Abstract
Background
Epirubicin is a first-line chemotherapeutic drug for the clinical treatment of diffuse large B cell lymphoma (DLBCL), but the overexpression of multidrug resistance (MDR) transporter proteins, especially P-glycoprotein (P-gp), renders epirubicin ineffective. Some studies reveal the potential role of melatonin in chemotherapeutic synergy and MDR.
Methods
The cell viability and apoptosis were determined by CCK-8 assay and acridine orange/ethidium bromide (AO/EB) fluorescence staining assay. Immunofluorescence and immunohistochemical staining were used to detect the expression of P-gp in DLBCL cells and tissues. Rhodamine-123 accumulation assay was used to evaluate the pump function of P-gp. The possible mechanisms of melatonin sensitize DLBCL cells to epirubicin were explored by western blotting, cytochrome C release, and pulldown assay.
Results
Melatonin significantly enhanced the epirubicin-induced cell proliferation suppression, epirubicin-induced apoptosis, and reduced the IC50 value of epirubicin. Further, melatonin synergized with epirubicin to promote the activation of the mitochondria-mediated apoptosis pathway and increased the accumulation of epirubicin in DLBCL cells by inhibiting the expression and function of P-gp. Immunohistochemical staining studies revealed that P-gp expression was positively correlated with P65 expression. Epirubicin was subsequently discovered to upregulate the expression of P-gp by activating the NF-κB pathway in the DLBCL cells. Melatonin reduced the amount of P65 protein in the nucleus and abrogated the ability of P65 to bind to the ABCB1 promoter, decisively suppressing P-gp expression.
Conclusions
Our results demonstrated that melatonin inactivates the NF-κB pathway and downregulates the expression of P-gp, ultimately sensitizing DLBCL cells to the epirubicin that suppresses their growth.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common B-cell non-Hodgkin lymphoma (NHL), composing about 30%–35% of all NHLs [1]. More than half of DLBCL patients can be cured by using anthracycline-based chemotherapy regimens, even in advanced stages [2]. However, DLBCL is a heterogeneous diagnostic category, which many subtypes and subpopulations are at high-risk for standard immune-chemotherapy failure [3,4]. About one-third of patients have refractory disease or replase after treatment, which remains a major cause of morbidity and mortality [5].
Epirubicin is a cell-permeable antitumor drug belonging to the anthracycline family, widely used in the treatment of DLBCL [6,7]. Similar to other anthracyclines, epirubicin act by intercalating with cell DNA or binding to DNA topoisomerase II, ultimately leads to hinders DNA/RNA synthesis and proliferation of the tumor cells [8,9]. Despite epirubicin being potent anticancer therapeutic agents, it's clinical usefulness is limited due to chemotherapy resistance [10].
Melatonin is a highly conserved indoleamine that rhythmic secreted from the pineal gland and other organs, including the retina, bone marrow and the gastrointestinal tract [11]. Endogenous oscillators within the suprachiasmatic nucleus (SCN) control the circadian rhythm (light-dark cycle) production of melatonin [12]. Previous reports have indicated that high melatonin levels play positive and important roles in health and anti-aging [13,14], however, the production of melatonin gradually decreases with age [15]. Melatonin exerts numerous physiological functions through receptor-dependent and receptor-independent mechanisms [16]. In mammals, three binding receptors for melatonin have been identified: the transmembrane receptors (MT1 and MT2), MT3 receptor located in the cytosol and the nuclear retinoid orphan receptor/retinoid Z receptors (ROR/RZR) [17,18]. Melatonin helps coordinate circadian rhythms and endocrine processes via activation of MT1 and MT2, which belong to G protein-coupled receptors [19,20]. MT3 may be a detoxification enzyme and exhibits a low affinity for iodomelatonin [18]. Furthermore, melatonin could participate in immunological processes by interacting with ROR/RZR [21]. Besides, melatonin directly detoxifies reactive oxygen species (ROS) and reactive nitrogen species (RNS) by receptor-independent pathway [22]. In addition to its abundant actions described above, various studies investigated the effects of melatonin against cancer, including antiproliferative, proapoptotic and regulate epigenetic responses [[23], [24], [25]]. Meanwhile, melatonin protects the normal cells from the harmful effects of chemotherapy by its antioxidant properties and by reducing the therapeutic doses of anticancer drugs [26]. Melatonin may be a promising supplementary component in chemotherapy.
The problem of chemotherapy resistance comes along with the use of cytotoxic agents [27]. In clinical situations, differences and change were observed in the chemotherapy-sensitive of certain cancer cells. Resistance could be divided into two types: single-agent resistance and multidrug resistance (MDR). The former resistance limited to the drugs to which patients were exposed. The phenomenon that simultaneous insensitivity to multiple drugs with different mechanisms of action called multidrug resistance (MDR), and has been recognized as a major reason for the failure of cancer treatment [28]. The mechanism of MDR has always been a hotspot of cancer research. Based on extensive studies, the expression of members of the ATP-binding cassette (ABC) family of drug efflux transporters, especially P-glycoprotein was frequently considered to be the cause of MDR [29].
P-glycoprotein is a multidomain polytopic membrane protein encoded by the ABCB1 gene located on chromosome 7, and it utilizes the energy from ATP binding and hydrolysis to perform a vast array of transport functions. [30,31]. The P-glycoprotein substrates include a broad spectrum of antitumor drugs, such as anthracyclines, vinca alkaloids, podophyllotoxins, and taxanes [32]. Normal expression of P-glycoprotein is observed in the transport epithelium of the kidney, liver, and gastrointestinal tract [33,34].P-gp in normal tissues can influence the absorption, tissue distribution, and/or elimination of drug and other xenobiotics. Nevertheless, overexpression of P-gp is usually found in drug-resistance human tumors, in particular acute myelogenous leukemia and breast cancer, causing a reduction of the intercellular drug concentrations through an ATP-dependent pump [35,36]. Despite enormous and sustained efforts to develop and implement effective P-gp modulators, subject to the difficulty in designing clinical trials and the unacceptable patients toxicities due to adverse pharmacokinetic interactions with coadministered anticancer drugs, still, no effective P-gp modulators could be used in clinical treatment [36,37].
The NF-κB pathway is an important crossroad where other pathways converge as a phenotype of resistance that emerges in patients who fail frontline and salvage immune-chemotherapy and its activation was considered to be the major mechanism of drug resistance in DLBCL [38,39]. Previous studies have demonstrated that inactivating the NF-κB pathway could inhibit P-gp expression [40,41]. Melatonin also has been reported to have an influence on the expression of P-gp and then increase the chemotherapy-sensitive of colon cancer cells [42]. There are still unknowns about the mechanism of melatonin reversing drug resistance.
