Abstract
Climate change is likely to influence livestock production by increasing the prevalence of diseases, including parasites. The traditional practice of controlling nematodes in livestock by the application of anthelmintics is, however, increasingly compromised by the development of resistance to these drugs in parasite populations. This study used a previously developed simulation model of the entire equine cyathostomin lifecycle to investigate the effect a changing climate would have on the development of anthelmintic resistance. Climate data from six General Circulation Models based on four different Representative Concentration Pathways was available for three New Zealand locations. These projections were used to estimate the time resistance will take to develop in the middle (2040–49) and by the end (2090–99) of the century in relation to current (2006–15) conditions under two treatment scenarios of either two or six yearly whole-herd anthelmintic treatments. To facilitate comparison, a scenario without any treatments was included as a baseline. In addition, the size of the infective and parasitic stage nematode population during the third simulation year were estimated. The development of resistance varied between locations, time periods and anthelmintic treatment strategies. In general, the simulations indicated a more rapid development of resistance under future climates coinciding with an increase in the numbers of infective larvae on pasture and encysted parasitic stages. This was especially obvious when climate changes resulted in a longer period suitable for development of free-living parasite stages. A longer period suitable for larval development resulted in an increase in the average size of the parasite population with a larger contribution from eggs passed by resistant worms surviving the anthelmintic treatments. It is projected that climate change will decrease the ability to control livestock parasites by means of anthelmintic treatments and non-drug related strategies will become increasingly important for sustainable parasite control.
Keywords: Climate, Climate change, Anthelmintic resistance, Drug resistance, Cyathostomin, Parasitic nematodes
Graphical abstract
Highlights
-
•
The development of anthelmintic resistance under climate change was simulated.
-
•
Climate can become more suitable for parasite development, increasing population size.
-
•
The time resistance took to develop was linked to changes in parasite population size.
-
•
Non-drug related strategies will become increasingly important for parasite control.
1. Introduction
Climate change is likely to influence livestock production globally and challenge many existing production systems. The impacts on livestock could be direct such as heat stress on the animal, and/or indirect, such as greater exposure to infectious diseases (Rojas-Downing et al., 2017). Recent reports by the Intergovernmental Panel on Climate Change (2019) and the Food and Agriculture Organization of the United Nations (FAO, 2018) identified an increase in livestock diseases, including parasites, as a result of changing climate to have a negative impact on future food security. A common argument is that a rise in global temperature will lead to increased occurrence and alterations in the distribution of many infectious diseases (Epstein, 2001; Lafferty, 2009).
In many parts of the world dynamics of parasite populations are expected to change due to environmental conditions becoming more or less suitable for the development, survival and transmission of their infective stages (Hudson et al., 2006; Morgan et al., 2013; Pickles et al., 2013; Rose et al., 2015). An increased exposure of domestic animals to nematode parasite challenge will presumably put increased emphasis on control measures, which today are largely dependent on the administration of broad-spectrum anthelmintic drugs (Molento, 2009; Morgan et al., 2012). Currently, however, a serious problem for the control of nematode parasites is the development of resistance to these drugs, with resistance now common worldwide in parasites of livestock (Eddi et al., 1996; Van Wyk et al., 1999; Waghorn et al., 2006; Demeler et al., 2009; Sales and Love, 2016; McKenna, 2018) and other grazing animals such as horses (Peregrine et al., 2014). If parasite challenge increases concurrently with escalating issues of anthelmintic resistance, then the continued utility of drug-based parasite control must become doubtful. A greater understanding of the relationship between climate change, parasite populations and the development of drug resistance is, therefore, needed.
The recent development of a whole lifecycle simulation model for the cyathostomin species complex infecting horses Leathwick et al., 2019; Leathwick et al., 2015a, Leathwick et al., 2015b; Sauermann et al., 2019) enabled the investigation of the development of anthelmintic resistance under different treatment regimens and for different climatic locations (Leathwick et al., 2019; Nielsen et al., 2019). In these modelling studies the size of the parasite population increased, and anthelmintic resistance developed more rapidly in warmer climates when compared to colder climates. Here we extend that work to investigate the effect of climate change projections in climatically different locations to explore the likely effect of climate change on parasite population size and the development of anthelmintic resistance.
2. Materials and methods
2.1. The model
The development of the model and incorporation of genetic mechanisms for anthelmintic resistance have been described in detail elsewhere ( Leathwick et al., 2019; Leathwick et al., 2015; Sauermann et al., 2019). Briefly, the model replicates the dynamics of the cyathostomin free-living and parasitic stages, the administration of anthelmintics and the subsequent build-up of resistant genotypes. The dynamics of the free-living stages is subject to the influence of temperature and rainfall, while the parasitic stages are influenced by the host age (as a proxy for immunity) and exposure to infection (infective stage larvae ingested and adult worm burden). Little is known of the genetic mechanisms involved in anthelmintic resistance in equine cyathostomins (Nielsen et al., 2014), however, previous work has indicated that irrespective of the assumptions made regarding the number of genes involved and their dominance treatment strategies can consistently be ranked in order of rate of resistance development (Sauermann et al., 2019) i.e. the most selective strategy is always the most selective. For simplicity in the current study a single anthelmintic was utilised and resistance was assumed to be the result of a single gene mutation, represented by three genotypes, a homozygous susceptible (SS), a homozygous resistant (RR) and a heterozygote (RS) with an initial resistant R-allele frequency of 10−6. The anthelmintic efficacy was assumed to be 99%, 50% and 5% against adults and luminal larval stages of the SS, RS and RR genotypes, defining the inheritance of the resistance as intermediate under treatment. The efficacy against the SS genotype was based on data for ivermectin reported by Klei and Torbert (1980), Klei et al. (2001), and Lyons et al. (1980).
