Abstract
Previous studies of correlations of microRNA (miR)-499 rs3746444 and miR-196a-2 rs11614913 polymorphisms with glioma risk have yielded inconsistent results. In this study, relationships between these two polymorphisms and glioma risk and survival were evaluated. In total, 605 patients and 1,300 controls were genotyped. rs3746444 increased glioma risk in five genetic models (GA versus AA, odds ratio [OR], 95% confidence interval [CI] = 1.31 [1.05–1.66], p = 0.02; GG versus AA, OR [95% CI] = 10.70 [6.13–18.69], p < 0.0001; GA + GG versus AA, OR [95% CI] = 1.82 [1.47–2.24], p < 0.0001; GG versus AA + GA, OR [95% CI] = 9.99 [5.74–17.40], p < 0.0001; G versus A, OR [95% CI] = 2.18 [1.82–2.60], p < 0.0001). rs11614913 decreased glioma risk in a recessive model (OR [95% CI] = 0.79 [0.64–0.97], p = 0.03). No relationships between either SNP and survival were found. rs3746444 in the miR-499 seed region could affect target recognition. Bioinformatics analyses indicated that miR-499 rs3746444 is involved in various biological processes and pathways, including “cell adhesion molecule binding,” “positive regulation of catabolic process,” “NF-kappa B pathway,” and “PI3K-Akt pathway,” by targeting mRNAs. Our results suggested that miR-499 rs3746444 and miR-196a-2 rs11614913 have crucial roles in glioma susceptibility.
Keywords: glioma, miR-499, miR-196a-2, single nucleotide polymorphism, susceptibility, survival
Graphical Abstract
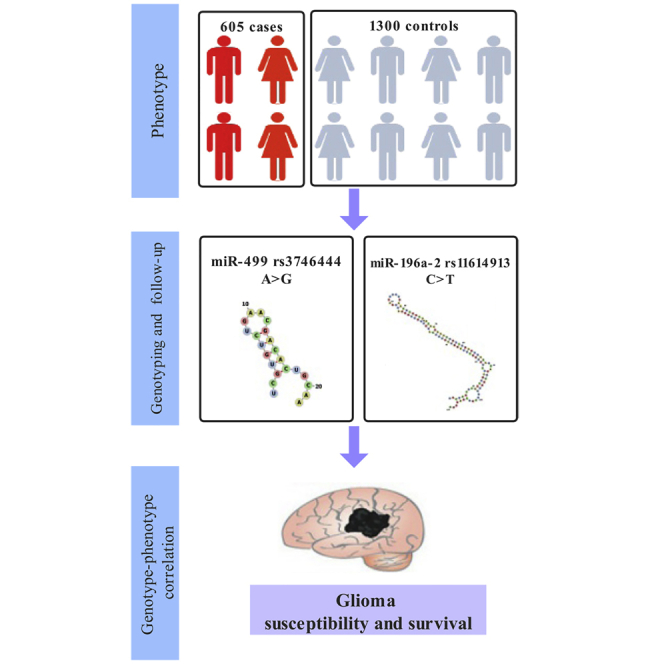
Relationships between miR-499 rs3746444 and miR-196a-2 rs11614913 polymorphisms and glioma susceptibility and survival were investigated. rs3746444 increased glioma risk, whereas rs11614913 decreased glioma risk. However, no relationships between either SNP and survival were found. These results first highlight that miR-499 rs3746444 and miR-196a-2 rs11614913 have crucial roles in glioma susceptibility.
Introduction
As the most prevalent central nervous system tumors, gliomas account for 27% of all brain tumors and 80% of all malignant brain tumors.1 According to the World Health Organization (WHO), gliomas are classified into four grades.2 Multiple molecular biomarkers have been identified for the diagnosis and treatment of glioma. Despite extensive studies and therapeutic advances, the etiology of this tumor remains unclear, and its survival is unsatisfactory, with a median overall survival (OS) of about 16 months.3 A more comprehensive understanding of the molecular and histological features of glioma is needed for the development of novel and effective therapeutic approaches.
MicroRNAs (miRNAs) are a family of small noncoding RNAs that regulate gene expression at the post-transcriptional level.4,5 They generally recognize target genes by base pairing between nucleotides 2–8 from the 5′ end (seed region) and the complementary nucleotides in the 3′ untranslated region of target mRNAs.6,7 There is substantial evidence indicating that aberrant miRNAs act as oncogenes or tumor suppressors in various types of cancers.8, 9, 10
Single-nucleotide polymorphisms (SNPs) can affect phenotypes and disease susceptibility.11 SNPs in miRNAs may have complex effects. SNPs in primary miRNAs (pri-miRNAs) or precursor miRNAs (pre-miRNAs) can affect stability or processing.12 SNPs in the promoters of pri-mRNAs may influence mature miRNA expression.13 SNPs in the seed region can affect target gene identification.14 Extensive research has linked miRNA SNPs to cancer susceptibility.15 Several studies have revealed correlations between microRNA (miR)-196a-2 rs11614913 and miR-499 rs3746444 and the risk of glioma. The rs11614913 polymorphism is located in the mature region of miR-196a-2, which is highly expressed in glioblastoma.16 rs3746444 is an A-to-G variant in the seed region of miR-499.11 However, inconsistent results have been obtained regarding the effects of these polymorphisms. Some research suggests that miR-196a-2 rs11614913 decreases the risk of glioma,17 whereas other work suggests that it increases susceptibility to glioma.18 Another study reported that there is no relationship between miR-196a-2 rs11614913 and glioma risk.19 An association between miR-499 rs3746444 and glioma susceptibility has not been observed in previous studies.18,20 However, many studies have demonstrated that this polymorphism is related to susceptibility in various cancers.21,22 The small sample sizes may explain the differences among studies. Thus, to further explore the correlations between these two SNPs and the risk of glioma, a larger cohort of patients is needed. Furthermore, few studies have explored whether miR-196a-2 rs11614913 and miR-499 rs3746444 are related to OS and progression-free survival (PFS) in patients with glioma.
In this study, we first explored associations between these two miRNA SNPs and glioma risk and survival in a Chinese Han population. We also used bioinformatics approaches to evaluate the functional significance of miR-499 rs3746444, which could affect miRNA-mRNA interactions.
Results
Characteristics of the Study Subjects
In total, 605 patients with glioma and 1,300 control subjects were enrolled in our study. The participants were Chinese Han. No significant differences between cases and controls were found with regard to age or gender (p > 0.05). Patients with glioma were divided into WHO I–II (n = 382) and III–IV groups (n = 223). The characteristics of patients and control subjects are provided in Table 1.
Table 1.
