Abstract
Magnesium oxide remained interesting from long time for several important phenomena like; defect induced magnetism, spin electron reflectivity, broad laser emission etc. Moreover, nanostructures of this material exhibited suitability for different kinds of applications ranging from wastewater treatment to spintronics depending upon their shape and size. In this way, researchers had grown nanostructures in the form of nanoparticles, thin films, nanotubes, nanowalls, nanobelts. Though nanoparticles and thin films are well known form of nanostructures and wide variety of synthesis approaches are available, however, limited methodology for other nanostructures are available. In order to grow these nanostructures in an optimized way an understanding of these methods is essential. Thus, this review article depicts an overview of various approaches for design of different kinds of nanostructures.
Keywords: Materials science, Nanotechnology, MgO, Thin films, Nanotubes, Nanoparticles, Nanobelts, Nanowalls
Materials science, Nanotechnology, MgO, Thin films, Nanotubes, Nanoparticles, Nanobelts, Nanowalls
1. Introduction
With the discovery of carbon nanotubes [1], development of nanostructures of different materials have evoked much attention for their fabrication, characterization and applications in various fields [2, 3, 4]. The unique properties of these nanostructures are not only intriguing for investigation of underlying phenomena but also opened several dimensions for their utilization in different technologies [5, 6, 7, 8]. Nanostructured oxides of different shape gain significant attentions in last two decades. Apart from nanoparticles, and thin films, most desirable form of nanostructures are nanocubes, nanorod and nanoflowers. These kinds of nanostructures and their applications are studied for numerous oxide systems. Depending upon the morphology, these form of nanostructures exhibit superiority over the nanoparticles and thin films. Hence, this review article is motivated to understand the growth behavior of different kind of nanostructures by considering MgO as a prototype system.
1.1. Nanostructures
Nanostructures represent a wide category of materials having dimensions less than 100 nm, which includes nanoparticles, thin films, nanotubes and nanorods [9, 10, 11]. Figure 1 depicts various category of nanostructures. It is clear from the figure that nanostructures have at least one dimension of the order of nm.
Figure 1.
Category of nanostructures: (a) nanoparticles, (b) thin film, (c) nanorod, (d) nanoplate, (e) nanotube and (f) tetrapod.
Due to small dimension of nanostructures, some exciting phenomena like quantum confinement [12, 13, 14, 15] and phonon confinement in these nanostructures take place [16, 17, 18]. This causes modulation in the optical behavior of nanostructures of various materials like Au, Ag [19], ZnO [20] and similar other systems [21,22]. Thus, these nanostructures exhibit completely different behavior compared to bulk counterpart. The modulated behavior of nanostructures is not limited to the optical properties but also reported for magnetic [23,24], electrical [25,26] and thermal properties [27,28]. For example, paramagnetic zinc ferrite (with large particle size) transforms into ferromagnetic when synthesized in the form of nanoparticles [29,30] or thin films [31]. Nanoparticles and thin films of several non-magnetic oxides are also reported to exhibit ferromagnetic ordering [32, 33, 34].
1.2. Specific phenomena observed in MgO nanostructures
Among the other non-magnetic oxides, magnesium oxide has become interesting from last two decades due to existence of several phenomena in it's nanostructures. Figure 2 summarizes phenomena that usually observed in the nanostructures of this material.
Figure 2.
Some interesting phenomena observed in MgO nanostructures.
Among the various non-magnetic oxide system, MgO is a technologically important material because of its simple crystal structure and complete absence of d orbital electrons, which persists way of understanding several physical [35, 36, 37] and chemical behavior [38, 39, 40, 41].
Bulk MgO is highly insulating material with a very high optical band-gap of ~7.6 eV [42], however, nanostructures of this material exhibit modified optical band-gap. The optical band-gap of MgO nanoparticles of size 7 nm is 2.8 eV [43]. One-dimensional nanostructure of this material shows an optical band-gap of 3.2 eV [44]. Similar value of optical band-gap is observed for MgO nanocubes [45].
Though, bulk MgO and thin films exhibit dielectric constant of 10 [46, 47, 48], but this material attains high value of dielectric constant depending upon the morphology of nanostructure [49, 50, 51].
Spin-dependent electron reflection is reported in MgO thin films grown on Fe substrate. The electron reflectivity exhibits quantum interference from which two MgO energy bands with Δ1 symmetry were determined in the experiment [52, 53, 54].
Resistive switching is observed in MgO based structures [55,56]. Ferromagnetism combining with multilevel switching characteristics is also reported in MgO capacitor [57,58]. Room-temperature broadband laser emission in the near ultraviolet to the blue-green spectral range was observed in MgO microcrystals obtained through a solid phase reaction between SiO and Mg at 450 °C in an Ar atmosphere [59,60]. Luminescence is another phenomenon observed in nanocrystals of MgO [61, 62, 63]. Other categories of luminescence like photoluminescence [64,65], electroluminescence [66], radioluminescence [67] and thermoluminescence [68] are also reported in MgO nanostructures.
It is observed that the presence of these phenomena do not only exist at reduced dimensions, but also reflect when defects accumulate in this material. For example; thin films and nanoparticles of magnetism oxide show magnetism associated with surface and extended defects [69, 70, 71]. Moreover, luminescent behavior of nanostructured MgO is tailored by controlling the defect states in the material [72, 73, 74].
Cataluminescence is another important phenomenon that occurs in MgO based nanostructures and persists a way for utilization as gas sensor [75, 76, 77].
1.3. Application of MgO nanostructures
Nanostructures of MgO are not only known for the observation of numerous phenomena as elaborated in section 1.2 but also because of significant enhancement of their applications in a variety of fields. MgO nanostructures are used as ultra-violet (UV) photodetector [78]. The photocatalytic activity of MgO nanoparticles are being utilized for degradation of methyl orange and methylene blue dyes under UV light irradiation [79].
It is used as protective layer in alternating current (AC) plasma display [80] and as a substrate for chemical reactions [81, 82, 83]. The catalytic [84,85] and antibacterial activities [86,87] of MgO have attracted a significant scientific interest in recent years.
MgO also finds applications in pharmaceutical preparation, when mixed with drug that is unstable in acid, demonstrates high stabilizing effects on the drug [88]. Moreover, shape of nanostructures plays important role for diverse therapeutic applications [89]. It is used as an antiseptic compound while mixed with iodine in ratio of 97:3 [90]. The nanoparticles of MgO exhibit potential for environmental and biomedical applications [91, 92, 93]. MgO nanoparticles also show toxicity (17%) towards the cancer cell, which enables their possible utilization for cancer treatment [94, 95, 96].
MgO is also utilized in wastewater treatment for removal of chloride ions [97] as well as precipitation of phosphors and nitrogen [98,99]. Porous MgO microrods are effective for removal of Pb and Cr [100,101]. The capacity of removal of similar heavy metal ions is influenced by morphology of MgO nanostructures [102, 103, 104, 105]. Incorporation of MgO nanoparticles helps to carbon, graphene oxide and Si to improve it's efficiency for CO2 capture [106, 107, 108]. CO2 capture efficiency is influenced by the shape of nanostructure [109].
It is being utilized for electrical insulation [110,111]. Magnetic property of this material make enable its use in low-field magnetic sensor [70]. Nevertheless, utilization of MgO as a barrier layer in magnetic tunnel junction has increased its importance in the field of spintronics [112,113].
Surface of this material has become promising for splitting of organic molecules like ethanol and methanol, which is helpful for production of H2 [114,115]. Prediction of water splitting on MgO thin film/nanoparticle surface is also made [116,117], which upon realization will be a boon for the production of clean energy.
MgO coating on cathode materials is effective to enhance electrochemical performance of rechargeable batteries [118, 119, 120]. Similarly, it's coating on selected anode materials for various rechargeable batteries is found effective to improve the performance [121,122]. In addition to above applications, this material is widely studied for understanding behaviors of materials under planetary conditions [123,124].
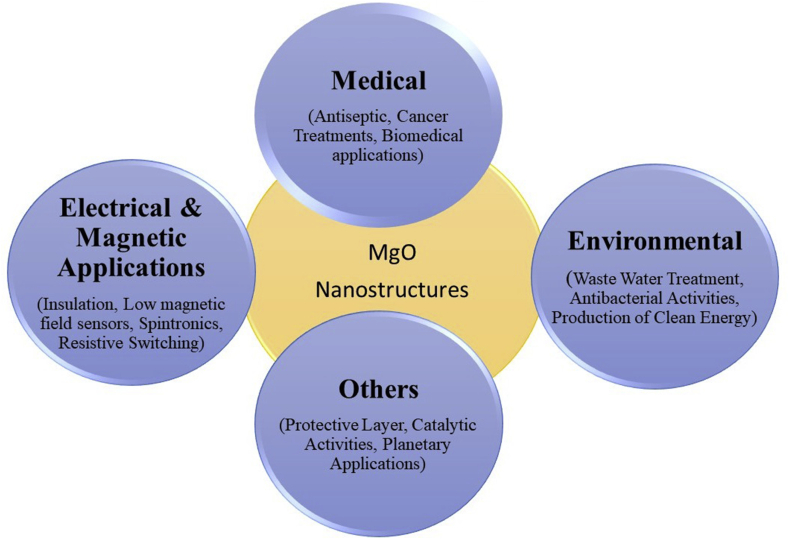
Figure 3 depicts the important field where nanostructures of this material play important role.
Figure 3.
The schematic is depicting the importance of MgO nanostructures in numerous field.
Thus, this material exhibits interesting behavior and diverse applications depending on nature of nanostructures. The deposition or synthesis methods play important role in determining the nature of nanostructures [125, 126, 127]. Thus, there is need to get profound understanding of these methods. Hence, this review article covers the approaches to design various types of nanostructures of MgO for their utilization in diverse applications.
2. Nanoparticles
Nanoparticles are synthesized using various approaches, here; some processes are depicted in the following paragraphs. The objective of these synthesis methods is to get control over size, size distribution and shape of nanoparticles [128, 129, 130].
2.1. Sol-gel process
This method involves any water-soluble salt of magnesium. The solution of this slat is mixed with appropriate organic host and is converted to gel by stirring or annealing. The gel is further heated overnight at temperature of around 100 °C to form precursor. This precursor is annealed at various temperatures to get nanoparticles of different size. Wahab et al. utilizes this approach for synthesizing MgO nanoparticles of size ranging from 50-70 nm. These authors used magnesium nitrate as starting material and sodium hydroxide as organic host [131]. The same approach was used by Fernandez et al. by utilizing magnesium citrate as host [132]. Our group utilizes magnesium nitrate and host citric acid for synthesis of MgO nanoparticles in the size ranging from 9-35 nm [133]. Variation of annealing temperature provide a way to control size of nanoparticles [133,134]. We have depicted the simple process to get these nanoparticles from magnesium nitrate. SEM analysis these nanoparticles have size of almost 30 nm (see Figure 4).
Figure 4.
SEM Image of MgO nanoparticles synthesized from magnesium nitrate. Adapted from Ref. [133] with permission from The Royal Society of Chemistry.
2.2. Ultrasonic methods
These methods utilize ultrasonic waves to make solution homogenous. The other advantage of ultrasound radiation is that, it yields smaller particles. The effects of ultrasonic radiation on chemical reactions are due to the very high temperatures and pressures that develop during the sono-chemical cavity collapse by acoustic cavitation [135, 136, 137]. Figure 5 shows a scheme that takes place during the formation of MgO nanoparticles under influence of ultrasonic irradiation [136]. MgO nanoparticles with different sizes are obtained through calcining.
Figure 5.
The mechanism of MgO formation. Reprinted from Ultrasonics Sonochemistry, 17/2, Mohammad Amin Alavi, Ali Morsali, Syntheses and characterization of Mg(OH)2 and MgO nanostructures by ultrasonic method, 441–446, Copyright (2020), with permission from Elsevier [136].
