifera. Biomass accumulation pattern showed that foliar application of 500 mg l−1ethrel at 50, 65, 85, 105, and 120 days after sowing (DAS) restricted phenological progression and reduced berry weight by 61% as comparted to the control at 160 DAS. 500 mg l−1 succinic acid foliar application resulted in maximum plant height (56.4 cm), maximum dry stem weight (DWS) and dry root weight (DRW) whereas 500 mg l−1 ethrel had resulted in minimum plant height and DRW at 180 DAS. During last 50 days of crop growth, the accumulation pattern drastically changed with more than 60% of the biomass allotment to the reproductive part, the berries. The WD in roots ranged between 0.325 mg g−1and 0.342 mg g−1 during all growth stages. WA content decreased with increase in progression of crop growth and reached the lowest at 180–190 DAS. In a pot experiment, ethrel application up regulated DWF-5 by 2.44, SQE by 3.79 and CYP450s by 1.17 log2fold in roots 8 h after treatment and succinic acid had up regulated the expression of all these genes by nearly 3 log2fold change. This is in accordance with the withanoloide accumulation pattern in field condition under foliar application of these molecules.
Keywords: Biomass partitioning, Gene expression, Residue analysis, W. somnifera, Withanoloides, Horticulture, Plant physiology, Phytochemistry, Root, Metabolite, Chromatography, Plant biology, Agriculture
Biomass partitioning, Gene expression, Residue analysis, W. somnifera, Withanoloides; Horticulture; Plant physiology; Phytochemistry; Root; Metabolite; Chromatography, Plant biology, Agriculture.
1. Introduction
Ashwagandha (Withania somnifera Dunal. Linn.), the Indian ginseng is an important commercial medicinal crop cultivated to harvest mainly roots for their medicinal values. As per ayurveda, ashwagandha roots fall under rasayana category. The literal meaning of rasayana is obtaining the optimum nourishment to the dhatu, the fundamental tissues in human body (National Health Portal Government of India, 2019; http://www.nhp.gov.in/). Rasayanas are known to promotes health and longevity through enhanced defence against disease, slowing down the process of ageing, revitalising the body in debilitated conditions. In Ayurvedic practice, it is used to calm the mind, relieve weakness and nervous exhaustion. It is also used to promote sexual energy and warrant healthy sleep (Narinderpal et al., 2013). The pharmacological effects of the roots of this plant are attributed to the presence of withanolides, a group of steroidal lactones (Budhiraja and Sudhir, 1987). Leaves of this plants are used in Ayurvedic and Unani systems for treatment of tumors and tubercular glands (Chopra, 1994). For neurodegenerative diseases such as Parkinson's, Huntington's and Alzeimer's diseases, the use of ashwagandha was found to be promising (Singh et al., 2011). Withaferin-A (WA), 12-deoxywithastramonolide (WO) and withanoloide A (WD) are the major bioactive compounds found in different plant parts of W. somnifera. This herb is a part of more than 100 formulations for health perspectives and therefore, there is a huge pharmaceutical demand making this crop a potential crop with significant industrial supplies.
The crop Withania somnifera passes through various growth stages. The phenological progression leads to morphological and quality changes in roots (Dhar et al., 2016). The normal vegetative growth of 60 days is required for flower bud initiation in W. somnifera. Therefore, changes in phenological progression may alter biomass partitioning as well as the quality parameters in W. somnifera. Paclobutrazol (PBZ) had reported to limit production of gibberellin which is required for cell elongation and growth in plants. There are reports in which ethylene had inhibited vegetative growth in many plants. Increased content of the immediate precursors of the ethylene and decrease in plant growth is well correlated. Succinic acid is a key intermediate in a primary metabolic pathway leading production of chemical energy in the presence of O2. Application of PBZ both as a soil drench and foliar application were effective in growth regulation in various crops (Kishore et al., 2015). In India, PBZ and ethephon have been registered as a plant growth regulator under section 9 (3) of Insecticides Act, 1968 in November 2009 by Central Insecticides Board & Registration and is available in the market with various trade names.
The biomass allocation which is the relative amount of biological yield of different plant parts like leaves, stem, roots and reproductive parts is not fixed. The biomass allocation may vary due to crop growth stage, environment condition and species differentiation (Reich, 2002). In ashwagandha, the later growth phase is very much important to get higher biomass production.
Thus, a field experiment was conducted with an objective to study the impact of foliar application of ethrel, PBZ and succinic acid on growth and quality parameters in W. somnifera. To study the impact foliar application of ethrel, PBZ and succinic acid at gene expression level, minimum impact of environmental condition is required. Hence, a separate pot experiment under control condition was conducted. Salicylic acid (SA) and methyl jasmonate (JA) are most widely used elicitor molecules in gene expression studies and therefore, they were also included in addition to ethrel, PBZ and succinic acid to study the gene expression pattern.