The Characteristics of Glioma Cases and Cancer-Free Controls
| Characteristics | Group | Cases (n = 605) | Controls (n = 1,300) | p Valuea |
|---|---|---|---|---|
| Age (mean ± SD) | 40.71 ± 18.28 | 41.68 ± 13.54 | 0.20 | |
| Age (years) | 0.69 | |||
| <40 | 267 (44.13%) | 561 (43.15%) | ||
| ≥40 | 338 (55.87%) | 739 (56.85%) | ||
| Sex | 0.53 | |||
| male | 335 (55.37%) | 700 (53.85%) | ||
| female | 270 (44.63%) | 600 (46.15%) | ||
| WHO Grade | ||||
| I–II | 382 (63.14%) | |||
| III–IV | 223 (36.86%) | |||
| Surgery | ||||
| STR and NTR | 189 (31.24%) | |||
| GTR | 416 (68.76%) | |||
| Radiotherapy | ||||
| none | 60 (9.92%) | |||
| conformal radiotherapy | 162 (26.78%) | |||
| gamma knife | 383 (63.31%) | |||
| Chemotherapy | ||||
| none | 355 (58.68%) | |||
| platinum | 124 (20.50%) | |||
| temozolomide | 52 (8.60%) | |||
| nimustine | 74 (12.23%) | |||
SD, standard deviation; STR, subtotal resection; NTR, near-total resection; GTR, gross total resection; WHO, World Health Organization.
t test or two-sided χ2-test.
Associations between rs3746444 and rs11614913 Polymorphisms and the Risk of Glioma
Significant correlations between miR-499 rs3746444 and a high risk of glioma were observed in the heterozygote, homozygote, dominant, recessive, and allele models (GA versus AA, odds ratio [OR], 95% confidence interval [CI] = 1.31 [1.05–1.66], p = 0.02; GG versus AA, OR [95% CI] = 10.70 [6.13–18.69], p < 0.0001; GA + GG versus AA, OR [95% CI] = 1.82 [1.47–2.24], p < 0.0001; GG versus AA + GA, OR [95% CI] = 9.99 [5.74–17.40], p < 0.0001; G versus A, OR [95% CI] = 2.18 [1.82–2.60], p < 0.0001, respectively). miR-196a-2 rs11614913 was identified as a protective factor for glioma in the recessive model (CC + CT versus TT, OR [95% CI] = 0.79 [0.64–0.97], p = 0.03). The results are displayed in Table 2.
Table 2.
Genotype Frequencies of miR-196a-2 rs11614913 and miR-499 rs3746444 Polymorphisms in Cases and Controls
| miRNA | Model | Genotype | Controls (n, %) | Cases (n, %) | OR (95% CI) | p Value | FPRP (Prior Probability = 0.1) |
|---|---|---|---|---|---|---|---|
| miR-196a-2 | |||||||
| rs11614913 | HWE: p = 0.69 | ||||||
| codominant | CC | 295 (22.69) | 139 (22.98) | 1.00 (reference) | |||
| heterozygote | CT | 656 (50.46) | 274 (45.29) | 0.89 (0.69–1.13) | 0.34 | ||
| homozygote | TT | 349 (26.85) | 192 (31.74) | 0.86 (0.66–1.12) | 0.26 | ||
| dominant | CC | 295 (22.69) | 139 (22.98) | 1.00 (reference) | |||
| CT + TT | 1005 (77.31) | 466 (77.02) | 1.02 (0.81–1.28) | 0.89 | |||
| recessive | CC + CT | 951 (73.15) | 413 (68.26) | 1.00 (reference) | |||
| TT | 349 (26.85) | 192 (31.74) | 0.79 (0.64– 0.97) | 0.03 | 0.189 | ||
| allele | C | 1,846 (71.00) | 552 (45.62) | 1.00 (reference) | |||
| T | 1,354 (52.08) | 658 (54.38) | 0.91 (0.80–1.05) | 0.19 | |||
| miR-499-3p | |||||||
| rs3746444 | HWE: p = 0.389 | ||||||
| codominant | AA | 999 (76.85) | 391 (64.63) | 1.00 (reference) | |||
| heterozygote | GA | 285 (21.92) | 147 (24.30) | 1.31 (1.05–1.66) | 0.02 | 0.21 | |
| homozygote | GG | 16 (1.23) | 67 (11.07) | 10.70 (6.13–18.69) | <0.0001 | <0.0001 | |
| dominant | AA | 999 (76.85) | 391 (64.63) | 1.00 (reference) | |||
| GA + GG | 301 (23.15) | 214 (35.37) | 1.82 (1.47–2.24) | <0.0001 | <0.0001 | ||
| recessive | AA + GA | 1,284 (98.77) | 538 (88.93) | 1.00 (reference) | |||
| GG | 16 (1.23) | 67 (11.07) | 9.99 (5.74–17.40) | <0.0001 | <0.0001 | ||
| allele | A | 2,283 (87.81) | 929 (76.78) | 1.00 (reference) | |||
| G | 317 (12.19) | 281 (23.22) | 2.18 (1.82–2.60) | <0.0001 | <0.0001 | ||
HWE, Hardy-Weinberg equilibrium; OR, odds ratio; CI, confidence interval; FPRP, false-positive report probability.
False-Positive Report Probability (FPRP) Test Results
We further validated the statistically significant findings using the FPRP test. The results revealed that four genetic models (GG versus AA, GA + GG versus AA, GG versus AA + GA, G versus A) of the miR-499 rs3746444 increased glioma risk (all FPRP < 0.2; Table 2) at a prior probability of 0.1 with an OR of 1.5. Moreover, we confirmed that miR-196a-2 rs11614913 decreased glioma risk in the recessive model (FPRP = 0.189; Table 2) at a prior probability of 0.1 with an OR of 0.67.
Associations between rs3746444 and rs11614913 Polymorphisms and Clinical Characteristics of Patients with Glioma
The genotype frequencies of miR-499 rs3746444 and miR-196a-2 rs11614913 were evaluated with regard to clinical variables, such as patient age, sex, and WHO grade. There were no relationships between genotypes and any of these variables. These results suggested that the genotype distribution was balanced for rs3746444 and rs11614913. The data are summarized in Table 3.
Table 3.
The Associations between rs3746444 and rs11614913 Polymorphisms and Clinical Characteristics of Glioma Patients
| Characteristics | rs3746444 |
rs11614913 |
||||||
|---|---|---|---|---|---|---|---|---|
| AA | GA | GG | GA + GG | CC | CT | TT | CT + TT | |
| Age | ||||||||
| <40/≥ 40 | 175/216 | 61/86 | 31/36 | 92/122 | 60/79 | 123/151 | 84/108 | 207/259 |
| OR (95% CI) | ref. | 1.14 (0.78–1.68) | 0.94 (0.56–1.59) | 1.07 (0.77–1.51) | ref. | 0.93 (0.62–1.41) | 0.98 (0.63–1.52) | 0.95 (0.65–1.39) |
| p valuea | 0.50 | 0.82 | 0.68 | 0.74 | 0.92 | 0.79 | ||
| Sex | ||||||||
| Male/female | 212/179 | 85/62 | 38/29 | 123/91 | 75/64 | 160/114 | 100/92 | 260/206 |
| OR (95% CI) | ref. | 0.86 (0.59–1.27) | 0.90 (0.53–1.52) | 0.88 (0.63–1.23) | ref. | 0.83 (0.55–1.26) | 1.08 (0.70–1.67) | 0.93 (0.64–1.36) |
| p valuea | 0.45 | 0.71 | 0.44 | 0.39 | 0.74 | 0.70 | ||
| WHO Grade | ||||||||
| I + II/III + IV | 250/141 | 83/64 | 49/18 | 132/82 | 81/58 | 169/105 | 132/60 | 301/165 |
| OR (95% CI) | ref. | 1.37 (0.93–2.01) | 0.65 (0.36–1.14) | 1.10 (0.78–1.55) | ref. | 0.87 (0.57–1.32) | 0.63 (0.40–1.00) | 0.77 (0.52–1.13) |
| p valuea | 0.11 | 0.15 | 0.58 | 0.50 | 0.05 | 0.18 | ||
OR, odds ratio; CI, confidence interval; WHO, World Health Organization; ref., reference.