2.2. Herd structure and treatments
All simulations assumed a group of 16 horses (1–19.75 years old in increments of 1.25 years). Age is used as a proxy for horse immune status, which influences the values of several parameters (Leathwick et al., 2019), including the establishment of new infection and egg count levels. Thus, the 16 horses used in the simulations represent a range in immune status, between that of a naïve foal or yearling to a mature, immune competent horse. The age structure remained unchanged, assuming the composition of the herd stayed consistent throughout the 40-year simulation period. The model assumes a single grazing area of 1 ha per horse, cumulatively grazed throughout the year with a predetermined pasture cover and fixed daily dry matter intake of 8 kg by each horse.
The treatment strategies were chosen to represent a lower application scenario with two yearly treatments, administered every six months (days 180, 360), i.e. summer and winter, and a more intensive application scenario with six yearly treatments administered every second month (days 60, 120, 180, 240, 300, 360). To facilitate comparison, a scenario without any treatments was included as a baseline. Furthermore, there was no persistent activity of the anthelmintic included in the model and efficacy was only applied on the day of treatment. No mitigation strategies to delay the development of resistance were included to keep the focus solely on the effect of future climate conditions as such strategies have been investigated with the current model by the authors previously (Leathwick et al., 2019; Nielsen et al., 2019).
2.3. Climate data
Future climate projections (Tait et al., 2016) each spanning from 2006 to 2100 for three New Zealand locations were chosen, i.e. with increasing latitude the Waikato (warm humid summer, mild winter), Hawkes Bay (warm dry summer, mild winter) and Southland (mild summer, cool winter) regions. The available projections were based on six different General Circulation Models (GCM), which model the general circulation of earth's oceans and atmosphere and are used to forecast climate; i.e. CESM1-CAM5 (Neale et al., 2010), BCC-CSM1.1 (Wu et al., 2014), GFDL-CM3 (Griffies et al., 2011), GISS-E2-R (Schmidt et al., 2014), HadGEM2-ES (Martin et al., 2011) and NorESM1-M (Bentsen et al., 2013). These GCMs considered four different Representative Concentration Pathways (RCP) for radiatively active constituents (Moss et al., 2010), which describe different future pathways for the concentration of greenhouse gases, each representative for a larger set of published scenarios, and were agreed upon as the basis of climate change research by the IPCC and the science community in 2007 (van Vuuren et al., 2011); i.e. a scenario with high mitigation resulting in a peak and decline before 2100 (RCP2.6), two increasing scenarios of stabilization without overshoot after 2100 (RCP4.5 and RCP6.0), and a high emission scenario with continuous rise during the 21st century (RCP8.5). From the total 72 climate datasets (3 locations, 6 GCMs and 4 RCPs), 10-year subsets including daily min-max temperatures and rainfall for the periods 2006–2015, 2040–2049 and 2090–2099 were extracted, to investigate the climate effects for different time periods. Each of these subsets was repeated four times to allow for a simulation period of 40 years. A general summary of the forecasted climate change for each location and period under the various RCPs can be found in Table 1, which also includes the number of days being with a freeze-thaw cycle increasing the mortality of infective stage larvae in the model (Leathwick et al., 2015a). (See supplementary data for more information).
Table 1.
Overview of current and projected climate conditions. Yearly mean temperature and rainfall data are given for three New Zealand locations and four different Representative Concentration Pathways (RCP2.6 to RCP8.5) averaged across six General Circulation Models (GCM). Number of days with a freeze-thaw cycle, i.e. daily maximum above and minimum below 0 °C, resulting in a raised mortality rate of infective stage larvae. Means are calculated based on all RCP and GCM data for current (2006–15), and for all GCM data within each RCP for mid-century (2040–49) and end-century (2090–99) decades. Changes are given in brackets as difference to current condition.
| Location and Period | RCP | Temperature [°C] | Rainfall [mm] | days with freeze/thaw |
|---|---|---|---|---|
| Waikato | ||||
| Current | 14.1 | 1,217 | 8 | |
| Mid | 2.6 | 14.5 (0.4) | 1,197 (-20) | 5.7 (-2.2) |
| 4.5 | 14.7 (0.6) | 1,222 (5) | 5.8 (-2.1) | |
| 6.0 | 14.6 (0.5) | 1,219 (2) | 5.9 (-2) | |
| 8.5 | 14.8 (0.7) | 1,202 (-15) | 5.1 (-2.8) | |
| End | 2.6 | 14.4 (0.3) | 1,207 (-10) | 6.3 (-1.6) |
| 4.5 | 15.1 (1.0) | 1,177 (-40) | 3.9 (-4) | |
| 6.0 | 15.6 (1.5) | 1,178 (-39) | 2.6 (-5.3) | |
| 8.5 | 16.8 (2.7) | 1,167 (-50) | 1.3 (-6.6) | |
| Hawkes Bay | ||||
| Current | 13.9 | 749 | 12.4 | |
| Mid | 2.6 | 14.3 (0.4) | 754 (5) | 11.1 (-1.3) |
| 4.5 | 14.5 (0.6) | 749 (0) | 9.7 (-2.7) | |
| 6.0 | 14.3 (0.4) | 772 (23) | 11.3 (-1.1) | |
| 8.5 | 14.6 (0.7) | 737 (-12) | 10.7 (-1.7) | |
| End | 2.6 | 14.2 (0.3) | 756 (7) | 11.2 (-1.2) |
| 4.5 | 14.7 (0.8) | 724 (-25) | 9.9 (-2.5) | |
| 6.0 | 15.2 (1.3) | 744 (-5) | 7.2 (-5.2) | |
| 8.5 | 16.1 (2.2) | 695 (-54) | 5.2 (-7.2) | |
| Southland | ||||
| Current | 10.4 | 966 | 23.4 | |
| Mid | 2.6 | 10.7 (0.3) | 991 (25) | 19.2 (-4.3) |
| 4.5 | 10.8 (0.4) | 994 (28) | 19.1 (-4.4) | |
| 6.0 | 10.8 (0.4) | 979 (13) | 18.6 (-4.8) | |
| 8.5 | 10.9 (0.5) | 999 (13) | 17.1 (-6.4) | |
| End | 2.6 | 10.6 (0.2) | 971 (5) | 21 (-2.5) |
| 4.5 | 11.2 (0.8) | 990 (24) | 14.9 (-8.5) | |
| 6.0 | 11.5 (1.1) | 1,043 (77) | 12.1 (-11.3) | |
| 8.5 | 12.4 (2.0) | 1,090 (124) | 5.7 (-17.8) | |
2.4. Impact on the development of resistance
The main output of interest for each simulation was the time (in model years) until the efficacy fell below 90% for a period of at least 30 days. As the model allows for multiple parasitic worm populations (i.e. horses) and to avoid complications of short-term changes in allele frequency in the host following anthelmintic treatment, the calculation of efficacy was based on the R-allele frequency of the third stage larval population on pasture as outlined previously (Sauermann et al., 2019). The calculations followed the same assumptions about the genetic mechanisms of resistance as described above, with resistance defined as a <90% efficacy against the treated population.