The simple process of synthesizing these nanoparticles using this method is adopted by number of researchers [138, 139, 140, 141].
2.3. Green synthesis
Green synthesis techniques utilize pollutant free chemicals like as water, natural extracts for the synthesis of nanoparticles [142]. Morrthy et. al reported synthesis of MgO nanoparticles using Neem leaves [143]. Figure 6 depicts procedure for the synthesis of MgO nanoparticles using Artemisia abrotanum Herba Extract [144].
Figure 6.
Synthesis of MgO nanoparticles using Artemisia abrotanum Herba Extract. Redrawn from Iranian Journal of Science and Technology, Transactions A: Science, 42, Renata Dobrucka, Synthesis of MgO Nanoparticles Using Artemisia abrotanum Herba Extract and Their Antioxidant and Photocatalytic Properties, 547–555, Copyright (2016), from Springer Nature [144].
Some other host which are effectively utilized for synthesis of MgO nanoparticles are Nephelium lappaceum L. peels [145], orange fruit [146], Aqueous Eucalyptus globulus leaf [147] and Medicinal Plant Pisonia grandis R. Br. Leaf [148].
Other chemical methods which are widely adopted for synthesizing MgO nanostructure are facile [149,150] and micro-emulsion [151, 152, 153]. Though, chemical methods are cost effective and known to provide better control over particle size and size distribution but the particles obtained by these methods are expected to have carbecanous impurities. These kinds of impurities are reflected from near edge X-ray fine structure [154] and Fourier transform infrared spectroscopy [155, 156, 157]. Though specific methodology is adopted by researchers to remove these nanoparticles to enhance their performance [158] but still their effect cannot be neglected.
Other approaches, which are used to make nanoparticles free from carbecanous impurities, are based on solid-state reaction [159], however, synthesis of MgO nanoparticles using these methods are very limited. Kamrulzaman et. al. reported synthesis of MgO nanoparticles of size ranging from 20 to 135 nm from solid state reaction method by employing magnesium acetate tetrhydrate as starting material [160]. Similar approaches for synthesis of MgO nanoparticles were utilized by Zhang et al. (2019) [161] and Guo et al. (2020) [162].
3. Thin films
The other well-known form of nanostructure is thin film. Thin films are layer of material supported on other material, known as substrate. The property of thin film is determined by stoichiometric proportion, lattice mismatch [163, 164, 165, 166], nature of growth [167] and stress developed film substrate [168]. Thus, numerous methods have been developed to grow thin films of MgO by researchers, which are discussed in recent work from our group and summarized as below.
E-beam evaporation method utilizes evaporation of material target with e-beam energy. This method is used to grow MgO thin films on air-cleaved (001) surfaces of LiF, NaCI, and KCI using a 6-kW electron gun [169, 170, 171]. It observed that MgO on (001) surfaces of LiF, NaCI, and KCI grows with parallel orientation in the temperature range 25–250 °C [172]. e-beam evaporation was used to grow MgO dielectric layer on glass substrate [173] as well as to grow MgO barrier layer for MgO based magnetic tunnel junctions [174] and multilayer structure [175]. This method is utilized by our group for growth of MgO thin films on quartz substrate [176] and Si substrate having thickness 5 and 50 nm [177]. The films grown on Si substrate are amorphous (5 nm) and crystalline (50 nm), in nature respectively [178]. Though films grown on quartz substrate are amorphous in nature but near edge X-ray absorption fine structure measurements revealed spectral features associated with MgO formation (Figure 7). The spectral features at 532, 538, 542 and 552 eV in the O K-edge spectra at different angles, 10, 45 and 70 °C are characteristics of MgO.
Figure 7.
O K-edge NEXAFS spectra of MgO thin film on quartz substrate in total fluorescence mode at 10, 45 and 70o.
Molecular beam epitaxy (MBE) provides better control over stoichiometry ratio but also helpful in epitaxial growth (Figure 7b). This technique is effectively used to grow MgO single crystals [179], epitaxial MgO films on single crystal Si(001) [180,181].
Pulsed Laser Deposition (PLD) utilizes laser beam to sputter molecules from target and used for growing epitaxial growth mode of MgO (111) films on yttrium stabilized zirconia (111) substrates [182], MgO films on Si [183] and Al2O3 substrates [184].
Presently most desired application of MgO is its utilization as a barrier for magnetic tunnel junction. RF sputtering method is preferred choice for this [185,186] as well as for other applications [80,187]. These films were prepared on a Si(001) substrate by the rf sputtering method at low ambient pressure using a metal target [186] and from MgO target [188,189]. Our group has successfully utilized sputtering method to grow MgO thin films on quartz [190] and Si substrate [191,192]. Deposition time plays important role for controlling film thickness along with sputtering power. On the other hand, annealing improves surface of these films [193]. The nature of these films is influenced by ageing [194], annealing environment [195], irradiation [196] and implantation [197].
Deposition methods such as chemical vapor deposition [198, 199, 200] and atomic layer deposition methods [201,202] are also being utilized to grow MgO thin films. However, these methods provide better control over stoichiometry and growth, but need a dedicated instrument. Thus, cost-effective approaches for growing MgO thin films are also being developed. In this context, Yoon et al. utilized sol-gel method to grow MgO thin films on Si substrate [183].
4. Nanocubes
MgO nanocubes are prepared using domestic microwave oven operated at 2.45 GHz and 1000 W [203]. To synthesize these nanocubes, Mg chips and steel-wool were used as starting materials (Figure 8).
Figure 8.
The schematic of MgO nanocube formation. Drawn on the basis of the experimental procedure depicted in reference [203].
Another approach, which is utilized to synthesize MgO nanocubes is arc discharge method. Figure 9 shows schematic of arc discharge apparatus [204].
Figure 9.
The schematic of arc discharge methods. Reprinted from Materials Letters, 65/1, Yanjie Su, Hao Wei, Zhihua Zhou, Zhi Yang, Liangmin Wei, Yafei Zhang, Rapid synthesis and characterization of magnesium oxide nanocubes via DC arc discharge, 100–103, Copyright (2011), with permission from Elsevier [204].
Figure 10.
Schematic representation of the flow reactor system for the production of highly dispersed metal oxides. Redrawn from Surface Science, 290/3, E. Knözinger, Karl-Heinz Jacob, S. Singh, P. Hofmann, Hydroxyl groups as IR active surface probes on MgO crystallites, 388–402, Copyright (1993), with permission from Elsevier [209].
MgO nanocubes are extensively studied by group of O. Diwald [205, 206, 207, 208] by chemical vapor synthesis (CVS). This method involves a reactor consists of two concentrical quartz glass tubes placed inside a cylindrical furnace, termed as CVS reactor (Figure 10) and utilized long-back a group of researcher long back to grow highly dispersed MgO particles [209].
Nanocubes of MgO were also synthesized by burning Mg chips and Mg ribben in air [210, 211, 212]. These nanocubes can be further transformed into nanobars under water exposure followed by vacuum annealing [213].
5. Hollow-spheres
MgO hollow nanospheres were produced via one-step laser synthesis in both gas and liquid media. In situ Kirkendall effect is responsible for the formation of the hollow nanospheres [214].
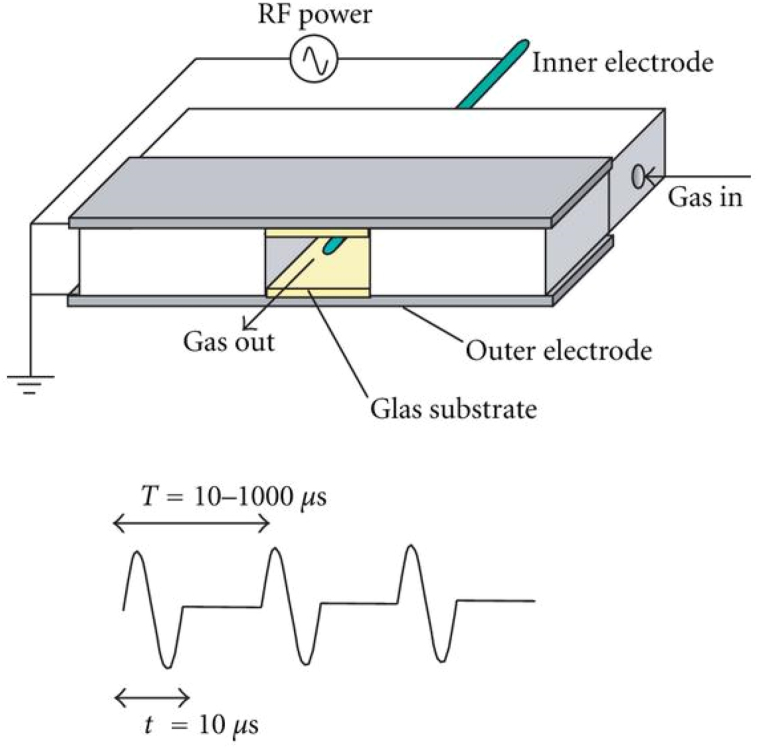
Nanoparticle-built MgO hollow microspheres were synthesized through a template-free hydrothermal route using citrate as a structural director. Zn was introduced into MgO to improve the surface charge [215]. Lizuka and Muroka use RF impulse discharge plasma for synthesis of MgO hollow sphere [216]. Figure 11 shows the schematic of equipment used for synthesizing hollow sphere using this method.
Figure 11.
The experimental configuration RF impulse discharge plasma method. Adapted from S. Iizuka, T. Muraoka, Single-crystal MgO hollow nanospheres formed in RF impulse discharge plasmas, J. Nanomater, 2012 (2012) 691874. Copyright © 2012 Satoru Iizuka and Takumasa Muraoka [216].
6. Nanowires
Nanowires are most common form other than nanoparticles and thin films, which get more attention by researchers. Yin et al. reported a vapor phase method for generation of MgO nanowires from MgB2 source. Figure 12 shows the schematic of formation of MgO nanowires by this group [217].
Figure 12.
Schematic illustration of the apparatus used for the synthesis of single-crystalline nanowires. Redrawn from Advanced Functional Materials, 12/4, Y. Xia, G. Zhang, Y. Yin, Synthesis and Characterization of MgO Nanowires Through a Vapor-Phase Precursor Method, 293–298, Copyright (2002), with permission from John Wiley and Sons [217].
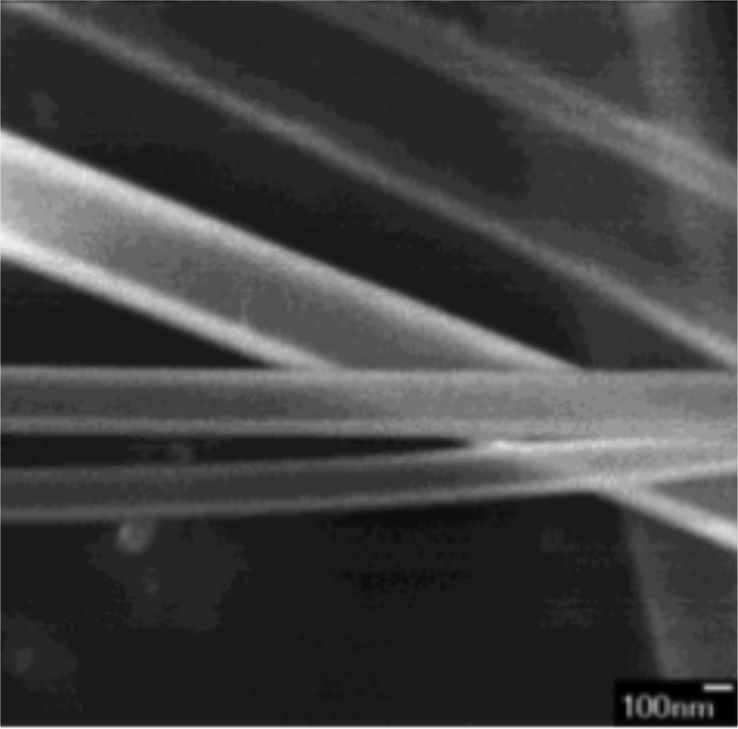
The growth of MgO nanowires from the vapor liquid-solid (VLS) growth mechanism with Mg3N2 as a precursor [218] as well as from MgO/Si with Au catalysts [219]. Figure 13 shows the MgO nanowire grown using boron oxide-assisted catalytic method from metal magnesium [220].