Univariate logistic regression analysis for the distributions of genotype frequencies.
Relationship among Genotypes, Clinical Variables, and OS
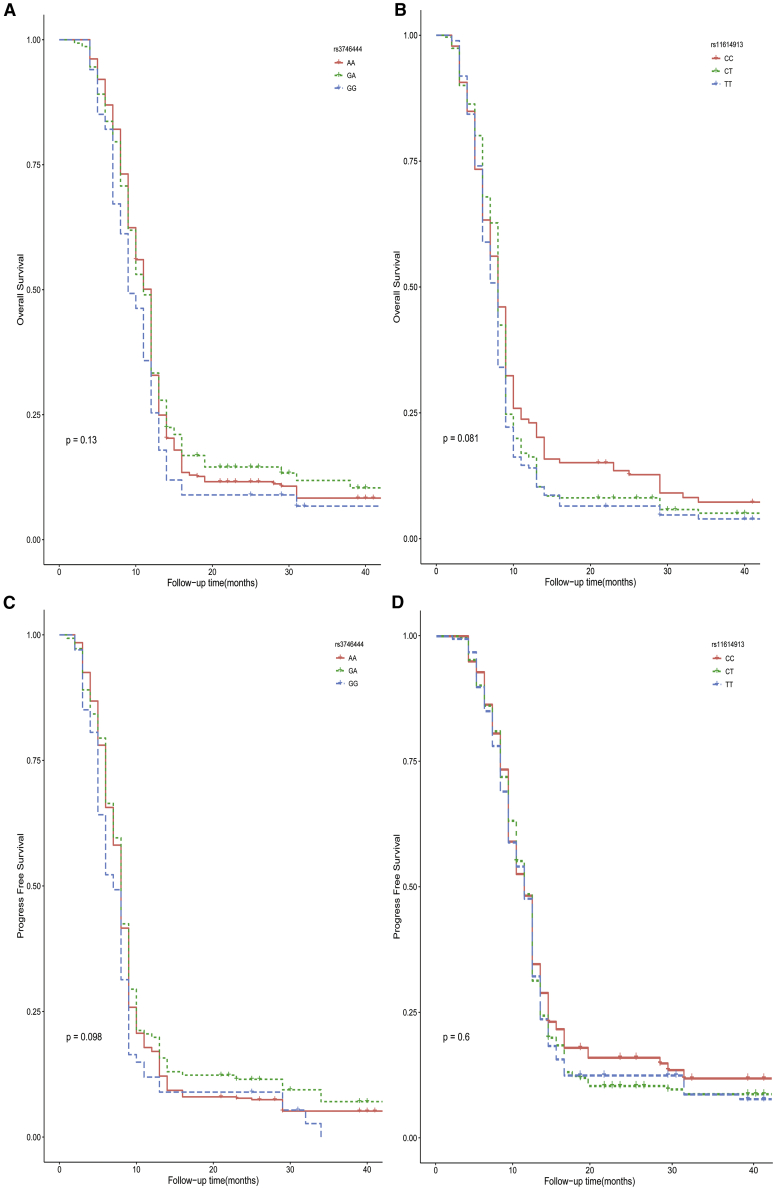
As shown in Table 4, age, surgery, and chemotherapy were significant variables in the univariate analysis and were therefore included in a multivariate analysis. The multivariate analysis revealed that age, surgery, and chemotherapy could serve as independent predictive indicators for OS. Patients with age ≥40 years had poorer survival (hazard ratio [HR] [95% CI] = 1.21 [1.02–1.44], p = 0.029) than that of patients younger than 40 years. The OS was better for patients who underwent gross total resection than for those who underwent subtotal resection or near-total resection (HR [95% CI] = 0.62 [0.51–0.75], p < 0.001). Similarly, survival was better in patients administered either temozolomide or nimustine than in those who did not receive chemotherapy (HR [95% CI] = 0.36 [0.24–0.52], p < 0.001; HR [95% CI] = 0.74 [0.56–0.97], p = 0.030, respectively). Significant associations between the two variants (rs11614913 and rs3746444) and OS were not observed. Based on Kaplan-Meier survival analyses of each variant, there were no differences in OS among the three genotypes (Figures 1A and 1B). This result was consistent with the results of the univariate analysis.
Table 4.
Univariate and Multivariate Analyses of Associations among Genotypes, Various Factors, and OS
| Characteristics | Patients (n) | Events (n) | Rate (%) | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Valuea | HR (95% CI) | p Valuea | ||||
| Age (Years) | |||||||
| <40 | 267 | 229 | 85.77 | ref. | ref. | ref. | ref. |
| ≥40 | 338 | 310 | 91.72 | 1.20 (1.01–1.42) | 0.039 | 1.21 (1.02–1.44) | 0.029 |
| Sex | |||||||
| Male | 335 | 297 | 88.66 | ref. | ref. | ||
| Female | 270 | 242 | 89.63 | 1.08 (0.91–1.28) | 0.355 | ||
| WHO Grade | |||||||
| I–II | 382 | 336 | 87.96 | ref. | ref. | ||
| III–IV | 223 | 206 | 92.38 | 1.18 (0.98–1.40) | 0.063 | ||
| Surgery | |||||||
| STR and NTR | 189 | 186 | 98.41 | ref. | ref. | ref. | ref. |
| GTR | 416 | 353 | 84.86 | 0.59 (0.49–0.71) | <0.001 | 0.62 (0.51–0.75) | <0.001 |
| Chemotherapy | |||||||
| None | 355 | 333 | 93.80 | ref. | ref. | ref. | ref. |
| Platinum | 124 | 112 | 90.32 | 0.84 (0.68–1.04) | 0.116 | 0.82 (0.66–1.02) | 0.072 |
| Temozolomide | 52 | 30 | 57.69 | 0.32 (0.22–0.48) | <0.001 | 0.36 (0.24–0.52) | <0.001 |
| Nimustine | 74 | 64 | 86.49 | 0.65 (0.49–0.85) | 0.001 | 0.74 (0.56–0.97) | 0.030 |
| Radiotherapy | |||||||
| None | 60 | 49 | 81.67 | ref. | ref. | ||
| Conformal radiotherapy | 162 | 133 | 82.10 | 1.08 (0.77–1.50) | 0.622 | ||
| Gamma knife | 383 | 357 | 93.21 | 1.17 (0.86–1.58) | 0.303 | ||
| rs3746444 | |||||||
| AA | 391 | 349 | 89.26 | ref. | ref. | ||
| GA | 147 | 128 | 87.07 | 0.97 (0.79–1.19) | 0.747 | ||
| GG | 67 | 62 | 92.54 | 1.28 (0.98–1.68) | 0.071 | ||
| rs11614913 | |||||||
| CC | 139 | 119 | 85.61 | ref. | ref. | ||
| CT | 274 | 246 | 89.78 | 1.10 (0.89–1.37) | 0.375 | ||
| TT | 192 | 169 | 88.02 | 1.14 (0.90–1.44) | 0.281 | ||
OS, overall survival; HR, hazard ratio; CI, confidence interval; STR, subtotal resection; NTR, near-total resection; GTR, gross total resection; WHO, World Health Organization; ref., reference.