Herein, we explored whether melatonin affects the chemotherapy-sensitive of epirubicin in DLBCL cells or not and further investigated the detailed underlying mechanisms. We find that such sensitization may through activation of the mitochondria-related apoptosis pathway and inhibiting P-gp expression via the NF-κB signaling pathway.
Materials and methods
Cell lines and cell culture
The human DLBCL cell lines SUDHL-10 and SUDHL-6 were obtained from the China Center for Type Culture Collection (CCTCC, Wuhan, China). All cells were grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Biological Industries, Inc., Israel), 100 μg/mL penicillin and 100 μg/mL streptomycin. Cells were incubated at 37 °C in an incubator with 5% CO2.
Reagents and antibodies
Melatonin was purchased from J&K, Chemical Ltd., and epirubicin was purchased from Pfizer, pharmaceutical Ltd. Melatonin and epirubicin were dissolved in dimethyl sulphoxide (DMSO) and made into stock solution before adding into the complete culture medium. The maximum concentration of DMSO in the culture medium did not exceed 0.1%. The primary antibodies for GAPDH, LaminB1, and P-glycoprotein were purchased from Proteintech (Proteintech, Inc., USA). The antibodies for Bcl-2, cleaved-PARP, IKKβ, p-IKKα/β, NF-κB P50, and P65 were purchased from Cell Signaling Technology (Cell Signaling Technology, Inc., USA). The antibody for cytochrome c was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell viability assay
Cell viability was determined using the CCK-8 assay. Briefly, SUDHL-10 and SUDHL-6 cell lines were seeded at 104 cells/well in 96-well plates. In this study, three different groups contained cells suspended in medium containing various concentrations of melatonin and epirubicin, and six replicated wells put up for each group. The control cells received 0.1% DMSO. Following a continuous 48 h of incubation, add 10 μL Cell Counting Kit-8 (CCK-8) per 100 μL culture medium. After incubated 4 h at 37 °C, record results using a microplate reader to measure the absorbance of the wells at 450 nm. The drug concentration required to cause 50% cell growth inhibition (IC50) was determined by interpolation from the dose-response curves.
Western blot analysis
After treatment, the cells were collected and washed with phosphate-buffered saline (PBS). Lysis buffer was used to extract protein and the protein concentration was determined using a BCA protein assay kit. Protein samples were separated by electrophoresis in a 10% or 12% sodium dodecyl sulfate-polyacrylamide mini gel (SDS-PAGE), electrophoretically transferred to polyvinylidene fluoride membranes (Millipore, USA). After membranes were blocked 2 h with skim milk, the specific primary antibodies were used to immunoblotted. The protein bands were detected by enhanced chemiluminescence.
Acridine orange/ethidium bromide (AO/EB) fluorescence staining
The cells were grown on glass coverslips with poly-l-lysine and treated with indicated doses of epirubicin with or without melatonin. After 48 h, cells were washed with PBS to remove detached cells and then fixed with 95% ethanol for 15 min. After slightly drying cells, 5 μL AO/EB (50 μg/mL) was added with gently pipetted to mix before imaging by Leica DM 14000B microscope with a digital camera.
Rhodamine-123 accumulation assay
Cell lines were incubated in 6-well plates and pretreated with 0.1% DMSO or 1 mM melatonin for 48 h. After pretreatment, add 1 μg/mL Rhodamine-123 (Rh-123) in culture medium and incubated in the dark at 37 °C in 5% CO2 for 90 min. The cells were harvested and washed twice with ice-cold PBS, then analyzed immediately using flow cytometry (EPICS XL; Beckman Coulter) equipped with 488 nm excitation and 525 nm emission wavelengths.
Intracellular accumulation of epirubicin
Briefly, cell lines were treated as Rhodamine-123 accumulation assay described above. Then cells were incubated with epirubicin at a final concentration of 1 μg/mL at 37 °C in 5% CO2 for 90 min. After incubation, cells were harvested and washed twice with ice-cold PBS, and then analyzed immediately using flow cytometry (EPICS XL; Beckman Coulter) equipped with 488 nm excitation and 575 nm emission wavelengths.
Immunofluorescence
For immunofluorescence analysis, cells were grown on glass coverslips with poly-l-lysine were washed by PBS and fixed for 20 min at room temperature (RT) with 4% paraformaldehyde. Incubating the samples for 3 min with PBS containing 0.2% Triton X-100 as needed, then blocked with 10% bovine serum albumin (BSA) in PBS for 30 min. Antibodies against Cytochrome-c or P-gp was diluted in the 1% blocking solution and then were used to incubate the sample in a humidified chamber overnight at 4 °C. Following wash the cells three times in PBS, 5 min each wash, fluorescein isothiocyanate, and rhodamine-conjugated secondary antibodies were added in 1% blocking solutions and incubated for 90 min at room temperature in dark. Finally, the stained samples were mounted with 4′6-diamidino-2-phenylindole (DAPI)-containing Vectashield solution (Vector Laboratories Inc.) and examined with a Leica DM 14000B confocal microscope.
Immunohistochemistry (IHC)
P-gp and P65 protein expression were analyzed in archived tissue of DLBCL patients from The Second Hospital of Dalian Medical University pathologically diagnosed with DLBCL. Immunohistochemical analysis of P-gp and P65 was performed on formalin-fixed, paraffin-embedded tissue sections. After deparaffinized and antigen retrieval performed, tissue sections were endogenous peroxidase blocked and incubated with a primary polyclonal antibody against P-gp (1:100) and P65 (1:100). In every case tissue, both P-gp and P65 proteins were determined.
Nuclear protein extraction
The cells were lysed in 250 μL cytoplasmic lysis buffer (10 mM Hepes, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.5% NP-40, 300 mM Sucrose) with multiple protease inhibitors (1 mM Na3VO4, 10 mM NaF, 2.5 mM β-glycerophosphate, 0.1 mM PMSF, 1 g/ml leupeptin and 0.5 mM dithiothreitol) on ice for 10 min, and then add 10% NP-40. The mixture was vortexed briefly and centrifuged at 3000 rpm for 10 min at 4 °C. The supernatant was transferred to a new tube and stored at −80 °C. The pellet was resuspended and extracted with 50 μL nuclei lysis buffer (20 mM Hepes, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, 2.5% Glycerol) with multiple protease inhibitors and kept on ice for 30 min. Nuclear proteins were extracted by centrifugation at 14,000 rpm for 30 min at 4 °C. The supernatant was then nuclei extract and protein concentration was determined by BCA assay.