Figure 13.
TEM image of MgO nanowires with curved and branchy morphologies. Reprinted from The Journal of Physical Chemistry B, 106, C. Tang, Y. Bando, T. Sato, Oxide-assisted catalytic growth of MgO nanowires with uniform diameter distribution, 7449–7452, Copyright (2002) American Chemical Society [220].
7. Nanotubes
Crystalline tubular magnesium oxide nanostructures were obtained through carbon-thermal evaporation of a MgO powder while adding Gallium oxide into the mixture of MgO and carbon (Figure 14) [221]. Zn- assisted catalysts-free method is utilize to grow MgO nanotubes by thermal evaporation of mixed Zn and Mg powders [222]. These nanotubes are also grown by other researchers with various methods of depositions [223, 224, 225].
Figure 14.
High-resolution SEM image of tubular MgO nanostructures Redrawn from Inorganic Chemistry, 43, Jinhua Zhan, Yoshio Bando, Junqing Hu, et al, Bulk Synthesis of Single-Crystalline Magnesium Oxide Nanotubes, 2462–2464, Copyright (2004) American Chemical Society [221].
8. Nanorods
MgO nanorods with spherical particles at the tips and having diameters ranging from 20 to 50 nm were fabricated through direct reaction of Mg and oxygen. The lengths of these nanorods are observed up to 10 μm [226]. The controlled growth of MgO nanorods was investigated under electron irradiation in TEM [227]. Synthesis of magnesium oxide (MgO) nanorods was achieved by simply heating MgCl2 powder at 750 °C for 1.5 h in a constant flow of mixture gas (90% pure argon and 10% pure hydrogen) as shown in Figure 15 given below [228]. Vapor-liquid-solid (VLS) mechanism is also MgO nanorods have been grown on Si (100) by using thermal evaporation of Mg3N2 powders [229].
Figure 15.
Schematic illustration of the synthesis of MgO nanorod. Redrawn from Materials Research Bulletin, 35/10, Z Cui, G.W Meng, W. D Huang, G. Z Wang,L.D Zhang, Preparation and characterization of MgO nanorods, 1653–1659, Copyright (1993), with permission from Elsevier [228].
9. Nanoflower
Nanoflowers are among the most studied nanostructure of MgO and receive significant interest for understanding of growth phenomena [230]. In a recent study, these nanoflowers are grown using Rosmarinus officinalis L. extracts (Figure 16) [231] as well as from magnesium chloride with the help of acacia gum [232].
Figure 16.
MgO nanoflowers grown using Rosmarinus officinalis L. extracts. Reprinted from Y. Abdallah, S. O. Ogunyemi, A. Abdelazez, M. Zhang, X. Hong, E. Ibrahim, A. Hossain, H. Fouad, B. Li, J. Chen, The green synthesis of MgO nano-flowers using rosmarinus officinalis L. (Rosemary) and the antibacterial activities against Xanthomonas oryzae pv. oryzae, BioMed Res. Inter. 2019 (2019) 5620989. Copyright © 2019 Yasmine Abdallah et al [231].
Zheng et al. grown MgO nanoflowers by taking magnesium nitrate and sodium carbonate as starting material [233]. Nano-MgO films prepared using sol-gel spin coating method converted to flower-like structure after annealing [234].
10. Some other special structure
Li et al. reported the growth of MgO nanobelts by the decomposition of magnesium nitrate in direct current arc plasma jet chemical vapor deposition process with Mo substrate at 950 °C. The process results in the formation of nanobelts after the process 0.5 min (Figure 17) [235].
Figure 17.
(a) SEM and (b) TEM images of MgO Nanobelts. Reprinted from Materials Letters, 102, Hongji Li, Mingji Li, Xiufeng Wang, Xiaoguo Wu, Fude Liu, Baohe Yang, Synthesis and optical properties of single-crystal MgO nanobelts, 80–82, Copyright (2013), with permission from Elsevier [235].
First observation of MgO nanowall structures were reported on the glass substrate. These nanostructures are grown using successive ionic layer adsorption and reaction (SILAR) method followed by annealing [236]. MgO based nanoplates [237, 238, 239], nanosheet [240,241], nanofibers [242,243] are other form of nanostructures of significant interest.
11. Synthesis of nanoparticles using advanced techniques
Thus, variety of methods have been discussed for synthesis of MgO nanostructures, however, nanostructures grown using chemical methods usually contain carbonaceous impurities. These nanostructures when grown using physical methods contain surface defects as well as stress. These several effects lead to tailored behavior of these nanoparticles. Hence, attempts are also made by researchers to utilize self-sustained growth of nanoparticles under influence of sufficient energy. The energy to initiate grain growth is provided either by X-ray source [244], or heavy ion irradiation [245].
These methods are able to make these nanostructures free from carbonaceous impurities as well as strain. Thus, monochromatic X-ray by Bharti et al. [246] reported synthesis of plasmonic nanostructures. However, reports depicting the formation of oxides using these methods are hardly reported. Thus, these tools open pathways to synthesize oxide nanoparticles.
12. Conclusion(s)
In this review, description of underlying phenomena observed in MgO nanostructures are briefly discussed. A description of their applications in different field of these nanostructures is described. It is contemplated that these nanostructures have different categories like nanoparticles, thin films, nanowall, nanobelt etc depending upon method of synthesis. Thus, a precise control over method of synthesis is highly desirable. Though, chemical methods are cost effective and able to design nanostructure of desired category but they lead to presence of carbonaceous impurities. Nanostructures in the form of thin films are grown using deposition methods like pulsed laser deposition, radio frequency sputtering etc. However, these methods of depositions induces strain in the films. From the future development in this research area, some advanced techniques based on synchrotron radiation and swift heavy ion irradiation may be the effective synthetization tools for these nanostructures.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research was supported by the MOTIE (Ministry of Trade, Industry & Energy) (10080526) and the KSRC (Korea Semiconductor Research Consortium) support program for the development of the future semiconductor device.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Jitendra Pal Singh, Email: jitendra2029@postech.ac.kr.
Keun Hwa Chae, Email: khchae@kist.re.kr.
Sangsul Lee, Email: sangsul@postech.ac.kr.
References
- 1.Iijima S., Ichihashi T. Single-shell carbon nanotubes of 1-nm diameter. Nature. 1993;363:603–605. [Google Scholar]
- 2.Huang X., Gonzalez-Rodriguez R., Rich R., Gryczynski Z., Coffer J.L. Fabrication and size dependent properties of porous silicon nanotube arrays. Chem. Comm. 2013;49:5760–5762. doi: 10.1039/c3cc41913d. [DOI] [PubMed] [Google Scholar]
- 3.Yourdkhania A., Caruntu G. Highly ordered transition metal ferrite nanotube arrays synthesized by template-assisted liquid phase deposition. J. Mater. Chem. 2011;21:7145–7153. [Google Scholar]
- 4.Huang X., Li J. From single to multiple atomic layers: A unique approach to the systematic tuning of structures and properties of inorganic−organic hybrid nanostructured semiconductors. J. Am. Chem. Soc. 2007;129:3157–3162. doi: 10.1021/ja065799e. [DOI] [PubMed] [Google Scholar]
- 5.Pathakoti K., Manubolu M., Hwang H.-M. Nanostructures: current uses and future applications in food science. J. Food Drug Anal. 2017;25:245–253. doi: 10.1016/j.jfda.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., Feng J., Bai Y., Zhang Q., Yin Y. Synthesis, properties, and applications of hollow micro-/nanostructures. Chem. Rev. 2016;116:10983–11060. doi: 10.1021/acs.chemrev.5b00731. [DOI] [PubMed] [Google Scholar]
- 7.J -h. Lee, Y. J. Choi, Y.-b. Lim, Self-assembled filamentous nanostructures for drug/gene delivery applications, Expert Opin. Drug Deliv. 2010;7:341–351. doi: 10.1517/17425240903559841. [DOI] [PubMed] [Google Scholar]
- 8.Weber J., Singhal R., Zekri S., Kumar A. One-dimensional nanostructures: fabrication, characterisation and applications. Int. Mater. Rev. 2008;53:235–255. [Google Scholar]
- 9.Nasrollahzadeh M., Issaabadi Z., Sajjadi M., Sajadi S.M., Atarod M. Types of nanostructures. Interface Sci. Tech. 2019;28:29–80. [Google Scholar]
- 10.Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakharova G.S., Volkov V.L., Ivanovskaya V.V., Ivanovskii A.L. Nanotubes and related nanostructures of d-metal oxides: synthesis and computer design. Russ. Chem. Rev. 2005;74:587–618. [Google Scholar]
- 12.Manzoor U., Islam M., Tabassam L., Rahman S.U. Quantum confinement effect in ZnO nanoparticles synthesized by co-precipitate method. Phys. E Low-dimens. Syst. Nanostruct. 2009;9:1669–1672. [Google Scholar]
- 13.Mohammad N.S. Understanding quantum confinement in nanowires: basics, applications and possible laws. J. Phys. Condens. Matter. 2014;26:423202. doi: 10.1088/0953-8984/26/42/423202. [DOI] [PubMed] [Google Scholar]
- 14.Singh J.P., Srivastava R.C., Agrawal H.M. Optical behaviour of zinc ferrite nanoparticles. AIP Conf Proc. 2010;1276:137–143. [Google Scholar]
- 15.Singh J.P., Park J.Y., Singh V., Kim S.H., Lim W.C., Kim Y.H., Kumar H., Lee S., Chae K.H. Correlating the size and cation inversion factor in context of magnetic and optical behavior of CoFe2O4 nanoparticles. RSC Adv. 2020;10:21259–21269. doi: 10.1039/d0ra01653e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begum N., Bhatti A.S., Jabeen F., Rubini S., Martelli F. Phonon Confinement Effect in III-V Nanowires, Nanowires, Paola Prete. IntechOpen; 2010. Phonon confinement effect in III-V nanowires. [Google Scholar]
- 17.Osswald S., Mochalin V.N., Havel M., Yushin G., Gogotsi Y. Phonon confinement effects in the Raman spectrum of nanodiamond. Phys. Rev. B. 2009;80:075419. [Google Scholar]