2.5. Impact on parasite populations
To better understand the interplay between climate change, development of resistance, treatment strategy and parasite population size, additional model outputs were summarised and compared. For a single year (the third) of each simulation, two variables were tabulated; 1) the mean daily number of third stage larvae (L3) in faeces and on herbage (a measure for the free-living population) and 2) the mean daily number of early third stage larvae (EL3) encysted in the intestinal mucosal walls averaged across all horses as a measure of the parasitic phase population. The EL3 was chosen over the adult stage, as adult populations undergo considerable and rapid fluctuations with treatments and adult population size is limited by density dependent effects. Summarizing the results for the third simulation year ensures that the efficacy of the treatments is still relatively high, i.e. above the selected 90% efficacy threshold for resistance, and represent the effect of the treatment scenario. Calculating the results for later timepoints has the potential to confound the results as anthelmintic efficacy declines.
3. Results
3.1. Impact of current climate (between regions)
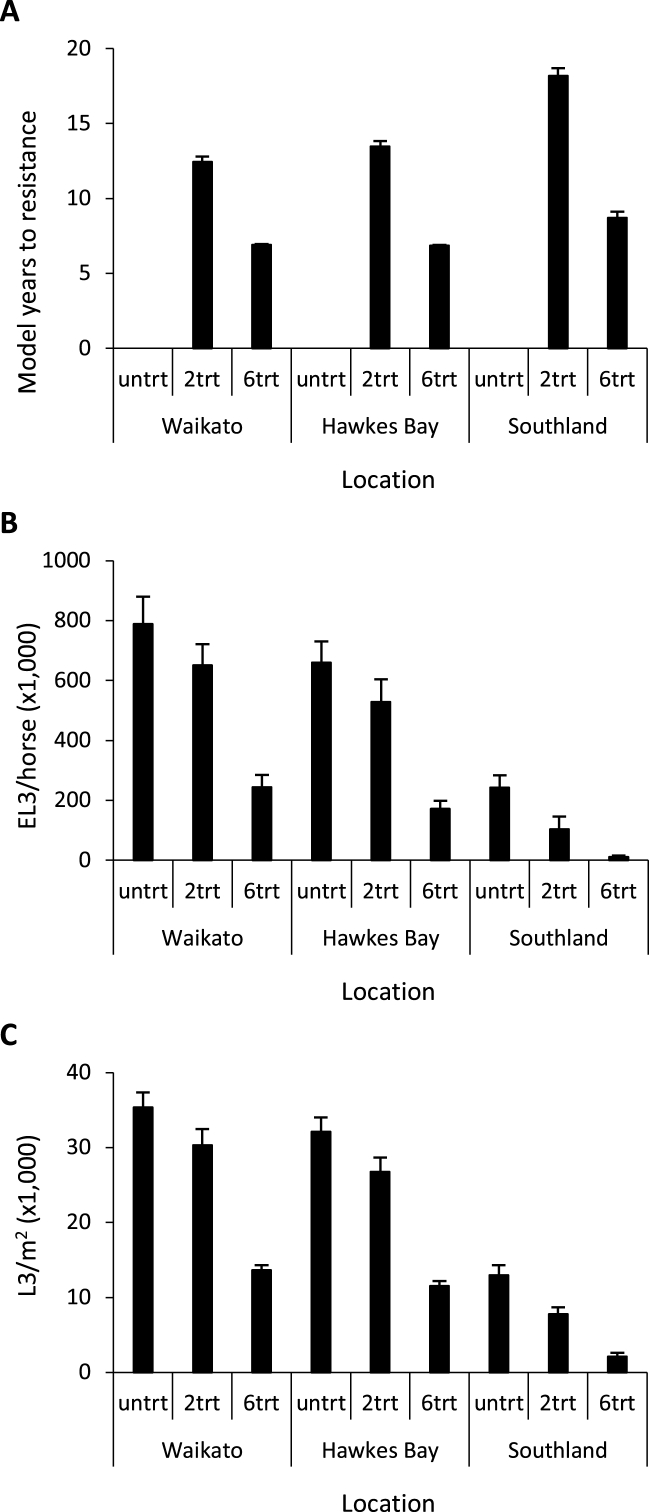
The development of resistance varied between locations, time periods and anthelmintic treatment strategies. Overall, when the horses were treated six times per year, anthelmintic resistance developed considerably faster than when the number of annual treatments was only two. For both anthelmintic treatment scenarios, resistance developed more slowly in Southland than in Waikato and Hawkes Bay (Fig. 1 A).