2. Materials and methods
2.1. Field experiment
A field experiment was conducted during two consecutive winter to spring seasons in year 2016 and 2017 (October 2016–April 2017and October 2017–April 2018). The experimental location is geographically located on N 22○ 55′, E 072○ 66’, MSL – 45 m at the Directorate of Medicinal and Aromatic Plants Research (DMAPR), Boriavi, Anand, Gujarat, India. The average rainfall, maximum and minimum temperature are 800 mm, 42 °C and 12.7 °C, respectively. The water holding capacity and the pH of the experimental sandy loam soil was 20–22% and the 7–7.5, respectively. Total eight treatments were repeated in four replications in randomized block design. Authentic seeds of W. somnifera Dunal cultivar JA 134 were procured from Mandsor centre of All India Co-ordinated Research Project on Medicinal and Aromatic Plants (AICRP on MAP), Vijaya Raje Scindia, Krishi Vishwa Vidhyalaya, Mandsor, Madhya Pradesh, India. Healthy seeds were sown in lines at 30 cm distance. To ascertain maximum germination and full plant strength, the first irrigation was given immediately after sowing. All agronomic practices including weeding, inter culturing, irrigation, fertilizer application etc., were followed as per recommended package of practices of this crop. Among the different treatments, there were no foliar application in T1: control whereas in T2: water, T3: 200 mg l−1 paclobutrazol (PBZ), T4: 500 mg l−1 PBZ, T5: 200 mg l−1ethrel, T6: 500 mg l−1ethrel, T7: 200 mg l−1 succinic acid and T8: 500 mg l−1 succinic acid was foliar applied at vegetative phase 50 and 65 days after sowing (DAS) and initial reproductive phase (85, 105, and 120 DAS). These growth regulators/retardants were foliar applied during morning hours in such a way to cover different crop growth stage. The spray periods were selected to cover different growth phases i.e. the first spray during vegetative growth period, second and third spray during flowering and berry initiation, respectively, fourth and the fifth spray during berry development phase. Observations on various growth parameters were recorded at four different growth periods to cover different growth stages i.e., stage I (110–115 DAS-berry development), stage II (125–130 DAS- end of berry development to initiation of berry maturity, green berry), stage III (155–160 DAS-berry maturity, green to red berry) and stage IV (180–190 DAS-harvest maturity with brown calyx).
2.2. Measurement of growth parameters
Plant height and root length were measured from freshly harvested plants. Sampled plants were separated into leaves, stem, roots and berries and dried under shade condition. Total arial biomass (TABM) and total biomass (TBM) were calculated based on the leaf dry weight (DWL), stem dry weight (DWS), root dry weight (DWR) and berry dry weight (BDW) measured after proper drying until constant moisture content. Based on the data, the biomass fraction of different organs like leaf mass fraction (LMF), shoot mass fraction (SMF), root mass fraction (RMF) and berry mass fraction (BMF) were calculated. Properly dried plant samples were ground to fine powder for chemical analysis.
2.3. Sample preparation for major withanoloides extraction
One-gram oven dried powdered samples were extracted with methanol (3 × 75 mL, 60 min), under reflux, on water bath maintained at 90 °C. The extracts were filtered, combined and then evaporated to dryness by rotary evaporator under reduced pressure. The dried extract samples were thereafter dissolved in 25 mL methanol and filtered through a 0.45-μm nylon membrane filter (M/s Whatman, India). The 10 μl filtrate samples were injected in UHPLC for analysis.
2.4. UHPLC condition
Ultimate 3000 UHPLC (M/s Thermo Fisher scientific) used for identification and quantification of withanoloides viz withanoloide-A, 12-deoxy withastramonolide and withaferin A. The reference standards were purchased from ChromaDex (CA, USA). The chromatographic separations were carried out by using RP- C18 column (Lichrocart, Merck, 250 × 4.6 mm, 5 μm). Mobile phase, flow rate, column temperature and peaks identification and content quantification were as per method given by Joshi et al. (2010).
2.5. Pesticide residual analysis
2.5.1. Chemicals and reagents
Methanol, water and acetonitrile (MS- grade) were procured from Merck (Darmstadt, Germany) and Formic Acid (99.5+%, Optima™) LC/MS Grade was purchased from Fisher Chemical (Fair lawn, NJ, USA). Paclobutrazol with purity 99.9 % was purchased from Sigma Aldrich (Germany). Stock solution of paclobutrazol (238.72 μg/ml) prepared in methanol and stored at-20.0 °C. The fresh working standards prepared in the range of 1–500 ng/mL from intermediate standard by suitably diluting with MeOH-Water (4:1:v/v).
2.5.2. Sample preparation and extraction procedure
Paclobutrazol residues from ashwagandha roots were prepared as per the method described by Mastovska et al. (2010). In final step, 1 ml of supernatant transferred and evaporated near to dryness and reconstituted with 2 ml of the methanol: water (80:20) for LC-MS/MS analysis.
2.5.3. UHPLC-MS/MS detection
The quantitative analysis of paclobutrazol was performed on Thermo Scientific made TSQ Quantum Access MAX equipped with Dionex made ultra-high performance liquid chromatograph (UHPLC) system (model: Dionex Ultimate 3000 RS). The separation was achieved on Hypersil Gold C18 column (150 × 4.6 mm, 5 μm particle size) with flow rate 0.3 ml/min and column oven temperature 30 °C. An elution gradient was used with solvents A (water with 5mM Ammonium format, 0.1%formic acid) and B (acetonitrile): 0–0.5 min, 98% B; 0.5–2 min, 98-60% B; 2–5 min, 60 -2 % B. The MS parameters of paclobutrazol were optimized in positive ionization mode with capillary voltage 4500 V; vaporizer temperature 350 °C; sheath gas (N2) 48 unit; aux gas (N2) 18 unit and capillary temperature 325 °C. The precursor ion of paclobutrazol was m/z 294.19, and production ion was m/z 125.0, 70.32 for paclobutrazol. The collision energy for product ion m/z 124.99 was 36 eV and for m/z 70.32 was 22 eV. Tube lens value was 145 V for both the product ions.
2.6. Data analysis and presentation
The data were subjected to analysis of variance for the randomized block design. The DSAASTAT Onofri (2007) in MS Excel based add-in module was used for statistical analysis and the least significant differences were calculated to assess the significance of treatment means where the “F” test was found significant at P ˂ 0.05.
2.7. Gene expression study
2.7.1. Pot experiment
A pot trial was conducted under control condition for q-PCR gene expression analysis of genes of withanoloide biosynthesis pathway. One healthy plant of ashwagandha was maintained per pot under poly carbonate house. The foliar chemical treatments were given to seedlings as (i) 500 μmethyl jasmonate (MJA) (ii) 500 μM salicylic acid (SA) (iii) 3460 μM ethrel (iv) 1700 μM paclobutrazol (PBZ) (v) 4235 μM succinic acid and distilled water spray as control treatment. The leaves were collected after 8 h of treatments and snap chilled in liquid nitrogen for RNA extraction.