Pulldown assay
The biotin-labeled double-stranded oligonucleotide probe of the ABCB1 promoter sequence was synthesized by PCR using biotin-labeled primers from Sangon Biotech company (sense,5′-TGCACT-GTTTAGGGAGGGTT-3′; antisense,5′-ACTCCG-AC CTCTCCAATTCT-3′). The nuclear proteins (400 μg) were mixed with double-strand biotinylated ABCB1 promoter probe (4 ugs), streptavidin agarose beads (50 mL) in 500 mL PBSI buffer containing 0.5 mM PMSF, 10 mM NaF, 25 mM, β-glycerophosphate and rotated overnight at 4 °C. The beads were centrifuged, washed with PBSI buffer two times, then the beads were resuspended by loading buffer, and cooked at 100 °C for 10 min. The supernatant was analyzed by western blot.
Densitometric analysis
Scion Image Software (Frederick, MD, USA) was used to determine the density of the protein bands from Western blot analysis. The data are expressed as an arbitrary unit.
Statistical analysis
Analysis of variance and Student's t-test was used to compare the values of the test and control samples. Spearman's rank correlation analyses were used to confirm the relation between the level of the P-gp protein expression and NF-κB P65 expression level in DLBCL patients. All P-values are given as two-sided, P < 0.05 was considered to be a statistically significant difference. Data are represented as mean ± standard deviation (SD). Graphpad Prism software was used for statistical analysis, and all the experiments were done three times.
Results
Melatonin potentiated the epirubicin-mediated inhibition of cell proliferation
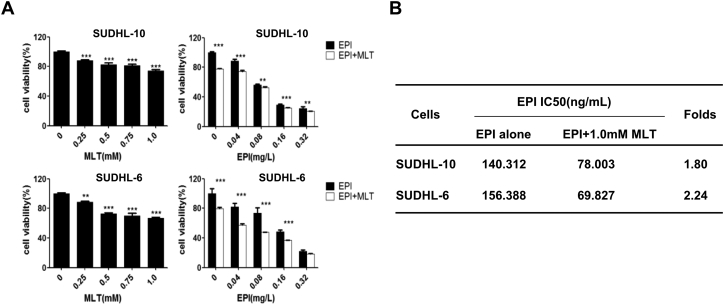
To investigate whether melatonin affects the cell proliferation inhibitor mediated by epirubicin, we examined the effects of melatonin or epirubicin alone or their combination on cell viability in SUDHL-10 and SUDHL-6 cell lines by CCK-8 assay. As shown in Fig. 1A, treatment with melatonin alone at the doses from 0 mM to 1.0 mM significantly inhibited cell viability in a dose-dependent manner in SUDHL-10 and SUDHL-6 cell lines respectively. Treatment of cells with epirubicin alone at the doses from 0 mg/L to 0.32 mg/L also suppressed DLBCL cell viability in a concentration-dependent manner (Fig. 1A). Besides, the co-treated with melatonin (1.0 mM) and various concentrations of epirubicin (0 mg/L to 0.32 mg/L) for 48 h markedly increased the epirubicin-mediated suppression of DLBCL cells viability as compared with epirubicin alone (Fig. 1A).
Fig. 1.
Effect of melatonin (MLT) and epirubicin (EPI) combination of DLBCL cells proliferation. (A) Human SUDHL-10 and SUDHL-6 cells were treated with MLT or EPI alone or their combination at the indicated doses. At 48 h after treatment, the cell viability was determined by a CCK8 assay. The cells treated with vehicle control DMSO were used as the referent group with cell viability set at 100%. (B) The IC50 values of EPI for cell viability inhibition in cells treated with or without MLT determined. The data were presented as mean ± SD of three separate experiments. The level of significant was indicated by *P < 0.05, **P < 0.01, ***P < 0.001.
Next, to evaluate the degree of melatonin and epirubicin combination-mediated the enhancement of the anti-proliferative effect, we analyzed the IC50 values of epirubicin in SUDHL-10 and SUDHL-6 cell lines treated with or without melatonin (1.0 mM). As shown in Fig. 1B, a combination of epirubicin with melatonin significantly decreased the IC50 value compared to the epirubicin action alone, IC50 folds reached 1.8 and 2.24 times in SUDHL-10 cells and SUDHL-6 cells respectively. The SUDHL-6 cells are more sensitive to melatonin than the SUDHL-10 cells. These results demonstrated that the combinational use of melatonin and epirubicin resulted in improved anti-proliferative effects in DLBCL cells compared with epirubicin treatment alone.
Melatonin increased epirubicin-induced apoptosis
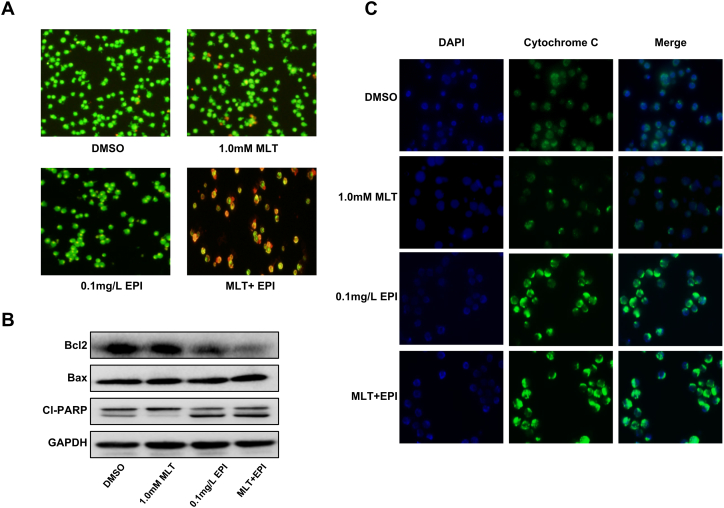
To determine whether the synergistic inhibition of cell proliferation induced by epirubicin and melatonin is associated with the enhanced activation of the apoptosis pathway, we assess the effect of the combinational use of melatonin and epirubicin on apoptosis by AO/EB staining. As shown in Fig. 2A, co-treatment of epirubicin (0.1 mg/L) with melatonin (1.0 mM) for 48 h induced apoptosis in SUDHL-6 cells. In the control group, the uniform green cells had normal morphology. In a single drug treatment group, mild yellow-green staining began to appear in the cells. However, much brighter orange-red fluorescence accumulated in the cell was observed in the group treated with combined compounds and cell morphology changes. We can also see the red-stained necrotic cells. These results indicated that more apoptosis was induced in DLBCL cells when they were exposed to melatonin and epirubicin simultaneously but not to a single compound alone.
Fig. 2.
Effect of melatonin and epirubicin combination on SUDHL-6 cells apoptosis. Human SUDHL-6 cells were treated with MLT (1.0 mM) or EPI (0.1 mg/L) alone or their combination for 48 h. (A) Acridine orange/ethidium bromide (AO/EB) fluorescence staining assay was used to evaluate the appearance and extent of apoptosis in SUDHL-6 cells. (B) The expression of Bcl-2, Bax, cleaved PARP proteins in SUDHL-6 cells were analyzed by Western blot. (C) The release of cytochrome c (cyto-c) was monitored by immunofluorescence imaging analysis from the inter-mitochondrial space into the cytosol.