Fig. 1.
Summarised model output (+sd) based on current climate conditions (2006–2015) for the time anthelmintic resistance takes to develop (A), average number of encysted early third stage larvae (EL3) (B) and average number of infective stage larvae (L3) (C). Results were averaged across different Representative Concentration Pathways and General Circulation Models. For larvae the results were limited to the third simulation year as number of encysted early third stage larvae per horse per day or number of infective stage larvae in faeces and on herbage per m2 per day.
The comparison of the parasite populations also showed a pronounced difference in the parasitic EL3 and free-living L3 stages between locations (Fig. 1 B&C). In Southland, the population sizes were substantially lower than in the Waikato and Hawkes Bay.
3.2. Impact of future climate
When results for the periods of 2040–2049 and 2090–2099 were compared to 2006–2015, the time for resistance to develop was generally reduced, most notably for Southland (Fig. 2 A). In one case (the RCP2.6 scenarios) resistance developed faster for the 2040–2049 than for the 2090–2099 climate projections, but for all other RCPs development time decreased towards the end of the century. For Waikato and Hawkes Bay, this did not occur in the six annual treatment scenarios with changes being minimal and inconsistent.
Fig. 2.
Summarised model output (+/-sd) for future climate conditions (2040–2049 and 2090–2099) relative to current climate conditions (2006–2015) for the time anthelmintic resistance takes to develop (A), number of encysted third stage larvae (EL3) (B) and number of infective stage larvae (L3) (C). Representative Concentration Pathway (RCP) Relative results were calculated for individual General Circulation Models before averaged and for larvae limited to the third simulation year as average number of encysted third stage larvae per horse per day or average number of infective stage larvae per m2 per day.
Comparison of the parasitic EL3 and free-living L3 stages indicated an increasing trend towards the end of the century for all climate scenarios (Fig. 2 B, C). For the free-living L3 the data showed a consistent pattern of an increase in parasite numbers with increasing emission scenarios, which were highest for the Southland with up to 110% for the untreated and 370% for the six treatment per year scenario for RCP8.5 in 2090–2099. However, for the parasitic EL3, this pattern indicated some inconsistencies mainly around the RCP4.5 and RCP6.0 projections which were caused by large variation of the individual results of single GCMs. The Southland simulations showed the largest increase in population size for RCP8.5 in the 2090–2099 period, with 127% for the untreated scenario and a maximum increase of more than 1200% when applying six treatments per year.
Combining the results for all the simulations involving two yearly treatments and comparing the time to resistance versus the parasitic EL3 and free-living L3 populations shows a clear link between the size of the parasite population and the time resistance takes to develop (Fig. 3). The data indicates that the time for resistance to develop declines exponentially with the average size of the parasite population. However, there were differences between locations and between time periods within locations. The population size was greatest in the Waikato region, which at the same time showed the fastest development of resistance. When the population size was small, such as in Southland, the development of resistance was delayed. However, for all three locations a similar pattern was observed regarding how predicted climate change will influence the development of resistance in the three locations. The future climate projections indicated an increase in population sizes as well as an increase in the development of resistance. These changes were particularly notable with the higher RCP scenarios, i.e. RCP8.5 showed the highest increase in population sizes and development of resistance, whereas in case of RCP2.6 the increase was largest during the mid-century period.
Fig. 3.
T ime for anthelmintic resistance (<90% efficacy) to develop versus average daily numbers (year three) of encysted early third stage (EL3) larvae per horse (top row) or third stage larvae (L3) in the herbage per m2 grazing area (bottom row) for three different locations (increasing latitude to left), when all animals receive two treatments per year. Individual data points represent individual simulation results for different Representative Concentration Pathways and General Circulation Models f or three time-periods of either 2006–2015 (circle), 2040–2049 (plus) or 2090–2099 (triangle). Inserts display the complete datasets for all three locations with the same axis-limits.
4. Discussion
The purpose of this study was to investigate the likely influence of climate change scenarios on the dynamics of cyathostomin parasites of horses and the development of anthelmintic resistance. This analysis indicates that without mitigation, climate change is likely to accelerate development of anthelmintic resistance (Fig. 2 A). The data also indicated that this is likely to be more pronounced in some locations than in others.
Studies on the effects of climate change on pathogens or parasites predict a modification of occurrence and distribution range (Epstein, 2001; Lafferty, 2009; Molnár et al., 2013; Pickles et al., 2013). Overall, studies largely indicate a shift of parasite distribution into areas where climatic conditions are currently limiting. For parasitic nematodes with direct lifecycles, which are dependent on environmental conditions for the development and survival of their free-living stages, similar changes are likely (van Dijk et al., 2010; Hoar et al., 2012; Morgan et al., 2013). For example, predictions suggest an increase of infection pressure with the ruminant parasite Haemonchus contortus in northern Europe, with an extended yearly transmission period compared to current climate conditions (Rose et al., 2016). Other parasites, however, such as Ostertagia ostertagi, benefit from the current climate in some locations and may become adversely effected by predicted conditions, resulting in a drop of infection pressure (Rose et al., 2015). Nevertheless, the current study indicates that an increase in average temperature resulting in a longer period where conditions are suitable for development of free-living parasite stages will not only lead to a larger annual average parasite population, but also to a more rapid development of anthelmintic resistance. This will occur even if anthelmintic treatment intensity is unchanged in response to the higher worm challenge.