2.7.2. RT-qPCR gene expression analysis of withanoloides biosynthesis pathway
Quantitative real-time PCR was conducted to evaluate the expression levels of sterol Δ7 reductase (DWF-5), squalene epoxidase (SQE) and cytochrome P450 reductase (CYP450s) genes in roots of treated ashwagandha plants. Total RNA was isolated using total RNA purification kit (Sigma-Aldrich) from roots. The primers were designed from sequences available at NCBI database using primer3 and the quality of primers were checked using oligo analyzer. Total RNA isolation, cDNA synthesize and RT-qPCR conditions and calculation of Log2fold changes are as per described by Kalariya et al. 2019.
3. Results
3.1. Plant growth characteristics
The climatic conditions during both crop period (CP I and CP II) are given in Figure 1 and the field view of the experiment is depicted in Figure 2. The visual observation of different treatments during both the crop periods showed that the plants sprayed with paclobutrazol had leathery dark green leaves whereas plants sprayed with 500 mg l−1ethrel became stunted with close canopy architecture having arrested berry formation (Figure 3) (till stage III) limiting the berry dry weight by 61% lesser than the control. Plants sprayed with 500 mg l−1 succinic acid were vigorous in growth. The analysis of variance (ANOVA) and least significance difference (LSD) at P = 0.05% for different parameters studied are presented in Table 1.
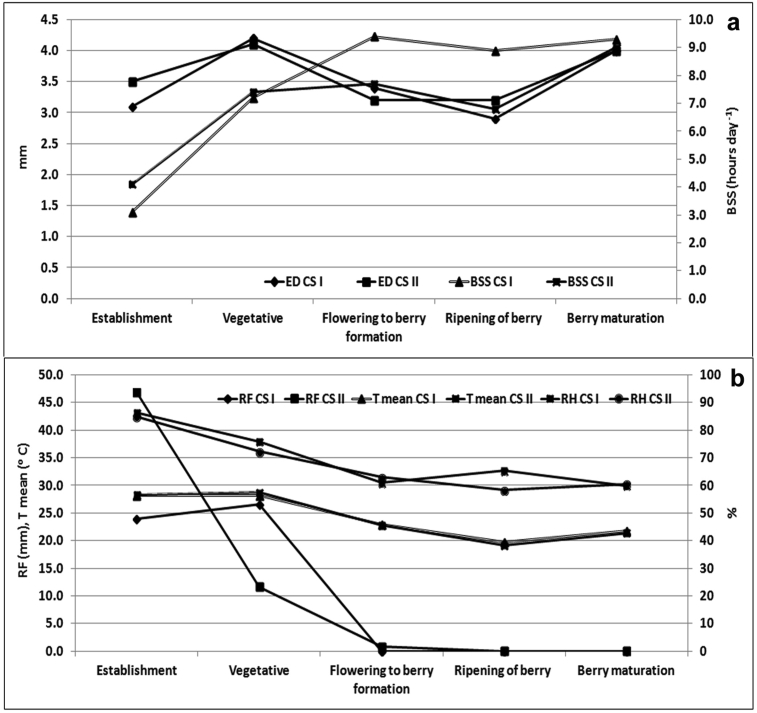
Figure 1.
Climatic conditions during crop growth period. (a) evaporative demand (ED) in mm and bright sun shine hours (BSS) (b) rainfall in mm, mean temperature in 0C and relative humidity (RH) in % during crop season (CS) I and II.
Figure 2.
Ashwagandha plants at 75 DAS. (a) field view of control plants (b) close-up view of control plants (c) field view of plants sprayed with 500 mg l−1 PBZ (d) close-up view of plants sprayed with 500 mg l−1 PBZ (e) field view of plants sprayed with 500 mg l−1 ethrel (f) close-up view of plants sprayed with 500 mg l−1 ethrel (g) field view of plants sprayed with 500 mg l−1 succinic acid (h) close-up view of plants sprayed with 500 mg l−1 succinic acid.
Figure 3.
Growth behaviour of ashwagandha at 155 DAS under different treatment. T1: control T2: water, T3: 200 mg l−1 paclobutrazol (PBZ), T4: 500 mg l−1 PBZ, T5: 200 mg l−1ethrel, T6: 500 mg l−1ethrel, T7: 200 mg l−1 succinic acid and T8: 500 mg l−1 succinic acid foliar applied at 50, 65, 85, 105, and 120 DAS.
Table 1.
Analysis of variance (ANOVA) and least significance difference (LSD) at P = 0.05% for different parameters studied.
| EFFECT | DF | PLH | RTL | DWL | DWS | DWR | DWB | TABM | TBM | LMF | SMF | RMF | BMF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage | 3 | 4.89 | 1.91 | 1.02 | 1.54 | 0.36 | 2.32 | 4.15 | 4.40 | 0.018 | 0.019 | 0.008 | 0.026 |
| Year X Stage | 3 | NS | 2.70 | 1.44 | 2.18 | NS | 3.27 | 5.86 | NS | 0.025 | 0.027 | NS | 0.037 |
| Treatment | 7 | 2.71 | NS | 0.76 | 1.86 | 0.32 | 3.69 | 5.46 | 5.62 | 0.017 | 0.021 | 0.011 | 0.027 |
| Year X Treatment | 7 | NS | NS | 1.07 | 2.63 | NS | NS | NS | NS | 0.024 | 0.029 | NS | 0.038 |
| Stage X Treatment | 21 | NS | NS | 1.52 | NS | NS | NS | NS | NS | 0.034 | 0.042 | 0.021 | 0.054 |
| Year X Stage X Treatment | 21 | NS | NS | 2.15 | 5.25 | NS | NS | NS | NS | NS | 0.059 | 0.030 | 0.076 |
PLH-Plant height, RTL-root length DWS-dry shoot weight, DWR-dry root weight, DWL-dry leaf weight, DWB-dry berry weight, TABM-total aerial biomass, TBM-total biomass, LMF-leaf mass fraction, SMF-shoot mass biomass, RFM-root mass biomass, BMF- berry mass fraction.