- 18.Singh J.P., Srivastava R.C., Agrawal H.M., Kumar R. Micro-Raman investigation of nanosized zinc ferrite: effect of crystallite size and fluence of irradiation. J. Raman Spectrosc. 2011;42:1510–1517. [Google Scholar]
- 19.Zube A., Purdey M., Schartner E., Forbes C., van der Hoek Be., Giles D., Abell A., Monro T., Heidepriem H.E. Detection of gold nanoparticles with different sizes using absorption and fluorescence based method. Sensor. Actuator. B Chem. 2016;227:117–127. [Google Scholar]
- 20.Wasly H.S., Abd El-Sadek M.S., Batoo K.M. Novel synthesis, structural, optical properties and antibacterial activity of ZnO nanoparticles. Mater. Res. Express. 2019;6:055003. [Google Scholar]
- 21.Kelly K.L., Coronado E., Zhao L.L., Schatz G.C. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B. 2003;107:668–677. [Google Scholar]
- 22.Guglietta G.W., Diroll B.T., Gaulding E.A., Fordham J.L., Li S., Murray C.B., Baxter J.B. Lifetime, mobility, and diffusion of photoexcited carriers in ligand-exchanged lead selenide nanocrystal films measured by time-resolved terahertz spectroscopy. ACS Nano. 2015;9:1820–1828. doi: 10.1021/nn506724h. [DOI] [PubMed] [Google Scholar]
- 23.Ko S.P., Soh J.Y., Kim Y. Control of magnetic behavior in Fe3O4 nanostructures. IEEE Trans. Magn. 2005;41:3304–3306. [Google Scholar]
- 24.Leslie-Pelecky D.L., Rieke R.D. Magnetic properties of nanostructured materials. Chem. Mater. 1996;8:1770–1783. [Google Scholar]
- 25.Baxter J.B., Schmuttenmaer C.A. Conductivity of ZnO nanowires, nanoparticles and thin films using time-resolved terahertz spectroscopy. J. Phys. Chem. B. 2006;110:25229–25239. doi: 10.1021/jp064399a. [DOI] [PubMed] [Google Scholar]
- 26.Alkhatib A., Souier T., Chiesa M. Morphology dependent electrical transport behavior in gold nanostructures. Thin Solid Films. 2011;520:656–661. [Google Scholar]
- 27.Kwon S., Zheng J., Wingert M.C., Cui S., Chen R. Unusually high and anisotropic thermal conductivity in amorphous silicon nanostructures. ACS Nano. 2017;11:2470–2476. doi: 10.1021/acsnano.6b07836. [DOI] [PubMed] [Google Scholar]
- 28.Termentzidis K. Thermal conductivity anisotropy in nanostructures and nanostructured materials. J. Phys. D Appl. Phys. 2018;51:094003. [Google Scholar]
- 29.Singh J.P., Dixit G., Srivastava R.C., Agrawal H.M., Reddy V.R., Gupta A. Bulk like magnetic ordering in nanosized zinc ferrite below blocking temperature. J. Magn. Magn Mater. 2012;324:2553–2559. [Google Scholar]
- 30.Yao C., Zeng Q., Goya G.F., Torres T., Liu J., Wu H. M.Ge, Y. Zeng , Y. Wang, J. Z. Jiang, ZnFe2O4 Nanocrystals: Synthesis and magnetic properties. J. Phys. Chem. C. 2007;111:12274–12278. [Google Scholar]
- 31.Kumar Y., Lorite I., Lorenz M., Esquinazi P., Grundmann M. Effect of annealing on the magnetic properties of zinc ferrite thin films. Mater. Lett. 2017;195:89–91. [Google Scholar]
- 32.Coey J.M.D. Magnetism in d0 oxides. Nat. Mater. 2019;18:652–656. doi: 10.1038/s41563-019-0365-9. [DOI] [PubMed] [Google Scholar]
- 33.Curnan M.T., Kitchin J.R. Effects of concentration, crystal structure, magnetism, and electronic structure method on first-principles oxygen vacancy formation energy trends in perovskites. J. Phys. Chem. C. 2014;118:28776–28790. [Google Scholar]
- 34.Singh J.P., Lim W.C., Chae K.H. An interplay among the Mg2+ ion coordination, structural order, oxygen vacancies and magnetism of MgO thin films. J. Alloys Compd. 2019;806:1348–1356. [Google Scholar]
- 35.Manzetti S., Yakovlev A. Quantum chemical study of regular and irregular geometries of MgO nanoclusters: effects on magnetizability, electronic properties and physical characteristics. Mater. Chem. Phys. 2017;199:7–17. [Google Scholar]
- 36.Pacchioni G., Freund H. Electron transfer at oxide surfaces. The MgO paradigm: from defects to ultrathin films, Chem. Rev. 2013;113:4035–4072. doi: 10.1021/cr3002017. [DOI] [PubMed] [Google Scholar]
- 37.Sternig A., Koller D., Siedl N., Diwald O., Mc Kenna K. Exciton formation at solid–solid interfaces: a systematic experimental and ab-Initio study on compressed MgO nanopowders. J. Phys. Chem. C. 2012;116:10103–10112. [Google Scholar]
- 38.Bailly M.L., Costentin G., Pernot H.L., Krafft J.M., Che M. Physicochemical and in-situ photoluminescence study of the reversible transformation of oxide ions of low coordination into hydroxyl groups upon interaction of water and methanol with MgO. J. Phys. Chem. B. 2005;169:2404–2413. doi: 10.1021/jp048760+. [DOI] [PubMed] [Google Scholar]
- 39.Malykhin S.E., Volodin A.M., Bedilo A.F., Zhidomirov G.M. Generation of O− radical anions on MgO surface: long-distance charge separation or homolytic dissociation of chemisorbed water? J. Phys. Chem. C. 2009;113:10350–10353. [Google Scholar]
- 40.Hemmingson S.L., Feeley G.M., Miyake N.J., Campbell C.T. Energetics of 2D and 3D gold nanoparticles on MgO(100): influence of particle size and defects on gold adsorption and adhesion energies. ACS Catal. 2017;7:2151–2163. [Google Scholar]
- 41.Petitjean H., Guesmi H., Lauron-Pernot H., Costentin G., Loffreda D., Sautet P., Delbecq F. How surface hydroxyls enhance MgO reactivity in basic catalysis: the case of methylbutynol conversion, ACS Catal. 2014;4:4004–4014. [Google Scholar]
- 42.Mellor A. IV. Longmans; London: 1924. pp. 280–284. (Comprehensive Treatise on Inorganic and Theoretical Chemistry). [Google Scholar]
- 43.Singh J.P., Won S.O., Lim W.C., Chae K.H. Optical behavior of MgO nanoparticles investigated using diffuse reflectance and near edge X-ray absorption spectroscopy. Mater. Lett. 2017;198:34–37. [Google Scholar]
- 44.Yin D., Chen C., Saito M., Inoue K. Yuichi Ikuhara, Ceramic phases with one-dimensional long-range order. Nat. Mater. 2019;18:19–23. doi: 10.1038/s41563-018-0240-0. [DOI] [PubMed] [Google Scholar]
- 45.Müller M., Stankic S., Diwald O., Knözinger E., Sushko P.V., Trevisanutto P.E., Shluger A.L. Effect of protons on the optical properties of oxide nanostructures. J. Am. Chem. Soc. 2007;129:12491–12496. doi: 10.1021/ja0736055. [DOI] [PubMed] [Google Scholar]
- 46.Subramanian M.A., Shannon R.D., Chai B.H.T., Abraham M.M., Wintersgill M.C. Dielectric constants of BeO, MgO, and CaO using the two-terminal method. Phys. Chem. Miner. 1989;16:741–746. [Google Scholar]
- 47.Talukdar T.K., Liu S., Zhang Z., Harwath F., Girolami G.S., Abelson J.R. Conformal MgO film grown at high rate at low temperature by forward-directed chemical vapor deposition. J. Vac. Sci. Technol. 2018;A 36:051504. [Google Scholar]
- 48.Thorp J.S., Enayati-Rad N. The dielectric behaviour of single-crystal MgO, Fe/MgO and Cr/MgO, J. Math. Sci. 1981;16:255–260. [Google Scholar]
- 49.Liu J., Wang W., Guo Z., Zeng R., Dou S., Chen X. Peashell-like nanostructure—a new kind of one-dimensional nanostructure: the case of magnesium oxide. Chem. Commun. 2010;46:3887–3889. doi: 10.1039/c0cc00167h. [DOI] [PubMed] [Google Scholar]
- 50.Hornak J., Trnka P., Kadlec P., Michal O., Mentlik V., Sutta P., Csanyi G.M., Tamus Z.A. Magnesium oxide nanoparticles: dielectric properties, surface functionalization and improvement of epoxy-based composites insulating properties. Nanomaterials. 2018;8:381. doi: 10.3390/nano8060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhargava R., Khan S. Superior dielectric properties and bandgap modulation in hydrothermally grown Gr/MgO nanocomposite. Phys. Lett. 2019;383:1671–1676. [Google Scholar]
- 52.Wu Y.Z., Schmid A.K., Qiu Z.Q. Spin-dependent quantum interference from epitaxial MgO thin films on Fe(001) Phys. Rev. Lett. 2006;97:217205. doi: 10.1103/PhysRevLett.97.217205. 1-4. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y.Z., Qiu Z.Q., Schmid A.K. Spin-dependent quantum interference from MgO thin films grown on Fe(0 0 1) J. Magn. Magn Mater. 2007;310:1629–1631. [Google Scholar]
- 54.Bertacco R., Ciccacci F. Large spin asymmetry in electron absorption and reflection from oxidized single crystal Fe/MgO(001) films. Surf. Sci. 1999;419:265–271. [Google Scholar]
- 55.Loy D.J.J., Danajaya P.A., Hong X.L., Shum D.P., Lew W.S. Conduction mechanisms on high retention annealed MgO-based resistive switching memory devices. Sci. Rep. 2018;8:14774. doi: 10.1038/s41598-018-33198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo Y., Song Q., Yan H. The influence of interaction between oxygen vacancies on set process in resistive switching: a case of MgO, AIP Adv. 2019;5:055230. [Google Scholar]
- 57.Jambois O., Carreras P., Antony A., Bertomeu J., Martínez-Boubeta C. Resistance switching in transparent magnetic MgO films. Solid State Commun. 2011;151:1856–1859. [Google Scholar]
- 58.Dias C., Guerra L.M., Bordalo B.D., Lv H., Ferraria A.M., do Rego A.M.B., Cardoso S., Freitas P.P., Ventura J. Voltage-polarity dependent multi-mode resistive switching on sputtered MgO nanostructures. Phys. Chem. Chem. Phys. 2017;19:10898–10904. doi: 10.1039/c7cp00062f. [DOI] [PubMed] [Google Scholar]
- 59.Uchino T., Okutsu D. Broadband laser emission from color centers inside MgO microcrystals. Phys. Rev. Lett. 2008;101:117401–117404. doi: 10.1103/PhysRevLett.101.117401. [DOI] [PubMed] [Google Scholar]
- 60.Uchino T., Okutsu D., Katayama R., Sawai S. Mechanism of stimulated optical emission from MgO microcrystals with color centers. Phys. Rev. B. 2009;79:165107. [Google Scholar]
- 61.Kumar A., Thota S., Verma S., Kumar J. Sol-gel synthesis of highly luminescent magnesium oxide nanocrystallites. J. Luminscence. 2011;131:640–648. [Google Scholar]
- 62.Debaraja P.B., Abadhani D.N., Prashantha S.C., Nagabhushana H., Sharma S.C., Nagabhushan B.M., Nagaswarup H.P. Synthesis, structural and luminescence studies of magnesium oxide nanopowder. Spectrochim. Acta: Mol. Biomolecular Spect. 2014;118:847–851. doi: 10.1016/j.saa.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 63.Nikiforov S.V., Kortov V.S., Petrov M.O. Luminescent and dosimetric properties of ultrafine magnesium oxide ceramics after high dose irradiation. Radiat. Meas. 2016;90:252–256. [Google Scholar]