Changes in the Southland region were notably larger than those predicted in the more northerly (generally warmer) regions. The large increase in EL3 in Southland reflects the increase in larval ingestion due to the much better development of eggs on pasture. In the model the EL3 population is strongly influenced by the number and seasonal pattern of larvae on pasture (Leathwick et al., 2019) while the adult worm burden remains relatively constant. EL3 populations are known to be highly seasonally variable between animals and can reach enormous numbers (Nielsen et al., 2010). Thus the large effect in Southland likely reflects the fact that the initial average population sizes of the free-living herbage L3 and parasitic EL3 were lower than in the northern locations and, therefore, showed a larger increase under climate change, i.e. the more severe winters typical of this region were initially more limiting to parasite development and survival. By the end of the century the RCP8.5 climate prognosis for Southland specifies that the number of days per year with a minimum temperature of below 0 °C will change from currently 37 to 7 and for days with a maximum temperature above 25 °C from 7.6 to 24 (Ministry for the Environment, 2018), and days with a freeze-thaw cycle, causing increased mortality of infective larvae in terms of the model mechanics, will on average reduce from currently 23.4 to 5.7 (Table 1). The predicted milder Southland winters with less cold day events will create environmental conditions more suitable for the free-living parasite stages for a longer part of the year and give any worms that survive treatment a greater opportunity to successfully produce viable infective stage offspring (Leathwick et al., 2019). It follows then that the increased rate of development in anthelmintic resistance is linked to the changes in parasite dynamics, as shown in Fig. 3.
This may stand in contrast to some current perceptions about the role of the free-living parasite population as a source of refugia, e.g. that in practice an increase of the rate resistance develops is mainly due to a greater treatment frequency and that an increase in the size of the free-living population size should increase the refugia capacity counteracting the development of resistance. However, any parasite on pasture is only in refugia, if it successfully develops to the adult stage and produces viable offspring, otherwise it does not contribute to the population genetics and must be disregarded in terms of refugia. The increase in average pasture contamination over a 12-month period observed in the current simulations reflects improving conditions for the development of infective stage larvae. This applies to all genotypes equally. Under anthelmintic treatment the reproductive advantage to surviving adult worms is greater because the period over which eggs successfully develop is longer, hence there is a shift of successful reproduction in favour of resistant genotypes. Under the more favourable projected climate conditions and unchanged management regimes, the ratio of the reproductive rate of susceptible versus resistant parasites will shift towards the resistant parasites and the refugia capacity of the free-living parasite population will decrease.
As with previous studies (Leathwick et al., 2019) there was an association between treatment frequency and the development of anthelmintic resistance (Fig. 1), with resistance developing faster under six annual treatments than under two. With more anthelmintic treatments the proportion of resistant adult parasites in the host is increased as is the time over which no susceptible eggs are passed onto pasture. Furthermore, with an extended transmission period these resistant adult worms also have a higher likelihood to reproduce successfully to infective stage offspring, thereby escalating development of anthelmintic resistance. Because adult worm burden increases with the number of infective stage larvae ingested (Leathwick et al., 2019), higher survival and therefore numbers of free-living stages on pasture also increases the number of adult worms available to be selected for resistance by anthelmintic treatments. This can be seen in the positive relationship between the time resistance took to develop and the number of parasitic EL3 (as a measure for the parasitic phase) or free-living L3 population (as a measure for the free-living phase and reproductive success) (Fig. 3).
The likely dilemma ahead will be that if parasite populations, in many areas, are going to increase in size under climate change, horse owners and veterinarians are likely to respond by increasing the number of administered anthelmintic treatments, thereby making an already increasing problem of anthelmintic resistance, even worse. It is widely accepted that higher usage of anthelmintic drugs never has nor ever will constitute a sustainable solution, but only results in a faster selection for resistance (Kaplan and Vidyashankar, 2012). The solution will be to find ways in which parasite populations can be managed without more frequent administration of anthelmintic products. Mitigation strategies, that need to be explored and/or implemented (Cooper et al., 2015), include communication of revised and evidence based management strategies, e.g. maintaining an adequate parasite refugia (Barnes et al., 1995; Kaplan and Nielsen, 2010; Miller et al., 2012;Leathwick et al., 2012) and utilize selective treatment (Gomez and Georgi, 1991; Nielsen et al., 2019), ensuring the current resistance status is identified and effective treatments are used, including combination products (Leathwick et al., 2009; Dobson et al., 2011; Bartram et al., 2012; Leathwick, 2012; Miller et al., 2012), avoiding unnecessary use of drugs, e.g. long acting anthelmintics or prophylactic applications without evidence of necessity (Sutherland et al., 1997; Leathwick et al., 2009, Leathwick et al., 2015a, Leathwick et al., 2015b).
In conclusion, the study indicated that climate change will influence both the level of parasitism and development of anthelmintic resistance, however, the scale will vary with the magnitude of climate change and between climatically different locations. The results suggest that the changing environmental conditions in temperate climates will further lessen the ability to sustainably control livestock parasites merely by means of anthelmintic treatment. Non-drug related parasite control strategies will be increasingly necessary with predicted climate changes.
Declaration of competing interest
The project was supported by AgResearch SIFF funding through project number PRJ0155808. The authors declare that they have no conflict of interest.