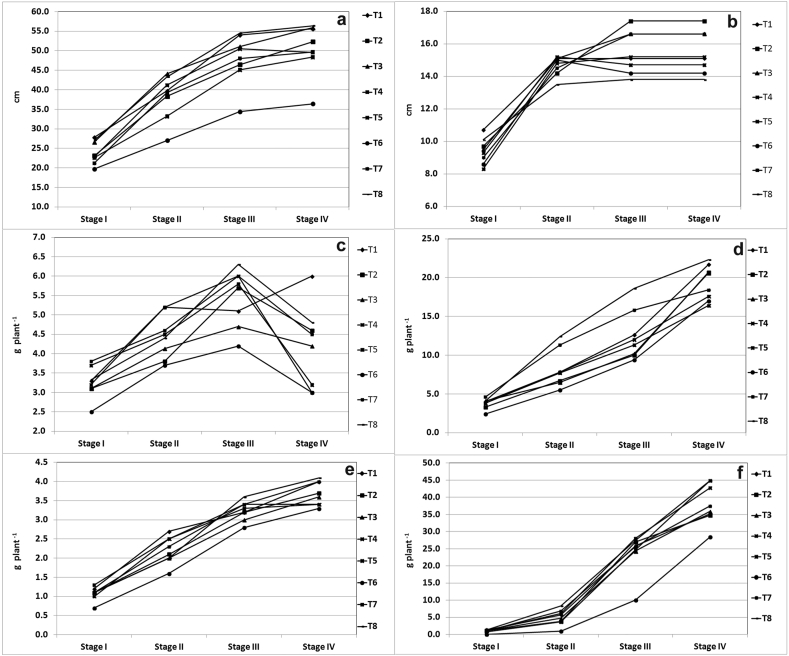
Plant height steadily increased from 23.9cm to 50.5 cm from stage I to stage IV. Plant height was minimum in T6 treated plants across different growth stages (Figure 4 a). Root length increased from 9.4 cm–14.1 cm from stage I to stage IV. The root length became steady after growth stage III (Figure 4b). Mean leaf dry weight was maximum at stage III. Minimum leaf dry weight was noted in T6 at all growth stages (Figure 4c). Stem dry weight continuously increased from stage I to stage IV. The stem dry weight was maximum in T8 at stage IV (Figure 4d). Root dry weight and berry dry weight continuously increase with increase in growth stage. Root dry weigh was maximum in T8 and minimum in T6 (Figure 4e). The berry dry weight was 0.9 g plant−1 at stage I and reached as high as 38.1 g plant−1 at stage IV (Figure 4f). Treatment T6 restricted the increase in berry weight leading to only 10.0 g plant−1 as compared to the maximum berry weight of 28.0 g plant−1 in T4 at stage III.
Figure 4.
Grwoth parameters in W. Somnifera during different growth stages. (a) plant height (b) root length (c) dry leaf weight (d) dry stem weight (e) dry root weight (f) dry berry weight.
3.2. Biomass partitioning
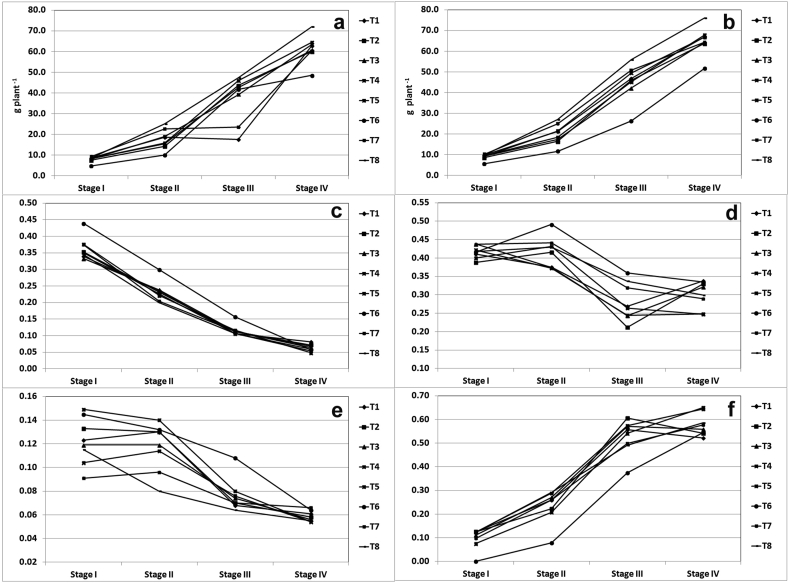
The TABM and TBM were 7.9 and 9.0 g plant −1 respectively, at stage I and increased to 61.6 and 65.3 g plant −1 respectively at stage IV (Figure 5 a,b). The organ specific biomass accumulation pattern changed with progression of growth stage. The LMF which was 0.364 at stage I decreased continuously and reached as low as to 0.063 at stage IV. The LMF was maximum in T6 at stage I, II and III (Figure 5 c). The SMF was 0.416 at stage I and stage II and decreased to 0.301 at stage IV. The SMF was maximum in T6 at stage II and III (Figure 5 d). The RMF was decreased continuously across the stages. It was 0.122 at stage I and decreased to 0.058 at stage IV (Figure 5 e). The berry mass fraction was 0.097 at stage I and reached to the maximum of 0.578 at stage IV. It is interesting to note that the stem was active sink till stage II whereas stage III onwards the active sink was berry which resulted in as high as nearly 60% of the total biomass allocation in berry in just 80 days period (Figure 5 f). The BMF was less than 37% at stage III in T6 treated plants.