Then we detected the expression of some key proteins (Bcl-2, Bax, cleaved PARP) involved in apoptosis induced by compound treatment in SUDHL-6 cells by western blot analysis. As shown in Fig. 2B, the level of Bcl-2 was obviously attenuated in the combinational treatment group than the single drug treatment group. By contrast, the expressions of Bax and cleaved PARP were improved in the combinational treatment group with epirubicin and melatonin. Besides, we also performed immunofluorescence (IF) analysis to monitor the subcellular localization of cytochrome c, which is an upstream molecule of the caspase cascade-dependent apoptotic signaling pathway. In SUDHL-6 cells, treatment with epirubicin (0.1 mg/L) or melatonin (1.0 mM) alone for 48 h triggered the release of cytochrome c from the inter-mitochondrial space into the cytosol, but the combined treatment with these two drugs markedly elevated the release of cytochrome c (Fig. 2C).
Melatonin inhibits epirubicin-induced P-glycoprotein expression
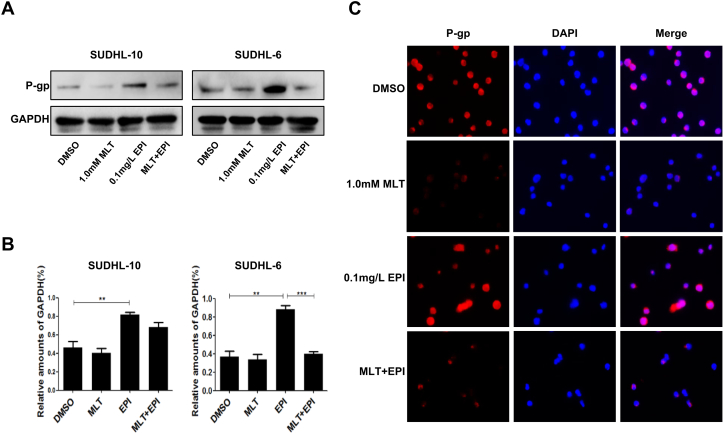
In order to identify the effect of melatonin on the expression of P-gp, we firstly analyzed the expression of P-gp in different drug treatment groups by western blot and found that epirubicin (0.1 mg/L) alone higher the expression of p-gp. However, co-treatment with melatonin (1.0 mM) and epirubicin (0.1 mg/L) almost diminished the expression of p-gp compared with epirubicin itself (Fig. 3A). Quantitative analysis of the protein bands also showed that the treatment of epirubicin alone significantly promoted the expression levels of p-gp (P < 0.05), on this base, co-treatment with melatonin inhibited the expression of p-gp and the inhibition effect is more pronounced in SUDHL-6 cells (Fig. 3B). The immunofluorescence assay reconfirmed the effect of melatonin on the expression of P-gp (Fig. 3C).
Fig. 3.
Melatonin and epirubicin combination impact the expression of P-glycoprotein (P-gp). Human SUDHL-10 and SUDHL-6 cells were treated with DMSO (0.1%), MLT (1.0 mM) or EPI (0.1 mg/L) alone or their combination for 48 h. (A) Cells were lysed, and the expression of P-gp was analyzed by Western blotting. (B) And the quantitative analysis of the proteins was performed beside. (C) The membrane location of P-gp in SUDHL-6 cells was indicated by immunofluorescence imaging analysis. The phase contrast, P-gp (red), and DAPI nuclear counterstain (blue). The data were presented as mean ± SD of three separate experiments. (**P < 0.01, ***P < 0.001).
Melatonin inhibits the activity of the P-glycoprotein pump
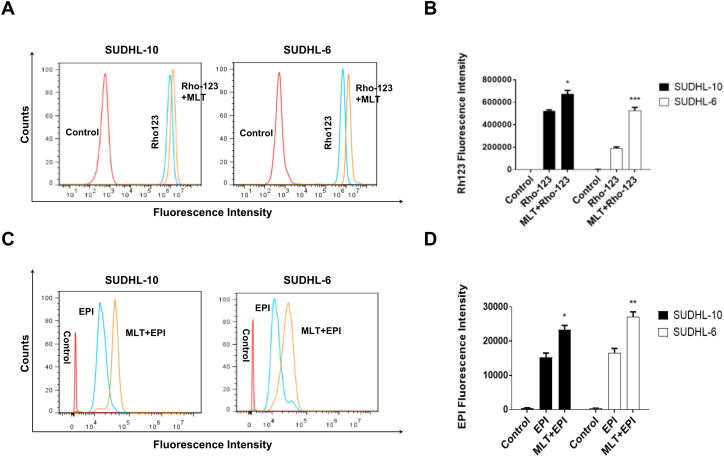
Therefore, the activity of the P-gp drug pump can be gauged by the degree of intracellular accumulation of Rho-123, which can in turn be determined by the measurement of intracellular fluorescence. As shown in Fig. 4A, the intracellular accumulation of Rho-123 was detected using flow cytometry. Compared with the melatonin untreated group, the fluorescence curve of the melatonin treatment group shifted to the right in SUDHL-10 cells and SUDHL-6 cells. As shown in Fig. 4B, quantitative analysis of the intracellular accumulation of Rho-123 showed that the fluorescence intensity of the melatonin treatment group significantly enhanced than the melatonin untreated group (P < 0.05).
Fig. 4.
Melatonin suppressed the function of P-glycoprotein in DLBCL cells. SUDHL-10 and SUDHL-6 cells were treated with MLT or RPMI 1640 (control) for 48 h. Then cells were incubated with 1 μg/mL Rhodamine-123 (Rho-123) dye or EPI for 90 min in dark. (A) The effect of MLT on intracellular Rho-123 accumulation in SUDHL-10 and SUDHL-6 cells was measured by flow cytometry. (B) Quantitatively analysis of intracellular Rho-123 fluorescence. (C) The effect of MLT on intracellular EPI accumulation in SUDHL-10 and SUDHL-6 cells was measured by flow cytometry. (D) Quantitatively analysis of intracellular EPI fluorescence. Each column shows the mean ± SD of three independent experiments. (*P < 0.05, **P < 0.01, ***P < 0.001).
The fluorescence characteristic of epirubicin was directly utilized to determine intracellular drug concentration by flow cytometry and intracellular fluorescence was indirectly representative of epirubicin concentration. The fluorescence curve of the melatonin treatment group shifted to the right and the intracellular accumulation of epirubicin was increased in the melatonin treatment group than the melatonin untreated group (Fig. 4C, D). These results suggested that the function of P-gp-dependent efflux could be inhibited by melatonin.