Acknowledgements
The authors wish to acknowledge Abha Sood (NIWA) for compiling the initial climate datasets. Two anonymous referees made helpful comments on the draft manuscript. This project was supported by AgResearch SIFF funding through project number PRJ0155808.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2020.09.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Barnes E.H., Dobson R.J., Barger I.A. Worm control and anthelmintic resistance: adventures with a model. Parasitol. Today. 1995;11:56–63. doi: 10.1016/0169-4758(95)80117-0. [DOI] [PubMed] [Google Scholar]
- Bartram D.J., Leathwick D.M., Taylor M.A., Geurden T., Maeder S.J. The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet. Parasitol. 2012;186:151–158. doi: 10.1016/j.vetpar.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Bentsen M., Bethke I., Debernard J.B., Iversen T., Kirkevåg A., Seland O., Drange H., Roelandt C., Seierstad I.A., Hoose C., Kristjánsson J.E. The Norwegian earth system model, NorESM1-M – Part 1: description and basic evaluation of the physical climate. Geosci. Model Dev. (GMD) 2013;6:687–720. [Google Scholar]
- Cooper K.M., McMahon C., Fairweather I., Elliott C.T. Potential impacts of climate change on veterinary medicinal residues in livestock produce: an island of Ireland perspective††This paper is one of a series of reviews on “Climate Change and Food Safety – an Island of Ireland perspective”. Trends Food Sci. Technol. 2015;44:21–35. [Google Scholar]
- Demeler J., Van Zeveren A.M.J., Kleinschmidt N., Vercruysse J., Höglund J., Koopmann R., Cabaret J., Claerebout E., Areskog M., von Samson-Himmelstjerna G. Monitoring the efficacy of ivermectin and albendazole against gastro intestinal nematodes of cattle in Northern Europe. Vet. Parasitol. 2009;160:109–115. doi: 10.1016/j.vetpar.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Dobson R.J., Hosking B.C., Besier R.B., Love S., Larsen J.W.A., Rolfe P.F., Bailey J.N. Minimising the development of anthelmintic resistance, and optimising the use of the novel anthelmintic monepantel, for the sustainable control of nematode parasites in Australian sheep grazing systems. Aust. Vet. J. 2011;89:160–166. doi: 10.1111/j.1751-0813.2011.00703.x. [DOI] [PubMed] [Google Scholar]
- Eddi C., Caracostantogolo J., Peña M., Schapiro J., Marangunich L., Waller P.J., Hansen J.W. The prevalence of anthelmintic resistance in nematode parasites of sheep in Southern Latin America: Argentina. Vet. Parasitol. 1996;62:189–197. doi: 10.1016/0304-4017(95)00905-1. [DOI] [PubMed] [Google Scholar]
- Epstein P.R. Climate change and emerging infectious diseases. Microb. Infect. 2001;3:747–754. doi: 10.1016/s1286-4579(01)01429-0. [DOI] [PubMed] [Google Scholar]
- FAO . Food and Agriculture Organization of the United Nations; 2018. The Impact of Disasters and Crises on Agriculture and Food Security 2017. [Google Scholar]
- Gomez H.H., Georgi J.R. Equine helminth infections: control by selective chemotherapy. Equine Vet. J. 1991;23:198–200. doi: 10.1111/j.2042-3306.1991.tb02754.x. [DOI] [PubMed] [Google Scholar]
- Griffies S.M., Winton M., Donner L.J., Horowitz L.W., Downes S.M., Farneti R., Gnanadesikan A., Hurlin W.J., Lee H.C., Liang Z., Palter J.B., Samuels B.L., Wittenberg A.T., Wyman B.L., Yin J., Zadeh N. The GFDL CM3 coupled climate model: characteristics of the ocean and sea ice simulations. J. Clim. 2011;24:3520–3544. [Google Scholar]
- Hoar B.M., Ruckstuhl K., Kutz S. Development and availability of the free-living stages of Ostertagia gruehneri, an abomasal parasite of barrenground caribou (Rangifer tarandus groenlandicus), on the Canadian tundra. Parasitology. 2012;139:1093–1100. doi: 10.1017/S003118201200042X. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Cattadori I.M., Boag B., Dobson A.P. Climate disruption and parasite-host dynamics: patterns and processes associated with warming and the frequency of extreme climatic events. J. Helminthol. 2006;80:175–182. doi: 10.1079/joh2006357. [DOI] [PubMed] [Google Scholar]
- Intergovernmental Panel on Climate Change . 2019. Climate Change and Land - an IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems (Geneva, Switzerland) [Google Scholar]
- Kaplan R.M., Nielsen M.K. An evidence-based approach to equine parasite control: it ain't the 60s anymore. Equine Vet. Educ. 2010;22:306–316. [Google Scholar]
- Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Klei T.R., Rehbein S., Visser M., Langholff W.K., Chapman M.R., French D.D., Hanson P. Re-evaluation of ivermectin efficacy against equine gastrointestinal parasites. Vet. Parasitol. 2001;98:315–320. doi: 10.1016/s0304-4017(01)00436-8. [DOI] [PubMed] [Google Scholar]
- Klei T.R., Torbert B.J. Efficacy of ivermectin (22,23-dihydroavermectin B1) against gastrointestinal parasites in ponies. Am. J. Vet. Res. 1980;41:1747–1750. [PubMed] [Google Scholar]