Figure 5.
Biomass partitioning in W. Somnifera during different growth stages. (a) total arial biomass (b) arial biomass (c) leaf mass fraction (d) stem mass fraction (e) root mass fraction (f) berry mass fraction.
3.3. Major withanoloides content in roots at different growth stages
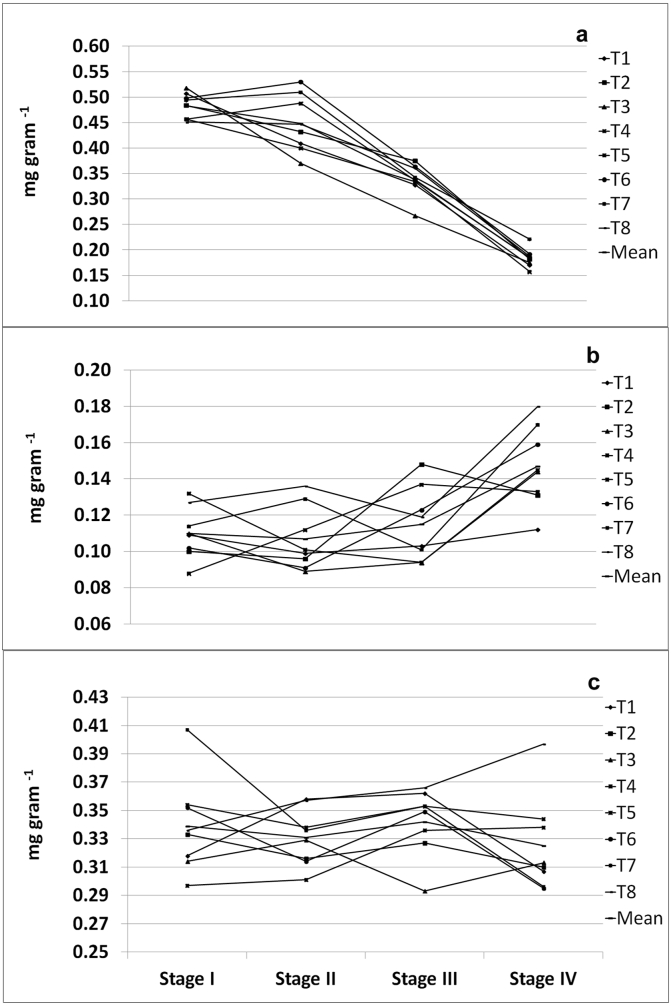
The HPLC chromatogram of major withanoloides are given in Figure 6. Two years mean WA content in roots decreased with increase in progression of crop growth and reached the lowest at stage IV. A very slow rate of increase in WO in roots is noted till stage III which then reached to the maximum at stage IV. Mean WA in roots was 0.483 mg g-1 at stage I, 0.448 mg g-1 at stage II and decreased to 0.338 mg g−1 stage III and further drastically decreased to 0.184 mg g−1 at stage IV accounting 62% reduction as compared to the stage III. Among all the treatments, T3 had the highest WA in roots at stage I whereas it was maximum in T7 at stage IV. The highest WO in roots was in T8 (0.180 mg g−1) followed by T7 (0.170 mg g−1) at stage IV. The mean WD in roots ranged between 0.325 mg g−1and 0.342 mg g−1 during all growth stages. There was a mix response in WD content with growth stage except in T8 in which the WD continuously increased from 0.336 mg g−1 stage I to 0.397 mg g−1 stage IV (Figure 7).
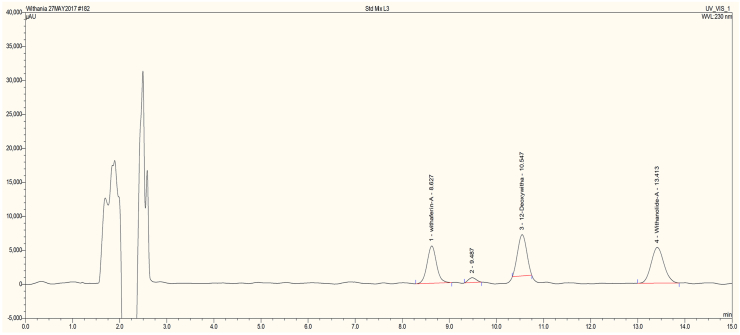
Figure 6.
HPLC chromatogram of major withanoloides standards.
Figure 7.
Content and accumulation pattern of (a) WA, (b) WO and (c) WD in roots of W. somnifera at different growth stages. (a).
3.4. Pesticide residue
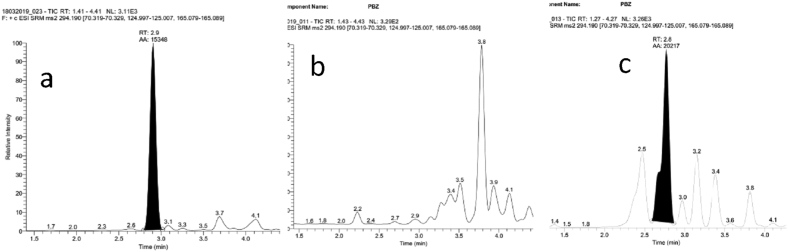
The chromatograph of paclobutrazol showing paclobutrazol standard and sample is given in Figure 8. The residues of paclobutrazol in ashwagandha roots were below quantification limit (BQL) i. e 0.006 mg/kg at stage I. During second and third stage the residues of paclobutrazol were in the range of 0.06–0.09 mg/kg at either dose (Table 2). Subsequently, the concentration of paclobutrazol slowly and steadily declined with the time, which has been reflected from the BQL values observed at stage-IV on both doses. However, the samples from remaining treatments were free from paclobutrazol residues.
Figure 8.