Epirubicin promotes the expression of P-gp by activating NF-κB pathway
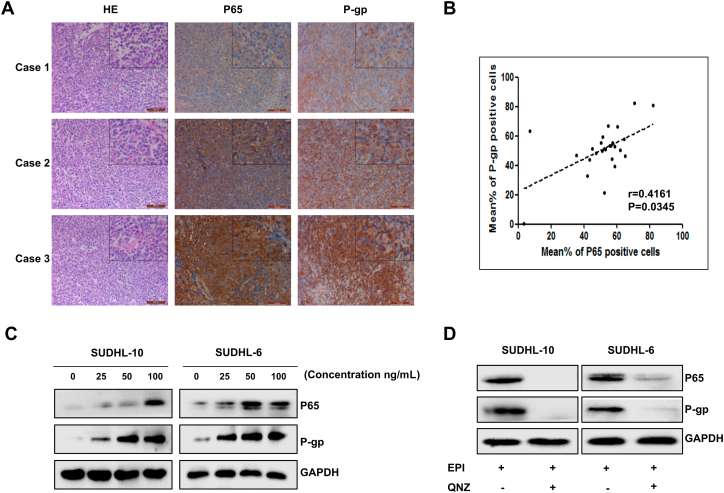
In order to explore the relationship between P-gp protein and NF-κB pathway in DLBCL, immunohistochemistry was performed on paraffin sections of DLBCL patients (n = 26) using the anti-P-gp and anti-P65. And, the HE staining showed the pathological structure of DLBCL (Fig. 5A). Subsequently, we found that the expression level of P-gp was positively correlated with NF-κB P65 expression in DLBCL patients by spearman's rank correlation analyses (Fig. 5B). To investigate whether epirubicin treatment regulating the expression of P-gp by activating the NF-κB pathway, different concentrations of epirubicin were used to treat SUDHL-10 cells and SUDHL-6 cells and then detected the expression of P65 and P-gp. As shown in Fig. 5C, both expressions of P65 and P-gp were up-regulated as drug concentrations increased. In order to further confirm the expression of P-gp is regulated by the NF-κB pathway, SUDHL-10 cells, and SUDHL-6 cells were treated with an NF-κB-selective inhibitor QNZ (500 nM) after pretreatment with a higher concentration of epirubicin (100 ng/mL). After continuous incubation of 8 h, the expression of P65 and P-gp was detected by western blot assay. As shown in Fig. 5D, the expression of P-gp was inhibited after blocking the NF-κB pathway with specific inhibitors. All of these results supported that epirubicin could induce the expression of P-gp by activating the NF-κB pathway.
Fig. 5.
Epirubicin induced the up-regulation of P-glycoprotein expression by activating the NF-κB signaling pathway. (A) HE staining and IHC analysis of P-gp and P65 in DLBCL tissue; representative pictures of each antibody staining are shown. (B) Scatterplot showing the positive correlation between P-gp and P65 expression in patients; Spearman's coefficient tests were performed to assess statistical significance. (C) P-gp and P65 protein expression were determined by Western blotting after 48 h treatment with EPI at various indicated concentrations. (D) Cells were treated with or without NF-κB-selective inhibitor QNZ (500 nM) for 8 h after pretreatment with a higher concentration of EPI (100 ng/mL) for 48 h. P-gp and P65 protein expression were determined by Western blotting.
Melatonin inhibited epirubicin-mediated activating of NF-κB signaling and the expression of P-gp
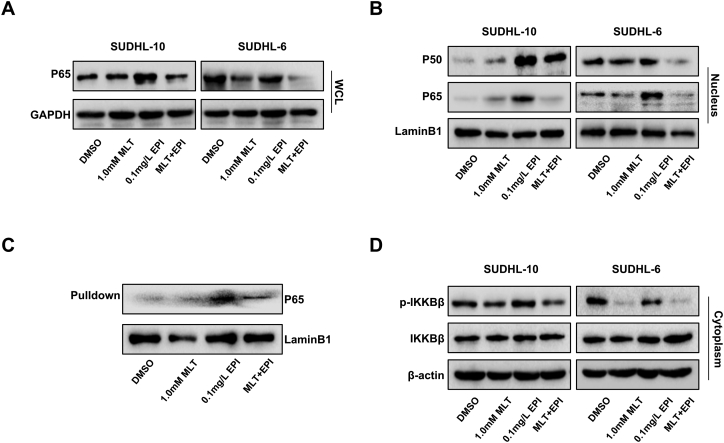
To explore whether the expression inhibition of P-gp by co-treatment with melatonin and epirubicin through inhibiting the activation of NF-κB pathway, we first determined the level of p65 in the whole cell lysate and found that the treatment with melatonin and epirubicin markedly inhibited the expression of P65 in SUDHL-10 cells and SUDHL-6 cells (Fig. 6A). Further, western blot assay was performed to examine the expression of p50/p65 in the nuclei of cells and found that the co-treatment of melatonin and epirubicin similarly reduced the expression of P50/P65 in the nuclei (Fig. 6B). We determined the effect of the co-treatment on the binding of P65 at the ABCB1 promoter region. Pulldown assay indicated that the binding of P65 to the ABCB1 promoter was reduced after co-treatment with melatonin and epirubicin compared to epirubicin treatment alone in SUDHL-6 cells (Fig. 6C). These results indicated that the inhibition of P-gp expression by co-treatment with melatonin and epirubicin might be mediated by inhibiting the expression of NF-κB P65 in the cell nuclei and further inhibiting its binding at ABCB1 promoter.
Fig. 6.
Melatonin inhibits the epirubicin-induced increase of P-glycoprotein expression by inhibiting the NF-κB signaling pathway. Human SUDHL-10 and SUDHL-6 cells were treated with DMSO (0.1%), MLT (1.0 mM) or EPI (0.1 mg/L) alone or their combination for 48 h. (A) The expression level of the P65 protein was detected by Western blot in the whole cell lysate. (B) The expression level of P50 and P65 protein were detected by Western blot in the nuclei. (C) The streptavidin-biotin pulldown assay was performed to analyze the binding of P65 protein to the ABCB1 promoter. Nuclear extracts prepared from human SUDHL-6 cells were incubated with biotin-labeled ABCB1 promoter probe and streptavidin agarose beads. The DNA-protein complexes were separated by SDS-PAGE, and the P65 protein bands were determined by Western blot. (D) The expression level of p-IKKβ and IKKβ protein were detected by Western blot in the cytoplasm.
We then investigated the influence of the combinational use of melatonin and epirubicin on the IKKβ protein and its phosphorylated forms in the cytoplasm. Fig. 6D shows that co-treatment with melatonin and epirubicin significantly suppressed the phosphorylation of IKKβ in SUDHL-10 cells and SUDHL-6 cells without affecting its overall expression. All of these results supported that the NF-κB pathway was a potential target of co-treatment with melatonin and epirubicin in DLBCL cells to finally suppress P-gp expression.
