Abstract
Intravitreal injections of anti-vascular endothelial growth factor drugs have become the gold standard treatment for diabetic retinopathy (DR). However, several patients are classified as non-responders or poor responders to treatment. Therefore, it is essential to study alternative target molecules. We have previously shown that the progression of DR in the Ins2Akita mouse reflects the imbalance between pro- and anti-angiogenic molecules found in the human retina. We report, for the first time, the therapeutic potential of a dual-acting antiangiogenic non-viral gene therapy. We have used an expressing vector encoding both the pigment epithelium-derived factor gene and a short hairpin RNA (shRNA) targeted to the placental growth factor to restore the balance between these factors in the retina. Twenty-one days after a single subretinal injection, we observed a marked decrease in the inflammatory response in the neural retina and in the retinal pigment epithelium, together with reduced vascular retinal permeability in the treated diabetic mouse. These results were accompanied by the restoration of the retinal capillary network and regression of neovascularization, with significant improvement of DR hallmarks. Concomitant with the favorable therapeutic effects, this approach did not affect retinal ganglion cells. Hence our results provide evidence toward the use of this approach in DR treatment.
Keywords: diabetic retinopathy, subretinal delivery, retina, retinal pigment epithelium, vascular plexus, neovascularization, gene therapy, placental growth factor, pigment epithelium-derived factor
Graphical Abstract
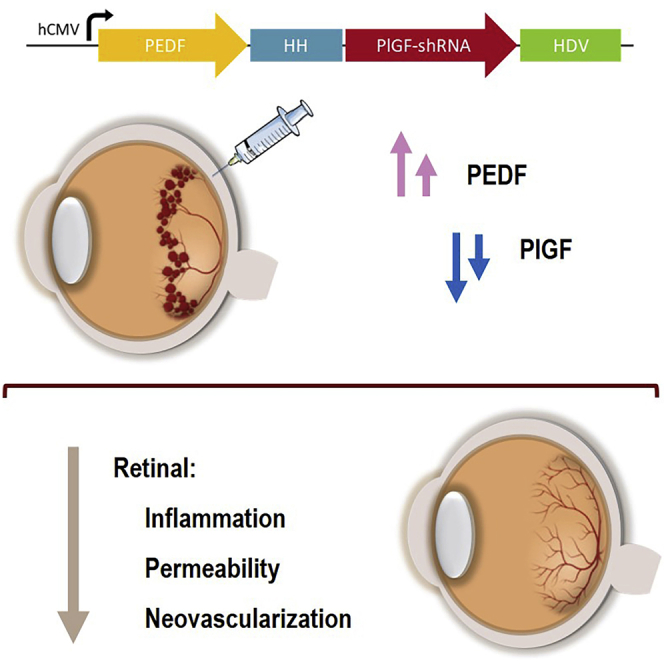
This study presents a promising therapeutic strategy for diabetic retinopathy. Araújo et al. developed a non-viral approach with dual anti-angiogenic action that efficiently reduces retinal inflammation and vascular permeability in the diabetic retina, accompanied by the restoration of the vascular network.
Introduction
It is estimated that 422 million people around the world have been diagnosed with diabetes.1 Approximately 35% of those with diabetes have diabetic retinopathy (DR).1 The prevalence of DR increases with duration of diabetes, and almost all type 1 diabetic patients and 60% of those with type 2 diabetes have some form of retinopathy 20 years after the onset of diabetes.2
DR is characterized by progressive damage in the retinal microvasculature caused by the chronic exposure to hyperglycemia. The earliest clinical signs of DR include thickening of the basement membrane, loss of pericytes, breakdown of the blood-retinal barrier (BRB), and appearance of microaneurysms.3, 4, 5 As the disease progresses, patients with pre-proliferative retinopathy display increased intra-retinal hemorrhages accompanied by cotton-wool spots, indicative of failure in microvascular circulation, resulting in ischemia.4 Later stages of DR, known as proliferative DR (PDR), are associated with pathological neovascularization, i.e., the formation of new blood vessels that develop from the retinal circulation. These new blood vessels can erupt through the surface of the retina, extend into the vitreous cavity, and hemorrhage, resulting in blindness.6, 7, 8 Besides PDR, a common cause of vision loss in patients with DR is diabetic macular edema (DME).9 DME can occur at any stage of DR and cause distortion of visual images, as well as a decrease in visual acuity. DME is characterized by swelling or thickening of the macula caused by the accumulation of intracellular and extracellular fluid within the macular retina, caused by the breakdown of the BRB.10
Current treatment options for patients with DR are limited. Laser photocoagulation effectively slows the loss of vision in patients with PDR, but it does not represent a cure and is in itself a destructive therapy.11,12 Pharmacological inhibition of vascular endothelial growth factor A (VEGF-A) has shown promising results regarding the prevention of neovascularization.13 However, the therapeutic outcome is not always satisfactory, with about 30%–50% of DR patients classified as non-responders or poor responders.14, 15, 16, 17 Moreover, persistent inhibition of VEGF-A may be associated with the risk for formation of fibrovascular membranes and retinal detachment.18, 19, 20, 21 Furthermore, apoptosis of retinal ganglion cells (RGCs) was reported in a patient with DME undergoing repeated administration of anti-VEGF treatment for 2 years.22 Additionally, substantial reduction of amacrine cell and RGC densities, upon sustained intraocular VEGF-A neutralization, has been reported in the Ins2Akita diabetic mouse model23 and in streptozotocin (STZ)-induced diabetic rats.24
Angiogenesis and vascularization are promoted by angiogenic factors, namely, VEGF family members, which are composed of six secreted proteins: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor (PlGF).25 Although not essential for physiological angiogenesis, PlGF seems to be an important player in pathological angiogenesis.26 Using a mouse model of oxygen-induced retinopathy (OIR), PlGF-deficient mice demonstrated decreased neovascularization and arterial tortuosity, whereas embryonic angiogenesis was not affected.27 Additionally, the ablation of PlGF in a mouse model of diabetes decreased diabetes-related retinal capillary degeneration and pericyte death.28 In accordance with findings from animal models, studies in DR patients have shown increased PlGF levels in the retina, vitreous, and aqueous humors.29, 30, 31, 32, 33, 34 In fact, the unbalanced expression levels of pro-angiogenic factors, such as PlGF and VEGF-A, and angiogenesis inhibitors, such as the pigment epithelium-derived factor (PEDF), are being pointed out as the leading cause of neovascularization in DR patients.35,36 PEDF, which is mainly produced by the retinal pigment epithelium (RPE) in the retina, is a potent antiangiogenic molecule that counteracts angiogenesis inducers.37 Additionally, PEDF has neuroprotective, anti-inflammatory, and anti-oxidative properties.37,38 The retinal transduction of PEDF by an adeno-associated virus (AAV) decreased the expression of angiogenesis-related fibrosis factors, as well as the intraocular levels of VEGF-A, in transgenic mice overexpressing insulin-like growth factor-1 (IGF-1) in the retina.39 In fact, management of DR through gene therapy has been studied in recent years in animal models, providing confidence that it is an efficient treatment modality. The therapeutic effects of viral- and non-viral-mediated expression of neuroprotective, anti-apoptotic, and antiangiogenic molecules in the retina have shown promising results.40 Previous studies from our group have reported the effectiveness of a self-replicating episomal vector, pEPito, in the long-term gene expression in the mouse retina.41,42 Based on that, in this study, we further addressed the therapeutic potential of a dual-acting antiangiogenic gene therapy, in the retina of a mouse model of type 1 diabetes, Ins2Akita mice, using the pEPito expressing system (Figure 1). This expressing vector encoded both PEDF transgene and a short hairpin RNA (shRNA) targeting the PlGF gene (pPEDF-shPlGF) with the aim of restoring the balance between both factors, which is deeply impaired in diabetic Ins2Akita mouse retina with disease progression, as we previously reported.43 The rationale behind this combinatory treatment was based on the well-established role of PlGF in DR pathology, on the strong antiangiogenic action of PEDF in the retina, and on the inverse correlation observed in their expression levels in DR conditions. For the first time, the rescue of the equilibrium between an angiogenesis inducer and an angiogenesis inhibitor, using a non-viral vector, was studied as a potential therapeutic strategy for DR.