Cox’s proportional hazard regression analysis for univariate and multivariate analysis.
Figure 1.
Kaplan-Meier Analyses for OS and PFS of Two miRNA SNPs
(A–D) Overall survival (OS) of rs3746444 (A) and rs11614913 (B), and progression-free survival (PFS) of rs3746444 (C) and rs11614913 (D).
Relationships among Genotypes, Clinical Variables, and PFS
As shown in Table 5, age, surgery, and chemotherapy were correlated with PFS in a univariate analysis. Therefore, these variables were included in a multivariate analysis. As a result, age, surgery, and chemotherapy were still identified as independent prognostic indicators for PFS in patients with glioma. Compared with those aged <40 years, patients who were older than 40 years exhibited a worse PFS (HR [95% CI] = 1.21 [1.02–1.43], p = 0.030). The patients who received gross total resection had an improved PFS (HR [95% CI] = 0.61 [0.51–0.74], p < 0.001) than that of patients who received subtotal resection or near-total resection. Patients treated with temozolomide also had a better PFS (HR [95% CI] = 0.38 [0.26–0.55], p < 0.001) than that of patients who did not undergo chemotherapy. Significant associations between the two variants (rs11614913 and rs3746444) and PFS were not observed in the univariate Cox regression analysis. Consistent with these findings, there was no difference in PFS among the different genotypes in Kaplan-Meier survival analyses (Figures 1C and 1D).
Table 5.
Univariate and Multivariate Analyses of Associations among Genotypes, Various Factors, and PFS
| Characteristics | Patients (n) | Events (n) | Rate (%) | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Valuea | HR (95% CI) | p Valuea | ||||
| Age (Years) | |||||||
| <40 | 267 | 239 | 89.51 | ref. | ref. | ref. | ref. |
| ≥40 | 338 | 324 | 95.86 | 1.19 (1.00–1.40) | 0.047 | 1.21 (1.02–1.43) | 0.030 |
| Sex | |||||||
| Male | 335 | 310 | 92.54 | ref. | ref. | ||
| Female | 270 | 253 | 93.70 | 1.10 (0.93–1.30) | 0.263 | ||
| WHO Grade | |||||||
| I–II | 382 | 353 | 92.41 | ref. | ref. | ||
| III–IV | 223 | 210 | 94.17 | 1.15 (0.97–1.36) | 0.116 | ||
| Surgery | |||||||
| STR and NTR | 189 | 183 | 96.83 | ref. | ref. | ref. | ref. |
| GTR | 416 | 380 | 91.35 | 0.58 (0.48–0.69) | <0.001 | 0.61 (0.51–0.74) | <0.001 |
| Chemotherapy | |||||||
| None | 355 | 351 | 98.87 | ref. | ref. | ref. | ref. |
| Platinum | 124 | 116 | 93.55 | 0.99 (0.80–1.22) | 0.916 | 0.98 (0.79–1.21) | 0.850 |
| Temozolomide | 52 | 32 | 61.54 | 0.35 (0.24–0.50) | <0.001 | 0.38 (0.26–0.55) | <0.001 |
| Nimustine | 74 | 64 | 86.49 | 0.73 (0.56–0.96) | 0.022 | 0.83 (0.63–1.10) | 0.189 |
| Radiotherapy | |||||||
| None | 60 | 55 | 91.67 | ref. | ref. | ||
| Conformal radiotherapy | 162 | 137 | 84.57 | 1.13 (0.83–1.56) | 0.436 | ||
| Gamma knife | 383 | 371 | 96.87 | 1.21 (0.91–1.60) | 0.199 | ||
| rs3746444 | |||||||
| AA | 391 | 366 | 93.61 | ref. | ref. | ||
| GA | 147 | 132 | 89.80 | 0.92 (0.76–1.13) | 0.434 | ||
| GG | 67 | 65 | 97.01 | 1.27 (0.97–1.65) | 0.078 | ||
| rs11614913 | |||||||
| CC | 139 | 131 | 94.24 | ref. | ref. | ||
| CT | 274 | 254 | 92.70 | 1.00 (0.81–1.24) | 0.985 | ||
| TT | 192 | 173 | 90.10 | 1.06 (0.84–1.32) | 0.641 | ||
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; STR, subtotal resection; NTR, near-total resection; GTR, gross total resection; WHO, World Health Organization; ref., reference.
Cox’s proportional hazard regression analysis for univariate and multivariate analysis.
Enrichment Analyses
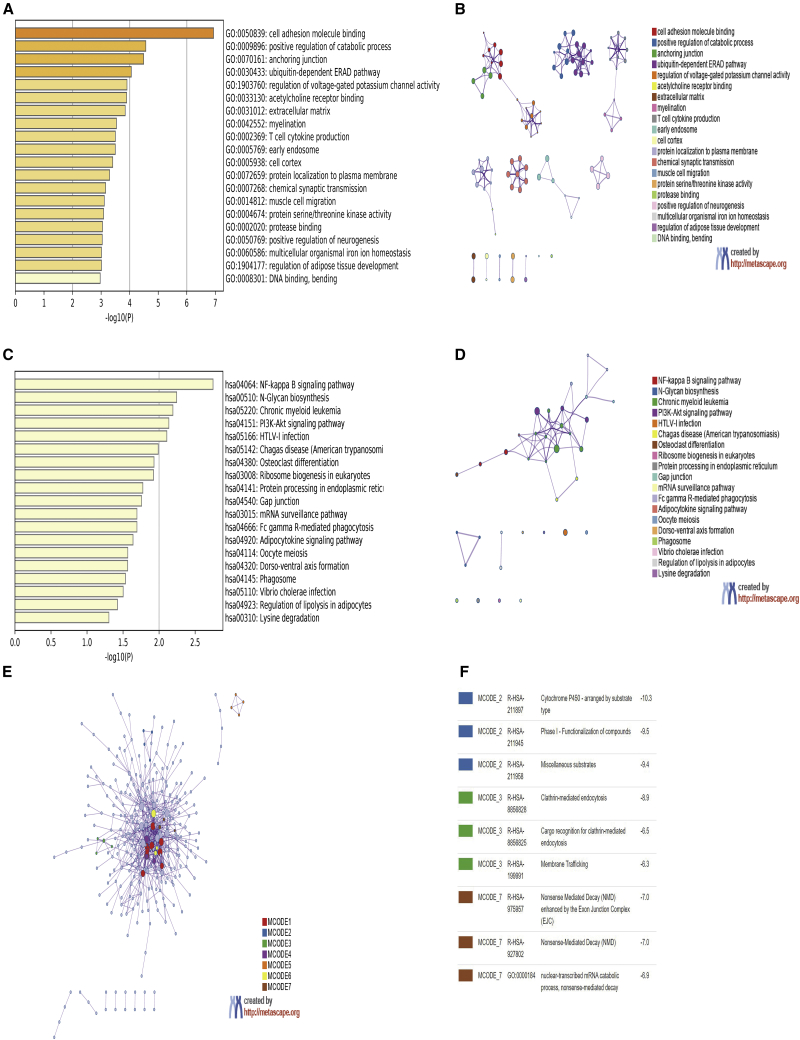
SNPs in miRNA seed sites may alter the target profile, including losses of original targets and gains of new targets. With the use of miRNASNP-v3, we found that rs3746444 in the seed region of miR-499 recognized 573 new target genes (so-called “targets gained”) and lost 5,392 original target genes (so-called “targets lost”). To reveal the potential functional significance of miR-499 rs3746444, enrichment analyses were conducted based on the list of target genes gained. The top 20 enriched functions and pathways are displayed in Figures 2A–2D. A Gene Ontology (GO) analysis revealed that “cell adhesion molecule binding,” “positive regulation of catabolic process,” and “anchoring junction” were the most highly enriched terms. A Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that the targets gained by the rs3746444 polymorphism were mainly involved in the “NF-kappa B pathway,” “PI3K-Akt pathway,” and “mRNA surveillance pathway,” which are cancer-related pathways. A protein-protein interaction (PPI) network and Molecular Complex Detection (MCODE) components are presented in Figures 2E and 2F. The three most significant MCODE components determined from the PPI network were related to “cytochrome P450 arranged by substrate type,” “clathrin-mediated endocytosis,” and “nonsense-mediated decay enhanced by the exon junction complex.”