Discussion
In our studies, we found that epirubicin had a much greater impact on DLBCL cells than melatonin. In addition, melatonin sensitized epirubicin-mediated anti-tumor growth in DLBCL cells, which was represented by the improved cell viability suppression and apoptosis induction. The IC50 value of epirubicin was dramatically decreased under co-treatment with melatonin in comparison with epirubicin treatment alone. Furthermore, this joint scheme resulted in more activation of the mitochondria-mediated apoptosis pathway, which through the release of cytochrome c from the mitochondrial intermembrane space and modulation of several pro- and antiapoptotic proteins including Bcl-2, Bax, and cleaved caspase PARP, leading then to cell death. Some studies agree with our finding that the inhibitory effect of melatonin on cell growth by inducing apoptosis [43,44].
Our studies showed that the sensitivity of DLBCL cells to epirubicin may be affected by the melatonin influence on the expression or function of P-gp. With the use of the cytostatic, the number of DLBCL cells sensitive to epirubicin decreased and intracellular MDR proteins increased, including P-gp. Evidence of this, as seen in our research, the expression of P-gp was significantly increased after epirubicin alone treatment. P-gp mainly locates in the cytomembrane and nuclear envelope, which could weaken the antitumor effect of epirubicin by reducing the concentration of drugs in the cytoplasm and nuclear. However, in the case of melatonin co-treatment with epirubicin, the expression of P-gp was significant decreases on the basis of epirubicin alone promoting the increase of P-gp expression. And we clearly demonstrated that treatment with melatonin increased the intracellular levels of rhodamine 123 or epirubicin by inhibiting the function of P-gp. Previous reports have described the impact of melatonin and cytostatic on the presence of P-gp in tumor cells [42,45]. The mechanism of such an impact has not been elucidated.
Our further molecular mechanism studies indicated that epirubicin-induced the increase of P-gp protein expression may be associated with activation of the NF-κB pathway. In some publications, it has been suggested that the upregulation of the NF-κB pathway as a possible mechanism for the development of MDR in resistant cancer [41]. Immunohistochemistry results in archived tissue of DLBCL patients also showed that the expression of P-gp is a positive correlation with the expression of the NF-κB P65. The cytotoxic effects of epirubicin on tumor cells are linked primarily to directly damage the DNA strand. And NF-κB is deemed as DNA damage-responsive transcription factor [46,47]. In our vitro experiment, with an increased concentration of epirubicin, intracellular DNA damage gradually accumulates and the expression of P-gp and P65 increased. Western blot data further confirmed that the down-regulation of P-gp is associated with inhibition of the NF-κB pathway. Kim JH et al. [48] carried out in vitro studies similar to us and noted that inhibition of the NF-κB pathway may sensitize lymphoma cells to cytostatic.
In the present study, we also found that melatonin sensitizes DLBCL cells to epirubicin may through inhibiting P-gp expression via the NF-κB pathway. There are reports that melatonin has a varying physiological effect by suppressing the NF-κB pathway, including immunomodulatory and anti-inflammatory [[49], [50], [51]]. We found that melatonin could reduce the amount of NF-κB P65 protein in the nucleus and abrogating P65's binding to ABCB1 promoter to finally suppressing P-gp expression. Reducing of NF-κB P65 protein in the nucleus may rely on the suppression of total P65 expression and the phosphorylation of IKKβ, the latter inhibiting the translocation of NF-κB P65 proteins from cell cytoplasm to the nucleus.
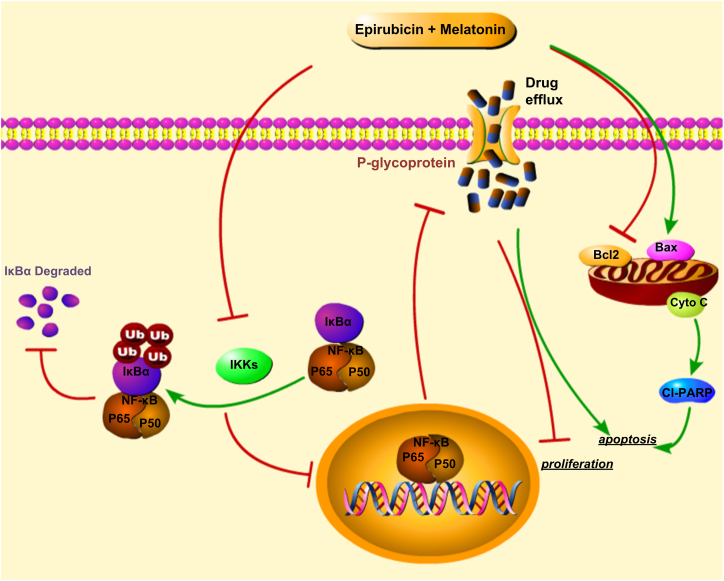
Based on the obtained results, the combinations of melatonin and epirubicin increased the sensitivity of DLBCL cells to epirubicin, including increase cell viability suppression and apoptosis induction. Furthermore, we elucidated the underlying molecular mechanisms of such enhanced action may be related to mitochondria-mediated apoptosis pathway and P-gp (Fig. 7). The IHC studies demonstrated that the expression of P-gp was positively correlated with NF-κB P65 expression. And epirubicin, a DNA-damaging cytotoxic, induces the expression of P-gp increased by activating the NF-κB pathway. The co-treatment of melatonin was found could inhibit the function of P-gp and the expression of P-gp through suppressing the NF-κB pathway. There are still many unknown effects of melatonin on tumors. But, our results might not only provide a theoretical basis for melatonin as a potential chemotherapy adjuvant, and provide a new direction for the clinic treatment of DLBCL, especially refractory/recurrent DLBCL.
Fig. 7.
The schematic diagram of the molecular mechanisms of melatonin enhancing the sensitivity of DLBCL cells to epirubicin. The co-treatment of epirubicin and melatonin increased proliferation suppression and apoptosis induction might be achieved through activating the cytochrome c/caspase-dependent apoptotic signaling and inhibiting the NF-κB signaling. The red symbol (┤) indicates negative regulation. Black colored arrow (→) indicates direct or indirect positive regulation.
Abbreviations
- DLBCL
Diffuse large B cell lymphoma
- MDR
Multidrug resistance
- P-gp
P-glycoprotein
- EPI
Epirubicin
- MLT
Melatonin
- NHL
Non-Hodgkin lymphoma
- SNC
Suprachiasmatic nucleus
- ROR/RZR
Retinoid orphan receptor/Retinoid Z receptors
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- ABC
ATP-binding cassette
- DMSO
Dimethyl sulphoxide
- CCK-8
Cell Counting Kit-8
- PBS
Phosphate-buffered saline
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide mini gel
- AO/EB
Acridine orange/ethidium bromide
- Rh-123
Rhodamine-123
- IHC
Immunohistochemistry
- IF
Immunofluorescence
Funding
This work was supported by the funds from the Liaoning Provincial Natural Science Fund Guidance Program (20170540281) and the National Natural Science Foundation of China (81772975, 81772925).