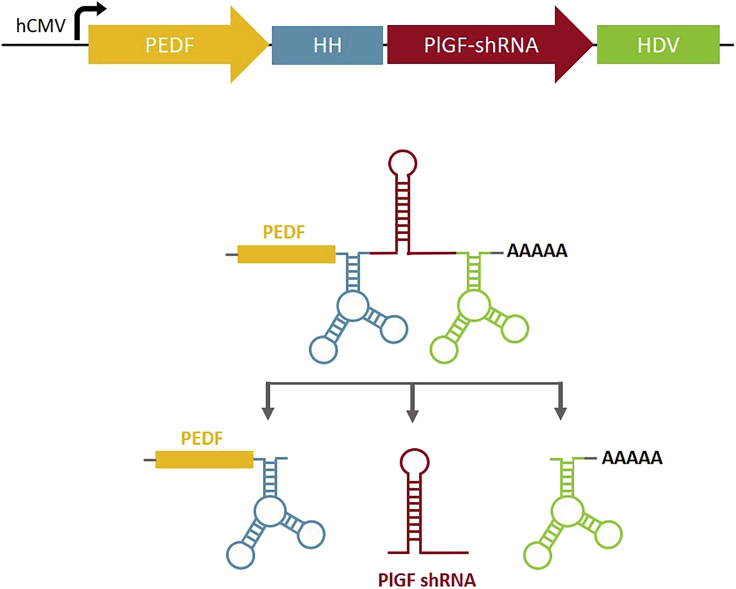
Figure 1.
Representation of the Construct for the Dual-Acting Antiangiogenic Approach
An expression cassette to drive the balance between angiogenesis mediators was based on a ribozyme architecture, allowing the encoding of the PEDF gene at the same time as the production of PlGF shRNA. cis-Acting ribozymes (5′ end hammerhead [HH] and 3′ end hepatitis delta virus [HDV]) were employed to flank nucleotides 5′ and 3′ of antisense and sense sequences comprising dsRNAs. Upon expression of the hCMV-driven construct, the cis-acting ribozymes self-cleave, releasing the PlGF shRNA.
Results
The Administration of the Anti-angiogenic Dual-Acting Therapy Did Not Induce Adverse Reactions
The animals injected subretinally with the therapy were followed for 21 days post-administration. No changes in grooming, behavior, or food or water consumption were observed. Additionally, visual inspection of the eyes of animals within the experimental groups revealed no signs of ocular inflammation or changes in overall health status.
The Balance between Angiogenesis Inducers and Inhibitors Is Restored in RPE and Neural Retina with the Administration of pPEDF-shPlGF
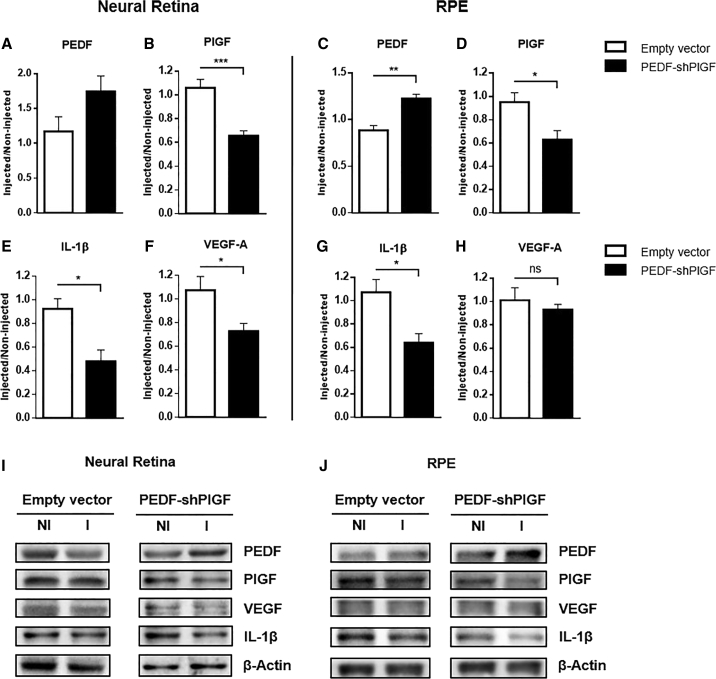
We have previously shown that similarly to DR patients, the progression of the disease in the Ins2Akita mouse model reflects the imbalance in the expression of pro- and anti-angiogenic factors in the neural retina and RPE.43 Therefore, to explore whether our dual-acting anti-angiogenic strategy would be effective in restoring the equilibrium between PEDF and PlGF in vivo, we have analyzed the protein levels of angiogenic factors in animals injected with the control (empty vector) or with pPEDF-shPlGF, 21 days following subretinal injection. We have observed a marked increase in PEDF expression, by approximately 50%, in the neural retina following administration of the dual-acting anti-angiogenic strategy (Figure 2A) and a significant reduction in PlGF expression by approximately 40% (Figure 2B) when compared with the control group. Similarly, in the RPE, we have detected a significant increase in PEDF levels (mean increase of 39%; Figure 2C) and a significant decrease in PlGF (mean decrease of 34%; Figure 2D) upon comparison with the control group.
Figure 2.
Expression of Diabetic Retinopathy-Associated Molecules in the Neural Retina and the RPE of Ins2Akita Mice 21 Days after Subretinal Injection of the Empty Vector or pPEDF-shPlGF
(A–H) Quantification of protein levels of the (A and C) angiogenesis inhibitor PEDF, of the pro-angiogenic factors (B and D) PlGF and (F and H) VEGF-A, and the pro-inflammatory cytokine (E and G) IL-1β, in the neural retina and in the RPE layer. (I and J) Representative western blot data for the studied key molecules in the neural retina and RPE. Protein levels were normalized to β-Actin, followed by a ratio between the expression of the correspondent protein in the injected eye (I) over the non-injected (NI) one. Data are expressed as mean ± SEM (n = 4 mice/group). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Student’s t test.
We have also analyzed the protein levels of VEGF-A, a major angiogenesis inducer overexpressed in DR conditions and deeply involved in the development of vascular permeability and pathological neovascularization.44 The results show a significant decrease in VEGF-A in the neural retina when compared with the control group (Figure 2E), but no significant changes were detected in the RPE layer (Figure 2G).
Several inflammatory cytokines have been shown to be critical to the development of DR. Interleukin-1β (IL-1β), among others, has been found in high concentrations in the vitreous of DR patients.45,46 Therefore, we have assessed the protein levels of this pro-inflammatory cytokine in the neural retina and the RPE of diabetic mice injected with pPEDF-shPlGF or control. As shown in Figure 2F, there is a significant decrease of almost 50% in IL-1β in the neural retina and approximately 40% in the RPE layer of the pPEDF-shPlGF-injected animals, when compared with the control group.