Figure 2.
Enrichment Analyses of Target Genes Gained by miR-499 rs3746444
(A) Heatmap of top 20 Gene Ontology (GO)-enriched terms. (B) Network of top 20 GO-enriched terms. (C) Heatmap of top 20 Kyoto Encyclopedia of Genes and Genomes (KEGG)-enriched pathways. (D) Network of top 20 KEGG-enriched pathways. (E) A protein-protein interaction (PPI) network and seven most significant MCODE components from the PPI network. (F) Independent functional enrichment analyses of three MCODE components.
Identification of Differentially Expressed Genes between Glioma and Normal Samples
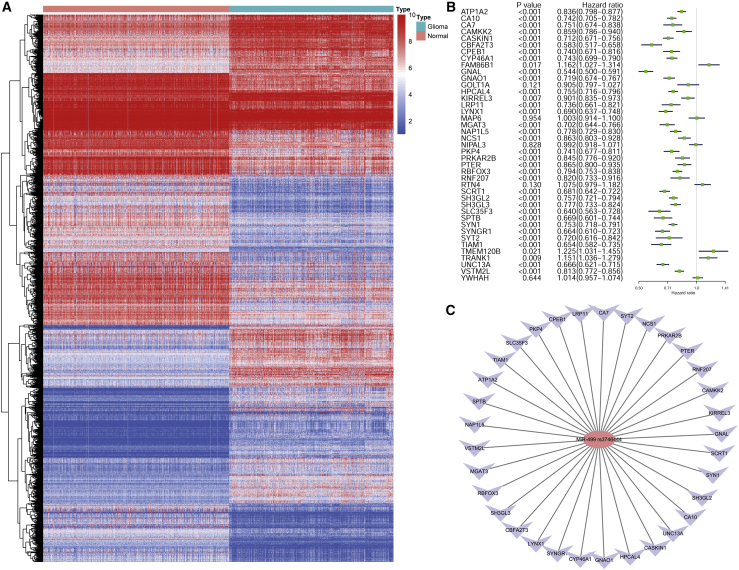
Based on the adjusted p value <0.05 and |log2 (fold change)| >1, a total of 2,158 differentially expressed genes were identified: 1,172 were upregulated, and 986 were downregulated (Figure 3A). By intersecting the 573 predicted target genes of miR-499 rs3746444 and 2,158 differentially expressed genes, 68 genes (27 upregulated genes and 41 downregulated genes) that may play crucial roles in glioma were identified. Since miR-499 can negatively regulate expression levels of target genes, we speculated that 41 downregulated genes were more likely to be target genes of miR-499 rs3746444. To explore the prognostic value of the 41 downregulated genes, a univariate Cox regression analysis was performed, and 36 genes were significantly linked to the OS of glioma patients (p < 0.05; Figure 3B). Among these 36 genes, three genes (TRANK1, FAM86B1, and TMEM120B) were associated with high risk (HR > 1; Figure 3B), and the remaining 33 genes were associated with low risk (HR < 1; Figure 3B). These 36 genes (except for TRANK1, FAM86B1, and TMEM120B) were protective factors for glioma patients. We thus constructed a miRNA-mRNA network (Figure 3C), which provided preliminary insight into the connections between the miR-499 rs3746444 and the 33 mRNAs.
Figure 3.
Identification of Differentially Expressed Genes between Glioma and Normal Samples
(A) Heatmap of differentially expressed genes in glioma and normal samples based on data from the CGGA and GTEx. (B) Forest plot illustrating the hazard ratios, 95% confidence intervals of 41 differentially expressed genes calculated by univariate Cox regression analysis. (C) A regulatory network based on miR-499 rs3746444 and 33 targeted genes. The red node reflects miR-499 rs3746444. Purple nodes reflect 33 targeted genes.
Relationship between Genotype and miRNA Expression
To further explore the effect of rs11614913 and rs3746444 on corresponding miRNA expression, the Genotype-Tissue Expression (GTEx) database (https://www.gtexportal.org/home/index.html) was used. The results demonstrated that the rs3746444 genotype was significantly correlated with miR-499 expression in two brain tissues (all p < 0.001; Figure S1). However, there was no relevant information in brain tissues for rs11614913 in the GTEx database.
Discussion
As the most common type of brain tumor with a poor prognosis and high mortality, glioma has become a serious public health problem. Thus, novel biomarkers for glioma are needed to improve diagnosis and treatment. Emerging evidence indicates that SNPs in the pre-miRNA or seed region of miRNAs are linked to susceptibility in some cancers. We investigated the relationships between miR-196a-2 rs11614913 and miR-499 rs3746444 and glioma risk. miR-196a-2 rs11614913 was correlated with decreased glioma risk, whereas miR-499 rs3746444 was associated with a high risk.
miR-196a-2 belongs to the class miR-196a. SNPs in miR-196a-2 affect the mature expression of miRNA and are implicated in tumor development and progression. Several studies have investigated the role of miR-196a-2 rs11614913 in various cancers. For instance, miR-196a-2 rs11614913 can increase the expression level of mature miR-196a and contribute to colorectal cancer susceptibility.23 Moreover, miR-196a-2 rs11614913 is correlated with breast cancer,24 lung cancer,25 liver cancer,26 and head and neck cancer.27 The effect of miR-196a-2 SNP in glioma has been explored in three studies of Chinese and Indian populations.17, 18, 19 In a southern Chinese population, the SNP was found to be related to a decreased glioma susceptibility (p = 0.035, OR = 0.74).17 In contrast, Hu et al.18 evaluated a northeast Chinese population and found that this SNP increases glioma susceptibility (p = 0.003, OR = 1.25). Sibin et al.19 reported that there is no association between this SNP and glioma susceptibility in the Indian population. In our study, rs11614913 polymorphism was related to decreased glioma risk in the Han Chinese population. These inconsistent findings indicate that the effects of the SNP may depend on the population and ethnicity.