- Lafferty K.D. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M. Modelling the benefits of a new class of anthelmintic in combination. Vet. Parasitol. 2012;186:93–100. doi: 10.1016/j.vetpar.2011.11.050. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Donecker J.M., Nielsen M.K. A model for the dynamics of the free-living stages of equine cyathostomins. Vet. Parasitol. 2015;209:210–220. doi: 10.1016/j.vetpar.2015.02.031. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Hosking B.C., Bisset S.A., McKay C.H. Managing anthelmintic resistance: is it feasible in New Zealand to delay the emergence of resistance to a new anthelmintic class? N. Z. Vet. J. 2009;57:181–192. doi: 10.1080/00480169.2009.36900. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Miller C.M., Fraser K. Selection for anthelmintic resistant Teladorsagia circumcincta in pre-weaned lambs by treating their dams with long-acting moxidectin injection. Int. J. Parasitol.: Drugs and Drug Resistance. 2015;5:209–214. doi: 10.1016/j.ijpddr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathwick D.M., Sauermann C.W., Nielsen M.K. Managing anthelmintic resistance in cyathostomin parasites: investigating the benefits of refugia-based strategies. Int. J. Parasitol.: Drugs and Drug Resistance. 2019;10:118–124. doi: 10.1016/j.ijpddr.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leathwick D.M., Sauermann C.W., Reinemeyer C.R., Nielsen M.K. A model for the dynamics of the parasitic stages of equine cyathostomins. Vet. Parasitol. 2019;268:53–60. doi: 10.1016/j.vetpar.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Drudge J.H., Tolliver S.C. Antiparasitic activity of ivermectin in critical tests in equids. Am. J. Vet. Res. 1980;41:2069–2072. [PubMed] [Google Scholar]
- Martin G.M., Bellouin N., Collins W.J., Culverwell I.D., Halloran P.R., Hardiman S.C., Hinton T.J., Jones C.D., McDonald R.E., McLaren A.J., O'Connor F.M., Roberts M.J., Rodriguez J.M., Woodward S., Best M.J., Brooks M.E., Brown A.R., Butchart N., Dearden C., Derbyshire S.H., Dharssi I., Doutriaux-Boucher M., Edwards J.M., Falloon P.D., Gedney N., Gray L.J., Hewitt H.T., Hobson M., Huddleston M.R., Hughes J., Ineson S., Ingram W.J., James P.M., Johns T.C., Johnson C.E., Jones A., Jones C.P., Joshi M.M., Keen A.B., Liddicoat S., Lock A.P., Maidens A.V., Manners J.C., Milton S.F., Rae J.G.L., Ridley J.K., Sellar A., Senior C.A., Totterdell I.J., Verhoef A., Vidale P.L., Wiltshire A. The HadGEM2 family of met office unified model climate configurations. Geosci. Model Dev. (GMD) 2011;4:723–757. [Google Scholar]
- McKenna P. VetScript (New Zealand Veterinary Association) 2018. Update anthelmintic reistance; pp. 44–45. [Google Scholar]
- Miller C.M., Waghorn T.S., Leathwick D.M., Candy P.M., Oliver A.M.B., Watson T.G. The production cost of anthelmintic resistance in lambs. Vet. Parasitol. 2012;186:376–381. doi: 10.1016/j.vetpar.2011.11.063. [DOI] [PubMed] [Google Scholar]
- Ministry for the Environment . second ed. Ministry for the Environment); Wellington: 2018. Climate Change Projections for New Zealand: Atmosphere Projections Based on Simulations from the IPCC Fifth Assessment. [Google Scholar]
- Molento M.B. Parasite control in the age of drug resistance and changing agricultural practices. Vet. Parasitol. 2009;163:229–234. doi: 10.1016/j.vetpar.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Molnár P.K., Dobson A.P., Kutz S.J. Gimme shelter - the relative sensitivity of parasitic nematodes with direct and indirect life cycles to climate change. Global Change Biol. 2013;19:3291–3305. doi: 10.1111/gcb.12303. [DOI] [PubMed] [Google Scholar]
- Morgan E.R., Charlier J., Hendrickx G., Biggeri A., Catalan D., Von Samson-Himmelstjerna G., Demeler J., Müller E., Van Dijk J., Kenyon F., Skuce P., Höglund J., Kiely P., Van Ranst B., De Waal T., Rinaldi L., Cringoli G., Hertzberg H., Torgerson P., Wolstenholme A., Vercruysse J. Global change and helminth infections in grazing ruminants in Europe: impacts, trends and sustainable solutions. Agriculture. 2013;3:484–502. [Google Scholar]
- Morgan E.R., Hosking B.C., Burston S., Carder K.M., Hyslop A.C., Pritchard L.J., Whitmarsh A.K., Coles G.C. A survey of helminth control practices on sheep farms in Great Britain and Ireland. Vet. J. 2012;192:390–397. doi: 10.1016/j.tvjl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Moss R.H., Edmonds J.A., Hibbard K.A., Manning M.R., Rose S.K., Van Vuuren D.P., Carter T.R., Emori S., Kainuma M., Kram T., Meehl G.A., Mitchell J.F.B., Nakicenovic N., Riahi K., Smith S.J., Stouffer R.J., Thomson A.M., Weyant J.P., Wilbanks T.J. The next generation of scenarios for climate change research and assessment. Nature. 2010;463:747–756. doi: 10.1038/nature08823. [DOI] [PubMed] [Google Scholar]
- Neale R.B., Chen C.-C., Gettelman A., Lauritzen P.H., Park S., Williamson D.L., Conley A.J., Garcia R., Kinnison D., Lamarque J.-F. vol. 1. Note NCAR/TN-486+ STR; 2010. Description of the NCAR community atmosphere model (CAM 5.0) pp. 1–12. (NCAR Tech). [Google Scholar]