pPEDF-shPlGF Injection Reduces Microglia Activity
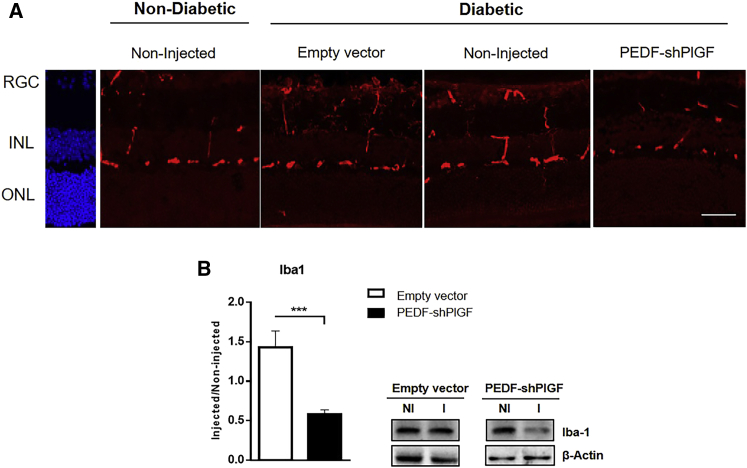
The increase in the expression of cytokines observed in DR potentiates microglia activation, which in turn results in its proliferation and migration, causing tissue damage.47 To analyze microglial activity, we sacrificed animals 21 days post-injection and sectioned their retinas for immunohistochemical analysis. As evidenced in Figure 3A, the analysis of the immunolabeling of the microglia/macrophage marker, ionized calcium-binding adaptor molecule 1 (Iba1), in retinal sections shows an increase in the density of microglia in diabetic mice when compared with non-diabetic animals (wild-type [WT] animals). Following the injection of pPEDF-shPlGF in diabetic animals, we have observed a marked decrease in the microglia labeling, similar to non-diabetic mice, when compared with control groups. Furthermore, we also verified a decrease in the number of vertically oriented processes that represents the microglia infiltration on multiple retinal layers, compared with non-injected and control-injected groups (Figure 3A). These results suggest that activation of the microglia observed in untreated diabetic animals is reduced in treated animals.
Figure 3.
Microglia Activity in the Retina of Non-diabetic and Diabetic Mice 21 Days after Subretinal Injection of the Empty Vector or pPEDF-shPlGF
(A) Iba1 (microglia/macrophage marker) immunolabeling (red) of Ins2Akita retinal sections. DAPI (blue) stains nuclei. Scale bar: 50 μm. (B) Quantification of Iba1 protein expression levels in the retina and representative western blot data for Iba1. Protein levels were normalized to β-actin, followed by a ratio between the expression of the correspondent protein in the injected (I) eye over the NI one. Data are expressed as mean ± SEM (n = 4 mice/group). ∗∗∗p < 0.001, Student’s t test. Scale bars: 50 μm. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
In agreement with the immunohistochemistry results, we have found a significant decrease in Iba1 protein levels (mean decrease of 58%) in pPEDF-shPlGF-injected retinas, compared with the control group, as assessed by western blot (Figure 3B).
Vascular Leakage Is Decreased upon pPEDF-shPlGF Administration
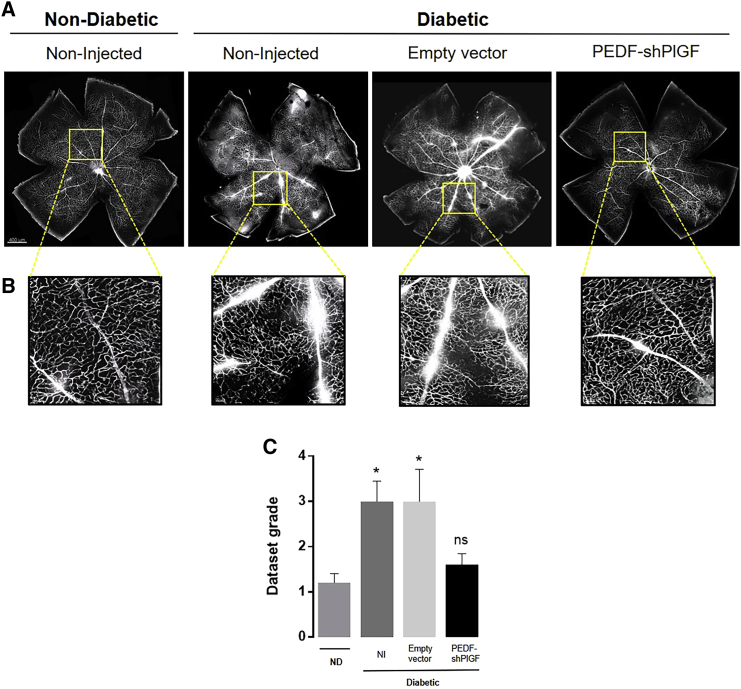
The breakdown of the BRB is an early sign of DR, which further results in vascular leakage.4 Mouse perfusion with the Evans blue dye is an efficient method to observe retinal blood vascular leakage,48 and it was used to assess the impact of pPEDF-shPlGF therapy on this DR hallmark. In non-diabetic retinas, almost all Evans blue dye remained within the intact blood vessels, which contrasts with the non-injected or empty vector-injected diabetic retinas of Ins2Akita mice, where a pronounced leakage of the dye was observed for both capillaries and larger-caliber vessels (Figures 4A and 4B). In contrast, the vascular permeability was remarkedly decreased in pPEDF-shPlGF-treated retinas, with a visible reduction of dye extravasation from the vessels, compared with control groups, as observed in the whole-mount images (Figure 4A).
Figure 4.
Retinal Vascular Permeability in the Retina of Non-diabetic and Diabetic Mice 21 Days after Subretinal Injection of the Empty Vector or pPEDF-shPlGF
(A) Representative images of Evans blue retinal fluorescence in vessels of whole-mount retinas of non-diabetic and diabetic mice. Each retinal flat mount was stitched from 64 images acquired with 20× objective. (B) Magnified images of areas of vascular leakage at specific points of the vessels. (C) Quantification of the vascular permeability (the dataset was graded in a scale of 1 to 5, depending on the severity of the Evans blue leakage, by a masked investigator). n = 5 mice/group. Scale bars indicate 400 μm (A) or 50 μm (B).
pPEDF-shPlGF Restores the Vascular Network
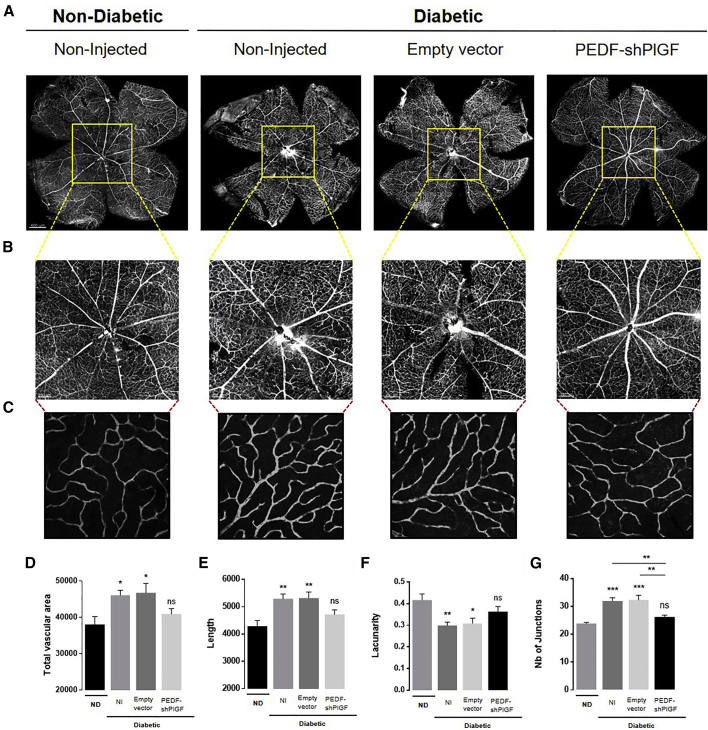
To analyze the vascular organization, we labeled whole-mount retinas of WT, treated, and non-treated diabetic mice with Isolectin B4 (Figure 5). The vasculature from non-diabetic (WT) animals shows normal vessel structure, as well as a uniformly distributed capillary network (Figure 5A). As predicted, the diabetic non-injected and the empty vector-injected retinas revealed abnormal and disorganized vasculature, with points of vessel dilation and vessel attenuation (Figures 5A and 5B). By contrast, the pPEDF-shPlGF-injected diabetic retinas displayed a solid capillary network, with retinal vessels radiating from well-defined optic nerve heads (Figure 5B). To obtain a measure of the retinal vascularity in all tested conditions, we have quantified eight images of the deep vascular plexus (Figure 5C), captured in the four quadrants, of each of the experimental conditions, which were processed using the AngioTool software. Retinas from non-injected and empty vector-injected diabetic mice exhibited a significant increase in deep vascular density, as demonstrated by the increase in total vascular area, vessel length, and number of junctions, as well as a decrease in lacunarity, when compared with non-diabetic mice (Figures 5D–5G). In striking contrast, the vascular status of pPEDF-shPlGF-injected retinas was similar to the non-diabetic group, with similar values in total vascular area, vessel length, number of junctions, and lacunarity. Moreover, the number of junctions was significantly decreased in pPEDF-shPlGF-injected retinas compared with both diabetic control groups (Figure 5G).