- 64.Chaudhri M.M., Sands H.S. Photoluminescence from indented MgO crystals using a near ultraviolet/visible Raman microscope. J. Appl. Phys. 1997;82:785–791. [Google Scholar]
- 65.Halder R., Bandyopadhyay S. Spark plasma sintering of nano magnesia: processing parameters influencing optical properties. Mater. Chem. Phys. 2019;228:51–59. [Google Scholar]
- 66.Benia H.M., Lin X., Gao H.-J., Nilius N., Freund H.-J. Nucleation and growth of gold on MgO thin films: A combined STM and luminescence study. J. Phys. Chem. C. 2007;111:10528–10533. [Google Scholar]
- 67.V Skvortsova, L.Trinkler, Luminescence of impyrity and radiation defects in magnesium oxide irradiated by fast neutrons. Phys. Proced. 2009;2:567–570. [Google Scholar]
- 68.Abramishvili M., Akhvlediani Z., Galustashvili M., Dekanozishvili G., Kalabegishvili T., Kvatchadze V., Tavkhelidze V. Peculiarities of radiation effects in MgO: Mn2+ crystals. J. Mod. Phys. 2011;2:841–844. [Google Scholar]
- 69.Pathak N., Gupta S.K., Prajapat C.L., Sharma S.K., Ghosh P.S., Kanrar B., Pujari P.K., Kadam R.M. Defect induced ferromagnetism in MgO and its exceptional enhancement upon thermal annealing: a case of transformation of various defect states. Phys. Chem. Chem. Phys. 2017;19:11975–11989. doi: 10.1039/c7cp01776f. [DOI] [PubMed] [Google Scholar]
- 70.Rao C.N., Nakate U.T., Choudhary R.J., Kale S.N. Defect-induced magneto-optic properties of MgO nanoparticles realized as optical-fiber-based low-field magnetic sensor. Appl. Phys. Lett. 2013;103:151107. [Google Scholar]
- 71.Narayan J., Nori S., Pandya D., Avasthi D.K. Defect dependent ferromagnetism in MgO doped with Ni and Co. Appl. Phys. Lett. 2008;93:082507. [Google Scholar]
- 72.Jain N., Marwaha N., Verma R., Gupta B.K., Srivastava A.K. Facile synthesis of defect-induced highly-luminescent pristine MgO nanostructures for promising solid-state lighting applications. RSC Adv. 2016;6:4960–4968. [Google Scholar]
- 73.Siedl N., Koller D., Sternig A.K., Thomelea D., Diwald O. Photoluminescence quenching in compressed MgO nanoparticle systems. Phys. Chem. Chem. Phys. 2014;16:8339–8345. doi: 10.1039/c3cp54582b. [DOI] [PubMed] [Google Scholar]
- 74.Sellaiyan S., Uedono A., Sivaji K., Janet Priscilla S., Sivasankari J., Selvalakshmi T. Vacancy defects and defect clusters in alkali metal ion-doped MgO nanocrystallites studied by positron annihilation and photoluminescence spectroscopy. Appl. Phys. A. 2016;122:920. [Google Scholar]
- 75.Cao X., Dai H., Chen S., Zeng J., Zhang K., Sun Y. A high selective cataluminescence sensor for the determination of tetrahydrofuran vapor, Meas. Sci. Technol. 2013;24:025103. [Google Scholar]
- 76.Chu Y., Zhang Q., Zhang W., Zhang G., Zhu S. Highly sensitive dimethyl ether gas sensor utilizing cataluminescence on nanosized MgO/In2O3. Meas. Sci. Technol. 2014;25:085105. [Google Scholar]
- 77.Meng F., Lu Z., Zhang R., Li G. Cataluminescence sensor for highly sensitive and selective detection of iso-butanol. Talanta. 2019;194:910–918. doi: 10.1016/j.talanta.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 78.Zhou C., Ali Q., Chen X., GaO X., Liu K., Shen D. Ultraviolet photodetectors based on wide bandgap oxide semiconductor films. Chin. Phys. B. 2019;28:048503. [Google Scholar]
- 79.Mageshwari K., Mali S.S., Sathyamoorthy R., Patil P.S. Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technol. 2013;249:456–462. [Google Scholar]
- 80.Lee J.H., Eun J.H., Park S.Y., Kim S.G., Kim H.J. Hydration of r.f. magnetron sputtered MgO thin films for a protective layer in AC plasma display panel. Thin Solid Films. 2003;435:95–101. [Google Scholar]
- 81.Shin H., Jung J., Motobayashi K., Yanagisawa S., Morikawa Y., Kim Y., Kawai M. State-selective dissociation of a single water molecule on an ultrathin MgO film. Nat. Mater. 2010;9:442–447. doi: 10.1038/nmat2740. [DOI] [PubMed] [Google Scholar]
- 82.Xu L., Henkelman G. Calculations of Li adsorption and diffusion on MgO(100) in comparison to Ca. Phys. Rev. B. 2010;82:115407. 1-7. [Google Scholar]
- 83.Pacchioni G., Giordano L., Baistrocchi M. Charging of metal atoms on ultrathin MgO/Mo(100) films. Phys. Rev. Lett. 2005;94:226104. doi: 10.1103/PhysRevLett.94.226104. 1-4. [DOI] [PubMed] [Google Scholar]
- 84.Zavyalova U., Geske M., Horn R., Weinberg G., Frandsen W., Schuster M., Schlög R. Morphology and microstructure of Li/MgO catalysts for the oxidative coupling of methane. ChemCatChem. 2011;3:949–959. [Google Scholar]
- 85.Gandhi S., Abiramipriya P., Pooja N., Latha Jeyakumari J.J., Yelil Arasic A., Dhanalakshmia V., Gopinathan Naird M.R., Anbarasana R. Synthesis and characterizations of nano sized MgO and its nano composite with poly(vinyl alcohol) J. Non-Cryst. Solids. 2011;357:181–185. [Google Scholar]
- 86.Huang L., Li D.-Q., Lin Y.-J., Wei M., Evans D.G., Duan X. Controllable preparation of Nano-MgO and investigation of its bactericidal properties. J. Inorg. Biochem. 2005;99:986–993. doi: 10.1016/j.jinorgbio.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 87.Tang Z.X., Lv B.F. MgO nanoparticles as antibacterial agent: preparation and activity. Braz. J. Chem. Eng. 2014;31:591–601. [Google Scholar]
- 88.Konishi M., Oonishi S., Ookubo A., Yunoki M., Kawamoto A. Magnesium oxide for pharmaceutical preparation. US 20130059151 A1. 2013 [Google Scholar]
- 89.Dipak S.P., Kumar C.A., Arnab M., Das D.B., Samanta A., Hardy J.G., Ghosh C.K. Effect of morphology and concentration on crossover between antioxidant and pro-oxidant activity of MgO nanostructures. Inorg. Chem. 2018;57:12727–12739. doi: 10.1021/acs.inorgchem.8b01938. [DOI] [PubMed] [Google Scholar]
- 90.Schwartz S.H., Buchwald A. Magnesium oxide and iodine. US4147775 A. 1979 [Google Scholar]
- 91.Mazaheri N., Naghsh N., Karimi A., Salavati H. In vivo toxicity investigation of magnesium oxide nanoparticles in rat for environmental and biomedical applications. Iran. J. Biotechnol. 2019;17 doi: 10.21859/ijb.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karthik K., Dhanuskodi S., Gobinath C., Prabukumar S., Sivaramakrishnan S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J. Photochem. Photobiol. B Biol. 2019;190:8–20. doi: 10.1016/j.jphotobiol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Wong C.W., Chan Y.S., Jeevanandam J., Pal K., Bechelany M., Elkodous M.A., Sayyad G.S.E. Response surface methodology optimization of mono-dispersed MgO nanoparticles fabricated by ultrasonic-assisted sol–gel method for outstanding antimicrobial and antibiofilm activities. J. Cluster Sci. 2020;31:367–389. [Google Scholar]
- 94.Krishnamoorthy K., Moon J.Y., Hyun H.B., Cho S.K., Kim S.-J. Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells J. Mater. Chem. 2012;22:24610–24617. [Google Scholar]
- 95.Sudakaran S.V., Venugopal J.R., Vijayakumar G.P., Abisegapriyan S., Grace A.N., Ramakrishna S. Sequel of MgO nanoparticles in PLACL nanofibers for anti-cancer therapy in synergy with curcumin/β-cyclodextrin. Mater. Sci. Eng. C. 2017;71:620–628. doi: 10.1016/j.msec.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 96.Mangalampalli B., Dumala N., Grover P. Toxicity assessment of magnesium oxide nano and microparticles on cancer and non-cancer cell lines. Nucleus. 2019;62:227–241. [Google Scholar]
- 97.Kameda T., Miyano Y., Yoshioka T., Uchida M., Okuwaki A. New treatment methods for waste water containing chloride ion using magnesium–aluminum oxide. Chem. Lett. 2000:1136–1137. [Google Scholar]
- 98.Quintana M., Colmenarejo M.F., Barrera J.S., Garciaa G., Garciaa E.A., Bustos A. Use of a byproduct of magnesium oxide production to precipitate phosphorus and nitrogen as struvite from wastewater treatment liquors. J. Agric. Food Chem. 2004;52:294–299. doi: 10.1021/jf0303870. [DOI] [PubMed] [Google Scholar]
- 99.Plaza C., Sanz R., Clemnte C., Ernandez J.M., Gonzalez R., Polo A., Colomenarejo M.F. Greenhouse evaluation of struvite and sludges from municipal wastewater treatment works as phosphorus sources for plants. J. Agric. Food Chem. 2007;55:8206–8212. doi: 10.1021/jf071563y. [DOI] [PubMed] [Google Scholar]
- 100.Zhang L., Zhu W., Zhang H., Bi S., Zhang Q. Hydrothermal–thermal conversion synthesis of hierarchical porous MgO microrods as efficient adsorbents for lead(II), chromium(VI) and Arsenate removal, RSC. Advances. 2014;4:0542–30550. [Google Scholar]
- 101.Ghosh A., Biswas S., Sikdar S., Saha R. Morphology controlled fabrication of highly permeable carbon coated rod-shaped magnesium oxide as a sustainable arsenite adsorbent. Ind. Eng. Chem. Res. 2019;58:10352–10363. [Google Scholar]
- 102.Karacabey P., Döven S., Uzunoğlu D., Özer A. Synthesis of 3D hierarchical flower-like MgO microstructure: investigation of its adsorption and antibacterial properties. Arabian J. Sci. Eng. 2019;44:9951–9964. [Google Scholar]
- 103.Kuang M., Shang Y., Yang G., Liu B., Yang B. Facile synthesis of hollow mesoporous MgO spheres via spray-drying with improved adsorption capacity for Pb(II) and Cd(II) Environ. Sci. Pollut. Res. 2019;26:18825–18833. doi: 10.1007/s11356-019-05277-w. [DOI] [PubMed] [Google Scholar]
- 104.Cao C.Y., Qu J., Wei F., Liu H., Song W.G. Superb adsorption capacity and mechanism of flowerlike magnesium oxide nanostructures for lead and cadmium ions. ACS Appl. Mater. Interfaces. 2012;4:4283–4287. doi: 10.1021/am300972z. [DOI] [PubMed] [Google Scholar]
- 105.Wang J., Zhang J., Chen R., Yang C., Xiang L., Yi M. A vacuum calcination route to high-surface-area MgO nanoplates for superior arsenate adsorption and catalytic properties, Vacuum. 2018;158:231–235. [Google Scholar]
- 106.Liu W.J., Jiang H., Tian K., Ding Y.W., Yu H.Q. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ. Sci. Technol. 2013;47:9397–9403. doi: 10.1021/es401286p. [DOI] [PubMed] [Google Scholar]