With respect to the miR-499 rs3746444 polymorphism, previous research has established that it is correlated with susceptibility to multiple types of cancer.28, 29, 30 However, two studies have indicated that miR-499 rs3746444 might not be correlated with susceptibility to glioma.18,20 Our results revealed that the rs3746444 SNP increases glioma risk in five genetic models. FPRP analyses were performed to avoid false-positive findings. The results demonstrated that the miR-499 rs3746444 actually increased glioma risk in four genetic models, and miR-196a-2 rs11614913 decreased glioma risk in the recessive model.
The rs3746444 polymorphism is located in the seed region, which could affect miRNA-mRNA interactions and its function. With the use of miRNASNP-v3, an in silico investigation of miRNA-mRNA interactions indicated that target mRNAs are gained or lost in the comparison between the mutant and wild-type genotypes. Therefore, enrichment analyses were conducted based on the 573 target genes gained of miR-499 rs3746444 to determine the possible functional significance of rs3746444 in miR-499. The results indicated that miR-499 rs3746444 plays key roles in various biological processes and pathways correlated with cancer, such as cell adhesion molecule binding, positive regulation of catabolic process, anchoring junction, NF-kappa B pathway, PI3K-Akt pathway, and mRNA surveillance pathway, by targeting mRNAs. Alterations of miRNA-mRNA interactions can affect the expression levels of target mRNAs, thereby contributing to the development of cancer. Therefore, we reasoned that miR-499 rs3746444 might increase glioma susceptibility through the above-mentioned biological processes and pathways via targeting mRNAs. Differential expression and univariate Cox regression analyses were conducted to screen the differentially expressed genes associated with OS from the 573 targeted genes of miR-499 rs3746444. Through these analyses, we found that 33 targeted genes that downregulated between glioma and brain samples were protective factors for glioma patients. A miR-499 rs3746444-mediated network was constructed in this study. It was suggested that miR-499 rs3746444 might promote the progression of glioma by negatively regulating the expression of the 33 genes that could be considered as tumor suppressors. However, given that the results were on the basis of computational biology, further studies are indispensable to verify comprehensive mechanisms of these 33 miRNA-mRNA interactions in glioma. Several studies have focused on the roles and mechanisms of action of SNPs in noncoding regions in the development of cancer.31, 32, 33 For instance, miR-499 rs3746444 contributes to a poor prognosis in lung cancer by regulating cancer-related gene expression and thus is involved in tumorigenesis and cisplatin resistance.33 Further functional experiments are required to verify our hypothesis.
Given the importance of miR-499 rs3746444, we further explored the role of rs3746444 on miR-499 expression by searches against the GTEx database. The expression of miR-499 was higher for the rs3746444 AG genotype than for the rs3746444 AA genotype.
The relationships among genotypes, clinical variables, and prognosis were further investigated. Contrary to our expectation, there were no differences in OS and PFS among the different genotypes of the two miRNA SNPs. In addition, we found that age, surgery, and chemotherapy could serve as independent predictive indicators for OS and PFS.
As we know, this is the first study of relationships between miR-196a-2 rs11614913 and miR-499 rs3746444 and both glioma susceptibility and survival in the Chinese Han population. The strengths of our study include the large sample size and the use of the FPRP test to avoid false-positive findings. Moreover, target gain and loss information for rs3746444 in the miR-499 seed region provides a basis for research on the function and mechanisms of action of miR-499 rs3746444. Our study had some limitations. First, only bioinformatics analyses were performed to predict the functional significance of miR-499 rs3746444 based on miRNA target binding and selection. Further experiments are required to validate the effects of the polymorphism on biological processes and pathways in glioma. Second, all of the participants were enrolled from a hospital located in northwestern China, and all participants were Chinese Han. Therefore, our findings might not apply to other ethnicities. To establish the generalizability of our findings, further studies of different ethnic groups with larger cohorts of subjects are needed.
In summary, our results suggested that miR-499 rs3746444 and miR-196a-2 rs11614913 are biomarkers with effects on glioma susceptibility.
Materials and Methods
Study Participants
A total of 605 glioma patients and 1,300 healthy controls were consecutively enrolled between September 2010 and May 2014. All patients with glioma were diagnosed and confirmed by the histopathological examination of specimens. Moreover, all participants had not previously received chemotherapy or radiotherapy. The clinical characteristics of all subjects were collected, including ethnicity, age, sex, WHO grade, radiotherapy, and chemotherapy. In addition, the patients were followed up for about 5 years by telephone interviews and/or outpatient records. Written, informed consent was acquired from all participants before sampling. By complying with the Declaration of Helsinki, the study was conducted in the Second Affiliated Hospital of Xi’an Jiaotong University and approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University.
Selection of SNPs
miR-499 rs3746444 and miR-196a-2 rs11614913 have been found to be associated with cancer susceptibility in several studies.34,35 Their associations with glioma susceptibility and survival have not been thoroughly investigated. miR-196a-2 rs11614913 and miR-499 rs3746444 were selected for further analysis. Information for the SNPs is presented in Table S1. The exact locations of rs11614913 and rs3746444 were identified using miRNASNP-v3.36 The rs11614913 is located in the mature region of hsa-mir-196a-2. The rs3746444 is located in the seed region of hsa-miR-499-3p.
DNA Extraction and Genotyping
Peripheral blood samples were collected from the subjects. Genomic DNA was extracted from peripheral blood using the Universal Genomic DNA Extraction Kit (TaKaRa, Kyoto, Japan)37 and measured by spectrophotometry (DU530 UV-visible [vis] spectrophotometer; Beckman Instruments, Fullerton, CA, USA).38 The Multiplexed SNP Mass Extend assay was designed using Sequenom Mass Array Assay Design (version 3.0; Agena Bioscience, San Diego, CA, USA).39 Sequenom Mass Array RS1000 and Sequenom Typer 4.0 were used for SNP genotyping and data analyses.40,41 The primers for each SNP are provided in Table S2.