Figure 5.
Isolectin-GS IB4 Staining of Retinal Flat Mounts of Non-diabetic and Diabetic Mice 21 Days after Subretinal Injection of the Empty Vector or pPEDF-shPlGF
(A) Representative images of whole-mount retinas of non-diabetic or diabetic retina. Each retinal flat mount was stitched from 64 images acquired with the 20× objective using a confocal microscope. (B) Magnified image of the central area of the optic disk. (C) Representative confocal images of the retina deep plexus (original magnification 40×). (D–G) Images of the indexes of vascularity: (D) vascular area, (E) vessel length, (F) lacunarity, and (G) number of junctions. Data are expressed as mean ± SEM (n = 5 mice/group) analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, compared with control. Scale bars indicate 400 μm (A), 150 μm (B), and 50 μm (C). ND, non-diabetic; ns, not statistically significant.
pPEDF-shPlGF Does Not Affect RGCs’ Density
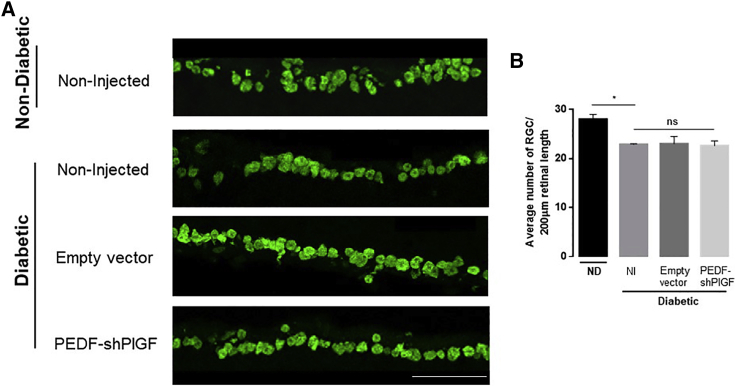
Neuronal loss, in particular of RGCs, is a consequence of the diabetic condition, which is mainly caused by neuroinflammation and microglia activation.47 In order to analyze the RGCs’ density in mice retinas after treatment, 21 days post-administration we have labeled retinal sections with anti-gamma synuclein, as previously described.49 RGCs counts were significantly higher in non-diabetic mice (28 ± 1/200 μm retinal length) when compared with diabetic retinas (22.8 ± 0.11/200 μm retinal length) (Figure 6). Our results show that RGCs counts of pPEDF-shPlGF-injected retinas (22.6 ± 1/200 μm retinal length) were not significantly different from non-injected (22.9 ± 0.12 /200 μm retinal length) and control-injected retinas (23 ± 1.5/200 μm retinal length) (Figure 6).
Figure 6.
Retinal Ganglion Cells’ Density in Retinal Sections of Non-diabetic or Diabetic Mice, 21 Days after Subretinal Injection of Empty Vector or pPEDF-shPlGF
(A) Representative images of the RGC marker gamma-synuclein in control and treatment retinas and (B) quantification of RGC counts over a 200-μm retinal length. Data are expressed as mean ± SEM (n = 4 mice/group) analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test. ∗p < 0.05. Scale bar indicates 50 μm.
Discussion
With the growing burden of diabetes over the last few decades, the incidence of DR has been steadily increasing. Furthermore, as the average life expectancy increases and with the duration of diabetes being the strongest predictor for development and progression of retinopathy, it is crucial to find effective therapies to treat chronic ocular pathologies for longer periods of time.
Currently, the gold standard in clinical practice for DR is the intravitreal injection of neutralizing antibodies against VEGF-A, which decreases vascular lesion and improves visual acuity after repeated injections.50, 51, 52 Although VEGF-A is an essential contributor to the pathogenesis of DR, it is also essential for neuronal function and survival,53 with reported neuronal loss of bipolar and amacrine cells upon sustained depletion of VEGF-A, in a diabetic rat model.24 Therefore, it is essential to find therapeutic alternatives able to restore the vascular network and, at the same time, preserve the neuronal cells. In this regard, gene therapy offers the possibility of a long-term, sustained, and fine-tuned therapeutic.
We have recently described that the expression of pro- and anti-angiogenic factors is dysregulated in the retina of the Ins2Akita mouse model.43 Specifically, we have observed increased levels of PlGF and decreased levels of PEDF, concomitant with disease progression, contributing to features of both early and late stages of DR.43 Although the Ins2Akita mouse does not present the severe phenotype seen in advanced stages of DR in human patients, in the present study we have confirmed that there is, in fact, the development of vascular leakage and neovascularization in the deep plexus at 11 months of age. These results are in accordance with studies performed in 9- and 10.5-month-old animals.54,55 Therefore, in this work, we aimed to restore the retinal network physiopathology by restoring the balance between players of angiogenesis, through the upregulation of PEDF and silencing of PlGF. The potent anti-angiogenic action of PEDF in combination with its anti-inflammatory and neuroprotective effects has been shown to have protective effects against retinal cell death.56 In contrast with PEDF, PlGF is considered not only an inducer of angiogenesis but also a modulator of inflammation associated with pathological angiogenesis. PlGF promotes inflammation by recruiting and activating macrophages via VEGFR-1 signaling, inducing the secretion of pro-inflammatory cytokines.57,58 Therefore, PlGF has a preponderant role in the context of inflammation, compared with VEGF-A. In fact, a study conducted by Van Bergen et al.59 demonstrated that PlGF neutralization showed comparable efficacy as anti-VEGF therapy regarding vascular permeability; however, whereas PlGF inhibition reduced inflammation, anti-VEGF therapy did not have an effect on the inflammatory response. Therefore, PlGF inhibition combined with PEDF overexpression could potentially offer additional benefit in the inflammatory context compared with anti-VEGF treatment, which could be of relevance especially in patients with DME. In fact, in all studied models of pathological angiogenesis, the deletion of PlGF impaired the associated inflammation and angiogenesis.27 Moreover, a preclinical study revealed that the injection of an antibody against PlGF in three different mouse models, STZ-diabetic, Akimba, and the non-diabetic laser-induced choroidal neovascularization model, reduced inflammation and vascular leakage.59 The transition from animal to human studies was already done, between 2017 and 2019, in the form of two phase II clinical trials to assess the therapeutic potential of an anti-PlGF recombinant monoclonal antibody in DR/DME patients, which demonstrated safety and efficacy (ClinicalTrials.gov: NCT03071068 and NCT03499223; ThromboGenics). We have recently described the enhanced antiangiogenic effect of PEDF overexpression in combination with PlGF silencing compared with single approaches, in RPE cells, in vitro.60
Herein, we have observed that a single subretinal injection of pPEDF-shPlGF significantly increased the protein levels of PEDF and decreased the expression of PlGF in both the neural retina and RPE. Additionally, we have observed a significant decrease in the levels of the pro-inflammatory and pro-angiogenic molecules VEGF-A and IL-1β. Previous reports correlate increased levels of VEGF-A and IL-1β with a pathological increase of vascular permeability.61,62 Our results are supported by these findings, because we have observed, upon treatment of diabetic mice with pPEDF-shPlGF, decreased levels of these cytokines along with the decrease in retinal vascular permeability, as shown by the decrease of Evans blue vessels extravasation. Although we did not study directly the impact of our strategy in DME pathology, the decrease in the vascular permeability, observed after treatment, suggests that this approach could benefit patients with this devastating condition. In fact, increased levels of PlGF33 and decreased levels of PEDF29 have been reported in patients with DME. Moreover, it was described that the injection of PlGF into rat eye vitreous resulted in the impairment of RPE tight junctions and subsequent subretinal fluid accumulation, suggesting that PlGF could be a key target for the control of DME.63
We have also detected increased microglia activity in 11-month-old animals, correlated with previous observations in 6-month-old Ins2Akita mice,64 thus suggesting that the pro-inflammatory state persists with the chronic exposure to hyperglycemia, contributing to vascular permeability and neuronal apoptosis. Nevertheless, we have observed reduced Iba1 levels by immunofluorescence and immunoblotting, upon pPEDF-shPlGF injection, which indicates reduced microglial activity. Additionally, it is visible in diabetic control retinas that some microglia cells migrated or extended their processes to the photoreceptor layer (outer nuclear layer), which is not observed in treated retinas. This observation is consistent with the hypothesis that photoreceptors are also negatively impacted in DR.