CRediT authorship contribution statement
Kaili Liu: Formal analysis, Data Curation, Writing-Original Draft; Jincheng Song: Writing - Review & Editing; Yue Yan: Investigation; Kun Zou: Data Curation; Yuxuan Che: Data Curation; Beichen Wang: Formal analysis; Zongjuan Li: Visualization; Wendan Yu: Resources; Wei Guo: Supervision; Lijuan Zou: Conceptualization, Methodology; Wuguo Deng: Term, Conceptualization, Methodology; Xiuhua Sun: Term, Conceptualization, Methodology, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Yue Yan, Email: yanyue@sysucc.org.cn.
Wendan Yu, Email: yuwendan@dmu.edu.cn.
Wei Guo, Email: wei1015@dmu.edu.cn.
Lijuan Zou, Email: lijuanzou1963@163.com.
Wuguo Deng, Email: dengwg@sysucc.org.cn.
Xiuhua Sun, Email: 3038668@vip.sina.com.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Armitage J.O. My treatment approach to patients with diffuse large B-cell lymphoma. Mayo Clin. Proc. 2012;87(2):161–171. doi: 10.1016/j.mayocp.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Paepe P., De Wolf-Peeters C. Diffuse large B-cell lymphoma: a heterogeneous group of non-Hodgkin lymphomas comprising several distinct clinicopathological entities. Leukemia. 2007;21(1):37–43. doi: 10.1038/sj.leu.2404449. [DOI] [PubMed] [Google Scholar]
- 4.Roschewski M., Staudt L.M., Wilson W.H. Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat. Rev. Clin. Oncol. 2014;11(1):12–23. doi: 10.1038/nrclinonc.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedberg J.W. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- 6.Bonfante V., Bonadonna G., Villani F., Martini A. Preliminary clinical experience with 4-epidoxorubicin in advanced human neoplasia. Recent Results Cancer Res. 1980;74:192–199. doi: 10.1007/978-3-642-81488-4_24. [DOI] [PubMed] [Google Scholar]
- 7.Prusila R.E.I., Peroja P., Jantunen E., Turpeenniemi-Hujanen T., Kuittinen O. Treatment of diffuse large B-cell lymphoma in elderly patients: replacing doxorubicin with either epirubicin or etoposide (VP-16) Hematol. Oncol. 2018 doi: 10.1002/hon.2572. [DOI] [PubMed] [Google Scholar]
- 8.Erdem A. Interaction of the anticancer drug epirubicin with DNA. Analytica Chimica Acta. 2001;(1):107–114. [Google Scholar]
- 9.Wang H., Mao Y., Zhou N., Hu T., Hsieh T.S., Liu L.F. Atp-bound topoisomerase II as a target for antitumor drugs. J. Biol. Chem. 2001;276(19):15990–15995. doi: 10.1074/jbc.M011143200. [DOI] [PubMed] [Google Scholar]
- 10.Hofman J., Malcekova B., Skarka A., Novotna E., Wsol V. Anthracycline resistance mediated by reductive metabolism in cancer cells: the role of aldo-keto reductase 1C3. Toxicol. Appl. Pharmacol. 2014;278(3):238–248. doi: 10.1016/j.taap.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 11.Siu A.W., Maldonado M., Sanchez-Hidalgo M., Tan D.X., Reiter R.J. Protective effects of melatonin in experimental free radical-related ocular diseases. J. Pineal Res. 2006;40(2):101–109. doi: 10.1111/j.1600-079X.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 12.Foulkes N.S., Whitmore D., Sassone-Corsi P. Rhythmic transcription: the molecular basis of circadian melatonin synthesis. Biol. Cell. 1997;89(8):487–494. doi: 10.1016/s0248-4900(98)80004-x. [DOI] [PubMed] [Google Scholar]
- 13.Majidinia M., Reiter R.J., Shakouri S.K., Mohebbi I., Rastegar M., Kaviani M.…Yousefi B. The multiple functions of melatonin in regenerative medicine. Ageing Res. Rev. 2018;45:33–52. doi: 10.1016/j.arr.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 2016;61(3):253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 15.Tan D.X., Xu B., Zhou X., Reiter R.J. Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules. 2018;23(2) doi: 10.3390/molecules23020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiter R.J., Tan D.X., Galano A. Melatonin: exceeding expectations. Physiology (Bethesda) 2014;29(5):325–333. doi: 10.1152/physiol.00011.2014. [DOI] [PubMed] [Google Scholar]
- 17.Dubocovich M.L., Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27(2):101–110. doi: 10.1385/endo:27:2:101. [DOI] [PubMed] [Google Scholar]
- 18.Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009;35(2):183–192. doi: 10.1002/biof.23. [DOI] [PubMed] [Google Scholar]
- 19.Dubocovich M.L., Delagrange P., Krause D.N., Sugden D., Cardinali D.P., Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010;62(3):343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lacoste B., Angeloni D., Dominguez-Lopez S., Calderoni S., Mauro A., Fraschini F.…Gobbi G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015;58(4):397–417. doi: 10.1111/jpi.12224. [DOI] [PubMed] [Google Scholar]
- 21.Jetten A.M. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl. Recept. Signal. 2009;7 doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiter R.J., Paredes S.D., Manchester L.C., Tan D.X. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol. 2009;44(4):175–200. doi: 10.1080/10409230903044914. [DOI] [PubMed] [Google Scholar]
- 23.Chuffa L.G.A., Reiter R.J., Lupi L.A. Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis. 2017;38(10):945–952. doi: 10.1093/carcin/bgx054. [DOI] [PubMed] [Google Scholar]
- 24.Pariente R., Bejarano I., Rodriguez A.B., Pariente J.A., Espino J. 440(1–2) 2018. Melatonin Increases the Effect of 5-Fluorouracil-based Chemotherapy in Human Colorectal Adenocarcinoma Cells in Vitro; pp. 43–51. [DOI] [PubMed] [Google Scholar]
- 25.Posadzki P.P., Bajpai R., Kyaw B.M., Roberts N.J., Brzezinski A., Christopoulos G.I.…Car J. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med. 2018;16(1):18. doi: 10.1186/s12916-017-1000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhelev Z., Ivanova D., Bakalova R., Aoki I., Higashi T. Synergistic cytotoxicity of melatonin and new-generation anticancer drugs against leukemia lymphocytes but not normal lymphocytes. Anticancer Res. 2017;37(1):149–159. doi: 10.21873/anticanres.11300. [DOI] [PubMed] [Google Scholar]