Chromatograph of paclobutrazol showing (a) Paclobutrazol standard 50 ppb (b) sample blank (c) sample.
Table 2.
Residue of paclobutrazol in the roots of Ashwagandha.
| Treatment Detail | Stage-I |
Stage-II |
Stage-III |
Stage-IV |
|---|---|---|---|---|
| 110-115 DAS | 125-130 DAS | 155-160 DAS | 180-190 DAS | |
| T1 | BQL# | BQL | BQL | BQL |
| T2 | BQL | BQL | BQL | BQL |
| T3 | BQL | 0.06ppm | 0.05ppm | BQL |
| T4 | BQL | 0.09ppm | 0.08ppm | BQL |
| T5 | BQL | BQL | BQL | BQL |
| T6 | BQL | BQL | BQL | BQL |
| T7 | BQL | BQL | BQL | BQL |
| T8 | BQL | BQL | BQL | BQL |
| Stage-I | Stage-II | Stage-III | Stage-IV |
|---|---|---|---|
| had 04 sprays at 50,65,85,105 DAS | had 05 sprays at 50,65,85,105 and 120 DAS | had 05 sprays at 50,65,85,105 and 120 DAS | had 05 sprays at 50,65,85,105 and 120 DAS |
| Samplings at 110–115 days | Samplings at 125–130 days | Samplings at 155–160 days | Samplings at 180–190 days |
| Sampling time from last spray-05 days | Sampling time from last spray-05 days | Sampling time from last spray-35 days | Sampling time from last spray-60 days |
BQL-below quantification limit, DAS- days after sowing.
BQL=<LOQ i.e. 0.006 mg/kg.
3.5. Differential expression of genes involved in withanoloides biosynthesis in response to foliar application
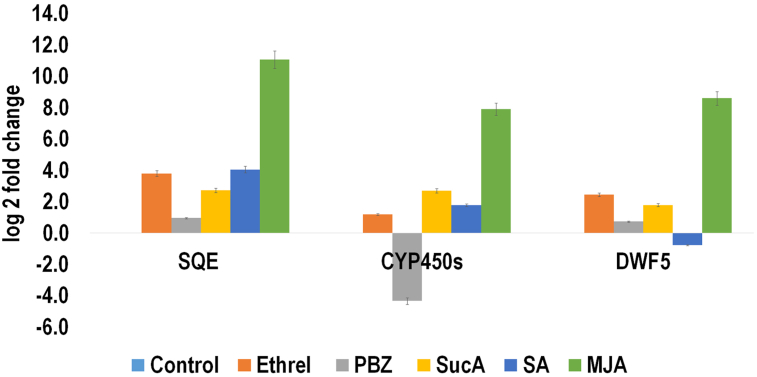
The primer sequences used to monitor expression profiling of different genes on real time basis are mentioned in Table 3. The expression levels in terms of log2fold changes of sterol Δ7 reductase (DWF-5), squalene epoxidase (SQE) and cytochrome P450 reductase (CYP450s) conferring increase in withanoloides contents in roots varied under different treatments. The result showed that the expression of SQE, CYP450s and DWF-5 genes in roots increases due to application of all tested elicitors except CYP450 under PBZ application and DWF-5 under SA application after 8 h of foliar application (Figure 9). Ethrel application increased expression of DWF-5 by 2.44 log2fold, SQE by 3.79 log2fold and CYP450s by 1.17 log2fold in roots 8 h after treatment. The MJA had maximum positive impact with more than 8 log2fold change during expression profiling of these genes on real time basis. Succinic acid had also positively up regulated the expression of theses by nearly 3 log2fold changes.
Table 3.
qRT-PCR primer sequences for gene expression analysis.
| Gene | Sequences | Tm | GC content (%) |
|---|---|---|---|
| CYP450s | CCTGCTGACCCTTCAACTCC | 60.32 | 60 |
| CTTTCTGTGGCCCTTCCCTT | 59.89 | 55 | |
| DWF-5 | CTTAGCTCGCCACTTCCACT | 59.75 | 55 |
| GCATCGGTCGTCATCCCTTT | 60.46 | 55 | |
| SQE | GCTACAACCACCACTACCACTACA | 57.38 | 50 |
| GTAGCCACCAGGTTGTAGGAGTTC | 59.09 | 54 |
Figure 9.
Log2fold changes in expression of DWF-5, SQE and CYP450s in roots of W. somnifera.
4. Discussion
PBZ application prevents synthesis of Gibberellins (GAs) by its interaction with kaurene oxidase, a cytochrome P-450 oxidase, and inhibits the microsomal oxidation of kaurene, kaurenal, and kaurenol. Decrease cell division and elongation at the apical meristem of the shoot are results of reduced GAs but, has little effect on the production of leaves or root growth (Lambers et al., 1995). In our study, the reduction in dry weight per plant under 500 mg l−1 PBZ applications seems to be the results of antagonistic effects of GAs. The ethylene had been reported to act as both stimulator as well as an inhibitor in plants but, largely was as a growth inhibitor at later growth stage and as growth promoter at early growth stage Sharp and Le Noble (2002). The PBZ treated plants senesced slowly compared to the control plants. The delayed onset of senescence in withania leaves seems probably by enhancement effects of PBZ on endogenous levels of cytokinins promoting chlorophyll formation and increased activity of certain antioxidant enzyme (Synkova et al., 2006).
High SMF and LMF in T6 during stage I to III was because the ehtrel application had restricted berry growth and hence, the photosynthates was incorporated in active sinks like leaf and stem. Decrease in RMF and increase in BMF towards maturity progression showed that berry became the active sinks.
Ethrel, after penetration into the plant tissues, is translocated and progressively decomposed to ethylene which reduces apical dominance and thereby increases axillary branching. The ethylene causes opposite effects of gibberellins in plants and thus changes the source–sink relationship. The delayed berry formation was because of the ethylene had led to the arrested vegetative growth. Prior to flowering, application of ethylene can result in flower abortion and delayed flowering.