- 107.Li P., Zeng H.C. Hierarchical nanocomposite by the integration of reduced graphene oxide and amorphous carbon with ultrafine MgO nanocrystallites for enhanced CO2 capture. Environ. Sci. Technol. 2017;51:12998–13007. doi: 10.1021/acs.est.7b03308. [DOI] [PubMed] [Google Scholar]
- 108.Hanif A., Sun M., Tao Z., Liu L., Tsang D.C.W., Gu Q., Shang J. Silica supported MgO as an adsorbent for precombustion CO2 capture, ACS App. Nanostruct. Mater. 2019;2:6565–6574. [Google Scholar]
- 109.Hu Y., Guo Y., Sun J., Liu W. Progress in MgO sorbents for cyclic CO2 capture: a comprehensive review. J. Mater. Chem. 2019;7:20103–20120. [Google Scholar]
- 110.Elsey H.M. Magnesium oxide insulation. US 2292065 A. 1940 [Google Scholar]
- 111.Jeong I.B., Kim J.S., Lee J.Y., Hong J.W., Shin J.Y. Electrical insulation properties of nanocomposites with SiO2 and MgO filler. Trans. Elect. Elect. Mater. 2010;11:261–265. [Google Scholar]
- 112.Ikeda S., Miura K., Yamantoo H., Gan H.D., Endo M., Kanai S., Hayakawa J., Matsukara F., Ohno H. A perpendicular anisotropy CoFeB-MgO magnetic tunnel junction. Nat. Mater. 2010;9:721–724. doi: 10.1038/nmat2804. [DOI] [PubMed] [Google Scholar]
- 113.Singh J.P., Kaur B., Gautam S., Lim W.C., Asokan K., Chae K.H. Chemical effects at interfaces of Fe/MgO/Fe magnetic tunnel junction. Superlattice. Microst. 2016;100:560–586. [Google Scholar]
- 114.Wurzlera G.T., Neto R.C.R., Mattos L.V., Fraga M.A., Noronha F.B. Steam reforming of ethanol for hydrogen production over MgO—supported Ni-based catalysts. Appl. Catal. Gen. 2016;518:115–128. [Google Scholar]
- 115.Liu Z., Yin Z., Cox C., Bosman M., Qian X., Li N., Zhao H., Du Y., Li J., Nocera D.G. Room temperature stable COx-free H2 production from methanol with magnesium oxide nanophotocatalysts. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1501425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hollerer M., Prochinig D., Puschnig P. E.r Carrasco, H. J. Freund, M. Sterrer, Scanning tunneling microscopy of the ordered water monolayer on MgO(001)/Ag(001) ultrathin films. J. Phys. Chem. C. 2019;123:3711–3718. [Google Scholar]
- 117.Kim Y.D., Stultz J., Goodmann D.W. Dissociation of water on MgO(100) J. Phys. Chem. B. 2002;106:1515–1517. [Google Scholar]
- 118.Shim J.-H., Lee S., Park S.S. Effects of MgO coating on the structural and electrochemical characteristics of LiCoO2 as cathode materials for lithium ion battery. Chem. Mater. 2014;26:2537–2543. [Google Scholar]
- 119.Hwang J.Y., Yu T.Y., Sun Y.K. Simultaneous MgO coating and Mg doping of Na[Ni0.5Mn0.5]O2 cathode: facile and customizable approach to high-voltage sodium-ion batteries. J. Mater. Chem. 2018;6:16854–16862. [Google Scholar]
- 120.Ma F., Wu Y., Wei G., Qiu S., Qu J. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via wet-chemical coating of MgO, J. Solid State Electrochemistry. 2019;23:2213–2224. [Google Scholar]
- 121.Wang Y., Zhang Y.F., Liu H.R., Yu S.J., Qin Q.Z. Nanocrystalline NiO thin film anode with MgO coating for Li-ion batteries. Electrochim. Acta. 2003;48:4253–4259. [Google Scholar]
- 122.Petnikota S., Rotte N.K., Reddy M.V., Srikanth V.V.S.S., Chowdari B.V.R. MgO-decorated few-layered graphene as an anode for Li-ion batteries. ACS Appl. Mater. Interfaces. 2015;7:2301–2309. doi: 10.1021/am5064712. [DOI] [PubMed] [Google Scholar]
- 123.Musella R., Mazevet S., Guyot F. Physical properties of MgO at deep planetary conditions. Phys. Rev. B. 2019;99:64110. [Google Scholar]
- 124.Root S., Shulenburger L., Lemke R.W., Dolan D.H., Mattsson T.R., Desjarlais M.P. Shock response and phase transitions of MgO at planetary impact vonditions. Phys. Rev. Lett. 2015;115:198501. doi: 10.1103/PhysRevLett.115.198501. [DOI] [PubMed] [Google Scholar]
- 125.Swetha J.V., Parse H., Kakade B., Geetha A. Morphology dependent facile synthesis of manganese oxide nanostructures for oxygen reduction reaction. Solid State Ionics. 2018;328:1–7. [Google Scholar]
- 126.Agrawal S., Rai P., Gatell E.N., Llobet E., Guell F., Kumar M., Awasthi K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sensor. Actuator. B Chem. 2019;292:24–31. [Google Scholar]
- 127.Sarkar S., Pal T. Noble metal-metal oxide hybrid nanoparticles. 2019. Theoretical aspects of synthesis for controlled morphological nanostructures; pp. 7–50. [Google Scholar]
- 128.Zhang F., Jin Q., Chan S.W. Ceria nanoparticles: size, size distribution, and shape. J. Appl. Phys. 2004;95:4319–4326. [Google Scholar]
- 129.Singh J.P., Kim S.H., Won S.O., Lim W.C., Lee I.-J., Chae K.H. Covalency, hybridization and valence state effects in nano-and micro-sized ZnFe2O4. CrystEngComm. 2016;18:2701–2711. [Google Scholar]
- 130.House S.D., Bonifacio C.S., Grisehabr R.V., Li L., Zhang Z., Ciston J., Stach E.A., Yang J.C. Statistical analysis of support thickness and particle size effects in HRTEM imaging of metal nanoparticles. Ultramicroscopy. 2016;169:22–29. doi: 10.1016/j.ultramic.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 131.Wahab R., Ansari S.G., Dar M.A., Kim Y.S., Shin H.S. Synthesis of magnesium oxide nanoparticles by sol-gel process. Mater. Sci. Forum. 2007;558–559:983–986. [Google Scholar]
- 132.Fernandez A.S., De la Rosa-García S.C., Gomez-Villalba L.S., Gomez-Cornelio S., Rabanal M.E., Fort R., Quintana P. Synthesis, photocatalytic, and antifungal properties of MgO, ZnO and Zn/Mg oxide nanoparticles for the protection of calcareous stone heritage. ACS Appl. Mater. Interfaces. 2017;9:24873–24886. doi: 10.1021/acsami.7b06130. [DOI] [PubMed] [Google Scholar]
- 133.Singh J.P., Kim S.H., Kang H.K., Won S.O., Lee I.J., Chae K.H. Are organic templates responsible for the optical and magnetic response of MgO nanoparticles? Mat. Chem. Front. 2018;2:1707–1715. [Google Scholar]
- 134.Rani N., Chahal S., Chauhan A.S., Kumar P., Shukla R., Singh S.K. X-ray analysis of MgO nanoparticles by modified Scherer’s Williamson-Hall and size-strain method. Mater. Today: Proc. 2019;12:543–548. [Google Scholar]
- 135.Gedanken A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004;11:47–55. doi: 10.1016/j.ultsonch.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 136.Alavi M.A., Morsali A. Syntheses and characterization of Mg(OH)2 and MgO nanostructures by ultrasonic method. Ultrason. Sonochem. 2010;17:441–446. doi: 10.1016/j.ultsonch.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 137.Tang Z.X., Shi L.E. Preparation of nano-MgO using ultrasonic method and its characteristics, Ecl. Quím. São Paulo. 2008;33:15–20. [Google Scholar]
- 138.Karimi M.A., Ardakani M.M., Asadiniya R., Roozbahani S.H. Synthesis and characterization of ZnO and MgO nanoparticles and ZnO/MgO nanocomposite and their application for preparation of zinc phosphate dental cement. Nanosci. Nanotech.: Ind. J. 2010;4:11–16. [Google Scholar]
- 139.Gajengi A.L., Sasaki T., Bhanage B.M. Mechanistic aspects of formation of MgO nanoparticles under microwave irradiation and its catalytic application. Adv. Powder Technol. 2017;28:1185–1192. [Google Scholar]
- 140.Bhatte K., Sawant D., Deshmukh K., Bhanage B.M. Additive free microwave assisted synthesis of nanocrystalline Mg(OH)2 and MgO. Particuology. 2012;103:384–387. [Google Scholar]
- 141.Zahran Y.H., Shneouda S.S., Yahia I.S., El-Tantaway F. Facile and rapid synthesis of nanoplates Mg(OH)2 and MgO via Microwave technique from metal source: structural, optical and dielectric properties. J. Sol-gel Sci. Tech. 2018;86:104–111. [Google Scholar]
- 142.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. [Google Scholar]
- 143.Moorthy S.K., Ashok C., Rao K., Viswanathana C. Synthesis and characterization of Mgo nanoparticles by neem leaves through green method. Mater. Today Proc. 2015;2:4360–4368. [Google Scholar]
- 144.Dobrucka R. Synthesis of MgO nanoparticles using Artemisia abrotanum Herba Extract and their antioxidant and photocatalytic properties. Iran. J. Sci. Technol. Trans. A-Science. 2018;42:547–555. [Google Scholar]
- 145.Suresh J., kumar R.Y., Sundrarajan M., Hong S.I. Green synthesis of magnesium oxide nanoparticles. Adv. Mater. Res. 2014;952:141–144. [Google Scholar]
- 146.Rao K.G., Ashok C., Rao K.V., Chakra C.S. Akshaykranth. A, Eco-friendly synthesis of MgO nanoparticles from orange fruit waste. Int. J. Adv. Res. Phys. Sci. (IJARPS) 2015;2:1–6. [Google Scholar]
- 147.Jeevanandam J., Chan Y.S., Ku Y.H. Aqueous Eu calyptus globulus leaf extract-mediated biosynthesis of MgO nanorods. Appl. Biol. Chem. 2018;61:197–208. [Google Scholar]
- 148.Joghee S., Ganeshan P., Vincent A. Ecofriendly biosynthesis of zinc oxide and magnesium oxide particles from medicinal plant Pisonia grandis R.Br. Leaf extract and their antimicrobial activity. BioNanoScience. 2019;9:141–154. [Google Scholar]
- 149.Marwaha N., Gupta B.K., Verma R., Srivastava A.K. Facile synthesis and characterization of pH-dependent pristine MgO nanostructures for visible light emission. J. Math. Sci. 2017;52:10480–10484. [Google Scholar]
- 150.Chamack M., Mahjoub A.R., Hosseinian A. Facile synthesis of nanosized MgO as adsorbent for removal of Congo red dye from wastewater. Nanochem Res. 2018;3:85–91. [Google Scholar]
- 151.He Y. MgO nanostructured microspheres synthesized by an interfacial reaction in a solid-stabilized emulsion. Mater. Lett. 2006;60:3511–3513. [Google Scholar]
- 152.Li S., Zhou B., Ren B., Xing L., Dong L., Li J. Preparation of MgO nanomaterials by microemulsion-based oil/water interface precipitation. Mater. Lett. 2016;171:204–207. [Google Scholar]
- 153.Bumajdad A., Al-Ghareeb S., Madkour M., Al Sagheer F., Zaki M.I. Synthesis of MgO nanocatalyst in water-in-oil microemulsion for CO oxidation. React. Kinet. Mech. Catal. 2017;122:1213–1229. [Google Scholar]