Unlike previous studies reporting the absence of neovascularization in the Ins2Akita mouse, following 6 months of hyperglycemia,65 we have observed an increase in total vessel length and area in the deep vascular plexus of diabetic animals, as well as an increase in number of junctions and a decrease in lacunarity when compared with non-diabetic mice. These results are similar to those described by Carroll et al.54 in a study with 10.5-month-old Ins2Akita mice. The contradictory results could be because of the discrepancy in the age of the animals analyzed, because most reports do not go beyond 6 months of hyperglycemia, the time at which the first signs of vascular damage are visible.64,66 In fact, the described capillary loss and deep retinal ischemia in Ins2Akita mice at 6 months might give rise to vascular remodeling to restore blood flow, as observed in older animals in later stages of the disease.
Because DR patients typically present no symptoms during the early stages of the disease, the condition is often at an advanced stage when detected, so the development of therapeutic strategies that are effective in the proliferative stages of the disease is crucial. Upon treatment with pPEDF-shPlGF, we here demonstrate a notable regression of neovascularization in 11-month-old proliferative Ins2Akita retinas. In fact, there was a visible improvement in the retinal vasculature network in treated animals, displaying solid, functional blood vessels radiating from the center of the optic nerve.
Along with the favorable therapeutic effects on vascular permeability and neovascularization of pPEDF-shPlGF, we did not observe any impact on RGC density, when compared with diabetic animals. This result suggests that this strategy does not trigger a neurodegenerative response. Nevertheless, we observed a decrease in RGC density in diabetic animals, in accordance with a previous report.65 Although future studies in younger animals are essential, we hypothesize that it could be possible to delay the progression of the disease even before the proliferative phase. Neurodegeneration and inflammation are early events in DR. Considering that there is some evidence on the neuroprotective effect of PlGF inhibition28,67 and given the well-established role of PEDF on retinal protection, we could predict that treatment administration in early stages of DR could retard the neurodegenerative process, preserving the retinal tissue. Moreover, given the anti-inflammatory properties of PEDF and the inflammatory action of PlGF, the studied approach, by overexpressing PEDF and silencing PlGF, could represent a beneficial treatment for patients diagnosed in earlier stages, without the need of frequent injections typical of conventional treatments.
As already described by our group, the pEPito vector is less affected by epigenetic silencing and consequently is able to promote sustained gene expression in the retina for at least 3 months.42 Specifically, pEPito vector contains a scaffold/matrix attachment region (S/MAR) derived from the human interferon β-gene, enabling its replication and co-segregation with the chromosomes upon mitosis. Additionally, it was shown that the S/MAR-containing vectors prevent epigenetic silencing of gene expression by shielding the transgene sequence from adjacent regulatory sequences and heterochromatinization, maintaining the vector in a transcriptionally active state. Furthermore, pEPito vector was constructed by cloning the pEPI-1 plasmid replicon in a plasmid backbone containing 60% less CpG motifs, and so is less affected by epigenetic silencing events.68 Therefore, the success of this dual-acting approach in rescuing the balance between PEDF and PlGF has potential for a long-term therapeutic effect.
The majority of studies describing therapeutic approaches for DR focused on a single gene, targeting a specific pathological change over a relatively short time frame, which is not adequate to the complex pathophysiology of DR. The strategy described in this work combines the potential of gene therapy in long-term therapeutic action, with the dual modulation of genes deeply involved in DR progression. Therefore, this strategy could represent an innovative and effective therapy not only for DR but possibly for other retinal neovascular diseases.
Materials and Methods
Design of the Anti-angiogenic Dual-Acting Vector
To simultaneously express PEDF and PlGF shRNA, we have used a combination of the polymerase II promoter (human cytomegalovirus [hCMV] enhancer/human elongation factor 1 alpha promoter) in conjunction with cis-acting ribozymes. cis-acting ribozymes (5′ end hammerhead [HH] and 3′ end hepatitis delta virus [HDV]) were employed to cleave nucleotides 5′ and 3′ of antisense and sense sequences comprising double-stranded RNAs (dsRNAs). Upon expression of the hCMV-driven construct, the cis-acting ribozymes self-cleave, releasing the shRNA to produce functional small interfering RNAs (siRNAs) to suppress the PlGF gene (Figure 1).
Animal Husbandry
The type 1 diabetic Ins2Akita mouse with a C57BL/6J background was used to model DR. This animal harbors a point mutation in the Insulin 2 gene that prevents proper insulin secretion leading to pancreatic β cell toxicity.69,70
The Ins2Akita heterozygous male and female breeder pairs (Jackson Laboratory, USA) were maintained and handled in accordance with the Portuguese and European Laboratory Animal Science Association (FELASA) Guide for the Care and Use of Laboratory Animals, the European Union Council Directive 2010/63/EU for the use of animals in research, and the Association for Research in Vision and Ophthalmology (ARVO) for the use of animals in ophthalmic and vision research. Animal experiments have been performed under the approval of the Portuguese national body DGAV (Direção Geral de Alimentação e Veterinária) and the institutional Ethics Review Board (CEFCM).
Animals were housed in a pathogen-free environment, with controlled temperature and a 12-h light/dark cycle with food and water ad libitum.
Only heterozygous male mice were included in this study, because mice homozygous for Ins2Akita mutant allele exhibit post-natal lethality, and disease progression in females is slower and less uniform than in males.70 The diabetic phenotype was confirmed 2 months after birth from a tail puncture with blood glucose levels higher than 250 mg/dL (Contour TS Blood Glucose Meter; Bayer, Germany).
Vector Construction
The pEPito-hCMV-EGFP plasmid containing the human CMV enhancer/human elongation factor 1 alpha promoter was used as backbone.41 The sequence PEDF-HH-PlGFshRNA-HDV, flanked by Nhe I (5′) and Acc III (3′) restriction sites (synthetized by Genecust, France), was digested with Nhe I and Acc III restriction enzymes and subcloned into the pEPito-hCMV backbone. The resulting construct was confirmed by restriction pattern and Sanger sequencing.