- 27.Goodman L.S., Wintrobe M.M., Dameshek W., Goodman M.J., Gilman A., McLennan M.T. Landmark article Sept. 21, 1946: nitrogen mustard therapy. Use of methyl-bis(beta-chloroethyl)amine hydrochloride and tris(beta-chloroethyl)amine hydrochloride for Hodgkin's disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. By Louis S. Goodman, Maxwell M. Wintrobe, William Dameshek, Morton J. Goodman, Alfred Gilman and Margaret T. McLennan. Jama. 1984;251(17):2255–2261. doi: 10.1001/jama.251.17.2255. [DOI] [PubMed] [Google Scholar]
- 28.Gottesman M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 29.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 30.Dean M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005;400:409–429. doi: 10.1016/s0076-6879(05)00024-8. [DOI] [PubMed] [Google Scholar]
- 31.Gottesman M.M., Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580(4):998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 32.Abolhoda A., Wilson A.E., Ross H., Danenberg P.V., Burt M., Scotto K.W. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin. Cancer Res. 1999;5(11):3352–3356. [PubMed] [Google Scholar]
- 33.Nakamura M., Abe Y., Katoh Y., Oshika Y., Hatanaka H., Tsuchida T.…Ueyama Y. A case of pulmonary adenocarcinoma with overexpression of multidrug resistance-associated protein and p53 aberration. Anticancer Res. 2000;20(3b):1921–1925. [PubMed] [Google Scholar]
- 34.Wijnholds J., deLange E.C., Scheffer G.L., van den Berg D.J., Mol C.A., van der Valk M.…Borst P. Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J. Clin. Invest. 2000;105(3):279–285. doi: 10.1172/jci8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amiri-Kordestani L., Basseville A., Kurdziel K., Fojo A.T., Bates S.E. Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Update. 2012;15(1–2):50–61. doi: 10.1016/j.drup.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaffer B.C., Gillet J.P., Patel C., Baer M.R., Bates S.E., Gottesman M.M. Drug resistance: still a daunting challenge to the successful treatment of AML. Drug Resist. Updat. 2012;15(1–2):62–69. doi: 10.1016/j.drup.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu M., Ocana A., Tannock I.F. Reversal of ATP-binding cassette drug transporter activity to modulate chemoresistance: why has it failed to provide clinical benefit? Cancer Metastasis Rev. 2013;32(1–2):211–227. doi: 10.1007/s10555-012-9402-8. [DOI] [PubMed] [Google Scholar]
- 38.Nagel D., Vincendeau M., Eitelhuber A.C., Krappmann D. Mechanisms and consequences of constitutive NF-kappaB activation in B-cell lymphoid malignancies. Oncogene. 2014;33(50):5655–5665. doi: 10.1038/onc.2013.565. [DOI] [PubMed] [Google Scholar]
- 39.Turturro F. Constitutive NF-kappa B activation underlines major mechanism of drug resistance in relapsed refractory diffuse large B cell lymphoma. Biomed. Res. Int. 2015;2015:484537. doi: 10.1155/2015/484537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H.G., Hien T.T., Han E.H., Hwang Y.P., Choi J.H., Kang K.W.…Jeong H.G. Metformin inhibits P-glycoprotein expression via the NF-kappaB pathway and CRE transcriptional activity through AMPK activation. Br. J. Pharmacol. 2011;162(5):1096–1108. doi: 10.1111/j.1476-5381.2010.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X., Ding Y., Xiao M., Liu X., Ruan J., Xue P. Anti-tumor compound RY10-4 suppresses multidrug resistance in MCF-7/ADR cells by inhibiting PI3K/Akt/NF-kappaB signaling. Chem. Biol. Interact. 2017;278:22–31. doi: 10.1016/j.cbi.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Fic M., Gomulkiewicz A., Grzegrzolka J., Podhorska-Okolow M., Zabel M., Dziegiel P., Jablonska K. The impact of melatonin on colon cancer cells' resistance to doxorubicin in an in vitro study. Int. J. Mol. Sci. 2017;18(7) doi: 10.3390/ijms18071396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bizzarri M., Proietti S., Cucina A., Reiter R.J. Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin. Ther. Targets. 2013;17(12):1483–1496. doi: 10.1517/14728222.2013.834890. [DOI] [PubMed] [Google Scholar]
- 44.Proietti S., Cucina A., Minini M., Bizzarri M. 74(21) 2017. Melatonin, Mitochondria, and The Cancer Cell; pp. 4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granzotto M., Rapozzi V., Decorti G., Giraldi T. Effects of melatonin on doxorubicin cytotoxicity in sensitive and pleiotropically resistant tumor cells. J. Pineal Res. 2001;31(3):206–213. doi: 10.1034/j.1600-079x.2001.310303.x. [DOI] [PubMed] [Google Scholar]
- 46.Dunphy G., Flannery S.M., Almine J.F., Connolly D.J., Paulus C., Jonsson K.L.…Unterholzner L. Non-canonical activation of the DNA sensing adaptor STING by ATM and IFI16 mediates NF-kappaB signaling after nuclear DNA damage. Mol Cell. 2018;71(5):745–760. doi: 10.1016/j.molcel.2018.07.034. e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piret B., Schoonbroodt S., Piette J. The ATM protein is required for sustained activation of NF-kappaB following DNA damage. Oncogene. 1999;18(13):2261–2271. doi: 10.1038/sj.onc.1202541. [DOI] [PubMed] [Google Scholar]
- 48.Kim J.H., Kim W.S., Hong J.Y., Ryu K.J., Kim S.J., Park C. Epstein-Barr virus EBNA2 directs doxorubicin resistance of B cell lymphoma through CCL3 and CCL4-mediated activation of NF-kappaB and Btk. Oncotarget. 2017;8(3):5361–5370. doi: 10.18632/oncotarget.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bora N.S., Mazumder B., Mandal S. 41(1) 2019. Protective Effect of a Topical Sunscreen Formulation Fortified With Melatonin Against UV-induced Photodermatitis: An Immunomodulatory Effect via NF-kappaB Suppression; pp. 130–139. [DOI] [PubMed] [Google Scholar]
- 50.Chinchalongporn V., Shukla M., Govitrapong P. 64(4) 2018. Melatonin Ameliorates Abeta42-induced Alteration of betaAPP-processing Secretases via the Melatonin Receptor Through the Pin1/GSK3beta/NF-kappaB Pathway in SH-SY5Y Cells. [DOI] [PubMed] [Google Scholar]
- 51.Liu Z., Gan L., Xu Y., Luo D., Ren Q., Wu S., Sun C. 63(1) 2017. Melatonin Alleviates Inflammasome-induced Pyroptosis Through Inhibiting NF-kappaB/GSDMD Signal in Mice Adipose Tissue. [DOI] [PubMed] [Google Scholar]