- Nielsen M.K., Baptiste K.E., Tolliver S.C., Collins S.S., Lyons E.T. Analysis of multiyear studies in horses in Kentucky to ascertain whether counts of eggs and larvae per gram of feces are reliable indicators of numbers of strongyles and ascarids present. Vet. Parasitol. 2010;174:77–84. doi: 10.1016/j.vetpar.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Reinemeyer C.R., Donecker J.M., Leathwick D.M., Marchiondo A.A., Kaplan R.M. Anthelmintic resistance in equine parasites-Current evidence and knowledge gaps. Vet. Parasitol. 2014;204:55–63. doi: 10.1016/j.vetpar.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Sauermann C.W., Leathwick D.M. The effect of climate, season, and treatment intensity on anthelmintic resistance in cyathostomins: a modelling exercise. Vet. Parasitol. 2019;269:7–12. doi: 10.1016/j.vetpar.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic resistance in important parasites of horses: does it really matter? Vet. Parasitol. 2014;201:1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Pickles R.S.A., Thornton D., Feldman R., Marques A., Murray D.L. Predicting shifts in parasite distribution with climate change: a multitrophic level approach. Global Change Biol. 2013;19:2645–2654. doi: 10.1111/gcb.12255. [DOI] [PubMed] [Google Scholar]
- Rojas-Downing M.M., Nejadhashemi A.P., Harrigan T., Woznicki S.A. Climate change and livestock: impacts, adaptation, and mitigation. Climate Risk Management. 2017;16:145–163. [Google Scholar]
- Rose H., Caminade C., Bolajoko M.B., Phelan P., van Dijk J., Baylis M., Williams D., Morgan E.R. Climate-driven changes to the spatio-temporal distribution of the parasitic nematode, Haemonchus contortus, in sheep in Europe. Global Change Biol. 2016;22:1271–1285. doi: 10.1111/gcb.13132. [DOI] [PubMed] [Google Scholar]
- Rose H., Wang T., van Dijk J., Morgan E.R. GLOWORM-FL: a simulation model of the effects of climate and climate change on the free-living stages of gastro-intestinal nematode parasites of ruminants. Ecol. Model. 2015;297:232–245. [Google Scholar]
- Sales N., Love S. Resistance of Haemonchus sp. to monepantel and reduced efficacy of a derquantel/abamectin combination confirmed in sheep in NSW, Australia. Vet. Parasitol. 2016;228:193–196. doi: 10.1016/j.vetpar.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Sauermann C.W., Nielsen M.K., Luo D., Leathwick D.M. Modelling the development of anthelmintic resistance in cyathostomin parasites: the importance of genetic and fitness parameters. Vet. Parasitol. 2019;269:28–33. doi: 10.1016/j.vetpar.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Schmidt G.A., Kelley M., Nazarenko L., Ruedy R., Russell G.L., Aleinov I., Bauer M., Bauer S.E., Bhat M.K., Bleck R., Canuto V., Chen Y.H., Cheng Y., Clune T.L., Del Genio A., De Fainchtein R., Faluvegi G., Hansen J.E., Healy R.J., Kiang N.Y., Koch D., Lacis A.A., Legrande A.N., Lerner J., Lo K.K., Matthews E.E., Menon S., Miller R.L., Oinas V., Oloso A.O., Perlwitz J.P., Puma M.J., Putman W.M., Rind D., Romanou A., Sato M., Shindell D.T., Sun S., Syed R.A., Tausnev N., Tsigaridis K., Unger N., Voulgarakis A., Yao M.S., Zhang J. Configuration and assessment of the GISS ModelE2 contributions to the CMIP5 archive. J. Adv. Model. Earth Syst. 2014;6:141–184. [Google Scholar]
- Sutherland I.A., Leathwick D.M., Brown A.E., Miller C.M. Prophylactic efficacy of persistent anthelmintics against challenge with drug-resistant and susceptible Ostertagia circumcincta. Vet. Rec. 1997;141:120–123. doi: 10.1136/vr.141.5.120. [DOI] [PubMed] [Google Scholar]
- Tait A., Sood A., Mullan B., Stuart S., Bodeker G., Kremser S., Lewis J. 2016. Updated Climate Change Projections for New Zealand for Use in Impact Studies. Synthesis Report RA1, Climate Changes, Impacts and Implications (CCII) for New Zealand to 2100. [Google Scholar]
- van Dijk J., Sargison N.D., Kenyon F., Skuce P.J. Climate change and infectious disease: helminthological challenges to farmed ruminants in temperate regions. Animal. 2010;4:377–392. doi: 10.1017/S1751731109990991. [DOI] [PubMed] [Google Scholar]
- van Vuuren D.P., Edmonds J., Kainuma M., Riahi K., Thomson A., Hibbard K., Hurtt G.C., Kram T., Krey V., Lamarque J.-F., Masui T., Meinshausen M., Nakicenovic N., Smith S.J., Rose S.K. The representative concentration pathways: an overview. Climatic Change. 2011;109:5. [Google Scholar]
- Van Wyk J.A., Stenson M.O., Van Der Merwe J.S., Vorster R.J., Viljoen P.G. Anthelmintic resistance in South Africa: surveys indicate an extremely serious situation in sheep and goat farming. Onderstepoort J. Vet. Res. 1999;66:273–284. [PubMed] [Google Scholar]
- Waghorn T.S., Leathwick D.M., Rhodes A.P., Jackson R., Pomroy W.E., West D.M., Moffat J.R. Prevalence of anthelmintic resistance on 62 beef cattle farms in the North Island of New Zealand. N. Z. Vet. J. 2006;54:278–282. doi: 10.1080/00480169.2006.36711. [DOI] [PubMed] [Google Scholar]
- Wu T., Song L., Li W., Wang Z., Zhang H., Xin X., Zhang Y., Zhang L., Li J., Wu F., Liu Y., Zhang F., Shi X., Chu M., Zhang J., Fang Y., Wang F., Lu Y., Liu X., Wei M., Liu Q., Zhou W., Dong M., Zhao Q., Ji J., Li L., Zhou M. An overview of BCC climate system model development and application for climate change studies. Journal of Meteorological Research. 2014;28:34–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.