Subretinal Injection
Ins2Akita mice were randomly assigned to an experimental treatment group: pEPito-hCMV (reporter) or pEPito-hCMV-PEDF-shPlGF (pPEDF-shPlGF). WT littermates did not receive any treatment. Ten-month-old Ins2Akita mice were injected with 1 μL of reporter plasmid or pPEDF-shPlGF into the subretinal space (1 μg/μL), with the contralateral eye used as non-injected control. Subretinal injections were performed in animals anesthetized by intraperitoneal (i.p.) injection of Avertin, and pupils dilated by using 1% tropicamide. A 30G needle was used to puncture a hole approximately 1 mm below the limbus, followed by the use of an automatic pump injector (WPI) to inject either treatment. The injected eye was then electroporated using 7-mm tweezer electrodes (Tweezertodes; Harvard Apparatus, USA) connected to a BTX ECM 830 (Harvard Apparatus), as previously described.71
Western Blot Analysis
Twenty-one days post-injection, the animals were humanely sacrificed by cervical dislocation, and the eyes were enucleated. The neural retina and the RPE were isolated by dissection of the eyeball. The tissue was homogenized in ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40 [NP-40], 0.25% Na-deoxycholate, 150 mM NaCl, and 1 mM EDTA) containing a protease inhibitor cocktail (Roche, Germany). The cell lysates were then centrifuged at 13,000 rpm for 20 min at 4°C to remove cellular debris.
Protein content was determined by the Bradford assay, using the Dye Reagent Concentrate assay (Bio-Rad, UK) and bovine serum albumin (BSA) (Nzytech, Portugal) as a standard curve. A total of 30 μg of protein was separated by a SDS-PAGE gel, transferred to a nitrocellulose membrane (GE Healthcare, UK), and blocked in 5% BSA in Tris-buffer saline (20 mM Tris base, 150 mM NaCl, pH 7.6) with 0.1% Tween 20 (TBS-T) (Sigma-Aldrich, USA) for 1 h at room temperature. The following primary antibodies were incubated overnight at 4°C: rabbit polyclonal anti-PEDF (1:500; Millipore, USA), rabbit polyclonal anti-VEGF-A (1:500; Abcam, UK), rabbit polyclonal anti-PlGF (1:500; Abcam), goat polyclonal anti-Iba1 (1:500; Sigma-Aldrich), and rabbit polyclonal anti-IL-1β (1:1,000; Abcam). β-Actin, used as a control, was incubated for 1 h at room temperature (1:10,000; Sigma-Aldrich). The membrane was probed with horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology) in a 1:5,000 dilution for 1 h at room temperature. The immunoreactive bands were detected by chemiluminescence, using the ECL Blotting Reagent (GE Healthcare, UK).
Immunofluorescent Labeling of Retinal Cryosections
To evaluate the effect of our therapeutic approach on retinal inflammation in the Ins2Akita mouse model of DR, we evaluated the number of microglia cells and macrophages in the diabetic retina 21 days post-injection. In brief, the eyes were fixed in ice-cold 4% paraformaldehyde (PFA) in PBS overnight at 4°C. Afterward, the eyes were cryoprotected in a gradient of 10%–30% sucrose in PBS (1 h in sucrose 10%, 1 h in sucrose 20%, and overnight in sucrose 30%) and then embedded in OCT mounting medium (Tissue-Tek). Serial sections of 10 μm were blocked in 1% goat serum/PBS at room temperature for 1 h. Primary antibodies, mouse anti-Iba1 (1:500; Wako, USA) and rabbit anti-gamma synuclein (1:500; Abcam, USA), were incubated overnight at 4°C. Samples were washed three times in 0.1% Triton X-100/PBS and incubated with respective secondary antibodies (Alexa Fluor 488 and 594; 1:500; Life Technologies, USA) for 1 h at room temperature. Slides were washed three times and were mounted with Fluoromount G (Southern Biotech, USA) containing 4’,6’-diamidino-2-phenylindole (DAPI). Images were acquired using a Zeiss 710 confocal microscope, using appropriate filter sets (LSM 710; Carl Zeiss, Germany).
Retinal Vascular Leakage Assay
Retinal vascular leakage was evaluated using the Evans blue assay, as previously described.48 Twenty-one days after subretinal injection, anesthetized mice were administered Evans blue (2% in PBS) by i.p. injection, with visual confirmation of the uptake and distribution of the dye. Two hours later, mice were sacrificed by cervical dislocation; the eyes were removed and immediately immersed on 4% PFA. After 2 h, the retinas were carefully dissected and flat mounted in glass slides. Retinal flat mounts were analyzed under a confocal microscope (LSM 710; Carl Zeiss, Germany) to visualize retinal vessel leakage.
Immunofluorescent Labeling of Retinal Flat Mounts
For flat-mount preparation, mice were sacrificed 21 days post-injection, and retinas were carefully dissected from enucleated ocular globes and fixed in 4% PFA/PBS overnight at 4°C. After washing in 1× PBS, retinas were permeabilized in 1% Triton in 1× PBS for 2 h at room temperature and then blocked in 10% goat serum, 1% BSA in 1× PBS overnight at 4°C. Retinas were incubated with Griffonia simplicifolia Lectin I, Isolectin B4 (Sigma-Aldrich, USA) diluted in blocking buffer to a final concentration of 1:200 and incubated for 3 days at 4°C. Samples were washed, incubated with streptavidin (1:100; Vector Laboratories, UK), and then incubated with fluorescein (1:80; Perkin Elmer, USA) at room temperature, according to the manufacturers’ instructions. After the final washing steps, retinas were flattened with four equidistant incisions toward the optic disk and mounted with Fluoromount G (Southern Biotech, USA). All sections were viewed and imaged using a Zeiss 710 confocal microscope, with appropriate filter sets (LSM 710; Carl Zeiss, Germany) at 40× magnification. Eight images per retina were acquired (two images in each quadrant). The eight values representing eight separate peripheral retinal fields were averaged for each eye. Described statistics are for total length of vessels, lacunarity (an index of “gappiness” within the image), number of vessel junctions, and area of vessels, processed through the AngioTool Software.72
Image Analysis
All images were analyzed using ImageJ software (Fiji image processing package). Two-dimensional (2D) images of each retinal flat mount were reconstructed from z stacks. For retinal vascular leakage analysis and Isolectin B4 staining, a composite picture of the whole retina vasculature was obtained by overlapping confocal images (×20 lens) in a tile scan of an 8 × 8 grid, for a total of 64 images.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism software. The Student’s t test with a significance level of 0.05 was used to compare differences between two groups. One-way ANOVA was used to analyze differences between treatment groups and was followed by Tukey’s honest significant difference (HSD) test for pairwise comparisons. Differences were considered statistically significant at p < 0.05 in all experiments: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. All data are expressed as mean ± standard error of the mean (SEM).
Author Contributions
Conceptualization, R.S.A. and G.A.S; Investigation, R.S.A. and D.B.B.; Writing – Original Draft, R.S.A.; Writing – Review & Editing, R.S.A. and G.A.S.; Funding Acquisition, G.A.S.; Supervision, G.A.S.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The authors acknowledge the financial support of Fundação para a Ciência e a Tecnologia (SFRH/BD/114051/2016 individual fellowship to R.S.A.), Projetos de Investigação Científica e Desenvolvimento Tecnológico (IC&DT, grant 02/SAICT/2017/028121 to G.A.S.), and the Marie Curie Reintegration Grant (PIRG-GA-2009-249314 to G.A.S.) under the FP7 program. iNOVA4Health, UID/Multi/04462/2013, a program financially supported by Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência through national funds and co-funded by FEDER under the PT2020 Partnership Agreement, is also acknowledged.