Succinate, a key intermediate in the tricarboxylic acid cycle, is considered as the mimetic of salicylic acid for imparting resistance to abiotic stresses in plants (Gupta et al., 2017). Exogenous application of succinic acid had beneficial impacts on mineral nutrient uptake and caused increase in biomass in alfafa plants under aluminium toxicity (An et al., 2014). Foliar application of succinic acid successively at different growth stages would have facilitated biomass partitioning along with quality partitioning in terms of high WD accumulation in roots.
Paclobutrazol was reportedly absorbed through the roots and transported primarily through stem (via xylem) before accumulating in the leaves (Wang et al., 1986). Thus, the plants roots are the main avenue of PBZ absorption storage and distribution. The results obtained in the study reveals that the residues of paclobutrazol were BQL i.e. 0.06 mg/kg from the root samples of ashwagandha collected at first stage which was just after the second spray of paclobutrazol applied at the rate of 200 and 500 mgl-1. It is worth to mention that the PBZ application had not any beneficial effects on root weight or withanolides content. The residues of paclobutrazol recorded on first stage were on expected line because paclobutrazol is believed to be readily absorbed by plants through roots but due to foliar spray its residues were not detected in roots during the first stage. However, on later stage root system of ashwagandha had absorbed the paclobutrazol residues deposited on soil due to foliar spray losses. This might be a probable reason of detection of paclobutrazol residues on II&III growth stage of ashwagandha. The rate of loss of paclobutrazol residue dissipation is quite slow as its residues detected on II &III growth stage of ashwagandha were quantitatively more or less similar but were BQL (<0.006 mg/l) on stage IV. It was reported that paclobutrazol residues in roots of Nerium oleander L. seedlings remained constant during the experimental period. The high level of PBZ residues detected in the substrate 30 days after application had decreased significantly (by 67%) by the end of the experiment (Ochoa Rego et al., 2009).
According to article 12 of Regulation (EC) No 396/2005, the European Food Safety Authority (EFSA) has reviewed the maximum residue levels (MRLs) for the pesticide active substance paclobutrazol by assessing the occurrence of paclobutrazol residues in plants, processed commodities, rotational crops and livestock. Based on the assessment of the available residue data and a consumer risk assessment, no apparent risk to consumers due to paclobutrazol was identified and established (Brancato et al., 2017) Although, the MRL value of the paclobutrazol is not available for Ashwagandha (Withania somnifera Dunal. Linn.) but the EU (EU, 2019) has suggested a default value of 0.02 mg/kg for roots of under the section teas, coffee, herbal infusions, cocoa and carobs (http://ec.europa.eu/food/plant/pesticides/eu-pesticides database/public). Consumption of ashwagandha could be safe although the residues of paclobutrazol were detected on II & III stage were above MRL value 0.02 mg/kg as farmers are predominantly harvest the ashwagandha crop after 4th stage which lies after 180 DAS when the residues of paclobutrazol were BQL (<0.006 mg/kg). Further, as a farmers practice, the harvested roots of ashwagandha are subjected to partial shade for proper drying could provide ample time where the concentration of trace amount of paclobutrazol could be reduced further.
Withanoloides are synthesized through sterol pathway the first step of which is conversion of squalene into 2,3 Oxidosqualene under the action of SQE gene. The conversion of 5-dehydroavenosterol to Δ5-avenosterol/isofucosterol is carried out by DWF 5. Cyclization of 24-methylene cholesterol compounds into withanoloides is mediated by CYP450s catalyzed (Gupta et al., 2013). In the current pot study, we found increased expression of important enzymes involved in withanoloides biosynthesis pathway under elicitor molecules i.e. SA and JA as well as ethrel and succinic acid application. This increased regulation of DWF-5 and SQE in roots up to four-fold after 8 h after treatment of foliar ethrel and SQE and CYP450s gene under succinic acid application after 8 h of treatment established their role in withanaloide biosynthesis pathway.
In green plants, photosynthesis is the sole source of energy and thus a plant has a limited amount of energy to divide between the multiple functions at a time. Plants have an option to put this energy in different proportion into different plant parts besides fulfilling the energy required for maintenance, storage and defence mechanism. Plants can decide priority in partitioning of this energy among various organs. In a medicinal plant the yield of an active principal compound depends on the content of the compound per unit weight of different plant parts. Thus, the quantitative understanding of functional partitioning is more complex in a medicinal plant than other crops. The rate of structural growth, storage, respiration and exudation of a root together decide the phloem import capacity to transport photosynthates from leaf to the roots. But reports are there in which roots have been shown as a poor sink (Brouwer, 1983). Reports are there in which exogenous application of succinic acid had positive impacts on mineral nutrient uptake and thereby increased biological yield in plants (An et al., 2014).
Organ specific biomass accumulation pattern changed with progression of growth stage in ashwagandha. It is interesting to note that the stem was active sink till stage II whereas stage III onwards the active sink was berry which resulted in as high as nearly 60% of the total biomass allocation in berry in last 80 days crop period. 500 mg l−1 can be used to delay berry formation and 500 mg l−1 succinic acid foliar application can be used to harvest greater biomass including root yield along with higher WO in roots. The PBZ application had no beneficial effects on root weight or withanolides content however, if applied as fungicide the residues of paclobutrazol in ashwagandha roots remains below the quantification limit 70 days after foliar application. Over expression of DWF-5, SQE and CYP450s in roots 8 h after treatment of ethrel and succinic acid are in conformity with the improved quality of ashwagandha in field condition due to foliar application of these molecules.
Declarations
Author contribution statement
Kuldeepsingh A. Kalariya: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Narendra A. Gajbhiye: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Dipal Minipara, Sushil Kumar, Vanrajsinh Solanki, Susheel Singh: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Parmeshwar L. Saran: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Prince Choyal: Contributed reagents, materials, analysis tools or data.