- 154.Singh J.P., Chae Keun Hwa. Local electronic structure perspectives of nanoparticle growth: the case of MgO, ACS Omega. 2019;4:7140–7150. doi: 10.1021/acsomega.9b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhardwaj R., Bharti A., Singh J.P., Chae K.H., Goyal N. Sa. Gautam, Structural and electronic investigation of ZnO nanostructures synthesized under different environments. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Singh J.P., Dixit G., Srivastava R.C., Negi P., Agrawal H.M., Kumar R. HRTEM and FTIR investigation of nanosized zinc ferrite irradiated with 100 MeV oxygen ions. Spectrochem. Acta A. 2013;107:326–333. doi: 10.1016/j.saa.2012.12.095. [DOI] [PubMed] [Google Scholar]
- 157.Singh J.P., Dixit G., Srivastava R.C., Agrawal H.M., Kumar R. Raman and Fourier-transform infrared spectroscopic study of nanosized zinc ferrite irradiated with 200 MeV Ag15+ beam. J. Alloys Compd. 2013;551:370–375. [Google Scholar]
- 158.Oh C., Streller F., Ashurst W.R., W Carpick R., P de Boer M. In situ oxygen plasma cleaning of microswitch surfaces—comparison of Ti and graphite electrodes. J. Micromech. Microeng. 2016;26:115020. [Google Scholar]
- 159.Salah N., Habib S.S., Khan Z.H., Memic A., Azam A., Alarfaj E., Zahed N., Hamed S.A. High-energy ball milling technique for ZnO nanoparticles as antibacterial material. Int. J. Nanomed. 2011;6:863–869. doi: 10.2147/IJN.S18267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kamarulzaman N., Chayed N.F., Badar N. MgO nanoparticles via a simple solid-state reaction. AIP Conf. Proc. 2016;1711:040004. [Google Scholar]
- 161.Zhou H., Hu J., Xe J., Wang S., Cao Y. A solid –state chemical raction method for synthesizing MgO nanoparticles with superior adsorption properties, RSC Adv. 2019;9:2011–2017. doi: 10.1039/c8ra09199d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo Y., Tan C., Wang P., Sun J., Li W., Zhao C., Lu P. Structure-performance relationships of magnesium-based CO2 adsorbents prepared with different methods. Chem. Eng. J. 2020;379:122277. [Google Scholar]
- 163.Hu L., Hecht D.S., Grüner G. Carbon nanotube thin films: fabrication, properties, and applications. Chem. Rev. 2010;110:5790–5844. doi: 10.1021/cr9002962. [DOI] [PubMed] [Google Scholar]
- 164.Loureiro J., Santos J.R., Nogueira A., Wyczisk F., Divay L., Reparaz S., Alzina F., Torres C.M.S., Cuffe J., Montemor F., Martinsa R., Ferreira I. Nanostructured p-type Cr/V2O5 thin films with boosted thermoelectric properties. J. Mater. Chem. 2014;2:6456–6462. [Google Scholar]
- 165.Kolhatkar A.G., Jamison A.C., Litvinov D., Willson R.C., Randall Lee T. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013;14:15977–16009. doi: 10.3390/ijms140815977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ma C., Liu M., Chen C., Lin Y., Li Y., Horwitz J.S., Jiang J., Meletis E.I., Zhang Q. The origin of local Strain in highly epitaxial oxide thin films, Sci. Rep. 2013;3:3092. doi: 10.1038/srep03092. 1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wu H.C., Mauit O., Coileáin C.Ó., Syrlybekov A., Khalid A., Mouti A., Abid M., Zhang H.-Z., Abid M., Shvets I.V. Magnetic and transport properties of epitaxial thin film MgFe2 O4 grown on MgO (100) by molecular beam epitaxy. Sci. Rep. 2014;4:7012. doi: 10.1038/srep07012. 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rao A., Dsa J., Goyal S., Singh B.R. Stress induced degradation in sputtered ZrO2 thin films on Silicon for nano-MOSFET’s. Phys. Semicond. Dev. Environ. Sci. Eng. 2014:555–558. [Google Scholar]
- 169.Matthews J.W., Griinbaum E. The structure of gold films grown in ultra-high vacuumon sodium chloride substrates. Philos. Mag. 1965;11:1233–1234. [Google Scholar]
- 170.Buer E., Green A.K., Kunz K.M. On the epitaxial growth of single crystal metal films free of impurities. Appl. Phys. Lett. 1966;8:248–249. [Google Scholar]
- 171.Maksimova O., Fisher P., Skowronski M., Salvador P.A., Snyder M., Xu J., Weng X. MgO films grown on yttria-stabilized zirconia by molecular beam epitaxy. J. Cryst. Growth. 2008;310:2760–2766. [Google Scholar]
- 172.Aboelfotoh M.O. Epitaxy of MgO on alkali halides with NaCI-type structure. J. Appl. Phys. 1978;49:2770–2776. [Google Scholar]
- 173.Lee M.J., Park S.Y., Kim S.G., Kim H.J., Moon S.H., Kim J.K. Effect of stress and density on the electrical and physical properties of MgO protecting layer for alternating current-plasma display panels. J. Vac. Sci. Technol. 2005;23:1192–1196. [Google Scholar]
- 174.Diao Z., Feng J.F., Kurt H., Feng G., Coey J.M.D. Reduced low frequency noise in electron beam evaporated MgO magnetic tunnel junctions. Appl. Phys. Lett. 2010;96:202506. 1-3. [Google Scholar]
- 175.Singh J.P., Lim W.C., Chae Keun Hwa, Gautam Sanjeev, Asokan K. Swift heavy ion irradiation induced effects in Fe/MgO/Fe/Co multilayer. Mater. Des. 2016;101:72–79. [Google Scholar]
- 176.Singh J.P., Sulania I., Prakash J., Gautam S., Chae K.H., Kanjilal D., Asokan K. Study of surface morphology and grain size of irradiated MgO thin films. Adv. Mat. Lett. 2012;3:112–117. [Google Scholar]
- 177.Singh J.P., Gupta L.K. Annealing effects on MgO films grown using e-beam evaporation. Int. J. Math., Eng. Manag. Sci. 2019;4:619–626. [Google Scholar]
- 178.Singh J.P., Chen C.L., Dong C.L., Prakash J., Kabiraj D., Kanjilal D., Pong W.F., Asokan K. Role of surface and subsurface defects in MgO thin film: XANES and magnetic investigations. Superlattice. Microst. 2015;77:313–324. [Google Scholar]
- 179.Yadavalli S., Yang M.H., Flynn C.P. Low-temperature growth of MgO by molecular-beam epitaxy. Phys. Rev. B. 1990;41:7961–9963. doi: 10.1103/physrevb.41.7961. [DOI] [PubMed] [Google Scholar]
- 180.Niu F., Hoerman B.H., Wessels B.W. Epitaxial thin films of MgO on Si using metalorganic molecular beam epitaxy. J. Vac. Sci. Technol. B. 2000;18:2146–2152. [Google Scholar]
- 181.Niu F., Meier A.L., Wessels B.W. Epitaxial growth and strain relaxation of MgO thin films on Si grown by molecular beam epitaxy. J. Vac. Sci. Technol. B. 2006;24:2586–2591. [Google Scholar]
- 182.Matsuzak K., Hosono H., Susak T. Layer-by-layer epitaxial growth of polar MgO(111) thin films. Phys. Rev. B. 2010;82 033408-1-4. [Google Scholar]
- 183.Yoon J.G., Kim K. Growth of (111) oriented MgO film on Si substrate by the sol-gel method. Appl. Phys. Lett. 1995;66:2661–2663. [Google Scholar]
- 184.Susaki T., Kumada S., Katase T., Matsuzaki K., Miyakawa M., Hosono H. Fabrication of flat MgO(111) films on Al2O3(0001) substrates by pulsed laser deposition. Appl. Phys. Exp. 2009;2 091403–1-4. [Google Scholar]
- 185.VillegleP J.C., Radparvar M., Yu L.S., Faris S.M. RF-sputter-deposited magnesium oxide films as high-quality adjustable tunnel barriers. IEEE Trans. Magn. 1989;25:1227–1230. [Google Scholar]
- 186.Chen X. Paulo. P. Freitas, Magnetic Tunnel Junction Based on MgO barrier prepared by natural oxidation and direct sputtering deposition. Nano-Micro Lett. 2012;4:25–29. [Google Scholar]
- 187.Park C.S., Tae H.S. Improvement of MgO characteristics using RF-plasma treatment in AC plasma display panel. Mol. Cryst. Liq. Cryst. 2010;531:373–381. [Google Scholar]
- 188.Kaneko S., Funakubo H., Kadowaki T., Hirabayashi Y., Akiyama K. Cubic-on-cubic growth of a MgO(001) thin film prepared on Si(001) substrate at low ambient pressure by the sputtering method. Euro. Phys. Lett. 2008;81:46001. [Google Scholar]
- 189.Nakano T., Fujimoto T., Baba S. Measurement of surface roughness and ion-induced secondary electron emission coefficient of MgO films prepared by high-pressure sputter deposition. Vacuum. 2004;74:595–599. [Google Scholar]
- 190.Singh J.P., Kim S.H., Won S.O., Lee I.J., Chae K.H. Atomic-scale investigation of MgO growth on fused quartz using angle-dependent NEXAFS measurements. RSC Adv. 2018;8:31275–31286. doi: 10.1039/c8ra02873g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singh J.P., Kumar M., Lee I.J., Chae K.H. X-ray reflectivity and near edge X-ray absorption fine structure investigations of MgO thin films. Appl. Sci. Lett. 2017;3:47–52. [Google Scholar]
- 192.Singh J.P., Kumar M., Lim W.C., Lee H.H., Lee Y.M., Lee S., Chae K.H. MgO Thin Film Growth on Si(001) by Radio-Frequency Sputtering Method. J. Nanosci. Nanotechnol. 2020;20(12):7530–7534. doi: 10.1166/jnn.2020.18613. [DOI] [PubMed] [Google Scholar]
- 193.Singh J.P., Lim W.C., Lee I.J., Won S.O., Chae K.H. Surface structure of MgO thin films revealed from X-ray reflectivity and near-edge X-ray absorption fine structure measurements. Sci. Adv. Mater. 2018;10:1372–1376. [Google Scholar]
- 194.Singh V., Singh J.P., Chae K.H. 2019. Aging assisted hydroxide formation onto the surface of MgO thin films. 2019 International Forum on Functional Materials (IFFM 2019) 199-199, June 23-26, Gangneung City, Korea. [Google Scholar]
- 195.Singh J.P., Ji M.J., Kumar M., Lee I.J., Chae K.H. Unveiling the nature of adsorbed species onto the surface of MgO thin films during prolonged annealing. J. Alloys Compd. 2018;748:355–362. [Google Scholar]
- 196.Nelson R.L., Tench A.J. Chemisorption and surface defects in irradiated magnesium oxide. J. Chem. Phys. 1964;40:2736. [Google Scholar]
- 197.Singh J.P., Lim W.C., Lee J., Song J., Chae K.H. Surface and local electronic structure modification of MgO film using Zn and Fe ion implantation. Appl. Surf. Sci. 2018;432:131–139. [Google Scholar]
- 198.Wang W.B., Yang Y., Gil A.Y., Chang N.N., Girolami G.S., Abelson J.R. Highly conformal magnesium oxide thin films by low-temperature chemical vapor deposition from Mg(H3BNMe2BH3)2 and water. Appl. Phys. Lett. 2013;102:101605. [Google Scholar]
- 199.Ko J.B., Kim S.M. Preparation and electric characteristics of MgO films deposited by plasma-enhanced chemical vapor deposition. J. Ceram. Process. Res. 2009;10:643–646. [Google Scholar]
- 200.Sartori A., Habra N.E., Bolzan M., Rossetto G., Sitran S., Barreca D., Gasparotto A., Casarin M. Stability study of a magnesium β-diketonate as precursor for chemical vapor deposition of MgO. Chem. Mater. 2011;23:1113–1119. [Google Scholar]