References
- 1.World Health Organization . WHO; 2016. Global Report on Diabetes.https://apps.who.int/iris/handle/10665/204871 [Google Scholar]
- 2.Williams R., Airey M., Baxter H., Forrester J., Kennedy-Martin T., Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond.) 2004;18:963–983. doi: 10.1038/sj.eye.6701476. [DOI] [PubMed] [Google Scholar]
- 3.Abcouwer S.F., Gardner T.W. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N Y Acad. Sci. 2014;1311:174–190. doi: 10.1111/nyas.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank R.N. Diabetic retinopathy. N. Engl. J. Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 5.Roy S., Sato T., Paryani G., Kao R. Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes. 2003;52:1229–1234. doi: 10.2337/diabetes.52.5.1229. [DOI] [PubMed] [Google Scholar]
- 6.Moreno A., Lozano M., Salinas P. Diabetic retinopathy. Nutr. Hosp. 2013;28(Suppl 2):53–56. doi: 10.3305/nh.2013.28.sup2.6714. [DOI] [PubMed] [Google Scholar]
- 7.Kusuhara S., Fukushima Y., Ogura S., Inoue N., Uemura A. Pathophysiology of Diabetic Retinopathy: The Old and the New. Diabetes Metab. J. 2018;42:364–376. doi: 10.4093/dmj.2018.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins P.J. Retinopathy. BMJ. 2003;326:924–926. doi: 10.1136/bmj.326.7395.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero-Aroca P. Managing diabetic macular edema: The leading cause of diabetes blindness. World J. Diabetes. 2011;2:98–104. doi: 10.4239/wjd.v2.i6.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandello F., Battaglia Parodi M., Tremolada G., Lattanzio R., De Benedetto U., Iacono P. Steroids as part of combination treatment: the future for the management of macular edema? Ophthalmologica. 2010;224(Suppl 1):41–45. doi: 10.1159/000315161. [DOI] [PubMed] [Google Scholar]
- 11.Dowler J.G.F. Laser management of diabetic retinopathy. J. R. Soc. Med. 2003;96:277–279. doi: 10.1258/jrsm.96.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak I., Luttrull J.K. Modern retinal laser therapy. Saudi J. Ophthalmol. 2015;29:137–146. doi: 10.1016/j.sjopt.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osaadon P., Fagan X.J., Lifshitz T., Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond.) 2014;28:510–520. doi: 10.1038/eye.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massin P., Bandello F., Garweg J.G., Hansen L.L., Harding S.P., Larsen M., Mitchell P., Sharp D., Wolf-Schnurrbusch U.E., Gekkieva M. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen Q.D., Brown D.M., Marcus D.M., Boyer D.S., Patel S., Feiner L., Gibson A., Sy J., Rundle A.C., Hopkins J.J., RISE and RIDE Research Group Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell P., Bandello F., Schmidt-Erfurth U., Lang G.E., Massin P., Schlingemann R.O., Sutter F., Simader C., Burian G., Gerstner O., Weichselberger A., RESTORE study group The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen Q.D., Shah S.M., Khwaja A.A., Channa R., Hatef E., Do D.V., Boyer D., Heier J.S., Abraham P., Thach A.B., READ-2 Study Group Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Arevalo J.F., Maia M., Flynn H.W.J., Jr., Saravia M., Avery R.L., Wu L., Eid Farah M., Pieramici D.J., Berrocal M.H., Sanchez J.G. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br. J. Ophthalmol. 2008;92:213–216. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 19.Falavarjani K.G., Nguyen Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27:787–794. doi: 10.1038/eye.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moradian S., Ahmadieh H., Malihi M., Soheilian M., Dehghan M.H., Azarmina M. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2008;246:1699–1705. doi: 10.1007/s00417-008-0914-4. [DOI] [PubMed] [Google Scholar]
- 21.Van Geest R.J., Lesnik-Oberstein S.Y., Tan H.S., Mura M., Goldschmeding R., Van Noorden C.J.F., Klaassen I., Schlingemann R.O. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br. J. Ophthalmol. 2012;96:587–590. doi: 10.1136/bjophthalmol-2011-301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filek R., Hooper P., Sheidow T.G., Gonder J., Chakrabarti S., Hutnik C.M. Two-year analysis of changes in the optic nerve and retina following anti-VEGF treatments in diabetic macular edema patients. Clin. Ophthalmol. 2019;13:1087–1096. doi: 10.2147/OPTH.S199758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hombrebueno J.R., Ali I.H.A., Xu H., Chen M. Sustained intraocular VEGF neutralization results in retinal neurodegeneration in the Ins2(Akita) diabetic mouse. Sci. Rep. 2015;5:18316. doi: 10.1038/srep18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H.Y., Kim J.H., Park C.K. Neuronal cell death in the inner retina and the influence of vascular endothelial growth factor inhibition in a diabetic rat model. Am. J. Pathol. 2014;184:1752–1762. doi: 10.1016/j.ajpath.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N., Gerber H.-P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen Q.D., De Falco S., Behar-Cohen F., Lam W.-C., Li X., Reichhart N., Ricci F., Pluim J., Li W.W. Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol. 2018;96:e1–e9. doi: 10.1111/aos.13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmeliet P., Moons L., Luttun A., Vincenti V., Compernolle V., De Mol M., Wu Y., Bono F., Devy L., Beck H. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 28.Huang H., He J., Johnson D., Wei Y., Liu Y., Wang S., Lutty G.A., Duh E.J., Semba R.D. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1α-VEGF pathway inhibition. Diabetes. 2015;64:200–212. doi: 10.2337/db14-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonas J.B., Jonas R.A., Neumaier M., Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina. 2012;32:2150–2157. doi: 10.1097/IAE.0b013e3182576d07. [DOI] [PubMed] [Google Scholar]
- 30.Khaliq A., Foreman D., Ahmed A., Weich H., Gregor Z., McLeod D., Boulton M. Increased expression of placenta growth factor in proliferative diabetic retinopathy. Lab. Invest. 1998;78:109–116. [PubMed] [Google Scholar]
- 31.Yamashita H., Eguchi S., Watanabe K., Takeuchi S., Yamashita T., Miura M. Expression of placenta growth factor (PIGF) in ischaemic retinal diseases. Eye (Lond.) 1999;13(Pt 3a):372–374. doi: 10.1038/eye.1999.95. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs K., Marra K.V., Yu G., Wagley S., Ma J., Teague G.C., Nandakumar N., Lashkari K., Arroyo J.G. Angiogenic and Inflammatory Vitreous Biomarkers Associated With Increasing Levels of Retinal Ischemia. Invest. Ophthalmol. Vis. Sci. 2015;56:6523–6530. doi: 10.1167/iovs.15-16793. [DOI] [PubMed] [Google Scholar]
- 33.Ando R., Noda K., Namba S., Saito W., Kanda A., Ishida S. Aqueous humour levels of placental growth factor in diabetic retinopathy. Acta Ophthalmol. 2014;92:e245–e246. doi: 10.1111/aos.12251. [DOI] [PubMed] [Google Scholar]
- 34.Spirin K.S., Saghizadeh M., Lewin S.L., Zardi L., Kenney M.C., Ljubimov A.V. Basement membrane and growth factor gene expression in normal and diabetic human retinas. Curr. Eye Res. 1999;18:490–499. doi: 10.1076/ceyr.18.6.490.5267. [DOI] [PubMed] [Google Scholar]
- 35.Gao G., Li Y., Zhang D., Gee S., Crosson C., Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–276. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 36.Ogata N., Nishikawa M., Nishimura T., Mitsuma Y., Matsumura M. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am. J. Ophthalmol. 2002;134:348–353. doi: 10.1016/s0002-9394(02)01568-4. [DOI] [PubMed] [Google Scholar]
- 37.Dawson D.W., Volpert O.V., Gillis P., Crawford S.E., Xu H., Benedict W., Bouck N.P. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 38.Yamagishi S., Matsui T., Nakamura K., Yoshida T., Takeuchi M., Inoue H., Yoshida Y., Imaizumi T. Pigment-epithelium-derived factor suppresses expression of receptor for advanced glycation end products in the eye of diabetic rats. Ophthalmic Res. 2007;39:92–97. doi: 10.1159/000099244. [DOI] [PubMed] [Google Scholar]