Bioinformatics Analyses
miRNASNP-v3 (http://bioinfo.life.hust.edu.cn/miRNASNP/#!/) provides information about SNPs, as well as genes with conserved sites that are lost and gained by SNPs in the seed region of miRNA.36 An enrichment analysis of target genes gained by SNPs in the seed region may provide insight into the mechanisms underlying the effects of the SNP. GO and KEGG enrichment analyses were conducted using Metascape (http://metascape.org).42 Terms with p <0.05, a minimum count of 3, and an enrichment factor of >1.5 were considered statistically significant. If >20 GO terms or pathways were determined, then the top 20 were selected for visualization. Moreover, to understand the relationships between target mRNAs, a PPI network was constructed and visualized using Metascape, and the MCODE algorithm was employed to determine densely connected regions.42
RNA sequencing data of Chinese glioma patients were downloaded from Chinese Glioma Genome Atlas (CGGA; http://www.cgga.org.cn/). RNA sequencing data of normal brain tissue from the GTEx were downloaded from University of California Santa Cruz (UCSC) Xena (https://xena.ucsc.edu/). The CGGA database included 1,018 glioma samples without normal brain samples, and the GTEx database contained 1,152 normal brain samples. These data were merged to obtain differentially expressed genes between tumor and normal samples. By using R, a differential expression analysis was conducted, with an adjusted p value <0.05 and |log2 (fold change)| >1 as the thresholds. Univariate Cox regression analyses were used to identify the differentially expressed genes associated with OS (p < 0.05). To further explore the role of the SNP on miRNA expression, the GTEx database was used for searches.
Statistical Analyses
Data analyses were conducted using R (version 3.5.1). Hardy-Weinberg equilibrium (HWE) was assessed using χ2 tests,38 where p >0.05 indicated a balanced equilibrium. Five genetic models were used to evaluate relationships between SNPs and glioma risk. In addition, FPRP values were calculated to validate significant associations in the study.43 We assigned a prior probability of 0.1 to detect an OR of 1.5 (or 0.67, for protective effect) for each SNP, according to the previous study.44 Only FPRP <0.2 was considered “noteworthy.” Univariate and multivariate Cox regression analyses were performed to explore relationships among genotypes, clinical variables, and prognosis. To evaluate the effects of the genotypes on the OS and PFS in patients with glioma, Kaplan-Meier survival analyses were further performed. All statistical tests were two sided, and the results were considered statistically significant at p <0.05.
Author Contributions
S.Y., Y.Z., and L.Z. collected the data and performed the statistical analysis. S.Y. wrote the manuscript. J.J. and Y.D. conducted some experiments and prepared the figures and tables. J.Y., P.Y., and L.Y. conducted some experiments. Y.W., Z.Z., N.L., and L.L. performed some experiments. Z.D. designed the study and revised the manuscript. All authors read, reviewed, and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We thank the members of our study team for their support.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2020.08.038.
Supplemental Information
References
- 1.Porter K.R., McCarthy B.J., Freels S., Kim Y., Davis F.G. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro-oncol. 2010;12:520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 3.Weller M., van den Bent M., Tonn J.C., Stupp R., Preusser M., Cohen-Jonathan-Moyal E., Henriksson R., Le Rhun E., Balana C., Chinot O., European Association for Neuro-Oncology (EANO) Task Force on Gliomas European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30194-8. [DOI] [PubMed] [Google Scholar]
- 4.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai E.C. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 6.Hausser J., Syed A.P., Bilen B., Zavolan M. Analysis of CDS-located miRNA target sites suggests that they can effectively inhibit translation. Genome Res. 2013;23:604–615. doi: 10.1101/gr.139758.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lytle J.R., Yario T.A., Steitz J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA. 2007;104:9667–9672. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent O.A., Mendell J.T. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene. 2006;25:6188–6196. doi: 10.1038/sj.onc.1209913. [DOI] [PubMed] [Google Scholar]
- 9.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 10.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Ryan B.M., Robles A.I., Harris C.C. Genetic variation in microRNA networks: the implications for cancer research. Nat. Rev. Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slaby O., Bienertova-Vasku J., Svoboda M., Vyzula R. Genetic polymorphisms and microRNAs: new direction in molecular epidemiology of solid cancer. J. Cell. Mol. Med. 2012;16:8–21. doi: 10.1111/j.1582-4934.2011.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georges M., Coppieters W., Charlier C. Polymorphic miRNA-mediated gene regulation: contribution to phenotypic variation and disease. Curr. Opin. Genet. Dev. 2007;17:166–176. doi: 10.1016/j.gde.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Zorc M., Skok D.J., Godnic I., Calin G.A., Horvat S., Jiang Z., Dovc P., Kunej T. Catalog of microRNA seed polymorphisms in vertebrates. PLoS ONE. 2012;7:e30737. doi: 10.1371/journal.pone.0030737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicoloso M.S., Sun H., Spizzo R., Kim H., Wickramasinghe P., Shimizu M., Wojcik S.E., Ferdin J., Kunej T., Xiao L. Single-nucleotide polymorphisms inside microRNA target sites influence tumor susceptibility. Cancer Res. 2010;70:2789–2798. doi: 10.1158/0008-5472.CAN-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan Y., Mizoguchi M., Yoshimoto K., Hata N., Shono T., Suzuki S.O., Araki Y., Kuga D., Nakamizo A., Amano T. MiRNA-196 is upregulated in glioblastoma but not in anaplastic astrocytoma and has prognostic significance. Clin. Cancer Res. 2010;16:4289–4297. doi: 10.1158/1078-0432.CCR-10-0207. [DOI] [PubMed] [Google Scholar]
- 17.Dou T., Wu Q., Chen X., Ribas J., Ni X., Tang C., Huang F., Zhou L., Lu D. A polymorphism of microRNA196a genome region was associated with decreased risk of glioma in Chinese population. J. Cancer Res. Clin. Oncol. 2010;136:1853–1859. doi: 10.1007/s00432-010-0844-5. [DOI] [PubMed] [Google Scholar]
- 18.Hu E., Wang D., Zhang X., Li J., Hu Y., Gong H., Liu E. Four common polymorphisms in microRNAs and the risk of adult glioma in a Chinese case-control study. J. Mol. Neurosci. 2013;51:933–940. doi: 10.1007/s12031-013-9980-0. [DOI] [PubMed] [Google Scholar]
- 19.Sibin M.K., Harshitha S.M., Narasingarao K.V., Dhananjaya I.B., Dhaval P.S., Chetan G.K. Effect of rs11614913 Polymorphism on Mature miR196a2 Expression and its Target Gene HOXC8 Expression in Human Glioma. J. Mol. Neurosci. 2017;61:144–151. doi: 10.1007/s12031-016-0855-z. [DOI] [PubMed] [Google Scholar]