- 201.Putkonen M., Sajavaara T., NiinistoÈ L. Enhanced growth rate in atomic layer epitaxy deposition of magnesium oxide thin films. J. Mater. Chem. 2000;10:1857–1861. [Google Scholar]
- 202.Vangelista S., Mantovan R., Lamperti A., Tallarida G., Kutrzeba-Kotowska B., Spiga S., Fanciulli M. Low-temperature atomic layer deposition of MgO thin films on Si. J. Phys. D Appl. Phys. 2013;46:485304. [Google Scholar]
- 203.Takahashi N. Simple and rapid synthesis of MgO with nano-cube shape by means of a domestic microwave oven. Solid State Sci. 2007;9:722–724. [Google Scholar]
- 204.Su Y., Wei H., Zhou Z., Yang Z., Wei L., Zhang Y. Rapid synthesis and characterization of magnesium oxide nanocubes via DC arc discharge. Mater. Lett. 2011;65:100–103. [Google Scholar]
- 205.Schneider J., Kollhoff F., Schindler T., Bichlmaier S., Bernardi J., Unruh T., Libuda J., Berger T., Diwald O. Adsorption, ordering, and metalation of porphyrins on MgO nanocube surfaces: the directional role of carboxylic anchoring groups. J. Phys. Chem. C. 2016;120:26879–26888. [Google Scholar]
- 206.Kollhoff F., Schneider J., Berger T., Diwald O., Libuda J. Thermally activated self-metalation of carboxy-functionalized porphyrin films on MgO nanocubes. ChemPhysChem. 2018;19:2272–2280. doi: 10.1002/cphc.201800152. [DOI] [PubMed] [Google Scholar]
- 207.Baumann S.O., Schneider J., Sternig A., Thomele D., Stankic S., Berger T., Grönbeck H., Diwald O. Size effects in MgO cube dissolution. Langmuir. 2015;192:2770–2776. doi: 10.1021/la504651v. [DOI] [PubMed] [Google Scholar]
- 208.McKenna K.P., Koller D., Sternig A., Siedl N., Govind N., Sushko P.V., Diwald O. Optical properties of nanocrystal interfaces in compressed MgO nanopowders. ACS Nano. 2011;5:3003–3009. doi: 10.1021/nn200062d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Knözinger E., Jacob K.H., Singh S., Hofmann P. Hydroxyl groups as IR active surface probes on MgO crystallites. Surf. Sci. 1993;290:388–402. [Google Scholar]
- 210.Issa I., Amodeo J., Re´thore´ J., Joly-Pottuz L., Esnouf C., Morthomas J., Perez M., Chevaliera J., Masenelli-Varlot K. In situ investigation of MgO nanocube deformation at room temperature. Acta Mater. 2015;86:295–304. [Google Scholar]
- 211.Heidenreich R. Electron reflections in MgO crystals with the electron microscope. Phys. Rev. 1942;62:291–292. [Google Scholar]
- 212.Senapati S., Nanda K.K. MgO nanocubes as self-calibrating optical probes for efficient ratiometric detection of picric acid in the solid state. ACS Sustain. Chem. Eng. 2018;6:13719–13729. [Google Scholar]
- 213.Thomele D., Bourret G.R., Bernardi J., Bockstedte M., Diwald O. Hydroxylation induced alignment of metal oxide nanocubes. Angew. Chem. Int. 2017;56:1407–1410. doi: 10.1002/anie.201608538. [DOI] [PubMed] [Google Scholar]
- 214.Niu K.Y., Yang J., Sun J., Du X.-W. One-step synthesis of MgO hollow nanospheres with blue emission. Nanotech. 2010;21:295604. doi: 10.1088/0957-4484/21/29/295604. 1-5. [DOI] [PubMed] [Google Scholar]
- 215.Dai D., Ma Q., Pei Y., Zhang Z., Yuan L. Template-free synthesis of nanoparticle-built MgO and Zn-doped MgO hollow microspheres with superior performance for Congo red adsorption from water. Dalton Trans. 2018;47:17421–17431. doi: 10.1039/c8dt03803a. [DOI] [PubMed] [Google Scholar]
- 216.Iizuka S., Muraoka T. Single-crystal MgO hollow nanospheres formed in RF impulse discharge plasmas. J. Nanomater. 2012;2012:691874. [Google Scholar]
- 217.Yin Y., Zhang G., Xia Y. Synthesis and characterization of MgO nanowires through a vapor phase precursor method. Adv. Funct. Mater. 2002;12:293–298. [Google Scholar]
- 218.Han S., Li C., Liu Z., Lei B., Zhang D., Jin W., Liu X., Tang T., Zhou C. Transition metal oxide core−shell nanowires: Generic synthesis and transport studies. Nano Lett. 2004;4:1241–1246. [Google Scholar]
- 219.Kim G., Martens R.L., Thompson G.B., Kim B.C., Gupta A. Selective area synthesis of magnesium oxide nanowires. J. Appl. Phys. 2007;102:104906. [Google Scholar]
- 220.Tang C., Bando Y., Sato T. Oxide-assisted catalytic growth of MgO nanowires with uniform diameter distribution. J. Phys. Chem. B. 2002;106:7449–7452. [Google Scholar]
- 221.Zhan J., Bando Y., Hu J., Golberg D. Bulk synthesis of single-crystalline magnesium oxide nanotubes. Inorg. Chem. 2004;43:2462–2464. doi: 10.1021/ic0351489. [DOI] [PubMed] [Google Scholar]
- 222.Lu B., Liao L., Li H., Tian Y., Li J.C., Wang D.F., Zhu B.P. Zn-assisted Synthesis and photoluminescence properties of MgO nanotubes. J. Phys. Chem. C. 2007;111:10273–10277. [Google Scholar]
- 223.Wang S.-F., Chen L.-Y., Zhang T., Xie Y. Charge modulation of magnetization in X-doped MgO nanotube clusters (X=C, N), Phys. E: low-dim. Syst. Nanostruct. 2016;76:135–139. [Google Scholar]
- 224.Janet M., Viswanathan B., Viswanath R.P., Varadarajan T.K. Characterization and photoluminescence properties of MgO microtubes synthesized from hydromagnesite flowers. J. Phys. Chem. C. 2007;111:10267–10272. [Google Scholar]
- 225.Peyghan A.A., Baei M.T., Hashemian S., Moghimi M. Adsorption of nitrous oxide on the (6,0) magnesium oxide nanotube. Chin. Chem. Lett. 2012;23:1275–1278. [Google Scholar]
- 226.Zhao M., Chen X.L., Wang W.J., Ma Y.J., Xu Y.P., Zhao H.Z. Self-catalyzed growth of magnesium oxide nanorods. Mater. Lett. 2016;60:2017–2019. [Google Scholar]
- 227.Nasibulin A.G., Sun L., Hämäläinen S., Shandakov S.D., Banhart F., Kauppinen E.I. In-situ TEM observation of MgO nanorod growth, Cryst. Growth Des. 2010;10:414–417. [Google Scholar]
- 228.Cui Z., Meng C.W., Huang W.D., Wang G.Z., Zhang L.D. Preparation and characterization of MgO nanorods. Mater. Res. Bull. 2000;35:1653–1659. [Google Scholar]
- 229.Jin C., Park S., Kim H., Lee C. Preparation and characterization of MgO nanorods sheathed with ZnS. J. Kor. Phys. Soc. 2012;61:847–851. [Google Scholar]
- 230.V S.Cvetković, N. M.Vukićević, N. D.Nikolić, Z. Baščarević, T. S.Barudžij, J. N. Jovićević, A possible mechanism of formation of flower-like MgO/Mg(OH)2 structures by galvanostatic molten salt electrolysis: the concept of local diffusion fields, J. Electroanal. Chem. 2019;842:168–175. [Google Scholar]
- 231.Abdallah Y., Ogunyemi S.O., Abdelazez A., Zhang M., Hong X., Ibrahim E. A.Hossain, H. Fouad, B. Li, J. Chen, the green synthesis of MgO nano-flowers using rosmarinus officinalis L. (Rosemary) and the antibacterial activities against Xanthomonas oryzae pv. oryzae, BioMed Res. Int. 2019;2019:5620989. doi: 10.1155/2019/5620989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Srivastava V., Sharma Y.C., Sillanpää M. Green synthesis of magnesium oxide nanoflower and its application for the removal of divalent metallic species from synthetic wastewater. Ceram. Int. 2015;41:6702–6709. [Google Scholar]
- 233.Zhang Y., CaO L., Xing G., Bai Z., Huang J., Zhang Z. Microscale flower like magnesium oxide for highly efficient photocatalytic degradation of organic dyes in aqueous solution. RSC Adv. 2019;9:7338–7348. doi: 10.1039/c8ra10385b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zulkefle Habibah, Arshad Adillah Nurashikin, Ismail Lyly Nyl, Bakar Rosli Abu, Rusop Mohamad. Synthesis and characterizations of sol-gel derived nano-MgO thin films. https://www.researchgate.net/publication/288412557_
- 235.Li H., Li M., Wang X., Wu X., Liu F., Yang B. Synthesis and optical properties of single-crystal MgO nanobelts. Mater. Lett. 2013;102–103:80–82. [Google Scholar]
- 236.İskenderoğlu D., Güney H. Effect of annealing on the structural, morphological and optical Properties of MgO nanowall structures grown by SILAR method. J. Electon. Mater. 2019;48:5850–5856. [Google Scholar]
- 237.Ling Z., Zheng M., Du Q., Wang Y., Song J., Dai W., Zhang L., Ji G., Cao J. Synthesis of mesoporous MgO nanoplate by an easy solvothermal–annealing method. Solid State Sci. 2011;13:2073–2079. [Google Scholar]
- 238.Duong T.H.Y., Nguyen T.N., Oanh H.T., Thi T.A.D., Giang L.N.T., Phuong H.T., Anh N.T., Nguyen B.M., Quang V.T., Le G.T., Nguyen T.V. Synthesis of magnesium oxide nanoplates and their application in nitrogen dioxide and sulfur dioxide adsorption. J. Chem. 2019;2019:4376429. [Google Scholar]
- 239.Hu J., Lifang Z.S., Yang C.H., Li J., Richards R. Adsorption properties of MgO(111) nanoplates for the dye pollutants from wastewater, Chem. Eng. Data. 2010;55:3742–3748. [Google Scholar]
- 240.Liu S., Wang Z., Han T., Fei T., Zhang T. Mesoporous magnesium oxide nanosheet electrocatalysts for the detection of Lead(II) ACS Appl. Nano Mater. 2019;2:2606–2611. [Google Scholar]
- 241.Yan C., Xue D. Novel self-assembled MgO nanosheet and its precursors. J. Phys. Chem. B. 2005;109:12358–12361. doi: 10.1021/jp050644z. [DOI] [PubMed] [Google Scholar]
- 242.Jin X., Yuan K., Lin X., Wang X., Zhang G., Zhu L., Que N., Xu D. Effects of water vapor on the crystallization and microstructure manipulation of MgO ceramic fibers. Ceram. Int. 2018;44:5257–5265. [Google Scholar]
- 243.Xu C., Yuan K., Jin X., Yu Z., Zheng L., Lü Y., Wang X., Zhu L., Zhang G., Xu D. High-temperature stable electrospun MgO nanofibers, formation mechanism and thermal properties. Ceram. Int. 2017;43:16210–16216. [Google Scholar]
- 244.Lee M.S., Lee S.Y., Noh D.Y., Kang H.C. X-ray-induced synthesis of Au nanoparticles on ZnO nanowire arrays. Mater. Chem. Phys. 2019;229:257–262. [Google Scholar]
- 245.Dhanunjaya M., Khan S.A., Pathak A.P., Avasthi D.K., Rao S.V.S.N. Ion induced crystallization and grain growth of hafnium oxide nano-particles in thin-films deposited by radio frequency magnetron sputtering. J. Phys. D Appl. Phys. 2017;50:505301. [Google Scholar]
- 246.Bharti A., Bhardwaj R., Agrawal A.K., Goyal N., Gautam S. Monochromatic X-ray induced novel synthesis of plasmonic nanostructure for photovoltaic application. Sci. Rep. 2016;6:22394. doi: 10.1038/srep22394. [DOI] [PMC free article] [PubMed] [Google Scholar]