Ponnuchamy Manivel: Analyzed and interpreted the data.
Funding statement
This work was supported by Indian Council of Agricultural Research, New Delhi.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the Director, ICAR-Directorate of Medicinal and Aromatic Plants Research, Boriavi, Gujarat for providing all basic facilities. We are also thankful to AICRP on MAP, Mandsor, Centre, VRSKVV, Madhya Pradesh, India for providing seed material used in this study.
References
- An Y., Zhou P., Xiao Q., Shi D. Effects of foliar application of organic acids on alleviation of aluminum toxicity in alfalfa. J. Plant Nutr. Soil Sci. 2014;177(3):421–430. [Google Scholar]
- Brancato A., Brocca D., De Lentdecker C., Erdos Z., Ferreira L., Greco L., Janossy J., Jarrah S., Kardassi D., Leuschner R. European Food Safety Authority (EFSA), Review of the existing maximum residue levels for paclobutrazol according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal. 2017;15(8):e04974. doi: 10.2903/j.efsa.2017.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer R. Functional equilibrium: sense or nonsense? NJAS - Wageningen J. Life Sci. 1983;31(4):335–348. [Google Scholar]
- Budhiraja R.D., Sudhir S. Review of biological activity of withanolides. J. Sci. Ind. Res. 1987 [Google Scholar]
- Chopra R.N. Academic Publishers India; New Delhi: 1994. Glossary of Indian Medicinal Plants. [Google Scholar]
- Dhar R.S., Khan S., Khajuria R.K., Bedi Y.S. Dynamics of squalene content in different tissues of Ashwagandha (Withania somnifera L. Dunal) during its growth phases. Ind. Crop. Prod. 2016;84:375–380. [Google Scholar]
- U Pesticide database. 2019. http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue Downloaded from. CurrentMRL&language=EN Date :17-07-2019.
- Gupta P., Goel R., Pathak S., Srivastava A., Singh S.P., Sangwan R.S., Asif M.H., Trivedi P.K. De novo assembly, functional annotation and comparative analysis of Withania somnifera leaf and root transcriptomes to identify putative genes involved in the withanolides biosynthesis. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0062714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Hisano H., Hojo Y., Matsuura T., Ikeda Y., Mori I.C., Senthil-Kumar M. Global profiling of phytohormone dynamics during combined drought and pathogen stress in Arabidopsis thaliana reveals ABA and JA as major regulators. Sci. Rep. 2017;7(1):1–13. doi: 10.1038/s41598-017-03907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C., Gajbhiye N., Phurailatpam A., Geetha K.A., Maiti S. Comparative morphometric, physiological and chemical studies of wild and cultivated plant types of Withania somnifera (Solanaceae) Curr. Sci. 2010:644–650. [Google Scholar]
- Kalariya Kuldeepsingh, Gajbhiye N., Minipara D., Meena R.P., Kumar S., Saha A., Trivedi A., Manivel P. Deep sequencing-based de novo transcriptome analysis reveals biosynthesis of gymnemic acid in Gymnema sylvestre (Retz.) SchultD. Ecol. Genet. Genom. 2019 [Google Scholar]
- Kishore K., Singh H.S., Kurian R.M. Paclobutrazol use in perennial fruit crops and its residual effects: a review. Indian J. Agric. Sci. 2015;85(7):863–872. [Google Scholar]
- Lambers H., Nagel O.W., Van Arendonk J.J.C.M. The control of biomass partitioning in plants from favourable and stressful environments: a role for gibberellins and cytokinins. Bulg. J. Plant Physiol. 1995;21(2-3):24–32. [Google Scholar]
- Mastovska K., Dorweiler K.J., Lehotay S.J., Wegscheid J.S., Szpylka K.A. Pesticide multiresidue analysis in cereal grains using modified QuEChERS method combined with automated direct sample introduction GC-TOFMS and UPLC-MS/MS techniques. J. Agric. Food Chem. 2010;58(10):5959–5972. doi: 10.1021/jf9029892. [DOI] [PubMed] [Google Scholar]
- Narinderpal K., Junaid N., Raman B. A review on pharmacological profile of Withania somnifera (Ashwagandha) Res. Rev. J. Bot. Sci. 2013;2:6–14. [Google Scholar]
- National health portal, government of India. 2019. https://www.nhp.gov.in/rasayana_mtl2019
- Ochoa Rego J., Leemhuis F., Antonio J., Bañón Arias S.D.P., Fernández Hernández J.A. 2009. Distribution in Plant, Substrate and Leachate of Paclobutrazol Following Application to Containerized Nerium Oleander L. Seedlings. [Google Scholar]
- Onofri . 2007. Data Analysis and Presentation. Chapter: 6 Data Analysis and Interpretation. [Google Scholar]
- Reich P.B. 2002. Root-shoot Relations: Optimality in Acclimation and Adaptation or the ‘Emperor, S New Clothes. Plant Roots: the Hidden Half; pp. 205–220. [Google Scholar]
- Sharp R.E., LeNoble M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002;53(366):33–37. [PubMed] [Google Scholar]
- Singh N., Bhalla M., de Jager P., Gilca M. An overview on ashwagandha: a rasayana (Rejuvenator) of ayurveda. Afr. J. Tradit., Complementary Altern. Med. 2011;8(5S) doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synkova H., Semoradova S., Schnablová R., Witters E., Husak M., Valcke R. Cytokinin-induced activity of antioxidant enzymes in transgenic Pssu-ipt tobacco during plant ontogeny. Biol. Plantarum. 2006;50(1):31–41. [Google Scholar]
- Wang S.Y., Sun T., Faust M. Translocation of paclobutrazol, a gibberellin biosynthesis inhibitor, in apple seedlings. Plant Physiol. 1986;82(1):11–14. doi: 10.1104/pp.82.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]