- 20.Lim J., Kim J.O., Park H.S., Han I.B., Kwack K., Kim N.K., Cho K. Associations of miR-146aC>G, miR-149C>T, miR-196a2C>T and miR-499A>G polymorphisms with brain tumors. Oncol. Rep. 2018;40:1813–1823. doi: 10.3892/or.2018.6557. [DOI] [PubMed] [Google Scholar]
- 21.Rogoveanu I., Burada F., Cucu M.G., Vere C.C., Ioana M., Cîmpeanu R.A. Association of microRNA Polymorphisms with the Risk of Gastric Cancer in a Romanian Population. J. Gastrointestin. Liver Dis. 2017;26:231–238. doi: 10.15403/jgld.2014.1121.263.rog. [DOI] [PubMed] [Google Scholar]
- 22.Omrani M., Hashemi M., Eskandari-Nasab E., Hasani S.S., Mashhadi M.A., Arbabi F., Taheri M. hsa-mir-499 rs3746444 gene polymorphism is associated with susceptibility to breast cancer in an Iranian population. Biomarkers Med. 2014;8:259–267. doi: 10.2217/bmm.13.118. [DOI] [PubMed] [Google Scholar]
- 23.Zhan J.F., Chen L.H., Chen Z.X., Yuan Y.W., Xie G.Z., Sun A.M., Liu Y. A functional variant in microRNA-196a2 is associated with susceptibility of colorectal cancer in a Chinese population. Arch. Med. Res. 2011;42:144–148. doi: 10.1016/j.arcmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Dai Z.J., Shao Y.P., Wang X.J., Xu D., Kang H.F., Ren H.T., Min W.L., Lin S., Wang M., Song Z.J. Five common functional polymorphisms in microRNAs (rs2910164, rs2292832, rs11614913, rs3746444, rs895819) and the susceptibility to breast cancer: evidence from 8361 cancer cases and 8504 controls. Curr. Pharm. Des. 2015;21:1455–1463. doi: 10.2174/1381612821666141208143533. [DOI] [PubMed] [Google Scholar]
- 25.Tian T., Shu Y., Chen J., Hu Z., Xu L., Jin G., Liang J., Liu P., Zhou X., Miao R. A functional genetic variant in microRNA-196a2 is associated with increased susceptibility of lung cancer in Chinese. Cancer Epidemiol. Biomarkers Prev. 2009;18:1183–1187. doi: 10.1158/1055-9965.EPI-08-0814. [DOI] [PubMed] [Google Scholar]
- 26.Akkız H., Bayram S., Bekar A., Akgöllü E., Ulger Y. A functional polymorphism in pre-microRNA-196a-2 contributes to the susceptibility of hepatocellular carcinoma in a Turkish population: a case-control study. J. Viral Hepat. 2011;18:e399–e407. doi: 10.1111/j.1365-2893.2010.01414.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., He A., Liu B., Zhong Y., Liao X., Yang J., Chen J., Wu J., Mei H. rs11614913 polymorphism in miRNA-196a2 and cancer risk: an updated meta-analysis. OncoTargets Ther. 2018;11:1121–1139. doi: 10.2147/OTT.S154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Yang B., Ren X. Hsa-miR-499 polymorphism (rs3746444) and cancer risk: a meta-analysis of 17 case-control studies. Gene. 2012;509:267–272. doi: 10.1016/j.gene.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Min K.T., Kim J.W., Jeon Y.J., Jang M.J., Chong S.Y., Oh D., Kim N.K. Association of the miR-146aC>G, 149C>T, 196a2C>T, and 499A>G polymorphisms with colorectal cancer in the Korean population. Mol. Carcinog. 2012;51(Suppl 1):E65–E73. doi: 10.1002/mc.21849. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Y., Fan S., Cao J., Huang S., Zhang L.P. Association of the microRNA-499 variants with susceptibility to hepatocellular carcinoma in a Chinese population. Mol. Biol. Rep. 2012;39:7019–7023. doi: 10.1007/s11033-012-1532-0. [DOI] [PubMed] [Google Scholar]
- 31.Hoffman A.E., Zheng T., Yi C., Leaderer D., Weidhaas J., Slack F., Zhang Y., Paranjape T., Zhu Y. microRNA miR-196a-2 and breast cancer: a genetic and epigenetic association study and functional analysis. Cancer Res. 2009;69:5970–5977. doi: 10.1158/0008-5472.CAN-09-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng J., Huang X., Tan W., Yu D., Du Z., Chang J., Wei L., Han Y., Wang C., Che X. Pancreatic cancer risk variant in LINC00673 creates a miR-1231 binding site and interferes with PTPN11 degradation. Nat. Genet. 2016;48:747–757. doi: 10.1038/ng.3568. [DOI] [PubMed] [Google Scholar]
- 33.Qiu F., Yang L., Ling X., Yang R., Yang X., Zhang L., Fang W., Xie C., Huang D., Zhou Y., Lu J. Sequence Variation in Mature MicroRNA-499 Confers Unfavorable Prognosis of Lung Cancer Patients Treated with Platinum-Based Chemotherapy. Clin. Cancer Res. 2015;21:1602–1613. doi: 10.1158/1078-0432.CCR-14-1174. [DOI] [PubMed] [Google Scholar]
- 34.Yan W., Gao X., Zhang S. Association of miR-196a2 rs11614913 and miR-499 rs3746444 polymorphisms with cancer risk: a meta-analysis. Oncotarget. 2017;8:114344–114359. doi: 10.18632/oncotarget.22547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu K., Wu Z.Z., Yu J.P., Guo W., Wu N., Wei L.J., Zhang H., Zhao J., Liu J.T. Meta-analysis of the association between three microRNA polymorphisms and breast cancer susceptibility. Oncotarget. 2017;8:68809–68824. doi: 10.18632/oncotarget.18516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong J., Liu C., Liu W., Wu Y., Ma Z., Chen H., Guo A.-Y. An update of miRNASNP database for better SNP selection by GWAS data, miRNA expression and online tools. Database (Oxford) 2015;2015:bav029. doi: 10.1093/database/bav029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng Y., Zhou L., Li N., Wang M., Yao L., Dong S., Zhang M., Yang P., Hao Q., Wu Y. Impact of four lncRNA polymorphisms (rs2151280, rs7763881, rs1136410, and rs3787016) on glioma risk and prognosis: A case-control study. Mol. Carcinog. 2019;58:2218–2229. doi: 10.1002/mc.23110. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L., Dong S., Deng Y., Yang P., Zheng Y., Yao L., Zhang M., Yang S., Wu Y., Zhai Z. GOLGA7 rs11337, a Polymorphism at the MicroRNA Binding Site, Is Associated with Glioma Prognosis. Mol. Ther. Nucleic Acids. 2019;18:56–65. doi: 10.1016/j.omtn.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai Z., Tian T., Wang M., Yang T., Li H., Lin S., Hao Q., Xu P., Deng Y., Zhou L. Genetic polymorphisms of estrogen receptor genes are associated with breast cancer susceptibility in Chinese women. Cancer Cell Int. 2019;19:11. doi: 10.1186/s12935-019-0727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabriel S., Ziaugra L., Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr. Protoc. Hum. Genet. 2009 doi: 10.1002/0471142905.hg0212s60. Chapter 2, Unit 2.12. [DOI] [PubMed] [Google Scholar]
- 41.Thomas R.K., Baker A.C., Debiasi R.M., Winckler W., Laframboise T., Lin W.M., Wang M., Feng W., Zander T., MacConaill L. High-throughput oncogene mutation profiling in human cancer. Nat. Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y., Zhou B., Pache L., Chang M., Khodabakhshi A.H., Tanaseichuk O., Benner C., Chanda S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019;10:1523. doi: 10.1038/s41467-019-09234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wacholder S., Chanock S., Garcia-Closas M., El Ghormli L., Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J. Natl. Cancer Inst. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelemen L.E., Sellers T.A., Schildkraut J.M., Cunningham J.M., Vierkant R.A., Pankratz V.S., Fredericksen Z.S., Gadre M.K., Rider D.N., Liebow M., Goode E.L. Genetic variation in the one-carbon transfer pathway and ovarian cancer risk. Cancer Res. 2008;68:2498–2506. doi: 10.1158/0008-5472.CAN-07-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.