- 39.Haurigot V., Villacampa P., Ribera A., Bosch A., Ramos D., Ruberte J., Bosch F. Long-term retinal PEDF overexpression prevents neovascularization in a murine adult model of retinopathy. PLoS ONE. 2012;7:e41511. doi: 10.1371/journal.pone.0041511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J.-H., Ling D., Tu L., van Wijngaarden P., Dusting G.J., Liu G.-S. Gene therapy for diabetic retinopathy: Are we ready to make the leap from bench to bedside? Pharmacol. Ther. 2017;173:1–18. doi: 10.1016/j.pharmthera.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Calado S.M., Oliveira A.V., Machado S., Haase R., Silva G.A. Sustained gene expression in the retina by improved episomal vectors. Tissue Eng. Part A. 2014;20:2692–2698. doi: 10.1089/ten.tea.2013.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calado S.M., Diaz-Corrales F., Silva G.A. pEPito-driven PEDF expression ameliorates Diabetic Retinopathy hallmarks. Hum. Gene Ther. Methods. 2016;27:79–86. doi: 10.1089/hgtb.2015.169. [DOI] [PubMed] [Google Scholar]
- 43.Araújo R.S., Silva M.S., Santos D.F., Silva G.A. Dysregulation of trophic factors contributes to diabetic retinopathy in the Ins2Akita mouse. Exp. Eye Res. 2020;194:108027. doi: 10.1016/j.exer.2020.108027. [DOI] [PubMed] [Google Scholar]
- 44.Miller J.W., Adamis A.P., Shima D.T., D’Amore P.A., Moulton R.S., O’Reilly M.S., Folkman J., Dvorak H.F., Brown L.F., Berse B. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994;145:574–584. [PMC free article] [PubMed] [Google Scholar]
- 45.Ting D.S.W., Tan K.-A., Phua V., Tan G.S.W., Wong C.W., Wong T.Y. Biomarkers of Diabetic Retinopathy. Curr. Diab. Rep. 2016;16:125. doi: 10.1007/s11892-016-0812-9. [DOI] [PubMed] [Google Scholar]
- 46.Sasongko M.B., Wong T.Y., Jenkins A.J., Nguyen T.T., Shaw J.E., Wang J.J. Circulating markers of inflammation and endothelial function, and their relationship to diabetic retinopathy. Diabet. Med. 2015;32:686–691. doi: 10.1111/dme.12640. [DOI] [PubMed] [Google Scholar]
- 47.Altmann C., Schmidt M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018;19:110. doi: 10.3390/ijms19010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Q., Qaum T., Adamis A.P. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest. Ophthalmol. Vis. Sci. 2001;42:789–794. [PubMed] [Google Scholar]
- 49.Surgucheva I., Weisman A.D., Goldberg J.L., Shnyra A., Surguchov A. Gamma-synuclein as a marker of retinal ganglion cells. Mol. Vis. 2008;14:1540–1548. [PMC free article] [PubMed] [Google Scholar]
- 50.Avery R.L., Pearlman J., Pieramici D.J., Rabena M.D., Castellarin A.A., Nasir M.A., Giust M.J., Wendel R., Patel A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1–1695.e15. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 51.Mason J.O., 3rd, Nixon P.A., White M.F. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am. J. Ophthalmol. 2006;142:685–688. doi: 10.1016/j.ajo.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 52.Hattori T., Shimada H., Nakashizuka H., Mizutani Y., Mori R., Yuzawa M. Dose of intravitreal bevacizumab (Avastin) used as preoperative adjunct therapy for proliferative diabetic retinopathy. Retina. 2010;30:761–764. doi: 10.1097/IAE.0b013e3181c70168. [DOI] [PubMed] [Google Scholar]
- 53.Storkebaum E., Lambrechts D., Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. BioEssays. 2004;26:943–954. doi: 10.1002/bies.20092. [DOI] [PubMed] [Google Scholar]
- 54.Carroll L.S., Uehara H., Fang D., Choi S., Zhang X., Singh M. Intravitreal AAV2.COMP-Ang1 attenuates deep capillary plexus expansion in the aged diabetic mouse retina. Invest. Ophthalmol. Vis. Sci. 2019;60:2494–2502. doi: 10.1167/iovs.18-26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han Z., Guo J., Conley S.M., Naash M.I. Retinal angiogenesis in the Ins2(Akita) mouse model of diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2013;54:574–584. doi: 10.1167/iovs.12-10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He X., Cheng R., Benyajati S., Ma J.X. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin. Sci. (Lond.) 2015;128:805–823. doi: 10.1042/CS20130463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang S.X., Wang J.J., Gao G., Parke K., Ma J.X. Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J. Mol. Endocrinol. 2006;37:1–12. doi: 10.1677/jme.1.02008. [DOI] [PubMed] [Google Scholar]
- 58.Mori K., Duh E., Gehlbach P., Ando A., Takahashi K., Pearlman J., Mori K., Yang H.S., Zack D.J., Ettyreddy D. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J. Cell. Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 59.Van Bergen T., Hu T.T., Etienne I., Reyns G.E., Moons L., Feyen J.H.M. Neutralization of placental growth factor as a novel treatment option in diabetic retinopathy. Exp. Eye Res. 2017;165:136–150. doi: 10.1016/j.exer.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Araújo R.S., Silva G.A. PlGF silencing combined with PEDF overexpression: Modeling RPE secretion as potential therapy for retinal neovascularization. Mol. Biol. Rep. 2020;47:4413–4425. doi: 10.1007/s11033-020-05496-2. [DOI] [PubMed] [Google Scholar]
- 61.Murata T., Nakagawa K., Khalil A., Ishibashi T., Inomata H., Sueishi K. The relation between expression of vascular endothelial growth factor and breakdown of the blood-retinal barrier in diabetic rat retinas. Lab. Invest. 1996;74:819–825. [PubMed] [Google Scholar]
- 62.Carmo A., Cunha-Vaz J.G., Carvalho A.P., Lopes M.C. Effect of cyclosporin-A on the blood—retinal barrier permeability in streptozotocin-induced diabetes. Mediators Inflamm. 2000;9:243–248. doi: 10.1080/09629350020025764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyamoto N., de Kozak Y., Jeanny J.C., Glotin A., Mascarelli F., Massin P., BenEzra D., Behar-Cohen F. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461–470. doi: 10.1007/s00125-006-0539-2. [DOI] [PubMed] [Google Scholar]
- 64.Barber A.J., Antonetti D.A., Kern T.S., Reiter C.E.N., Soans R.S., Krady J.K., Levison S.W., Gardner T.W., Bronson S.K. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest. Ophthalmol. Vis. Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 65.Wright W.S., Yadav A.S., McElhatten R.M., Harris N.R. Retinal blood flow abnormalities following six months of hyperglycemia in the Ins2(Akita) mouse. Exp. Eye Res. 2012;98:9–15. doi: 10.1016/j.exer.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rakoczy E.P., Ali Rahman I.S., Binz N., Li C.R., Vagaja N.N., de Pinho M., Lai C.M. Characterization of a mouse model of hyperglycemia and retinal neovascularization. Am. J. Pathol. 2010;177:2659–2670. doi: 10.2353/ajpath.2010.090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Izawa H., Inoue Y., Ohno Y., Ojino K., Tsuruma K., Shimazawa M., Hara H. Protective Effects of Antiplacental Growth Factor Antibody Against Light-Induced Retinal Damage in Mice. Invest. Ophthalmol. Vis. Sci. 2015;56:6914–6924. doi: 10.1167/iovs.15-16748. [DOI] [PubMed] [Google Scholar]
- 68.Haase R., Argyros O., Wong S.-P., Harbottle R.P., Lipps H.J., Ogris M., Magnusson T., Vizoso Pinto M.G., Haas J., Baiker A. pEPito: a significantly improved non-viral episomal expression vector for mammalian cells. BMC Biotechnol. 2010;10:20. doi: 10.1186/1472-6750-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Takeuchi T., Tanaka S., Kubo S.K., Kayo T., Lu D., Takata K., Koizumi A., Izumi T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Invest. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshioka M., Kayo T., Ikeda T., Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 71.Matsuda T., Cepko C.L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zudaire E., Gambardella L., Kurcz C., Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS ONE. 2011;6:e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]